Abstract
Regulatory T cells (TReg cells), a specialized T cell lineage, have a pivotal function in the control of self-tolerance and inflammatory responses. Recent studies have revealed a discrete mode of TCR signaling that regulates Treg cell differentiation, maintenance and function and that impacts on gene expression, metabolism, cell adhesion and migration of these cells. Here, we discuss the emerging understanding of TCR-guided differentiation of Treg cells in the context of their function in health and disease.
Introduction
As a major tenet of modern immunology, Burnet’s clonal selection theory postulated that differentiating lymphocytes adopt distinct cell fates when they encounter self or foreign antigens in the primary lymphoid organs or peripheral tissues1. Whereas foreign antigens induce population expansion and effector differentiation of mature lymphocytes, self antigens were proposed to trigger clonal deletion of immature lymphocytes as a means to weed out potentially damaging autoreactive cells. Although the basic principles of the clonal selection theory have stood the test of time, both self and foreign agonist antigens are now known to also promote alternative T cell fates, including the differentiation of regulatory T (Treg) cells in the thymus (tTreg cells) and in the periphery (pTreg cells) (for reviews see2, 3).
Thymic escape of pathogenic self-reactive T cells and generation of Treg cells that are capable of preventing disease was first revealed in neonatal thymectomy studies performed half a century ago4. Subsequent efforts at identifying Treg cells capable of suppressing autoimmune inflammation revealed their high expression of T cell receptor (TCR)-induced CD5, CTLA4 and CD255–7, and low expression of TCR-repressed CD45RB8, 9. The subsequent identification of the X chromosome-encoded transcription factor Foxp3 as a dedicated Treg cell lineage specification factor enabled stringent characterization of Treg cell differentiation and function10–12. Analysis of mice expressing a functional Foxp3 reporter or a reporter of nonfunctional Foxp3 expression demonstrated a requirement for TCR signaling for Foxp3 expression and showed that TCR signaling precedes the induction of Foxp3 gene transcription13–15. Notably, TCR stimulation not only activates transcriptional programs, including the IκB kinase (IKK)-associated NF-κB and calcium-dependent NFAT programmes, but also represses the activity of the Foxo family of transcription factors via the Akt kinase16 (Box 1). In this review, we discuss the emerging understanding of the role of TCR specificity and signaling in the differentiation and function of Treg cells and review the molecular mechanisms underlying these processes.
Box 1. Antigen Recognition and T Cell Receptor Signaling.
T cell receptor (TCR) signaling has a central role in the control of T cell differentiation, homeostasis and function.
TCR priming
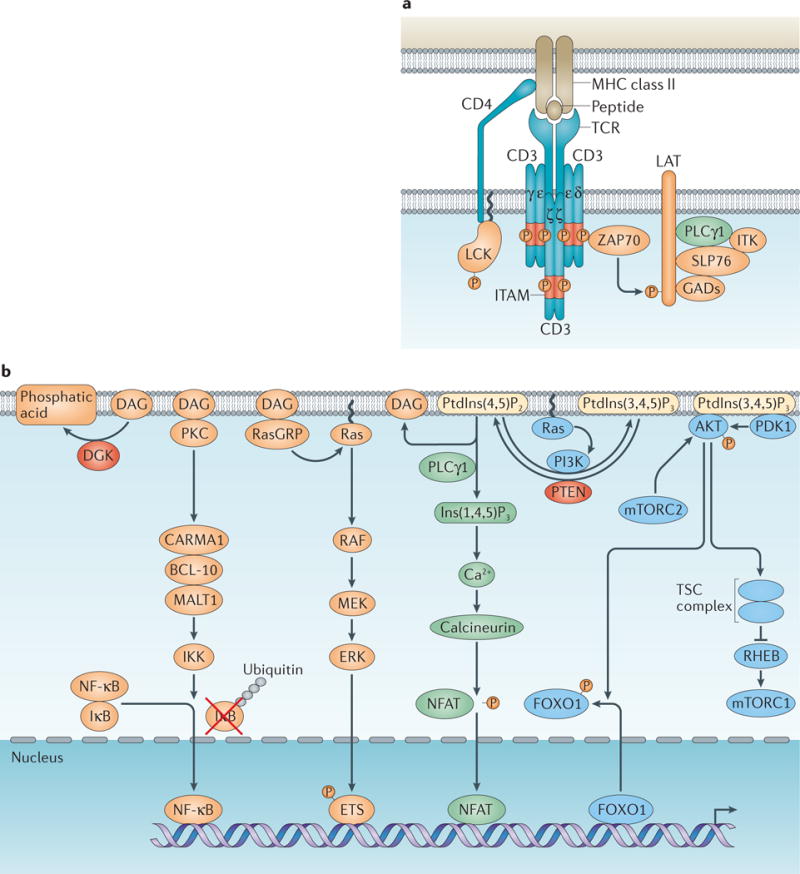
The extracellular portion of TCR interacts with peptide–MHC complexes, which is facilitated by co-receptors CD4 and CD8 that bind to membrane proximal domains of MHC class II and class I molecules, respectively. The intracellular domain of CD4 associates with the Src family kinase Lck, which “primes” TCR signaling upon recruitment to the TCR-CD3 complex. The CD3 δ-, γ-, ɛ- and ζ-chains contain the immunoreceptor tyrosine-based activation motifs (ITAMs) that are phosphorylated by Lck, and recruit the Syk family kinase Zeta-associated protein 70 kDa (Zap70) to the TCR–CD3 complex. Zap70 propagates TCR signaling by phosphorylating multiple targets including the membrane-associated scaffold molecule activation of T cells (Lat). Phosphorylated Lat recruits another scaffold protein SH2-domain-containing leukocyte protein of 76 kDa (Slp76) via Grb2-related adapter proteins (GADs). Slp76 is subsequently phosphorylated by Zap70, and together with Lat, amplifies TCR-induced signaling by recruitment of effector molecules including phospholipase Cγ (PLCγ1) and the Tec family kinase interleukin-2-inducible T-cell kinase (Itk) (see part a of figure).
Propagation of TCR signaling
This is largely controlled by lipid second messengers (see part b of figure). PLCγ1 hydrolyzes phosphatidylinositol (4,5)-bisphosphate (PtdIns(4,5)P2) to generate the membrane-associated diacylglycerol (DAG) and the diffusible inositol-(1,4,5)-triphosphate (Ins(1,4,5)P3). Ins(1,4,5)P3 triggers an increase of calcium (Ca2+) by releasing Ca2+ from endoplasmic reticulum and subsequent influx of extracellular Ca2+ mediated by the Ca2+ sensor stromal interaction molecule (STIM) and the Ca2+ channel Orai1. Ca2+ binding to calmodulin activates the phosphatase calcineurin that dephosphorylates the transcription factor NFAT and induces its nuclear import. DAG recruits a number of effector proteins to the plasma membrane including protein kinase C-θ (PKCθ) and RAS guanyl nucleotide-releasing protein (RasGRP). PKCθ activates the adapter protein complex made of caspase recruiting domain-containing membrane-associated guanylate kinase protein 1 (CARMA1), B-cell lymphoma 10 (Bcl-10) and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1). This complex promotes the activation of the IκB kinase (IKK) that phosphorylates the IκB protein leading to its ubiquitination (Ub) and degradation, and allows translocation of the transcription factor NK-κB to the nucleus. RasGRP is a guanine nucleotide-exchange factor for the small GTPase Ras that activates the mitogen-activated protein kinase (MAPK) pathways including Raf–MEK–ERK. ERK phosphorylates the ETS family transcription factors to induce the expression of immediate early genes such as Fos, which is a component of the AP-1 transcription complex.
PtdIns(4,5)P2 can also be modified by phosphatidylinositol 3-kinase (PI3K) that is activated by TCR signaling effectors including Ras. PI3K phosphorylates PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 functions as a lipid second messenger that recruits protein kinases Akt and PDK1 to the plasma membrane. Phosphorylation of Akt by PDK1 and the mechanistic target of rapamycin complex 2 (mTORC2) triggers its activation. Akt modulates the activities of multiple downstream targets including the Foxo family of transcription factors and the tuberous sclerosis complex (TSC). Akt phosphorylation of Foxo proteins triggers their nuclear export and transcriptional inactivation, while Akt phosphorylation of the TSC represses its function as a GTPase-activating protein (GAP) towards the small GTPase Rheb. Rheb activates mTORC1, which regulates cell growth and metabolic responses. To downregulate TCR-induced signaling, the lipid second messengers are metabolized by lipid enzymes including diacylglycerol kinase (DGK) and Pten that converts DAG to phosphatidic acid and PtdIns(3,4,5)P3 to PtdIns(4,5)P2, respectively.
