Abstract
Natural killer (NK) cells are specialized innate lymphoid cells that survey against viral infections and malignancy. Numerous advances have improved our understanding of the molecular mechanisms that control NK cell development and function over the past decade. These include both studies on the regulatory effects of transcription factors and translational repression via microRNAs. In this review, we summarize our current knowledge of DNA-binding transcription factors that regulate gene expression and thereby orchestrate NK cell development and activation, with an emphasis on recent discoveries. Additionally, we highlight our understanding of how RNA-bindings microRNAs fine tune the NK cell molecular program. We also underscore the large number of open questions in field that are now being addressed using new technological approaches and genetically engineered model organisms. Ultimately, a deeper understanding of the basic molecular biology of NK cells will facilitate new strategies to manipulate NK cells for the treatment of human disease.
Keywords: NK cells, transcription factors, microRNAs, mRNA, molecular biology, innate lymphoid cells
1. Transcription factors
1.1 Introduction
Natural killer (NK) cells are innate lymphocytes critical for host protection against viral infections and immune malignancy surveillance [1,2]. NK cells participate in early immune responses through the production of cytokines and chemokines, including IFN-γ, and through cytotoxic effector functions through the directed release of apoptosis-inducing granule proteins [2]. Triggering of an NK cell response is mediated by an imbalance of activating to inhibitory signals delivered via cell surface receptors [3,4]. In the healthy state, inhibitory signals are constitutively provided by self-defining ligands, usually through cell-surface major histocompatibility complex (MHC) class I molecules. NK cell activation is triggered during inflammatory conditions when host cells upregulate stress ligands that engage NK cell activating receptors or cytokines bind to constitutively expression receptors. Alternatively, loss of class I MHC expression, which occurs as a result of viral infection or malignant transformation, can release inhibition of NK cell activity [5].
In mice, NK cells are identified by the expression of the activating receptors NK1.1 (in certain strains including C57Bl/6) and NKp46 and lack of T cell (CD3, TCR) and B cell (e.g. CD19) lineage marker expression. In humans, NK cells can be identified by the expression of CD56, and further subdivided by CD16 expression into CD56bright and CD56dim subsets. NK cells originally develop within the bone marrow and undergo terminal differentiation in both the bone marrow and peripheral lymphoid tissues [6]. Like T and B cells, NK cells originate from the common lymphoid progenitor but, during differentiation, express both shared and distinct molecular programs that direct NK cell development [7]. The progression of NK cell development can be followed by changes in surface marker expression. For example, murine NK cells can be divided into discrete stages based on the expression of CD27 and CD11b [8]. Similarly, human NK cells have been divided into several stages marked by the acquisition of CD56, CD117, and CD94 [9,10]. Terminal differentiation of NK cells is accompanied by the expression of NK cell receptors, specifically Ly49 receptors in mice and killer-cell immunoglobulin-like receptors (KIRs) in humans. Furthermore, human NK cells are thought to transition from the CD16− CD56bright NK cells to CD16+ CD56dim NK cells [11]. Recently, it has been appreciated that distinct, tissue specific NK cell subsets also contribute to the heterogeneity of this innate lymphoid lineage [12].
As NK cells mature, they acquire the capacity for effective functional responses, marked by the acquisition of a set of activating and inhibitory receptors, production of effector granule proteins and cytokines, and the altered expression of cytokine and chemokine receptors. The effector proteins produced by NK cells include granzymes, perforin, IFN-γ, TNF, MIP1α, Rantes, and other cytokines and chemokines [7]. The development and functionality of NK cells are under tight regulatory control by both transcription factors and post-transcriptional mechanisms, including that of microRNAs (miRNAs). The gene expression profiles of both human and mouse NK cells have been recently characterized and have largely confirmed the expression of transcription factors and genes previously reported to be important in the NK cell lineage [13,14]. However, cross-interactions between transcription factors make defining the individual roles of each transcription factor difficult [15]. Furthermore, transcription factors acting early during NK cell lineage commitment have been a challenge to identify, in part due to difficulties in defining the earliest committed NK cell progenitors. In this review, we highlight recent findings in the regulation of NK cell gene expression, focusing on work elaborating the functions of novel and previously described transcription factors. The developmental stages and functional responses these transcription factors control are summarized in Figures 1 and 2 for mouse and human NK cells, respectively.
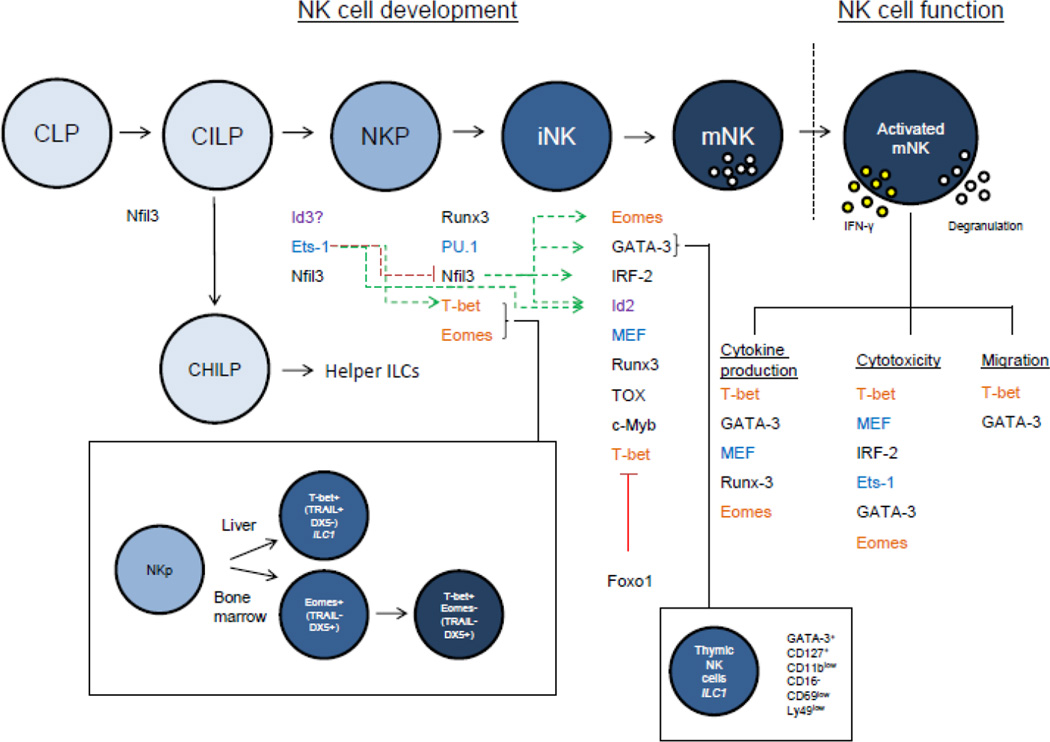
Figure 1.
Regulation of murine NK cell development and effector functions by transcription factors. Transcription factors are listed between stages in which they are thought to regulate, or under the effector function they control. Question marks indicate that its role in NK cells is unclear. Green and red arrows indicate positive and negative regulation between transcription factors, respectively; dotted lines indicate an indirect or unknown mechanism of interaction, and solid lines indicate direct interactions between transcription factors. Related family members are color coded. Given the relatively recent characterization of the CILP, the precise role of many of these transcription factors in governing helper ILC vs. NK cell development has not been fully elucidated. Here, NKP includes the described pre-pro NK and rNKP precursor/progenitor populations.
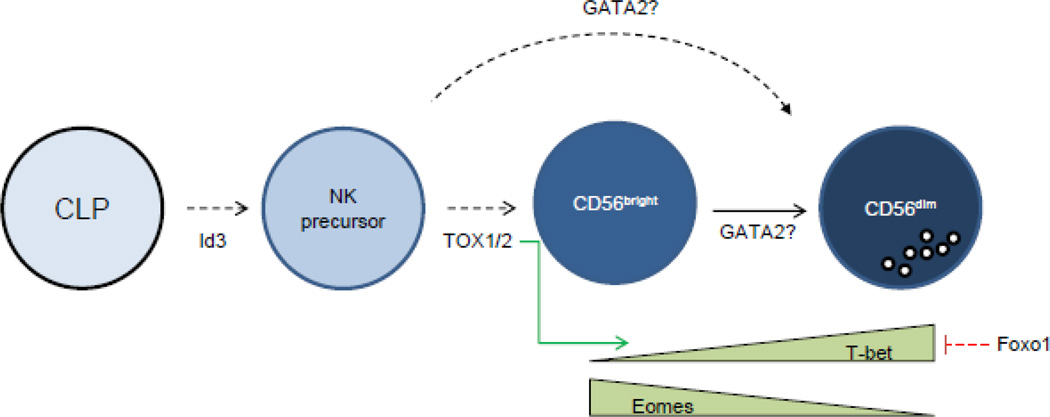
Figure 2.
