Review on the biological roles of glycans in infection and immunity.
Keywords: selectin, siglec, galectin, lectin, sialic acid, fucose
Abstract
Glycans and complementary glycan-binding proteins are essential components in the language of cell-cell interactions in immunity. The study of glycan function is the purview of glycobiology, which has often been presented as an unusually complex discipline. In fact, the human glycome, composed of all of its glycans, is built primarily from only 9 building blocks that are combined by enzymes (writers) with specific and limited biosynthetic capabilities into a tractable and increasingly accessible number of potential glycan patterns that are functionally read by several dozen human glycan-binding proteins (readers). Nowhere is the importance of glycan recognition better understood than in infection and immunity, and knowledge in this area has already led to glycan mimetic anti-infective and anti-inflammatory drugs. This review includes a brief tutorial on human glycobiology and a limited number of specific examples of glycan-binding protein-glycan interactions that initiate and regulate inflammation. Examples include representatives from different glycan-binding protein families, including the C-type lectins (E-selectin, P-selectin, dectin-1, and dectin-2), sialic acid-binding immunoglobulin-like lectins (sialic acid-binding immunoglobulin-like lectins 8 and 9), galectins (galectin-1, galectin-3, and galectin-9), as well as hyaluronic acid-binding proteins. As glycoscience technologies advance, opportunities for enhanced understanding of glycans and their roles in leukocyte cell biology provide increasing opportunities for discovery and therapeutic intervention.
Introduction
Glycobiology, the study of the structures and functions of glycans (also called carbohydrates, saccharides, or simply sugars), has been deemed to be a uniquely complex discipline [1, 2]. A recent article starts with the observation that “Glycans are among the most complex biological molecules found in nature” [3], a sentiment repeated in many reviews in the field. This concept is technically true but may be unnecessarily daunting to those seeking to grasp the essentials required to incorporate human (or vertebrate) glycobiology into their biomedical worldview. This is especially important in the study of the human immune system, where glycans have major roles in the control of both innate and adaptive immunity [4–7]. In this arena, glycobiology is, in essence, pattern recognition, and the patterns are both understandable and tractable. In some cases, glycan pattern recognition is broad and structurally flexible, such as the variety of microbial mannosylated glycans that bind to mannose-binding lectins as pathogen-associated molecular patterns [8]. In other cases, glycan pattern recognition is highly specific, requiring precise spacing of multiple atomic-binding determinants in a distinct glycan structure. This review examines glycobiology in the human immune system, providing a perspective on some of the molecular structures and mechanisms involved in glycan function and a few specific roles that glycans have in the control of inflammation. With apologies to those well versed in the broader discipline, this review focuses mainly on major human glycan structures and functions. Most of the references are themselves reviews and provide deeper and broader coverage of knowledge in the field.
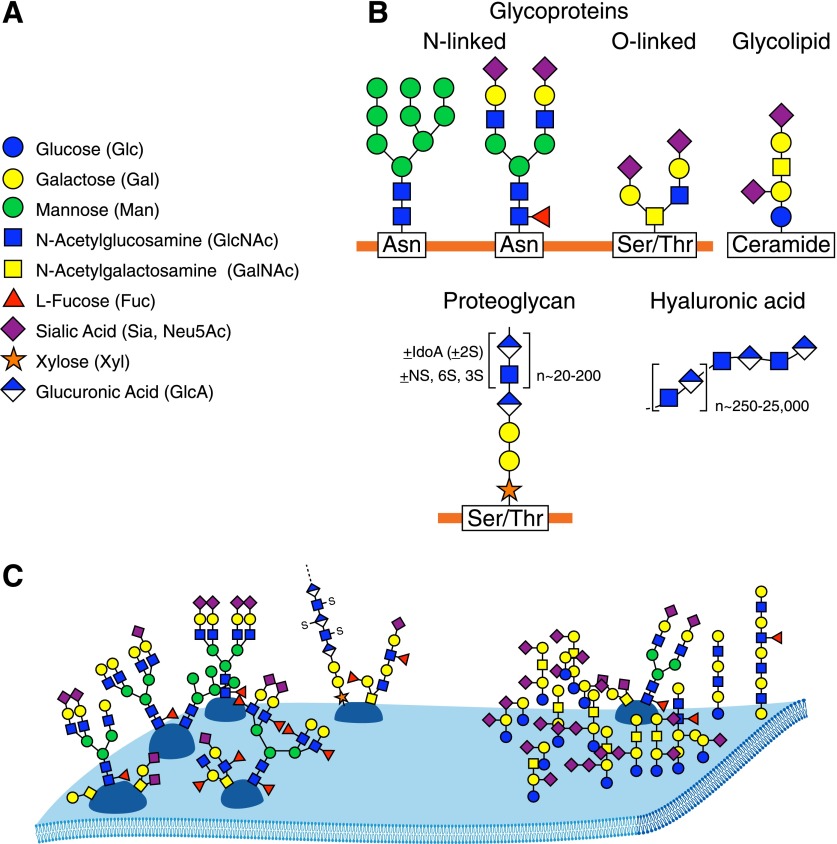
Every living cell, eukaryotic and prokaryotic, is endowed with a rich and diverse sugar coat—its glycocalyx [9, 10]. Glycans are the dominant face of each cell to its immediate environment. In vertebrates, the glycocalyx comprises primarily glycoproteins, glycolipids, and proteoglycans (Fig. 1B and C). Nearly all cell-surface and secreted proteins are glycoproteins. Glycolipids are components of all vertebrate plasma membranes, and the extracellular matrix is rich in both glycoproteins and proteoglycans. Asking “What are the functions of glycans?” is rather like asking “What are the functions of proteins?” in that glycans have a wide variety of molecular, cellular, and biophysical roles [11]. It is noteworthy that because cell surfaces are a major site of glycan expression, their roles include mediating cell-cell and pathogen-cell interactions. This is nowhere more evident than in the immune system where cell-surface glycans are broadly involved in immune cell activation, trafficking, and regulation.
Figure 1. Major human glycans.
(A) The 9 sugars that comprise most of the human glycome, with their broadly accepted symbol representations [3]. (B) Major classes of human glycans. Linkage details (hydroxyl attachment sites and anomeric configurations at each glycosidic bond) that are keys to structural recognition (as described elsewhere) are omitted here for simplicity. Representative asparagine (N-linked) and serine or threonine (O-linked) glycoprotein structures, a glycosphingolipid (ceramide-linked), a proteoglycan (most frequently O-linked), and HA (unlinked) are shown. (C) A schematic representation of glycans on a cell surface. Notable features important for understanding glycan recognition include varied glycan branching patterns, variations in terminal glycan structures, and the tendency of glycans to form distinctive, lateral glycan patches.
Regulatory functions of glycans are often mediated by their interactions with complementary glycan binding proteins (GBPs), each of which carries a carbohydrate recognition domain (CRD) that binds specific groupings of ∼2–7 sugars in more or less precise configurations [12]. In this context, glycobiology is pattern recognition, with glycans constituting the patterns, glycan metabolic enzymes (glycosyltransferases, glycosidases, and glycan modifying enzymes) as the pattern “writers,” and GBPs as the pattern “readers.” In humans, there are ≥80 GBPs (lectins) that fall into about a dozen structural families and, perhaps, an equal number of HA and glycosaminoglycan binding proteins (see below). GBPs serve in both self and pathogen recognition. They initiate inflammation, regulate ongoing inflammatory responses, and recognize and destroy pathogens, and yet, pathogens can subvert them for immune evasion. A deeper and broader understanding of glycan and GBP functions in inflammation has provided new insights into human disease and has led to novel mechanism-based proinflammatory and anti-inflammatory therapeutics.
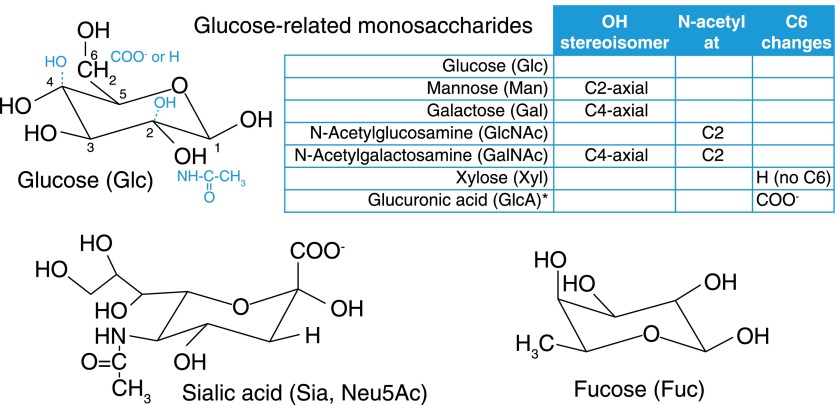
Understanding glycan pattern recognition requires knowledge of glycan components and the rules for their semiotic organization. In the diversity of nature, the numbers and complexities of glycans are truly staggering—microbes, in particular, have been delightfully creative in this regard. One database (http://monosaccharidedb.org) lists >700 monosaccharide building blocks. In humans, however, the situation is much simpler. Human glycans are built primarily from only 9 monosaccharides (Fig. 1A), most of which are structurally related, varying from one another at a single carbon (Fig. 2). Another level of pattern variability in glycans arises from the multiple ways in which monosaccharides attach to one another. Whereas the 20 amino acids in proteins or the 4 nucleotides in nucleic acids combine in linear sequences using a single type of intersubunit bond, monosaccharides bind to one another in 2 possible configurations (α or β) at any of 3–4 hydroxyl groups on each monomer in linear or branched groupings. Mathematically, this means that 3 different amino acids (or nucleotides) combine to make 6 distinct structures, whereas 3 different sugars can combine to make >1000 structures. Because the possibilities increase exponentially, one calculation of possible distinct hexasaccharides [1] placed the number at >1012. Although this number is also staggering, in humans, the biosynthetic combinations of glycans are strictly limited by the capabilities of the enzymes responsible for their biosynthesis (the writers). The outcome is a handful of common core structures that carry a large, but limited, set of terminal glycan structures (glycan determinants) that vary within defined parameters. It has been estimated that glycan determinants in humans that might function in molecular recognition number ∼3000 on glycoproteins and glycolipids with, perhaps, another ∼4000 within proteoglycans [13]. These numbers are tractable in terms of “omics” considerations and are increasingly accessible chemically, with some printed glycan arrays used for determining GBP specificity having >600 distinct glycan structures [14, 15]. It is time to turn the argument about the intractable complexity of glycobiology on its head and demystify what has been presented at times as a stubbornly inaccessible body of knowledge.
