SUMMARY
The high-mannose patch on HIV Env is a preferred target for broadly neutralizing antibodies (bnAbs), but to date, no vaccination regimen has elicited bnAbs against this region. Here, we present the development of a bnAb lineage targeting the high-mannose patch in an HIV-1 subtype-C-infected donor from sub-Saharan Africa. The Abs first acquired autologous neutralization, then gradually matured to achieve breadth. One Ab neutralized >47% of HIV-1 strains with only ~11% somatic hypermutation and no insertions or deletions. By sequencing autologous env, we determined key residues that triggered the lineage and participated in Ab-Env coevolution. Next-generation sequencing of the Ab repertoire showed an early expansive diversification of the lineage followed by independent maturation of individual limbs, several of them developing notable breadth and potency. Overall, the findings are encouraging from a vaccine standpoint and suggest immunization strategies mimicking the evolution of the entire high-mannose patch and promoting maturation of multiple diverse Ab pathways.
Graphical Abstract
INTRODUCTION
The isolation of potent broadly neutralizing antibodies (bnAbs) to HIV-1 has driven efforts to design immunogens and improve vaccination protocols to induce similar antibody (Ab) responses and protect against HIV infection (reviewed in Burton et al., 2012). These bnAbs target multiple regions on the viral spike that are relatively conserved in either sequence or character despite the tremendous global diversity of the HIV-1 Env protein. These regions include the CD4 binding site, the high-mannose patch centered on the glycan at N332 at the base of variable loop 3 (V3), a quaternary epitope in variable loops 1 and 2 (V1–V2) at the Env apex, the membrane-proximal external region of gp41 (reviewed in Kwong and Mascola, 2012), and several epitopes recently identified at the gp120-gp41 interface (Blattner et al., 2014; Falkowska et al., 2014; Huang et al., 2014; Scharf et al., 2014). The N332 supersite is commonly targeted by Abs from HIV-infected individuals (Walker et al., 2010; Landais et al., 2016), highlighting it as a favored region by the immune system and therefore potentially amenable to vaccination to elicit bnAbs. Abs targeting this region, including PGT121 and PGT128, are among the most potent bnAbs isolated to date (Walker et al., 2011), and passive administration of bnAbs targeting this region has been shown to prevent infection (Moldt et al., 2012) and strongly impact ongoing infection in a non-human primate model (Barouch et al., 2013). The N332-supersite-targeting bnAbs exhibit differential glycan usage for binding to this region given that they approach Env from various angles (Kong et al., 2013; Sok et al., 2014a), unlike the CD4 binding site, which has a more restricted angle of approach (Scheid et al., 2011; Wu et al., 2011). Together, these features make the N332 glycan region an attractive target for HIV vaccine design.
Although the natural development of bnAbs in humans provides strong proof of concept that they could be elicited, no vaccine has been able to induce such Abs. The major barriers appear to be related to one or more unusual features found in all bnAbs isolated to date. High levels of somatic hypermutation (SHM), as well as insertions and deletions (indels) to the germline (GL) immunoglobulin (Ig) genes, suggest that an elaborate maturation process might be required for development of these bnAbs (Klein et al., 2013). Some have exceptionally long CDRH3 regions, which might result from rare recombination events (Briney et al., 2012). Furthermore, polyreactivity and autoreactivity have been reported for some bnAbs (Haynes et al., 2005; Liu et al., 2015), possibly due to relaxed B cell tolerance checkpoints in HIV-infected donors (Haynes et al., 2012). To what extent these features are required for the acquisition of neutralization breadth and potency of bnAbs remains poorly understood, yet such an understanding is critical for the design of vaccine strategies aiming to elicit such Abs.
Studying natural Ab responses in HIV-infected individuals during the course of infection and learning how B cells naturally engage and mature in response to constantly evolving viral epitopes might inform vaccine design strategies. The evolution of bnAb lineages in longitudinal samples targeting the CD4 binding site (Liao et al., 2013b) and the V2 apex region (Doria-Rose et al., 2014) has been described. Key changes in the autologous virus at specific times during Ab development appear to be critical for driving the acquisition of neutralization breadth and potency. It has been previously reported that development of broad serum neutralization targeting the N332 glycan might be triggered in some donors by the shift of a glycan from the N334 to N332 position (Moore et al., 2012). However, isolation and characterization of the maturation of Abs targeting the N332 supersite in longitudinal samples from an HIV-infected individual has not yet been described.
Here, we describe the development of a bnAb lineage that targets the N332 glycan supersite in a donor from sub-Saharan Africa. We isolated a lineage of Abs from multiple time points during the maturation from non-broadly neutralizing Abs to bnAbs through interactions with the autologous virus as it mutates to escape recognition. Next-generation sequencing (NGS) of the Ab repertoire revealed an early diversification of the lineage followed by parallel maturation of various Ab limbs, several of which led to neutralization breadth. In contrast to all Abs to the N332 region previously isolated, these new Abs were capable of significant breadth despite relatively low levels of SHM and no indels, suggesting that extraordinary features are not an absolute requirement for bnAbs.
RESULTS
A Broad Neutralizing Response Targeting the N332 Supersite Was First Detected at 33 Months Post-Infection in Donor PC76
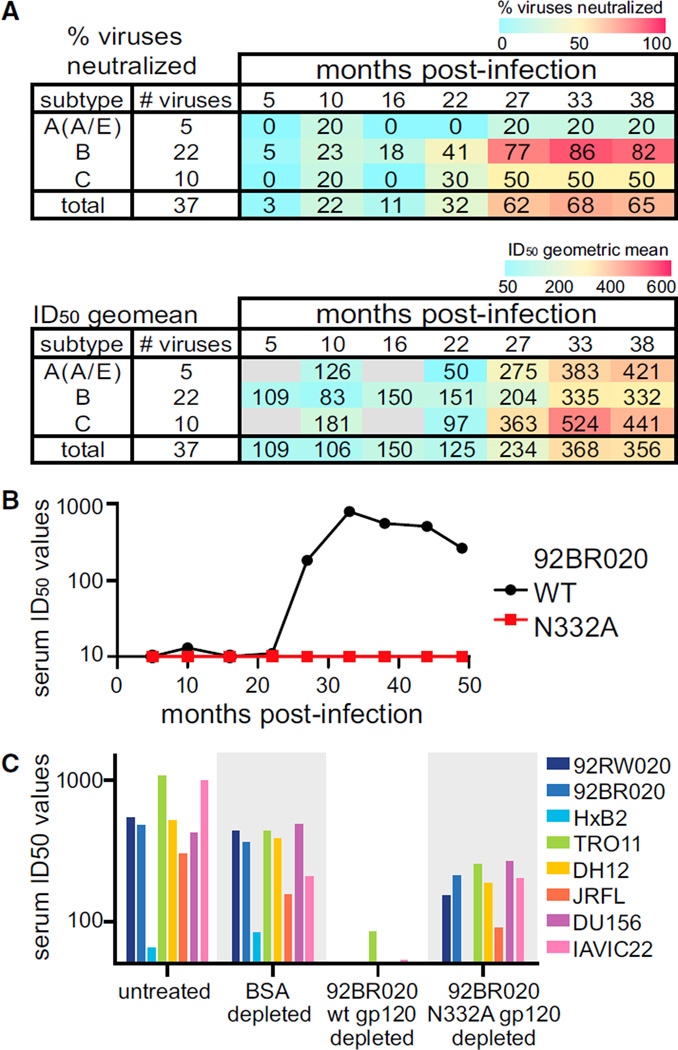
We characterized the breadth and potency of the serum neutralizing activity in 439 of a total of 613 IAVI (International Aids Vaccine Initiative) Protocol C participants (Landais et al., 2016). Development of neutralizing activity in donor PC76 was examined with serum samples from 5 to 38 months post-infection (mpi) tested against a cross-subtype panel of 37 viruses. Neutralization of heterologous viruses was observed starting at 5 mpi and reached a peak of 68% of viruses neutralized by 33 mpi (Figures 1A and S1A). Serum neutralizing activity was N332 dependent for most of the viruses tested (Figure S1B) and throughout this time course (Figure 1B), confirming that N332-dependent Ab responses were predominantly responsible for the broad neutralizing activity developing in this donor.
Figure 1. Development of N332-Glycan-Specific Neutralizing Serum Response in Donor PC76.
(A) Neutralization assays were performed with serum isolated from donor PC76 at various times post-infection, and multiple HIV-1 pseudoviruses were used. Neutralization inhibitory dilution 50 (ID50) values calculated as 1/(serum dilution at 50% inhibition of infection). Samples for which 50% neutralization was not reached at a dilution of 1/50, were determined as having no neutralization. The percent of viruses neutralized and ID50 geometric mean values are shown for each subtype and overall values at the bottom.
(B) Neutralization assays were performed at the time points indicated; 92BR020 WT or N332A pseudovirus with PC76 donor serum was used.
(C) Serum from donor PC76 at 33 mpi was depleted of Abs binding to the proteins listed on the x axis. This depleted serum was used in neutralization assays with the eight pseudoviruses listed. Experiments were performed in duplicate, and the means were used to calculate ID50 values.
