Abstract
Cell division in the fission yeast Schizosaccharomyces pombe yields two equal-sized daughter cells. Medial fission is achieved by deposition of a primary septum flanked by two secondary septa within the dividing cell. During the final step of cell division, cell separation, the primary septum is hydrolyzed by an endo-(1,3)-β-glucanase, Eng1p. We reasoned that the cell wall material surrounding the septum, referred to here as the septum edging, also must be hydrolyzed before full separation of the daughter cells can occur. Because the septum edging contains (1,3)-α-glucan, we investigated the cellular functions of the putative (1,3)-α-glucanases Agn1p and Agn2p. Whereas agn2 deletion results in a defect in endolysis of the ascus wall, deletion of agn1 leads to clumped cells that remained attached to each other by septum-edging material. Purified Agn1p hydrolyzes (1,3)-α-glucan predominantly into pentasaccharides, indicating an endo-catalytic mode of hydrolysis. Furthermore, we show that the transcription factors Sep1p and Ace2p regulate both eng1 and agn1 expression in a cell cycle-dependent manner. We propose that Agn1p acts in concert with Eng1p to achieve efficient cell separation, thereby exposing the secondary septa as the new ends of the daughter cells.
INTRODUCTION
Cell separation is the final step of the cell cycle in yeasts and results in an irreversible separation of two independent cells, a mother and daughter cell in budding yeasts or two equal-sized daughter cells in fission yeasts. Cell separation occurs after division of the nucleus (mitosis) and division of the cytoplasm (cytokinesis) have successively been completed. In yeasts, cytokinesis involves formation of a division septum, which is synthesized within the confines of the dividing cell. Subsequent cell separation involves partial dissolution of this division septum. Importantly, the division septum is composed mainly of the same types of polysaccharides that constitute the structural framework of the extracellular matrix (cell wall), which maintains cellular integrity (Hochstenbach et al., 1998; Klis et al., 2002). The molecular mechanisms that are responsible for dissolution of the division septum while maintaining structural integrity are not understood. An attractive model yeast to study this question is the fission yeast Schizosaccharomyces pombe, because its complete genome has been sequenced, and mutants with defects in cell separation can be isolated or engineered.
Septum formation requires two separate but interdependent processes, namely, formation of a contractile actomyosin ring in the equatorial plane of the dividing cell and deposition of the septum (Chang, 2001; McCollum and Gould, 2001). At the end of anaphase, after formation of the actomyosin ring and segregation of the sister chromatids, a signaling cascade called the septation initiation network in S. pombe initiates constriction of the actomyosin ring and concomitant synthesis of the primary septum in a centripetal direction (Le Goff et al., 1999; McCollum and Gould, 2001). In S. pombe, the primary septum consists of linear (1,3)-β-glucan, and its synthesis involves the (1,3)-β-glucan synthase Cps1p (Liu et al., 1999; Humbel et al., 2001), whereas in the budding yeast Saccharomyces cerevisiae, the primary septum consists of chitin, and its synthesis involves the chitin synthase Chs2p (Cabib et al., 2001; Roh et al., 2002). Secondary septa are deposited at both sides of the growing primary septum, starting at the base and becoming progressively thicker as the primary septum develops (Johnson et al., 1973).
The molecular mechanisms of cell separation and their regulation are poorly understood. In S. cerevisiae, an endo-chitinase, ScCts1p, is involved in hydrolysis of the primary septum, whereas an endo-(1,3)-β-glucanase, ScEng1p, also has been shown to be involved in septum hydrolysis (Kuranda and Robbins, 1991, Baladrón et al., 2002). Expression of both hydrolases is regulated by a cascade of transcription factors, including two members of the HNF-3/forkhead family, ScFkh1p, and ScFkh2p (Zhu et al., 2000), in addition to the C2H2 zinc-finger transcription factor ScAce2p (Dohrmann et al., 1992; Spellman et al., 1998; Baladrón et al., 2002). In S. pombe, a homolog of ScFkh1p and ScFkh2p, as well as a homolog of ScAce2p, have been identified, denoted Sep1p and Ace2p, respectively (Sipiczki et al., 1993; Ribár et al., 1997; Martín-Cuadrado et al., 2003). Deletion of sep1+ or ace2+ caused a severe cell-separation defect with cells displaying a hyphal morphology. Furthermore, Martín-Cuadrado et al. (2003) showed recently that S. pombe expresses a homolog of ScEng1p, Eng1p, which is involved in hydrolysis of the primary septum and is regulated by Ace2p. Whether eng1+ expression is regulated also by Sep1p is not known.