Figure 2. Monosaccharides of the human glycome.
The human glycome is constructed mainly using 9 monosaccharide building blocks, 7 of which are denoted as “glucose related” in embedded chart. The structure of glucose (black) is shown with modifications relating that structure to 6 of the other monosaccharides (blue) as detailed in the chart. The lower panels are Sia and Fuc, which are often involved in GBP recognition. *Glucuronic acid is sometimes enzymatically modified after incorporation into GAG chains by epimerization around the C5 carbon. Its epimer, iduronic acid, can be considered an important tenth monosaccharide not further discussed here.
This review covers the basics of major human glycans and GBPs, describes some of the functions of GBP-glycan recognition in inflammation, and discusses ways in which knowledge in the field is leading to new drugs and therapeutic targets. To simplify its scope, functions of glycans unrelated to GBP-mediated cell regulation and GBP-glycan recognition not directly related to inflammation are not addressed. For broader considerations, the reader is directed to excellent texts [11, 16] and compendia [17] in the area.
THE HUMAN GLYCOME—SIMPLIFIED
The glycome, analogous to genome or proteome, comprises all of the glycans in a particular cell, tissue, or organism [2]. The human glycome (with 1 important exception, HA) comprises groupings of sugars covalently bound to proteins or lipids (Fig. 1B). The proportion of these varies among tissues. In many tissues, most glycans are carried on proteins, whereas in some (such as brain), glycolipids dominate [18]. Glycans carried on proteins and lipids are both involved in GBP recognition, cell-cell and pathogen-cell recognition, and cell regulation.
Proteins that carry covalently bound glycans are conveniently classified in 2 subgroups, rather arbitrarily called glycoproteins and proteoglycans. Glycoproteins typically carry linear or branched glycans composed of 1–20 sugar units. By comparison, proteoglycans (such as heparan sulfate and chondroitin sulfate) are distinguished by having 1 or more long, linear chains of repeated disaccharides (glycosaminoglycans, GAGs) that reach >100 sugar units in length and that are decorated with sulfate groups. A single protein may carry as few as a single glycan chain or up to 100s and may carry short glycans, GAG chains, or both. An important exceptional glycan is HA [19], an unsubstituted linear disaccharide that is not covalently bound to either lipid or protein (Fig. 1B). It consists of repeating GlcNAc and Glucuronic acid residues and reaches enormous lengths, typically ≥20,000 sugar residues (several million daltons). In addition to its important gel-like biophysical properties, smaller fragments of HA are targets of glycan recognition and regulate inflammation.
Most of the human glycome is built from only 9 sugars (Figs. 1A and 2). As with other biopolymer components, it is worth considering these on 2 levels, the atomic and pattern-recognition levels. Glycan recognition by GBPs is accomplished by the arrangement of protein amino acid side chains that engage multiple chemical moieties (e.g., hydroxyl groups, N-acetyl groups, and carboxylic acids) on glycans. The chemical nature and stereochemical placement of each moiety drive recognition, so even small atomic changes are often important. Seven of the 9 monosaccharides that make up most of the human glycome, those designated as glucose-related in Fig. 2, differ from one another in the chemical moiety or stereochemistry at a single carbon. Although the differences appear subtle, they generate highly specific, atomic-level driving forces for differential recognition by GBPs. The 2 remaining monosaccharides, Fuc and Sia are unusual both in their structures and functions. Sia stands out as distinct among human glycan monosaccharides as a 9-carbon sugar that carries a wealth of chemical moieties that enhance glycan recognition (see more below). Both Sia and Fuc are often found on the termini of glycans (Fig. 1B and C), where they are intimately involved in GBP-glycan recognition. In the human glycome, there are other important monosaccharides (iduronic acid and glucosamine) and glycans (glycophosphatidylinositol anchors and O-linked GlcNAc) that are not discussed here; readers interested in delving more deeply into these glycans are directed to excellent reviews [20–22].
Although atomic level interactions drive GBP-glycan binding specificity, much of the pattern recognition of glycans can be understood at a higher level of organization. GBP recognition of glycoprotein and glycolipid glycans typically involves the outermost terminal groupings of 2–7 specifically arranged sugars, whereas GAG-binding proteins recognize similarly sized linear stretches of sugars decorated by defined patterns of sulfation [23]. Among these moderately sized glycan signatures are the determinants for GBP recognition and related glycan functions. To make recognition patterns more intuitive, the glycobiology community has agreed on a symbol nomenclature (Fig. 1A) that uses colors and shapes to represent monosaccharides [3].
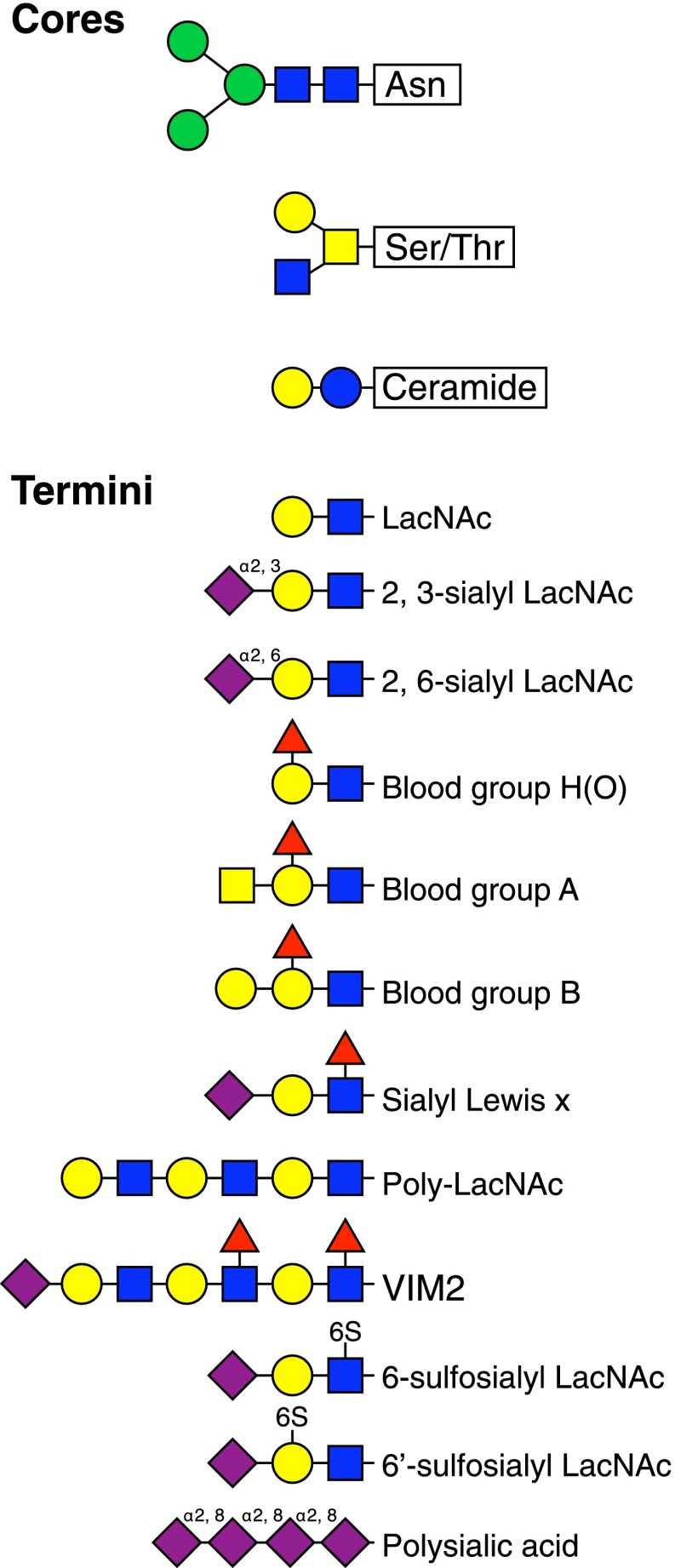
Monosaccharides are linked to one another enzymatically to create linear or branched oligosaccharides. The incoming (donor) sugar in an activated form (nucleotide sugar or dolichol phosphate sugar) is enzymatically attached via a glycosidic bond in 1 of 2 stereochemical configurations—α or β—to a particular hydroxyl on the acceptor sugar. This process is repeated, 1 by 1, by highly specific glycosyltransferases until a precise target glycan is created. There are ∼200 human glycosyltransferases [2], each of which is more or less stringent for the sugar donor, anomeric linkage, and hydroxyl on the acceptor sugar. The resulting variety of glycans are most readily understood as a set of core structures (closest to the protein or lipid attachment site) decorated with variable termini that extend outward and are common targets of GBP binding (Fig. 3).
Figure 3. Theme and variation in the human glycome.
Glycan recognition often involves variations of terminal glycan structures that are attached to core structures (linkage details are omitted for simplicity). The upper panel provides examples of the invariant, N-linked glycoprotein pentasaccharide core, one of several serine/threonine-linked glycoprotein cores, and a common glycosphingolipid (ceramide-linked) core. The lower panel provides a sampling of terminal structures that are referred to elsewhere in the text. A representation of how these might be grouped on a cell surface is shown in Fig. 1C.
In some cases, a biosynthetic target glycan is partly depolymerized and rebuilt with different terminal sugars or is enzymatically derivatized with functional groups, such as sulfates or phosphates. A circumscribed number of glycosyltransferases, glycosidases, and glycan-modifying enzymes in the human genome, each with specific and limited capabilities, define the structures of the human glycome. Biosynthesis typically occurs in the endoplasmic reticulum and Golgi apparatus, where the expression levels of biosynthetic enzymes define the distinctive glycomes of each cell type. Glycans cover the intraluminal surfaces of the Golgi apparatus, endosomes, and lysosomes and are displayed on the outer leaflet of the plasma membrane (Fig. 1C). On the cell surface, the glycans form a dense covering that has been likened to a forest, with the carrier proteins being the tree trunks, the branches being the core structures, and the outermost leaves and flowers are the termini [10]. As depicted in Fig. 1C, glycans may associate to form distinctive glycan areas on the surface, which are akin to different types of forests (boreal, deciduous, and tropical) with different functions. If one imagines flying over the cell surface, one would look down on a variety of dense forests; incoming pathogens and other cells have the ability to read the terrain and respond accordingly.
Humans (and other vertebrates) express a tiny fraction of the vast array of complex glycans found in nature. Each distinctive glycan has key roles in the organisms in which it is found, and in terms of human health, some have important roles in cross-species interactions [9, 24]. Although investigating the broader library of glycans in nature provides fascinating biologic and medical insights, this review is primarily limited to major structures of the human glycome.