Next, serum from 33 mpi was adsorbed on wild-type (WT) recombinant gp120 (rgp120) and rgp120 with a point mutation eliminating the glycan at position N332 (N332A) (Figures 1C and S1C). Serum depleted of Abs that bound WT rgp120 lost neutralizing activity, whereas N332A-rgp120 adsorbed serum was comparable to the negative control BSA adsorbed serum. This result confirmed that neutralizing Abs present in the serum exhibited differential binding to WT and N332A rgp120 proteins.
A Lineage of N332-Glycan-Targeting Abs
On the basis of the above results, we designed a strategy to isolate B cells expressing B cell receptors specific to the N332 glycan from peripheral blood mononuclear cell (PBMC) samples of donor PC76 (Figure S2). To exclude strain-specific or non-specific binding, WT rgp120 probes from virus strains 92BR020 (subtype B) and IAVIC22 (subtype C), which both depleted neutralizing Abs from the PC76 serum (Figures 1C and S1C), were used to identify B cells binding to both proteins. The 92BR020 N332A rgp120 probe was used to isolate cells that bound only when the N332 glycan was present. Single live N332-specific IgG+ B cells identified as described in Figure S2 were sorted into plates for single-cell PCR amplification and sequencing.
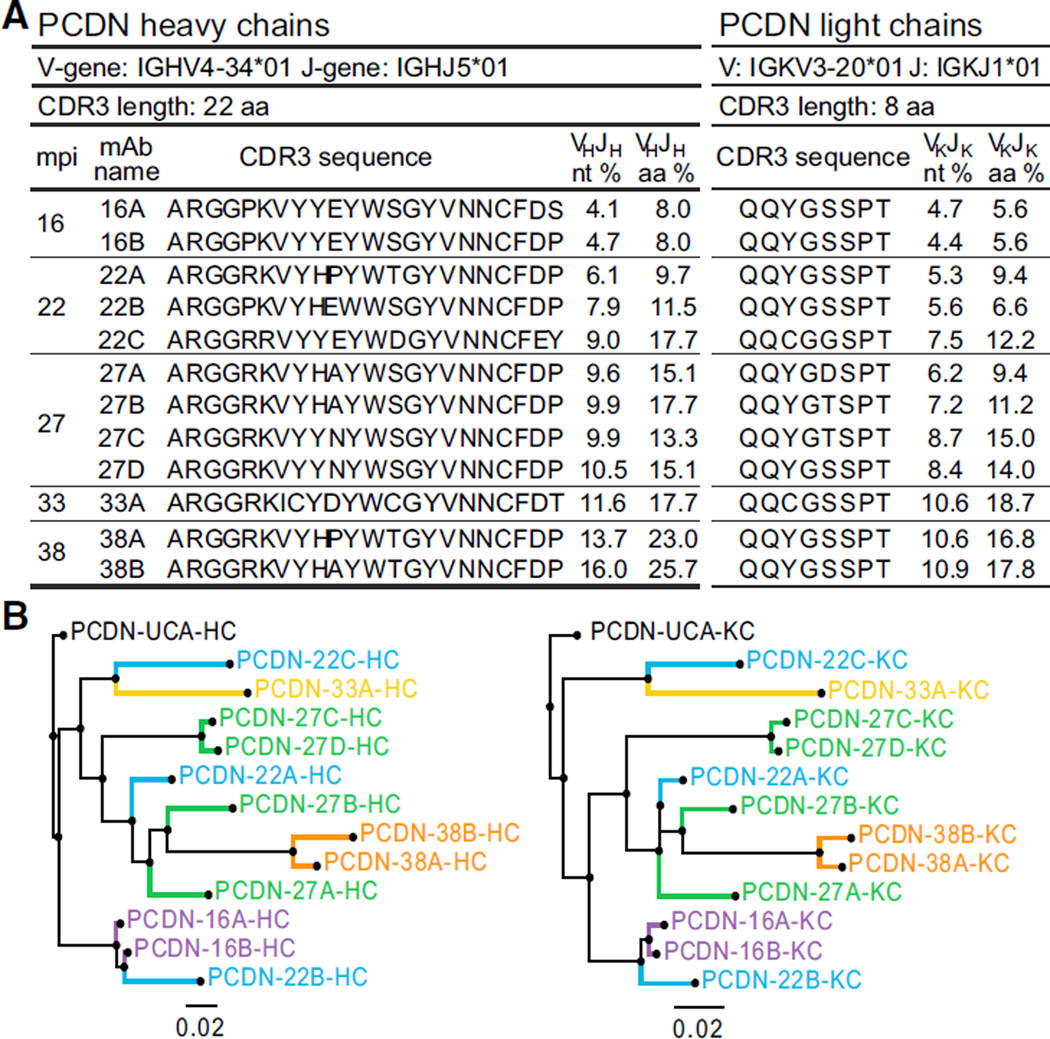
A lineage of 12 somatically related monoclonal Abs (mAbs) from a total of five time points from 16 to 38 mpi was identified from donor PC76. Selected features of PCDN (Protocol C, lab code DN) mAbs are summarized in Figure 2A. This Ab lineage used the IGHV4–34*01 heavy chain (HC) variable gene segment (V-gene) and IGHJ5*01 joining gene segment (J-gene). The diversity gene segment (D-gene) usage could not be reliably determined. The isolated mAbs had CDRH3 lengths of 22 amino acids (aa). The HCs of the mAbs showed ~4%–5% nucleotide (nt) mutation from the GL (~8% aa) at 16 mpi and matured to ~16% nt mutation (~26% aa) for a mAb isolated at 38 mpi. Light chains (LCs) used genes IGKV3–20*01 and IGKJ1*01 and ranged from ~5%–11% nt mutation. Phylogenetic analysis of the mAb HC and LC sequences (Figure 2B) and aa sequence alignments (Figure S3) showed the sequences maturing correspondingly over time. In contrast to all previously isolated bnAbs that target the high-mannose patch, no indels were introduced into either the HC or LC sequences during affinity maturation.
Figure 2. PCDN mAb Gene Usage, Evolutionary Tree, and Relative Maturation.
(A) PCDN HC and LC V- and J-gene usage, CDR3 length and sequences, and percent mutation for nt or aa sequences are tabulated for the 12 mAbs isolated at 16 to 38 mpi. The number in each mAb name corresponds to the isolation time point.
(B) The evolutionary distance between the mAbs is illustrated as a phylogenetic tree for both HC and LC nt sequences. Each color corresponds to a time point reflected in the mAb name.
The PCDN Ab Lineage Matures toward Heterologous Neutralization and is Dependent on Specific Glycans
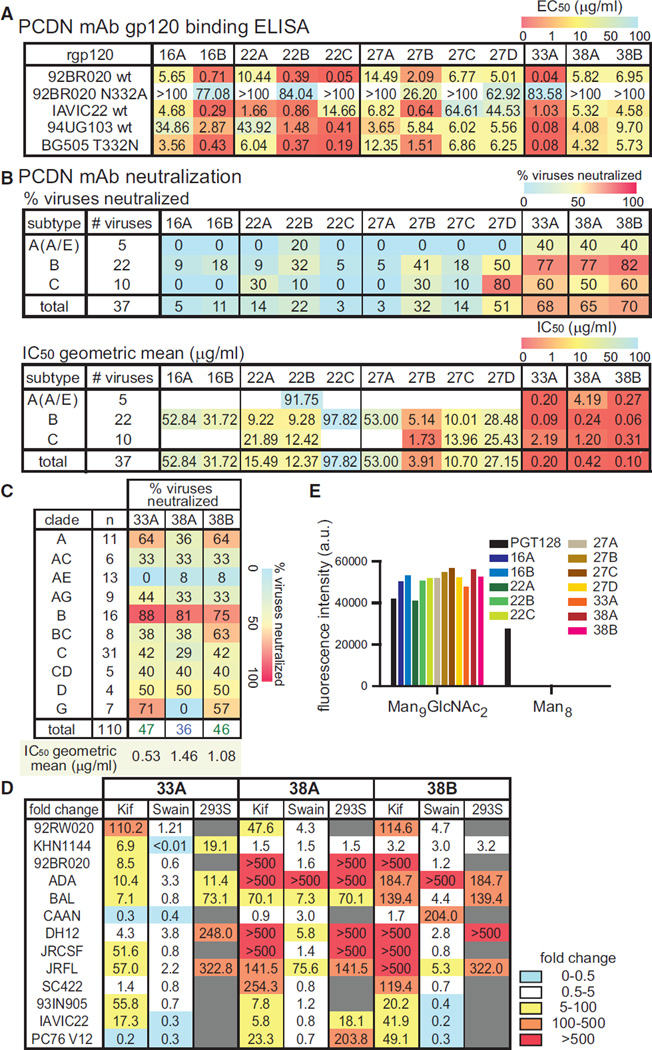
The PCDN mAbs were characterized for binding to rgp120s of various isolates and different subtypes. All mAbs exhibited preferential binding to WT rgp120 and reduced affinity for N332A rgp120, as measured by ELISA (Figure 3A). The Abs were then tested for neutralization of a cross-subtype panel of 37 pseudoviruses (Figures 3B and S4A). As anticipated, Abs isolated at the earliest time point, PCDN-16A and 16B, had limited neutralization breadth, 5% and 11% of viruses, respectively. However, consistent with the kinetics of the development of serum neutralization (Figure 1A), Abs isolated at 33 and 38 mpi neutralized 65%–70% of HIV-1 strains.