TCR signaling and Treg cell differentiation
Antigen affinity and Treg cell differentiation
Following the identification of CD25 and Foxp3 as markers for Treg cells, multiple lines of investigation have supported a role for high-affinity TCR signaling in tTreg cell development. Using mice that express green fluorescent protein (GFP) driven by the nuclear receptor subfamily 4 group A member 1 (Nr4a1) locus, the expression of which is driven by TCR signaling, investigators have shown that tTreg cells exhibit a greater activation phenotype than conventional CD4+ T cells17. Furthermore, Treg cells expressed higher levels of GFP in the periphery17, suggesting that tTreg cells continuously sample high-affinity antigens. These findings are in line with the earlier observation that conventional T cells engineered to express transgenic TCRs isolated from Treg cells are more likely to proliferate in an MHC class II-dependent manner in a lymphopenic host18. Importantly, in models of transgenic expression of TCRs with defined antigen specificity, Treg cells appear to require distinct specificities compared with conventional T cells for their differentiation or function19, and expression of cognate antigens triggers tTreg cell differentiation20, 21. In one such transgenic system, expression of an agonist but not a partial agonist induced tTreg cell generation, implying a stringent antigen affinity requirement in tTreg cell differentiation22. In another study, a panel of TCRs with a wide range of reactivity to a model self antigen were evaluated for their ability to drive tTreg cell development23. Although the spectrum of antigen reactivity that could trigger Foxp3 expression appears to be broad, the efficiency of tTreg cell generation is positively associated with TCR affinity for the model self antigen, suggesting that tTreg cell differentiation is preferentially induced under conditions of heightened TCR reactivity.
In addition to the thymic development of Treg cells, antigen stimulation of mature naive T cells induces pTreg cell differentiation24. This pathway is a possible means to re-direct potentially pathogenic T cells that fail to undergo clonal deletion in the thymus into anti-inflammatory protective cells and to regulate immune responses to “non-self” antigens, including commensal microbiota-derived antigens. Administering cognate antigens under sub-immunogenic conditions, as well as chronic antigen exposure, triggers pTreg cell differentiation25, 26 and although a high dose of a weak agonist could promote Foxp3 expression, stable pTreg cells are induced by a low dose of a strong agonist27.
The emerging consensus that a broad range of high affinity antigens drives tTreg and pTreg cell differentiation highlights the idea that the Treg cell fate is an essential, biologically critical addition to the classical T cell fates of clonal deletion or effector T cell differentiation induced by agonist antigens (Figure 1). Such consideration poses an important question whether the distinct T cell fates are determined by unique modes of TCR signaling, by the presence of cell fate-specifying accessory signals or a combination thereof.
Figure 1. Treg cell differentiation as an alternative agonist antigen-induced cell fate.
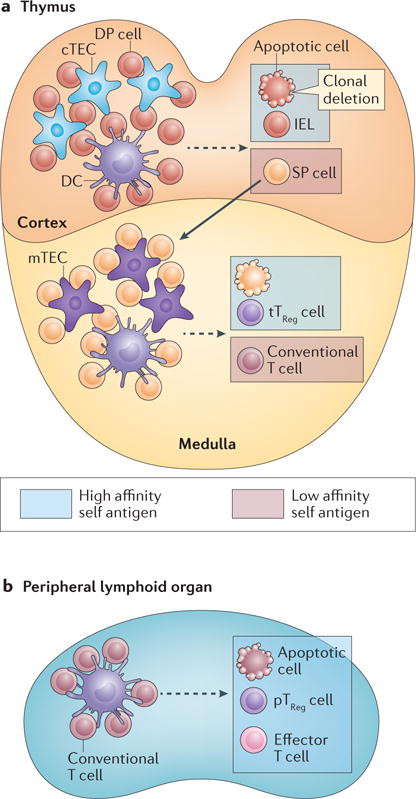
(a) Following rearrangement of T cell receptors (TCRs), thymocytes undergo selection and maturation processes in the cortex and medulla based on their TCR reactivity. Immature CD4+CD8+ double-positive (DP) T cells interact with the cortical thymic epithelial cells (cTECs) and bone marrow-derived antigen-presenting cells, such as dendritic cells (DCs). T cells that recognize high affinity self peptide–MHC complexes, designated as high-affinity self antigen, classically undergo apoptosis (that is clonal deletion). High-affinity antigen can also induce DP cells to differentiate to intestinal intraepithelial lymphocytes (IELs). T cells with low-affinity to self peptide–MHC complexes are positively selected and differentiate to CD4+ or CD8+ single-positive (SP) cells while they migrate from the cortex to the medulla. SP cells continue to sample antigens in the medulla presented by DCs and medullary TECs (mTECs). mTECs express the nuclear factor Aire that promotes the expression of tissue-specific antigens. Stimulation of SP cells by high-affinity self antigen can induce clonal deletion of T cells. Alternatively, strong TCR signals can induce CD4+ SP cells to differentiate to thymic Treg (tTreg) cells. (b) Conventional T cells emigrate from the thymus and circulate as a pool of naïve T cells in peripheral lymphoid tissues. Recognition of agonist antigen presented by antigen-presenting cells such as DCs induces distinct T cell fates including clonal deletion as well as differentiation of effector T cells and peripherally derived Treg (pTreg) cells.
Stage-dependent tTreg cell differentiation
The pro-apoptotic protein Bim is induced by TCR stimulation, and promotes clonal deletion of autoreactive T cells in the thymus. Quantitative studies of autoreactive thymocytes in Bim-deficient mice have revealed that approximately 50% of TCR-signalled CD4CD8 double-positive (DP) and 50% of the positively selected single-positive (SP) T cells are deleted in the cortex and medulla, respectively28, 29. Thus, developing thymocytes are continuously exposed to self antigens that can trigger T cell clonal deletion. Developing tTreg cells, however, reside almost exclusively in the medulla, and the later appearance of tTreg cells compared with conventional (SP) T cells in neonatal mice is associated with the delayed medulla maturation30. Indeed, tTreg cell generation is impaired in the absence of RelB31, which is an NF-κB family transcription factor crucial for the differentiation of medullary thymic epithelial cells (mTECs). Autoimmune regulator (Aire) is expressed in mTECs, and triggers the ectopic expression of genes encoding tissue-specific antigens32. Antigen presentation by Aire-positive mTECs promotes tTreg cell differentiation33, and Treg cells generated in an Aire-dependent manner early after birth have a specific function in maintaining T cell self tolerance34. Taken together, these findings support the idea that Treg cells are specifically induced by medulla-associated agonist antigens.
Why the medulla, but not the cortex, is permissive for Treg cell differentiation is not completely understood. A recent study showed that strong TCR stimulation can induce Bim expression in CCR7− cortical and CCR7+ medullary thymocytes28. However, CCR7+ cells, but not CCR7− cells, can mount an NF-κB-dependent transcriptional response that opposes Bim-mediated apoptosis28, allowing the medullary thymocytes to survive. Considering the important functions of NF-κB family members in Foxp3 expression (see below), it is conceivable that a finely tuned NF-κB signaling pathway accounts in part for the selective tTreg cell induction in the medulla by coupling T cell survival signals with Treg cell differentiation. Future studies will determine how TCR-induced NF-κB signaling is differentially regulated at distinct stages of T cell development. These findings imply that tTreg cell differentiation is better viewed as a cell fate diversification of CD4+ T cells subsequent to their positive selection rather than as a cell fate choice that is alternative to naïve CD4+ T cell differentiation and concomitant with positive selection. In contrast, clonal deletion may both precede and coincide with Treg cell differentiation, and may represent an alternative cell fate choice during the differentiation of CD4 SP thymocytes in the medulla (Figure 1a).
Inducing TCR signaling modules
In line with a critical role for agonist-driven TCR stimulation in tTreg cell generation, mutations in several TCR signaling molecules lead to impaired Treg cell differentiation. The tyrosine kinase Zap70 propagates antigen-initiated signals by bridging the TCR complex and downstream signaling pathways (Box 1). SKG mice, which express a phosphorylation-defective Zap70 mutant, have reduced numbers of tTreg cells35. Similar observations were made in mice harboring Zap70 mutations that affect its kinase activity or its recruitment of the adaptor protein LAT36, 37. However, positive and negative selection of T cells were equally affected in these mice, suggesting that the Zap70 mutants do not selectively impair the TCR signaling involved in tTreg cell differentiation.
The transmembrane protein LAT resides in a major complex that amplifies TCR-induced signals (Box 1). Mice expressing a LAT mutant that is defective in binding to phospholipase-γ1 (PLCγ1) were nearly completely devoid of tTreg cells, while the development of conventional CD4+ T cell was only partially impaired38. Similar observations made upon analysis of mice with a T cell-specific ablation of PLCγ139 revealed a crucial role for the LAT–PLCγ1 signaling axis in tTreg cell differentiation.