Regulation of human NK cell development by transcription factors. Transcription factors are listed between stages in which they are thought to regulate. Green and red arrows indicate positive and negative regulation between transcription factors, respectively; dotted lines indicate an indirect or unknown mechanism of interaction, and solid lines indicate direct interactions between transcription factors.
1.2 ETS family
The ETS family of transcription factors are winged helix-turn-helix proteins with reported functions in cellular development and activation [16]. Although the family is composed of a large number of members, only a few have been implicated in NK cell development, including PU.1, Ets-1, and MEF [17–19]. Elf-1, an ETS family member related to MEF, was shown not to impact NK cell development [20]. All ETS family members contain a conserved ETS domain that facilitates DNA binding.
Barton et al. reported the first phenotype of NK cells in Ets-1 deficient mice, which have reduced splenic NK cell numbers likely due to impaired NK cell development, yet intact development of other lymphocyte lineages [17]. The NK cells that do develop have defective cytotoxic activity against tumor cells, fail to clear NK cell sensitive RMA-S tumor challenge, and exhibit reduced in vivo production of IFN-γ. Additional analysis of the Ets-1−/− mice by Ramirez et. al., confirmed the NK cell developmental defects, showing that Ets-1 is required for early stages of development during the transition between the common lymphoid progenitor (CLP) to the NK precursor (NKP). Consequently, Ets-1−/− mice have a significant reduction in both the immature and mature NK cell pools. Furthermore, Ets-1−/− NK cells have defects in activating receptor expression, including NKp46, Ly49H, and Ly49D [21]. However, these NK cells paradoxically appear to be chronically activated and have increased expression of inhibitory Ly49 receptors, including Ly49G2 and Ly49E. Additionally, Ets-1−/− NK cells have increased proliferative responses and granzyme B induction following IL-15 stimulation. This suggests that Ets1 is likely required for modulating IL-15 responsiveness during homeostasis and may thereby mechanistically explain the defect in NK cell development. However, defining the precise role of Ets-1 in NK cell biology is complicated by its direct regulation of the transcription factors T-bet and Id2, two transcription factors involved in NK cell development, and by the presence of other partially homologous ETS family members that may have redundant functions [21].
1.3 NFIL3
Nfil3, also known as E4bp4, is a basic leucine zipper transcription factor important for promoting NK cell developmental processes. With the exception of other innate lymphoid cells and CD8α conventional dendritic cell, Nfil3 does not appear to play a role in the development of other leukocytes, but regulates cell-specific functions such as IgE class switching in B cells and the control of Th2 responses [22]. Earlier studies demonstrated an essential requirement for Nfil3 in the generation of immature NK cells and their optimal cytotoxic function and IFN-γ production [23,24]. Nfil3 acts downstream of the IL-15 receptor through the IL-15-PI3K-AKT-mTOR pathway [25], and thus the severe NK cell reduction in Nfil3−/− mice phenocopies that of the IL-15−/− or IL-15Rα−/− mice [26,27]. Nfil3 is necessary for the progression from an NKP to an immature NK cell (iNK) and this is reflected in the significantly increased Nfil3 expression in immature NK cells compared to precursors [23]. However, it is now clear that Nfil3 is not essential past this developmental checkpoint, as NKp46icre or tamoxifen-inducible Cre crossed with the Nfil3flox mice contain normal numbers of NK cells [28]. Since NK cells are dependent on IL-15 throughout their development, IL-15 signaling at maturation stages beyond the iNK appears to be Nfil3 independent. Furthermore, Nfil3 is important for the generation of all ILCs, including the common innate lymphoid progenitor (CILP) [29,30] but does not affect the development of some tissue-specific NK cells, including salivary gland and certain liver and thymic NK cells [31–33].Specifically, in the liver, thymus, and spleen,Nfil3 deficiency appears to most severely impact Eomeshigh DX5high NK cells, which constitute the vast majority of splenic NK cells but are present to a more variable extent in the thymus and liver [32,33], Nfil3−/− mice also contain a small population of Ly49H+ cells that is both functionally competent and able to generate memory NK cells [28]. Thus, Nfil3 appears to have pleiotropic effects not uniformly applicable to all NK cell subtypes. Additional studies have identified both Eomes and Id2 as targets of Nfil3, and retroviral overexpression of either factor largely rescued in vitro Nfil3−/− NK cell development [34].
1.4 T-bet and Eomes
T-bet and Eomes are highly related family members of the T-box transcription factor family. Considerable work has been done establishing their roles in regulating lineage commitment and functional responses in T cells. In particular, T-bet is known to be the master transcription factor driving T helper type 1 (TH1) cell development and IFN-γ production downstream of IL-12 signaling [35]. In NK cells, T-bet−/− mice have reduced numbers of peripheral NK cells due to a maturation block between stage III (CD27+CD11b+) and stage IV (CD27−CD11b+) NK cells [36]. T-bet−/− NK cells produce IFN-γ normally in short-term 6 hour activation assays, but IFN-γ+ NK cells are reduced at 24 hours in T-bet−/− mice, consistent with increased NK cell death in those cultures. Cytotoxicity assays also demonstrated reduced killing by cytokine-activated T-bet−/− NK cells in vitro, and MCMV-activated NK cells ex vivo. While there were clear functional impairments in NK cells from T-bet−/− mice, there were no differences in early MCMV viral titers, and consequently similar host protection.
Eomes-deficient mice show a greater reduction of splenic NK cells compared to T-bet-deficiency, and combined deletion of Eomes and T-bet results in a near total loss of immature and mature NK cells [37]. That Eomes and T-bet play both unique and redundant roles in NK cell development was further clarified by examination of the liver, which contains a distinct population of liver resident NK cells (also termed ILC1) defined by Eomes− TRAIL+ DX5− expression[37–39]. Initially, TRAIL+ DX5− (Eomes−) NK cells were shown to be precursors to TRAIL− DX5+ (Eomes+) NK cells [37]. However, a subsequent study using sorted NK cells from Eomes-GFP reporter mice demonstrated that Eomes− and Eomes+ NK cells within the liver are stable populations [40]. Additional work is necessary to clarify whether certain time-dependent environmental cues during either neonatal or adult hematopoiesis may induce this conversion or maintains these separate lineages. Cross-regulation of T-bet and Eomes by other transcription factors may also influence their role during development. For example, Foxo1 negatively regulates late-stage NK cell maturation and IFN-γ production through T-bet repression [41]. The role of Eomes in the regulation of effector functions is more consistent than its role in development. Eomes deficiency does not appear to impact degranulation or cytokine production to a major degree. However, Eomes− NK cells primarily located in the liver appear to be a functionally distinct NK subset from Eomes+ NK cells. Indeed, the decreased perforin expression, increased granzyme B/C expression, and production of IL-2 by this Eomes− subset makes them more akin to NK T cells than to Eomes+ NK cells [37,40].
Several target genes of T-bet and Eomes have been identified, including IFN-γ, granzyme B, perforin, and Runx1 [36]. Additionally, T-bet promotes the expression of PRDM1, a transcription factor that is necessary for appropriate NK cell development and selective regulation of effector functions, such as augmentation of NK cell proliferation, but dispensable for cytokine production and cytotoxicity [42]. However, NK cells from PRDM1-deficient mice do not completely recapitulate the phenotype of T-bet−/− mice, suggesting that additional T-bet regulated factors contribute to the developmental block. The direct transcriptional targets of Eomes in NK cells have not been characterized in depth, although there is evidence that it regulates perforin and granzyme B in CD8+ T cells [43].
T-bet has additionally been linked to the expression of the chemokine receptor sphingosine-1-phosphate receptor 5 (S1P5) [44]. NK cells from a mouse with a point mutation in T-bet (Duane) were preferentially localized in the bone marrow and lymph nodes due to an inability to migrate towards the S1P gradient in the peripheral blood. Thus, in addition to its role in maturation, T-bet appears to have roles in regulating the selective expression of a chemokine receptor.
In human NK cells, both CD56bright and CD56dim NK cells highly express T-bet and Eomes, and the majority of mature NK cells co-express both [45]. However a fraction of CD56bright NK cells express lower T-bet levels than CD56dim NK cells. Eomes expression shows an inverse pattern in which CD56bright NK cells are nearly all Eomes+, but a fraction of CD56dim NK cells express less Eomes. It is unclear whether this represents a maturation-associated switch in T-box transcription factor expression or whether these are distinct subsets of mature NK cells, as has been described in the mouse setting [40]. Further, there has been limited investigation to date of the key targets of T-bet and Eomes important for human NK cell development and function.