SELECTINS INITIATE INFLAMMATION
The best-understood cell-cell interaction in which glycans participate is the initiation of inflammation [25]. Knowledge gained about the glycans, GBPs, and the structural biology involved in their binding led to enhanced understanding of human diseases and development of new glycan-based natural and glycomimetic therapies [26, 27].
Neutrophil extravasation at sites of infection is most often initiated when 2 GBPs—P-selectin and E-selectin—are mobilized by vascular endothelial cells to the cell surface facing the blood vessel lumen [28]. In response to inflammatory mediators (bacterial lipopolysaccharide, histamine, and TNF-α) and within minutes, P-selectin is deployed to the luminal surface from prepackaged granules, whereas E-selectin biosynthesis is up-regulated to provide a response over hours. Once on the vessel lumen, selectins bind to preexisting, complementary glycans on the surface of passing neutrophils. Selectins and their glycan targets on apposing membranes cooperate to snag neutrophils from the circulation, where they roll on the endothelium until other cell activation and adhesion mechanisms are triggered that direct neutrophil extravasation into infected tissues.
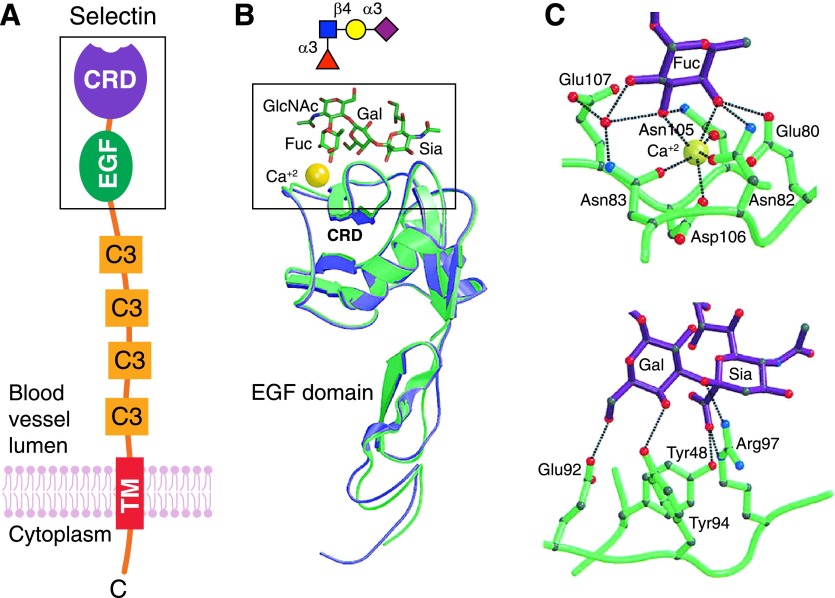
P-selectin and E-selectin are structurally similar (Fig. 4) and bind to closely related, but not identical, glycans on neutrophil surfaces [25]. The glycans have Sia and Fuc residues that are appropriately arranged to engage complementary amino acids on the selectin CRDs [29]. The prototype terminal tetrasaccharide that binds both E-selectin and P-selectin is SLex (Figs. 3 and 4). Crystal structures of E-selectin and P-selectin bound to SLex (Fig. 4) reveal a network of hydrogen bonds between amino acid side chains and moieties on 3 of the 4 sugars, with 2 key Fuc hydroxyl groups coordinated to a tightly bound calcium ion on the protein [29]. The fourth sugar (GlcNAc) does not directly engage the protein CRD but is a structural component that keeps the other sugars in proper alignment. This general model holds for other GBP-glycan binding interactions in humans. Proteins have evolved to have appropriately spaced amino acid side chains, often on a shallow pocket, that engage precisely spaced moieties on target glycans. These atomic-level interactions provide specificity but often do not provide sufficient avidity by themselves to support GBP functions. Enhanced avidity is acquired by at least 2 mechanisms: auxiliary binding sites and multivalency.
Figure 4. E-selectin and P-selectin binding to SLex tetrasaccharide.
(A) Schematic generalized structure of selectins with an outermost N-terminal, C-type lectin CRD attached to an epidermal growth factor (EGF) domain, several complement regulatory (C3) repeats (6 in E-selectin, 9 in P-selectin), and a single transmembrane domain leading to a cytoplasmic tail. Image from [95] with permission (copyright The Consortium of Gycobiology Editors, La Jolla, CA). The structure of the boxed area is shown in panel B. (B) Ribbon representations of the crystal structures of the N-terminal domains of E-selectin (green) and P-selectin (blue) superimposed on the common-bound calcium ion (yellow). An energy-minimized molecular model and symbol cartoon structure of SLex is represented above the CRD positioned with the Fuc over the bound calcium. Atomic details of the boxed area are shown in panel C. (C) Atomic interactions of Fuc (top) and Siaα2,3Gal (bottom) from the crystal structure of SLex bound to E-selectin. Dotted lines represent ligation to the bound calcium (yellow) and hydrogen bonds. Crystallographic images are from [29], with permission (Elsevier Limited). The energy-minimized SLex structure was created using GLYCAM-Web (http://glycam.org).
The natural glycan on humans (and mice) responsible for P-selectin binding is the glycoprotein PSGL-1 [30]. An O-linked glycan, linked to a threonine near the outermost end of this neutrophil cell surface glycoprotein, carries the key SLex sequence that is required for glycan recognition. Within a few amino acids of this glycan, 3 closely spaced tyrosine residues on PSGL-1 are sulfated to create a negatively charged surface patch. Elegant synthetic studies [30] demonstrated that the nearby sulfated tyrosines increased the binding avidity of P-selectin to SLex by 1000-fold.
Unlike the ligand for P-selectin, the natural human ligand for E-selectin has not been established. Data suggested that the E-selectin ligand on mouse neutrophils was a glycoprotein because it was protease sensitive, whereas the ligand on human neutrophils was not [31]. This implicated glycolipids on human neutrophils as natural E-selectin ligands. Extraction and in vitro functional analyses [32] revealed a family of glycolipids with several Gal-GlcNAc repeats (poly-LacNAc; Fig. 3) with a terminal Sia and multiple Fuc residues on internal sites (e.g., VIM2; Fig. 3). Whether these neutrophil glycolipids are necessary and sufficient for binding of circulating human neutrophils to E-selectin on the endothelium has not been established. If they are, it is likely that multivalency, a well-established mechanism that enhances GBP-glycan binding avidity [33], has a role in enhancing the interaction. Glycosphingolipids tend to self-associate on the cell surface (Fig. 1C) [34], where they might engage multiple E-selectin molecules on the endothelium to form a Velcro-like adherence.
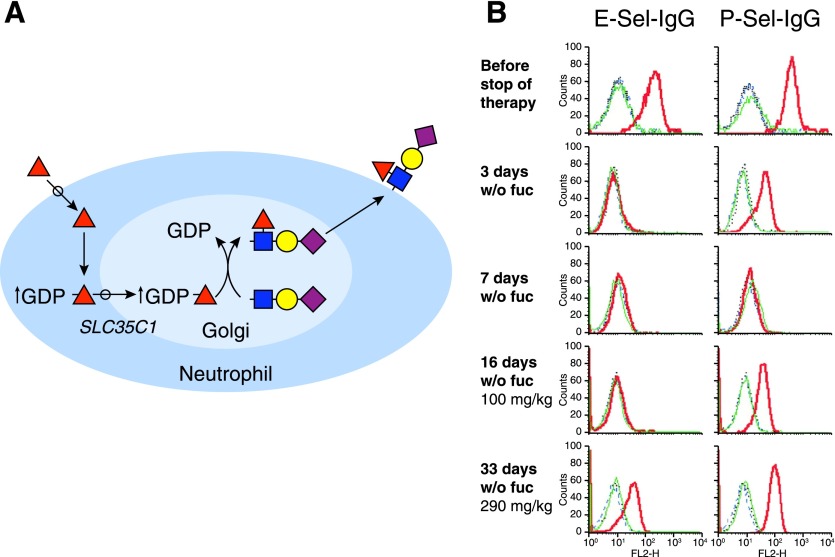
A requirement for selectin-glycan binding in initiating human inflammation was confirmed by studies of a rare congenital leukocyte adhesion deficiency (LADII) marked by failure to mount effective inflammatory responses and neutrophilia [35]. Patients with LADII suffer from repeated infections requiring administration of systemic antibiotics. These patients have a rare blood group type, the Bombay phenotype, which is not O, A, or B [36]. Because selectin-binding glycans and blood-group glycans (see Fig. 3) both require Fuc, the hypothesis that LADII is a Fuc-deficiency disease was tested by administering oral Fuc. Remarkably, Fuc administration resolved the LAD, reinstating effective inflammation and bacterial clearance [26]. The genetic mutation responsible was found to encode the Golgi membrane transporter (SLC35C1) that moves the donor form of Fuc (GDP-Fuc) from the cytoplasm to the Golgi lumen, where fucosyltransferases reside [37]. Because the mutation was hypomorphic, oral Fuc administration resulted in increased GDP-Fuc in the cytoplasm, increased transport of GDP-Fuc into the Golgi, and sufficient transfer of Fuc onto acceptor glycans to support neutrophil adherence to the vascular endothelium at sites of infection (Fig. 5A). Subsequent proof of concept was obtained when a patient with LADII was removed from oral Fuc therapy (Fig. 5B). Within days of withdrawing Fuc both E-selectin and P-selectin binding were reduced to background levels, blood neutrophil counts spiked, infection ensued, and antibiotics were administered [38]. Only after oral Fuc was restarted, did inflammation return to normal, along with E-selectin and P-selectin binding. These data demonstrate the requirement for specific glycan expression supporting glycan recognition, cell adhesion, and biologic function in human inflammation. Notably, LADII is a congenital disorder of glycosylation (CDG-IIc, carbohydrate-deficient glycoprotein type IIc), one of a family of >100 inborn errors of glycosylation [39].
Figure 5. Oral Fuc corrects E-selectin and P-selectin binding deficiency in patient with LADII.
(A) Schematic demonstrating how oral Fuc overcomes a hypomorphic mutation in the Golgi GDP-Fuc transporter (SLC35C1) responsible for LADII. Oral Fuc uptake into neutrophils results in an increase in the cytoplasmic concentration of the activated Fuc donor GDP-Fuc, resulting in more GDP-Fuc entering the Golgi apparatus. The higher GDP-Fuc concentration results in synthesis of fucosylated glycans, including SLex and VIM2 (not shown) on glycoproteins and glycolipids that are transported to the plasma membrane, providing ligands for E-selectin and P-selectin. (B) Expression of selectin ligands during discontinuation and resumption of oral Fuc therapy. Neutrophils were isolated at the time points before (row 1), during discontinuation (rows 2 and 3), and during resumption of oral Fuc therapy (rows 4 and 5), as indicated on the left. Expression levels of selectin ligands were analyzed by flow cytometry, using E-selectin-IgG (E-Sel-IgG) or P-selectin-IgG (P-Sel-IgG) in the presence of calcium (red line) or EDTA (green line). Replacement of the selectin chimera with VE-cadherin-IgG (blue) serves as a control. w/o, without. Data are from [38], with permission (copyright American Society of Hematology).