PLCγ1 hydrolyzes phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to generate the diffusible second messenger inositol-1,4,5-triphosphate (Ins(1,4,5)P3) and the membrane-associated second messenger diacylglycerol (DAG). Ins(1,4,5)P3 mobilizes calcium from the endoplasmic reticulum, and calcium signaling facilitated by sensor stromal interaction molecule (STIM) proteins is required for tTreg cell differentiation40. DAG enables the recruitment of several signaling-related proteins to the plasma membrane including protein kinase-θ (PKCθ) and RAS guanyl nucleotide-releasing protein (RasGRP). PKCθ induces activation of IKK via the CARMA1, BCL-10 and MALT1 signaling complex and the resulting activation of NF-κB family members is required for tTreg cell differentiation41–44. RasGRP activates the small GTPase Ras, which is a crucial activator of mitogen-activated protein kinase (MAPK) signaling pathways. Thymic Treg cell differentiation is compromised in RasGRP-deficient mice or in mice expressing a transgene encoding a dominant negative form of the downstream effector molecule Raf45, 46. DAG is metabolized by DAG kinase (DGK) as a means to attenuate PLCγ1-induced signaling. Mice devoid of the ζ isoform of DGK have increased numbers of tTreg cells47, 48, which is associated with enhanced IKK and ERK activities. Indeed, ectopic activation of IKK or ERK in T cells promotes tTreg cell generation47, 49, demonstrating that these signaling pathways comprise limiting steps of TCR-induced tTreg cell differentiation.
Inhibitory TCR signaling modules
Mice deficient in Bim or the transcription factor Nur77 (encoded by the Nr4a1 gene) are defective in T cell negative selection. However, the majority of medullary autoreactive T cells rescued from clonal deletion in these mice are not diverted to the Treg cell lineage29, 50. Thus, strong TCR stimulation per se does not always initiate the tTreg cell differentiation program. Intriguingly, recent studies have revealed inhibitory functions of distinct TCR signaling modules in tTreg cell generation, suggesting a requirement for elaborately measured TCR stimulation for Treg cell fate specification.
TCR signaling is initiated by tyrosine phosphorylation at ITAM motifs in the CD3 chains of the TCR complex (Box 1). Mice expressing a phosphorylation-deficient CD3ζ mutant have attenuated TCR signaling, but exhibit enhanced Treg cell differentiation51. While diminished negative selection in these mice likely spares self-reactive precursor cells, which in turn may account for their increased tTreg cell numbers, these findings suggest that there are distinct signaling requirements for negative selection and Treg cell differentiation, and that certain aspects of CD3ζ signaling may interfere with tTreg cell differentiation. In support of this conclusion, a recent study of mice deficient in SHARPIN, a component of the linear-ubiquitin-chain-assembly complex, showed diminished tTreg cell generation in association with enhanced anti-CD3-triggered phosphorylation of CD3ζ52.
While the IKK and calcium signaling pathways are largely unperturbed in CD3ζ mutant T cells, activation of Akt is substantially attenuated51. Early studies showed that ectopic expression of an active form of Akt in T cells inhibited tTreg cell generation53. Akt signaling is induced by PI3K, which generates the lipid messenger phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) and recruits Akt to the plasma membrane, as well as by the mTOR complex 2 (mTORC2), which activates Akt at the plasma membrane (Box 1). Mice expressing a kinase-defective p110δ isoform of PI3K and mice devoid of the mTORC2 component Sin1 have increased numbers of tTreg cells54, 55. Together, these findings indicate that the Akt signaling pathway inhibits tTreg cell differentiation.
A “hit and run” model of tTreg cell differentiation
The divergent sensitivity of tTreg cell-promoting or -inhibitory signaling pathways that is observed in the context of attenuated TCR signaling caused by the CD3ζ mutation raises an intriguing possibility that tTreg cell generation is facilitated by antigens that induce distinct or partial TCR activation. Considering that high affinity antigens are most efficient in inducing tTreg cell differentiation, partial TCR signaling is likely a consequence of transient engagement of antigens. Indeed, transient, but not continuous, TCR stimulation induces robust Foxp3 expression in CD4+ SP T cells56, 57. Likewise, TCR signaling is superfluous for CD4+ SP T cells that express CD25 and have experienced prior TCR stimulation to differentiate into Treg cells58, 59. In addition, studies of differentiation of thymic precursor cells expressing transgenic Treg cell-derived TCRs have revealed substantial intraclonal competition60, 61, suggesting that tTreg cell differentiation is facilitated by ligands present in limiting amounts in the medulla. In contrast, multiple encounters with antigen are required for negative selection of developing thymocytes62. Consistent with the idea of transient high affinity TCR engagement favoring Treg cell differentiation, diminished MHC class II expression on mTECs in mice co-expressing a transgenic TCR and a transgene-encoded cognate antigen under the control of the Aire regulatory elements favors Treg cell differentiation over clonal deletion63. Taken together, these findings support a “hit and run” signaling model of tTreg cell differentiation64, in which transient TCR stimulation creates a time window for the activation of the signaling pathways that promote tTreg cell differentiation, but not for the activation of the opposing signaling pathways (for example, strong Akt activation), whereas continuous antigen stimulation induces both signaling pathways and results in clonal deletion (Figure 2a). Future dissection of key signaling pathways induced downstream of TCR engagement will help test this hypothesis.
Figure 2. tTreg cell fate specification by TCR and accessary signals.
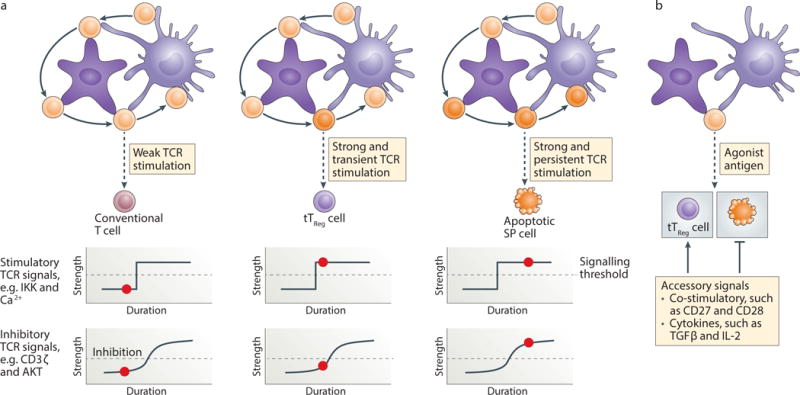
(a) CD4 single-positive (SP) thymocytes are educated in the medulla by sampling antigens presented by medullary thymic epithelial cells (mTECs) and bone marrow-derived antigen-presenting cells such as dendritic cells (DCs). Antigen-triggered TCR signaling plays a principal role in dictating the SP cell fates. Weak TCR stimulation promotes the continuous maturation of SP cells into conventional T cells. Transient stimulation of SP cells by high-affinity antigens is likely sufficient to activate the Treg cell-stimulatory signaling pathways including the IκB kinase (IKK) and Ca2+, while the activities of Treg cell-inhibitory signaling modules, including CD3ζ and Akt, may not reach an optimal level (signaling threshold, demarcated by the dotted line). Persistent stimulation of SP cells by high-affinity antigens activates Treg cell stimulatory as well as inhibitory signaling pathways, which is not permissive for tTreg cell differentiation, but may trigger T cell clonal deletion. The red dot depicts the level of antigen engagement (duration) and the signaling activity of the indicated modules (strength). (b) Despite a relatively distinct mode of TCR signaling being involved in the control of tTreg cell differentiation and T cell deletion, agonist antigen recognition can induce overlapping T cell fates under certain conditions. Accessory signals provided by co-stimulatory receptors such as CD28 and CD27 as well as cytokines including TGF-β and IL-2 promote tTreg cell differentiation by suppressing T cell clonal deletion.
The Goldilocks Principle of Treg cell differentiation
pTreg cell differentiation is induced upon naïve T cell activation under sub-immunogenic conditions with a low dose of high affinity agonist antigen27. The role of individual TCR signaling pathways in pTreg cell generation in vivo remains largely unexplored. Nevertheless, a number of studies have examined TCR signaling pathways in the control of in vitro-induced Treg (iTreg) cell differentiation and revealed several parallels with tTreg cell differentiation. Whereas IKK and calcium signaling pathways promote iTreg cell differentiation49, 65, 66, strong Akt activation antagonizes Foxp3 induction in peripheral T cells53, 56. In line with these findings, strong, but not weak, TCR stimulation of CD4+ T cells represses the expression of the PtdIns(3,4,5)P3 phosphatase Pten67, which is an inhibitor of the Akt signaling pathway, and this may in part explain how suboptimal TCR stimulation promotes iTreg cell generation. TCR-induced Pten repression involves the LAT-dependent activation of the Tec-family tyrosine kinase Itk (Box 1). Accordingly, Itk deficiency results in enhanced iTreg cell differentiation67. The absence of Itk also results in an increased tTreg cell frequency in vivo68, but it remains to be determined whether Pten-mediated repression of Akt is affected. An important downstream target of Akt is the tuberous sclerosis (TSC) complex that suppresses mTOR complex 1 (mTORC1) activation by functioning as a GTPase-activating protein for the small GTPase Rheb69. T cell-specific deletion of mTOR, but not Rheb or Sin1 alone, leads to enhanced iTreg cell generation55, 70, suggesting that both mTORC1- and mTORC2-dependent signaling pathways repress iTreg cell differentiation. The shared theme of restrained TCR stimulation in tTreg, pTreg and iTreg cell differentiation implies that TCR-triggered Treg cell lineage commitment follows the “Goldilocks Principle”, as previously proposed for T cell positive selection71, with TCR signaling being not too high, not too low, but just right.