1.5 RUNX proteins
The RUNX (runt-related transcription factor) protein family is composed of three highly homologous members (Runx1, Runx2, and Runx3). Runx proteins heterodimerize with the protein CBFβ and bind to DNA via a conserved Runt domain [46]. Runx3 is the dominant family member expressed by NK cells and CD8 T cells, although Runx1 is also expressed to a lesser extent [47,48]. Runx2 is likely involved in osteochondrogenesis rather than hematopoiesis [49]. Runx3 is abundantly expressed upon differentiation to an immature NK cell (NK1.1+ CD3− DX5-) and is maintained in mature NK cells (NK1.1+ CD3− DX5+) [48]. A number of important NK cell genes have been found to have Runt binding motifs, including the promoters of murine CD122, Ly49 receptors, NKp46, and human KIR genes, including KIR3DL1 and KIR2DL4 [48,50–54]. CD2-cre-directed dominant-negative Runx transgenic mice (that have reduced Runx3 binding in iNK and mNK) have an overall reduction in NK cell maturity and reduced Ly49 expression [48]. However, NK cells that develop in Runx-dominant-negative mice have enhanced cytokine-induced IFN-γ production but unchanged cytotoxicity against tumor targets, suggesting a decoupling between maturation and functional attributes in this setting. The impact of genetic deletion of Runx3 on NK cell development and function was reported [55]. Runx3−/− mice had normal numbers of NK cells at baseline, and modest alteration in CD27/CD11b maturation, with increased stage I (CD27−CD11b−) and stage II (CD27+CD11b−), and decreased stage III (CD27+CD11b+) frequencies. Runx3−/− splenic NK cells had decreased proliferative responses to IL-15R based stimulation in vitro and in vivo. Analysis of relevant targets of Runx3 were performed using ChiP-seq and gene expression profiling [55]. Ninety-percent of identified Runx3 peaks contained an RBS with the RCCRCA (R=A/G) consensus motif. Expression of NK cell regulated genes were both increased (e.g., Prdm1, CD226, CXCR4, CX3CR1, Itgb7) and decreased (e.g., c-kit, TNFRSF9, CD96, ITGAX) with deletion of Runx3. Significant enrichment of ETS, T-box, and AP-1 consensus motifs were found in NK cell Runx3 peaks, suggesting collaboration of these transcription factors in Runx3 function.
1.6 GATA proteins
The GATA family of transcription factors are broadly important in cellular differentiation and maturation [56]. Of the three hematopoietic GATA family members (GATA-1, 2, 3), GATA-3 has been best studied in NK cells. GATA-3 was originally described to be a critical regulator of thymic T cell development and T helper type 2 (TH2) differentiation [57]. However, GATA-3 expression in the NK cell compartment was largely restricted to thymic NK cells. Thus, GATA-3-deficient mice have grossly normal NK cell numbers in the majority of lymphoid tissues, but selectively lack thymic NK cells [58]. Despite normal NK cell numbers, GATA-3-deficient NK cells are phenotypically immature, produce less IFN-γ during Listeria monocytogenes infection, and have reduced cytotoxicity against Yac-1 target cells [58,59]. GATA-3 also controls the migration of liver-homing NK cells, but does not appear to affect NK cell distribution elsewhere [60]. GATA-3-dependent thymic NK cells represent a distinct lineage of NK cells expressing CD127 and low amounts of Ly49H receptors, but do not derive from T cell precursors [59]. Interestingly, for human NK cells there is significantly higher GATA-3 (and CD127) expression in the CD56bright compared to CD56dim subset, but it remains unknown whether GATA-3 plays a direct role in their functional or developmental differences [58].
GATA-2 is expressed widely among hematopoietic cell progenitors and is necessary for the maintenance of early hematopoietic cells and generation of mast cells [61]. Patients with heterozygous mutations in GATA-2 have a severe loss of dendritic cells, monocytes, B cells, and NK cells [62–64]. Indeed, the first reported case of NK cell deficiency is now known to be caused by a frame shift mutation in GATA-2 [63]. These patients are susceptible to human papillomaviruses, herpesviruses, and mycobacterial infections. The few GATA-2-deficient NK cells that are present in these patients display reduced NK cell cytotoxicity but largely normal NK cell surface receptor repertoire. GATA-2 haplo-insufficiency results in low numbers of all CD56+ NK cells, but, surprisingly, there is a near total loss of the CD56bright NK cell subset in the peripheral blood [63]. Correspondingly, GATA-2 is normally expressed at higher levels in CD56bright compared to CD56dim NK cells. The residual peripheral blood CD56dim NK cells in GATA-2-mutated individuals may either reflect the few NK cells that have differentiated from CD56bright NK cells or a separate lineage of NK cells not deriving from a CD56bright precursor [65]. NK cells were also reported to be profoundly decreased in the bone marrow of GATA2 mutated patients [66].
1.7 TOX proteins
The thymocyte selection-associated high mobility group box (TOX) proteins are a family of transcription factors shown to regulate NK cell [67], T cell [68,69], and NKT cell [68] development. Knockout mice of TOX1, the original member of the TOX family, have a severe, cell-intrinsic NK cell deficiency beginning at the immature NK cell stage [67]. In human NK cells, both TOX1 and the related family member TOX2 have been shown to regulate human NK cell development [70,71]. Using an in vitro differentiation system from umbilical cord blood CD34+ cells, lentiviral knockdown of TOX2 reduced the number of differentiated NK cells beginning at stage III NK cells (CD117+ CD94− CD56−), suggesting that lineage commitment was not affected by decreased TOX2 [70]. Furthermore, TOX2 knockdown using this in vitro NK cell differentiation approach repressed activating receptor expression, degranulation, and perforin expression, but this may be related to NK cell developmental defects. Notably, TOX2 directly binds to the human Tbx21 (T-bet) promoter region, and overexpression of T-bet in TOX2 knockdown cells rescues in vitro NK cell development. The role of TOX2 in mouse NK cell development has not been examined, but the near total loss of NK cells in TOX1−/− mice suggests independent, non-redundant functions [67].
1.8 Id and E proteins
Inhibitor of DNA-binding (Id) proteins are helix-loop-helix transcription factors important for cellular development [72,73]. The four Id proteins (Id1–4) cannot themselves bind DNA since they lack a basic DNA binding region; instead they regulate transcription by heterodimerizing with other DNA-binding proteins, such as E proteins, and preventing them from binding their cognate DNA elements. E proteins are class I basic helix-loop-helix transcription factors critical for B- and T-cell development. There are four E proteins (E12, E47, HEB, and E2-2) that regulate genes involved in lymphoid lineage commitment and antigen receptor gene rearrangement [74–76]. Id proteins repress E protein-induced transcriptional programs during NK cell development [77], and balanced E and Id protein expression permits the differentiation of the different lymphoid lineages. Id2 and Id3 are highly expressed in lymphocytes and are likely the major Id proteins in NK cells [78]. Id proteins are critical for NK cell development as reduced numbers of mature bone marrow and splenic NK cells are seen in Id2−/− mice [79,80]. However, Id2−/− mice have normal numbers of NK cell precursors in the bone marrow suggesting that Id2 is required for differentiation of immature into mature NK cells rather than NK cell lineage specification [80]. Id2 functions by repressing E protein activity, and deletion of E2A in Id2−/− mice is sufficient to restore NK cell maturation in the bone marrow. In contrast, E2A deletion does not restore NK cell maturation in the peripheral blood or spleen, and thus Id2 likely has additional functions or E protein targets [80]. Id proteins may still regulate NK cell precursor development, however, as Id3 may compensate for the loss of Id2 in Id2−/− mice [80]. Indeed, whereas Id3 is normally expressed in NKP but only minimally in mature NK cells, Id2 is expressed in both precursor and mature NK cell populations. In the setting of Id2 deletion, Id3 mRNA levels in NKP are increased [80]. Additionally, a role for both Id2 and Id3 has been described in determining the lineage specification of bi-potential human T/NK progenitor cells, since constitutive Id2 or Id3 expression was shown to block development of CD34+ cells into T cells and enhance NK cell development in a fetal thymic organ culture [81,82].