Cell separation must involve not only dissolution of the primary septum but also dissolution of the cell wall material that surrounds the septum, referred to here as the septum edging to distinguish it from the septum proper. Because the septum edging is cell wall material of the dividing cell, it consists mainly of (1,3)-β-glucan and (1,3)-α-glucan. Although dissolution of the primary septum is known to involve the (1,3)-β-glucanase Eng1p, it remains unclear how the septum edging is dissolved. As (1,3)-α-glucan is present in the septum edging but absent from the primary septum, we studied two genes encoding putative (1,3)-α-glucanases, denoted agn1+ (systematic gene name, SPAC14C4.09) and agn2+ (SPBC646.06c). We demonstrate that Agn1p functions as an endo-(1,3)-α-glucanase that hydrolyzes septum-edging material, and we conclude that localized hydrolysis of cell wall α-glucan plays an integral part in the cell cycle of S. pombe.
MATERIALS AND METHODS
Strains and Culture Media
Mutant strains were derived from S. pombe wild type, 972 (Table 1). Cells were grown in YEA medium (Hochstenbach et al., 1998) or in EMM2 medium (Moreno et al., 1991) with addition of 250 mg/l adenine sulfate (EMMA). GEMMA was EMMA with addition of 2% (wt/vol) sodium d-gluconic acid instead of d-glucose, adjusted to pH 4.0 by using 2 M phosphoric acid. In a conjugation and sporulation assay, cells were crossed on agar plates containing ME medium at 25°C for 2 d and treated with iodine vapor to test for ascospore formation. Diploids were selected on EMM2 medium (Moreno et al., 1991).
Table 1.
S. pombe strains used in this study
| Strain | Genotype |
|---|---|
| 972 | h- |
| cdc10ts | h- cdc10-129 |
| cdc25ts | h- cdc25-22 |
| FYC11 | h- ade6-M210 |
| FYC15 | h+ ade6-M216 |
| MBY580 | h- cps1-191 ade6-M210 leu1-32 ura4-D18 lys1-131 |
| ND001 | h- agn1Δ::kanMX4 |
| ND003 | h- agn1::13myc-kanMX6 |
| ND005 | h- eng1Δ::kanMX4 |
| ND007 | h- eng1::13myc-kanMX6 |
| ND019 | h- agn1Δ::kanMX4 eng1Δ::kanMX4 |
| ND028 | h- agn2Δ::kanMX4 |
| ND049 | h- cdc25-22 agn1::13myc-kanMX6 |
| ND051 | h- cdc25-22 eng1::13myc-kanMX6 |
| ND054 | h- cdc10-129 agn1::13myc-kanMX6 |
| ND061 | h- agn2::13myc-kanMX6 |
| ND064 | h- ace2Δ::kanMX4 |
| ND080 | h-/h+ ade6-M210/ade6-M216 |
| ND084 | h-/h+ agn1Δ::kanMX4/agn1Δ::kanMX4 ade6-M210/ade6-M216 |
| ND088 | h-/h+ agn2Δ::kanMX4/agn2Δ::kanMX4 ade6-M210/ade6-M216 |
| ND102 | h- sep1Δ::kanMX4 |
| ND111 | h- cdc25-22 ace2Δ::kanMX4 agn1::13myc-kanMX6 |
| ND132 | h- ace2Δ::kanMX4 agn1::13myc-kanMX6 |
| ND136 | h- ace2Δ::kanMX4 eng1::13myc-kanMX6 |
| ND140 | h- sep1Δ::kanMX4 agn1::13myc-kanMX6 |
| ND144 | h- sep1Δ::kanMX4 eng1::13myc-kanMX6 |
| ND148 | h- ace2Δ::kanMX4 sep1Δ::kanMX4 |
| ND154 | h- cps1-191 agn1::13myc-kanMX6 |
| ND172 | h- agn2::GFPS65T-kanMX6 ade6-M210 |
| ND218 | h- ace2Δ::kanMX4 sep1Δ::kanMX4 eng1::13myc-kanMX6 |
| ND234 | h- agn1Δ::kanMX4 ura4-D18 [pND02] |
| ND235 | h- agn1Δ::kanMX4 ura4-D18 [pSE01] |
| ND236 | h- ura4-D18 [pND02] |
All strains were constructed for this study, except strain 972 (P. Nurse) strains FYC11, FYC15, cdc10ts, and cdc25ts (R. Dahr), and strain MBY580 (M. Balasubramanian).