Figure 3. Binding and Neutralization of PCDN mAbs.
(A) PCDN Abs were tested by ELISA for binding to rgp120, and EC50 values (µg/mL) are listed. If 50% binding was not reached at 100 µg/mL of Ab, the Abs were considered non-binding (>100).
(B) A heterologous panel of 37 pseudoviruses was tested for neutralization by PCDN mAbs. The percent neutralization and geometric mean of IC50 values (µg/mL) are shown for each HIV-1 virus subtype; overall values are at the bottom. All samples for which 50% neutralization was not reached at 100 µg/mL were determined as having no neutralization. The geometric mean was calculated only with viruses that were neutralized.
(C) A heterologous virus panel of 110 pseudoviruses was tested for neutralization by the 3 most mature mAbs and the percentage of viruses neutralized is shown for each subtype of HIV-1 virus; overall values are listed at the bottom of the table.
(D) Neutralization assays were performed with PCDN Abs and the indicated pseudoviruses produced in HEK293T cells in the presence of kifunensine or swainsonine or in HEK293S cells. Neutralization IC50 values were calculated and presented as a fold increase relative to neutralization of WT pseudoviruses produced in untreated cells.
(E) Glycan array binding by PCDN mAbs and PGT128 for Man9GlcNAc2 and Man8. ELISA and neutralization experiments were performed in triplicate, and the means were used to calculate EC50 and IC50 values. Glycan array was performed in duplicate, and values are from a representative experiment.
We tested the three most mature Abs, PCDN-33A and PCDN-38A and 38B, on a 110-virus panel for a better representation of globally circulating HIV-1 strains. Potency and breadth were reduced overall, primarily due to the different composition of the large panel (Figures 3C and S4B), which is more diverse and includes a greater number of subtype AE recombinants. The majority of subtype AE viruses (91%) have an N334-glycan site instead of N332 (Sok et al., 2014a); <10% were neutralized by PCDN mAbs. PCDN Abs failed to neutralize all viruses that lacked the N332 glycan site, and viruses with a Tyr at position 330, instead of the more common His, were also not neutralized, pointing to N332 and H330 as critical residues for neutralization by these mAbs.
Specific glycan types are essential for binding of Abs targeting the N332 glycan region, and promiscuity in glycan usage has been observed for some bnAbs (Mouquet et al., 2012; Sok et al., 2014a). PCDN-33A and PCDN-38A and 38B were tested against pseudoviruses produced in cells treated with glycosidase inhibitors, kifunensine or swainsonine, or prepared in UDP-N-acetyl-D-glucosamine: α-3-D-mannoside β1 → 2-N-ace-tylglucosaminyltransferase I (GnTI)-deficient HEK293S cells to determine their dependency on certain glycan types. Although all three Abs were sensitive to kifunensine, neutralization by PCDN-38A and PCDN-38B was more severely affected than that by PCDN-33A (Figure 3D). Neutralization was lost for most HEK293S-produced viruses for all three Abs. Swainsonine treatments mostly had no effect, except on neutralization of a few viruses by PCDN-38A and PCDN-38B (Figure 3D). Swainsonine does not affect hybrid glycans but blocks processing to complex glycans, and GnTI deficiency leads to loss of both hybrid and complex glycans (Doores and Burton, 2010). Therefore, in most cases, PCDN mAbs seem to require hybrid glycans for neutralization. The direct binding of PCDN mAbs to glycans was also tested with a glycan array. Of the tested glycans, all PCDN mAbs bound only to Man9GlcNAc2 and no other detectable binding was observed (Figure 3E), whereas PGT128 bound to Man9GlcNAc2 and Man8 as previously reported (Walker et al., 2011). No binding to hybrid glycans was observed, suggesting that although they are required for neutralization, the affinity of the Abs for hybrid glycans is not high enough to detect their binding to the array. Thus, both high-mannose and hybrid glycans appear to be important for binding of the PCDN mAbs.
To map the residues involved in recognition of Env, Ala substitution mutants of JR-CSF Env were made and neutralization was assessed with the three most mature PCDN Abs because only these were capable of neutralizing JR-CSF. Mutations to the potential N-glycosylation sites (PNGSs) of N332 and N301 glycans completely abolished neutralization (Figure S4C). N156A, S158A, and F159A also led to loss of neutralization, suggesting that the N156 glycan is involved in Ab binding. In contrast, little to no sensitivity to mutations at the N295 PNGS was observed for JR-CSF. Similar glycan-site dependencies were observed for other heterologous viruses (Figure S4D). Mutations to Y177 and L179 in V2, as well as a number of residues in V3, also had moderate effects, including those of the 324GDIR327 motif, which has been reported to make extensive contacts with PGT124 and PGT128 (Garces et al., 2014), and nearby residues. Mutating I307 had a strong impact in particular on PCDN-38A and PCDN-38B; this residue has been suggested to be part of a conserved hydrophobic core structure with I309 and F317 (Jiang et al., 2010). The Ala scan results show that mutation of these residues can affect the ability of PCDN Abs to recognize Env.
Some HIV bnAbs have been shown to exhibit polyreactivity and/or autoreactivity, and it has been proposed that this unusual feature contributes to the development of broadly neutralizing activity (Haynes et al., 2005; Liu et al., 2015). We sought to determine whether the PCDN Abs exhibited auto- or polyreactivity as measured by ELISA against a panel of antigens previously described (Wardemann et al., 2003). Histones were bound by a few of the mAbs (22A and 27C), but the 33 mpi and later Abs did not show any detectable polyreactivity (Figure S4E). We also measured binding to HEp-2 cells, and PCDN-27D was the only one that showed detectable fluorescent signals (Figure S4F). Overall, the PCDN mAbs demonstrated weak to no auto- or poly-reactivity, with the exceptions described.
Autologous Virus and the bnAb Lineage Co-evolved within Donor PC76
Changes in Env sequence can provide information as to how the virus adapted to the immune response after infection and might have driven the maturation of a bnAb lineage. A total of 76 autologous env genes were cloned and sequenced from PC76 plasma at multiple time points. A phylogenetic analysis of the virus full-length env sequences shows their course of evolution (Figure S5A).
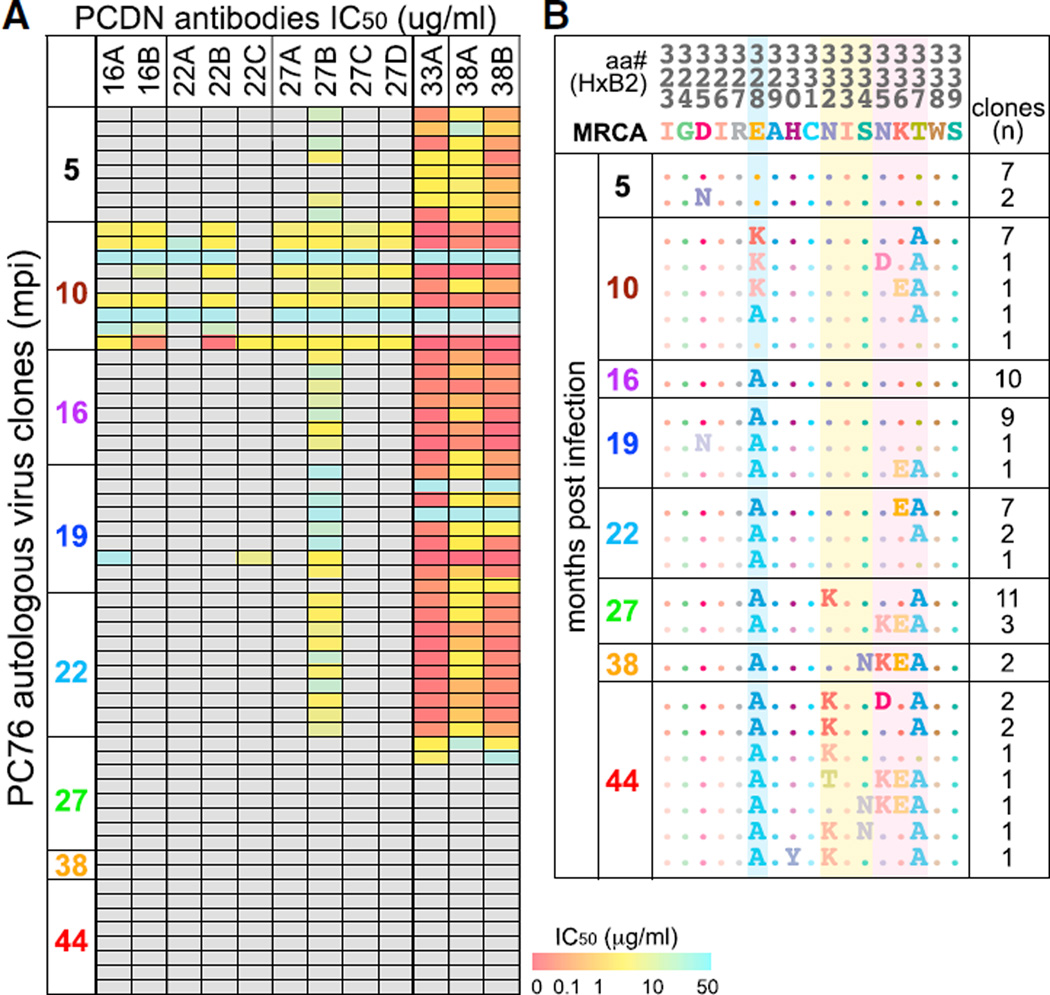
Viruses pseudotyped with the autologous env were tested for neutralization by the 12 mAbs (Figure 4A). The viruses from 5 mpi were not neutralized by any of the earliest mAbs (from months 16 and 22), which neutralized only viruses from 10 mpi. The month-16 viruses were no longer neutralized by these Abs, demonstrating viral escape. This suggests that the lineage was triggered by viruses emerging between months 5 and 10. When the 5 mpi virus was tested for neutralization by serum, all but the 5 mpi serum were able to neutralize the virus, supporting this time frame (Figure S5B). All Abs isolated past 33 mpi displayed both strong autologous breadth and potency, neutralizing almost all of the viruses tested up to month 22. The first mAb that showed autologous breadth but weak potency was isolated at 27 mpi (PCDN-27B). Interestingly, these Abs were able to neutralize the 5 mpi viruses, in contrast to the earliest Abs. However, from 27 mpi on, most of the viruses fully escaped all PCDN mAbs.
