Abstract
Cas9 is a bacterial RNA-guided endonuclease that uses base pairing to recognize and cleave target DNAs with complementarity to the guide RNA. The programmable sequence specificity of Cas9 has been harnessed for genome editing and gene expression control in many organisms. Here, we describe protocols for the heterologous expression and purification of recombinant Cas9 protein and for in vitro transcription of guide RNAs. We describe in vitro reconstitution of the Cas9-guide RNA ribonucleoprotein complex and its use in endonuclease activity assays. The methods outlined here enable mechanistic characterization of the RNA-guided DNA cleavage activity of Cas9 and may assist in further development of the enzyme for genetic engineering applications.
1. Introduction
The clusters of regularly interspaced short palindromic repeat (CRISPR)-associated protein Cas9 is an RNA-guided endonuclease that generates double-strand DNA breaks (DSBs) (reviewed in Hsu, Landers, & Zhang, 2014; Mali, Esvelt, & Church, 2013). Found in type II CRISPR systems, Cas9 functions in conjunction with CRISPR RNAs (crRNAs) and a transactivating crRNA (tracrRNA) to mediate sequence-specific immunity against bacteriophages and other mobile genetic elements (Barrangou et al., 2007; Deltcheva et al., 2011; Garneau et al., 2010). Cas9 associates with a partially base-paired crRNA-tracrRNA guide structure and the resulting ribonucleoprotein complex recognizes and cleaves DNA molecules containing sequences complementary to a 20-nucleotide guide segment in the crRNA (Gasiunas, Barrangou, Horvath, & Siksnys, 2012; Jinek et al., 2012; Karvelis et al., 2013).
Due to its programmability, Cas9 has been developed into a versatile molecular tool for genome editing in numerous organisms and cell types (reviewed extensively in Hsu et al., 2014; Mali, Esvelt, et al., 2013; Sander & Joung, 2014), including human cells (Cong et al., 2013; Jinek et al., 2013; Mali, Yang, et al., 2013), mice (Wang et al., 2013; H. Yang et al., 2013), zebrafish (Hwang et al., 2013), Drosophila melanogaster (Bassett & Liu, 2014; Gratz et al., 2013) , Caenorhabditis elegans (Cho, Lee, Carroll, Kim, & Lee, 2013; Friedland et al., 2013; Katic & Grosshans, 2013; Lo et al., 2013) and plants (Li et al., 2013; Nekrasov, Staskawicz, Weigel, Jones, & Kamoun, 2013; Shan et al., 2013; Xie & Yang, 2013). The sequence specificity of Cas9 permits the targeting of unique loci in a typical eukaryotic genome and can be readily altered in vitro and in vivo by supplying artificially designed guide RNAs either in the naturally occurring dual-RNA form or as single-molecule guide RNAs (sgRNAs) (Cong et al., 2013; Jinek et al., 2012, 2013; Mali, Yang, et al., 2013). Cas9 thus provides a superior alternative to existing protein-based approaches such as zinc finger nucleases and transcription activator-like effector nucleases. In eukaryotic cells, Cas9-generated DSBs are repaired by nonhomologous end joining or homologous recombination, which can be exploited to engineer insertions, deletions, and substitutions in the vicinity of the DSB. Furthermore, a catalytically inactive variant of Cas9 (the D10A/H840A mutant of Streptococcus pyogenes Cas9, referred to as dCas9) has been employed as an RNA-programmable DNA-binding protein for transcriptional regulation (Gilbert et al., 2013; Mali, Aach, et al., 2013; Qi et al., 2013). Variants of the basic targeting approach, including paired nickases (Mali, Aach, et al., 2013; Ran et al., 2013), dCas9-FokI fusion nucleases (Guilinger, Thompson, & Liu, 2014; Tsai et al., 2014) and 5’-truncated sgRNAs (Fu, Sanders, Reyon, Cascio, & Joung, 2014) have emerged recently to address the issue of off-targeting and to further improve Cas9 specificity.
Extensive biochemical and structural studies have illuminated many aspects of the molecular mechanism of Cas9. The two nuclease domains found in Cas9, HNH and RuvC domains, catalyze the cleavage of the complementary and noncomplementary DNA strands, respectively (Chen, Choi, & Bailey, 2014; Gasiunas et al., 2012; Jinek et al., 2012). Target DNA recognition is strictly dependent on the presence of a short protospacer adjacent motif (PAM) immediately downstream of the DNA region base-paired to the guide RNA (Gasiunas et al., 2012; Jinek et al., 2012). An 8-12 nt PAM-proximal “seed” region in the guide RNA-target DNA heteroduplex is critical for target binding by Cas9 (Jinek et al., 2012; Nishimasu et al., 2014). While seed region interactions are sufficient for target binding, DNA cleavage requires more extensive guide-target interactions (Wu et al., 2014). Nevertheless, Cas9 tolerates mismatches within the guide-target heteroduplex, which is the principal cause of off-target activity (Fu et al., 2013; Hsu et al., 2013; Mali, Aach, et al., 2013; Pattanayak et al., 2013). Recent crystal structures and electron microscopic reconstructions of Cas9 in its free and nucleic-acid-bound states have revealed that Cas9 undergoes a striking RNA-driven conformational rearrangement that results in the formation of the DNA-binding site (Anders, Niewoehner, Duerst, & Jinek, 2014; Jinek et al., 2014; Nishimasu et al., 2014). Additionally, single-molecule and ensemble biophysical studies of target recognition by the Cas9-guide RNA complex have indicated that target DNA binding is dependent on an initial recognition of the PAM, followed by local unwinding of the adjacent DNA duplex and directional formation of the guide RNA-target DNA heteroduplex (Sternberg, Redding, Jinek, Greene, & Doudna, 2014).
