Abstract
Monkeypox (MPXV) and cowpox (CPXV) are emerging agents that cause severe human infections on an intermittent basis, and variola virus (VARV) has potential for use as an agent of bioterror. Vaccinia immune globulin (VIG) has been used therapeutically to treat severe orthopoxvirus infections but is in short supply. We generated a large panel of orthopoxvirus-specific human monoclonal antibodies (Abs) from immune subjects to investigate the molecular basis of broadly neutralizing antibody responses for diverse orthopoxviruses. Detailed analysis revealed the principal neutralizing antibody specificities that are cross-reactive for VACV, CPXV, MPXV, and VARV and that are determinants of protection in murine challenge models. Optimal protection following respiratory or systemic infection required a mixture of Abs that targeted several membrane proteins, including proteins on enveloped and mature virion forms of virus. This work reveals orthopoxvirus targets for human Abs that mediate cross-protective immunity and identifies new candidate Ab therapeutic mixtures to replace VIG.
Keywords: smallpox vaccine, poxvirus infections, human monoclonal antibodies, antigen specificity, cross-neutralization, protective immunity
Graphical Abstract

Protective immunity against smallpox and other members of the orthopoxvirus family requires the cooperation of antibodies that target different viral proteins at distinct stages of maturation of the virus.
Introduction
Naturally occurring members of the Orthopoxvirus genus, cowpox virus (CPXV), monkeypox virus (MPXV), and variola virus (VARV), cause severe infections in humans. VARV exclusively causes human infections, with an estimated 300–500 million deaths during the 20th century before the initiation of the global smallpox vaccination campaign (Smith and McFadden, 2002). MPXV and CPXV are emerging zoonotic infections with a sporadic occurrence worldwide (McCollum et al., 2015, Reed et al., 2004, Vorou et al., 2008). There is no licensed specific treatment for these infections, and the only method of prevention is vaccination using vaccinia virus (VACV). Vaccination against smallpox was discontinued in the late 1970s, leaving a large proportion of the current human population vulnerable to orthopoxviruses. The fear that smallpox could potentially re-emerge following a bioterror or biowarfare action (Smith and McFadden, 2002), the sporadic outbreaks of zoonotic MPXV and CPXV, and the increasing prevalence of immunocompromised individuals who cannot be vaccinated safely (Kemper et al., 2002), has stimulated renewed interest in research on orthopoxvirus protective immunity and treatment.
Orthopoxviruses have a large and complex proteome containing over 200 proteins. During infection, the virus exists in two antigenically distinct forms, designated mature virions (MV) or enveloped virions (EV), which contain ∼25 or 6 surface proteins, respectively (Moss, 2011). MPXV and VARV are select agents and subject to the U.S. select agent regulation 42 CFR part 73. Various orthopoxvirus species share many genetic and antigenic features (Hughes et al., 2010, Ichihashi and Oie, 1988, Stanford et al., 2007), and an infection with an orthopoxvirus of any one species may confer substantial protection against infection with the other orthopoxviruses (McConnell et al., 1964). Vaccination with VACV protects against disease caused by VARV, MPXV, or CPXV (Hammarlund et al., 2005). The immunologic mechanisms underlying cross-protection by immunization with VACV likely are diverse, but include neutralizing antibodies (Moss, 2011). A critical role for antibodies (Abs) in orthopoxvirus immunity was suggested by historical cases in which passive transfer of serum from VARV- or VACV-immune subjects protected exposed individuals against smallpox (Kempe et al., 1961). Recent studies in non-human primate or murine models of experimental infection showed that polyclonal Abs are necessary and sufficient for protection against lethal challenge with MPXV or VACV (Belyakov et al., 2003, Edghill-Smith et al., 2005). The level of neutralizing activity in immune serum is thought to be the best laboratory predictor of protective immunity to orthopoxvirus infections in humans (Mack et al., 1972). Human vaccinia immune globulin (VIG) has been used for the prevention and treatment of some smallpox and vaccine-related complications with limited success (Wittek, 2006); Food and Drug Administration (FDA) indications only address use for complications following vaccinia inoculation. The level of efficacy is uncertain due to lot-to-lot variation in potency and a lack of understanding of the molecular determinants of protection.
Percutaneous inoculation with VACV elicits a broad and heterogeneous serum Ab response that targets a large number of antigenic determinants of VACV (Davies et al., 2005a, Davies et al., 2007). The viral inhibitory activity of serum from immune subjects with cross-neutralizing activity to VACV, MPXV, and VARV likely is composed of Abs to diverse specificities (Hughes et al., 2012, Kennedy et al., 2011). Abs in VIG recognize many antigen targets, including surface proteins of both EV and MV virion forms of VACV (Davies et al., 2005a). Study of polyclonal Abs in orthopoxvirus-immune sera of rabbits revealed the pattern of recognition for each orthopoxvirus was unique, but also suggested that different orthopoxvirus species shared common neutralizing determinants (Baxby, 1982). Studies in murine infection models identified targets for neutralizing and protective mouse monoclonal Abs (mAbs), which included the MV surface proteins A27, L1, H3, D8, A28, A13, A17, and the EV surface proteins B5 and A33 (Moss, 2011). Protection of mice against systemic and respiratory infection with murine Abs required clones specific to antigens of both MV and EV forms of VACV (Lustig et al., 2005). These studies suggest complex patterns of recognition by Abs protecting against infection and disease in experimental animal models, but the molecular basis for neutralization and cross-reactive orthopoxvirus immunity in humans is poorly understood. Moreover, the principal mAb specificities that are necessary and sufficient for protective immunity to orthopoxviruses in the human B cell response remain unknown.
Here, we report the isolation of large numbers of naturally occurring human mAbs from the blood cells of human subjects with a history of prior orthopoxvirus vaccination or infection. We used these mAbs to determine the basis for potent neutralizing Ab activity and breadth of cross-reactivity to VACV, CPXV, MPXV, and VARV. The data reveal Ab specificities contributing to optimal levels of cross-neutralizing potency. We also identified antibody activities in in vitro assays that serve as correlates of protection for the extent of in vivo protection observed in small animal models of lethal infection. The studies show that the critical features of Ab-mediated protection against orthopoxvirus infection include the need for a mix of Abs that recognizes both EV and MV forms of virion particles, a mixture of Abs to diverse proteins on each of those particle forms, and the presence of complement. The studies also suggest novel potent and broadly neutralizing candidate mixtures of mAbs for therapeutic use in humans to replace VIG.
Results
Orthopoxvirus Infection in Humans Elicits a Complex B Cell Response Encoding Large Numbers of Clones Reactive with Antigens from Diverse Orthopoxvirus Species
We obtained peripheral blood mononuclear cells (PBMCs) from a donor who had recovered from a naturally occurring MPXV infection or from otherwise healthy subjects previously immunized with one of three different vaccine formulations (Table S1), IMVAMUNE (live attenuated modified vaccinia Ankara virus), Dryvax (a freeze-dried calf lymph produced vaccinia virus), or ACAM2000 (Vero cell culture produced vaccinia virus) (Verardi et al., 2012). To identify orthopoxvirus-specific B cell cultures, PBMCs were transformed with Epstein-Barr virus, and the supernatants from the resulting lymphoblastoid cell lines were screened by ELISA for binding to orthopoxvirus antigens. Hybridomas secreting human antigen-specific mAbs were generated from B cell lines secreting virus-specific antibodies, as previously described (Crowe, 2009). Because orthopoxviruses have so many protein components, we used complementary screening approaches to identify orthopoxvirus-specific mAbs. In one approach, we assessed hybridoma cell line supernatants for reactivity to each of 12 recombinant VACV protein antigens designated A21, A27, A28, A33, B5, D8, F9, J5, H2, H3, L1, and L5. The A33 and B5 proteins are surface antigens on the EV form of virus, while the remaining ten proteins are surface antigens on MV particles. In addition, we also screened for mAbs that bind to inactivated lysates of VACV-, CPXV-, or MPXV-infected cell monolayer cultures.
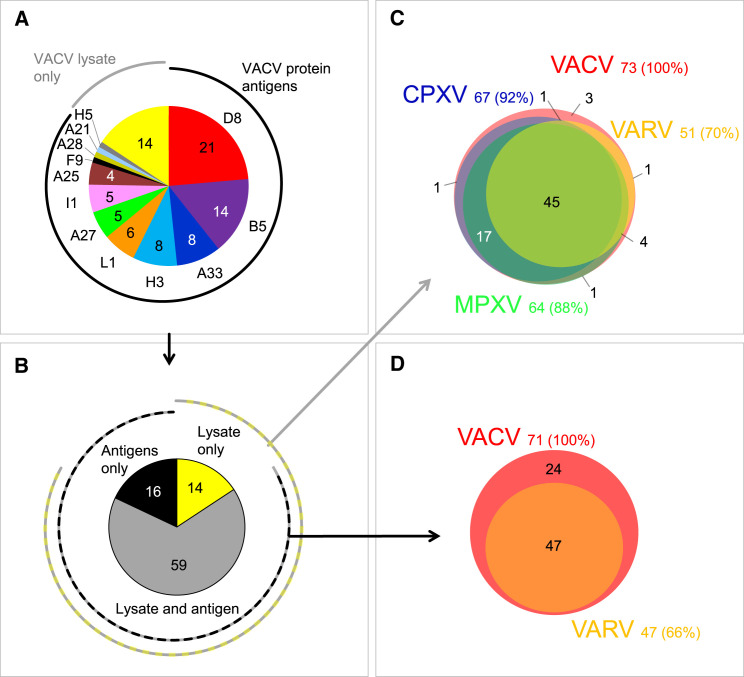
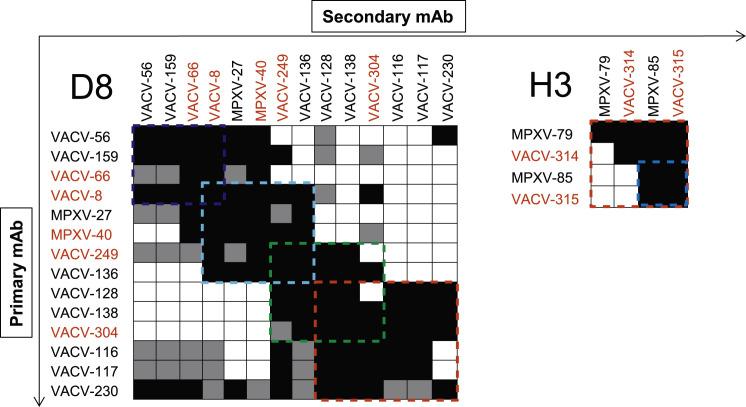
A total of 89 cloned hybridoma cell lines secreting human mAbs was isolated, including 44 lines from vaccinees and 45 from the donor with a history of MPXV infection (Table S1). The 89 mAbs were independent clones that displayed a high degree of sequence diversity, including a unique HCDR3 sequence for each mAb (Table S2). Thirty-two mAbs in the panel bound in ELISA to inactivated VACV-infected cell lysates only and thus their protein antigen specificity was uncertain initially. Binding of these mAbs was reassessed using VACV protein antigen microarrays, which revealed additional mAbs specific to D8, H3 A21, A25, H5, and I1 VACV proteins. Therefore, the mAb panel contained Abs to at least 12 antigens: D8, B5, A33, H3, L1, A27, I1, A25, F9, A28, A21, and H5 (Figure 1 A). The majority (62 of 89 [70%]) of purified mAbs reacted to one of six VACV antigens that were reported previously as major targets for neutralizing Abs in mice or humans (Moss, 2011), specifically A27, H3, D8, L1, B5, and A33. Sixteen percent (14 of 89) of mAbs in the panel reacted with VACV-infected cell lysate but not with a recombinant protein antigen, therefore they remain of unknown specificity (Figure 1A). MAbs that targeted the antigens VACV D8 and B5 were over-represented in the panel (35 of 75 mAbs) accounting for 47% of mAbs with known antigen specificity. Further analysis revealed several competition-binding groups among Ab specificities that bind to H3 or D8 antigens (Figure S1 ), indicating the presence of mAbs to several antigenic sites on these antigens.