Figure 4. Autologous Virus Neutralization and Development.
(A) 76 autologous virus clones from 8 time points were tested for neutralization by PCDN mAbs. The IC50 values (µg/mL) are represented in the color gradient. All samples for which 50% neutralization was not reached at 50 µg/mL were determined as having no neutralization (gray boxes). Neutralization matrix was performed once.
(B) Sequences of Env from 323 to 339 by HXB2 numbering of 76 of the virus clones are shown. MRCA (most recent common ancestor) is an inferred earliest ancestral sequence. The residues or PNGSs that change over time are shaded in blue, yellow, or pink. The number of isolated autologous virus clones with each sequence at each time point is shown on the right.
When the Env aa changes were examined, seven out of nine clones from 10 mpi simultaneously mutated E328 to a Lys while deleting a glycan site at N335 by mutating T337 to an Ala (Figure 4B). The N335 PNGS is present in <1% of virus strains on the Los Alamos HIV Sequence Database, making this PNGS a unique feature of the PC76 autologous virus. In contrast to N334, N335 could potentially be glycosylated together with N332. This E328K mutation was then further mutated to an Ala in all of the viruses isolated from 16 mpi and later. The N335 PNGS was initially reverted back in most clones at 16 and 19 mpi and then removed again in 26 of 28 virus clones from 22 mpi and later. All of the viruses from 27 mpi and later that were not neutralized by any of the PCDN Abs had removed the N332 PNGS (N332K or S334N), which was likely the final route of escape.
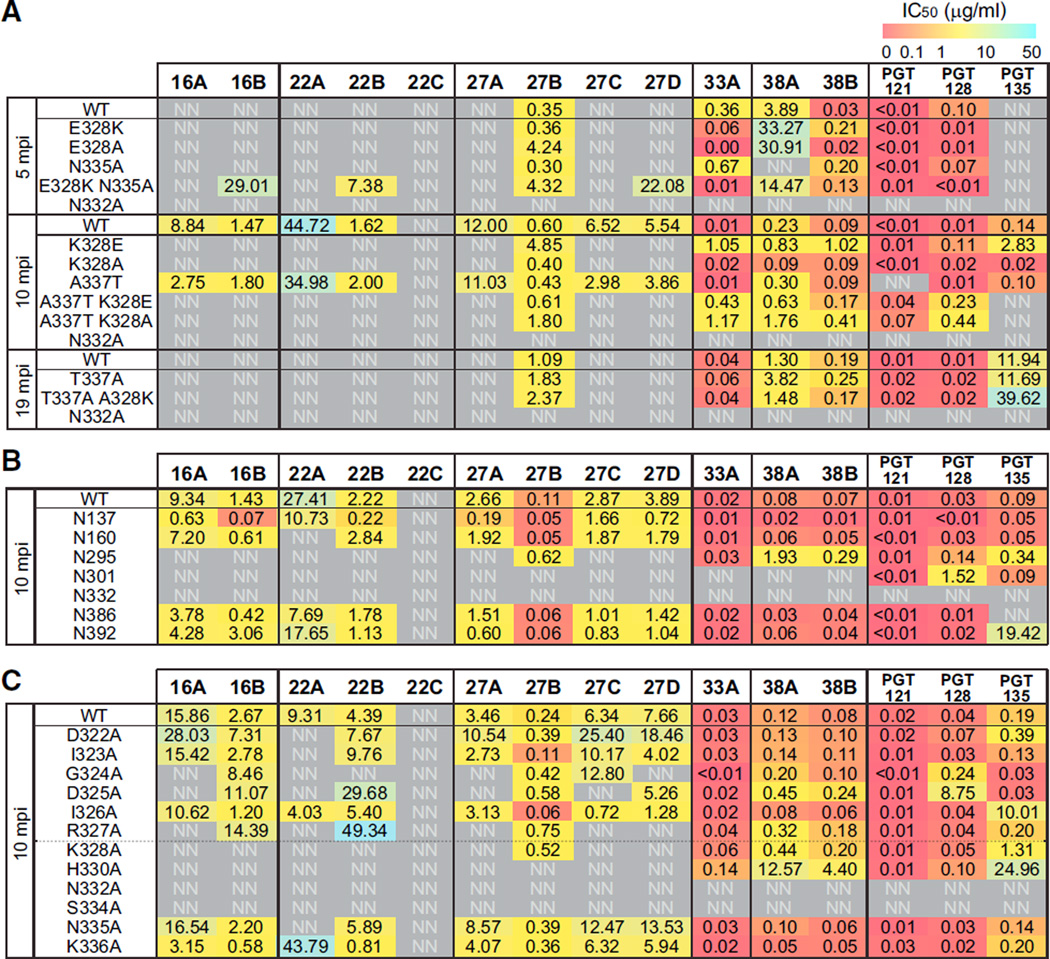
To test whether these residues indeed had a role, mutagenesis of the autologous virus from 5, 10, and 19 mpi was performed (Figure 5A). When the 5 mpi virus was mutated to have both E328K and N335A mutations, some early Abs were now able to neutralize the virus. When the mutations were introduced individually, this increased sensitivity was not observed, suggesting the combination of mutations contributed to the recognition of the virus by the early PCDN lineage Abs. If the 10 mpi virus was either reverted to the 5 mpi form, K328E, or mutated to the later form, K328A, neutralization by all the early Abs was knocked out, demonstrating escape, whereas PCDN-27B and later Abs were still able to neutralize these viruses. However, when the 19 mpi virus was reverted to A328K with the N335 glycan, neutralization was not restored. Reversal of an escape mutation is more difficult because there might be additional mutations involved that are not in the vicinity of the epitope and harder to pinpoint. However, when the N332 PNGS was removed from the 19 mpi virus, neutralization by all the PCDN Abs was completely lost, which was also the case for N332 PNGS mutations of earlier viruses. Once the N332 PNGS was eliminated, the virus completely escaped neutralization by the PCDN Ab lineage, consistent with all the heterologous virus neutralization results (Figure S4B).
Figure 5. Autologous Virus Mutagenesis and Mapping.
(A) The dominant autologous viruses from three critical time points were tested for neutralization by all PCDN mAbs. The unmutated virus is indicated as WT for each time point. Neutralization assays with pseudoviruses expressing Env with the specified mutations were performed in comparison to WT.
(B) PNGSs on the 10 mpi virus were mutated and tested for neutralization in comparison to the WT virus.
(C) Residues from 322 to 336 were mutated to Ala in the 10 mpi virus and tested for neutralization in comparison to WT. The numbers show IC50 values (µg/mL) and are shaded for potency as indicated in the color gradient bar. Neutralization experiments were performed in triplicate, and the means were used to calculate IC50 values.
The 10 mpi virus was neutralized by most of the PCDN Abs, allowing for mutagenesis studies to map the change in contact residues over time. Mutations to PNGSs showed that N295, N301, and N332 were critical (Figure 5B). Loss of glycosylation sites at N301 and N332 knocked out neutralization for all the Abs, although N295 PNGS mutation did not greatly affect PCDN-27B and late Abs. Loss of the N137 glycan site in the 10 mpi virus potentiated all the Abs except those that didn’t neutralize this virus to start with. An Ala scan of the N332-proximal V3 region was also performed. Mutations to residues D322 to K328 knocked out or weakened neutralization by the majority of the early Abs, with the exception of I323A and I326A, which had little to no effect. The 33 mpi and later Abs were largely unaffected by these mutations. The H330A mutation knocked out PCDN-27B neutralization and reduced the potency of later Abs. Similarly to the results from the 110-virus panel and JR-CSF alanine scan (Figures S4B and S4C), this suggests that the later Abs matured to become less dependent on residues in the GDIR motif but still rely on N332 and N301 glycans and H330 to interact with Env.