In this Chapter, we provide detailed protocols for the heterologous expression and purification of S. pyogenes Cas9, preparation of guide RNAs by in vitro transcription, and for the use of these reagents in endonuclease cleavage assays in vitro. The assays described here can be used to validate guide RNAs and target sites for in vivo gene targeting applications or to test the in vitro efficacy of new guide RNA structures and designs. Moreover, the described procedures can be implemented to utilize Cas9 as a programmable restriction enzyme for DNA manipulations in vitro. Although S. pyogenes Cas9 has been the mainstay of genome editing applications so far, the protocols are readily adaptable for Cas9 proteins and guide RNAs from other bacterial species and may aid in the rational design of novel Cas9 variants with altered specificity or PAM requirements.
2. Expression and Purification of Cas9
Cas9 from S. pyogenes (hereafter referred to as SpyCas9) is expressed from a pET-based T7 promoter-containing plasmid (pMJ806, available from Addgene, www.addgene.org) in the E. coli strain Rosetta 2 DE3. The expressed fusion protein construct contains an N-terminal His6-tag, followed by maltose-binding protein (MBP) polypeptide sequence, a tobacco etch virus (TEV) protease cleavage site, and the SpyCas9 sequence spanning residues 1-1368. We found that expression in the Rosetta 2 strain was necessary to overcome the unfavorable codon bias in the S. pyogenes genomic DNA sequence, while inclusion of the MBP tag further boosted expression levels. The purification protocol includes three chromatography steps: immobilized metal ion affinity chromatography (IMAC), followed by cation exchange chromatography (IEX), and a final purification by size exclusion chromatography (SEC). The protocol is generally based on previously published procedures, with minor modifications (Jinek et al., 2012, 2014; Sternberg et al., 2014). The procedure can be used for the expression and purification of mutant SpyCas9 proteins and can be adapted for the expression of Cas9 orthologs from other bacterial species.
Day 1: Cell transformation
-
1
Transform chemically competent Rosetta 2 DE3 cells (Novagen, Merck Millipore) according to the protocol supplied with the cells. Briefly, add ~200 ng of plasmid DNA (pMJ806) to 50 μl of freshly thawed competent cells and incubate on ice for 15 min. Heat-shock cells by incubation at 42 °C for 45 s, then place cells on ice for further 3 min. Add 500 μl of LB (Luria Broth) medium to the cells and incubate the culture at 37 °C for 1 h in a shaking incubator. Plate 100 μl of culture out on LB agar containing 50 μg ml-1 kanamycin and 33 μg ml-1 chloramphenicol. Incubate plates overnight at 37 °C.
Day 2: Culture growth and induction
-
2.
Pick one colony from the agar plate to inoculate 50 ml LB medium containing 50 μg ml-1 kanamycin and 33 μg ml-1 chloramphenicol. Incubate the preculture at 37 °C in a shaking incubator (250 rpm) for a minimum of 4–5 h or overnight.
-
3.
Use 7.5 ml of the preculture to inoculate 750 ml prewarmed LB medium supplemented with 50 μg ml-1 kanamycin and 33 μg ml-1 chloramphenicol in a 2 l baffled flask. We typically express 6 x 750 ml total culture volume at a time. Incubate the cultures at 37 °C in a shaking incubator (90 rpm) while monitoring the cell growth by measuring optical density at 600 nm (OD600). Reduce the temperature to 18 °C at an OD of ~ 0.8 and continue shaking for additional 30 min. Induce protein expression by the addition of 150 μl 1 M isopropyl-β-D-1-thiogalactopyranoside to each flask (200 μM final concentration). Continue shaking at 18 °C overnight for another 12-16 h.
Day 3: Cas9 purification by IMAC
-
4.
Harvest cells by centrifugation for 15 min at 3500 rpm (~2700 x g) in a swing-bucket rotor in 1 l bottles. Decant the supernatant and resuspend the cell pellets using ~15 ml ice-chilled lysis buffer (20 mM Tris-Cl, pH 8.0, 250 mM NaCl, 5 mM imidazole, pH 8.0, 1 mM phenylmethylsulfonyl fluoride) per cell pellet from 1 l culture. The resuspended cell pellets can either be used directly for further purification or flash frozen in liquid nitrogen and stored at -80 °C for several months without loss of Cas9 enzymatic activity.
-
5.
Lyse the resuspended cell pellets using a cell homogenizer (Avestin Emulsiflex). Pass the cell suspension through the homogenizer three times at ~1000 bar to ensure complete lysis. The lysate should be cooled on ice between passes.
-
6.
Clarify the lysate by centrifugation in 50 ml Nalgene Oak Ridge tubes at 18,000 rpm (~30,000 x g) in an SS-34 rotor (or equivalent) for 30 min at 4 °C. Collect the supernatant.
-
7.
All chromatographic steps should be performed at 4 °C. Equilibrate 10 ml of His-Select Ni resin (Sigma-Aldrich) packed in a XK 16/20 column housing (GE Healthcare) with 20 ml lysis buffer. Load the cleared lysate on the column using a peristaltic pump at ~1.5 ml min-1. Attach the column with bound protein to an FPLC system equilibrated in wash buffer (20 mM Tris-Cl, pH 8.0, 250 mM NaCl, 10 mM imidazole, pH 8.0).
-
8.
Wash with ~50 ml wash buffer at 1.5 ml min-1 until the absorbance nearly reaches the baseline again. Elute with ~50 ml elution buffer (20 mM Tris-Cl, pH 8.0, 250 mM NaCl, 250 mM imidazole, pH 8.0) and collect in 2 ml fractions. Analyze the peak fractions using SDS-PAGE and pool those containing Cas9 protein.
-
9.