Figure 1.
Panel of Orthopoxvirus-Specific Human MAbs
A panel of 89 human mAbs was generated based on reactivity to VACV-infected cell lysate or to VACV protein antigens. Individual mAbs were assessed for cross reactivity using CPXV, MPXV, and VARV-infected cell lysates or antigens.
(A) Antigen specificity of purified mAbs. Reactivity of Abs of unknown antigen specificity that bound to inactivated VACV-infected cell lysate only is designated as “VACV lysate only.”
(B) Representation of mAbs in the panel from (A) that bound only to VACV-infected cell lysate, recombinant VACV proteins, or both, VACV infected cell lysate and recombinant VACV proteins. See also Tables S3 and S4.
(C) Cross-reactivity of mAbs that bound to VACV lysates from (B) to VACV-, CPXV-, MPXV-, or VARV-infected cell lysates. See also Figure S2.
(D) Cross-reactivity of mAbs that bound to VACV antigens from (B) to the respective 12 ortholog proteins of VARV. Four mAbs with low expression were not tested.
Figure S1.
Human Anti-D8 and Anti-H3 MAbs Targeted Diverse Epitopes of the Major VACV Surface Antigens, Related to Figure 1
Anti-D8 and anti-H3 mAbs from our panel were assessed for competitive binding to D8 and H3 proteins by biolayer interferometry. MAbs were judged to compete for the same site if maximum binding of second antibody was reduced to ≤ 39% of its un-competed binding (shown in black boxes). The mAbs were considered non-competing if maximum binding of second mAb was ≥ 61% of its un-competed binding (shown in white boxes). Gray boxes indicate an intermediate phenotype (competition resulted in between 40% and 60% of un-competed binding). Blue, yellow, cyan, green, and red dashed lines indicate designated competition groups. Antibodies that were selected for in vivo protection studies shown in red color. Two anti-H3 mAbs, MPXV-72 and MPXV-1, did not bind to antigen in biolayer interferometry and were distinguished as separate group from other anti-H3 mAbs.
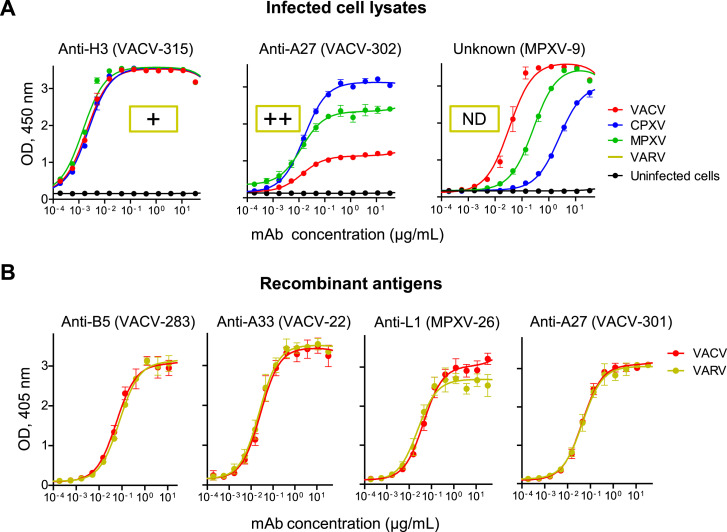
We next assessed the cross-reactivity of individual VACV-reactive mAbs to CPXV, MPXV, or VARV by testing binding to CPXV-, MPXV-, or VARV-infected cell lysates or to recombinant VARV protein antigens that are orthologs of the identified VACV targets. A large fraction (45 of 73 [62%]) of mAbs that bound to VACV antigens in virus-infected cell lysate (Figure 1B) bound in a cross-reactive manner to the virus-infected lysates of all four Orthopoxvirus species tested, and the majority (70 of 73 [96%]) of mAbs cross-reacted with at least two orthopoxviruses (Figure 1C; Table S3). Remarkably, a large fraction (47 of 71 tested [66%]) of the mAbs with an established protein antigen specificity for VACV cross-reacted with the orthologous VARV antigens (Figure 1D; Table S3). The mAbs bound to recombinant antigens and/or infected cell lysates in a concentration-dependent manner (Figure S2 and data not shown), and the majority of them possessed half maximal effective concentration (EC50) binding values of 1 μg/mL or lower, confirming their antigen-specific binding phenotype (Table S4). Therefore, the majority of mAbs in the panel exhibited binding patterns that suggested the potential to neutralize several Orthopoxvirus species that are infectious for humans.
Figure S2.
Cross-Reactivity of Human MAbs to Orthopoxviruses, Related to Figure 1
Cross-reactivity of individual mAbs to different orthopoxviruses were assessed by ELISA using infected cell lysates or purified recombinant protein antigens.
(A) Examples of mAbs within the panel that exhibited cross-reactivity to VACV, CPXV, MPXV, and VARV-infected cell lysates. Reactivity to VARV-infected cell lysate was measured at single mAb dilution as detailed in Table S3, ND indicates not determined.
(B) Examples of four mAbs within the panel that exhibited cross-reactivity to VACV and VARV protein antigen orthologs. These four mAbs were included in mAb mixtures Mix4 and Mix6, which later were assessed for protective capacity in vivo. Data represent one of two independent experiments, shown as mean ± SD of assay triplicates.
The Majority of Human Neutralizing mAbs Recognized One of Six Antigens and Exhibited Cross-Neutralization for Several Orthopoxvirus Species
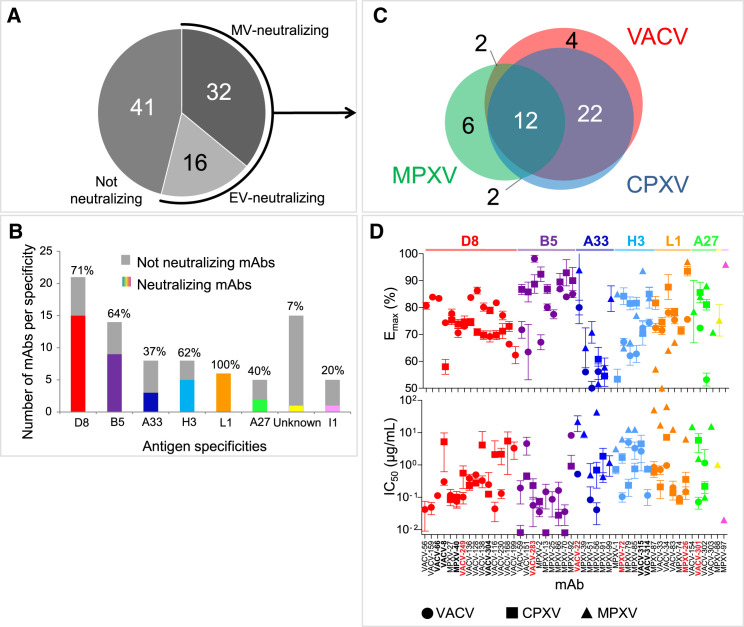
We next tested the mAbs in virus neutralization assays using MV or EV forms of VACV, CPXV, or MPXV. Neutralization potency of mAbs was assessed based on the half maximal inhibitory concentration (IC50) and the maximum of neutralization effect (Emax) values. More than half (48 of 89 [54%]) of the mAbs possessed neutralizing activity (Emax ≥ 50%) at 100 μg/mL or lower concentration for at least one orthopoxvirus; 16 or 32 mAbs neutralized the EV or MV form of VACV, respectively (Figure 2 A). Of note, neutralizing activity for the majority of these Abs required complement (Table S5). Most (46 of 48 [98%]) of the neutralizing mAbs recognized one of six proteins, D8, L1, B5 A33, A27, or H3 (Figure 2B). Two remaining mAbs were from the subject with prior wild-type MPXV infection and recognized I1 or an undetermined MPXV antigen (Table S5).
Figure 2.
Neutralizing and Cross-Neutralizing Potency of Human MAbs
Individual mAbs were assessed for neutralization using MV or EV forms of VACV, CPXV, or MPXV.
(A) Representation of individual mAbs within the panel that neutralized at least one of three Orthopoxvirus species. See also Table S5.
(B) Relative abundance (shown in colors and with percent on the top of each bar) and number of VACV-neutralizing mAbs for each antigen specificity from (A). Anti-I1 and unknown specificity mAbs neutralized MPXV only.