NGS of the PCDN Ab Lineage
To examine the longitudinal maturation of the PCDN Ab lineage, we performed NGS of Ab HCs by using RNA isolated from approximately 106 PBMCs from time points 5–66 mpi. Experimental details can be found in Supplemental Experimental Procedures and Figure S6A. Raw Ab sequences were corrected with molecular barcodes and GL gene assignments were made with AbStar (data not shown). Ab lineages were assigned with Clonify (Briney et al., 2016), and the PCDN lineage was identified by a homology search with the panel of PCDN mAbs.
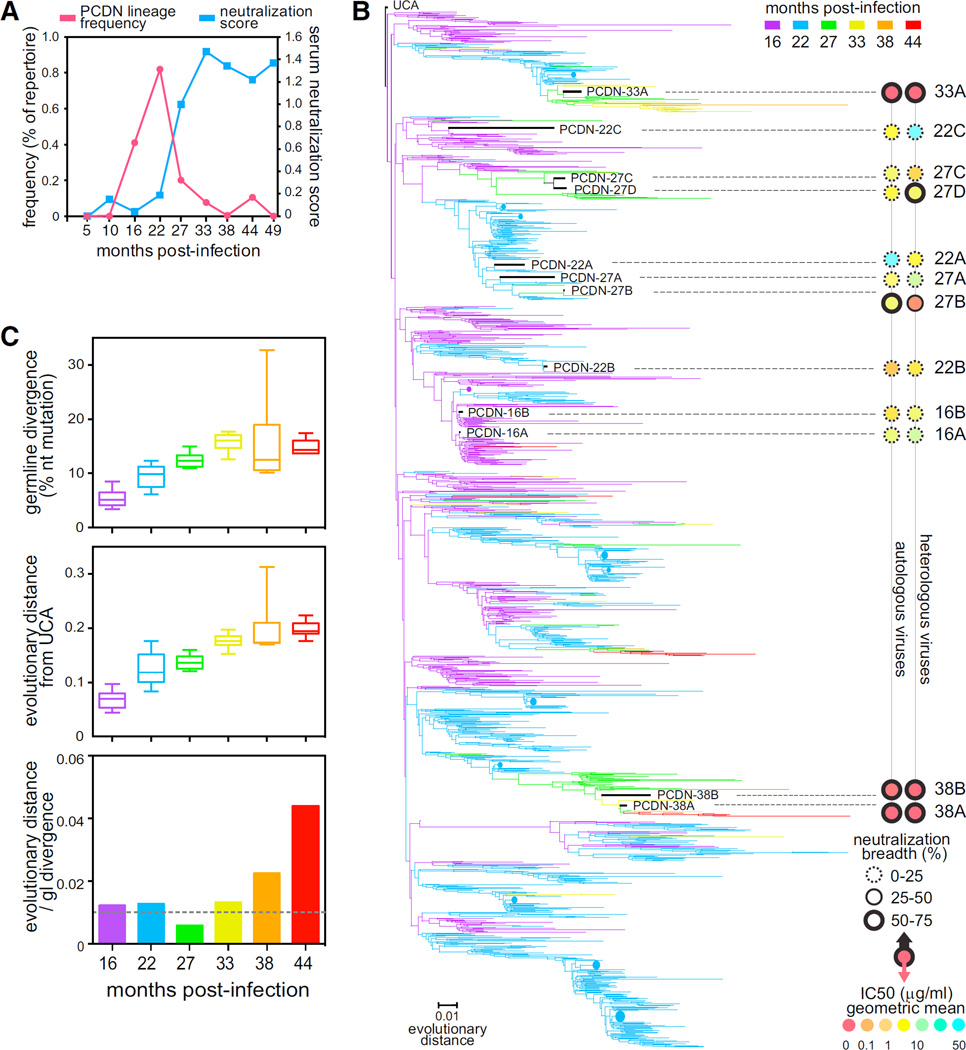
Although lineage members were identified at all time points between 16 and 44 mpi, there was a pronounced peak at 22 mpi, immediately prior to the development of neutralization breadth and potency (Figure 6A). The subsequent drop might be due to the virus escaping neutralization by this lineage around 27 mpi (Figure 4A). To verify that this frequency peak was not an artifact of sequencing or pre-sequencing amplification, we amplified and sequenced additional longitudinal samples by using different sample preparation methods (5′ rapid amplification of cDNA ends [RACE] and alternate gene-specific primers) and observed the same frequency pattern in each case (Figure S6B).
Figure 6. NGS of the PCDN mAb Lineage.
(A) The frequency of sequences from the PCDN lineage relative to the entire repertoire is shown in pink with circles for each time point. The neutralization score at each time point is indicated in blue with squares.
(B) The phylogenetic tree of PCDN Ab HC sequences from six different time points shaded in the colors as indicated. The relative evolutionary distance is indicated as a bar at the bottom. Collapsed sequences are shown in circles in sizes relative to the number of sequences. The PCDN mAb sequences are indicated in black with the Ab name. The neutralization breadth and IC50 values for autologous and heterologous viruses are illustrated as concentric circles on the right side of the tree after the dotted lines. The outside circle represents breadth; the center circle represents IC50 values; the color code is as depicted at the bottom right.
(C) SHM was calculated for each time point either as divergence (number of aa changes compared to UCA; top panel) or as sum of the evolutionary distance (middle panel). The rate increase of evolutionary distance normalized by the rate of GL divergence is shown in the bottom panel. The boxes show the first and third quartile as the lower and upper limits of the box with a horizontal line for the median, and the end of the vertical lines indicate the minimum and maximum values.
NGS identified a PCDN lineage sequence with high homology to the GL V- and J-genes, bearing a single aa difference in the J-gene. This least mutated sequence was selected as the unmutated common ancestor (UCA) for HC (Figure S3). A UCA IgG was expressed by pairing the HC with an inferred LC that was reverted directly to GL. The UCA IgG had only minimal binding to some heterologous rgp120 proteins and no binding to the autologous rgp120s tested by ELISA (Figures S6C and S6D). In addition, no neutralization was observed for the UCA for any of the viruses of the 37-virus panel or any autologous viruses tested (data not shown).
Phylogenetic analysis of the PCDN lineage (Figure 6B) revealed multiple large limbs that diverged early in the lineage lifespan, almost as soon as the lineage was initiated, and independently matured in parallel. A genetic divide was observed in the lineage phylogeny, beyond the peak of frequency described above, where most branches did not develop beyond 27 mpi and seemingly reached a dead end. This suggests the occurrence of a virus-induced lineage-sieving event near 27 mpi due to viral escape and a need for early intra-lineage diversification to achieve lineage longevity. Interestingly, more than one of the limbs led to Abs capable of heterologous neutralization, including the ones leading to the broad and potent Abs, PCDN-33A and PCDN-38A and 38B. In addition, sequencing identified another long-lived lineage branch that might have developed breadth and from which we were unable to isolate Abs by B cell sorting. Therefore, within this single donor, multiple developmental pathways led to Abs that acquired breadth. Together, early diversification and parallel maturation appear to be central to the development of a broad Ab response.
When analyzing the lineage phylogeny, we noticed a substantial discrepancy between the phylogenetic evolution of the lineage and the divergence from GL over time. Although GL divergence (GD), measured as the percent nucleotide change from UCA, appeared to plateau at 33 mpi (top panel, Figure 6C), the tree-adjusted evolutionary distance (ED) from UCA continued to increase after 33 mpi (middle panel, Figure 6C). To quantify this discrepancy, we calculated the ratio between the GD rate of mutation and tree-adjusted ED rate of increase (bottom panel, Figure 6C). Until 33 mpi, the GD:ED ratio remained relatively constant at approximately 0.01, suggesting that GD roughly approximated total ED. After 33 mpi, however, there was a sharp increase in the ED:GD ratio, which indicated that GD was underestimating total ED. Because both were calculated at the nucleotide level, this result suggests repeated somatic mutations at the same position; re-mutating a previously mutated site would increase ED but would not change GD. Presumably, the number of activation-induced cytidine deaminase (AID) hotspot sequences, WRCY (Martin et al., 2002), should decrease with continued SHM. When we assessed the number of AID hotspots for the PCDN lineage over time, we observed a steady decrease that was not seen for a random sample of Ab sequences (Figure S6E). Therefore, as an Ab lineage matures, SHM appears to continue to occur at previously mutated sites despite the loss of hotspot motifs, suggesting a strong selection pressure for these positions.