Estimate protein concentration by measuring the absorbance at 280 nm (use elution buffer as blank). Add 0.5 mg TEV protease per 50 mg of protein. Dilute the Cas9 sample to ~1 mg ml-1 with dialysis buffer (20 mM HEPES-KOH, pH 7.5, 150 mM KCl, 10 % (v/v) glycerol, 1 mM dithiothreitol (DTT), 1 mM EDTA) and dialyze the sample in dialysis tubing with a molecular weight cut off (MWCO) of 12-14 kDa against 2 l dialysis buffer at 4 °C overnight. Dialysis buffer (without DTT and glycerol) can be prepared as a 10 x stock, but DTT should be added immediately prior to use.
Day 4: IEX and SEC chromatographic steps
-
10.
Recover the dialyzed sample from the dialysis tubing. Typically, slight precipitation occurs after successful TEV protease cleavage. Centrifuge the sample at 3900 rpm (~3200 x g) for 5 min at 4 °C to remove the precipitate. Check the extent of TEV protease cleavage using SDS-PAGE.
-
11.
Equilibrate a 5-ml HiTrap SP FF column (GE Healthcare) with IEX buffer A (20 mM HEPES-KOH, pH 7.5, 100 mM KCl) and load the cleaved protein onto the column using a peristaltic pump or a sample loading superloop at a flow rate of ~2 ml min-1. Attach the column to the FPLC system. Set the flow rate to 2 ml min-1 and the pressure limit to 0.3 MPa for further steps using the HiTrap column. Collect 2 ml fractions throughout. Wash the column with 10 ml IEX buffer A and elute bound protein by applying a gradient from 0% to 50% IEX buffer B (20 mM HEPES-KOH, pH 7.5, 1 M KCl) over 60 ml. Cas9 typically elutes in two peaks with different ratios of the absorbances at 260 and 280 nm (A260/A280). The first peak starts eluting at ~15% IEX buffer B with its maximum at ~20%, the second peak elutes at ~25-40%, with a maximum at ~30%. Analyze all peak fractions for the presence of Cas9 using SDS-PAGE. Pool Cas9-containing fractions that have an A260/A280 of less than ~0.6. The pooled sample can be stored at 4 °C overnight or can be flash frozen in liquid nitrogen and stored at -80 °C.
-
12.
Exchange the buffer to SEC buffer (20 mM HEPES-KOH, pH 7.5, 500 mM KCl, 1 mM DTT) while concentrating the protein to <1.5 ml volume using a 30,000 MWCO centrifugal concentrator (Amicon) at 3900 rpm. The buffer exchange helps circumvent precipitation in the centrifugal concentrator. Recover concentrate in a 1.5 ml Eppendorf tube and centrifuge for 10 min at 14,000 rpm (16,900 x g) at 4 °C to remove any precipitated material.
-
13.
In the meantime, equilibrate a HiLoad 16/600 Superdex 200 PG gel filtration column (GE Healthcare) with the SEC buffer. Inject concentrated Cas9 onto column using a 2 ml sample loop. Elute with 120 ml SEC buffer at a flow rate of 1 ml min-1, collecting 2 ml fractions. Cas9 typically elutes at a volume of ~66 ml. Analyze the peak fractions using SDS-PAGE and pool those containing Cas9 protein.
Day 5: Concentration and storage
-
14.
Concentrate eluted Cas9 using a 30,000 MWCO centrifugal concentrator to a concentration required for further experiments. In the described SEC buffer, Cas9 can be concentrated up to ~30 mg ml-1 (189.3 μM) without precipitation. The concentration is determined based on the assumption that 1 mg ml-1 has an absorbance at 280 nm of 0.76 (based on a calculated extinction coefficient of 120,450 M-1 cm-1).
-
15.
Divide concentrated protein sample into 50 μl aliquots and flash freeze in liquid nitrogen. Frozen Cas9 can be stored at -80 °C for several months without loss of activity. Prior to use, thaw an aliquot and dilute to the required concentration with SEC buffer. We typically dilute Cas9 to 15 μM for endonuclease activity assays. To avoid unnecessary freeze-thaw cycles that can result in loss of enzymatic activity, Cas9 can be stored on ice or at 4 °C for at least 2 days without loss of activity if used for several experiments. However, it is recommended to check the stored sample periodically for the absence of any precipitated material and to monitor protein integrity by SDS-PAGE.