(C) Cross-neutralization of VACV, CPXV, or MPXV by individual mAbs from (A).
(D) Cross-neutralizing potency of individual neutralizing mAbs from (A). Each symbol represents the mean ± SD of triplicate Emax values of individual mAbs. Antibodies later tested for protection in vivo (detailed below) are indicated in red.
A majority (38 of 48 [79%]) of neutralizing mAbs cross-neutralized at least two Orthopoxvirus species (mainly VACV and CPXV) and 12 of 48 (25%) mAbs neutralized three orthopoxviruses—VACV, CPXV, and MPXV (Figure 2C). Regardless of their antigen specificity, the neutralizing mAbs varied widely in their neutralization potency. IC50 values of individual mAbs ranged from ∼0.02 to 100 μg/mL, and Emax values varied from 50% (the designated cut-off threshold to identify potent neutralizing clones) to 99.5% (Figure 2D; Table S5). Most of the neutralizing mAbs reduced plaque number only by ∼60%–80% at the highest tested concentration, regardless of antigen or form of virus targeted. The neutralizing activity of MV-targeted anti-D8, L1, A27, or H3, and EV-targeted anti-B5 mAbs were similar for VACV and CPXV, and two of six VACV and MPXV-neutralizing anti-A33 mAbs neutralized CPXV. None of the anti-D8 or anti-B5 mAbs neutralized MPXV, despite the ability of these mAbs to bind the corresponding MPXV ortholog protein (Tables S4 and S5). In contrast, the broadest cross-neutralizing activity (neutralization of VACV, CPXV, and MPXV), was detected in mAbs directed to A33, L1, A27, or H3 antigens (Figure 2D). Cross-neutralizing mAbs were isolated from most orthopoxvirus-immune subjects (Table S5). Together, our data indicate that mAbs induced by VACV immunization or MPXV infection that recognize any of six neutralizing determinants can inhibit several Orthopoxvirus species and also suggest that the broadest cross-neutralization is mediated predominantly by four Ab specificities.
Mixtures of Diverse mAb Specificities Possess Superior Cross-Neutralizing Activity for VACV, CPXV, MPXV, and VARV
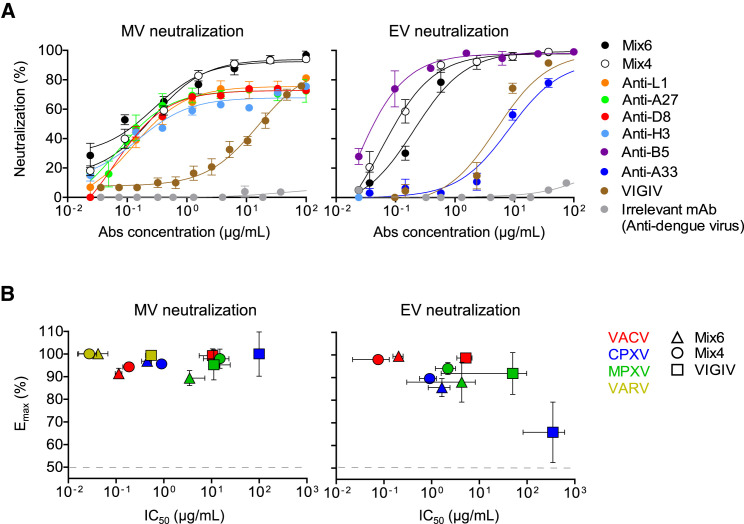
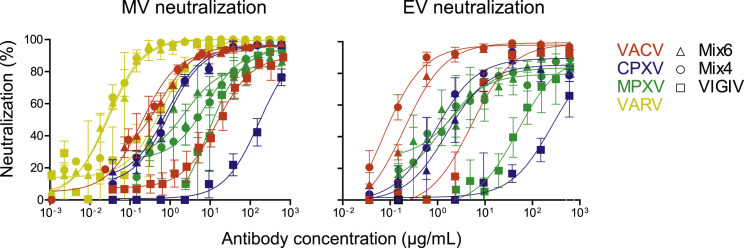
We next designed two mixtures of mAbs, designated Mix6 and Mix4, containing diverse specificities with high neutralizing (low IC50 and high Emax values) and cross-neutralizing activities to both MV and EV forms of virus (Figure 2D; Table S5). Mix6 contained single neutralizing mAbs directed to each of six antigens that targeted by neutralizing mAbs—MV proteins D8, A27, H3, and L1, and EV proteins B5 and A33. Mix4 was similar to Mix6, containing mAbs to A27, L1, B5, and A33, but lacked anti-D8 and H3 mAbs (Table S6) that were found to be non-protective in the lower respiratory tract infection model (detailed below). Both mixtures included four mAbs that exhibited similar binding for VACV proteins and the corresponding VARV protein orthologs (Figure S2). The neutralizing activity of Mix6 and Mix4 for VACV were higher than that of individual mAbs or VIGIV (Figure 3 A). Moreover, Mix6 and Mix4 cross-neutralized VACV, CPXV, MPXV, and VARV more potently than did VIGIV in EV and MV neutralization assays (Figures 3B and S3 ; VARV could only be tested in the MV assay, without complement). Therefore, neutralization and cross-neutralization are more efficiently achieved with mixtures of diverse mAbs specificities than with individual potently neutralizing mAbs.
Figure 3.
Mixtures of Four or Six MAbs Possess High Cross-Neutralizing Activity for VACV, CPXV, MPXV, and VARV
Neutralizing activity of mAbs or VIGIV was assessed using MV- and EV-neutralization assays. Mix6 included anti-L1, anti-H3, anti-A27, anti-D8, anti-B5, and anti-A33 mAbs. Mix4 included anti-L1, anti-A27, anti-B5, and anti-A33 mAbs.
(A) VACV neutralization by individual mAbs or their mixtures, compared with VIGIV. MAb mixtures designations are listed in Table S6.
(B) Cross-neutralizing activity of Mix4, Mix6 and VIGIV for VACV, CPXV, MPXV, or VARV (only the MV form was tested for VARV). Data represent one of two independent experiments, shown as mean ± SD of assay triplicates.
See also Figure S3.
Figure S3.
Cross-Neutralizing Activity of Human mAb Mixtures, Related to Figure 3
Cross-neutralizing activity of Mix6, Mix4, or VIGIV was assessed using MV-and EV-neutralization assays for VACV, CPXV, MPXV and VARV (MV form only for VARV). Data represent one of two independent experiments, shown as mean ± SD of assay triplicates.
Superior In Vivo Protection against VACV Infection Was Achieved by Administration of a Mixture of Human mAbs that Targeted Multiple Viral Antigens
We next evaluated the protective capacity of Mix6. Single-dose treatment with Mix6 one day before lethal intranasal (IN) challenge of C57BL/6 mice with VACV provided complete protection against weight loss and mortality (Figure 4 A). Mock-treated mice experienced severe illness and succumbed by day 7 post-inoculation (p.i.). The protection was associated with a profound (∼106-fold) reduction of viral load in the lungs on day 7 p.i., when compared to the mock-treated group (Figure 4B). Notably, the level of protection provided by Mix6 was comparable to, if not higher than, that provided by prior immunization with a sub-lethal dose of VACV. In contrast, pre-treatment with VIGIV did not protect mice under the challenge conditions used. These mice were unable to control VACV replication in the lungs and succumbed by day 7 p.i., similarly to the mock-treated group (Figure 4B). These data indicate a high prophylactic potency of Mix6 for prevention of respiratory tract infection.
Figure 4.
Mix6 Provides Superior Protection against Lethal VACV Infection In Vivo
Groups of C57BL/6 or BALB/c SCID mice representing, respectively, lower respiratory tract (A) or systemic dissemination (C) infection models, were inoculated i.p. with 1.2 mg of Mix6 or with 5 mg of VIGIV, or 1.2 mg of an irrelevant anti-dengue virus neutralizing mAb. The next day (d0) mice were challenged with a lethal dose of VACV and monitored for protection.
(A) Protection from respiratory VACV infection that was mediated by Mix6, VIGIV, or vaccination with live VACV 3 weeks prior with a sub-lethal dose of VACV.
(B) VACV titers assessed in the lungs of infected mice from (A) on day 7 p.i., shown as mean ± SEM; data represent one of two independent experiments with n = 5–10 mice per group. Dotted line indicates limit of detection (LOD) for the assay.
(C) Protection from systemically disseminated lethal VACV infection that was mediated by Mix6.
(D) Human mAb concentration in blood of treated mice from (C) at different times after treatment, shown as mean concentration ± SEM. One of two independent experiments, n = 3–5 mice per group.
To further characterize the protective efficacy of Mix6, we tested it in a lethal model of systemic VACV dissemination using severe combined immunodeficiency (SCID) mice that lack adaptive immune responses but retain a functional complement system (Bosma and Carroll, 1991). Initially, we assessed the prophylactic effect of Mix6 given to mice by the intraperitoneal (i.p.) route 1 day prior to lethal i.p. virus challenge. Remarkably, single-dose pre-treatment with Mix6 provided sterilizing immunity in this model (Figure 4C). Mice pre-treated with a human mAb of irrelevant specificity succumbed to the disease by day 20 p.i., when the group of animals pre-treated with Mix6 was completely protected from death and any signs of disease. Clearance of human mAbs from animal blood rendered healthy mice susceptible to VACV re-infection (Figures 4C and 4D), demonstrating that the sterilizing immunity observed during primary VACV infection was mediated solely by the administered Mix6.
In summary, these findings demonstrate the high prophylactic potency of Mix6 for prevention of respiratory and systemically disseminated VACV infections.