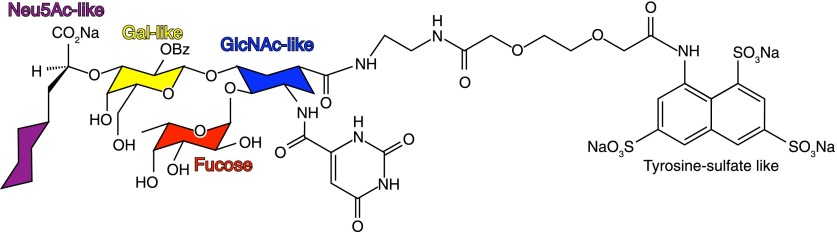
Knowledge of selectin functions and glycan-binding specificities provided opportunities for therapeutic development. A glycomimetic “pan-selectin” inhibitor (GMI-1070, Rivipansel, GlycoMimetics, Rockville, MD, USA; Fig. 6) that retains key chemical moieties from the 4 sugars of SLex-linked to a trisulfonate (to mimic the sulfated tyrosines of PSGL-1) completed successful phase 2 trials for vaso-occlusive crisis in sickle cell disease, an inflammatory clinical target [27]. Rivipansel treatment resulted in a remarkable 83% decrease (P < 0.01) in opioid analgesic use by hospitalized patients suffering from this painful inflammatory disorder, leading to the initiation of phase 3 trials (https://clinicaltrials.gov/ct2/show/NCT02187003). From a rational drug-design standpoint, Rivipansel (Fig. 6) retains the carboxylic acid and linkage oxygen from Sia; the galactose C4- and C6-hydroxyls; and the Fuc C2-, C3-, and C4-hydroxyls; all of which engage the binding sites on both E-selectin and P-selectin (Fig. 4C). These findings validate glycan recognition as a therapeutic target and rationally designed glycomimetics as viable drugs. Rivipansel [40], as well as the rationally designed anti-influenza Sia mimetics Relenza (zanamivir; GlaxoSmithKline, Brentford, United Kingdom) and Tamiflu (oseltamivir phosphate; Hoffmann-La Roche, Basel, Switzerland) [41], are models for the development of novel therapies based on glycan recognition. To the extent that the functions and atomic-level binding specificities of each human GBP are determined, the promise of glycomimetics as therapeutics advances [42].
Figure 6. Anti-inflammatory pan-selectin glycomimetic drug GMI-1070 (Rivipansel).
Rivipansel is closely based on the structure of SLex [40]; glycan-like constituents of the drug are color-coded according to accepted symbol nomenclature [3]. Note the leashed trisulfonate that mimics tyrosine sulfates near the SLex glycosylation site on PSGL-1, the key P-selectin ligand on neutrophils.
SIGLECS REGULATE INFLAMMATION
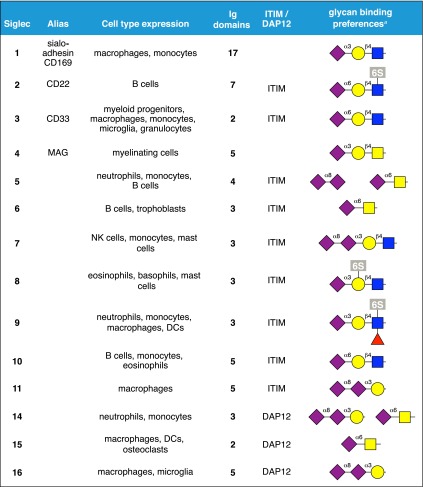
Sia, also called N-acetylneuraminic acid is the most-abundant terminal sugar on cell-surface glycans of humans [10]. It is unique among human monosaccharides for the diversity of its chemical moieties: a carboxylic acid, a glycerol side chain, and an N-acetyl group (Fig. 2), which provide opportunities for ionic, hydrogen bond, and hydrophobic interactions used by GBPs to enhance binding affinity and establish specificity. Many human and pathogen GBPs include Sia as part of their endogenous glycan ligands. E-selectin and P-selectin (see above) primarily engage the carboxylic acid on Sia (Fig. 4C) and have little requirement for its other moieties, which can be replaced with simpler structures (Fig. 6). In contrast, Siglec family members (14 functional members in humans) engage Sia more completely through a network of atomic interactions that recognize Sia in specific linkages and glycan contexts (Table 1) [43].
TABLE 1.
Human siglecs.
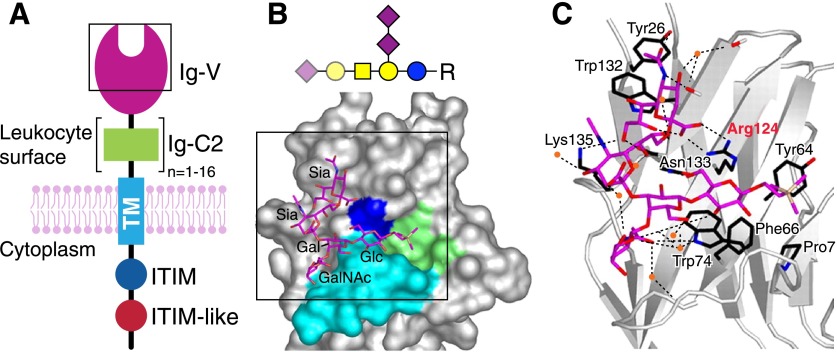
Siglecs are cell-surface proteins with common domain architectures (Fig. 7A) [44]. An outermost, N-terminal Ig V-set domain (Fig. 7B) harbors the main glycan-binding site, a shallow groove with an essential arginine residue that binds the Sia carboxylate at its center (Fig. 7C). The V-set domain is followed by a variable number of Ig C2-set domains, then a single transmembrane domain and a cytoplasmic C-terminal domain. The cytoplasmic domains of 9 of the human siglecs (siglecs 2, 3, and 5–11) carry immunoinhibitory (ITIM and ITIM-like) domains, which either have been directly demonstrated to suppress immune responses or are hypothesized to do so [43]. Three different human siglecs (siglecs 14–16) contain positively charged residues in their transmembrane domains, which bind to the immunoregulatory adapter protein DAP12 and may lead to immune cell activation or inhibition. The functions of each siglec in immunity depend on its glycan-recognition specificity, its immunoregulatory domain(s), the particular cells on which it is expressed, and the presence of appropriate glycan ligands in the immediate environment.
Figure 7. Sia binding Ig-like lectins (siglecs).
(A) Schematic generalized structure of siglecs; an outermost N-terminal Ig-V set domain (purple) attached to a variable number (1–16) of Ig-C2 like domains (green), a single transmembrane domain, and a cytoplasmic tail with ITIM and ITIM-like sequences. Image modified from [95] with permission (copyright The Consortium of Gycobiology Editors, La Jolla, CA). The structure of the boxed area is shown in panel B. (B) The glycan binding site surface of the Ig-V set domain of siglec-7 bound to the heptasaccharide from ganglioside GT1b (as a trimethylsilylethyl glycoside, R) is shown below the symbol structure of GT1b with structurally resolved, bound sugars highlighted. The ligand binding site is very open, and GT1b lies exposed to the solvent. A convex shelf (turquoise) forms the base of the binding site, over which, the terminal of the glycan lies. The trimethylsilyl aglycon lies in a hydrophobic cup (green). The Sia-binding Arg residue conserved in all siglecs (Arg124 in siglec-7) is blue. Atomic details of the boxed area are shown in panel C. (C) The potential hydrogen bonds responsible for glycan binding are shown as dashed lines. Several water molecules are stably associated with the ligand and are shown as orange spheres. The conserved Sia-binding Arg residue is highlighted in red. Crystallographic images are from [96] (copyright the American Society for Biochemistry and Molecular Biology).
Nearly all human siglecs are expressed on distinct subpopulations of leukocytes overlapping the innate and adaptive immune systems (Table 1) [43]. For example, siglec-1 is expressed on macrophages and activated monocytes, where it is involved both in clearance of sialylated human pathogens and antigen presentation; siglec-2 is expressed on human B cells, where it regulates immune tolerance; and siglec-8 is expressed on allergic inflammatory cells (eosinophils, mast cells, and basophils), where it inhibits ongoing inflammation (see below). Although knowledge of siglec-mediated regulation of the immune system is still at an early stage, important functions in inflammation are already apparent [43–47].
A notable aspect of the siglec family is the variety of their sialoglycan-binding specificities [43]. They have evolved to take advantage of the different ways Sia is presented in the context of larger glycans (Table 1). Structural variety in sialoglycans arises in part from the sugars and hydroxyl groups to which Sia is attached. The major types of Sia linkages in vertebrates are to the C3- or C6-hydroxyls of galactose, the C6-hydroxyl of N-acetylgalactosamine (or GlcNAc), or the C8-hydroxyl of another Sia. Different members of the siglec family bind preferentially to each of these linkages in the context of larger glycans.