1.9 IRF-1 and IRF-2
Interferon regulatory factor 2 (IRF-2) is a transcription factor that can function as both a transcriptional activator and inhibitor. It was originally discovered along with IRF-1 for its role in regulating the IFN-β gene by binding to interferon-sensitive response elements (ISREs) [83–86]. IRF-2 represses IRF-1-induced transcriptional activation of IFN-α/β [87,88] and other IFN-inducible genes [84]. However, IRF-2 can also upregulate transcription of certain genes, including histone 4 [89] and vascular cell adhesion molecule-1 (VCAM-1) in muscle cells [90]. In fact, IRF-2 is considered a potential oncogene since its overexpression leads to tumor formation in mice as well as anchorage-independent NIH 3T3 cell growth [91]. IRF-2 is expressed in most cell types and its expression is inducible by Type I IFNs as well as viral infection [84]. IRF-2−/− mice show defects in both Th1 differentiation and NK cell development [92]. NK cell numbers are profoundly reduced in the periphery but not the bone marrow of IRF-2−/− mice. Furthermore, peripheral NK cells of IRF-2−/− mice are immature, despite the ability of IRF-2−/− bone marrow to permit normal NK cell development and produce physiologically relevant levels of IL-15 [92]. The peripheral NK cell deficiency seen in IRF-2−/− mice is due to a selective loss of mature bone marrow NK cells due to accelerated apoptosis [93]. The peripheral (splenic) NK cells that do develop in these mice show intact cytotoxicity against Yac-1 targets in vitro, yet have reduced cytokine-induced IFN-γ production. Thus, loss of IRF-2 results in failure of mature NK cell homeostasis, with mature bone marrow NK cells exhibiting accelerated apoptosis, and residual peripheral NK cells having normal cytotoxicity but reduced IFN-γ production capacity. NK cell numbers are also reduced in IRF-1 deficient mice [94,95], but in this case, the defect is due to an altered bone marrow microenvironment and is not NK cell intrinsic [96]. IRF-1 induces IL-15, and exogenous IL-15 promotes functional NK cell development when IRF-1−/− bone marrow is cultured in vitro. [96]
1.10 MITF
Microphthalmia-associated transcription factor (MITF) is a basic helix-loop-helix leucine zipper important for the development of a variety of cell types, including mast cells, retinal epithelial cells, osteoclasts, and melanocytes [97]. In mice, MITF is encoded by mi and binds to DNA in complex with Tfeb, Tfec, or Tfe3 [98,99]. Several mutations in the murine mi gene have been reported, some of which produce an dominant negative form of MITF, such as MITFmi and MITFor [99,100]. Other mice have been generated containing a transgene insertion into the 5’ flanking region of mi (MITFtg) and consequently disrupting MITF expression [99]. Mice with either type of MITF mutation have normal NK cell development; however MITFmi/mi mice show impaired NK cell cytotoxicity [101,102]. Indeed, NK cells of MITFmi/mi but not MITFtg/tg mice show decreased perforin expression, and MITFmi/mi appears to impair the nuclear translocation of transcription factors responsible for the transactivation of the perforin gene [101]. In addition to impaired cytotoxicity, MITFmi/mi NK cells show impaired IFN-γ production in response to IL-12 and IL-18 stimulation [103]. This is due to reduced surface expression of IL-12 receptor β2 and IL-18 receptor α in MITFmi/mi NK cells, but not MITFtg/tg cells [103]. Thus, normal MITF protein likely does not participate in regulating cytokine production and cytotoxicity; rather, the mutant form of MITF (MITFmi/mi) interferes with other proteins that directly control NK cell function, postulated to be MEF [104].
2. MicroRNA regulation of NK cell gene expression and translation
2.1 Introduction
MiRNAs are small, 18–22 nucleotide noncoding RNAs that function as post-transcriptional repressors [105,106]. Both biological evidence and in silico analyses support the pervasiveness of miRNA regulation. Indeed, greater than 50% of all genes in mammals are thought to be regulated by miRNAs [107,108]. mRNA transcripts are regulated via miRNA binding to the 3’ untranslated region (UTR), thereby inducing mRNA degradation or blocking their translation. In some instances, miRNAs can increase mRNA translation via binding to AU-rich elements in the 3’UTR of the target mRNA, although it is presently unknown how frequently this mechanism occurs [109]. MiRNAs are processed through a canonical pathway involving sequential cleavage by the ribonuclease enzymes Drosha/Dgcr8 and Dicer [106]. Long primary transcripts called the primary miRNA (pri-miRNA) are “cropped” by the Drosha/Dgcr8 complex into a smaller stem-loop sequence known as a precursor miRNA (pre-miRNA) [110]. Pre-miRNAs are then exported out of the nucleus into the cytoplasm, where Dicer removes the hairpin, generating a miRNA duplex. Typically, one strand of the mature miRNA is preferentially loaded into an RNA-induced silencing complex (RISC), containing the miRNA and catalytic argonaute proteins [111–113]. This complex facilitates the translational repression or degradation of the target mRNA.
Each miRNA contains a specific “seed” sequence, comprised of the 5’ 2–7 nucleotides that is likely the most important determinant of mRNA target specificity [114]. For example, some miRNAs are grouped together into families due to the similarity of their seed sequence and regulate the same genes. Multiple miRNAs with distinct seed sites can also simultaneously regulate the same mRNA transcript through separate or overlapping 3’UTR binding sites. New evidence shows that multiple copies of miRNAs located in different genomic locations, presumably arising through gene duplication, can be differentially regulated during NK cell development. For example, the increase in miR-181a and miR-181b expression during NK cell differentiation is largely attributed to an increase in transcription from the MIR181A2B2 gene on chromosome 9 rather than MIR181A1B1 on chromosome 1, although the significance of this transcriptional switch is unknown [115]. The expression and regulation of miRNAs in NK cells appears complex and remains an area of active investigation, with open questions about differences in NK cell development, functional, and tissue-specific subsets.
Studies of the function of miRNAs in T and B lymphocyte biology have demonstrated broad involvement in cellular processes ranging from survival to control of effector functions [116,117]. The importance of miRNAs in NK cell regulation was first defined in several studies that globally deleted miRNA processing. Ablation of miRNA expression through Dicer1 deletion results in altered NK cell maturation and function, although the exact phenotype appears to differ depending on the mouse model used [118,119]. Additionally, deletion of the exoribonuclease Eri1, which results in a global increase in miRNA copy number, also perturbed NK cell functionality during triggering of ITAM-coupled receptors [120]. Taken together, these data suggest that miRNA abundance directly impacts NK cell homeostasis. Since then, efforts have been made to characterize the miRNA compartment of NK cells during both steady-state physiology and following activation, as well as to define the roles of specific miRNAs that target relevant NK cell functions. In this section, we highlight recent advances in our understanding of the miRNA network regulating NK cell developmental and functional pathways. The overall summary of miRNAs targeting NK cell relevant targets in human and mice are listed in Table I.
Table I.
Summary of microRNAs and their validated targets in either human or mouse NK cells, categorized by their role in effector functions or cellular development. MiRNAs that repress the expression of the listed gene are red, whereas miRNAs that promote target gene expression are in green.
| Regulation | Regulated gene |
Mouse | Human |
|---|---|---|---|
|
Effector Functions |
IFN-γ | miR-29 [157], miR-150 [128], miR- 155[144], miR-15/16 [158] |
miR-155 [132,144], miR-181 [127], HCMV-miR-122UL [148] |
| Perforin | miR-27a-5p [138], miR-30e [123], miR-150 [135], miR-378 [123] |
||
| Granzyme B | miR-223 [120] | miR-27a-5p [138], miR-30e [123], miR-378 [123] |
|
| DAP12 | miR-183 [142] | ||
| STAT4 | miR-132, miR-200a, miR-212 [140] | ||
| CYLD | miR-362-5p [122] | ||
| HMBOX | miR-30c-3p [139] | ||
| IL2RG | miR-583 [143] | ||
| NF-κB | miR-15b, miR-122 [141] (extracellular miRNAs acting on NK cell Toll-like receptors) |
||
| Development | IGF-1 | miR-483 [130] | |
| c-Myb | miR-15/16[129], miR-150 [128] | miR-15/16 [129] | |
| IL2Rγ | miR-583[143] |
2.2 Expression of miRNAs in NK cells
MiRNA transcriptomes of both mouse [121] and human NK cells [122–124] have been described using next generation sequencing. Small RNA sequencing identified >300 mature miRNA sequences in mouse splenic NK cells, although 85% of the miRNA sequence content was represented by the top 20 miRNAs [121]. Given the known heterogeneity of human NK subsets, it is remarkable that the general expression of highly abundant miRNAs is largely similar between different human NK cell studies. Moreover, many of these miRNAs appear to be evolutionarily conserved as they are highly abundant within mouse NK cells as well [121]. Additional work is needed to define miRNA expression in human NK subsets, particularly given their distinct roles in effector functions. For example, analysis of the CD56bright and CD56dim NK cell subsets, two developmentally-related yet functionally-independent mature NK cell populations, would likely yield miRNAs that regulate cytokine-induced NK cell cytokine production, which is a property attributed to CD56bright NK cells [125]. We now know based on mass cytometry studies that NK cells are extraordinarily heterogeneous at the population level, likely comprising thousands of distinct receptor-expression defined NK cell subsets [126]. Thus, probing differences in miRNA content between different NK cell populations is likely to reveal subset-specific regulatory roles for miRNAs. Recently, miRNA expression was compared among NKT cells, T cells, and three human NK pools from the peripheral blood, cord blood, and uterine decidua [123]. Not surprisingly, the largest number of altered miRNAs occurred between NK subsets and NKT or T cells, although a pool of miRNAs was also differentially expressed between the NK cell populations. This highlights the heterogeneity within NK cell pools of different origin and the clear need for in-depth profiling. Future studies will be needed to confirm the correlative findings that these miRNAs are important for NK cell development. There are strengths and limitations to different approaches to miRNA profiling in NK cells, with small RNA sequencing providing the most rigorous assessment of miRNA content and relative abundance. In contrast, while quantitative real-time PCR based assays do not measure absolute abundance, they are feasible to perform on limiting numbers of primary cells or subsets.