Accessory signals and Treg cell fate control
As discussed, clonal deletion and Treg cell differentiation represent two competing cell fates of a partially overlapping pool of self-reactive precursor cells29, 50. Hence, these distinct T cell fates are unlikely to be determined by TCR signaling alone. Indeed, the co-stimulatory receptor CD28 has a well-established role in tTreg cell differentiation. In the CD28 cytoplasmic tail, a sequence motif responsible for Lck binding and NF-κB activation, but not the motif involved in PI3K signaling, promotes tTreg cell generation72. In a transgenic model of agonist antigen-induced tTreg cell differentiation, CD28 deficiency results in increased clonal deletion73. This observation suggests an important function of CD28-dependent NF-κB activation in sparing self-reactive tTreg cell progenitors from apoptosis as well as in promoting Foxp3 expression (see below). The co-stimulatory receptor CD27 also prevents apoptosis of developing tTreg cells, and likely acts subsequently to CD28 engagement74. Furthermore, TCR and CD28 signaling induce the expression of TNF receptor superfamily proteins including GITR, OX40 and TNFR2, which collectively promote tTreg cell generation75.
However, in contrast to tTreg cells, strong co-stimulation through CD28 suppresses pTreg or iTreg cell differentiation76. In fact, signaling from various inhibitory receptors is essential for Treg cell generation from mature T cells. CD5 is induced by TCR stimulation and functions as a negative feedback regulator of TCR signaling. CD5 deficiency results in enhanced tTreg cell differentiation77, but impairs pTreg cell generation through the enhancement of mTORC1 signaling78. Likewise, the co-inhibitory receptor CTLA4 promotes iTreg cell generation79. In addition, the inhibitory ligand PDL1 is required for optimal iTreg and pTreg cell differentiation, which is associated with enhanced Pten expression and the suppression of Akt signaling80. How co-stimulation promotes and opposes thymic and peripheral Treg cell differentiation, respectively, is not well understood. One potential scenario to explore could be a differential sensitivity of co-stimulatory and co-inhibitory signaling pathways in thymic or peripheral Treg cell precursors.
In addition to co-stimulatory receptors, cytokines have important functions in controlling Treg cell differentiation. The immunoregulatory cytokine TGFβ inhibits Bim-mediated T cell clonal deletion81 and, therefore, likely preserves a larger pool of self-reactive thymic Treg precursor cells. In addition to specific TCR-induced signals, Foxp3 expression has been suggested to promote the death of thymocytes during Treg cell differentiation82, aside from its well established function of specifying the Treg cell lineage. Importantly, TCR stimulation and Foxp3 expression is associated with the induction of the high affinity IL-2 receptor CD25, and IL-2 signaling rescues developing Treg cells from Foxp3-induced death82, indicating a critical role for IL-2 in Treg cell survival. Combined deficiency of TGFβ and IL-2 almost completely abolishes tTreg cell generation83, supporting their crucial functions in diverting the T cell fate of clonal deletion to tTreg cell generation.
The TGFβ and IL-2 duo also plays an important role in pTreg and iTreg cell differentiation. In addition to inducing Foxp3 expression59, 84, 85, TGFβ and IL-2 potently inhibit alternative cell fate choices, namely, effector T helper (Th) cell differentiation into Th1 and Th2 (TGFβ), and Th17 lineages (IL-2)86, 87. Taken together, these studies reveal that prevention of alternative T cell fates through the engagement of accessory signals likely represents a major strategy underlying the TCR-driven differentiation of Treg cells, including tTreg cells (Figure 2b).
Transcriptional control of Treg cell differentiation
Study of Treg cell fate specification has mostly focused on transcriptional regulation of the Treg cell lineage factor Foxp3. The apparently opposing functions of TCR-induced signaling modules in Treg cell generation are in line with the involvement of several TCR-regulated transcription factors in Foxp3 induction. Both NF-κB and the NF-κB cofactor IκBNS, as well as Foxo proteins, promote Foxp3 expression by binding to regulatory elements in the Foxp3 locus49, 57, 65, 88–91. In addition, NFAT regulates Foxp3 expression, which is mediated in part by its binding to the Foxp3 conserved noncoding sequence 1 (CNS1) element, which is necessary for pTreg cell differentiation92. Furthermore, the TCR-induced Nr4a1 family of transcription factors transactivates Foxp393, suggesting an additional mechanism by which TCR-induced signaling cooperates to promote Treg cell differentiation.
Members of the E-protein family of transcription factors are important regulators of lymphocyte development. TCR signaling down-regulates the activity of E-box transcription factors in part due to the induction of Id proteins that serve as the E-protein inhibitors. Deletion of E-box proteins or overexpression of Id1 results in enhanced Treg cell differentiation94, 95, whereas Id protein deficiency inhibits Treg cell generation96. Mechanistically, TCR-induced E-protein repression appears to be crucial for optimal activation of several Treg cell-promoting signaling pathways including NF-κB94, and thus facilitates Treg cell generation.
Epigenetic control of Treg cell differentiation
In addition to the recruitment of transcription factors, deposition of active histone markers histone H3 lysine 4 trimethylation (H3K4me3) and H3K27ac occur at the Foxp3 promoter in Treg cells97. However, enrichment of H3K4me1 takes place at the Foxp3 promoter prior to Foxp3 induction in mature SP thymocytes and naïve CD4+ T cells, and is dependent on the intronic Foxp3 CNS388, 97. CNS3 deficiency alters the Treg cell TCR repertoire, with an enrichment of Treg cells that exhibit heightened TCR signaling97. These findings reveal an epigenetic mechanism via the cis CNS3 element in the Foxp3 locus that broadens the TCR repertoire of Treg cells.
In addition to Foxp3 expression, Treg cell differentiation is associated with the exploitation of a pre-existent enhancer landscape by Foxp398, and acquisition of a Treg cell-specific CpG DNA methylation patterns at a number of genomic loci99–101. The epigenetic specification of Treg cells is induced by TCR signaling, but is independent of Foxp3 expression98, 99. In fact, the CNS2 region in the Foxp3 locus itself is demethylated in differentiated Treg cells, which promotes heritable Foxp3 expression and maintenance of a stable Treg cell lineage88. Site-specific DNA methylation is controlled by the balanced activity of DNA methyltransferases and DNA demethylases, which likely act in concert with DNA-binding transcription factors and accessory proteins. T cell-specific deletion of the DNA methylation maintenance enzyme Dnmt1 results in enhanced Foxp3 expression even in CD8+ T cells, suggesting that Dnmt1 repression participates in DNA demethylation in Treg cells102. How Dnmt1 activity is inhibited at demethylation sites in Treg cells is unknown. Recent studies of epigenetic control of the myeloid cell-specific transcription factor CEBPA have revealed that Dnmt1 can be sequestered and inhibited by locus-specific non-coding RNA transcripts103, providing an intriguing mechanism for site-specific regulation of DNA methylation. Whether the Treg cell-specific epigenome is established through a similar mechanism, for example via TCR-induced regulatory RNA species in targeted loci that sequester Dnmt1, remains to be determined.
TCR signaling and Treg cell homeostasis and function
TCR signaling and Treg cell subsets
The involvement of strong self reactivity in tTreg cell differentiation has led to the classification of Treg cells as one of the agonist-selected T cell lineages, which also include CD8αα-expressing intestinal intraepithelial lymphocytes (CD8αα+ IELs)104. Although these T cell populations are all considered “antigen-experienced”, Treg cells are abundant in secondary lymphoid organs, whereas CD8αα+ IELs are preferentially tissue-resident. Based on the expression of T cell activation and homing molecules, including CD45RA in humans or CD62L and CD44 in mice, Treg cells can be divided into resting Treg cells and activated Treg cells105, 106, which exhibit distinct patterns of cell migration and tissue localization (Table 1). Treg cells among the recent thymic emigrants have been shown to display a resting Treg cell phenotype107. Considering that agonist-selected T cells, such as IELs, typically display an activated phenotype and reside in target tissues, the question arises how are lymphoid organ-homing resting Treg cells generated.
Table 1.