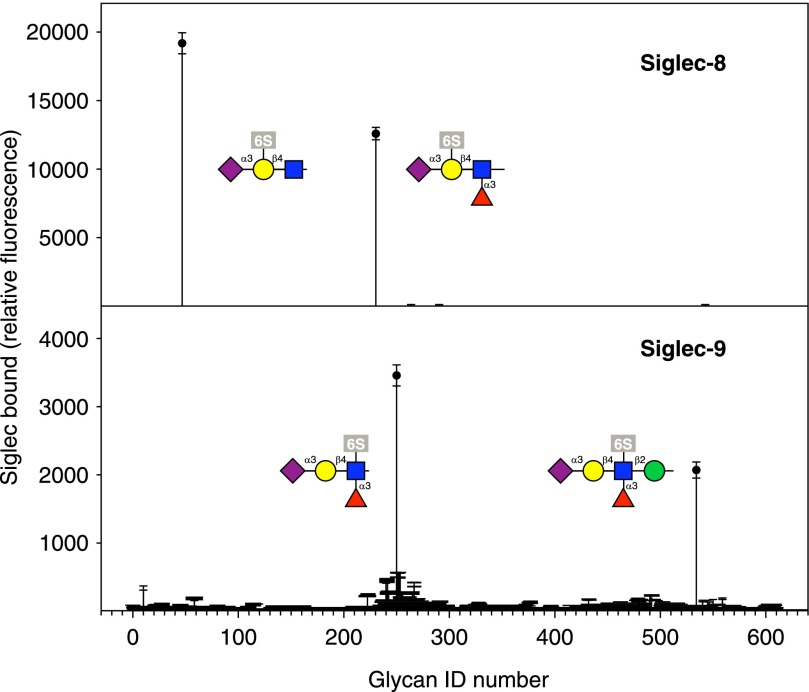
The glycan-binding specificities of siglecs and other GBPs are explored using printed glycan arrays, in which synthetic or purified glycans are covalently attached as microdots on a glass slide with a capacity of 1000s of spots [48, 49]. Current iterations of this technology have several 100 defined glycans that are simultaneously probed with tagged, expressed siglecs or other GBPs. The results of siglec-8 and siglec-9 binding to the latest glycan array from the Consortium for Functional Glycomics (http://functionalglycomics.org) provide excellent examples of differential glycan binding and GBP pattern recognition (Fig. 8). For example, the SLex tetrasaccharide, which is a primary ligand for E-selectin and P-selectin (Fig. 4), fails to bind siglec-8. However, adding a single sulfate to the galactose residue of the same SLex generates strong siglec-8 binding (Fig. 8). Siglec-9 fails to bind to SLex when there is a sulfate on the galactose but binds strongly when a sulfate is on the GlcNAc or when SLex is presented on a longer glycan containing an additional mannose residue. These observations demonstrate the subtle specificity of glycan pattern recognition. One can imagine that expression of alternative sulfotransferases in a tissue would signal differential engagement of either siglec-8 on allergic inflammatory cells or siglec-9 on neutrophils (see below).
Figure 8. Glycan array binding of siglec-8 and siglec-9.
The printed glycan array (version 5.1) of the Consortium for Functional Glycomics, which contains >600 defined glycans, was overlaid with the extracellular domain of the indicated siglec as a chimera with human Fc. Binding to each of the 600+ glycans was detected simultaneously using a fluorescent secondary antibody. Experimental details and data are freely available at the consortium website (http://functionalglycomics.org).
Although glycan arrays with 100s of glycans have been highly illuminating and are a major accomplishment, their value is limited by their coverage of the human glycome (∼600 of what may be ∼3000 terminal glycan determinants plus ∼4000 GAG recognition sequences), and their lack of a larger glycan (glycoprotein/glycolipid) context, such as the sulfated tyrosines on PSGL-1 that enhance P-selectin binding. Furthermore, the multivalency of lectin targets affects their avidity [33], and the chemically designed multivalency of glycan arrays is unlikely to reflect their presentation in cells and tissues. Therefore, the search for endogenous, intact glycan targets for GBPs remains a highly fruitful endeavor. In this respect, efforts to expand glycan arrays to include more naturally sourced, as well as synthetic, glycans and to include differentially sulfated GAG chain fragments are ongoing [50, 51].
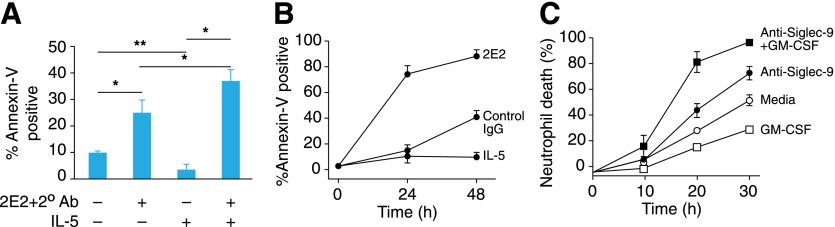
The roles of siglecs in immunology and human-pathogen interactions are many and varied, as recently reviewed elsewhere [43, 52]. Two examples of siglecs involved in the resolution of ongoing inflammation—siglec-8 and siglec-9—are included here. Siglec-8 and siglec-9 have the same domain architecture and share >65% amino acid sequence identity but are expressed on nonoverlapping subsets of leukocytes (Table 1) and bind to related, but nonoverlapping, glycans (Fig. 8). Siglec-8 appears to function in resolving allergic inflammation [45], whereas siglec-9 may function in resolving neutrophil-mediated inflammation [53, 54]. Evidence for function was obtained by clustering siglecs on the surfaces of human leukocytes using anti-siglec mAbs [55, 56]. When siglec-8 was clustered on cultured, primary human eosinophils, apoptosis was induced (Fig. 9A). It is noteworthy that when the cells were grown in the presence of IL-5, an IL that promotes growth and survival of eosinophils, the apoptotic effect of siglec-8 clustering was enhanced [57]. Eosinophils activated in vivo, freshly isolated from allergic, late-phase bronchoalveolar lavage, were remarkably sensitive to siglec-8 cross-linking (Fig. 9B), suggesting that engaging siglec-8 with its natural ligands in vivo results in potent eosinophil apoptosis. In a similar experimental scenario, apoptosis of primary human neutrophils in culture was increased by antibody-mediated clustering of siglec-9 on their surfaces [56], and the addition of the neutrophil growth-promoting cytokine GM-CSF, enhanced survival of untreated neutrophils and greatly increased their susceptibility to apoptosis when siglec-9 was clustered (Fig. 9C). A hypothesis supported by these data is that when ITIM-containing siglecs on these activated leukocytes engage their endogenous sialoglycan ligands on tissues, the siglecs are clustered, resulting in leukocyte apoptosis. Direct evidence supporting this hypothesis was obtained by treating human eosinophils with a synthetic polymer carrying multiple copies of a siglec-8-binding glycan, which increased eosinophil apoptosis [58]. As corollary supporting data, polymorphisms in the siglec-8 gene are associated with susceptibility to asthma [59].
Figure 9. Siglec-induced killing of human leukocytes.
(A) Antibody-mediated siglec-8 cross-linking on human eosinophils induces apoptosis, which is enhanced by IL-5 priming. Human eosinophils were cultured with an anti-siglec-8 mAb (2E2) precomplexed with a secondary antibody to cross-link siglec-8 in the presence or absence of IL-5 (30 ng/ml). After 6 h, apoptosis was quantified by annexin-V staining. In the absence of IL-5, siglec-8 cross-linking increased apoptosis. In the presence of IL-5, spontaneous apoptosis was reduced, but siglec-8 cross-linking induced significantly more apoptosis (*P = 0.05, **P = 0.005). (B) Eosinophils from late-phase bronchoalveolar lavage fluids undergo apoptosis when exposed to anti-siglec-8 antibody. Human eosinophils were incubated with IL-5 (30 ng/ml), with or without anti-siglec-8 antibody or the same concentration of isotype-matched control antibody, and apoptosis was quantified by annexin-V staining. (C) Antibody-mediated siglec-9 cross-linking on primary human neutrophils induces cell death. Addition of the cytokine GM-CSF (25 ng/ml) reduced spontaneous cell death in the absence of anti-siglec-9 antibody and significantly increased cell death when siglec-9 was cross-linked. Data in panels (A) and (B) are from [57] with permission (copyright American Thoracic Society), and data in panel (C) are from [56] with permission (copyright American Society of Hematology).
Additional support for a role of siglecs in regulating inflammation comes from animal models. However, siglecs have evolved so rapidly that there are not clear homologs for most of them between humans and mice. It has been hypothesized that targeting of host sialoglycans by pathogens led to rapid evolutionary sialoglycan changes, followed by changes in endogenous Sia-binding proteins to keep pace [60, 61]. Several potent pathogenic viruses bind to human cell surface Sia to gain access to cells [62], whereas other pathogens, such as group B Streptococcus, express their own sialoglycans (e.g., as capsular polysaccharides) that engage and subvert human GBPs for immune evasion [52]. Although mice do not express siglec-8 or siglec-9, they express paralogs or orthologs that appear to have similar physiologic roles. Mouse siglec-F, considered a functional paralog of siglec-8 [63], is expressed on eosinophils, as well as on some tissue macrophages, and binds to the same sulfated sialoglycans as siglec-8 (see Fig. 8) as well as to more-common sialoglycans found on N-linked glycoproteins (e.g., Fig. 1B) to which siglec-8 does not bind (glycan array, http://functionalglycomics.org). In support of the regulatory function of siglecs, in most models tested, mice engineered to lack siglec-F exhibit exacerbated eosinophilic infiltration [64, 65]. Consistent with these models, mice engineered to lack a sialyltransferase responsible for biosynthesis of siglec-F ligands in the lung (St3gal3-null mice) displayed increased lung infiltration of eosinophils in an asthma model [66, 67]. Mice lacking siglec-E, considered an ortholog of siglec-9, have exacerbated neutrophil infiltration in bacterial lipopolysaccharide-induced lung inflammation [68], and administration of siglec-E cross-linking sialylated nanoparticles reduces sepsis in mice [69]. Although these are imperfect animal models, they support a role for siglecs on leukocytes and their glycan ligands on target tissues in down-regulating ongoing inflammatory responses.
As regulators of inflammation, siglecs are inviting targets for drug development [70]. Commercial and academic efforts have focused on humanized anti-siglec antibodies as well as glycomimetics [45, 69, 71]. In the former, anti-siglec-8 antibodies are entering phase I clinical trials (https://clinicaltrials.gov/ct2/show/NCT02563938), and in the latter, nanoparticles carrying both natural and synthetic glycans have been shown to target specific siglecs with therapeutic benefits in preclinical models [69, 70]. Development of siglec-targeted therapeutics as anti-cancer therapy is much more advanced. Because siglecs are expressed on cells of the myeloid lineage, they are expressed on certain cancers. Humanized mAbs to siglec-2 and siglec-3, some conjugated to drugs or toxins, are in various stages of clinical evaluation [70]. Targeting siglecs and their glycan ligands for therapeutic benefit is an active current area of research with novel opportunities anticipated as additional siglec functions and sialoglycan targets are discovered.
GLYCOSYLATION AND INFLAMMATION—A WEALTH OF OPPORTUNITIES
Selectins and siglecs are examples of a growing body of knowledge relating glycans, glycosylation, and glycan recognition to pathogen-human interactions and inflammation. Other selected brief scenarios relating glycans to inflammation follow in less detail, with references to recent reviews included to direct those interested in more depth. Given the rapidly expanding knowledge of glycans and glycan recognition in the immune system, the following are but a handful of ongoing areas of investigation.
