Abstract
Background
Macrophages can imbibe low-density lipoprotein (LDL) through scavenger receptors to become foam cells, which is critical in the initiation and progression of atherosclerosis. Mounting evidence suggests that the anti-inflammatory nature of Chinese herbs have the capacity to halt the complex mechanisms underlying atherosclerosis. This study examined the effects of Chinese herbs on foam cell formation.
Methods
Chinese herbs were obtained from the Sun Ten pharmaceutic company. Using oxidized LDL (OxLDL) uptake and a cell toxicity assay, we screened more than 30 types of Chinese herbs. Western blotting was used to determine expressions of scavenger receptors (SRs) and extracellular-signal-regulated kinase (ERK) activities.
Results
We found that Gentiana scabra reduced oxidized LDL uptake effectively in THP-1 macrophages (p < 0.05 vs. OxLDL treated control). Moreover, treatment with Gentiana scabra in THP-1 macrophages resulted in decreased expression of scavenger receptor- A (SR-A) (p < 0.05 vs. control). Molecular investigation revealed that Gentiana scabra inhibited SR-A protein expression, possibly by regulating ERK signaling pathways (p < 0.05 vs. control).
Conclusions
By regulating SR-A expression, Gentiana scabra reduced oxidized LDL uptake in human macrophages. These results support the potential use of Gentiana scabra in treating atherosclerosis.
Keywords: Atherosclerosis, Foam cells, Gentiana scabra, Macrophage, Oxidized LDL
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease that originates in the walls of large- and medium-sized arteries, and causes luminal narrowing.1-5 One of the critical steps during the process of atherosclerosis is macrophages ingesting lipids through scavenger receptors (SRs) and becoming foam cells or lipid-laden macrophages in the arterial intimae. These foam cells can release pro-inflammatory cytokines and chemokines, which further activate vascular endothelial cells and cause progress of atherosclerosis.6 Despite the comprehensive understanding of the inflammatory events leading to atherogenesis, no specific and effective anti-inflammatory therapy has been described in the literature, and the disease remains the leading cause of death worldwide.
Herbal medicines are a common form of alternative therapy because of their supposed low toxicity and natural origins.7,8 Recently, advanced Western medical techniques have been used to study herbal medicine, particularly to determine their therapeutic efficacy and underlying mechanisms. Several herbal medicines have been reported to have cardioprotective effects and may facilitate preventing cardiovascular disease. Recently, Tsai et al. suggested that treatment with ginkgo biloba extract reduced foam cell formation and suppressed atherosclerosis in atherogenesis-prone mice.9 A study by Ling et al. demonstrated that Salvia miltiorrhiza and Panax notoginseng effectively inhibited tumor necrosis factor-α-induced adhesion molecule expressions in human umbilical vein endothelial cells.8 Moreover, Chinese herbs have been reported to have antioxidative effects, inhibit low density lipoprotein (LDL) oxidation, and improve endothelial dysfunction.10-12 Such evidence indicates the importance of Chinese herbs in treating cardiovascular disease, including atherosclerosis.
According to the current understanding of the chronic inflammatory nature of atherosclerosis, it is evident that Chinese herbs are beneficial in treating atherosclerosis. Berberine, the main alkaloid of Coptis chinensis, has been reported to exhibit its anti-atherosclerotic effects by regulating nuclear factor-κB and cyclooxygenase-2 (COX-2) signaling.13-15 Tanshinone IIA, a pharmacologically active component of Salvia miltiorrhiza Bunge (Danshen), reduces atherosclerosis by inhibiting macrophage cholesterol accumulation and proinflammatory cytokine expression.16 In addition to berberine and tanshinone IIA, other anti-inflammatory herbs may produce anti-atherosclerotic effects.
In this study, more than 30 types of anti-inflammatory Chinese herbs were screened. By using an oxidized LDL (OxLDL) uptake assay and cell cytotoxicity assay, we observed that Gentiana scabra effectively reduced OxLDL uptake in nontoxic therapeutic concentrations, possibly through the suppression of scavenger receptor-A (SR-A) expression. Moreover, Gentiana scabra reduced SR-A expression by regulating extracellular signal-regulated kinase (ERK) signaling. Although further in vivo and human studies are required, these results indicate the therapeutic potential of Gentiana scabra in treating atherosclerosis and related cardiovascular disorders.