Resting and activated Treg cells
| Treg cell subsets | Markers | Distribution | Generation | Homeostasis | Function |
|---|---|---|---|---|---|
| Resting Treg cells | CD62LhiCCR7hiCD44lo (mouse) CD45RA+ (human) |
Reside at secondary lymphoid tissues and circulate through lymph and blood | Recent thymic emigrants display a resting phenotype | IL-2, but not TCR, is required; low level cell proliferation and cell death | May inhibit T cell priming and differentiation by consuming IL-2 |
| Activated Treg cells | CD62LloCCR7loCD44hi (mouse) CD45RA− (human) |
Reside at nonlymphoid tissues and circulate through lymph and blood |
TCR stimulation is essential for their differentiation | Both TCR and cytokine are required; high level cell proliferation and cell death | Suppress differentiation and function of effector T cells, e.g. CD8+ CTLs |
Firstly, unlike IELs whose differentiation commence at the immature DP stage with predominant specificity towards MHC class I molecule-associated antigens108, tTreg cells are differentiated from MHC class II-restricted CD4+ SP T cells in the medulla. Therefore, tTreg cells acquire properties associated with T cell maturation including the expression of lymph node homing molecules such as the chemokine receptor CCR728. Secondly, TCR signaling sensitivity decreases as cells mature from cortical DP cells to medullary SP cells109, and as discussed above, intermediate level of TCR signaling is required for Treg cell differentiation. Thus, TCR stimulation is probably not strong enough to induce an overtly activated phenotype in tTreg cells. Thirdly, TCR stimulation of Treg cells results in attenuated signaling including calcium flux and activation of Akt compared with conventional T cells110, 111. Indeed, among Foxp3 target genes are molecules of the TCR signaling pathway. For instance, whereas TCR stimulation induces Zap70 expression in conventional T cells, Foxp3 binds to the promoter region of the Zap70 gene and inhibits its expression99. Antigen-induced signaling in Treg cells may be further suppressed by high expression of inhibitory receptors such as CTLA4 and CD55, 6, 112. Notably, the Ctla4 locus is demethylated in tTreg cells99, which promotes its high expression and therefore supports a role for the Treg cell-specific epigenome in signal modulation. Such genetic and epigenetic mechanisms of TCR signal reprogramming likely dampen antigen-induced Treg cell activation in the periphery, and promote the maintenance of resting Treg cells (Figure 3).
Figure 3. tTreg cells egressed from the thymus display a resting Treg cell phenotype.
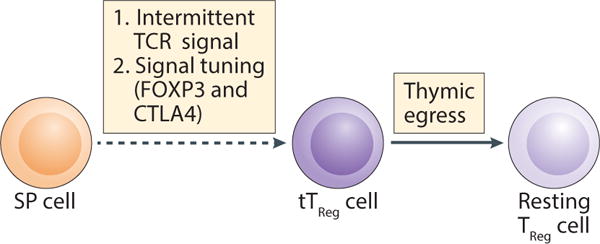
tTreg cell differentiation in the medulla is triggered by intermittent TCR signals that, coupled with Foxp3- and CTLA-4-mediated tuning of the TCR signal, promote recent thymic emigrant tTreg cells to exhibit a resting Treg cell phenotype.
Despite attenuated responses to TCR stimulation, Treg cells with an activated phenotype are present in non-lymphoid tissues as well as tissue-draining lymph nodes as a likely consequence of resting Treg cell activation by self antigens (Figure 4a) and, probably by cytokines113. In addition, inflammatory signals augment activated Treg cell differentiation from resting Treg cells107 possibly by enhancing antigen presentation and supplying T cells with accessory signals of activation. The homeostatic properties of activated Treg cells in the steady state have been examined in mouse parabiosis experiments, which showed that these cells recirculate and are not locally maintained long term in tissues such as the liver and intestine107, 114. Nonetheless, the half-life of activated Treg cells is substantially higher than that of resting Treg cells114. Furthermore, activated Treg cells can proliferate in response to a neo-tissue-antigen, with a fraction of them becoming long-lived memory Treg cells115. Although the question remains whether activated Treg cells exhibit enhanced functional capabilities akin to conventional memory T cells, TCR stimulation and probably cell division facilitates resting Treg cell differentiation into activated Treg cells.
Figure 4. Treg cell recirculation and transcriptional control of Treg cell function.
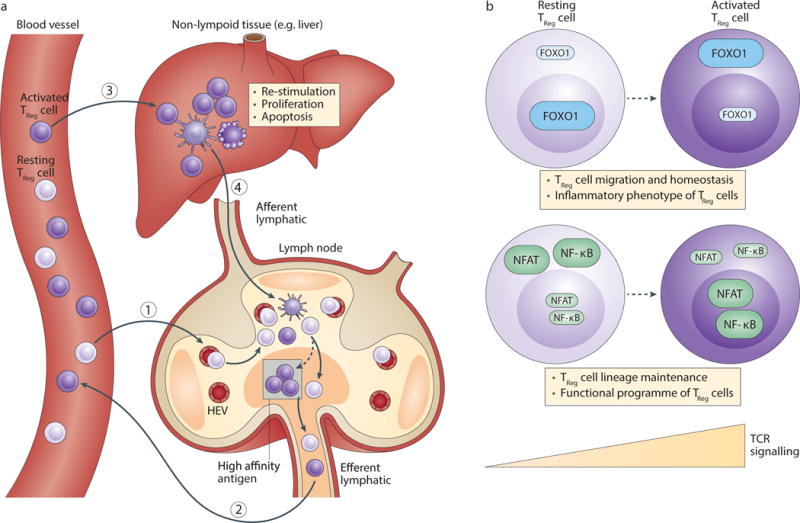
(a) Similar to conventional naïve T cells, resting Treg cells circulate between lymph nodes, lymph and blood. Resting Treg cells enter lymph nodes via high endothelial venues (HEVs) present within the paracortical regions. They further migrate to the T cell zone and scan for antigens presented by dendritic cells (DCs) that are recruited from target tissues via the afferent lymphatic. Resting Treg cells that fail to detect high affinity antigens recirculate back to the blood through the efferent lymphatic, whereas agonist antigen-stimulated resting Treg cells differentiate into activated Treg cells, proliferate and enter the circulation. Activated Treg cells migrate to non-lymphoid tissues where they can be re-stimulated, proliferate or undergo apoptosis. Activated Treg cells may leave the tissue via the afferent lymphatic and recirculate through lymph nodes, lymph and blood. (b) Resting Treg cell homeostasis is promoted by the Foxo family of transcription factor Foxo1 that suppresses the inflammatory phenotype of Treg cells and induces the expression of lymphoid organ homing molecules including the chemokine receptor CCR7. Foxo1 and CCR7 are required for Treg cell function, which may be mediated by resting Treg cell-specific suppressive mechanisms. While resting Treg cells experience some level of TCR signaling, they are converted to activated Treg cells following strong antigen stimulation. Multiple TCR-induced signaling pathways, including Ubc13-dependent activation of NF-κB and STIM-dependent activation of NFAT, are crucial for maintaining the Treg cell identity via Foxp3 induction as well as promoting the activated Treg cell-mediated control of immune tolerance and homeostasis. Furthermore, TCR-triggered Foxo1 inactivation supports activated Treg cell trafficking to target tissues for the control of immune tolerance.
Recent studies of mice with Treg cell-specific deletion of the TCRα chain have indeed revealed critical functions for TCR signaling pathways in the generation of activated Treg cells116, 117. TCR-depleted activated Treg cells fail to acquire T cell activation markers or repopulate non-lymphoid tissues, whereas the transcriptome of resting Treg cells is minimally affected by the absence of TCRα. In fact, TCRα-deficient resting Treg cells maintain high Foxp3 expression and the Treg cell-specific epigenome116, 117. Thus, continuous TCR signaling is dispensable for preserving the two defining characteristics of Treg cells at the resting state.
Notably, the ability of resting Treg cells to sense IL-2 is unperturbed116, suggesting that the Treg cell identity is promoted by both IL-2 and TCR signaling. The hypomethylated CNS2 element in the Foxp3 locus is required for sustaining Foxp3 expression in TCR-stimulated Treg cells that undergo cell division88. Importantly, CNS2 is crucial to preserve the Treg cell identity under the in vivo condition of chronic inflammation, in particular when IL-2 amounts are limiting118, 119. In line with these observations, STAT5 recruitment to the Foxp3 locus, including CNS2, is observed following IL-2 stimulation. Furthermore, CNS2 contains binding sites for a number of TCR-induced transcription factors including NFAT, and NFAT activation has been implicated in stabilization of Foxp3 expression in activated Treg cells119. Together, these findings suggest that although cytokine, but not TCR, signaling is important for resting Treg cell maintenance, both signals likely participate in preserving the identity of activated Treg cells (Figure 4b).