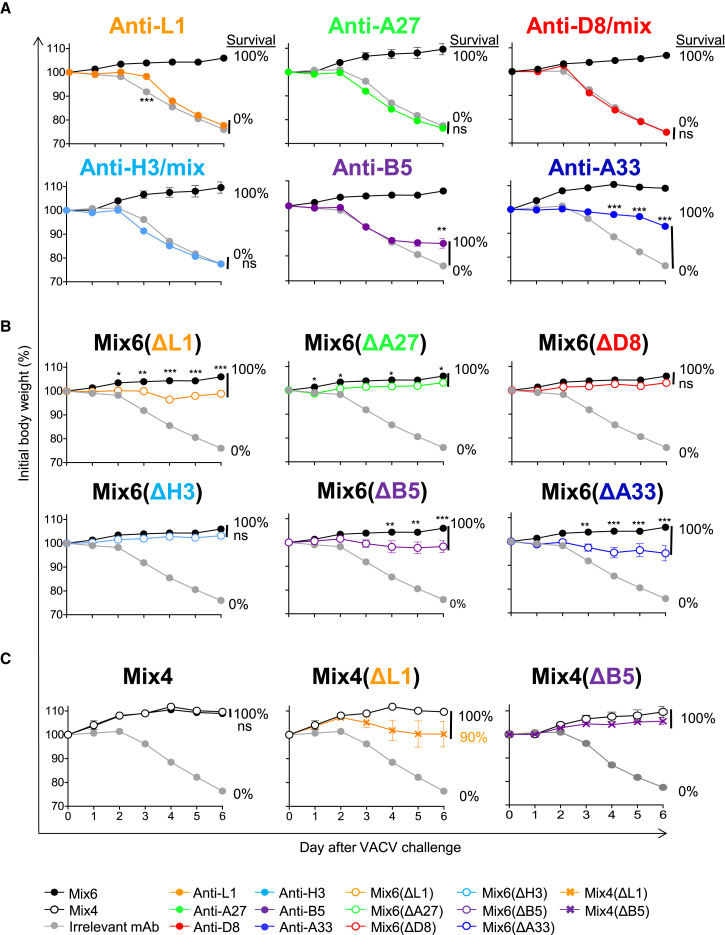
Four Principal Antibody Specificities Participated in Protection against Respiratory VACV Challenge when Used in Mixture
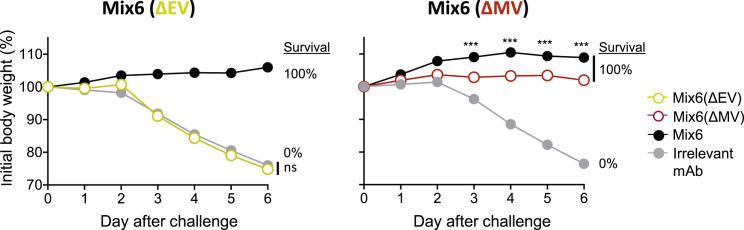
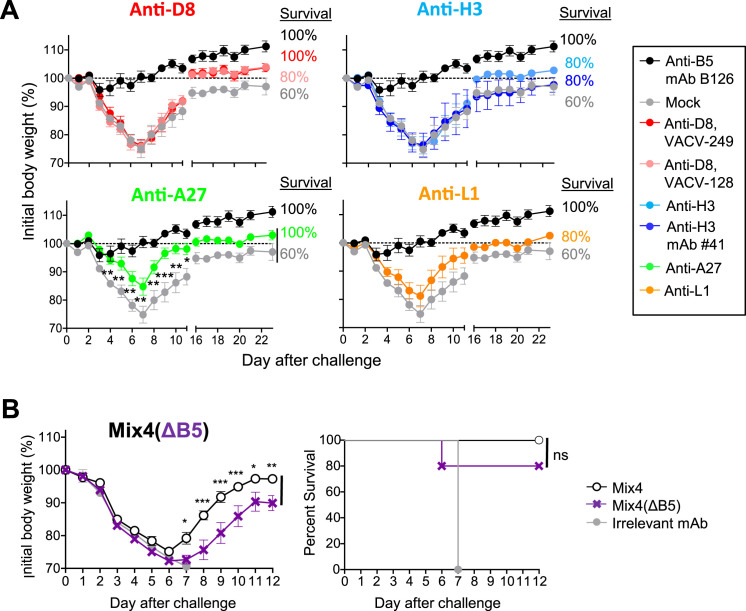
We next determined the contribution of individual mAbs within Mix6 by assessing the protective capacity of single mAbs or their mixtures (Table S6). Both of the EV-targeted antibodies, anti-A33 and -B5, protected B6 mice from death and severe weight loss when administered alone or as a mixture (Mix6(ΔMV)) 1 day before IN VACV challenge. In contrast, none of the MV-targeted mAbs, or their mixture (Mix6(ΔEV)), protected mice in the same conditions (Figures 5 A and S4 ). A possible explanation for this result was that the VACV challenge conditions used in this model are quite stringent and likely do not allow detection of moderate levels of protection by some mAbs. The other possibility was that the selected mAb clones may bind to non-protective epitopes of their antigens. To investigate further, we assessed protection using a less severe upper airways infection mouse model (Figure S5 A). These conditions resulted in milder disease and less mortality. In addition, anti-D8 and H3 mAbs were tested as mixtures of five or three different epitope specificities that incorporated mAbs from different competition-binding groups for D8 or H3 antigens, respectively. These single antigen-specific mixtures thus recognized diverse epitopes in D8 or H3 antigen. In this less stringent challenge setting, anti-A27 mAbs prevented mortality and severe weight loss, showing these mAbs may contribute to the protective efficiency by Mix6. Anti-L1 mAbs and mixtures of anti-D8 or anti-H3 mAbs still were not protective (Figure S5A). Therefore, these monotherapy studies suggested three protective human mAbs specificities in this model—anti-B5, anti-A33, and anti-A27.
Figure 5.
Human mAb Specificities that Contribute to Protection against Lethal Respiratory VACV Infection
C57BL/6 mice were inoculated i.p. 1 day prior to VACV challenge with 0.2 mg of individual mAbs or one of several mixtures designed to de-convolute protective mAbs specificities within Mix6. The next day (d0), mice were challenged IN with VACV and monitored for protection.
(A) Protective capacity of individual mAbs of Mix6. Anti-D8 and -H3 specificities were inoculated as a mixture of three to five mAbs to those proteins from different competition-binding groups.
(B) Protective capacity of mixtures that were based on Mix6 but had removal of a mAb for a single specificity, either L1, A27, D8, H3, A33, or B5.
(C) Protective capacity of mixtures based on Mix4, Mix4 lacking anti-L1 mAb but with a 2-fold excess of anti-A27 mAb, or Mix4 lacking anti-B5 mAb but with a 2-fold excess of anti-A33 mAb. Data shown indicate mean ± SEM from one of two independent experiments using five to ten mice per group. mAb mixtures designations are listed in Table S6.
See also Figures S4 and S5.
Figure S4.
Protection against of Lethal Respiratory VACV Infection Is Mediated Principally by EV-Targeted MAbs, Related to Figure 5
Groups of C57BL/6 mice were inoculated IP with 1.2 mg of Mix6, or with 0.8 mg of Mix6 lacking two anti-EV mAbs (designated as Mix6(ΔEV)), or 0.4 mg of Mix6 lacking four anti-MV mAbs (designated as Mix6(ΔMV)), or 1.2 mg of an irrelevant anti-dengue virus neutralizing mAb. The next day (d0) mice were challenged by the IN route with a lethal dose of VACV and monitored for protection.
(A) Protection from respiratory VACV infection that was mediated by Mix6(ΔEV).
(B) Protection from respiratory VACV infection that was mediated by Mix6(ΔMV); Data represent one of two independent experiments with n = 5-10 mice per group. Percent (%) indicates survival by day 7.
Figure S5.
Human mAb Specificities that Participate in Protection against Lethal Respiratory VACV Infection, Related to Figure 5
(A) C57BL/6 mice were inoculated i.p. one day prior to VACV challenge with 0.2 mg of individual anti-D8, -H3, -A27, or –L1 mAbs. The next day (day 0), mice were anesthetized and challenged IN with VACV under conditions promoting less severe upper airway infection (2 × 105 pfu VACV in 10 μl of PBS) and monitored for protection. Previously reported protective mouse anti-B5 mAb B126 and anti-H3 #41 (Benhnia et al., 2009, McCausland et al., 2010) served as control treatment for protection.
(B) C57BL/6 mice received 400 μg of mAbs in the mixture designated as Mix4(ΔB5) (200 μg of anti-A33, 100 μg of anti-A27, and anti-L1) and was challenged with 10-fold higher (106 pfu) dose of VACV next day. Mice were monitored for protection and survival. Body weight is shown only for animals that survived. Percent figures near each curve indicate survival by day 7 based on endpoint criteria for euthanasia. Data showed mean ± SEM from one of two independent experiments using 5 to 10 mice per group.
It was possible that some of the six Ab specificities contributed to protection in mixtures only in a cooperative manner that would not be detected by monotherapy studies. To detect such activity, we designed mixtures that were variants of the Mix6 that each lacked one mAb specificity (Table S6). Each of the Mix6 variant mixtures lacking one of the mAbs was protective, although mixtures lacking anti-L1, anti-A27, anti-A33, and anti-B5 were less efficient in protection against weight loss than Mix6 ( Figure 5B). Removal of the protective MV-targeted anti-A27 mAb from Mix6 did not affect the outcome of challenge substantially. However, exclusion of the MV-targeted anti-L1 mAb from Mix6 resulted in detectable weight loss upon infection, which was comparable to that seen when mice were pre-treated with Mix6 lacking either of the most potent EV-targeted mAbs (anti-A33 or anti-B5) identified in the monotherapy studies (Figures 5A and 5B). Moreover, Mix4 containing anti-L1, anti-A27, anti-B5, and anti-A33 mAbs conferred a level of protection equivalent to that of Mix6 (Figure 5C). Therefore, the mAbs in Mix4 appear to cooperate in achieving their protective effect.