Crystal Structures of PCDN Fabs
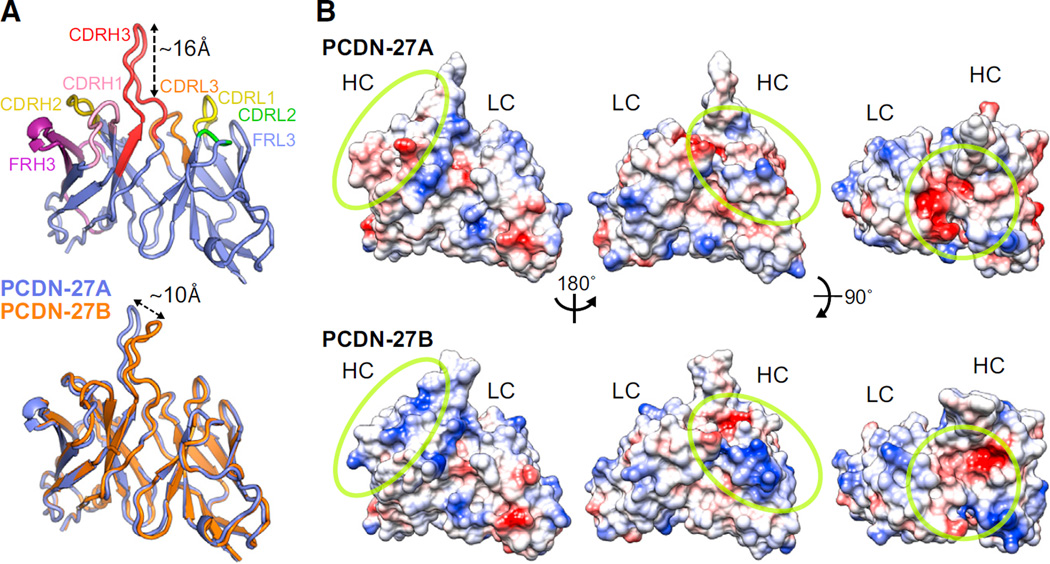
The structural evolution of the PCDN lineage Abs could provide explanation of how they mature to gain breadth or potency. PCDN-27A and PCDN-27B are situated on the same branch of Ab maturation (Figure 6B), yet 27A has limited heterologous neutralization in comparison to 27B (Figure 3B).
To understand this functional leap, we determined the crystal structures of PCDN-27A and PCDN-27B Fabs (Figure 7A). Both Fabs have a protruding “dagger”-like shape with the CDRH3 “blade” rising ~16 Å above the other CDR loops (Figure 7A). The extended CDRH3 will most likely penetrate the glycan shield and establish contacts with the underlying protein motif. The aromatic residues positioned on both sides of the CDRH3 loop (Figure S7A), Y103, Y106, and W107, could be interacting with the Env 324GDIR327 residues or the carbohydrates at the high-mannose patch through stacking interactions similar to PGT124 (Garces et al., 2014). The variable HC and LC regions of both PCDN-27A and PCDN-27B Abs align well with a Cα RMSD (root-mean-square deviation) of 0.49 Å (Figure 7A). However, the tip of the CDRH3 in PCDN-27B appears to be tilted ~10 Å in comparison to PCDN-27A. Before relating this difference to functionality, we investigated the crystal packing of both Fabs. When the structure of PCDN-27A is superimposed on that of PCDN-27B within the crystal lattice, the CDRH3 loop of 27A would clash with the constant region of a symmetry-related molecule of 27B (Figure S7B). Thus, the slight variation in the CDRH3 loops between the two Fabs is probably due to crystal packing and is likely not the cause of the observed greater breadth and potency of PCDN-27B.
Figure 7. Crystal Structures of PCDN-27A and PCDN-27B Fabs.
(A) The secondary structure of the PCDN-27A is illustrated in lavender with the CDR and FR regions highlighted in different colors. Beneath this, a superimposition of the PCDN-27A structure in lavender and the PCDN-27B structure in orange is shown.
(B) The electrostatic potential of the two Fabs is illustrated. Red indicates a negatively charged surface, and blue indicates a positive charge. Rotated views, shown with a yellow circle, highlight the areas with the most change.
According to the sequence alignments for PCDN-27A and PCDN-27B (Figure S3), there were no differences between the two in CDRH1 or CDRH3, whereas the FRH3s were distinct. Although this sequence variability was not translated into structural variation, the electrostatic surface indicated a shift from residues with negative charges in PCDN-27A to positive charges in PCDN-27B (Figure 7B). This suggests that FRH3 might be important for neutralization of both autologous and heterologous viruses. In addition, we noticed two PNGSs in FRH1 (N26) and FRH3 (N68) of PCDN-27B. Density was observed in the crystal structure at N26, confirming the presence of an N-linked glycan, but not at N68, due to either disorder or to non-use of the PNGS (Figure S7C). However, from the position of the N26 glycan in the Fab structure, it seems unlikely that this glycan would contribute to the greater breadth of PCDN-27B than PCDN-27A.
We compared the structural features of PCDN-27A against the other N332-targeting bnAbs for which crystal structures have been solved (Garces et al., 2014; Kong et al., 2013; Pejchal et al., 2011). The Cα RMSD was lowest for PGT135, showing overall the greatest structural similarity (Figure S7D). However, the CDRH3 of PCDN-27A (22 aa) is longer than that of PGT135 (18 aa), and PCDN-27A does not have any insertions, unlike PGT135, which has a 5 aa insertion in CDRH1.
DISCUSSION
The description herein of the developmental pathway of a broad neutralizing response to the N332 glycan supersite on HIV-1 Env is encouraging from a vaccine design standpoint. Although other high-mannose-patch-targeting bnAbs may be superior in both breadth and potency in comparison to the PCDN Abs, Abs of this lineage notably did not have any indels and, in the case of PCDN-33A, achieved close to 50% breadth (>85% and 60% for subtypes B and A, respectively) with ~11% overall SHM, a level that is achievable through vaccination (Liao et al., 2013a; Pappas et al., 2014; Wrammert et al., 2008). The PCDN mAbs therefore represent a finding that provides optimism for attempts to induce bnAbs to the high-mannose patch through vaccination.
Auto- or polyreactivity has been observed to be relatively more prevalent in HIV bnAbs (Liu et al., 2015) and has been suggested to be a hurdle for bnAb development. Although the PCDN Abs overall exhibited very low levels of auto- or polyreactivity, the GL VH gene of the PCDN lineage, IGHV4–34*01, is known to have autoreactive properties due to a hydrophobic motif (Ala-Val-Tyr; AVY) in FRH1 (Pascual et al., 1991; Pascual et al., 1992). The corresponding B cells are mostly suppressed or anergic (Pugh-Bernard et al., 2001), but in some circumstances might develop, as in patients with systemic lupus erythematosus, who have compromised B cell tolerance checkpoints (Cappione et al., 2005), or in healthy donors upon repetitive exposure to antigens (Sabouri et al., 2014). In HIV-infected donors, a higher prevalence of VH4–34 Abs has been reported, possibly due to a breakdown of tolerance, which correlates with broadly neutralizing serum activity (Kobie et al., 2012). Interestingly, some Abs of the lineage acquired a PNGS in FRH1 (most likely glycosylated, given that corresponding density was observed in the crystal structure) immediately downstream of the AVY motif, reminiscent of changes diminishing self-reactivity described by Sabouri et al. (2014). Furthermore, some of the PCDN Abs lost the CDRH2 PNGS at position 52, characteristic of the VH4–34 GL VH; glycosylation at this site has been speculated to decrease binding to foreign antigens. Together, the data suggest that as PCDN Abs mature, they mutate away from the features described to prevent the development of VH4–34 Abs. Further studies will need to determine whether and how the autoreactivity properties of VH4–34 played a role in the elicitation of the PCDN lineage and whether this might be a potential limitation to triggering this Ab lineage through vaccination.
Study of the autologous virus neutralization revealed that it was not an early virus but a virus emerging between 5 and 10 mpi that triggered the PCDN lineage, in part as a consequence of a E328K charge change and the removal of a PNGS at 335 that increased the sensitivity to early Abs. The N335 PNGS is rarely observed in global HIV strains, and it is not known whether N335 is indeed glycosylated or what specific type of glycosylation it might acquire. The E328K and T337A changes might have been selected through pressure from another earlier autologous non-broad Ab lineage—given that there was no evidence of breadth from serum neutralization at this stage— that targeted the same region as the PCDN lineage and possibly acted as a helper lineage (Gao et al., 2014). A further mutation at 328 led to escape from early and intermediate Abs and might have played a role in driving selection of the most mature broad Abs. A loss of the N332 PNGS past 27 mpi led to escape from all the PCDN Abs, suggesting the termination of antigenic stimulation at this point. However, PCDN-related Ab sequences were observed by NGS to mature up to 44 mpi, suggesting either the persistence of some viral antigenic stimulation or the existence of a delay between Ab-mediated pressure and the appearance of memory B cells in the peripheral blood, as seen in previous bnAb lineage studies (Doria-Rose et al., 2014; Liao et al., 2013b; Wu et al., 2015).