2.3 MicroRNA regulation of NK cell development and functional responses
Current miRNA studies in NK cells have largely focused on identifying those miRNAs that regulate NK cell development and those that regulate effector proteins. Several miRNAs have been found to promote (miR-181 [127], miR-150 [128], and miR-15/16 [129]) or repress (miR-483 [130] and miR-583 [131]) NK cell development. MiR-181 targets nemo-like kinase, an inhibitor of Notch signaling, and thereby impacts human NK cell development. miR-15/16 family miRNAs and miR-150 target c-Myb, a transcription factor that is down-regulated during the final steps of NK cell maturation. Global deletion of miR-150 [128] or NK cell-specific deficiency of miR-15/16 [129] resulted in impaired NK cell maturation into CD27−CD11b+ (stage IV) NK cells, while miR-150tg over-expression resulted in enhanced NK cell maturation that was phenocopied in Myb+/− mice [128]. Of note, NK cell-specific deletion of the mir-15a/16-1 gene that results in a 50% reduction in miR-15/16 family mature miRNA expression resulted in defective terminal NK cell maturation [129]. miR-15/16-deficient NK cells had increased expression of c-Myb, and restoration of miR-15/16 or knockdown of Myb in miR-15/16 immature NK cells restored NK cell maturation following adoptive transfer in vivo. Over-expression of Myb in immature primary NK cells also blocked terminal maturation in vivo. Further studies are needed to fully define the impact of complete miR-15/16 loss in NK cells, and define the role of c-Myb in early and terminal NK cell development, using genetic models.
miR-583 was found to be one of the most significantly downregulated miRNAs during in vitro differentiation of NK cells from umbilical cord blood stem cells [131]. The functional target of miR-583 was found to be the common gamma chain (IL2RG), an essential component of the IL-15 receptor and required for the survival of NK cells. The development of genetic models of miR-583 gain and loss of function will be informative to test whether this miRNA is globally required for other lymphocytes that depend on cytokine signaling through the IL2RG cytokines. miR-483 was also postulated to be important for promoting NK cell differentiation through targeting IGF-1, a growth factor secreted by NK cells that augments the number of NK cells generated during in vitro differentiation [130]. However, it remains unclear whether this miRNA drives NK cell proliferation or induces the commitment of NK cell precursors along the NK cell lineage.
2.3 MicroRNA Regulation of NK Cell Function
NK cells mediate functional responses through the release of apoptosis-inducing granule proteins and the production of effector cytokines such as IFN-γ and TNF. To date, NK cell effector functions have been linked with to a number of miRNAs, including miR-155[132–134], miR-150 [128,135], miR-181 [127], and miR-29 [136,137], miR-27a-5p [138], miR-30c-3p [139], miR-30e [124], miR-378 [124], miR-132 [140], miR-200a [140], miR-212 [140], miR-15b [141], miR-122 [141], and miR-362-5p [123] (Table 1). The majority of these miRNAs regulate classical effector molecules, including granzyme B and perforin, as well as the prototype NK cell cytokine IFN-γ. However, recent reports show that signaling proteins are also targeted by miRNAs and effect NK cell function. For example, signaling pathways downstream of NK cell activating and cytokine receptors are modulated by various miRNAs, including STAT4 (by miR-132, miR-200a, miR-212 [140]) and DAP12 (by miR-183 [142]). Other miRNAs negatively regulate the NF-kB pathway. These include miR-362-5p and its target CYLD, an inhibitor of NF-kB activation, and the extracellular miRNAs miR-15b and miR-122, which signal through NK cell-expressed Toll-like receptors to activate NF-kB [123,141]. Other miRNAs appear to have multiple functions in NK cell processes. By targeting the IL2RG, miR-583 likely regulates IL-15 responsiveness during inflammatory responses and homeostatic survival [131].
The best characterized miRNA in NK cells is miR-155, which was reported by three separate groups to regulate IFN-γ production [132–134,143]. MiR-155 is highly upregulated upon NK cell activation with exogenous cytokines, ITAM-coupled receptor stimulation, or MCMV infection. In these studies, miR-155 was found to have various targets, including SHIP1 [133], Noxa [134], SOCS1 [134] and multiple members of intracellular signaling pathways. The major phenotype observed in these studies was a link between miR-155 overexpression and increased IFN-γ production, attributed to a repression of SHIP-1 and components of signaling pathways upstream of IFN-γ transcription. miR-155−/− NK cells paradoxically also exhibited increased IFN-γ production, however, via a distinct cellular mechanism. While miR-155 overexpression resulted in an increase in per cell IFN-γ protein production, miR-155−/− NK cells had an increased percentage of IFN-γ producing NK cells. This latter finding was linked using RISC-seq miR-155 target identification in primary NK cells to the upregulation of multiple signaling pathway components in miR-155−/− NK cells, resulting in an overall lowering of the activation threshold of miR-155−/− NK cells. There were some discrepancies in miR-155−/− phenotypes and miR-155 transgenic or knock-in over-expression phenotypes that are likely linked to different stimulation methods tested and global versus NK cell-specific conditional modulation. Additional studies are needed to better define the impact of miR-155 knockout or over-expression on NK cell activation in vivo, in response to viral infection or tumor challenge.
2.4 Clinical implications of microRNAs in NK cell biology
NK cells are critically important in host defense against viral infections and malignancy. However, data regarding the role of miRNAs during protective responses, especially in viral infections, remains limited. One area of active investigation includes that of HCMV, where a number of studies have shown that miRNAs have NK cell extrinsic effects that impact NK cell functions. For example, HCMV-miR-UL112 represses fibroblast production of RANTES, a chemotactic factor for NK cells [144], and induces the downregulation of MICB, a stress ligand recognized by the NK cell activating receptor NKG2D [145]. HCMV-miR-UL112 also appears to have direct effects on NK cells via the downregulation of type I interferon production and abrogation of cytotoxicity [146].
Dysregulated miRNA levels have also been actively studied in NK cell malignancies. Although NK cell malignancies are relatively rare, the miRNA profiles of nasal-type T/NK lymphomas have been examined and have identified several altered miRNAs, including the overexpression of miR-21, miR-155, and several Epstein-Barr virus (EBV)-associated miRNAs [147–150]. In one study, miR-21 and miR-155 expression were dysregulated in a number of NK cell lymphoma lines [150]. Overexpression of these miRNAs is linked to alterations in the PI3-kinase-AKT signaling pathway through targeting of PTEN and SHIP-1, two common tumor suppressors. Other studies have also reported alterations in the PI3-kinase pathway. Chen et al. used NanoString to define the most highly expressed miRNAs in nasal NK cell lymphoma and found that the top 20 miRNAs constituted nearly 50% of total miRNA reads assessed [148]. Of these 20 miRNAs, the top 5 (miR-21, miR-142-3p, miR-126, miR-451, and miR-494-3p) had predicted targets within the PTEN-AKT-mTOR pathway. Furthermore, overexpression studies showed that miR-494-3p negatively regulated PTEN, and miR-142-3p inhibited RICTOR, a component of the mTORC2 complex. Downregulation of other miRNAs have also been reported in NK/T cell lymphoma cell lines, including miR-26ab, miR-28-5p, miR-101, miR-14a, and miR-363 [147]. The targets of these miRNAs are enriched in pathways related to cell cycle and MAPK activation and may indicate one mechanism by which NK cell lymphoma disease progression occurs. Furthermore, these studies indicate miRNA inhibition or overexpression as potential avenues for treatment.