MATERIALS AND METHODS
Reagents
The ERK inhibitor, PD98059, was purchased from Calbiochem (Darmstadt, Germany) and Dii-OxLDL was purchased from Intracel Resources (Frederick, MD, USA). Unless otherwise specified, all other reagents were purchased from Sigma-Aldrich Chemical Company.
Preparation of Chinese herb extracts
For this study, Chinese herbs were obtained from SunTen pharmaceutic company. To prepare the Chinese herb extracts, 1000 mL of distilled water was added to 20 grams of Chinese herbs and the solution was boiled for about 40 minutes until the total volume was 200 mL. The solutions were then filtered using a 0.22 μm filter (Millipore) and used for subsequent experiments. The extract was stored at 20 °C until use.
Cell culture
Human monocytic cell line THP-1 cells (Bioresource Collection and Research Center; Hsinchu, Taiwan) were cultured in a RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL). THP-1 cells at a density of 1 × 106/mL were incubated with 100 ng/mL PMA for 3 days to enable them to differentiate into macrophages.9
DiI-OxLDL uptake assays
THP-1 macrophages were incubated with 1,19-dioctadecyl-3,3,39,39-tetramethylindocarbocyane perchlorate (DiI)-labeled oxidized LDL (DiI-OxLDL) in RPMI 1640 containing 10% FBS at 37 °C for 24 hrs. Thereafter, DiI-OxLDL uptake was analyzed by flow cytometry and fluorescence microscopy.17,18
Measurement of nonspecific cytotoxicity of drugs
The release of lactate dehydrogenase (LDH), as an indicator of damage to the plasma membrane and cell death, was measured according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). The percentage of cytotoxicity was calculated as: ([sample value – medium control]/[high control – medium control]) × 100, where the sample values were the averages of the absorbance values from triplicates of indicated dose of various Chinese herb-treated THP-1 macrophage supernatants, after subtraction of the absorbance values from the background control. The average absorbance values of untreated cell culture supernatants, which were used as the medium control, were similarly calculated. Equal amounts of cells treated with 1% Triton X-100 were used as the high control.
Western blotting
Enhanced chemiluminescence Western blotting (Amersham) was performed as previously described.19 After extensive washing, the treated cells were pelleted and re-suspended in a lysis buffer. Protein concentrations were measured and equal amounts of whole cellular extracts were then analyzed on an 8-10% SDS-PAGE and transferred to a nitrocellulose filter. For immunoblotting, the nitrocellulose filter was incubated with TBS-T containing 5% nonfat milk (milk buffer) for 1 hour, and then blotted with primary antibodies against SR-A, CD36, total ERK (Santa Cruz Biotechnology, Dallas, TX, USA), actin (Chemicon, Temecula, CA, USA), or phosphorylated ERK (Cell Signaling, Danvers, MA, USA) at 4 °C overnight. After being washed with a TBS-T buffer, the filter was incubated with secondary antibodies conjugated to horseradish peroxidase at a concentration of 1:5000 for 1 hour. The filter was then incubated with the substrate and exposed to X-ray film.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with Bonferroni post-hoc tests for multiple comparisons and Student’s t-test for comparison between two groups. p < 0.05 was considered significant.
RESULTS
Screening effects of Chinese herbs on Dii-OxLDL uptake in THP-1 macrophages
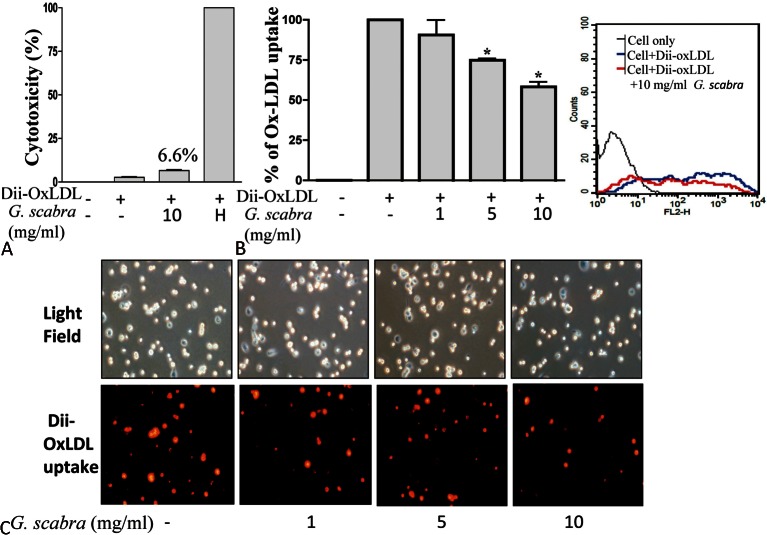
To examine the effects of Chinese herbs on Dii-OxLDL uptake in PMA-treated human THP-1 macrophages, THP-1 macrophages were pretreated with Chinese herbs for 2 hours, and then incubated with Dii-OxLDL for 24 hours. Gentiana scabra and some Chinese herbs inhibited Dii-OxLDL uptake significantly in THP-1 macrophages (data not shown). We then performed LDH assays to evaluate the cytotoxic effects of these potential therapeutic Chinese herbs on THP-1 macrophages. Only the concentrations of Gentiana scabra used in the experiments were not cytotoxic (Figure 1A).