Mapping of the PCDN mAbs on the 10 mpi autologous virus revealed a divide between early and late Abs. Many of the V3 residues upstream of N332, including previously reported G324, D325, and R327, severely affected neutralization of the early Abs. However, even some early Abs, such as PCDN-16B, were able to tolerate these mutations, showing that diversity in the mode of recognition of the base of the V3 loop started early. These V3 mutations, however, did not severely affect the 33 mpi and later Abs, other than the H330A mutation. H330 has been reported to be part of the epitopes for PGT135 (Kong et al., 2013) and PGT122 (Pancera et al., 2014), so it is conceivable that it could also affect the PCDN mAbs. The H330 residue also might have a role in positioning the N332 glycan in the correct orientation with its large aromatic ring as opposed to actually interacting with the mAbs for recognition. Overall, the results confirm that the V3 base is a protein anchor point recognized through various modes by Abs targeting the N332 supersite. The most mature and broad Abs are able to tolerate mutations in the protein anchor region, possibly due to recognition of the backbone, as seen in PGT128. However, because the V3 base is highly conserved (GDIR motif found in >78% viruses), backbone recognition should not presumably provide an advantage over side-chain recognition in terms of breadth. The evolution in the mode of recognition of the V3 base by the PCDN lineage might thus be a by-product of affinity improvement toward the N332 glycan and nearby protein anchors. This evolution might be allowing the most mature Abs to accommodate the diversity surrounding the conserved V3 base, coincidently granting heterologous neutralizing activity.
Glycans have a large role in determining PCDN mAb recognition of Env. Glycan array results suggested that high-mannose glycans are important for PCDN Ab binding. Although no affinity for processed glycans was detected on the array (data not shown), neutralization by the PCDN lineage appears to depend on the presence of hybrid glycans. Hybrid or complex glycans are mostly found at position N301 in the glycan patch of the Env, whereas high-mannose glycans are mostly found at N295 and N332 (Pritchard et al., 2015). Accordingly, mutation of the N301 PNGS in the autologous or heterologous viruses invariably led to greatly reduced neutralization. It is likely that binding to hybrid or complex glycans alone on an array does not provide enough affinity for detection. Differences between the mature Abs were noticeable given that PCDN-33A was not as dependent on hybrid or complex glycans as PCDN-38A and PCDN-38B were. Whereas N301 and N332 PNGSs were crucial for binding of all the PCDN Abs, the N295 PNGS was required for neutralization by all the Abs up to 27 mpi except for PCDN-27B. Tolerating the absence of the N295 glycan accompanies and might contribute to breadth. Similarly to the PGT121 family, neutralization by the PCDN Abs improved when the N137 PNGS was removed, suggesting a shielding effect by this glycan. The sensitivity to loss of the N156 glycan points to a possible interaction with this glycan as well. This is not surprising, given that proximity to this glycan has been seen for PGT122, which might be recognizing Env in a similar fashion to the PCDN Abs (Pancera et al., 2014).
According to the crystal structures of the PCDN Fabs, these Abs have more similarities with PGT135 than with PGT128 and PGT124. PGT135 recognizes different glycans at the high-mannose patch, interacting with glycans at N386 and N392. However, none of the PCDN mAbs are affected by the loss of PNGSs at N386 or N392, and the glycan dependency of these Abs appears more similar to that of the PGT121 and PGT128 families. The two Fab structures that we have determined, PCDN-27A and PCDN-27B, are on the same branch of the lineage in the affinity maturation process and are structurally very similar. It is intriguing that, given the limited number of aa changes, PCDN-27B is fairly broad compared to PCDN-27A, which exhibits no heterologous neutralization. Changes in electrostatic charge between the Fabs might help provide an explanation, although this might apply to just this single branch and might not be relevant to all Abs of the lineage that develop breadth.
The NGS results for the PCDN Ab lineage illustrate an Ab response that finds multiple approaches to attack an ever-changing target. The first PCDN-related sequences were observed at 16 mpi, although autologous virus escape suggests the lineage was triggered earlier, presumably between 5 and 10 mpi. This again shows a delay, although shorter than the time lag following complete lineage escape described above, between apparent Ab pressure on the virus and presence of the corresponding memory B cells in the peripheral blood. The phylogenetic tree of the PCDN-related sequences showed a burst of diversification at 16–22 mpi shortly after the lineage was elicited, Then, from 27 mpi and beyond, there seemed to be a focusing of maturation on a limited number of branches, with recurring replacement of residues at a limited number of sites within the Ab sequence, suggesting a fine-tuning of Abs for greater affinity after the initial diversification. Several branches continued to progress in evolutionary distance, but the majority were stunted and did not progress beyond 27 mpi, leading to apparent dead ends, as seen in previous bnAb lineage studies (Bhiman et al., 2015; Pappas et al., 2014). Of the branches that continued to evolve, two were able to develop Abs that had breadth and potency comparable to other previously described bnAbs, and two other branches led to Abs with moderate breadth. There was an additional branch with mature, potentially neutralizing Abs that were not isolated and hence not characterized. Therefore, in this donor, it was remarkable that several Ab branches diverged very early and evolved in parallel toward breadth and potency. This demonstrates that within a single donor and a single Ab lineage, several pathways can evolve for selection of bnAbs against the high-mannose patch and therefore enable multiple approaches for targeting this region. Development of other bnAb lineages reported also suggests an early diversification, but one followed by a more restricted B cell selection (Doria-Rose et al., 2014; Liao et al., 2013b). This might be related to the multiple modes of Ab recognition of the N332 region, in contrast to the CD4 binding site and the V1–V2 apex.
Our results support the idea that the N332 glycan region might be a favorable vaccine target. Contrary to other bnAbs targeting this site (Mouquet et al., 2012; Walker et al., 2011), the PCDN Abs attained broad neutralization at relatively low levels of SHM and without indels in either HCs or LCs. The multi-limb lineage development that leads to varied solutions to breadth suggests that rational vaccine design aiming at eliciting bnAbs targeting the N332 region, as with serial lineage-based immunogens, might be most efficient if not restricted to a precise narrow path of development. Immunization strategies would most likely benefit by promoting simultaneous maturation of multiple pathways through the use of immunogens that mimic the evolution of the entire N332 supersite rather than focusing on the precise epitope of a particular bnAb.
EXPERIMENTAL PROCEDURES
Human Specimens
Serum and PBMC samples were obtained from donor PC76, enrolled in the IAVI Protocol C longitudinal HIV-1 infection cohort. IAVI Protocol C enrolled 613 individuals from sub-Saharan Africa who were rapidly screened for HIV Abs after recent exposure to HIV-1. Samples were collected with written informed consent, and clinical protocols were approved by the appropriate ethics and research committees.
Neutralization Assays
Neutralization assays were performed by incubating Abs or sera with HIV-1 pseudoviruses that are capable of one round of infection to TZM-BL cells (Walker et al., 2009). Serum depletion was performed with Tosylactivated Dynabeads (Life Technologies) conjugated with BSA or with WT and N332A 92BR020 rgp120 proteins. Serum samples were depleted of Abs binding to these proteins through multiple rounds of immunoprecipitation (Li et al., 2009) as confirmed by ELISA, prior to use in neutralization assays.
Single Memory B Cell Sorting and Ab Isolation
Single antigen- and epitope-specific memory B cells were sorted into wells of 96-well PCR plates, RT-PCR was performed with multiplex PCR methods and primers, then products were sequenced and cloned into HC or LC constant-region-encoding vectors (Sok et al., 2014b; Tiller et al., 2008; Wu et al., 2010).
IgG Ab and Fab Fragment Production
Full-length or truncated Fab HC constructs and LC expression plasmids were transfected into HEK293F cells with the FreeStyle 293 Expression System (Life Technologies) to produce Abs and Fab fragments and were purified as described in the Supplemental Experimental Procedures.
Glycan Array Preparation and Ab Hybridization
40 glycans were printed by a robotic pin (SMP3; TeleChem International) and 0.6 nL of 100 µM concentration of amine-functionalized glycans in printing buffer ([pH 8.5] 300 mM phosphate buffer, 0.05% Tween-20) from a 384-well plate was deposited onto NHS-coated glass slides that were then blocked and washed before being incubated with Abs (10 µg in 50 µl PBS) pre-complexed with secondary Ab. The slides were washed and scanned on an Array-Worx microarray reader. Images were analyzed with Genepix Pro 6.0 (Molecular Devices Corporation).
Ab NGS and Computational Analysis
RNA was prepared either from 1 × 107 whole PBMCs or IgG+ memory B cells separated from PBMCs (Switched memory B cell isolation kit; Miltenyi Biotec). RNA was subjected to RT-PCR with barcoding primers that contain unique Ab identifiers, and Illumina sequencing adapters and sample-specific indexes were added during a second round of PCR; primer sequences can be found in Table S1. Samples were quantified with fluorometry (Qubit; Life Technologies) and pooled at approximately equimolar concentrations, and the sample pool was requantified to be loaded onto an Illumina MiSeq. Paired-end MiSeq reads were merged with PANDAseq (Masella et al., 2012). GL assignment, junction identification, and other basic Ab information was determined with AbStar, an antibody analysis package that uses BLASTn and Smith-Waterman alignment to calculate the most likely GL genes, identify the junctional region, and annotate SHM-induced substitutions and indels (B.B., unpublished data). Sequences were assigned to clonal lineages with Clonify, a software package specifically developed for Ab lineage assignment (Briney et al., 2016). We constructed the phylogenetic trees with FastTree by using maximum likelihood.