EBV has been associated with a number of malignancies, including a significant proportion of non-Hodgkin and Hodgkin lymphoma [151]. Additionally, there is a strong association between nasal NK/T cell lymphoma and EBV infection. To date, EBV is known to encode 50 unique miRNA species that could potentially have roles in disease pathogenesis [152]. Using microarray profiling of two NK/T cell lymphoma lines, 19 EBV miRNAs were found to be highly expressed. Of these miRNAs, miR-BART9 was validated to target LMP-1, an EBV latency protein with potential involvement in NK/T cell lymphoma development. Another EBV-associated miRNA, miR-BART20-5p was reported to target T-bet, which is lost in invasive type nasal NK/T cell lymphomas [149]. Transfection of miR-BART20-5p into T cell lymphoma lines resulted in repression of T-bet expression and a secondary effect of p53 suppression due to loss of T-bet-induced p53 expression. Additional studies are needed to determine if overexpression of miR-BART20-5p in primary NK/T lymphomas can convert disease subtype.
3. Concluding remarks
The transcriptional regulation of NK cell development and function has been under intense study over the past decade. Numerous advances have been made in identifying transcription factors selectively important for NK cell differentiation and the specific developmental or maturation stages at which they act. However, many of these studies have been evaluated using global genetic deletion models that include caveats of cell extrinsic phenotypes. To date a completely NK cell -specific transcription factor has not been identified. Furthermore, the complex interactions between related transcription factors limit our understanding of the biological processes that each transcription factor controls. These studies have been complicated by the discovery of miRNAs and other mechanisms of post-transcriptional regulation which remain poorly understood. Finally, the study of human NK cell development is further complicated by the fact that the genetic knockout models used to study NK cell transcriptional regulation in mice are not easily extendable to humans. Thus, the way in which many of these transcription factors regulate human NK cells specifically is not fully understood. Patients with mutations in NK cell-related transcription factors such as GATA-2 are particularly informative, but such patients are rarely seen.
Several advances will aid in our present and future understanding of gene expression in NK cells. First, the advent of high dimensional flow cytometry and mass cytometry (CyTOF) will allow refinement of specific subsets of NK cells and development intermediates, and, in particular, the early NK cell progenitors. Secondly, the use of relatively NK cell-specific Cre mice [153,154] combined with Cre-models expressed in NK cell progenitors such as Id2, will aid in evaluating intrinsic and extrinsic roles for each transcription factor. Finally, the study of miRNAs, though a relatively new and emerging field, has been strengthened by the development of new methodologies to analyze (e.g., CLIP-SEQ, small RNA sequencing), manipulate, and inhibit their expression.
Highlights.
A complex set of transcription factors and microRNAs orchestrate NK cell development
NK cell activation is regulated by a transcription factor-controlled mRNA program
mRNA translation in resting and activated NK cells are fine-tuned by microRNAs
Acknowledgments
The authors thank members of the Fehniger laboratory for critical review of this manuscript. This work was supported by R01AI102924 (T.A.F.) and the Howard Hughes Medical Institute Medical Research Fellows Award (J.A.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Ann Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 6.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Ann Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 8.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 9.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Chan A, Hong D, Atzberger A, Filer AD, Buckley CD, Mcmichael A, et al. CD56bright human nk cells differentiate into cd56dim cells: Role of Contact with Peripheral Fibroblasts. J Immumol. 2013;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin. Immunol. 2014:1–5. doi: 10.1016/j.smim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dybkaer K, Iqbal J, Zhou G, Geng H, Xiao L, Schmitz A, et al. Genome wide transcriptional analysis of resting and IL2 activated human natural killer cells: gene expression signatures indicative of novel molecular signaling pathways. BMC Genomics. 2007;8:230. doi: 10.1186/1471-2164-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a Bezman N, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, et al. Molecular definition of the identity and activation of natural killer cells. Nat. Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez K, Kee BL. Transcriptional regulation of natural killer cell development. Curr Opin Immunol. 2010;22:193–198. doi: 10.1016/j.coi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharrocks AD. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 17.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 18.Colucci F, Samson SI, DeKoter RP, Lantz O, Singh H, Di Santo JP. Differential requirement for the transcription factor PU.1 in the generation of natural killer cells versus B and T cells. Blood. 2001;97:2625–2632. doi: 10.1182/blood.v97.9.2625. [DOI] [PubMed] [Google Scholar]
- 19.Lacorazza HD, Miyazaki Y, Di Cristofano A, Deblasio A, Hedvat C, Zhang J, et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity. 2002;17:437–449. doi: 10.1016/s1074-7613(02)00422-3. [DOI] [PubMed] [Google Scholar]
- 20.Choi HJ, Geng Y, Cho H, Li S, Giri PK, Felio K, et al. Differential requirements for the Ets transcription factor Elf-1 in the development of NKT cells and NK cells. Blood. 2011;117:1880–1887. doi: 10.1182/blood-2010-09-309468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez K, Chandler KJ, Spaulding C, Zandi S, Sigvardsson M, Graves BJ, et al. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity. 2012;36:921–932. doi: 10.1016/j.immuni.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwada M, Levy DM, McKeag L, Murray K, Schröder AJ, Canfield SM, et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc. Natl. Acad. Sci. U. S. A. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 24.Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J. Exp. Med. 2015;212:253–265. doi: 10.1084/jem.20141703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy MK, Glaccum M, Brown SN, a Butz E, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.a Firth M, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, et al. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J. Exp. Med. 2013;210:2981–2990. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife. 2014;3:e04406. doi: 10.7554/eLife.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, et al. NFIL3 Orchestrates the Emergence of Common Helper Innate Lymphoid Cell Precursors. Cell Rep. 2015;10:2043–2054. doi: 10.1016/j.celrep.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting Edge: Salivary Gland NK Cells Develop Independently of Nfil3 in Steady-State. J. Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- 32.Crotta S, Gkioka A, Male V, Duarte JH, Davidson S, Nisoli I, et al. The Transcription Factor E4BP4 Is Not Required for Extramedullary Pathways of NK Cell Development. J. Immunol. 2014;192:2677–2688. doi: 10.4049/jimmunol.1302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seillet C, Huntington ND, Gangatirkar P, Axelsson E, Minnich M, Brady HJM, et al. Differential Requirement for Nfil3 during NK Cell Development. J. Immunol. 2014;192:2667–2676. doi: 10.4049/jimmunol.1302605. [DOI] [PubMed] [Google Scholar]
- 34.Male V, Nisoli I, Kostrzewski T, Allan DSJ, Carlyle JR, Lord GM, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J. Exp. Med. 2014;211:635–642. doi: 10.1084/jem.20132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 36.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, a Biron C, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 37.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 39.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:1–21. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Y, Kerdiles Y, Chu J, Yuan S, Wang Y, Chen X, et al. Transcription Factor Foxo1 Is a Negative Regulator of Natural Killer Cell Maturation and Function. Immunity. 2015;42:457–470. doi: 10.1016/j.immuni.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, et al. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 43.Pearce EL, Mullen AC, a Martins G, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 44.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knox JJ, Cosma GL, Betts MR, McLane LM. Characterization of T-bet and Eomes in peripheral human immune cells. Front. Immunol. 2014;5:1–13. doi: 10.3389/fimmu.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito Y, Bae S-C, Chuang LSH. The RUNX family: developmental regulators in cancer. Nat. Rev. Cancer. 2015;15:81–95. doi: 10.1038/nrc3877. [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Maillard I, Chakraborti S, V Rothenberg E, a Speck N. Core binding factors are necessary for natural killer cell development and cooperate with Notch signaling during T-cell specification. Blood. 2008;112:480–492. doi: 10.1182/blood-2007-10-120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno S, Sato T, Kohu K, Takeda K, Okumura K, Satake M, et al. Runx proteins are involved in regulation of CD122, Ly49 family and IFN-gamma expression during NK cell differentiation. Int. Immunol. 2008;20:71–79. doi: 10.1093/intimm/dxm120. [DOI] [PubMed] [Google Scholar]