3. Preparation of Guide RNAs
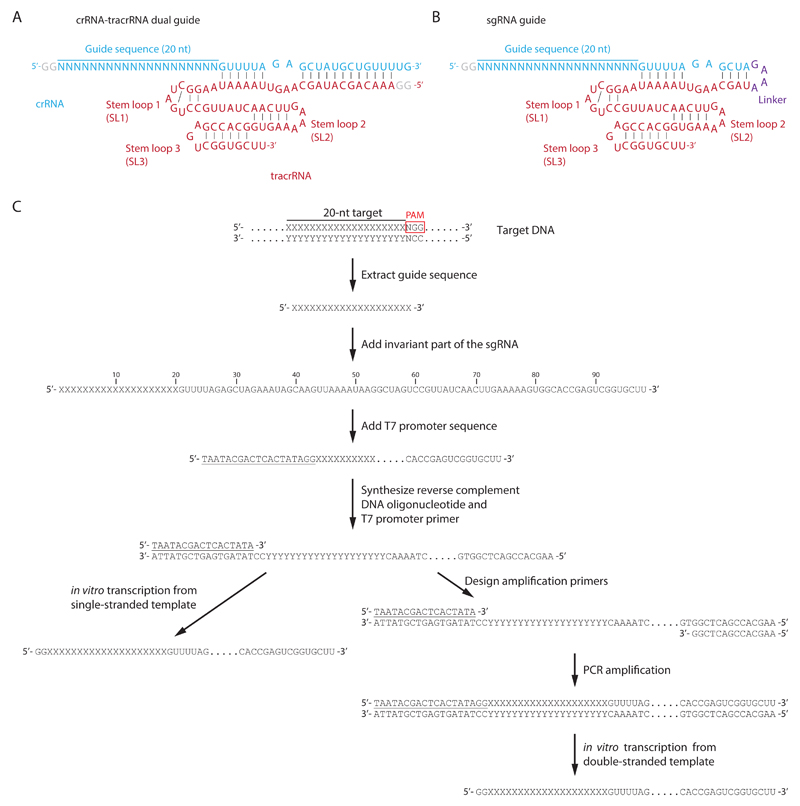
For sequence-specific DNA cleavage, Cas9 can be programmed either with a custom crRNA that is partially base-paired to an invariant tracrRNA molecule (i.e., the dual-RNA guide) or with chimeric sgRNAs that combine the essential parts of the crRNA and tracrRNA molecules in a single oligonucleotide chain (Jinek et al., 2012). When using dual-RNA guides, the crRNA guide is composed of a 5’-terminal 20-nt guide sequence, followed by an invariant 22-nt repeat-derived sequence at the 3’ end (5’-XXXXXXXXXXXXXXXXXXXX-GUUUUAGAGCUAUGCUGUUUUG-3’) that will ensure base pairing to the tracrRNA (Fig. 1.1A). The tracrRNA sequence remains identical for all guide crRNAs and corresponds to the sequence of the mature processed S. pyogenes tracrRNA (5’-AAACAGCAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCUU-3’) (Fig. 1.1A). Alternatively, a sgRNA can be used for Cas9 programming. The chimeric RNA is essentially composed of the desired 20-nt guide sequence at its 5’ end, followed by a segment corresponding to the 3’-terminal invariant sequence of the crRNA, and fused to a tracrRNA fragment with a GAAA tetraloop. The tracrRNA-derived part of the sgRNA consists of a region complementary to the repeat-derived part of crRNA and three additional stem-loops (SL1-3) at the 3’ end (Fig. 1.1B). Although 3’-terminally truncated sgRNAs containing just SL1 are functional in vitro (Jinek et al., 2012), inclusion of SL2 and SL3 increases the stability of the Cas9-crRNA-tracrRNA complex and enhances cleavage activity (Hsu et al., 2013; Nishimasu et al., 2014).
Figure 1.1.
(A) Schematic representation of the dual-RNA guide structure for programming SpyCas9. Blue: crRNA containing a 20-nt guide sequence and 22-nt invariant sequence. The GG dinucleotide at the 5’ end is appended during in vitro transcription. Red: tracrRNA base-pairing with the invariant sequence of the crRNA. tracrRNA contains three stem-loops (SL1, SL2, and SL3) at its 3’ end. Although SL1 is sufficient for Cas9-mediated cleavage in vitro (Jinek et al., 2012), inclusion of both SL2 and SL3 increases cleavage efficiency by increasing the stability of the Cas9-crRNA-tracrRNA complex (Hsu et al., 2013; Nishimasu et al., 2014). (B) Schematic representation of chimeric single-guide RNA (sgRNA). crRNA- and tracrRNA-derived sequences are connected by a 5’-GAAA-3’ tetraloop linker. (C) Outline of the procedure for sgRNA guide design and preparation by in vitro transcription.
Custom 42-nt crRNAs can be obtained as synthetic oligonucleotides, whereas tracrRNA and sgRNAs need to be prepared by in vitro transcription using T7 RNA polymerase and subsequently purified by denaturing polyacrylamide gel electrophoresis. The RNAs are sufficiently short to be transcribed using synthetic DNA oligonucleotides as transcription templates (Milligan & Uhlenbeck, 1989), without the need to clone the transcribed sequence into a DNA plasmid (Fig. 1.1C). The RNAs are transcribed as run-off products, so that no transcriptional terminator is required. Note that an optimal T7 promoter contains two G nucleotides that are required for efficient transcription and that will be appended to the 5’ end of the transcribed RNA upstream of the 20-nt guide sequence. Addition of the 5’-terminal GG dinucleotide to the guide RNA has little effect on Cas9 loading and enzymatic activity (Jinek et al., 2012; Sternberg et al., 2014). T7 polymerase can transcribe a single-stranded DNA template but requires a double-stranded promoter region for efficient template binding (Milligan, Groebe, Witherell, & Uhlenbeck, 1987). Such partially duplexed template can be prepared by annealing a T7 promoter oligonucleotide to a synthetic oligonucleotide consisting of the antisense sequence of the desired RNA followed by the reverse complement of the T7 promoter sequence (Fig. 1.1C). While this method is generally efficient and produces hundreds of micrograms of RNA from a 1 ml transcription reaction, the RNA yield can be low in some cases. Transcription efficiency can be improved by converting the template to fully double-stranded DNA by PCR amplification (Fig. 1.1C).
The following protocol is used to prepare the sgRNA used in the endonuclease activity assays described in Section 4. The protocol describes preparation of a fully double-stranded transcription templates in step 1; to prepare partially duplexed templates by annealing synthetic oligonucleotides, start at step 2. We recommend PAGE-purification of DNA oligonucleotides prior to annealing (Lopez-Gomollon & Nicolas, 2013). We typically perform in vitro transcription in a 5 ml reaction to obtain ~0.5-1 mg of pure RNA, but the reaction can be scaled down accordingly. A control transcription reaction in a total volume of 100 μl should be carried out first and analyzed by denaturing PAGE before scaling up to large volumes. T7 RNA polymerase is available from a number of commercial sources. Alternatively, several published protocols describe the expression and purification of recombinant T7 RNA polymerase (Ellinger & Ehricht, 1998; He et al., 1997; Li, Wang, & Wang, 1999; Rio, 2013).
Note: Working with RNA requires an RNase-free environment. Wear gloves and use RNase-free plasticware and filter pipette tips. Prepare all reagents using nuclease-free or DEPC-treated water (DEPC-H2O). Transcription reactions can be supplemented with RNase inhibitors if necessary.