Figure 1.
Gentiana scabra inhibited OxLDL uptake in THP-1 macrophages. (A) THP-1 macrophages were pretreated with Gentiana scabra at a concentration of 10 mg/ml for 2 h and were then incubated with 10 μg/ml Dii-OxLDL for another 24 h. The culture supernatants were collected to determine the possible cytotoxic effects of Gentiana scabra by using LDH release assays. Cells treated with 1% Triton X-100 were taken as the positive control (indicated as H). (B) THP-1 macrophages were pretreated with various doses of Gentiana scabra for 2 h, incubated with 10 μg/ml Dii-OxLDL for another 24 h, and then analyzed using flow cytometry. Representative and average percentages of Dii-OxLDL uptake from at least three independent experiments are presented. * p < 0.05 vs. OxLDL treated control. (B) After treatment, cells were observed using fluorescence microscopy. LDH, lactate dehydrogenase; OxLDL, oxidized low-density lipoprotein.
Gentiana scabra dose-dependently suppressed Dii-OxLDL uptake in THP-1 macrophages
To further confirm the effects of Gentiana scabra on Dii-OxLDL uptake, we treated THP-1 macrophages with various doses of Gentiana scabra and evaluated the effects on Dii-OxLDL uptake. By using flow cytometric analysis and microscopic examination, we determined that Gentiana scabra dose-dependently inhibited Dii-OxLDL uptake in THP-1 macrophages (Figure 1B and 1C).
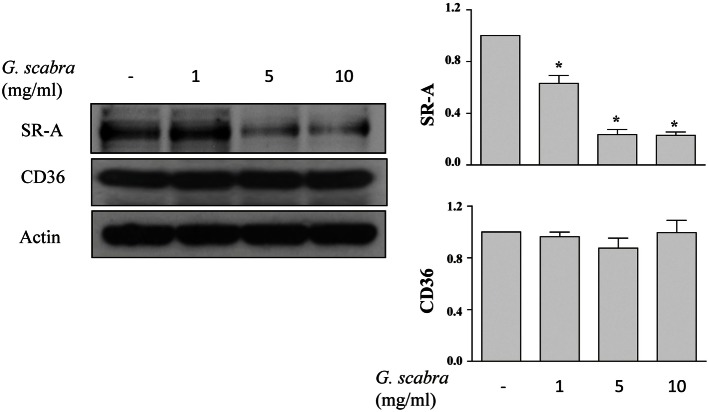
Gentiana scabra inhibited expression of SR-A, but not CD36, in THP-1 macrophages
Because the majority of modified lipoproteins are ingested in macrophages through SR-A and CD36,20 we evaluated the effects of Gentiana scabra on the expression of these two SRs. We found that Gentiana scabra significantly inhibited the expression of SR-A, but not CD36, in THP-1 macrophages (Figure 2).
Figure 2.
Gentiana scabra suppressed SR-A expression in THP-1 macrophages. THP-1 macrophages were treated with Gentiana scabra at indicated concentrations for 24 h. The expressions of SR-A, CD36, and actin were determined. Values representative and relative protein expressions of SR-A and CD36 from at least three independent experiments were presented after densitometric analysis. * p < 0.05 vs. control. SR-A, scavenger receptor-A.