DNA Sequencing
For agn1+ and agn2+ sequencing (GenBank accession nos. AY626901 and AY626902, respectively), total RNA was isolated from vegetatively grown 972 cells or sporulating ND080 cells, respectively (Koerkamp et al., 2002). cDNA of a part of the agn1+ 5′-untranslated region, the open reading frame (ORF), and 3′-untranslated region was synthesized by polymerase chain reaction (PCR), by using pfu polymerase (Stratagene, La Jolla, CA). For agn2+ sequencing, cDNA of the agn2+ 5′-untranslated region, ORF, and 3′-untranslated region was synthesized in three separate PCR amplification reactions. These PCR amplification products were used for direct DNA sequencing (BDT sequencing kit; Applied Biosystems, Foster City, CA). The sequence of the 3′-end of the agn2 ORF also was confirmed by direct DNA sequencing of several PCR amplification products obtained for the region of interest using genomic DNA from strain 972 as template (our unpublished data).
Disruption and Tagging
Using a PCR-mediated strategy (Bähler et al., 1998), ORFs of agn1+ (systematic gene name, SPAC14C4.09), agn2+ (SPBC646.06c), eng1+ (SPAC821.09), ace2+ (SPAC6G10.12C), and sep1+ (SPBC4C3.12), were replaced by a loxP-kanMX4-loxP cassette (pUG6; Güldener et al., 1996). agn1+ and eng1+ ORFs were tagged at their 3′ ends with the 13myc-kanMX6 cassette (Bähler et al., 1998). Hot-start PCR amplification products were obtained in 28 cycles by using Taq polymerase (TaKaRa). Transformations were performed using a lithium-acetate method (Bähler et al., 1998), and cells were allowed to recover for 30 h at 28°C, followed by replica plating onto YEA plates containing 200 μg/ml Geneticin (Invitrogen, Carlsbad, CA). PCR analyses were used to verify correct integration, and tetrad analyses were used to confirm single integration events. Double and triple mutants were generated using tetrad dissection, and genotypes were rechecked by PCR analyses.
Cloning and Overexpression of agn1+
For overexpression of agn1+ from its chromosomal locus, a kanMX6–3nmt1 cassette was integrated in front of the ORF by homologous recombination (Bähler et al., 1998). For agn1p-his overexpression from a plasmid, the agn1 ORF was amplified from genomic DNA of strain 972 and cloned into plasmid pSE01, a pREP4-based plasmid (Maundrell, 1993) containing sequences encoding a protease X cleavage site followed by six histidines. After verifying the agn1 ORF sequence, the resulting plasmid, pND02, was transformed into a strain with genotype h- ura4-D18 by using a lithium acetate method (Akada et al., 2000).
Synchronization and Immunoblotting
For synchronization, cdc25-22 strains were grown at 25°C to an optical density at 595-nm wavelength (OD595) of 0.3 and shifted to 37°C for 4 h. Then, cells were released into the cell cycle by quick transfer to 25°C. Septation index was determined by calculating the percentage of cells with a septum after calcofluor-white staining (Fluostain I; F-0386; Sigma-Aldrich, St. Louis, MO). Cells were taken up in 100 mM Tris-HCl, pH 7.6, containing 1 mM phenylmethylsulfonyl fluoride (PMSF), and broken with washed glass beads by using a FastPrep 120 (Bio 101, Vista, CA) at speed 5.5 for two intervals of 10 s. Protein concentrations were measured using the Bradford method with bovine serum albumin as a standard. Total cell lysates were resolved at 6 μg of protein per lane on a SDS/8% polyacrylamide gel under reducing conditions, blotted onto nitrocellulose membranes (0.45 μm; Schleicher & Schuell, Keene, NH), and probed with anti-myc monoclonal antibody (mAb) 9E10 (Evan et al., 1985) and an anti-α-tubulin antibody (T-5168; Sigma-Aldrich). Blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (BioRad, Hercules, CA) and developed by chemiluminescence (ECL kit; Amersham Biosciences, Piscataway, NJ).