Day 1: Preparation of transcription template
-
1
A double-stranded transcription template is prepared by amplifying a single-stranded oligonucleotide by PCR amplification of the antisense template oligonucleotide with the T7 promoter sequence as forward primer and the 3’ end of the antisense template as reverse primer (Fig. 1.1C). Mix reagents for PCR according to Table 1.1 and split into 50 μl aliquots into a 96-well plate suitable for PCR. Perform PCR cycling according to Table 1.2. After PCR, combine all reactions into 500 μl fractions in 2 ml tubes. Precipitate the PCR product by adding 50 μl 3 M sodium acetate, pH 5.2 and 1.45 ml ice-chilled 100 % ethanol to each 500 μl fraction and incubate for 1 h at -20 °C. Centrifuge the precipitated PCR product at 14,000 rpm (16,900 x g) for 40 min at 4 °C. Remove supernatant and wash the DNA pellet with 200 μl ice-chilled 70% ethanol. Centrifuge for 5 min, remove supernatant and air-dry the DNA pellet for 30 min. Dissolve the DNA pellet in 100 μl water. Measure the absorbance at 260 nm and calculate the concentration of the double-stranded DNA template. Extinction coefficients can be calculated using the OligoCalc server (Kibbe, 2007). Dilute the double-stranded template with 5 x transcription buffer (150 mM Tris-Cl, pH 8.1, 125 mM MgCl2, 0.05% Triton X-100, 10 mM spermidine) and water to a final concentration of 10 μM in a total volume of 1 ml. Incubate mixture at 75 °C for 5 min and allow it to cool down slowly to room temperature. Proceed with the transcription reaction described in step 3.
-
2
To prepare a partially single-stranded DNA template, mix 100 μl of 100 μM template oligonucleotide stock with 150 μl 100 μM (1.5-fold molar excess) complementary T7 promoter oligonucleotide (5’-TAATACGACTCACTATAGG-3’) and 200 μl 5 x transcription buffer. Add water to bring the total volume to 1 ml. Anneal the oligonucleotides at 75 °C for 5 min and let the mixture slowly cool down to room temperature.
Table 1.1.
PCR reaction for preparing double-stranded template for in vitro transcription
| Stock concentration | Final concentration | Volume (μl)* | |
|---|---|---|---|
| DEPC-H2O | - | - | 3810 |
| Phusion buffer | 5 x | 1 x | 1000 |
| dNTP mix | 10 mM each | 200 μM each | 100 |
| Primer forward | 100 μM | 0.5 μM | 25 |
| Primer reverse | 100 μM | 0.5 μM | 25 |
| Template | 1 μM | 4 nM | 20 |
| Phusion polymerase | 2 U μl-1 | 0.02 U μl-1 | 20 |
| Total volume | 5000 |
Master mix for a 96-well PCR plate. Aliquot 50 μl into each well. Scale down as appropriate.
Table 1.2.
PCR cycling program
| 98 °C | 30 s | |
| 98 °C | 5 s | Repeat 34 x |
| 42 °C | 20 s | |
| 72 °C | 10 s | |
| 72 °C | 1 min | |
| 4 °C | ∞ |
Day 2: In vitro transcription and gel purification
-
3.
Transcription reaction: Set up a transcription reaction by mixing the reagents according to Table 1.3. A 5 ml reaction is usually sufficient for ~0.5-1 mg of pure sgRNA. The reaction can be scaled down as needed.
Incubate the reaction at 37 °C for 1.5 h. During transcription, magnesium pyrophosphate precipitates from the reaction due to production of inorganic pyrophosphate upon NTP incorporation into nascent transcripts. To restore magnesium ion levels, add 25 μl 1 M MgCl2 after 1.5 h and continue with incubation at 37 °C for another 1.5 h. The optimal reaction times may vary as a function of length and RNA sequence of the transcribed RNA and should be determined empirically.
-
4.
Add 25 μl (25 U) of RNase-free RQ1 DNase (Promega) to the transcription reaction and incubate 15 min at 37 °C in order to remove the template DNA. Centrifuge the mixture for 5 min at 3900 rpm (~3200 x g) and 4 °C to pellet magnesium pyrophpsphate and remove the supernatant containing transcribed RNA. The supernatant can be stored at -20 °C at this point.
-
5.
Add 5 ml 2 x RNA loading dye (5% glycerol, 2.5 mM EDTA, pH 8.0, 90% formamide, trace bromophenol blue) to the sample. Separate the RNA by electrophoresis on a prewarmed 8% polyacrylamide, 7 M urea denaturing gel in 0.5 x TBE (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA, pH 8.0) until the bromophenol blue dye reaches the lower quarter of the gel. We typically use gels with the dimensions of 400 mm x 360 mm x 4 mm (h x w x d) and run them at 50 W for ~4-6 h. Visualize RNA by UV-shadowing over a silica glass TLC plate and excise the band corresponding to the correct RNA product using a disposable scalpel. Crush the gel slices in a 50-ml tube using a clean spatula or a plastic serological pipette and add water to 50 ml total volume. Incubate overnight at 4 °C while rocking.
Table 1.3.
In vitro transcription reaction
| Stock concentration | Final concentration | Volume (ml) | |
|---|---|---|---|
| DEPC-H2O | - | - | 1.95 |
| Transcription buffer | 5 x | 1 x | 1.00 |
| ATP | 100 mM | 5 mM | 0.25 |
| UTP | 100 mM | 5 mM | 0.25 |
| GTP | 100 mM | 5 mM | 0.25 |
| CTP | 100 mM | 5 mM | 0.25 |
| DTT | 1 M | 10 mM | 0.05 |
| Hybridized template | 10 μM | 1 μM | 0.50 |
| T7 RNA polymerase | 1 mg ml-1 | 0.1 mg ml-1 | 0.50 |
| Total volume | 5.00 |
Day 3: Gel purification - continued
-
6.