One possible explanation for the diminished protection observed when a mAb mixture lacked a single MV- or EV-targeted mAb specificity was the decrease in total amount of mAb per treatment. Therefore, we next examined whether the lack of one mAb specificity in protective Mix4 could be compensated by using a mixture containing the same total amount of Ab by adding an equivalent amount of one of the retained mAb specificities targeting the same virion form. The monotherapy results suggested higher potency of anti-A33 and anti-A27 mAbs when compared to the EV- or MV-specific anti-B5 and anti-A27 mAbs (Figures 5A and S5A). Therefore, groups of mice were treated before VACV challenge with a variant of Mix4 that contained a 2-fold higher amount of the anti-A27 or anti-A33 mAb and lacked anti-L1 (designated as Mix4(ΔL1)), or anti-B5 (designated as Mix4(ΔB5)) mAb, respectively. An excess of anti-A27 or anti-A33 mAb did not restore the initial activity of Mix4 in absence of mAbs with anti-L1 or anti-B5 specificities, although the effect was minor under the challenge conditions used (Figure 5C). However, in more stringent challenge conditions, mice pre-treated with Mix4 exhibited significantly higher resistance to the disease and recovered faster compared to mice that received the Mix4 (ΔB5) containing a 2-fold higher amount of anti-A33 mAb (Figure S5B). This finding suggested Mix4 as a potent therapeutic mixture. Together, these results showed that four principal mAb specificities in Mix4 contributed to, and were required for, efficient protection against lethal respiratory tract VACV infection in the mouse model.
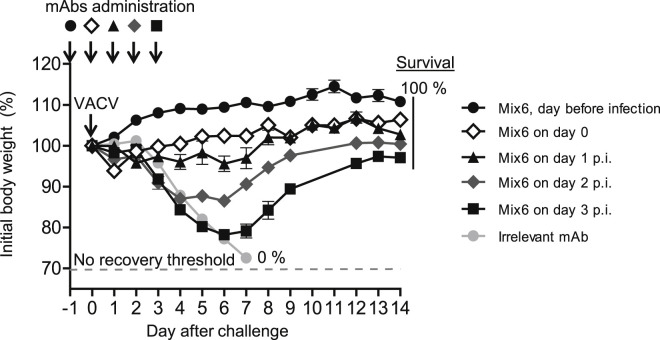
Therapeutic Effect of Mix6 when Given Up to 3 Days after Infection by the Respiratory Route
We next determined how long after respiratory infection Mix6 would exhibit a therapeutic effect, when treatment was delayed. For these studies, mice were immunized passively with Mix6 one day before or on the day of virus challenge, or 1, 2 or 3 days after virus challenge (Figure 6 ). As expected, the treatment was most efficient when administered before disease onset. Mice given Mix6 showed significant protection from weight loss if the treatment was given 1 day before, on the day of challenge or 1 day after infection. When the treatment was delayed until day 3, the time point when untreated animals developed disease due to profound virus burden in the lungs (data not shown), we observed protection from death, but only partial protection from weight loss (Figure 6). These data demonstrated that Mix6 mediated a therapeutic effect even when treatment was delayed, especially against lethality.
Figure 6.
Efficient Post-exposure Treatment Effect Mediated by Mix6 MAbs
C57BL/6 mice were inoculated i.p. with 1.2 mg of Mix6 on the day before (d-1), or on the day of (d0), or day 1 to day 3 (d1–d3) after lethal IN challenge with VACV. The control group included mice pre-treated one day before challenge with 1.2 mg of an anti-dengue virus mAb. The body weight loss kinetics are shown for 14 days around the time of infection and treatment. Data are presented as mean ± SEM, using five to ten mice per group. Percent indicate survival based on endpoint criteria for euthanasia.
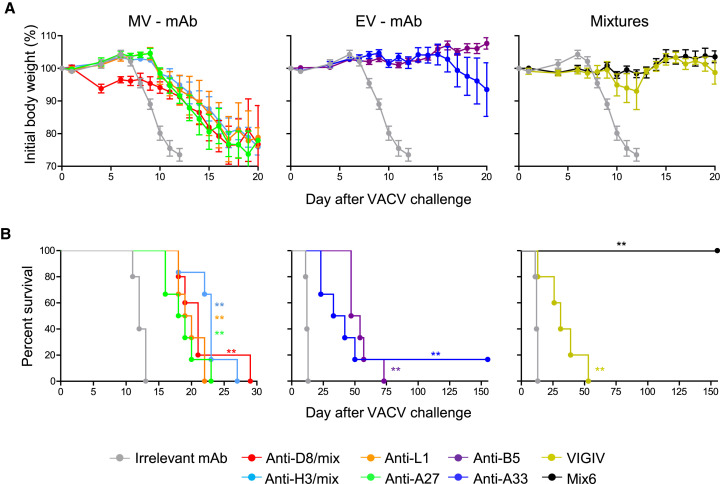
Diverse Human Ab Specificities Participate in Protection against Systemic VACV Infection
The experiments described above showed that a single dose of Mix6 given prior to systemic inoculation with a lethal dose of VACV conferred sterilizing protective immunity in SCID mice, which lack adaptive immunity (Figure 4C). Using this model, we next assessed the efficacy of monotherapy with individual mAbs, Mix6, or VIGIV that were given to mice 1 day after inoculation with a lethal dose of VACV (Figure 7 ). Similar to the respiratory challenge study, anti-H3 or anti-D8 mAbs were used as mixtures of several epitope specificities. Each of the Abs tested, including those identified as non-protective in respiratory tract infection, delayed morbidity and mortality in mice when compared to the animals in the mock-treated group. Moreover, delayed treatment with Mix6 conferred sterilizing immunity to inoculated mice, which all survived and lacked signs of illness for >155 days after VACV inoculation (Figures 7A and 7B). Together, these results demonstrated a high therapeutic potency of Mix6 and showed that diverse mAbs specificities may contribute to protection against systemically disseminated VACV infection.
Figure 7.
Human mAb Specificities that Contribute to Protection against Progressive Systemic VACV Infection
(A and B) BALB/c SCID mice were challenged i.p. with 105 plaque forming units (pfu) VACV. The next day, mice were inoculated i.p. with 1.2 mg of Mix6, 5 mg of VIGIV, or 0.2 mg anti-L1, anti-A27, anti-D8, anti-H3, anti-B5, anti-A33, or irrelevant mAb. Body weight loss kinetics (A) and survival (B). Data in (A) shows body weight only for the animals that survived. Mean ± SEM, n = 5–6 mice per group. Each curve was compared to that of the irrelevant mAb-treated group on (B).
Discussion
In this study, we elucidated the breadth and specificity of human cross-neutralizing mAbs against the clinically relevant orthopoxviruses VACV, CPXV, MPXV, and VARV. In addition, we identified the principal protective specificities for human mAbs and demonstrated that superior protection in mouse challenge models could be achieved with a defined mAb mixture that targeted a limited number of orthopoxvirus protein antigens.
Studying protective antibody-mediated immunity for orthopoxvirus infections has been challenging because of the lack of clonal human Abs representing the naturally occurring human B cell response to orthopoxvirus infection or immunization. In the current work, using a cohort of orthopoxvirus-immune subjects, we showed that orthopoxvirus infection elicits a complex B cell response encoding large numbers of clones reactive to antigens from diverse orthopoxvirus species. Further analysis of individual clones revealed the importance of six major neutralizing mAb specificities that targeted both MV (anti-H3, anti-A27, anti-D8, and anti-L1) and EV (anti-B5 and anti-A33) infectious forms of orthopoxvirus and required complement for optimal activity. The presence of complement enhanced the inhibitory activity of mAbs targeting most neutralizing determinants, suggesting a major component of complement-dependent mechanisms underlying the protection in vivo mediated by neutralizing Abs elicited in response to orthopoxvirus infection.
In studies of human mAbs to other viruses, such as HIV, influenza, or dengue virus, we have found that the percentage of neutralizing mAbs among the total number of mAbs induced by infection or vaccination varies according to the agent. For example, typically <1% of the mAbs induced by dengue virus infection neutralize virus (Smith et al., 2012), whereas a large proportion of influenza-specific mAbs neutralize (Thornburg et al., 2013). For orthopoxviruses, we found here that a high fraction of the mAbs from our panel (54%) possessed neutralizing activity. Given the high level of sequence homology among the surface proteins from VACV, CPXV, MPXV, and VARV (89%–100%), such a robust and diverse neutralizing Ab response likely explains the efficient cross-protection induced by VACV immunization against heterologous orthopoxvirus infections. Our finding that a large fraction of orthopoxvirus-specific mAbs of the panel exhibited cross-binding and/or cross-neutralizing activity for VACV, CPXV, MPXV, and VARV further substantiates this model. The broadest cross-neutralization was achieved by mAbs targeting four antigens in the MV or EV forms of VACV, namely A33, A27, L1, and H3 (or the ortholog proteins in the other three viruses), thus identifying the principal determinants of Ab-mediated cross-protective immunity to orthopoxviruses.
Information about the protective potential of human Abs has been limited mostly to the study of varying lots of VIGIV, which has been used with partial success for post-exposure treatment and for management of some severe adverse reactions to smallpox vaccination (Wittek, 2006). Multiple antigen specificities appear to contribute to neutralization of the MV form of VACV by VIGIV or immune serum IgG (Benhnia et al., 2008, Moss, 2011). Abs to B5 were thought responsible for much of the neutralization activity against VACV EV forms of virus (Bell et al., 2004). Animal studies suggested that protection is not readily achieved by administration of a single neutralizing mAb and requires both EV- and MV-targeted mAbs (Lustig et al., 2005). Reconstituting (or improving) the protective activity of VIGIV with mAbs has been attempted empirically, using a mixture of anti-H3 and anti-B5 mAbs (McCausland et al., 2010), or a complex mixture of 26 human mAbs directed to 14 antigens (Lantto et al., 2011, Zaitseva et al., 2011). Our data suggest that a mixture containing mAbs of only two specificities (anti-H3 and anti-B5) likely would fail to cross-protect efficiently, because we observed that anti-B5 mAbs fail to neutralize the EV form of MPXV. In contrast, the previous mixture of 26 mAbs likely includes redundant or noncontributory mAbs, because this composition contains a number of mAbs that are directed to antigenic specificities without an apparent role in cross-neutralization or protection. To make a potent neutralizing and protective human Ab mixture by rational design that recognizes the four major orthopoxvirus threats to humans, we combined potent cross-neutralizing human mAbs targeting six major orthopoxvirus antigenic proteins: the MV antigens H3, A27, D8, and L1 and the EV antigens B5 and A33. Remarkably, Mix6 or its derivative Mix4 cross-neutralized all four clinically relevant orthopoxviruses, including live VARV, and exhibited superiority compared to conventional VIGIV.