Microscopy
For light microscopy, cells were stained with calcofluor white and embedded in 2% (wt/vol) low-melting agarose. Micrographs were obtained using an Axiophot 2 microscope (Carl Zeiss, Thornwood, NY) equipped with a Coolsnap HQ digital camera (Photometrics, Tucson, AZ). For transmission electron microscopy, cells were fixed with potassium permanganate (Hochstenbach et al., 1998). Thin sections were stained with lead citrate (Reynolds, 1963) and uranyl acetate, and micrographs were obtained using a model 420 transmission electron microscope (Philips, Eindhoven, The Netherlands) operating at 80 kV.
Sedimentation Assay
Cells were grown in YEA medium in an orbital shaker at 200 rpm at 28°C to an OD595 of 1. Subsequently, 1 ml of culture was transferred into a cuvette (Sarstedt 67.742), gently mixed, and placed without further disturbance in an Ultrospec III spectrophotometer (Pharmacia AB, Uppsala, Sweden). OD595 was registered at 2.5-min intervals over a period of 30 min.
Purification of Agn1p-his
Agn1p-his expression was induced in GEMMA for 24 h. Culture medium was concentrated 10-fold under N2 pressure at 4°C by using a polyethersulfone filter (10 kDa cut-off; Millipore, Billerica, MA), and its pH was changed to 8.0 by addition of 100 mM MOPS buffer. Then, supernatant was incubated at 4°C for 1 h with nickel-nitrilotriacetic acid beads (QIAGEN, Valencia, CA). Beads were washed with 300 mM NaCl, 50 mM NaH2PO4, pH 8.0, buffer (buffer A), containing 15 mM imidazole. Agn1p-his was eluted with buffer A containing 250 mM imidazole, and fractions with (1,3)-α-glucanase activity were pooled. After exchanging buffer A for 20 mM sodium acetate, pH 5.0, by using a gel-filtration column (PD-10; Amersham Biosciences), the eluate was loaded onto an anion-exchange column (Mono-Q; Amersham Biosciences). Agn1p-his was eluted using a linear gradient of KCl to 250 mM, and fractions with highest (1,3)-α-glucanase activity were used for further study. Size exclusion chromatography was performed at a flow rate of 0.5 ml/min, by using a Superdex-75 column (Amersham Biosciences) equilibrated with 50 mM sodium acetate, pH 6.0, and calibrated with low-molecular-mass markers (Amersham Biosciences) and blue dextran.
Purification of MutAp
Fifty mg of Novozym (L-2265: Sigma-Aldrich) was dissolved in 50 mM Tris-HCl, pH 7.6, containing 1 mM PMSF and 10 μl of protease inhibitor cocktail (P-8215; Sigma-Aldrich) and run over an anion-exchange column (DEAE; Amersham Biosciences) equilibrated with 50 mM Tris-HCl, pH 7.6. Flow-through fractions containing (1,3)-α-glucanase activity were pooled. For adsorption chromatography, buffer was exchanged for 5 mM sodium azide, 50 mM sodium acetate, pH 5.6 (buffer B), and 0.85 mM PMSF and 10 μl of protease inhibitor cocktail were freshly added. Twenty mg of (1,3)-α-glucan (Pleurotus ostreatus) was dissolved in 10 M HCl, incubated at 37°C for 15 min, and reprecipitated overnight by addition of ice-cold 1.25 M sodium acetate and neutralization with 10 M NaOH. Then, MutAp was bound to (1,3)-α-glucan in buffer B containing 10% (vol/vol) ethylene glycol and 250 mM NaCl at 4°C for 45 min, washed, and eluted by a 5-min incubation in 80% (vol/vol) ethylene glycol in buffer B at room temperature. Finally, ethylene glycol was removed using a PD-10 column equilibrated with buffer B.