TCR signaling and Treg cell function
Treg cells deficient in several proximal TCR signaling molecules including Zap70 and Lat exhibit impaired suppressor activity37, 120. Furthermore, Treg cell-specific deletion of the TCRα chain results in a loss of Treg cell function in mice116, 117. Gene expression studies have revealed that TCR expression is required for maintaining approximately 25% of the Treg cell transcriptional signature116. In line with these observations, Treg cell-specific deletion of Ubc13, a Lys63-specific ubiquitin-conjugating enzyme involved in TCR-induced NF-κB activation, impairs the in vivo suppressive function of Treg cells121. Likewise, Treg cells devoid of the calcium sensor STIM proteins that promote NFAT activation are defective in repressing conventional T cells40. These observations suggest that TCR activation-dependent transcriptional programs are crucial for Treg cell function (Figure 4b).
Compared with conventional T cells, Treg cells have elevated steady-state mTORC1 activity that is dependent on TCR signaling117. Treg cell-specific deletion of the essential mTORC1 component Raptor results in loss of Treg cell suppressive activity122, which is associated with a loss mTORC1-mediated regulation of cholesterol metabolism in Treg cells122. TCR stimulation of Treg cells is required for their suppressive activity in vitro, which is associated with enhanced Treg cell adhesion to antigen-presenting cells123. The TCR-augmented interaction between Treg cells and antigen-presenting cells is mediated by membrane-proximal inside-out activation of integrins, which is largely independent of the enzymatic activity of Zap70124. Together, these observations suggest that TCR signaling may promote Treg cell suppressive functions by affecting gene expression, cell metabolism and cell adhesion. Although TCR expression is not required for the maintenance of resting Treg cells116, 117, resting Treg cells receive continuous TCR signaling as they express high levels of Nur77 in a TCR-dependent manner107, 116. Thus, it is possible that compromised Treg cell-mediated suppression in the aforementioned TCR signaling mutants can be due to a defective function of both resting Treg and activated Treg cells.
As discussed above, TCR stimulation not only induces signaling pathways to activate transcription factors including NF-κB and NFAT, but also suppresses the activity of Foxo proteins via Akt (Box 1). Mice with Treg cell-specific deletion of Foxo1 develop a fatal inflammatory disease125. Likewise, ablation of the Akt inhibitor Pten in Treg cells triggers Foxo1 hyperphosphorylation and the loss of Treg cell suppressive function126, 127. In line with a well-established role of Foxo1 in the control of homeostasis and migration of conventional naïve T cells64, resting Treg cells are depleted in the absence of Foxo1 (M.O.L., unpublished observations). Foxo1 suppresses the expression of the pro-inflammatory cytokine IFNγ, and promotes expression of several proteins involved in T cell trafficking including the transcription factor Klf2 and chemokine receptor CCR7125. Indeed, CCR7 is highly expressed in resting Treg cells107, and CCR7-dependent Treg cell migration is essential for its function in vivo128. Resting Treg cells express high levels of CD25 and are preferentially dependent on IL-2 to maintain their homeostasis107. A recent study showed that Treg cells expressing phosphorylated STAT5 reside in discrete clusters with IL-2+ conventional T cells within secondary lymphoid tissues129, and provide a local negative feedback mechanism, possibly involving IL-2 consumption, to repress autoreactive T cell responses. Thus, it is conceivable that CCR7 may be required for resting Treg cells to migrate to functional niches, where they promote immune tolerance by, among other mechanisms, consuming IL-2.
Compared with resting Treg cells, activated Treg cells exhibit predominant cytoplasmic Foxo1 localization, and enhanced Foxo1 phosphorylation at Akt target sites114. Treg cell-specific expression of an Akt-insensitive Foxo1 mutant prevents the downregulation of lymphoid organ homing molecules, and impedes activated Treg cell homing to non-lymphoid organs, causing CD8+ T cell-mediated autoimmune diseases114. Together, these findings imply complementary activities of resting Treg and activated Treg cells in the maintenance of immune tolerance, which are promoted and repressed by Foxo1, respectively, and are regulated by the Akt signaling pathway downstream of TCRs (Figure 4b).
Treg cell tolerance control beyond self
Tumors
In addition to the well-established function of Treg cells in promoting immunological self tolerance, Treg cells control immune responses to foreign antigens and antigens associated with the altered self, such as in the case of tumors. Increased frequencies of tumor Treg cells are often correlated with poor prognosis of cancer patients, as a likely consequence of Treg cell suppression of anti-tumor immunity130. Recent studies have started to unravel the origin and antigen specificity of tumor-associated Treg cells in mouse models.
TCR profiling studies have revealed that tumor-infiltrating Treg cells and conventional CD4+ T cells have a largely non-overlapping TCR repertoire131, 132, suggesting that it is unlikely for the Treg cells to have converted from conventional T cells. Likewise, in a transgenic model of prostate cancer, Treg cells from multiple individual tumors recurrently express several TCRs distinct from those of conventional T cells133. The differentiation of Treg cells from transgenic T cells expressing one such TCR was shown to take place in the thymus in an Aire-dependent manner, whereas mature conventional T cells failed to convert to Treg cells in tumor-bearing mice133. These findings suggest that most of the Treg cells that infiltrate at least some solid tumors are self antigen-specific tTreg cells in mice.
The enrichment of antigen-specific Treg cells in tumors implies that tumor progression may release a high levels of antigen that trigger Treg cell proliferation. Thus, interfering with the TCR signaling pathway might impair the homeostasis or function of tumor-associated activated Treg cells. Compared with activated Treg cells from healthy tissues, tumor-infiltrating activated Treg cells downregulate Foxo1 target genes more substantially114, rendering them more susceptible to Foxo1 repression of Treg cell migration to tumors. Indeed, expression of a Foxo1 mutant that is insensitive to AKT-mediated phosphorylation in Treg cells at a dose that does not cause overt autoimmune diseases is sufficient to deplete tumor-associated activated Treg cells, and to activate effector CD8+ T cells and inhibit tumor growth114. Interestingly, Treg cell specific-deletion of the leukocyte-specific PI3K isoform p110δ also potentiates anti-tumor immunity134, but whether p110δ blockade impairs the generation of tumor-associated activated Treg cells remains to be determined. Nevertheless, these findings suggest that the PI3K–Akt–Foxo1 signaling pathway in Treg cells might be manipulated to break tumor immune tolerance.
Pregnancy
Pregnancy poses a unique challenge to the maternal immune system in placental mammals as the fetus and placenta may harbor “foreign” antigens inherited from the paternal genome. Studies of pregnant mice exposed to conceptus-specific antigens have shown that conventional T cells become activated and proliferate, but do not differentiate into pathogenic effector cells135. Instead, Treg cells are enriched among antigen-specific CD4+ T cells, and their accumulation is due to both the peripheral conversion of convetional T cells and to expansion of the pre-existing Treg cell population136. Interestingly, conceptus-specific Treg cells persist after fetus delivery, and rapidly expand during secondary pregnancy136. Such an elevated memory-like Treg cell response may account for the enhanced fetal tolerance in recurrent human pregnancy. pTreg cell differentiation is dependent on the Foxp3 CNS1 enhancer element88. Remarkably, the presence of CNS1 appears to be unique among placental mammals and may have been introduced by retro-transposon insertion coinciding with eutherian evolution137. Female mice lacking the CNS1 element fail to induce conceptus-specific pTreg cells, and have higher embryo resorption rates when mated with allogenic males137. These findings have validated an important function for Treg cells in fetomaternal tolerance, as previously suggested (see refs 138, 139).
Close contact between tissues and blood supplies of fetus and mother makes the fetomaternal antigen exchange a two-way process. As the fetus harbors half of the maternal genome, many non-inherited maternal antigens (NIMA) are “foreign” to the fetus. Previous studies have revealed that maternal cells that cross the placenta induce Treg cell differentiation, and suppresses fetal anti-maternal immune responses in humans140. By tracking antigen-specific T cell responses, a recent study in mice has revealed that maternal cells that form microchimerism induce the differentiation of NIMA-specific pTreg cells in female offspring during development141. Importantly, these females exhibit increased numbers of NIMA-specific pTreg cells during pregnancy sired by allogeneic males with overlapping NIMA specificity, which promotes cross-generation fetal tolerance. Together, these findings imply that the pTreg cell differentiation pathway may have been selected during evolution to reinforce reproductive fitness of placental mammals.