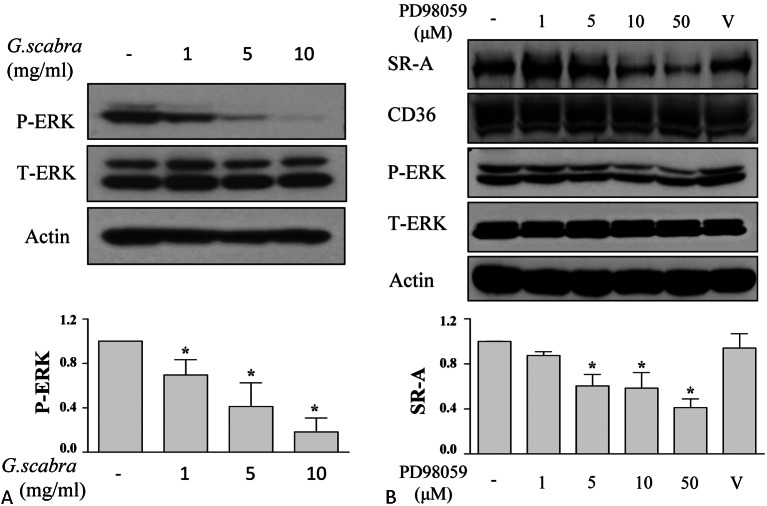
Gentiana scabra inhibited SR-A expression through ERK signaling
We previously demonstrated that ERK signaling is involved in PKCδ-regulated SR-A expression.21 Li et al. reported that ERK is crucial to the interferon (IFN)- γ-induced uptake of modified lipoproteins in human macrophages.22 We examined the effects of Gentiana scabra on ERK activation and demonstrated that treatment with Gentiana scabra reduced ERK activation in a dose-dependent manner (Figure 3A). Moreover, treatment with an ERK inhibitor, PD98059, reduced the expression of SR-A, but not CD36 (Figure 3B).21
Figure 3.
ERK pathway is involved in Gentiana scabra regulating SR-A expression. (A) THP-1 macrophages were treated with Gentiana scabra at indicated concentrations for 24 h. The expressions of p-ERK, T-ERK, and actin were determined. Values representative and relative protein expressions of p-ERK from at least three independent experiments were presented after densitometric analysis. * p < 0.05 vs. control. (B) THP-1 macrophages were treated with various doses of an ERK inhibitor, PD98059, for 16 h. The cell lysates were collected to determine SR-A, CD36, p-ERK, T-ERK and actin expressions. “v.” indicates vehicle control. Relative protein expressions of SR-A from at least three independent experiments were presented. * p < 0.05 vs. control.
Collectively, these results suggested that Gentiana scabra effectively reduced OxLDL uptake and SR-A expression, supporting its potential use as a therapeutic agent in treating atherosclerosis. Figure 4 summarizes the proposed mechanisms of Gentiana scabra suppressing OxLDL uptake and foam cell formation.
Figure 4.
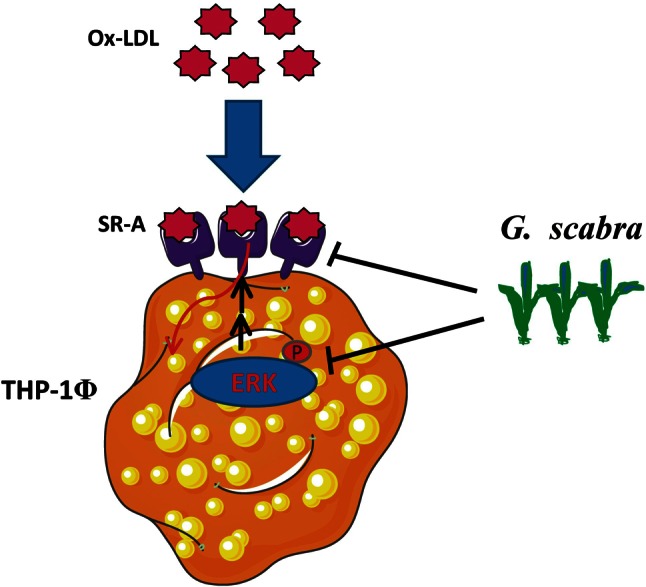
Proposed mechanisms of Gentiana scabra suppressing SR-A expression and OxLDL uptake. The results of this study suggested that Gentiana scabra inhibits OxLDL uptake by regulating SR-A expression. A possible underlying mechanism may be through ERK signaling pathways.