Isolation of (1,3)-α-Glucan
Fruiting bodies of P. ostreatus or Laetiporus sulfureus were minced in a blender and boiled in 2% (wt/vol) SDS, 40 mM 2-mercaptoethanol for 15 min to extract cytosolic contaminants. To precipitate the alkali-soluble, water-insoluble fraction, cell wall material was dissolved in 2 mM NaBH4, 2 M KOH for 30 min on ice and reprecipitated overnight at 4°C by adjusting the pH to 6 with acetic acid. Then, the pellet was resuspended in 5 mM sodium azide, 40 mM 2-mercaptoethanol, 50 mM citrate-phosphate buffer, pH 5.3, containing 45 mg of Zymolyase-100T (Seikagaku America, Rockville, MD) and incubated overnight at 37°C. After collection, the insoluble fraction was reextracted as described above and washed in 5 mM sodium azide.
Mass Spectrometry
For matrix-assisted laser desorption ionization analysis, protein spots were excised from a Coomassie-stained SDS/8% polyacrylamide gel and treated as described previously (Shevchenko et al., 1996). Dried peptides, cleaned with ZipTipC18 (Millipore), were resuspended in 5 μl 60% (vol/vol) acetonitrile, 1% (vol/vol) formic acid. Approximately 4 μg of protein also was digested directly with 2 μg of endoproteinase Glu-C (V8 protease) (Roche Diagnostics, Indianapolis, IN) overnight in 50 mM sodium phosphate, pH 7.9, with 5% (vol/vol) acetonitrile at 25°C. Subsequently, the peptide solution was alkylated (Shevchenko et al., 1996), cleaned, and concentrated on a ZipTipC18 (Millipore), and eluted in 10 μl 60% (vol/vol) acetonitrile, 1% (vol/vol) formic acid. Peptide solutions were mixed with an equal volume of 52 mM (aceton-prewashed) α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich) in 50% (vol/vol) ethanol, 48% (vol/vol) acetonitrile, 2% (vol/vol) trifluoroacetic acid, 1 mM ammonium acetate; spotted on a target plate; and dried at room temperature. Mass spectrometry spectra were collected on a MALDI R (Micromass, Wythenshawe, United Kingdom). For tandem mass spectrometry analysis, V8-protease digests were analyzed on a quadrupole time-of-flight (Q-TOF) mass spectrometer (Micromass). Low-energy collision-induced dissociation was performed using argon as a collision gas.
Enzyme Reactions
Substrate specificity was determined by an incubation at 37°C for 20 min of ∼2 pmol of Agn1p-his with substrates at a final concentration of 4 mg/ml in 5 mM sodium azide, 50 mM sodium acetate, pH 5.6. Reduced and carboxymethylated (1,3)-α-glucan (L. sulfureus), (1,4)(1,6)-α-glucan (starch; 85642; Fluka, Buchs, Switzerland), reduced (1,4)(1,6)-α-glucan (glycogen; G-8751; Sigma-Aldrich), reduced (1,4)(1,6)-α-glucan (pullulan; P-4516; Sigma-Aldrich), reduced (1,6)-α-glucan (dextran; D-9260; Sigma-Aldrich), reduced (1,3)-β-glucan (laminarin; 61430; Fluka), carboxymethylated (1,4)-β-glucan (cellulose; C-5678; Sigma-Aldrich), or reduced (1,3)-α-mannan (mannan; M-7504; Sigma-Aldrich) were used as substrates. O-(Carboxy)methylation and polysaccharide reduction were performed as described previously (Takasaki and Kobata, 1978; Kiho et al., 1989). Reaction products were analyzed using a colorimetric assay (Lever, 1972).
For high-performance anion-exchange chromatography (HPAEC), (1,3)-α-glucan (0.5 mg) purified from S. pombe cell walls was incubated with Agn1p-his or MutAp in 5 mM sodium azide, 50 mM sodium acetate, pH 5.6, at 37°C for 5 h. As standards, oligosaccharides from S. pombe (1,3)-α-glucan were prepared as described previously (Koizumi et al., 1989). HPAEC was performed using a CarboPac PA-1 column on a DX 500 system (Dionex, Sunnyvale, CA) by using a linear gradient from 0 to 500 mM sodium acetate in 0.1 M NaOH at a flow rate of 1.0 ml/min.