Orthopoxviruses transmit by several routes of infection and cause diverse clinical syndromes in humans (Smith and McFadden, 2002), which can be modeled in part using different animal models (Chapman et al., 2010). We sought here to compare the prophylactic and treatment efficiency of human mAbs and their mixtures in several well-established VACV lethal challenge murine models using either mild or severe respiratory tract infection or, alternatively, systemic inoculation resulting in disseminated infection (Belyakov et al., 2003, Flexner et al., 1987, Wyatt et al., 2004). The resulting data revealed that the contribution of individual specificities to protection varied depending on the route of virus inoculation. Four specificities (anti-A33, anti-B5, anti-L1, and anti-A27), contributed significantly to protection against respiratory tract infection, while in contrast, all six tested specificities contributed to protection in the model of systemic infection. Moreover, we observed that the major contribution to protection in both models was provided by EV-targeted anti-B5 and anti-A33 human mAbs, consistent with previous studies of mouse mAbs (Lustig et al., 2005). Thus, cross-protection against all clinically important orthopoxviruses is most likely achieved when incorporating both EV-neutralizing anti-B5 and anti-A33 mAbs, which may compensate for some species cross-neutralization deficiencies of the other. Mix6 and Mix4 exhibited superiority in protection against VACV compared to VIGIV, suggesting novel efficient mixtures of mAbs for therapeutic use in humans. In summary, the findings presented here reveal the fundamental mechanisms underlying efficient cross-protective antibody-mediated immunity to orthopoxviruses in humans.
Limitations, Caveats, and Open Questions
Using naturally occurring human mAbs isolated by hybridoma technology, this study revealed six principal cross-neutralizing human mAb specificities for VACV, CPXV, MPXV, and VARV. We next showed that these Ab specificities that are necessary and sufficient determinants of protection in murine challenge models. This work suggests that a mixture of these Abs could mediate cross-protective immunity to orthopoxviruses. As with most studies, there are several limitations of this work that we would like to point out.
The antibody discovery platform used likely allowed us to identify mAbs only from the most frequent classes of B cell memory clones that occur in human peripheral blood. Therefore, less frequent clones could be missing from our analysis.
It remains unknown to what extent the B cell memory repertoire in the blood that we have studied corresponds to the antigen-reactive antibody protein repertoire in the serum that is secreted by long-lived plasma cells in the bone marrow. Future proteomics studies using emerging technologies might be able to address this question.
Future development for use in humans of individual mAbs or mixtures described here against VACV, CPXV, and MPXV or VARV should include studies of larger animal models, such as non-human primates.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| HRP Anti-Human IgG | BD PharMingen | Cat# 555788 |
| Goat anti-Human IgG(Fc)-AP | Meridian Life Science | Cat# W99008A; RRID: AB_205090 |
| Mouse Anti-Human IgG1 Hinge-AP | Southern Biotech | Cat# 9052-04 |
| Mouse Anti-Human IgG2 Fc-AP | Southern Biotech | Cat# 9070-04 |
| Mouse Anti-Human IgG3 Hinge-AP | Southern Biotech | Cat# 9210-04 |
| Mouse Anti-Human IgG4 Fc-AP | Southern Biotech | Cat# 9200-04 |
| Mouse anti-B5 mAb, B126 | Dr. Shane Crotty Laboratory | N/A |
| Mouse anti-H3 mAb, #41 | Dr. Shane Crotty Laboratory | N/A |
| VIGIV | BEI Resources | Cat# NR-2632 |
| DENV 2D22, mAb | Dr. J. E. Crowe, Jr. Laboratory | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| VACV (WR) A27L protein | BEI Resources | Cat# NR-2622 |
| VACV (WR) A33R protein | BEI Resources | Cat# NR-2623 |
| VACV (WR) B5R protein | BEI Resources | Cat# NR-2624 |
| VACV (WR) L1R protein | BEI Resources | Cat# NR-2625 |
| VARV (Bangladesh-1975) B6R protein | BEI Resources | Cat# NR-10502 |
| VARV (Bangladesh-1975) M1R protein | BEI Resources | Cat# NR-10501 |
| VARV (India-1967) A36R protein | BEI Resources | Cat# NR-10503 |
| VACV D8 protein | Dr. D. M. Zajonc Laboratory | N/A |
| VACV H3 protein | Dr. Shane Crotty Laboratory | N/A |
| VACV H2 protein | Dr. G. H. Cohen Laboratory | N/A |
| VACV A28 protein | Dr. G. H. Cohen Laboratory | N/A |
| VACV A21 protein | Dr. G. H. Cohen Laboratory | N/A |
| VACV L5 protein | Dr. G. H. Cohen Laboratory | N/A |
| VACV F9 protein | Dr. G. H. Cohen Laboratory | N/A |
| VACV J5 protein | Dr. G. H. Cohen Laboratory | N/A |
| VARV A31 protein | Dr. G. H. Cohen Laboratory | N/A |
| VARV I2 protein | Dr. G. H. Cohen Laboratory | N/A |
| MPXV A27 ortholog, protein | Dr. J. E. Crowe, Jr. Laboratory, plasmid from BEI Resources | plasmid Cat# NR-3022 |
| VARV D8 ortholog, protein | This paper | N/A |
| VARV H3 ortholog, protein | This paper | N/A |
| Catalase | MP | Cat# 190311 |
| Guinea pig complement | Rockland | Cat# C200-0005 |
| Baby rabbit complement | Cedarlane | Cat# CL3441-S100 |
| Critical Commercial Assays | ||
| VACV (strain WR) protein array | Antigen Discovery | Cat# 13-MA-0005 |
| RTS 100 E. coli HY Kit | 5 Prime | Cat# 2401110 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | Jackson Laboratories | Cat# 000664 |
| Mouse: CBySmn.CB17-Prkdc SCID/J | Jackson Laboratories | Cat# 001803 |
| Experimental Models: Cell Lines | ||
| BSC-40 cells | ATCC | Cat# CRL-2761 |
| RK-13 | ATCC | Cat# CCL-37 |
| Vero cells | ATCC | Cat# CCL-81 |
| Vero E6 cells | ATCC | Cat# CRL-1586 |
| Experimental Models: Viruses | ||
| VACV Dryvax | NIH | Cat# 4008284 |
| VACV Western Reserve (WR) | ATCC | Cat# VR-119 |
| CPXV Brighton Red | BEI Resources | Cat# NR-88 |
| Monkeypox virus Zaire 79 | Dr. Mark Slifka Laboratory | N/A |
| VARV Bangladesh 1974 Solaiman strain | Centers for Disease Control and Prevention | N/A |
| Software and Algorithms | ||
| IMGT, the international ImMunoGeneTics information system | (Ruiz et al., 2000) | http://www.imgt.org |
| Prism 5.0 software | GraphPad | http://www.graphpad.com |
| GenePix Pro 5.0 software | Molecular Devices | https://www.moleculardevices.com |
| Other | ||
| Orthopoxvirus-specific human mAbs | This paper | Table S2 |
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to, and be fulfilled by the corresponding author: James E. Crowe, Jr. (james.crowe@vanderbilt.edu). Materials described in this paper are available for distribution under the Uniform Biological Material Transfer Agreement, a master agreement that was developed by the NIH to simplify transfers of biological research materials.
Experimental Model and Subject Details
Donors
PBMCs were obtained from subjects vaccinated with Dryvax (Wyeth), IMVAMUNE (Bavarian Nordic), or ACAM2000 (Acambis). The VRC 201 study was approved by the NIAID IRB under the intramural number 02-I-0316. The ClinicalTrials.gov number was NCT00046397. One sample was obtained from a U.S. survivor of naturally acquired MPXV infection (Lewis et al., 2007). The studies were approved by the Institutional Review Boards of Vanderbilt University Medical Center, Oregon Health Sciences University, and the National Institute of Allergy and Infectious Diseases.
Mice
C57BL/6 and CBy.Smn.CB17PRKdc SCID/J (BALB/c SCID) mice were purchased from Jackson Laboratories (Bar Harbor). BALB/c SCID mice received Laboratory Autoclavable Rodent Diet #5010 (LabDiet). Breeding, maintenance and experimentation complied with Institutional Animal Care and Use Committee regulations.
Cell Lines and Viruses
VACV Dryvax (NIH, Lot# 4008284), VACV Western Reserve (VACV-WR; ATCC VR-119) and CPXV Brighton Red (BEI Resources, NR-88) were propagated and titered in monolayer cultures of BSC-40 cells (ATCC CRL-2761). MPXV Zaire was propagated in BSC-40 cells and titered on Vero cells (ATCC CCL-81). Bangladesh 1974 Solaiman strain of VARV was propagated in monolayer cultures of Vero E6 cells (ATCC CRL-1586). VACV and CPXV were manipulated under BSL-2 conditions by vaccinated personnel. MPXV was manipulated under BSL-2 conditions with BSL-3 precautions by vaccinated personnel. All experiments with live VARV were reviewed and approved by the World Health Organization Advisory Committee on Variola Virus Research (WHO ACVVR). Experiments with VARV were conducted in accordance with WHO ACVVR guidelines and within a biosafety level 4 laboratory.
Antigens
Recombinant VACV proteins A27, A33, L1, B5, A28, L5, A21, H2, F9, J5, and VARV proteins I2, A31.5, A36, M1, B6, A31 were produced using a baculovirus expression system or purchased from BEI Resources. Truncated monomeric D8 protein was kindly provided by Dr. D. M. Zajonc and Dr. Y. Xiang. Recombinant VACV H3 protein was kindly provided by Dr. Crotty. DNA encoding the MPXV ortholog of the A27 VACV protein was purchased from BEI Resources. H3 and D8 protein orthologs of VARV were produced after WHO approval, as described previously (Davies et al., 2005b, Matho et al., 2012). Cell lysates infected with VACV (NYCBOH), CPXV, MPXV were prepared and inactivated as described previously (Amanna et al., 2012). For preparation of VARV-infected cell lysate, RK-13 cells were inoculated with VARV at the CDC at an MOI of 1 and incubated for 48 hrs at 55.5°C. Harvested cells were resuspended in 1 mM Tris buffer, lysed by two freeze/thaw cycles, followed by sonication three times at 160 watts for 1 min. VARV was inactivated by gamma irradiation using three times the kill dose on dry ice, where one kill dose equals 4.4 × 106 rads. A VACV-WR protein array was acquired from Antigen Discovery. The VARV protein microarray was prepared as described previously (Davies et al., 2005b).