Crystal Diffraction Data Collection and Computational Analysis
Data were collected at Stanford Synchrotron Radiation Lightsource (SSRL) 12-2 beamline. The best dataset for the PCDN-27A crystal was collected at 2.7 Å resolution, and the diffraction data were processed with HKL-2000 (Otwinowski and Minor, 1997) to an overall Rsym of 0.12% and completeness of 93.9% (Table S2) in space group P21 with unit cell parameters a = 41.8 Å, b = 120.4 Å, c = 111.1 Å, and β = 96.4°. Data were collected from a PCDN-27B crystal to 2.9 Å resolution and processed with HKL-2000 (Otwinowski and Minor, 1997) to an overall Rsym of 0.28% and completeness of 99.5% in space group P6122 with unit cell parameters a = b = 89.3 Å, c = 211.1 Å. The PCDN-27A Fab structure was solved by molecular replacement with Phaser and a complementarity determining region (CDR)-loop-deleted X5 Fab structure (PDB: 1RRH) was used as the initial model, whereas the PCDN-27B Fab structure was solved with the family-related PCDN-27A Fab structure. Model building was carried out as previously described (Garces et al., 2014). Final Rcryst and Rfree values for PCDN-27A and PCDN-27B Fabs structure are 21.3% and 24.7%, and 23.3% and 29.7%, respectively.
Detailed additional experimental procedures can be found in the Supplemental Experimental Procedures.
Supplementary Material
In Brief.
Exploring the development of broadly neutralizing antibodies (bnAbs) provides insight into improving HIV vaccination strategies. Poignard and colleagues describe the evolution of a bnAb lineage with diverse recognition of the conserved high-mannose patch on HIV Env and several promising features that provide optimism for eliciting similar responses through vaccination.
Highlights.
N332-site Abs with low somatic mutation and no indels achieve broad neutralization
N332-bnAb lineage diversifies early into multiple limbs
Independent maturation of lineage limbs leads to diverse N332-site bnAb recognition
Abs to the N332 site show CDRH3s with extended “dagger”-like shapes
Acknowledgments
This work was funded in part by IAVI and made possible by the support of many donors, including the Bill & Melinda Gates Foundation, the Ministry of Foreign Affairs of Denmark, Irish Aid, the Ministry of Finance of Japan in partnership with The World Bank, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the United Kingdom Department for International Development, and the United States Agency for International Development (USAID). The full list of IAVI donors is available at http://www.iavi.org. The contents of this manuscript are the responsibility of IAVI and do not necessarily reflect the views of USAID or the United States government. We would also like to thank all of the Protocol C clinical investigators, study participants, and research staff at each of the Protocol C clinical centers. This work was supported by grants U19AI090970 and UM1AI100663 from the National Institute of Allergy And Infectious Diseases. B.M. was supported by grants U01GM110749, AI100665, and K99AI120851 and I.A. by R01 AI084817. We thank Kay Limoli, Sam Jauregui, and Johnazen Perea at Monogram Biosciences for their contribution to this study and Olayinka Fagbayi for helping with the graphical summary. Finally, we are grateful to Shane Crotty (La Jolla Institute for Allergy and Immunology; LIAI), Colin Havenar-Daughton (LIAI), Devin Sok (IAVI), Matthias Pauthner (The Scripps Research Institute), and all researchers of the U19 group for generously providing reagents, thoughtful discussions, and technical help for this project.
CONSORTIA
The members of The IAVI Protocol C Investigators & The IAVI African HIV Research Network are Matt A. Price, Jill Gilmour, Pat Fast, Anatoli Kamali, Eduard J. Sanders, Omu Anzala, Susan Allen, Eric Hunter, Etienne Karita, William Kilembe, Shabir Lakhi, Mubiana Inambao, Vinodh Edward, and Linda-Gail Bekker.
Footnotes
ACCESSION NUMBERS
Accession numbers for HC and LC variable regions are deposited in the GenBank database under GenBank: KU200842–KU200867. NGS sequences are deposited in the NCBI Sequence Read Archive BioProject database under PRJNA: 304070.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2016.04.016.
AUTHOR CONTRIBUTIONS
D.T.M., N.M.C., E.L., and P.P. conceived and designed the experiments; D.T.M., N.M.C., B.B., F.G., L.S.V., E.L., T.W., C.H.L., A.R., C.B.B., L.W., C.Y.W., and C.H.W. performed the experiments; W.K. collected samples; C.Y.W. and C.H.W. provided the glycan array; D.T.M., N.M.C., B.B., F.G., B.M., L.K., K.E., S.L.K.P., D.R.B., I.A.W., and P.P. analyzed the data; D.T.M., N.M.C., and P.P. wrote the manuscript; D.T.M., N.M.C., I.A.W., D.R.B., and P.P. reviewed and edited the manuscript.
REFERENCES
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-WW, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat. Med. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Peña AT, Cupo A, Julien JP, van Gils M, Lee PS, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity. 2014;40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Crowe JE., Jr Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes. PLoS ONE. 2012;7:e36750. doi: 10.1371/journal.pone.0036750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney B, Le K, Zhu J, Burton DR. Clonify: unseeded antibody lineage assignment from next-generation sequencing data. Sci. Rep. 2016;6:23901. doi: 10.1038/srep23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. NISC Comparative Sequencing Program. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, et al. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces F, Sok D, Kong L, McBride R, Kim HJ, Saye-Francisco KF, Julien JP, Hua Y, Cupo A, Moore JP, et al. Structural evolution of glycan recognition by a family of potent HIV antibodies. Cell. 2014;159:69–79. doi: 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, Zolla-Pazner S, Kong X-P. Conserved structural elements in the V3 crown of HIV-1 gp120. Nat. Struct. Mol. Biol. 2010;17:955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobie JJ, Alcena DC, Zheng B, Bryk P, Mattiacio JL, Brewer M, Labranche C, Young FM, Dewhurst S, Montefiori DC, et al. 9G4 autoreactivity is increased in HIV-infected patients and correlates with HIV broadly neutralizing serum activity. PLoS ONE. 2012;7:e35356. doi: 10.1371/journal.pone.0035356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Lee JH, Doores KJ, Murin CD, Julien J-PP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse P-JJ, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, Wickramasinghe L, Ramos A, Bian CB, Simek M, Allen S, et al. Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog. 2016;12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, et al. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 2009;83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H-X, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013a;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H-XX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. NISC Comparative Sequencing Program. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013b;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, Kelsoe G. Polyreactivity and autoreactivity among HIV-1 antibodies. J. Virol. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Bardwell PD, Woo CJ, Fan M, Shulman MJ, Scharff MD. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature. 2002;415:802–806. doi: 10.1038/nature714. [DOI] [PubMed] [Google Scholar]
- Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. 2012;13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B, Rakasz EG, Schultz N, Chan-Hui P-YY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams M-RR, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat. Med. 2012;18:1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. USA. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang G-YY, Ofek G, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- Pascual V, Victor K, Lelsz D, Spellerberg MB, Hamblin TJ, Thompson KM, Randen I, Natvig J, Capra JD, Stevenson FK. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4–21 gene segment is responsible for the major crossreactive idiotype. J. Immunol. 1991;146:4385–4391. [PubMed] [Google Scholar]
- Pascual V, Victor K, Spellerberg M, Hamblin TJ, Stevenson FK, Capra JD. VH restriction among human cold agglutinins. The VH4–21 gene segment is required to encode anti-I and anti-i specificities. J. Immunol. 1992;149:2337–2344. [PubMed] [Google Scholar]
- Pejchal R, Doores KJ, Walker LM, Khayat R, Huang P-SS, Wang S-KK, Stanfield RL, Julien J-PP, Ramos A, Crispin M, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LK, Spencer DI, Royle L, Bonomelli C, Seabright GE, Behrens AJ, Kulp DW, Menis S, Krumm SA, Dunlop DC, et al. Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies. Nat. Commun. 2015;6:7479. doi: 10.1038/ncomms8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl. Acad. Sci. USA. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf L, Scheid JF, Lee JH, West AP, Jr, Chen C, Gao H, Gnanapragasam PNP, Mares R, Seaman MS, Ward AB, et al. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep. 2014;7:785–795. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien J-PP, Menis S, Wickramasinghe L, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci. Transl. Med. 2014a;6:236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, van Gils MJ, Pauthner M, Julien J-P, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. USA. 2014b;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui P-YY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Protocol G Principal Investigators. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-PP, Wang S-KK, Ramos A, Chan-Hui P-YY, Moyle M, et al. Protocol G Principal Investigators. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng N-Y, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang Z-YY, Li Y, Hogerkorp C-MM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. NISC Comparative Sequencing Program. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang Z, Schramm CA, Joyce MG, Kwon YD, Zhou T, Sheng Z, Zhang B, O’Dell S, McKee K, et al. NISC Comparative Sequencing Program. Maturation and Diversity of the VRC01-Antibody Lineage over 15 Years of Chronic HIV-1 Infection. Cell. 2015;161:470–485. doi: 10.1016/j.cell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.