Commensals
The intestine harbor trillions of commensal bacteria that form symbiotic relationship with mammalian hosts to promote nutrient extraction from food as well as organismal homeostasis. Thus, this symbiotic relationship can be considered as a distinct antigenic state. Indeed, although intestinal Treg cells are present in germ-free animals, recent studies have revealed that commensals can induce the accumulation of antigen-specific Treg cells142, 143. TCRs isolated from colonic Treg cells from specific pathogen-free (SPF) mice recognize commensal antigens in the intestines of these animals142. In addition, Treg cells isolated from mice exposed to Clostridium sp. are more suppressive in the presence of fecal material containing the same bacterial species than in its absence144, in line with antigen-specific stimulation of Treg cells. The origin of commensal-reactive Treg cells has also been investigated by creating retrogenic mice expressing the Treg cell TCRs. In contrast to TCRs from lymph node Treg cells, commensal-reactive TCRs from colonic Treg cells failed to promote thymic Treg cell differentiation, but facilitated pTreg cell generation in the presence of commensals142. These findings are corroborated by the observations that Treg cell frequencies are reduced in mice deficient for the Foxp3 CNS1 element88, or devoid of a binding site for TGFβ-induced SMAD transcription factors in CNS1145. Notably, CNS1-deficient mice develop colitis146, supporting an important function for pTreg cells in the control of immune tolerance to commensals.
Therapeutic implications and future perspective
The pivotal function of Treg cells in the control of immune tolerance and inflammatory responses renders them attractive therapeutic targets for many diseases including autoimmune disorders, graft-versus-host disease, and cancer. Understanding how the TCR signaling pathway is differentially wired in Treg cells could be exploited for disease therapy. For instance, compared to conventional T cells, Treg cells exhibit reduced activation of the Akt kinase111, 125, and the attenuated Akt-mTORC1 signaling supports the combined use of Treg cells and the mTORC1 inhibitor, rapamycin, for the treatment of graft-versus-host disease147. On the other hand, IL-2 signaling is elevated in Treg cells, and the use of low-dose IL-2 may selectively expand Treg cells in therapy of autoimmune disorders148. Furthermore, defining the resting and activated Treg cell subsets, and how their differentiation and homeostasis is modulated might be particularly instructive for manipulating tumor-associated Treg cells. For example, recent studies have revealed that activated Treg cells in tumors express high levels of CTLA4, and the anti-tumor activity of anti-CTLA4 antibody can be attributed to depletion of Treg cells149–151. Despite these attempts of Treg cell manipulation, the full potential of Treg cell therapy is far from being achieved. Future studies to define novel modulators of Treg cells and how their and TCR signals control Treg cell differentiation, homeostasis, and function will pave the way of harnessing the maximal power of Treg cells for therapy of a variety of diseases.
Key points.
-
➢
Treg cells are a distinct lineage of CD4+ T cells that differentiate in response to agonist self antigens in the thymus and to non-pathogenic foreign antigens in the periphery.
-
➢
The involvement of TCR signaling modules that have opposing activities in T cell lineage specification favors a Treg cell repertoire that in general reacts to low abundance but high affinity antigens.
-
➢
Compared to ubiquitous antigens, such antigens will likely induce inefficient clonal deletion of T cells, and thus the existence of these antigens justifies the necessity of Treg cell-mediated dominant immune tolerance.
-
➢
Depending on the expression of activation markers, mature Treg cells can be divided into resting and activated Treg cell subsets, and these discrete populations likely accompany conventional T cells to control their activation and effector functions in secondary lymphoid organs and target tissues.
-
➢
Distinct TCR signaling modules are selectively involved in the control of trafficking, maintenance and suppressive activities of resting and activated Treg cells.
Acknowledgments
We apologize to the authors whose work we could not cite due to space constraints. We thank former and current members of Li and Rudensky laboratories for discussions. This work was supported by the National Institutes of Health (RO1 AI102888-01A1 to M.O.L., R37 A134206 to A.Y.R., and the Memorial Sloan Kettering Cancer Center Core Grant P30 CA008748).
Glossary
- Clonal deletion
The process by which double-positive or single-positive thymocytes that express T cell receptors with high affinity for self antigens are induced to undergo apoptosis
- tTreg cell
Thymus-derived Treg cell, T cell with regulatory (suppressive) ability that acquires the transcriptional and epigenetic signature including Foxp3 expression in the thymus
- pTreg cell
Peripherally derived Treg cell, T cell with regulatory (suppressive) ability that acquires the transcriptional and epigenetic signature including Foxp3 expression in the peripheral tissue
- Autoimmune regulator (Aire)
Aire is expressed in medullary thymic epithelial cells, and facilitates expression of diverse set of transcripts characteristic of different non-lymphoid organs and affects cellular make-up and architecture of thymic medulla
- Positive selection
The process by which CD4 and CD8 double-positive immature thymocytes that express T cell receptors with intermediate affinity for self antigens are induced to differentiate into CD4 or CD8 single-positive thymocytes
- Mechanistic target of rapamycin (mTOR)
mTOR is a conserved serine/threonine protein kinase that regulates cell growth and metabolism, as well as cytokine and growth-factor expression, in response to environmental cues. mTOR receives stimulatory signals from RAS and phosphoinositide 3-kinase (PI3K) downstream of growth factors, as well as nutrients, such as amino acids and glucose
- iTreg cell
in vitro-induced Treg cell, T cell with regulatory (suppressive) ability that acquires the expression of Foxp3 under specific T cell culture conditions
- Intestinal intraepithelial lymphocyte (IEL)
T lymphocyte population found within the epithelial layer of gastrointestinal tract lining. Unlike conventional lineage T cells, IELs are selected by agonist antigens, and exert immediate effector functions in response to antigens and stress signals
- Resting Treg cell
Treg lineage cell phenotypically similar to conventional naïve CD4+ T cell with high expression of CD62L in mice or CD45RA in human
- Activated Treg cell
Treg lineage cell phenotypically similar to conventional effector/effector memory CD4+ T cell with low expression of CD62L in mice or CD45RA in human
- Recent thymic emigrant
T cell that has completed thymic development and has recently entered the lymphoid periphery. These young T cell undergoes a maturation process that includes changes in function and cell surface phenotype
- Retrogenic mice
Mice that express defined TCRα and TCRβ proteins from retroviral vectors following retrovirus-mediated hematopoietic stem cell gene transfer
Biographies
Ming Li is a Member of the Immunology program at Memorial Sloan Kettering Cancer Center (MSKCC), and a Professor at Weill-Cornell Medical School. Dr. Li is an international leader in fields of immune regulation and tumor immunology, where he has made seminal contributions in defining the regulatory mechanisms of T cell development, homeostasis, tolerance, and memory as well as elucidating the nature of tumor-elicited innate and adaptive immune responses. Dr. Li is a Rita Allen Foundation Scholar, and is the recipient of Louise and Allston Boyer Award in Basic Research and more recently the AAI-BD Bioscience Investigator Award.
Alexander Rudensky is Chairman of the Immunology Program and Director of the Ludwig Center for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center (MSKCC), an Investigator with the Howard Hughes Medical Institute, and a Tri-Institutional Professor at MSKCC, the Rockefeller University and Cornell University. Dr. Rudensky is an internationally-recognized leader in the field of immune regulation, where he has made numerous seminal discoveries including the identification of the molecular mechanisms of regulatory T cell differentiation. Dr. Rudensky was elected to the National Academy of Sciences in 2012 and the American Academy of Sciences and the National Academy of Medicine in 2015. He is the recipient of the Searle Scholar Award and more recently the Coley Award for Basic Science.
Footnotes
Competing interest statement
The authors declare no competing interests.