Dectin-1 and -2 in pathogen-induced immunity
The C-type lectins are the largest family of lectins in animals [72], with several dozen members in 15 structural subfamilies in humans (http://www.imperial.ac.uk/research/animallectins). C-type lectins typically bind glycans via coordination of key hydroxyls to a bound calcium ion (e.g., E-selectin and P-selectin; Fig. 4C). However, unlike the siglecs that share a common domain structure and terminal glycan (Sia) specificity, the C-type lectin family is highly diverse both in domain architecture and glycan recognition [72]. Each C-type lectin carries a C-type lectin-like domain identified by key amino acid residues. Evolution has placed these domains in different structural contexts for different functions. Examples include the soluble trimeric mannose-binding protein in blood that binds to pathogens and initiates the lectin complement pathway required for optimal pathogen clearance [73, 74], the single-pass membrane proteins E-selectin and P-selectin (Fig. 4A) involved in cell-cell adhesion and initiation of inflammation [25], and the macrophage mannose receptor that carries 8 C-type lectin-like domains on a single transmembrane protein and is involved in serum glycoprotein homeostasis [75]. Among the C-type lectins are dectin-1 and dectin-2, which bind to pathogen-associated molecular patterns and activate the immune system [76, 77].
All living organisms have their own glycocalyx, and differences between human and pathogen glycans can be the basis for innate immunity [7]. For example, glucose polymers are common in certain pathogenic microbes but are rare or nonexistent on the human cell surface. Dectin-1 is a C-type lectin expressed primarily on human myeloid cells, including macrophages, neutrophils, dendritic cells, and B cells and eosinophils [76, 77]. It binds to β-linked glucose polymers on pathogen cell walls, especially those on pathogenic fungi, including Candida, Aspergillus, and Pneumocystis species. Dectin-1 is a cell-surface protein with a single-pass transmembrane domain leading to a cytoplasmic domain with an immunoreceptor tyrosine-based activation domain. Upon clustering, dectin-1 recruits Syk-kinase, leading to NF-κB and immune activation, including phagocytosis, respiratory burst killing, and the production/release of cytokines and chemokines. Mice engineered to lack dectin-1 (Clec7a gene) suffer from uncontrolled fungal growth in models of candidiasis and aspergillosis. Likewise, human polymorphisms in the dectin-1 gene (CLEC7A) are prone to fungal infections [78]. Dectin-1 is the eponymous member of a group of 7 human C-type lectins clustered on chromosome 12, which may be functionally related.
Dectin-2 (human CLEC6A gene) is likewise the eponymous member of its own group of 5 C-type lectins separately clustered on chromosome 12 [77]. Unlike dectin-1, it does not have an immunoreceptor tyrosine-based activation domain sequence on its cytoplasmic tail but, instead, associates laterally with an adapter protein, FcRγ, to initiate a similar immune-activating signaling pathway by myeloid cells, including tissue macrophages, neutrophils, and certain dendritic cells. Dectin-2 binds to clustered mannose residues, a property shared by some other C-type lectins involved in innate immunity. Although human N-linked glycoproteins sometimes carry clustered mannoses (Fig. 1B), they are typically limited in size and density, whereas several pathogens, including mycobacteria and fungi, express large, branched mannose polymers, which are recognized as foreign by dectin-2 to initiate immune activation [77, 79]. Experiments with mice engineered to lack dectin-2 revealed altered responses to both fungal and mycobacterial challenges [80, 81]. In another side of this immune-activating axis, mice lacking dectin-2 were less susceptible to house dust mite-induced lung inflammation, suggesting that dust-mite allergies are exacerbated by the same glycan-mediated activating mechanism [82].
Galectin-1, -3, and -9 in inflammation
Galectins are a fascinating family of GBPs [83] produced as soluble proteins in the cytoplasm, where they have intracellular functions believed to be glycan independent, such as precursor mRNA splicing. However, they are transported across the plasma membrane (by nonclassic mechanisms) into the local extracellular milieu, where they bind to glycans bearing β-linked galactose residues, including galactose-terminated, branched, N-linked glycans and poly-LacNAc termini (Fig. 10). In the immune system, galectins are expressed by many immune cells, including activated macrophages, dendritic cells, B cells, and T cells. They are also expressed by endothelial cells as well as stromal cells in many tissues. When secreted, galectins bind to glycans on the surfaces of the cells in which they are produced, to glycans on other cells in the local environment, and to extracellular-matrix glycans to modulate both innate and adaptive immunity. Their physiologic effects are striking and broad, and excellent reviews provide more depth than can be included here [83–86].
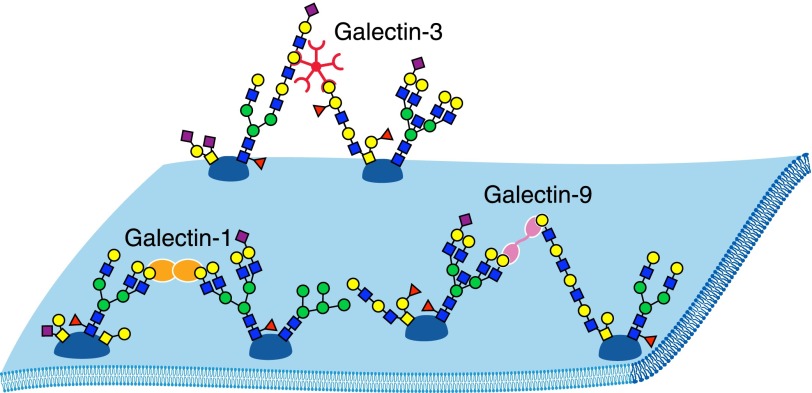
Figure 10. Galectin-mediated cell surface glycoprotein cross-linking.
Schematic representations of a spontaneously dimerizing, single-CRD galectin (galectin-1, orange); a tandem CRD galectin (galectin-9, purple); and a spontaneously pentamerizing, chimeric galectin (galectin-3, red) binding to cell-surface glycoproteins. Galectin-1 binds preferentially to branched N-glycans, galectin-3 to poly-LacNAc and blood group glycans, and galectin-9 to long poly-LacNAc repeats and branched N-glycans [83].
Galectins have a shared CRD that is evolutionarily ancient and is broadly distributed in the animal kingdom (Caenorhabditis elegans, e.g., has dozens). There are 11 human galectins that fall into 3 structural families: proteins consisting of a single CRD (∼15 kDa), those consisting of tandem CRDs connected by a short linker (∼35–40 kDa), and chimeric proteins with a single CRD attached to a multimerization domain (∼26 kDa). Single CRD galectins spontaneously form dimers, and chimeric galectins form pentamers. Because glycoproteins most often carry multiple glycans and all galectins are multivalent, the result is extended cross-linking of galectin target proteins into lattices that bring target glycoproteins into close association laterally on the cell surface [87]. This clustering can directly induce signaling, modify signaling pathways, or alter the residence time of proteins at the cell surface. A molecular dialog between glycosyltransferases that produce galectin-binding glycoproteins (writers) and galectin expression (readers) results in dynamic changes in cell-surface protein associations, turnover, and functions.
Among the galectins that modulate inflammation are galectin-1, galectin-3, and galectin-9 [86], examples of single CRD, chimeric, and tandem CRD galectins, respectively (Fig. 10). Depending on the context, each can be proinflammatory or anti-inflammatory. In some contexts, galectin-1 is immunosuppressive and anti-inflammatory. Exogenously added galectin-1 attenuates inflammation and autoimmunity in many mouse models, including induced arthritis, encephalomyelitis, uveitis, inflammatory bowel disease, graft vs. host disease, and autoimmune diabetes. In these models, galectin-1 administration is accompanied by T cell apoptosis, a loss of Th1 and Th17 cells, a skewing toward Th2-type cytokines and expansion of Foxp3+ T regulatory cells [7, 85]. Mice engineered to lack galectin-1 were more susceptible to experimental autoimmune inflammation.
Examples of a proinflammatory role for galectin-3 include the discovery of locally increased levels of galectin-3 in inflamed tissues in various mouse models and in human inflammatory disease (rheumatoid arthritis) [86]. Targeted knockout of galectin-3 in mice resulted in decreased Th1 and Th17 cells, expansion of Treg cells, and reduced severity in inflammatory models, including thioglycollate-induced peritonitis and Streptococcus pneumoniae induced lung inflammation [85].
Galectin-9 was discovered as a potent eosinophil attractant and activator and can also activate dendritic cells [84, 86]. However, in several different animal models of inflammation (including house dust mite-induced asthma), administration of galectin-9 reduced disease severity [86, 88, 89]. Among its varied functions, galectin-9 induces Treg cell differentiation and downstream suppression of inflammation. Mice lacking galectin-9 have decreased expression of Foxp3+ Treg cells, and exogenous galectin-9 stimulates Foxp3 expression in a mechanism that involves TGF-β receptor recruitment and Smad3 activation [90].
Galectins are secreted into the local inflammatory milieu, where they bind to select glycoprotein targets, induce clustering and lattice formation, and initiate downstream signaling cascades with distinctly different outcomes. Although all galectins engage β-linked galactose residues, there is considerable experimental focus on galectin specificity and endogenous glycoprotein targets, which include CD45, CD44, T cell receptor, integrins, and others [83]. During biosynthesis, these endogenous glycoprotein ligands of galectins are selectively acted upon by glycan biosynthetic enzymes to engineer galectin by glycans. The basis for selective pattern recognition by galectins is not fully understood. Both O-linked and N-linked glycans bind galectins, and branching of N-linked glycans is one mechanism that has been demonstrated to affect galectin binding [87, 91]. Attenuation of the branching of N-linked glycans by mutating key glycosyltransferases results in attenuation of galectin binding and altered physiologic outcomes. There are also differences in glycan-binding selectively [83], with galectin-1 preferring galactose-terminated, branched N-glycans, galectin-3 binding to blood group A and B structures among others, and galectin-9 preferring longer poly-LacNAc repeats. The galectin family of GBPs is an excellent example of the experimental goals of current research in functional glycobiology: 1) which glycans and glycan moieties are key to GBP specificity; 2) what are the endogenous glycoproteins, glycolipids, or proteoglycans that carry these glycans; 3) which glycosyltransferases, glycosidases, and glycan-modifying enzymes (writers) are involved in creating the target glycans, and how are these enzymes regulated; and 4) by what mechanisms does glycan binding regulate cell physiology and pathology. Given the breadth of important activities of galectins, there is much yet to learn, with clear opportunities for enhanced understanding and the discovery of new therapeutic targets.