Centrifuge the crushed gel suspension for 5 min at 3900 rpm (~3200 x g) and 4 °C and collect the supernatant. Filter supernatant through a 0.22-μm disposable filter (Steriflip, Millipore). Concentrate the eluted RNA in 3000 Da MWCO centrifugal concentrator to a final volume of ~2 ml. Aliquot the concentrate into 500 μl fractions in 2-ml tubes. Add 50 μl 3 M sodium acetate, pH 5.2 and 1.5 ml ice-chilled 100 % ethanol to each 500 μl fraction and incubate for 1 h at -20 °C. Centrifuge the precipitated guide RNA at 14,000 rpm (16,900 x g) for 40 min at 4 °C. Remove supernatant and wash the RNA pellet with 200 μl ice-chilled 70% ethanol. Air-dry the pellet and dissolve in 100 μl water. Determine RNA concentration by measuring absorbance at 260 nm.
4. Endonuclease Cleavage Assays
Endonuclease cleavage assays can be used to characterize the activity of purified Cas9 or test the in vitro efficacy of a particular guide RNA or a target DNA site. In these assays, the target DNA site, including its PAM motif, is either inserted into a plasmid or provided in the form of an oligonucleotide duplex. Cleavage of plasmid substrates is monitored by agarose gel electrophoresis and staining with an intercalating dye, whereas oligonucleotide substrates typically require labeling (with a radioisotope or a fluorophore) of one or both target DNA strands. Although we previously used 5’-radiolabeling with 32P phosphate, we recently switched to ATTO532-labeled oligonucleotides, choosing the fluorophore for its superior quantum yield and photostability. Custom 5’-ATTO532-labeled oligonucleotides are readily available from commercial sources.
In both plasmid and oligonucleotide cleavage assays, Cas9 and guide RNA are preincubated in a 1:1 molar ratio in the cleavage buffer to reconstitute the Cas9-guide RNA complex prior to the addition of target DNA. Preincubation is not strictly required for cleavage (Jinek et al., 2012). The cleavage reaction is started by the addition of DNA to the binary complex. As Cas9 is a single turnover enzyme (Sternberg et al., 2014), it is important to maintain the protein-RNA complex in excess over the DNA substrate. We recommend a molar ratio of 5:1 or higher to ensure complete cleavage. The reaction mixture is sampled at different time points and analyzed by gel electrophoresis. Since the reaction rate can strongly vary as a function of DNA source and length (oligonucleotide vs. plasmid, supercoiled circular vs. linear), optimal enzyme and substrate concentrations, and also reaction time points need to be determined empirically. In both assay types, product formation can be quantified by densitometry using a fluorescence scanner and curve fitting to extract pseudo-first-order rate constants.
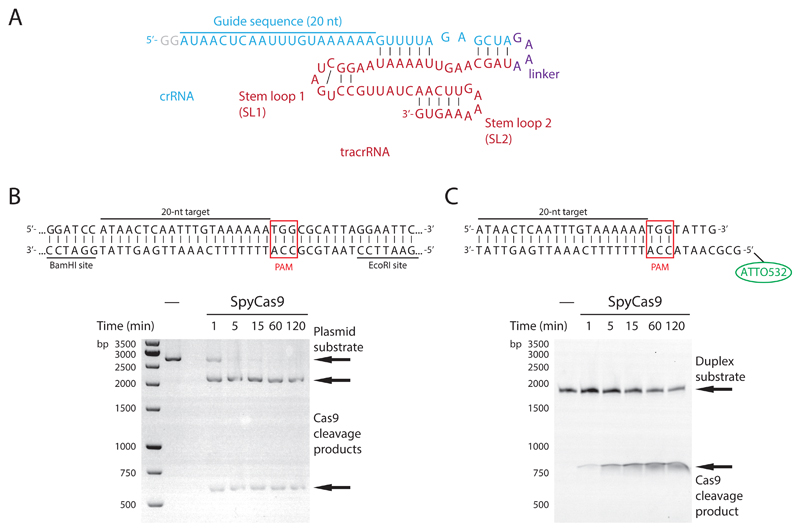
For endonuclease cleavage assays shown in Fig. 1.2, SpyCas9 was programmed with chimeric sgRNA guides to cleave either linearized plasmid DNA or a short oligonucleotide duplex substrate. The sgRNA guide included stem loops SL1 and SL2 in its 3’-terminal region (Fig. 1.2A), which were sufficient for Cas9 loading and robust DNA cleavage activity. Plasmid cleavage was carried out with a pUC19-derived plasmid in which the target site (including a 5’-NGG-3’ PAM) was inserted between the EcoRI and BamHI sites (Fig. 1.2B). The oligonucleotide duplex substrate contained a 5’-ATTO532-labeled target strand containing an 8-nt linker at the 5’ end, followed by the complementary PAM sequence (5’-CCN-3’) and the 20-nt target sequence. The target strand was annealed to a complementary unlabeled nontarget strand containing the 5’-NGG-’3 PAM (Fig. 1.2C). A 250-fold excess of Cas9-guide RNA complex over DNA substrate was used. The following protocol describes the technical details of the two assays.
Figure 1.2.
(A) sgRNA used in endonuclease activity assays in panels B and C. The sgRNA contains stem-loops SL1 and SL2 but lacks SL3 (total length of sgRNA is 83 nt). (B) Endonuclease activity assay of SpyCas9 using SspI-linearized plasmid (2702 bp). Samples were taken at indicated time points. Cleavage products (2104 and 598 bp) were resolved on a 1% agarose gel and stained with GelRed. The sequence of the target site in the plasmid substrate is shown above the gel image. (C) Endonuclease activity assay of SpyCas9 using a double-stranded oligonucleotide target. The oligonucleotide duplex substrate is shown above the gel image. The cleavage reaction was sampled the indicated time points and analyzed by electrophoresis on a denaturing (7 M urea) polyacrylamide gel. ATTO532 fluorescence was detected using a FLA9500 laser scanner.