Method Details
Generation of Human Hybridomas
Human hybridomas were generated as described previously (Crowe, 2009). Briefly, cryopreserved samples were transformed with Epstein-Barr virus. Cultures were incubated in 384-well culture plates for 10 days and then expanded using cell culture medium containing irradiated heterologous human PBMCs (Nashville Red Cross). Plates were screened for VACV recombinant antigen- or VACV-infected cell lysate-specific antibody secreting cell lines using ELISA. Cells from wells with supernatants containing Abs that reacted to antigen or infected cell lysate were fused with HMMA2.5 myeloma cells using an established electrofusion technique (Yu et al., 2008).
ELISA
For screening ELISA, plates were coated with antigen at 1 μg/mL, or 1:400 dilution of a lysate in PBS. After blocking, plates were incubated with culture supernatants followed by incubation with anti-human IgG conjugated with alkaline phosphatase (Meridian, Life Science) or HRP (BD Pharmingen). Plates were developed and supernatants were counted as VACV-reactive or recombinant protein antigen-reactive if their absorbance was 2.5-fold above the background from wells containing medium or coated with uninfected cell lysate, respectively. For binding kinetics and cross-reactivity assays, purified mAbs were assessed at concentrations ranging from 100 μg/mL to 20 pg/mL, in triplicate. EC50 values were determined using Prism 5.0 software (GraphPad) after log transformation of antibody concentration using sigmoidal dose-response nonlinear fit analysis with R 2 values greater than 0.85, as described previously (Thornburg et al., 2013). Binding of purified mAbs to VARV-infected cell lysate was determined at a single dilution of 100 μg/mL, in triplicate.
Protein Arrays and mAb Target Analysis
The Orthopoxvirus (VACV strain WR) protein array was acquired from Antigen Discovery (ADI). The VARV protein microarray was fabricated in a similar manner as described previously (Davies et al., 2005b). Briefly, individual open reading frames encoded by the viral genome were amplified and cloned into T7 expression vectors by homologous recombination. Proteins were produced using an Escherichia coli-based cell-free coupled transcription/translation reactions (RTS 100 kits; 5 Prime, Gaithersburg, USA) according to the manufacturer’s instructions. Proteins were printed without further purification on nitrocellulose-coated glass slides (Whatman). Protein expression was monitored using hemagglutinin or His tags present on the protein termini; quantification of the amount of protein spotted was not possible. No-DNA control spots containing the reaction mixture but lacking template DNA were included throughout the array to correct for background binding to E. coli proteins found in the transcription-translation mixture.
MAbs were probed on the VACV strain WR or VARV protein arrays at dilutions between 1:25 and 1:100, according to the manufacturer’s instructions and reagents (ADI). Briefly, arrays were probed with antibody overnight at 4°C, then with biotin-conjugated goat anti-human antibodies for 1 hr at RT, then with a streptavidin-conjugated fluorophore for 1 hr at RT. Arrays were scanned using a GenePix 4100A scanner (Molecular Devices) with laser setting at 100% and photomultiplier (PMT) gain of 400. Image analysis was performed with GenePix Pro 5.0 software (Molecular Devices). Spot intensity was calculated as the median spot value minus local spot background. A secondary correction for background binding to E. coli proteins in the reaction mixture was done by subtracting an average of the no-DNA spots from the background-corrected spot value. Since mAb affinity, protein sequence conservation, and protein expression levels vary, a simple evaluation for highest fluorescent intensity, and a correlation between the two chips, if needed, was used to identify protein targets.
Biolayer Interferometry Analysis
Experiments were performed on an Octet RED biosensor instrument (Pall ForteBio; Menlo Park) essentially as described previously (Smith et al., 2014). For competition-binding studies, mAb-antigen complexes were tested for the ability to bind a second mAb in sandwich assay. Briefly, biosensors were pre-wetted in running buffer containing DPBS, 0.1% BSA, and 0.05% Tween-20. Primary human mAbs were loaded onto Protein G biosensor tips (ForteBio) at a concentration of 105 μg/mL. After biosensors were incubated with recombinant protein solutions of D8 or H3 at concentration 90 μg/mL. Following by secondary human mAbs were loaded at concentration 105 μg/mL.
The extent of antibody-antigen association was determined as wavelength shift in nm and calculated as a percentage after normalization, where 0% was the wavelength shift in nm for self-blocking control and 100% was the maximal wavelength shift in nm. Experiments were performed in duplicate. Antibodies were considered to be members of the same competition-binding group if they competed for binding to antigen and exhibited a similar blocking pattern to other antibodies in the panel.
mAb Isotype and Gene Sequence Analysis
The isotype and subclass of secreted antibodies were determined using murine anti-human IgG1-IgG4 antibodies followed by secondary anti-mouse HRP-conjugated antibody (Southern Biotech). Nucleotide sequences of variable gene segments were determined by Sanger sequencing from cloned cDNA generated by reverse transcription PCR of mRNA, using variable gene-specific primers designed to amplify antibody genes from all gene families (Weitkamp et al., 2003). Identity of the gene segments and mutations from the germline sequences were determined by alignment using the ImMunoGeneTics database (http://www.imgt.org) (Ruiz et al., 2000).
mAb Production and Purification
Hybridoma cells secreting VACV-specific mAbs were grown in serum-free medium (GIBCO). MAbs were purified from culture supernatants using HiTrap MabSelect Sure or HiTrap Protein G columns (GE Healthcare).
Virus Neutralization Assays
Neutralizing activity of mAbs was determined using MV or EV forms of VACV strain NYCBOH, CPXV, or MPXV, or MV of VARV in a plaque reduction neutralization (PRNT) assay. Neutralization of VACV, CPXV, and MPXV MV particles, and MPXV EV particles was performed using 10% guinea pig complement (Rockland Inc.). Neutralization of VACV EV was performed using 10% baby rabbit complement (Cedarlane), and neutralization of VARV MV was performed without complement. For EV neutralization MV was depleted with blocking mAbs MPXV-26 and VACV-301, both at 20 μg/mL. For VACV and CPXV neutralization, 0.125 mL HBSS with 1% BSA containing 100 PFU of virus was incubated with 0.125 mL of serial two-fold dilutions of mAb for 60 min at 37°C and then applied to BSC-40 cell culture monolayers. Plates were incubated for 2 hrs, after which 0.5 mL of Opti-MEM I (Gibco) with 10% fetal calf serum was added. Plates then were incubated 24-48 hrs at 37°C, and plaques were visualized with crystal violet containing 3.7% formaldehyde. For MPXV neutralization 32-50 PFU of virus was incubated with serial two-fold dilutions of mAb for two hours at 37°C and then applied to Vero cell culture monolayers in 6 well plates. After a one-hour incubation, the cells were overlaid with 0.5% agarose in EMEM with 2.5% FBS, 20 mM glutamine and antibiotics and incubated for 4 days at 37°C, in 5% CO2, to allow for plaque formation. The agarose was removed and the monolayer was stained with 0.1% crystal violet in PBS containing 0.2% formaldehyde. Neutralization was performed in the presence of complement for all viruses except VARV MV. All experiments with live VARV were reviewed and approved by the World Health Organization Advisory Committee on Variola Virus Research (WHO ACVVR). Experiments with VARV were conducted in accordance with WHO ACVVR guidelines within a biosafety level 4 laboratory. Vero E6 cells were plated at 1 × 106 cells/well, in DMEM supplemented with 10% FBS, and incubated at 37°C with 6% CO2 for 48 hrs. Antibodies were diluted in RPMI supplemented with 2% low IgG FBS (Gibco). All antibodies underwent 18 two-fold serial dilutions starting at 75 μg/mL while VIGIV started at 0.15 mg/mL. Variola virus strain Solaimen (VARV_BSH74_sol) was diluted to 150 PFU/mL and sonicated in a cup horn sonicator set at 40% for 1.5 min in an ice bath. The viral inoculum was added to each antibody dilution tube and then incubated rocking at 35.5°C and 6% CO2 overnight. Plates were inoculated in duplicate and incubated for 1 hr at 35.5°C and 6% CO2 before addition 1 mL of fresh medium. Plates were incubated further at 35.5°C and 6% CO2 for five days and then fixed with crystal violet stain. Emax was determined as a maximum of neutralization mAb effect (%); IC50 and Emax values were determined using Prism 5.0 software (GraphPad) after log transformation of antibody concentration using a 3-parameter nonlinear fit analysis of antibody log10 concentration versus response with R 2 values greater than 0.85, as described previously (Thornburg et al., 2013).
In Vivo Protection Study
To test the effect of mAbs on respiratory tract infection, six- to eight-week old male C57BL/6 mice were injected i.p. with 100-200 μg of individual mAbs or designated mixtures of mAbs (100-200 μg of each mAb), or 5 mg of VIGIV (BEI Resources). Human anti-dengue virus mAb served as mock-vaccinated control. In ABSL-2 facilities, ketamine-xylazine anesthetized mice were inoculated IN with 105 PFU VACV-WR in 50 μL, or in some experiments in 10 μl of PBS. In some experiments, mice were inoculated with 106 PFU VACV. For virus titer determination, lungs from individual mice were homogenized and plated on confluent BSC-40 cell monolayer cultures. To test the effect of mAbs on disseminated VACV infection, eight- to ten-week old female BALB/c SCID mice were given Abs i.p. either prior to or after VACV inoculation, as detailed in the text. For lethal challenge, mice were inoculated i.p. with 105 PFU VACV-WR in 100 μl PBS. Mice were weighed and monitored daily for morbidity, and those losing over 30% of initial body weight were euthanized, per IACUC requirements.