HA-binding proteins in inflammation
Among the simplest glycan sequences in humans is HA, an unsubstituted, linear, repeating disaccharide of GlcNAc and glucuronic acid that reaches ∼4 × 106 Da [19]. HA is a prominent extracellular-matrix component throughout the body, which acts as a hydrated cushioning agent (e.g., in joints) and organizes other extracellular-matrix components [92]. HA is essential for development, tissue organization, and physiology; targeted mutation of 1 of the 3 HA biosynthetic genes (Has2) in mice results in embryonic lethality. The functions of HA are keenly dependent on its size, and tissue injury is accompanied by the accumulation of polydisperse HA fragments varying in size from thousands of daltons to hundreds of thousands [93, 94]. HA fragments are potent regulators, influencing inflammatory cell recruitment, differentiation, and activation.
HA fragmentation is characteristic of tissue pathology and may occur enzymatically by the actions of hyaluronidases or nonenzymatically by reactive oxygen species [93]. Whereas full length HA (>1000 kDa) is associated with immunosuppression, HA fragments (notably in the tens of thousands of daltons, several dozen disaccharides long) are decidedly proinflammatory [19]. They enhance the proliferation and migration of myeloid and lymphoid cells, promote maturation and inflammatory cytokine production by dendritic cells and chemokine and cytokine production by macrophages, and promote granulocyte phagocytosis and cell-mediated cytotoxicity by NK cells. These effects are mediated by a variety of leukocyte HA binding proteins, including CD44, RHAMM, and Toll-like receptors TLR2 and TLR4, among others.
A particularly fascinating aspect of HA function is the relationship between polymer length and biologic effect. Current research in this area is focused both on generation and analysis of defined-size HA fragments, as well as detailing the various receptors and mechanisms involved.
THE FUTURE IS SWEET
From initiation of inflammation to the regulation of innate and adaptive immunity at essentially every level, evolution has integrated glycans and GBPs intimately into immune responsiveness. Thorough understanding of the molecular language of immunity must include glycan-driven pattern recognition and its downstream effects. Glycoscience, which embodies both the chemistry and biology of glycans, is still at an early stage in its scientific maturity, practiced primarily by devotees of the field. Nevertheless, biologic and therapeutic opportunities continue to expand rapidly and major efforts are underway, including those by the U.S. National Institutes of Health Common Fund (https://commonfund.nih.gov/glycoscience), to enhance the tools needed to take advantage of glycobiology in medicine. A missing piece of the equation of transitioning glycobiology into the mainstream is broader knowledge of the field and its potential to address arising biomedical research challenges. The oft-repeated sentiment that glycan chemistry and biology are extraordinarily complex works against efforts to broaden knowledge and appreciation of the field. With its 9 major monosaccharide building blocks and the manageable variety of potential glycan determinants likely to be involved in recognition and regulation, the human glycome is becoming increasingly more accessible to the tools at hand. Although glycobiology affects every human tissue, nowhere is the role of glycans more evident than in infection and immunity. The tip of the spear of translational glycobiology is in this area. With glycoscience knowledge, tools, and abilities expanding, there is reason to expect that glycobiology will have an increasing role in biomedical discovery and therapeutic intervention.
AUTHORSHIP
R.L.S. wrote the manuscript.
Acknowledgments
The author was supported by the U.S. National Institutes of Health National Heart, Lung, and Blood Institute Grant P01 HL107151 through the Lung Inflammatory Disease Program of Excellence in Glycosciences (http://lidpeg.org).
Glossary
- CRD
carbohydrate recognition domain
- Fuc
fucose
- GAG
glycosaminoglycan
- Gal
galactose
- GBP
glycan-binding protein
- GlcNAc
N-acetylglucosamine
- HA
hyaluronic acid
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- LADII
leukocyte-adhesion deficiency type 2
- poly-LacNAc
poly-N-acetyllactosamine
- PSGL-1
P-selectin glycoprotein ligand 1
- Sia
sialic acid
- siglec
sialic acid-binding immunoglobulin-like lectin
- SLex
sialyl Lewis x
Disclosures
The author declares no conflicts of interest.
REFERENCES
- 1.Laine R. A. (1994) A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767. [DOI] [PubMed] [Google Scholar]
- 2.Cummings R. D., Pierce J. M. (2014) The challenge and promise of glycomics. Chem. Biol. 21, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A., Cummings R. D., Aebi M., Packer N. H., Seeberger P. H., Esko J. D., Stanley P., Hart G., Darvill A., Kinoshita T., Prestegard J. J., Schnaar R. L., Freeze H. H., Marth J. D., Bertozzi C. R., Etzler M. E., Frank M., Vliegenthart J. F., Lütteke T., Perez S., Bolton E., Rudd P., Paulson J., Kanehisa M., Toukach P., Aoki-Kinoshita K. F., Dell A., Narimatsu H., York W., Taniguchi N., Kornfeld S. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marth J. D., Grewal P. K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kooyk Y., Rabinovich G. A. (2008) Protein–glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J. L., Jones M. B., Ryan S. O., Cobb B. A. (2013) The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 34, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovich G. A., van Kooyk Y., Cobb B. A. (2012) Glycobiology of immune responses. Ann. N. Y. Acad. Sci. 1253, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzás E. I., György B., Pásztói M., Jelinek I., Falus A., Gabius H. J. (2006) Carbohydrate recognition systems in autoimmunity. Autoimmunity 39, 691–704. [DOI] [PubMed] [Google Scholar]
- 9.Varki A. (2011) Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect. Biol. 3, a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M., Varki A. (2010) The sialome—far more than the sum of its parts. OMICS 14, 455–464. [DOI] [PubMed] [Google Scholar]
- 11.Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E.. 2009. Essentials of Glycobiology. [Second Edition] Cold Spring Harbor Laboratory Press, New York. [PubMed] [Google Scholar]
- 12.Taylor M. E., Drickamer K. (2014) Convergent and divergent mechanisms of sugar recognition across kingdoms. Curr. Opin. Struct. Biol. 28, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings R. D. (2009) The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 5, 1087–1104. [DOI] [PubMed] [Google Scholar]
- 14.Rillahan C. D., Paulson J. C. (2011) Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 80, 797–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma A. S., Feizi T., Childs R. A., Chai W., Liu Y. (2014) The neoglycolipid (NGL)-based oligosaccharide microarray system poised to decipher the meta-glycome. Curr. Opin. Chem. Biol. 18, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor M. E., Drickamer K.. 2011. Introduction to Glycobiology. [Third Edition] Oxford University Press, Oxford., 283 pp. [Google Scholar]
- 17.Feizi T. E., Haltiwanger R. S. (2015) Editorial overview: carbohydrate-protein interactions and glycosylation: glycan synthesis and recognition: finding the perfect partner in a sugar-coated life. Curr. Opin. Struct. Biol. 34, vii–ix. [DOI] [PubMed] [Google Scholar]
- 18.Schnaar R. L., Gerardy-Schahn R., Hildebrandt H. (2014) Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra S., Hascall V. C., Markwald R. R., Ghatak S. (2015) Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 6, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomin V. H. (2015) Sulfated glycans in inflammation. Eur. J. Med. Chem. 92, 353–369. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita T., Fujita M. (2016) Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J. Lipid Res. 57, 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baudoin L., Issad T. (2014) O-GlcNAcylation and inflammation: a vast territory to explore. Front. Endocrinol. (Lausanne) 5, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarrazin S., Lamanna W. C., Esko J. D. (2011) Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anish C., Schumann B., Pereira C. L., Seeberger P. H. (2014) Chemical biology approaches to designing defined carbohydrate vaccines. Chem. Biol. 21, 38–50. [DOI] [PubMed] [Google Scholar]
- 25.McEver R. P. (2015) Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 107, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquardt T., Lühn K., Srikrishna G., Freeze H. H., Harms E., Vestweber D. (1999) Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 94, 3976–3985. [PubMed] [Google Scholar]
- 27.Telen M. J., Wun T., McCavit T. L., De Castro L. M., Krishnamurti L., Lanzkron S., Hsu L. L., Smith W. R., Rhee S., Magnani J. L., Thackray H. (2015) Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood 125, 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarbock A., Ley K., McEver R. P., Hidalgo A. (2011) Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somers W. S., Tang J., Shaw G. D., Camphausen R. T. (2000) Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell 103, 467–479. [DOI] [PubMed] [Google Scholar]
- 30.Leppänen A., White S. P., Helin J., McEver R. P., Cummings R. D. (2000) Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 275, 39569–39578. [DOI] [PubMed] [Google Scholar]
- 31.Kobzdej M. M., Leppänen A., Ramachandran V., Cummings R. D., McEver R. P. (2002) Discordant expression of selectin ligands and sialyl Lewis x–related epitopes on murine myeloid cells. Blood 100, 4485–4494. [DOI] [PubMed] [Google Scholar]
- 32.Nimrichter L., Burdick M. M., Aoki K., Laroy W., Fierro M. A., Hudson S. A., Von Seggern C. E., Cotter R. J., Bochner B. S., Tiemeyer M., Konstantopoulos K., Schnaar R. L. (2008) E-selectin receptors on human leukocytes. Blood 112, 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee R. T., Lee Y. C. (2000) Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconj. J. 17, 543–551. [DOI] [PubMed] [Google Scholar]
- 34.Regina Todeschini A., Hakomori S. I. (2008) Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 1780, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna S., Etzioni A. (2012) Leukocyte adhesion deficiencies. Ann. N. Y. Acad. Sci. 1250, 50–55. [DOI] [PubMed] [Google Scholar]
- 36.Etzioni A., Frydman M., Pollack S., Avidor I., Phillips M. L., Paulson J. C., Gershoni-Baruch R. (1992) Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N. Engl. J. Med. 327, 1789–1792. [DOI] [PubMed] [Google Scholar]
- 37.Lühn K., Wild M. K., Eckhardt M., Gerardy-Schahn R., Vestweber D. (2001) The gene defective in leukocyte adhesion deficiency II encodes a putative GDP–fucose transporter. Nat. Genet. 28, 69–72. [DOI] [PubMed] [Google Scholar]
- 38.Lühn K., Marquardt T., Harms E., Vestweber D. (2001) Discontinuation of fucose therapy in LADII causes rapid loss of selectin ligands and rise of leukocyte counts. Blood 97, 330–332. [DOI] [PubMed] [Google Scholar]
- 39.Freeze H. H., Chong J. X., Bamshad M. J., Ng B. G. (2014) Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 94, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang J., Patton J. T., Sarkar A., Ernst B., Magnani J. L., Frenette P. S. (2010) GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood 116, 1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Itzstein M. (2007) The war against influenza: discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 6, 967–974. [DOI] [PubMed] [Google Scholar]