Substrate preparation
-
1
For plasmid substrates, linearize the circular DNA by a restriction digest. Choose a restriction enzyme that cuts at a unique site away from the Cas9 target site so that Cas9-mediate cleavage of the linear DNA produces two well-separated fragments. For pUC19-based plasmids, use 50 units of SspI-HF (New England Biolabs) and 5 μg plasmid in 1 x CutSmart™ buffer in a total volume of 50 μl. Incubate the reaction for 1 h at 37 °C. Heat-inactivate SspI-HF by incubating for 20 min at 65 °C. Analyze plasmid cleavage by electrophoresis on a 1 % agarose gel in 1 x TAE buffer (40 mM Tris, pH 8.0, 20 mM glacial acetic acid, 1 mM EDTA, pH 8.0) stained with GelRed (Biotium) or similar nucleic acid stain. Complete digestion of pUC19-derived vectors yields a single band of ~2700 bp.
-
2
For oligonucleotide duplex substrate, the 5’-ATTO532-labeled target strand oligonucleotide should be PAGE-purified. To generate the oligonucleotide duplex, anneal target and nontarget strand by mixing the oligonucleotides in a molar ratio of 1:1.5. Prepare 100 μM stock solutions of the target and nontarget strand oligonucleotides. Mix 1.0 μl of the target strand with 1.5 μl of the nontarget strand and add water to a total volume of 25 μl. Heat the mixture to 75 °C for 5 min and cool slowly to room temperature (Table 1.5). Dilute the mixture with 225 μl DEPC-H2O to obtain 400 nM stock solution of the oligonucleotide duplex substrate.
Table 1.5.
Endonuclease activity assay using oligonucleotide duplex substrate
| Stock concentration | Final concentration | Volume (μl) | |
|---|---|---|---|
| DEPC-H2O | - | - | 1.5 |
| Cleavage buffer | 5 x | 1 x | 21.0 |
| SpyCas9 | 15 μM | 5 μM | 35.0 |
| Guide RNA | 15 μM | 5 μM | 35.0 |
| Incubate for 10-15 min at room temperature. Then add annealed DNA duplex. | |||
| Duplex substrate* | 400 nM | 20 nM | 12.5 |
| Total volume | 105.0 | ||
The target and nontarget DNA are annealed prior addition to the reaction. See step 2 for the annealing procedure.
Cleavage assay
-
3.
Prepare 5 x cleavage buffer (100 mM HEPES, pH 7.5, 500 mM KCl, 25% glycerol, 5 mM DTT, 2.5 mM EDTA, pH 8.0, 10 mM MgCl2).
-
4.
Dilute Cas9 to 15 μM with SEC buffer (see Seption 2). Dilute in vitro transcribed sgRNA to 15 μM with water.
-
5.
Anneal the guide RNA by heating to 90 °C for 5 min and slowly cooling to room temperature.
-
6.
Set up reaction mixes according to Table 1.4 (plasmid DNA substrates) or Table 1.5 (oligonucleotide DNA substrates). Add equimolar amounts of Cas9 protein and guide RNA in the reaction mix without DNA. Incubate for 10-15 min at room temperature.
-
7.
Start the cleavage reaction by adding the DNA target to the reaction mix and incubate at 37 °C immediately.
-
8.
Remove 10 μl aliquots at different time points and quench by adding to 1.0 μl of 500 mM EDTA, pH 8.0 (final concentration 50 nM) in a separate 1.5 ml tube. Mix by pipetting up and down and store at -80 °C until all time points are collected.
-
9.
Thaw the samples and add 1.0 μl Proteinase K (20 mg.ml-1) to digest DNA-bound Cas9. Incubate for 20 min at room temperature. Add 6 x plasmid DNA-loading buffer (10 mM Tris, pH 7.6, 60 mM EDTA, 60% glycerol, 0.03% bromophenol blue, 0.03% xylene cyanol FF) or 2 x oligonucleotide-loading buffer (90% formamide, 10% glycerol) to each sample.
-
10.
Plasmid cleavage assay: Analyze each plasmid sample on a 1% agarose gel in 1 x TAE stained with GelRed (Biotium) or compatible nucleic acid stains. For visualization with a Typhoon FLA 9500 scanner, loading 2 μl of sample was sufficient.
-
11.
Oligonucleotide cleavage assay: Resolve oligonucleotide duplex samples on a 16% polyacrylamide, 7 M urea denaturing gel (300 mm x 180 mm; 1 mm thick) in 0.5 x TBE buffer. Load 12.5 μl of each sample and run at 25 W for ~2 h until the bromophenol blue dye front has reached the bottom half of the gel. Scan the gel using a laser gel scanner (e.g., Typhoon FLA9500, GE Healthcare) with the appropriate excitation and emission wavelength settings (532 and 553 nm for ATTO532, respectively).
Table 1.4.
Endonuclease activity assay using linearized plasmid DNA substrate
| Stock concentration | Final concentration | Volume (μl) | |
|---|---|---|---|
| DEPC-H2O | - | - | 22.0 |
| Cleavage buffer | 5 x | 1 x | 11.0 |
| SpyCas9 | 15 μM | 1.5 μM | 5.5 |
| Guide RNA | 15 μM | 1.5 μM | 5.5 |
| Incubate for 10-15 min at room temperature. Then add the DNA. | |||
| Plasmid DNA | 100 ng μl-1 | 10 ng μl-1 | 5.5 |
| Total volume | 55.0 | ||
Interpretation of cleavage assays
-
12.