Quantification and Statistical Analysis
The descriptive statistics mean ± SEM or mean ± SD were determined for continuous variables as noted. Comparisons were performed using Wilcoxon rank sum test or the post hoc group comparisons in ANOVA; all tests were two-tailed and unpaired. Survival curves were estimated using the Kaplan Meier method and curves compared using the log rank test with subjects right censored, if they survived until the end of the study. ∗ -p < 0.05; ∗∗ - was used to reject a “null hypothesis.” ∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ - = p < 0.001; ns – non-significant. Statistical analyses were performed using Prism v5.0 (GraphPad).
Author Contributions
Conceptualization, I.G., P.G., and J.E.C.; Investigation, I.G., P.G., E.H., H.-P.R., A.V.K., Z.R., Z.W., L.J.H., M.B.T., and P.S.S.; Resources, G.S., R.L., V.S., N.K., D.L.B., B.S.G., K.M.E., R.J.E., S.J., M.B.T., and G.H.C.; Formal Analysis, I.G., P.G., and J.C.S.; Supervision, M.K.S., V.A.O., S.J., and J.E.C.; Writing – Original Draft, I.G., P.G., and J.E.C.; Writing – Review & Editing, I.G., P.G., and J.E.C.; all authors reviewed and approved the manuscript; Funding Acquisition, M.K.S., S.J., and J.E.C.
Acknowledgments
This project received support from the U.S. NIH (grants U01 AI48512 and contract HHSN272200900047C to J.E.C., and U19 AI109948 and 8P51 OD 011092-53 to M.K.S.). The project was supported by NCRR grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06, and VA Merit Award BX001444 (to S.J.). This work was supported by intramural funding from the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, NIH. Flow cytometry experiments were performed in the Vanderbilt University Medical Center Flow Cytometry Shared Resource, supported by NIH P30 CA68485 and DK058404. We thank Jill Janssen and the Vanderbilt Clinical Trials Center for regulatory support, and Yingchun Yu, Patricia McGraw, Natalie Thornburg, Shane Crotty, Chwan Hong Foo, J. Charles Whitbeck, and Stuart Isaacs for technical contributions and advice. We thank Y. Xiang and Dirk Zajonc for providing recombinant D8 protein and Shane Crotty for providing H3 protein. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Centers for Disease Control and Prevention. I.G. and J.E.C. are named as co-inventors on a patent applied for that is associated with some of the antibodies described in this manuscript.
Published: October 20, 2016
Footnotes
Supplemental Information includes five figures and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2016.09.049.
Supplemental Information
References
- Amanna I.J., Raué H.P., Slifka M.K. Development of a new hydrogen peroxide–based vaccine platform. Nat. Med. 2012;18:974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxby D. The surface antigens of orthopoxviruses detected by cross-neutralization tests on cross-absorbed antisera. J. Gen. Virol. 1982;58:251–262. doi: 10.1099/0022-1317-58-2-251. [DOI] [PubMed] [Google Scholar]
- Bell E., Shamim M., Whitbeck J.C., Sfyroera G., Lambris J.D., Isaacs S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., Snyder J.T., Ahlers J.D., Franchini G., Moss B., Berzofsky J.A. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia M.R., McCausland M.M., Su H.P., Singh K., Hoffmann J., Davies D.H., Felgner P.L., Head S., Sette A., Garboczi D.N., Crotty S. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 2008;82:3751–3768. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia M.R., McCausland M.M., Moyron J., Laudenslager J., Granger S., Rickert S., Koriazova L., Kubo R., Kato S., Crotty S. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 2009;83:1201–1215. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M.J., Carroll A.M. The SCID mouse mutant: definition, characterization, and potential uses. Annu. Rev. Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Chapman J.L., Nichols D.K., Martinez M.J., Raymond J.W. Animal models of orthopoxvirus infection. Vet. Pathol. 2010;47:852–870. doi: 10.1177/0300985810378649. [DOI] [PubMed] [Google Scholar]
- Crowe J.E., Jr. Recent advances in the study of human antibody responses to influenza virus using optimized human hybridoma approaches. Vaccine. 2009;27(Suppl 6):G47–G51. doi: 10.1016/j.vaccine.2009.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.H., Liang X., Hernandez J.E., Randall A., Hirst S., Mu Y., Romero K.M., Nguyen T.T., Kalantari-Dehaghi M., Crotty S., et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.H., McCausland M.M., Valdez C., Huynh D., Hernandez J.E., Mu Y., Hirst S., Villarreal L., Felgner P.L., Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 2005;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.H., Molina D.M., Wrammert J., Miller J., Hirst S., Mu Y., Pablo J., Unal B., Nakajima-Sasaki R., Liang X., et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330:259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- Hughes A.L., Irausquin S., Friedman R. The evolutionary biology of poxviruses. Infect. Genet. Evol. 2010;10:50–59. doi: 10.1016/j.meegid.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.M., Newman F.K., Davidson W.B., Olson V.A., Smith S.K., Holman R.C., Yan L., Frey S.E., Belshe R.B., Karem K.L., Damon I.K. Analysis of variola and vaccinia virus neutralization assays for smallpox vaccines. Clin. Vaccine Immunol. 2012;19:1116–1118. doi: 10.1128/CVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Oie M. Epitope mosaic on the surface proteins of orthopoxviruses. Virology. 1988;163:133–144. doi: 10.1016/0042-6822(88)90240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe C.H., Bowles C., Meiklejohn G., Berge T.O., St Vincent L., Babu B.V., Govindarajan S., Ratnakannan N.R., Downie A.W., Murthy V.R. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull. World Health Organ. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- Kemper A.R., Davis M.M., Freed G.L. Expected adverse events in a mass smallpox vaccination campaign. Eff. Clin. Pract. 2002;5:84–90. [PubMed] [Google Scholar]
- Kennedy J.S., Gurwith M., Dekker C.L., Frey S.E., Edwards K.M., Kenner J., Lock M., Empig C., Morikawa S., Saijo M., et al. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infect. Dis. 2011;204:1395–1402. doi: 10.1093/infdis/jir527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantto J., Haahr Hansen M., Rasmussen S.K., Steinaa L., Poulsen T.R., Duggan J., Dennis M., Naylor I., Easterbrook L., Bregenholt S., et al. Capturing the natural diversity of the human antibody response against vaccinia virus. J. Virol. 2011;85:1820–1833. doi: 10.1128/JVI.02127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.W., Graham M.B., Hammarlund E., Hanifin J., Slifka M.K. Monkeypox without exanthem. N. Engl. J. Med. 2007;356:2112–2114. doi: 10.1056/NEJMc062788. [DOI] [PubMed] [Google Scholar]
- Lustig S., Fogg C., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 2005;79:13454–13462. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack T.M., Noble J., Jr., Thomas D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 1972;21:214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- Matho M.H., Maybeno M., Benhnia M.R., Becker D., Meng X., Xiang Y., Crotty S., Peters B., Zajonc D.M. Structural and biochemical characterization of the vaccinia virus envelope protein D8 and its recognition by the antibody LA5. J. Virol. 2012;86:8050–8058. doi: 10.1128/JVI.00836-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland M.M., Benhnia M.R., Crickard L., Laudenslager J., Granger S.W., Tahara T., Kubo R., Koriazova L., Kato S., Crotty S. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir. Ther. (Lond.) 2010;15:661–675. doi: 10.3851/IMP1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum A.M., Nakazawa Y., Ndongala G.M., Pukuta E., Karhemere S., Lushima R.S., Ilunga B.K., Kabamba J., Wilkins K., Gao J., et al. Human Monkeypox in the Kivus, a Conflict Region of the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015;93:718–721. doi: 10.4269/ajtmh.15-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S., Herman Y.F., Mattson D.E., Huxsoll D.L., Lang C.M., Yager R.H. Protection of Rhesus Monkeys against Monkeypox by Vaccinia Virus Immunization. Am. J. Vet. Res. 1964;25:192–195. [PubMed] [Google Scholar]
- Moss B. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 2011;239:8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Ruiz M., Giudicelli V., Ginestoux C., Stoehr P., Robinson J., Bodmer J., Marsh S.G., Bontrop R., Lemaitre M., Lefranc G., et al. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2000;28:219–221. doi: 10.1093/nar/28.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.L., McFadden G. Smallpox: anything to declare? Nat. Rev. Immunol. 2002;2:521–527. doi: 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- Smith S.A., Zhou Y., Olivarez N.P., Broadwater A.H., de Silva A.M., Crowe J.E., Jr. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 2012;86:2665–2675. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.A., de Alwis A.R., Kose N., Jadi R.S., de Silva A.M., Crowe J.E., Jr. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J. Virol. 2014;88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M.M., McFadden G., Karupiah G., Chaudhri G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 2007;85:93–102. doi: 10.1038/sj.icb.7100033. [DOI] [PubMed] [Google Scholar]
- Thornburg N.J., Nannemann D.P., Blum D.L., Belser J.A., Tumpey T.M., Deshpande S., Fritz G.A., Sapparapu G., Krause J.C., Lee J.H., et al. Human antibodies that neutralize respiratory droplet transmissible H5N1 influenza viruses. J. Clin. Invest. 2013;123:4405–4409. doi: 10.1172/JCI69377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verardi P.H., Titong A., Hagen C.J. A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication. Hum. Vaccin. Immunother. 2012;8:961–970. doi: 10.4161/hv.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorou R.M., Papavassiliou V.G., Pierroutsakos I.N. Cowpox virus infection: an emerging health threat. Curr. Opin. Infect. Dis. 2008;21:153–156. doi: 10.1097/QCO.0b013e3282f44c74. [DOI] [PubMed] [Google Scholar]
- Weitkamp J.H., Kallewaard N., Kusuhara K., Feigelstock D., Feng N., Greenberg H.B., Crowe J.E., Jr. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J. Immunol. Methods. 2003;275:223–237. doi: 10.1016/s0022-1759(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int. J. Infect. Dis. 2006;10:193–201. doi: 10.1016/j.ijid.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., McGraw P.A., House F.S., Crowe J.E., Jr. An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J. Immunol. Methods. 2008;336:142–151. doi: 10.1016/j.jim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva M., Kapnick S.M., Meseda C.A., Shotwell E., King L.R., Manischewitz J., Scott J., Kodihalli S., Merchlinsky M., Nielsen H., et al. Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs. J. Virol. 2011;85:9147–9158. doi: 10.1128/JVI.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.