References
- 1.Burnet FM. The Clonal Selection Theory of Acquired Immunity. Vanderbilt University Press; Nashville, TN: 1959. [Google Scholar]
- 2.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Nishizuka Y. Thymus and Reproduction: Sex-linked dysgnesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–55. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 8.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 9.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–44. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 15.Lin W, et al. Regulatory T cell development in the absence of functional Foxp3. Nature Immunology. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 16.Luo CT, Li MO. Transcriptional control of regulatory T cell development and function. Trends Immunol. 2013;34:531–9. doi: 10.1016/j.it.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 19.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 20.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 21.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 22.Cozzo Picca C, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci U S A. 2011;108:14890–5. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–86. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paiva RS, et al. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc Natl Acad Sci U S A. 2013;110:6494–9. doi: 10.1073/pnas.1221955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunology. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 27.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. Journal of Experimental Medicine. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med. 2013;210:269–85. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci U S A. 2013;110:4679–84. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowan JE, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210:675–81. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 33.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 34.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–94. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka S, et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 36.Siggs OM, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–26. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527–41. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203:119–29. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu G, et al. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–18. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh-hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nature Immunology. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt-Supprian M, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–71. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta S, et al. Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Molecular Immunology. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medoff BD, et al. Differential requirement for CARMA1 in agonist-selected T-cell development. European Journal of Immunology. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes MJ, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Priatel JJ, Chow MT, Teh HS. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol. 2008;180:5973–82. doi: 10.4049/jimmunol.180.9.5973. [DOI] [PubMed] [Google Scholar]
- 46.Willoughby JE, et al. Raf signaling but not the ERK effector SAP-1 is required for regulatory T cell development. J Immunol. 2007;179:6836–44. doi: 10.4049/jimmunol.179.10.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt AM, et al. Diacylglycerol kinase zeta limits the generation of natural regulatory T cells. Sci Signal. 2013;6:ra101. doi: 10.1126/scisignal.2004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi RP, et al. The zeta isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling. Sci Signal. 2013;6:ra102. doi: 10.1126/scisignal.2004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear Factor-κB Modulates Regulatory T Cell Development by Directly Regulating Expression of Foxp3 Transcription Factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Fassett MS, Jiang W, D’Alise AM, Mathis D, Benoist C. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proceedings of the National Academy of Sciences. 2012;109:3891–3896. doi: 10.1073/pnas.1200090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang S, et al. Reduced TCR signaling potential impairs negative selection but does not result in autoimmune disease. J Exp Med. 2012;209:1781–95. doi: 10.1084/jem.20120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park Y, et al. SHARPIN controls regulatory T cells by negatively modulating the T cell antigen receptor complex. Nat Immunol. 2016 doi: 10.1038/ni.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. Journal of Experimental Medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patton DT, et al. Cutting edge: The phosphoinositide 3-kinase p110 delta is critical for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Journal of Immunology. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 55.Chang X, Lazorchak AS, Liu D, Su B. Sin1 regulates Treg-cell development but is not required for T-cell growth and proliferation. Eur J Immunol. 2012;42:1639–47. doi: 10.1002/eji.201142066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang W, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nature immunology. 2010;11:618–27. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 58.Lio CWJ, Hsieh CS. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. The Journal of Immunology. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 60.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–7. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–30. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Borgne M, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–30. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nature Immunology. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang W, Li MO. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends in immunology. 2011;32:26–33. doi: 10.1016/j.it.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruan Q, et al. Development of Foxp3+ Regulatory T Cells Is Driven by the c-Rel Enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaeth M, et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences. 2012;109:16258–16263. doi: 10.1073/pnas.1203870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Rodriguez J, et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211:529–43. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang W, Jeong AR, Kannan AK, Huang L, August A. IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function. J Immunol. 2014;193:2267–72. doi: 10.4049/jimmunol.1400968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delgoffe GM, et al. The mTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yun TJ, Bevan MJ. The Goldilocks conditions applied to T cell development. Nat Immunol. 2001;2:13–4. doi: 10.1038/83118. [DOI] [PubMed] [Google Scholar]
- 72.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature Immunology. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 73.Hinterberger M, Wirnsberger G, Klein L. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Front Immunol. 2011;2:30. doi: 10.3389/fimmu.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coquet JM, et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. 2013;210:715–28. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahmud SA, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–81. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. Journal of Experimental Medicine. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ordonez-Rueda D, et al. Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur J Immunol. 2009;39:2233–47. doi: 10.1002/eji.200839053. [DOI] [PubMed] [Google Scholar]
- 78.Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity. 2015;42:471–83. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 79.Zheng SG, et al. TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+ CD25+ regulatory cells. The Journal of Immunology. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 80.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tai X, et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–28. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isomura I, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. Journal of Experimental Medicine. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harada Y, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. The Journal of experimental medicine. 2010;207 doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuster M, et al. IkappaB(NS) protein mediates regulatory T cell development via induction of the Foxp3 transcription factor. Immunity. 2012;37:998–1008. doi: 10.1016/j.immuni.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 92.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunology. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 93.Sekiya T, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nature Immunology. 2013;14:230–237. doi: 10.1038/ni.2520. This study shows that the TCR-induced immediate early genes encoding the Nr4a nuclear receptors family members are crucial for Foxp3 expression, which implies that Treg cell differentiation is promoted by transcriptional factors activated downstream of TCR signaling. [DOI] [PubMed] [Google Scholar]
- 94.Gao P, et al. Dynamic changes in E-protein activity regulate T reg cell development. J Exp Med. 2014;211:2651–68. doi: 10.1084/jem.20132681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu C, et al. Id1 expression promotes T regulatory cell differentiation by facilitating TCR costimulation. J Immunol. 2014;193:663–72. doi: 10.4049/jimmunol.1302554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maruyama T, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng Y, et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature. 2015;528:132–6. doi: 10.1038/nature16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Samstein Robert M, et al. Foxp3 Exploits a Pre-Existent Enhancer Landscape for Regulatory T Cell Lineage Specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–99. doi: 10.1016/j.immuni.2012.09.010. References 98 and 99 report that during Treg cell lineage commitment Foxp3 expression is preceded by TCR-dependent establishment of key regulatory elements and changes in DNA methylation at several genomic loci. These changes prepare precursor cells for Foxp3 expression and its essential function in regulating gene expression in Treg cells. [DOI] [PubMed] [Google Scholar]
- 100.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–51. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–52. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 103.Di Ruscio A, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–6. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 106.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–36. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15:815–23. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 111.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–22. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 112.Tai X, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–63. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–81. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–6. doi: 10.1038/nature16486. This study demonstrates that activated Treg cell differentiation is associated with the repression of Foxo1-dependent gene transcription, concomitant with Akt kinase-induced Foxo1 phosphorylation. Foxo1 inactivation is essential for the migration of activated Treg cells to target tissues and the suppression of CD8+ T cell-dependent autoimmunity and tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–42. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–8. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vahl JC, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–36. doi: 10.1016/j.immuni.2014.10.012. References 116 and 117 demonstrate that TCR expression in mature Treg cells is dispensable for maintaining the expression of Foxp3 or the Treg cell-specific epigenome, but is required for the suppressor capacity of Treg cells. [DOI] [PubMed] [Google Scholar]
- 118.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–63. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158:734–48. doi: 10.1016/j.cell.2014.07.030. References 118 and 119 reveal that the hypomethylated intronic Foxp3 regulatory element CNS2 has an essential function in controlling the Treg cell identity in antigen-experienced Treg cells. CNS2 contain the cis regulatory motifs that sense cytokine and TCR signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol. 2010;184:2476–86. doi: 10.4049/jimmunol.0902876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang JH, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol. 2012;13:481–90. doi: 10.1038/ni.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–90. doi: 10.1038/nature12297. This study shows that Treg cells have elevated steady-state mTORC1 activity. mTORC1 deficiency results in compromised Treg cell function, which is partly due to the enhanced mTORC2 signaling and is further associated with the defective lipid metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–8. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Au-Yeung BB, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085–92. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. This study reports that Treg cell differentiation is associated with the attenuated Akt-triggered Foxo1 nuclear exclusion. Foxo1 controls a gene expression program distinct from that of Foxp3, which is indispensable for Treg cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–87. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huynh A, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–96. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. Journal of Experimental Medicine. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Z, et al. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–30. doi: 10.1038/nature16169. This study reports that Treg cells expressing phosphorylated STAT5 exist in clusters with IL-2+ conventional T cells in secondary lymphoid tissues, and such co-localization is dependent on Treg cell expression of TCR. These findings imply a negative feedback mechanism that promotes tolerance control of autoreactive T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 131.Hindley JP, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–46. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 2012;72:3557–69. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- 133.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. doi: 10.1126/science.1233913. This study reports that Treg cells reactive to a prostate-associated self-antigen are highly enriched in oncogene-induced prostate tumors. Differentiation of these Treg cells in the thymus is determined by Aire-dependent expression of prostate tissue antigens by mTECs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali K, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samstein Robert M, Josefowicz Steven Z, Arvey A, Treuting Piper M, Rudensky Alexander Y. Extrathymic Generation of Regulatory T Cells in Placental Mammals Mitigates Maternal-Fetal Conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. References 136 and 137 show that maternal Treg cells specific for fetal antigens are induced during pregnancy. pTreg cell differentiation is dependent on the Foxp3 intronic regulatory element CNS1 that has emerged in placental mammals during evolution. Furthermore, Treg cells persist at postpartum and rapidly expand during subsequent pregnancies. The robust recall (memory) Treg cell response promotes immune tolerance to the fetus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 139.Zenclussen AC, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006;36:82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 140.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kinder JM, et al. Cross-Generational Reproductive Fitness Enforced by Microchimeric Maternal Cells. Cell. 2015;162:505–15. doi: 10.1016/j.cell.2015.07.006. This study reports that exposure to maternal tissues induces Treg cell differentation and stable immune tolerance to non-inherited maternal antigens in female offspring. Such female offspring experience reduced fatal wasting when mated with males that share these antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. This study reports that colonic Treg cells express distinct TCRs, many of which react to antigens derived from commensal bacteria. These TCRs, when expressed in precursor cells, do not facilitate thymic Treg cell development, but promote microbiota-dependent pTreg cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cebula A, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–62. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 145.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. Journal of Experimental Medicine. 2012;209:1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeiser R, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–94. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 149.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bulliard Y, et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–93. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]