DISCUSSION
In the present study, by screening the effects of potential anti-inflammatory Chinese herbs on foam cell formation, we demonstrated that Gentiana scabra effectively suppressed OxLDL uptake in THP-1 macrophages. Moreover, Gentiana scabra inhibited SR-A expression by regulating ERK activity. These results indicate the therapeutic potential of Gentiana scabra in treating atherosclerosis.
Gentiana, an important genus of the Gentianaceae family, comprises more than 400 species that are extensively distributed worldwide. Gentiana scabra, also called “longdan”, has been used as a crude drug to treat inflammation and to stimulate appetite and gastric infections.23 Recent pharmacological research indicated that polysaccharides isolated from Gentiana scabra Bunge roots exhibit antioxidant and immunological effects.24 Gentiana scabra extracts, probably secoiridoid and its glycosides, significantly inhibit severe acute respiratory syndrome (SARS) – associated coronavirus replication and could be a potential therapeutic agent for SARS.25 Moreover, Ko et al. reported that the antioxidant effects of Gentiana scabra extracts produce hepatoprotective effects in the liver of carbon-tetrachloride-intoxicated mice.26 These effects make Gentiana scabra a common ingredient in Chinese herbal medicine and health products.23,24
The formation of foam cells is principally regulated by the uptake of OxLDL or cholesterol efflux in macrophages, which leads to excessive lipid accumulation inside the cells.27 The efflux of cholesterol is mediated by reverse cholesterol transporters including adenosine triphosphate (ATP)-binding cassette transporter A1 and G1 (ABCA1 and ABCG1), and it is generally accepted that SRs (SR-A and CD36) are responsible for the internalization of OxLDL.20,28 Because the uptake of cholesterol-rich LDL by macrophages is believed to be critical in mediating the development of atherosclerotic plaques, understanding and targeting the pathways mediating LDL uptake by macrophages are crucial in treating atherosclerosis.2,3,29,30 Moreover, Babaev et al. demonstrated that SR-A in macrophages contributes significantly to atherosclerotic lesion formation in an atherogenic mouse model.31 By showing down-regulation of SR-A expression, our results provided further evidence demonstrating that Gentiana scabra reduces foam cell formation by affecting OxLDL uptake in THP-1 macrophages.
Regarding the underlying molecular mechanisms through which Gentiana scabra regulates SR-A expression, we observed that Gentiana scabra significantly reduced ERK activity in THP-1 macrophages. ERK signaling is critical in the IFN-γ-induced expression of several proinflammatory genes, such as the chemokines of monocyte chemoattractant protein-1, macrophage inflammatory protein 1β, and IFN-γ-induced protein 10, adhesion molecules of intercellular adhesion molecule 1, and suppressor of cytokine signaling 1, all of which have been implicated in atherosclerosis.32 Our previous reports suggested that ERK is mediated in protein kinase Cδ-regulated foam cell formation and SR-A expression.21 Additionally, previous studies have suggested that ERK signaling is critical in the IFN-γ mediated OxLDL uptake and the efflux of cholesterol from foam cells through the regulation of ABCA1 expression in macrophages.22,33 Combined with the evidence that ERK is mediated in IFN-γ-induced STAT1 activation, the inhibitory effects of Gentiana scabra on ERK signaling in the current study indicate its anti-inflammatory and anti-atherogenic effects in treating atherosclerosis.22
Limitations
Although the current study revealed Gentiana scabra reduced OxLDL uptake significantly in THP-1 macrophages, some limitations should be noted before the findings are interpreted. First, atherosclerosis is a chronic inflammatory disease with a complex interplay of various cell types, such as endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and macrophages in vessel walls. However, this study did not evaluate the effects of Gentiana scabra on ECs, VSMCs or in vivo animal models. Moreover, we used a mixture instead of pure compounds of Gentiana scabra extracts, which is a common limitation in studies of Chinese herbs. Nevertheless, the so-called “Junn-Chenn-Zuou-SS” theory illustrates a concept of coordinated effects from a combination of compounds or Chinese herbs, which might make Chinese herbs less toxic and more highly tolerated than Western drugs.34
CONCLUSIONS
In conclusion, we demonstrated for the first time that Gentiana scabra inhibited OxLDL uptake and the expression of SR-A in THP-1 macrophages, at least in part via ERK signaling. Further investigations, including research into other vascular cells and in vivo atherogenic-prone model and human studies, are necessary to elucidate the underlying mechanisms and evaluate the effects of Gentiana scabra on atherosclerosis.