- 49.Otto F, Lübbert M, Stock M. Upstream and downstream targets of RUNX proteins. J. Cell. Biochem. 2003;89:9–18. doi: 10.1002/jcb.10491. [DOI] [PubMed] [Google Scholar]
- 50.Presnell SR, Zhang L, Chlebowy CN, Al-Attar A, Lutz CT. Differential transcription factor use by the KIR2DL4 promoter under constitutive and IL-2/15-treated conditions. J. Immunol. 2012;188:4394–4404. doi: 10.4049/jimmunol.1103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Presnell SR, Zhang L, Ramilo Ca, Chan HW, Lutz CT. Functional redundancy of transcription factor-binding sites in the killer cell Ig-like receptor (KIR) gene promoter. Int. Immunol. 2006;18:1221–1232. doi: 10.1093/intimm/dxl043. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Lozano N, Trompeter HI, de Pablo R, Estefanía E, Uhrberg M, Vilches C. Epigenetic silencing of potentially functional KIR2DL5 alleles: Implications for the acquisition of KIR repertoires by NK cells. Eur. J. Immunol. 2007;37:1954–1965. doi: 10.1002/eji.200737277. [DOI] [PubMed] [Google Scholar]
- 53.Trompeter H-I, Gómez-Lozano N, Santourlidis S, Eisermann B, Wernet P, Vilches C, et al. Three structurally and functionally divergent kinds of promoters regulate expression of clonally distributed killer cell Ig-like receptors (KIR), of KIR2DL4, and of KIR3DL3. J. Immunol. 2005;174:4135–4143. doi: 10.4049/jimmunol.174.7.4135. [DOI] [PubMed] [Google Scholar]
- 54.Lai CB, Mager DL. Role of runt-related transcription factor 3 (RUNX3) in transcription regulation of Natural Cytotoxicity Receptor 1 (NCR1/NKp46), an activating Natural Killer (NK) cell receptor. J. Biol. Chem. 2012;287:7324–7334. doi: 10.1074/jbc.M111.306936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levanon D, Negreanu V, Lotem J, Bone KR, Brenner O, Leshkowitz D. et al. Transcription Factor Runx3 Regulates Interleukin-15-Dependent Natural Killer Cell Activation, Mol. Cell. Biol. 2014;34:1158–1169. doi: 10.1128/MCB.01202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng R, a Blobel G. GATA Transcription Factors and Cancer. Genes Cancer. 2010;1:1178–1188. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tindemans I, Serafini N, Di Santo JP, Hendriks RW. GATA-3 Function in Innate and Adaptive Immunity. Immunity. 2014;41:191–206. doi: 10.1016/j.immuni.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Vosshenrich CaJ, García-Ojeda ME, Samson-Villéger SI, Pasqualetto V, Enault L, Richard-Le Goff O, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 59.Ribeiro VSG, Hasan M, Wilson A, Boucontet L, Pereira P, Lesjean-Pottier S, et al. Cutting edge: Thymic NK cells develop independently from T cell precursors. J Immunol. 2010;185:4993–4997. doi: 10.4049/jimmunol.1002273. [DOI] [PubMed] [Google Scholar]
- 60.Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CaJ, Colucci F, et al. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 61.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- 62.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2013;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56bright subset. Blood. 2013;121:2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spinner Ma, Sanchez La, Hsu AP, Shaw Pa, Zerbe CS, Calvo KR, et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fehniger TA. “AbroGATAed” human NK cell development (Inside Blood) Blood. 2013;121:2579–2580. doi: 10.1182/blood-2013-02-483362. [DOI] [PubMed] [Google Scholar]
- 66.a Ganapathi K, Townsley DM, Hsu AP, Arthur DC, Zerbe CS, Cuellar-Rodriguez J, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56–70. doi: 10.1182/blood-2014-06-580340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat. Immunol. 2010;11:945–952. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J. Exp. Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkinson B, Chen JY-F, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat. Immunol. 2002;3:272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 70.Vong QP, Leung W-H, Houston J, Li Y, Rooney B, Holladay M, et al. TOX2 regulates human natural killer cell development by controlling T-BET expression. Blood. 2014;124:3905–3913. doi: 10.1182/blood-2014-06-582965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yun S, Lee SH, Yoon S-R, Kim MS, Piao Z-H, Myung P-K, et al. TOX regulates the differentiation of human natural killer cells from hematopoietic stem cells in vitro. Immunol. Lett. 2011;136:29–36. doi: 10.1016/j.imlet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 73.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 74.Kee BL, Murre C. Induction of Early B Cell Factor (EBF) and Multiple B Lineage Genes by the Basic Helix-Loop-Helix Transcription Factor E12. J. Exp. Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu W, Carr T, Ramirez K, McGregor S, Sigvardsson M, Kee BL. E2Atranscription factors limit expression of Gata3 to facilitate T lymphocyte lineage commitment. Blood. 2013;121:1534–1542. doi: 10.1182/blood-2012-08-449447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inlay MA, Tian H, Lin T, Xu Y. Important roles for E protein binding sites within the immunoglobulin kappa chain intronic enhancer in activating Vkappa Jkappa rearrangement. J. Exp. Med. 2004;200:1205–1211. doi: 10.1084/jem.20041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun XH, Copeland NG, Jenkins NA, Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol. Cell. Biol. 1991;11:5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kee BL, Quong MW, Murre C. E2A proteins: essential regulators at multiple stages of B-cell development. Immunol. Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- 79.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S-I, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 80.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heemskerk MHM, Blom B, Nolan G, Stegmann APA, Bakker AQ, Weijer K, et al. Inhibition of T Cell and Promotion of Natural Killer Cell Development by the Dominant Negative Helix Loop Helix Factor Id3. J. Exp. Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schotte R, Dontje W, Nagasawa M, Yasuda Y, Bakker AQ, Spits H, et al. Synergy between IL-15 and Id2 promotes the expansion of human NK progenitor cells, which can be counteracted by the E protein HEB required to drive T cell development. J. Immunol. 2010;184:6670–6679. doi: 10.4049/jimmunol.0901508. [DOI] [PubMed] [Google Scholar]
- 83.Fujita T, Kimura Y, Miyamoto M, Barsoumian EL, Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989;337:270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 84.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, et al. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 85.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, et al. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 86.Fujita T, Sakakibara J, Sudo Y, Miyamoto M, Kimura Y, Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988;7:3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63:303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 88.Hida S, Ogasawara K, Sato K, Abe M, Takayanagi H, Yokochi T, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13:643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 89.Vaughan PS, Aziz F, van Wijnen AJ, Wu S, Harada H, Taniguchi T, et al. Activation of a cell-cycle-regulated histone gene by the oncogenic transcription factor IRF-2. Nature. 1995;377:362–365. doi: 10.1038/377362a0. [DOI] [PubMed] [Google Scholar]
- 90.Jesse TL, LaChance R, Iademarco MF, Dean DC. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J. Cell Biol. 1998;140:1265–1276. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, et al. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and −2. Science. 1993;259:971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 92.Lohoff M, Duncan GS, Ferrick D, Mittrücker HW, Bischof S, Prechtl S, et al. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J. Exp. Med. 2000;192:325–336. doi: 10.1084/jem.192.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taki S, Nakajima S, Ichikawa E, Saito T, Hida S. IFN regulatory factor-2 deficiency revealed a novel checkpoint critical for the generation of peripheral NK cells. J. Immunol. 2005;174:6005–6012. doi: 10.4049/jimmunol.174.10.6005. [DOI] [PubMed] [Google Scholar]
- 94.Duncan GS, Mittrucker HW, Kagi D, Matsuyama T, Mak TW. The transcription factor interferon regulatory factor-1 is essential for natural killer cell function in vivo. J Exp Med. 1996;184:2043–2048. doi: 10.1084/jem.184.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, et al. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 96.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 97.Hershey CL, Fisher DE. Mitf and Tfe3: Members of a b-HLH-ZIP transcription factor family essential for osteoclast development and function. Bone. 2004;34:689–696. doi: 10.1016/j.bone.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 98.Hughes MJ, Lingrel JB, Krakowsky JM, Anderson KP. A helix-loop-helix transcription factor-like gene is located at the mi locus. J. Biol. Chem. 1993;268:20687–20690. [PubMed] [Google Scholar]
- 99.Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 100.Steingrímsson E, Moore KJ, Lamoreux ML, Ferré-D’Amaré AR, Burley SK, Zimring DC, et al. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat. Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- 101.Ito A, Kataoka TR, Kim DK, Koma YI, Lee YM, Kitamura Y. Inhibitory effect on natural killer activity of microphthalmia transcription factor encoded by the mutant mi allele of mice. Blood. 2001;97:2075–2083. doi: 10.1182/blood.v97.7.2075. [DOI] [PubMed] [Google Scholar]