Plasmid-based assays: In the absence of Cas9-mediated cleavage, linearized plasmid yields a single band at 2702 bp. Cleavage by Cas9 leads to gradual emergence of two cleavage products at 2104 and 598 bp.
-
13.
Oligonucleotide-based assays: Cleavage of the oligonucleotide substrate by Cas9 leads to a mobility shift of the fluorescent signal. The 5’-labeled target strand of the substrate has a length of 31 nt, while the cleaved product runs as a 14-nt band.
5. Concluding Remarks
Thanks to its specificity and easy programmability, Cas9 represents a revolutionary advance in genetic engineering technologies for basic research, biomedicine, and biotechnology. In this chapter, we have outlined procedures for generating recombinant Cas9 and guide RNAs and for performing endonuclease cleavage assays with the reconstituted Cas9-guide ribonucleoprotein complex. These protocols provide the experimental framework for further in vitro mechanistic studies of Cas9 that will facilitate ongoing development of Cas9-based genetic engineering technologies. A number of questions pertaining to the molecular mechanism of Cas9 remain unanswered, in particular the nature of the conformational rearrangements that activate Cas9 nuclease domains prior to target cleavage. In the context of genome editing and regulation in eukaryotic cells, major outstanding questions concern the off-target activity of Cas9 and the effect of chromatin structure on Cas9 targeting and DNA cleavage. Therefore, further in vitro studies will be required to define the functional constraints of Cas9. Finally, although SpyCas9 has been extensively characterized, relatively little is known about the biochemical properties of other Cas9 orthologs. The protocols provided here can be readily applied to Cas9 proteins from other bacterial species or novel rationally designed Cas9 variants in order to expand the molecular toolbox for genome engineering.
Acknowledgments
We thank Ole Niewoehner and Alessia Duerst for technical assistance. This work was supported by the University of Zurich and the European Research Council (ERC) Starting Grant ANTIVIRNA (337284).
References
- Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–573. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- Bassett AR, Liu J-L. CRISPR/Cas9 and genome editing in Drosophila. J Genet Genomics. 2014;41:7–19. doi: 10.1016/j.jgg.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Choi J, Bailey S. Cut Site Selection by the Two Nuclease Domains of the Cas9 RNA-guided Endonuclease. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M113.539726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Lee J, Carroll D, Kim J-S, Lee J. Heritable Gene Knockout in Caenorhabditis elegans by Direct Injection of Cas9-sgRNA Ribonucleoproteins. Genetics. 2013 doi: 10.1534/genetics.113.155853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger T, Ehricht R. Single-step purification of T7 RNA polymerase with a 6-histidine tag. BioTechniques. 1998;24:718–720. doi: 10.2144/98245bm03. [DOI] [PubMed] [Google Scholar]
- Friedland AE, Tzur YB, Esvelt KM, Colaiácovo MP, Church GM, Calarco JA. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CrIsPr-Cas nucleases in human cells. Nat Biotechnol. 2013:1–6. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau JE, Dupuis M-È, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci US A. 2012;109:E2579–86. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O'Connor-Giles KM. Genome Engineering of Drosophila with the CRISPR RNA-Guided Cas9 Nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Rong M, Lyakhov D, Gartenstein H, Diaz G, Castagna R, McAllister WT, Durbin RK. Rapid mutagenesis and purification of phage RNA polymerases. Protein Expr Purif. 1997;9:142–151. doi: 10.1006/prep.1996.0663. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013 doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh J-RJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvelis T, Gasiunas G, Miksys A, Barrangou R, Horvath P, Siksnys V. crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA biology. 2013;10 doi: 10.4161/rna.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic I, Grosshans H. Targeted Heritable Mutation and Gene Conversion by Cas9-CRISPR in Caenorhabditis elegans. Genetics. 2013 doi: 10.1534/genetics.113.155754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35(Web Server issue):W43–6. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-F, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 2013;31:688–691. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang E, Wang Y. A modified procedure for fast purification of T7 RNA polymerase. Protein Expr Purif. 1999;16:355–358. doi: 10.1006/prep.1999.1083. [DOI] [PubMed] [Google Scholar]
- Lo T-W, Pickle CS, Lin S, Ralston EJ, Gurling M, Schartner CM, Bian Q, Doudna JA, Meyer BJ. Precise and Heritable Genome Editing in Evolutionarily Diverse Nematodes Using TALENs and CRISPR/Cas9 to Engineer Insertions and Deletions. Genetics. 2013 doi: 10.1534/genetics.113.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gomollon S, Nicolas FE. Purification of DNA Oligos by denaturing polyacrylamide gel electrophoresis (PAGE) Meth Enzymol. 2013;529:65–83. doi: 10.1016/B978-0-12-418687-3.00006-9. [DOI] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013a doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013b;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, Dicarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013c;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Meth Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Nekrasov V, Staskawicz B, Weigel D, Jones JDG, Kamoun S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:691–693. doi: 10.1038/nbt.2655. [DOI] [PubMed] [Google Scholar]
- Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell. 2014 doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013 doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin C-Y, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio DC. Expression and Purification of Active Recombinant T7 RNA Polymerase from E. coli. Cold Spring Harbor Protocols. 2013;2013 doi: 10.1101/pdb.prot078527. pdb.prot078527-pdb.prot078527. [DOI] [PubMed] [Google Scholar]
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu J-L, Gao C. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 2013;31:686–688. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014:1–17. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol. 2014;32:569–576. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, Jaenisch R, et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol. 2014 doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Yang Y. RNA-guided Genome Editing in Plants Using A CRISPR-Cas System. Mol Plant. 2013 doi: 10.1093/mp/sst119. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-Step Generation of Mice Carrying Reporter and Conditional Alleles by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]