- 42.Ernst B., Magnani J. L. (2009) From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 8, 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macauley M. S., Crocker P. R., Paulson J. C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266. [DOI] [PubMed] [Google Scholar]
- 45.Kiwamoto T., Kawasaki N., Paulson J. C., Bochner B. S. (2012) Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol. Ther. 135, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bochner B. S., Zimmermann N. (2015) Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J. Allergy Clin. Immunol. 135, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill A. S., van den Berg T. K., Mullen G. E. (2013) Sialoadhesin—a macrophage-restricted marker of immunoregulation and inflammation. Immunology 138, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L., Cummings R. D., Smith D. F., Huflejt M., Campbell C. T., Gildersleeve J. C., Gerlach J. Q., Kilcoyne M., Joshi L., Serna S., Reichardt N. C., Parera Pera N., Pieters R. J., Eng W., Mahal L. K. (2014) Cross-platform comparison of glycan microarray formats. Glycobiology 24, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur C. M., Cummings R. D., Stowell S. R. (2014) Using glycan microarrays to understand immunity. Curr. Opin. Chem. Biol. 18, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song X., Heimburg-Molinaro J., Cummings R. D., Smith D. F. (2014) Chemistry of natural glycan microarrays. Curr. Opin. Chem. Biol. 18, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monneau Y., Arenzana-Seisdedos F., Lortat-Jacob H. (2015) The sweet spot: how GAGs help chemokines guide migrating cells. J. Leukoc. Biol. doi:jlb.3MR0915-440R [Epub ahead of print]. [DOI] [PubMed]
- 52.Chang Y. C., Nizet V. (2014) The interplay between siglecs and sialylated pathogens. Glycobiology 24, 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schleimer R. P., Schnaar R. L., Bochner B. S. (2016) Regulation of airway inflammation by siglec-8 and siglec-9 sialoglycan ligand expression. Curr. Opin. Allergy Clin. Immunol. 16, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Reilly M. K., Paulson J. C. (2009) Siglecs as targets for therapy in immune-cell–mediated disease. Trends Pharmacol. Sci. 30, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nutku E., Aizawa H., Hudson S. A., Bochner B. S. (2003) Ligation of siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101, 5014–5020. [DOI] [PubMed] [Google Scholar]
- 56.von Gunten S., Yousefi S., Seitz M., Jakob S. M., Schaffner T., Seger R., Takala J., Villiger P. M., Simon H. U. (2005) Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106, 1423–1431. [DOI] [PubMed] [Google Scholar]
- 57.Nutku-Bilir E., Hudson S. A., Bochner B. S. (2008) Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am. J. Respir. Cell Mol. Biol. 38, 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson S. A., Bovin N. V., Schnaar R. L., Crocker P. R., Bochner B. S. (2009) Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J. Pharmacol. Exp. Ther. 330, 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao P. S., Shimizu K., Grant A. V., Rafaels N., Zhou L. F., Hudson S. A., Konno S., Zimmermann N., Araujo M. I., Ponte E. V., Cruz A. A., Nishimura M., Su S. N., Hizawa N., Beaty T. H., Mathias R. A., Rothenberg M. E., Barnes K. C., Bochner B. S. (2010) Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (siglec-8) gene are associated with susceptibility to asthma. Eur. J. Hum. Genet. 18, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padler-Karavani V., Hurtado-Ziola N., Chang Y. C., Sonnenburg J. L., Ronaghy A., Yu H., Verhagen A., Nizet V., Chen X., Varki N., Varki A., Angata T. (2014) Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related siglecs in primates. FASEB J. 28, 1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angata T., Margulies E. H., Green E. D., Varki A. (2004) Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. U. S. A. 101, 13251–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stencel-Baerenwald J. E., Reiss K., Reiter D. M., Stehle T., Dermody T. S. (2014) The sweet spot: defining virus–sialic acid interactions. Nat. Rev. Microbiol. 12, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tateno H., Crocker P. R., Paulson J. C. (2005) Mouse siglec-F and human siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis x as a preferred glycan ligand. Glycobiology 15, 1125–1135. [DOI] [PubMed] [Google Scholar]
- 64.Zhang M., Angata T., Cho J. Y., Miller M., Broide D. H., Varki A. (2007) Defining the in vivo function of siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 109, 4280–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMillan S. J., Richards H. E., Crocker P. R. (2014) Siglec-F-dependent negative regulation of allergen-induced eosinophilia depends critically on the experimental model. Immunol. Lett. 160, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiwamoto T., Brummet M. E., Wu F., Motari M. G., Smith D. F., Schnaar R. L., Zhu Z., Bochner B. S. (2014) Mice deficient in the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J. Allergy Clin. Immunol. 133, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzukawa M., Miller M., Rosenthal P., Cho J. Y., Doherty T. A., Varki A., Broide D. (2013) Sialyltransferase ST3Gal-III regulates siglec-F ligand formation and eosinophilic lung inflammation in mice. J. Immunol. 190, 5939–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McMillan S. J., Sharma R. S., McKenzie E. J., Richards H. E., Zhang J., Prescott A., Crocker P. R. (2013) Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood 121, 2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spence S., Greene M. K., Fay F., Hams E., Saunders S. P., Hamid U., Fitzgerald M., Beck J., Bains B. K., Smyth P., Themistou E., Small D. M., Schmid D., O’Kane C. M., Fitzgerald D. C., Abdelghany S. M., Johnston J. A., Fallon P. G., Burrows J. F., McAuley D. F., Kissenpfennig A., Scott C. J. (2015) Targeting siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 7, 303ra140. [DOI] [PubMed] [Google Scholar]
- 70.Angata T., Nycholat C. M., Macauley M. S. (2015) Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol. Sci. 36, 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rillahan C. D., Schwartz E., McBride R., Fokin V. V., Paulson J. C. (2012) Click and pick: identification of sialoside analogues for siglec-based cell targeting. Angew. Chem. Int. Ed. Engl. 51, 11014–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drickamer K., Taylor M. E. (2015) Recent insights into structures and functions of C-type lectins in the immune system. Curr. Opin. Struct. Biol. 34, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. (2009) Mannose-binding lectin and innate immunity. Immunol. Rev. 230, 9–21. [DOI] [PubMed] [Google Scholar]
- 74.De Pascale G., Cutuli S. L., Pennisi M. A., Antonelli M. (2013) The role of mannose-binding lectin in severe sepsis and septic shock. Mediators Inflamm. 2013, 625803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang W. H., Aziz P. V., Heithoff D. M., Mahan M. J., Smith J. W., Marth J. D. (2015) An intrinsic mechanism of secreted protein aging and turnover. Proc. Natl. Acad. Sci. U. S. A. 112, 13657–13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drummond R. A., Brown G. D. (2013) Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 9, e1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dambuza I. M., Brown G. D. (2015) C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferwerda B., Ferwerda G., Plantinga T. S., Willment J. A., van Spriel A. B., Venselaar H., Elbers C. C., Johnson M. D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morré S. A., Vriend G., Williams D. L., Perfect J. R., Joosten L. A., Wijmenga C., van der Meer J. W., Adema G. J., Kullberg B. J., Brown G. D., Netea M. G. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barreto-Bergter E., Figueiredo R. T. (2014) Fungal glycans and the innate immune recognition. Front. Cell. Infect. Microbiol. 4, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yonekawa A., Saijo S., Hoshino Y., Miyake Y., Ishikawa E., Suzukawa M., Inoue H., Tanaka M., Yoneyama M., Oh-Hora M., Akashi K., Yamasaki S. (2014) Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 41, 402–413. [DOI] [PubMed] [Google Scholar]
- 81.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S. H., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. (2010) Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. [DOI] [PubMed] [Google Scholar]
- 82.Norimoto A., Hirose K., Iwata A., Tamachi T., Yokota M., Takahashi K., Saijo S., Iwakura Y., Nakajima H. (2014) Dectin-2 promotes house dust mite–induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 51, 201–209. [DOI] [PubMed] [Google Scholar]
- 83.Rabinovich G. A., Toscano M. A. (2009) Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352. [DOI] [PubMed] [Google Scholar]
- 84.Rabinovich G. A., Toscano M. A., Ilarregui J. M., Baum L. G.. 2009. Galectins as novel regulators of immune cell homeostasis and inflammation. In Animal Lectins: a functional view. (Vasta G. R., Ahmed H., eds.) Taylor & Francis Group, LLC, Boca Raton, FL, 397–416. [Google Scholar]
- 85.Blidner A. G., Méndez-Huergo S. P., Cagnoni A. J., Rabinovich G. A. (2015) Re-wiring regulatory cell networks in immunity by galectin–glycan interactions. FEBS Lett. 589, 3407–3418. [DOI] [PubMed] [Google Scholar]
- 86.Norling L. V., Perretti M., Cooper D. (2009) Endogenous galectins and the control of the host inflammatory response. J. Endocrinol. 201, 169–184. [DOI] [PubMed] [Google Scholar]
- 87.Nabi I. R., Shankar J., Dennis J. W. (2015) The galectin lattice at a glance. J. Cell Sci. 128, 2213–2219. [DOI] [PubMed] [Google Scholar]
- 88.Liu F. T., Yang R. Y., Hsu D. K. (2012) Galectins in acute and chronic inflammation. Ann. N. Y. Acad. Sci. 1253, 80–91. [DOI] [PubMed] [Google Scholar]
- 89.Wiersma V. R., de Bruyn M., Helfrich W., Bremer E. (2013) Therapeutic potential of galectin-9 in human disease. Med. Res. Rev. 33(Suppl 1), E102–E126. [DOI] [PubMed] [Google Scholar]
- 90.Wu C., Thalhamer T., Franca R. F., Xiao S., Wang C., Hotta C., Zhu C., Hirashima M., Anderson A. C., Kuchroo V. K. (2014) Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41, 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miwa H. E., Song Y., Alvarez R., Cummings R. D., Stanley P. (2012) The bisecting GlcNAc in cell growth control and tumor progression. Glycoconj. J. 29, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monslow J., Govindaraju P., Puré E. (2015) Hyaluronan—a functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 6, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh S., Hoselton S. A., Dorsam G. P., Schuh J. M. (2015) Hyaluronan fragments as mediators of inflammation in allergic pulmonary disease. Immunobiology 220, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang J., Jiang D., Noble P. W. (2016) Hyaluronan as a therapeutic target in human diseases. Adv. Drug Deliv. Rev. 97, 186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varki A., Etzler M. E., Cummings R. D., Esko J. D.. 2009. Discovery and classification of glycan-binding proteins. In Essentials of Glycobiology. (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds.) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 375–386. [PubMed] [Google Scholar]
- 96.Attrill H., Imamura A., Sharma R. S., Kiso M., Crocker P. R., van Aalten D. M. (2006) Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. J. Biol. Chem. 281, 32774–32783. [DOI] [PubMed] [Google Scholar]