- 102.Kataoka TR, Morii E, Oboki K, Kitamura Y. Strain-dependent inhibitory effect of mutant mi-MITF on cytotoxic activities of cultured mast cells and natural killer cells of mice. Lab. Invest. 2004;84:376–384. doi: 10.1038/labinvest.3700040. [DOI] [PubMed] [Google Scholar]
- 103.Kataoka TR, Komazawa N, Oboki K, Morii E, Nakano T. Reduced expression of IL-12 receptor beta2 and IL-18 receptor alpha genes in natural killer cells and macrophages derived from B6-mi/mi mice. Lab. Invest. 2005;85:146–153. doi: 10.1038/labinvest.3700188. [DOI] [PubMed] [Google Scholar]
- 104.Hesslein DGT, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- 105.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 106.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 107.Lai EC. Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 108.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 109.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 110.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 111.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29:1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 112.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 113.Liu J. Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008;20:214–221. doi: 10.1016/j.ceb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 114.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Cell Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Presnell SR, Al-Attar A, Cichocki F, Miller JS, Lutz CT. Human natural killer cell microRNA: differential expression of MIR181A1B1 and MIR181A2B2 genes encoding identical mature microRNAs. Genes Immun. 2015;16:89–98. doi: 10.1038/gene.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Connell RM, Rao DS, Chaudhuri Aa, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 117.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 118.Sullivan RP, Leong JW, Schneider SE, Keppel CR, Germino E, French AR, et al. MicroRNA Deficient NK Cells Exhibit Decreased Survival but Enhanced Function. J Immunol. 2012 doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bezman NA, Cedars E, Steiner DF, Blelloch R, Hesslein DGT, Lanier LL. Distinct Requirements of MicroRNAs in NK Cell Activation, Survival, and Function. J Immunol. 2010;185:3835–3846. doi: 10.4049/jimmunol.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P, et al. Eri1 regulates microRNA homeostasis and mouse lymphocyte development and anti-viral function. Blood. 2012 doi: 10.1182/blood-2011-11-394072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fehniger TA, Wylie T, Germino E, Leong JW, Magrini VJ, Koul S, et al. Next-generation sequencing identifies the natural killer cell microRNA transcriptome. Genome Res. 2010;20:1590–1604. doi: 10.1101/gr.107995.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X, Wang Y, Sun Q, Yan J, Huang J, Zhu S, et al. Identification of microRNA transcriptome involved in human natural killer cell activation. Immunol. Lett. 2012;143:208–217. doi: 10.1016/j.imlet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Ni F, Guo C, Sun R, Fu B, Yang Y, Wu L, et al. MicroRNA transcriptomes of distinct human NK cell populations identify miR-362-5p as an essential regulator of NK cell function. Sci. Rep. 2015;5:9993. doi: 10.1038/srep09993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang P, Gu Y, Zhang Q, Han Y, Hou J, Lin L, et al. Identification of Resting and Type I IFN-Activated Human NK Cell miRNomes Reveals MicroRNA-378 and MicroRNA-30e as Negative Regulators of NK Cell Cytotoxicity. J. Immunol. 2012 doi: 10.4049/jimmunol.1200609. jimmunol.1200609–. [DOI] [PubMed] [Google Scholar]
- 125.Cooper MA, Fehniger TA, Turner SC, Chen KS, a Ghaheri B, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 126.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, et al. Cutting Edge: MicroRNA-181 Promotes Human NK Cell Development by Regulating Notch Signaling. J Immunol. 2011 doi: 10.4049/jimmunol.1100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bezman NA, Chakraborty T, Bender T, Lanier LL. miR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011:1–15. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sullivan R, Leong J, Schneider S, Ireland A, Berrien-Elliott M, Singh A, et al. Mir-15/16 antagonizes Myb to control natural killer cell maturation. J Immunol. 2015;195:2806–2817. doi: 10.4049/jimmunol.1500949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ni F, Sun R, Fu B, Wang F, Guo C, Tian Z, et al. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 2013;4:1479. doi: 10.1038/ncomms2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yun S, Lee SU, Kim JM, Lee H-J, Song HY, Kim YK, et al. Integrated mRNA-MicroRNA Profiling of Human NK Cell Differentiation Identifies MiR-583 as a Negative Regulator of IL2Rγ Expression. PLoS One. 2014;9:e108913. doi: 10.1371/journal.pone.0108913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sullivan RP, Fogel LA, Leong JW, Schneider SE, Wong R, Romee R, et al. miR-155 tunes both the threshold and extent of NK cell activation via targeting of multiple signaling pathways. J Immunol. 2013;191:5904–5913. doi: 10.4049/jimmunol.1301950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trotta R, Chen L, Costinean S, Josyula S, Mundy-Bosse BL, Ciarlariello D, et al. Overexpression of miR-155 causes expansion, arrest in terminal differentiation and functional activation of mouse natural killer cells. Blood. 2013 doi: 10.1182/blood-2012-12-467597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.L Zawislak C, Beaulieu AM, Loeb GB, Karo J, Canner D, a Bezman N, et al. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc Natl Acad Sci USA. 2013;110:6967–6972. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim N, Kim M, Yun S, Doh J, Greenberg PD, Kim T-D, et al. MicroRNA-150 regulates the cytotoxicity of natural killers by targeting perforin-1(*) J. Allergy Clin. Immunol. 2014 doi: 10.1016/j.jaci.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 137.Mikulak J, Bozzo L, Roberto a, Pontarini E, Tentorio P, Hudspeth K, et al. Dopamine Inhibits the Effector Functions of Activated NK Cells via the Upregulation of the D5 Receptor. J. Immunol. 2014;193:2792–2800. doi: 10.4049/jimmunol.1401114. [DOI] [PubMed] [Google Scholar]
- 138.Kim T-D, Lee SUH, Yun S, Sun H, Kim JW, Kim HM, et al. Human microRNA-27a * targets Prf1 and GzmB expression to regulate NK cell cytotoxicity. Blood. 2011;118:5476–5486. doi: 10.1182/blood-2011-04-347526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong J, Liu R, Zhuang R, Zhang Y, Fang L, Xu Z, et al. miR-30c-1* Promotes NK Cell Cytotoxicity against Human Hepatoma Cells via Targeting the Transcription Factor HMBOX1. Cancer Sci. 2012 doi: 10.1111/j.1349-7006.2012.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang Y, Lei Y, Zhang H, L Hou, Zhang M, Dayton AI. MicroRNA regulation of STAT4 protein expression: rapid and sensitive modulation of interleukin-12 signaling in human natural killer cells. Blood. 2011;118:6793–6802. doi: 10.1182/blood-2011-05-356162. [DOI] [PubMed] [Google Scholar]
- 141.He S, Chu J, Wu L-C, Mao H, Peng Y, a Alvarez-Breckenridge C, et al. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood. 2013;121:4663–4671. doi: 10.1182/blood-2012-07-441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donatelli SS, Zhou J-M, L Gilvary D, a Eksioglu E, Chen X, Cress WD, et al. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, et al. MiR-155 regulates IFN-γ production in natural killer cells. Blood. 2012:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim Y, Lee S, Kim S, Kim D, Ahn JH, Ahn K. Human cytomegalovirus clinical strain-specific microRNA miR-UL148D targets the human chemokine RANTES during infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, et al. Host immune system gene targeting by a viral miRNA. Science (80-. ) 2007;317:376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Y, Chen D, He J, Cai J, Shen K, Liu X, et al. Hcmv-miR-UL112 attenuates NK cell activity by inhibition type I interferon secretion. Immunol. Lett. 2015;163:151–156. doi: 10.1016/j.imlet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 147.Ng S-B, Yan J, Huang G, Selvarajan V, Tay JL-S, Lin B, et al. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood. 2011;118:4919–4929. doi: 10.1182/blood-2011-07-364224. [DOI] [PubMed] [Google Scholar]
- 148.Chen H-H, Huang W-T, Yang L-W, Lin C-W. The PTEN-AKT-mTOR/RICTOR Pathway in Nasal Natural Killer Cell Lymphoma Is Activated by miR-494-3p via PTEN But Inhibited by miR-142-3p via RICTOR. Am. J. Pathol. 2015;185:1487–1499. doi: 10.1016/j.ajpath.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 149.Lin TC, Liu TY, Hsu SM, Lin CW. Epstein-barr virus-encoded miR-BART20-5p inhibits t-bet translation with secondary suppression of p53 in invasive nasal NK/T-cell lymphoma. Am. J. Pathol. 2013;182:1865–1875. doi: 10.1016/j.ajpath.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 150.Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo Y-M, Iwamoto K, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–3275. doi: 10.1182/blood-2009-06-222794. [DOI] [PubMed] [Google Scholar]
- 151.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 152.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Eckelhart E, Warsch W, Zebedin E, Simma O, Stoiber D, Kolbe T, et al. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood. 2011;117:1565–1573. doi: 10.1182/blood-2010-06-291633. [DOI] [PubMed] [Google Scholar]
- 154.Narni-mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci USA. 2011;108:18324–18329. doi: 10.1073/pnas.1112064108. doi:10.1073/pnas.1112064108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1112064108. [DOI] [PMC free article] [PubMed] [Google Scholar]