FUNDING
This work was supported by grants from Taiwan Society of Cardiology, Taiwan, R.O.C.
REFERENCES
- 1.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 8.Ling S, Nheu L, Dai A, et al. Effects of four medicinal herbs on human vascular endothelial cells in culture. Int J Cardiol. 2008;128:350–358. doi: 10.1016/j.ijcard.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 9.Tsai JY, Su KH, Shyue SK, et al. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–423. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- 10.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 11.Quettier-Deleu C, Voiselle G, Fruchart JC, et al. Hawthorn extracts inhibit LDL oxidation. Pharmazie. 2003;58:577–581. [PubMed] [Google Scholar]
- 12.Chan K, Chui SH, Wong DY, et al. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004;75:3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Wu M, Wang J, Liu LT. Advance of studies on anti-atherosclerosis mechanism of berberine. Chin J Integr Med. 2010;16:188–192. doi: 10.1007/s11655-010-0188-7. [DOI] [PubMed] [Google Scholar]
- 15.Hsiang CY, Wu SL, Cheng SE, Ho TY. Acetaldehyde-induced interleukin-1beta and tumor necrosis factor-alpha production is inhibited by berberine through nuclear factor-kappaB signaling pathway in HepG2 cells. J Biomed Sci. 2005;12:791–801. doi: 10.1007/s11373-005-9003-4. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Liu Z, Li H, et al. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 17.McLaren JE, Michael DR, Salter RC, et al. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–1229. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 18.Zhao ZZ, Wang Z, Li GH, et al. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 19.Lai JH, Ho LJ, Lu KC, et al. Western and Chinese antirheumatic drug-induced T cell apoptotic DNA damage uses different caspase cascades and is independent of Fas/Fas ligand interaction. J Immunol. 2001;166:6914–6924. doi: 10.4049/jimmunol.166.11.6914. [DOI] [PubMed] [Google Scholar]
- 20.Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 21.Lin CS, Lin FY, Ho LJ, et al. PKC delta signalling regulates SR-A and CD36 expression and foam cell formation. Cardiovasc Res. 2012;95:346–355. doi: 10.1093/cvr/cvs189. [DOI] [PubMed] [Google Scholar]
- 22.Li N, McLaren JE, Michael DR, et al. ERK is integral to the IFN-gamma-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 23.Kim JA, Son NS, Son JK, et al. Two new secoiridoid glycosides from the rhizomes of Gentiana scabra Bunge. Arch Pharm Res. 2009;32:863–867. doi: 10.1007/s12272-009-1608-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Wang C, Su T, Zhang J. Antioxidant and immunological activities of polysaccharides from Gentiana scabra Bunge roots. Carbohydr Polym. 2014;112:114–118. doi: 10.1016/j.carbpol.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 25.Wen CC, Shyur LF, Jan JT, et al. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J Tradit Complement Med. 2011;1:41–50. doi: 10.1016/S2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko HJ, Chen JH, Ng LT. Hepatoprotection of Gentiana scabra extract and polyphenols in liver of carbon tetrachloride-intoxicated mice. J Environ Pathol Toxicol Oncol. 2011;30:179–187. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i3.10. [DOI] [PubMed] [Google Scholar]
- 27.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerterp M, Bochem AE, Yvan-Charvet L, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 29.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 31.Babaev VR, Gleaves LA, Carter KJ, et al. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- 32.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Yin Z, Guo X, et al. Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J Biol Chem. 2010;285:6316–6326. doi: 10.1074/jbc.M109.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho LJ, Chang WL, Chen A, et al. Differential immunomodulatory effects by Tripterygium wilfordii Hook f-derived refined extract PG27 and its purified component PG490 (triptolide) in human peripheral blood T cells: potential therapeutics for arthritis and possible mechanisms explaining in part Chinese herbal theory “Junn-Chenn-Zuou-SS”. J Transl Med. 2013;11:294. doi: 10.1186/1479-5876-11-294. [DOI] [PMC free article] [PubMed] [Google Scholar]