RESULTS
Agn1p Contains a Signal Sequence for Secretion, whereas Agn2p Does Not
Annotation of the S. pombe genome sequence suggested that the agn1+ and agn2+ genes each contain one continuous ORF (Wood., 2002). This prediction was confirmed by our analysis of the transcripts. agn1+ cDNA contained a 5′-untranslated region of at least 136 base pairs and a 3′-untranslated region of 422 base pairs, whereas agn2+ cDNA contained a 5′-untranslated region of 157 base pairs and a 3′-untranslated region of 122 base pairs. Analysis of their ORFs showed that agn1+ and agn2+ encode polypeptides of 424 and 433 amino acids, respectively, with calculated molecular masses of 46.9 and 48.0 kDa (Figure 1). These predicted polypeptides contain 22 additional amino acids at the amino terminus of Agn1p and 26 additional amino acids at the carboxy terminus of Agn2p beyond those predicted by the original genome-project annotation. These polypeptides share 39% amino acid sequence identity, suggesting that they are paralogs. Furthermore, Agn1p and Agn2p show 41 and 34% amino acid sequence identity, respectively, to the catalytic domain (residues 38–461) of the known Trichoderma harzianum (1,3)-α-glucanase MutAp, the founding member of glycoside hydrolase family 71 (Fuglsang et al., 2000), identifying Agn1p and Agn2p as putative (1,3)-α-glucanases. This prediction is consistent with the absence of Agn homologues in S. cerevisiae and Candida albicans (our unpublished data), both of which lack cell wall (1,3)-α-glucan. However, Agn1p and Agn2p lack sequences corresponding to the carboxy-terminal Pro-Ser-Thr-rich linker (residues 462–550) and polysaccharide-binding domain (residues 551–634) present in MutAp (Figure 1).
Figure 1.
S. pombe Agn1 and Agn2 polypeptides show amino acid sequence identities to the catalytic domain of T. harzianum MutAp. cDNA-derived sequences for Agn1p and Agn2p were aligned with that of MutAp (AAF27911). Solid arrowheads indicate cleavage sites in Agn1p and MutAp for signal peptidase predicted by the SIGNALP algorithm, whereas the open arrowhead indicates the start of mature MutA protein observed by Fuglsang et al. (2000). Boxes indicate sequence identities and dashes indicate gaps in the PILEUP alignment.
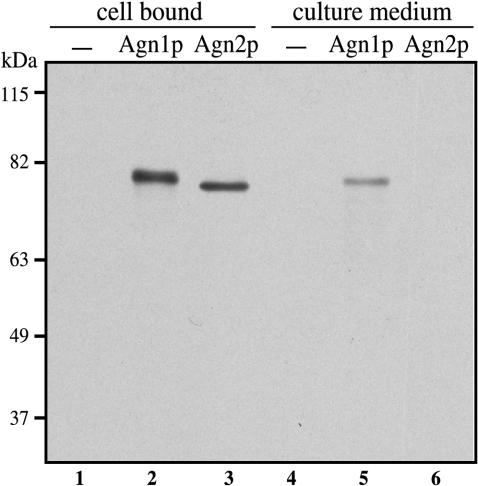
A hydropathy profile of the Agn1 polypeptide reveals a hydrophobic region at its amino terminus (residues 1–20) that is predicted to function as a cleavable signal sequence (Kyte and Doolittle, 1982; Nielsen et al., 1997). However, a signal sequence was found to be absent from the Agn2 polypeptide. To study secretion of Agn1p and Agn2p directly, we tagged these proteins at their carboxy terminus and performed an immunoblot analysis on exponentially growing agn1-myc and agn2-myc cells. In total cell lysates, Agn1-myc and Agn2-myc protein bands resolved at apparent molecular masses of ∼78 and ∼76 kDa, respectively (Figure 2, lanes 2 and 3), which are in agreement with the calculated molecular masses of 67.4 and 68.5 kDa. Immunoblot analysis on corresponding culture media revealed the presence of Agn1-myc protein in culture medium of agn1-myc cells, whereas no Agn2-myc protein was observed in culture medium of agn2-myc cells (Figure 2, compare lanes 5 and 6). Comparison of the cell-bound fraction with the culture-medium fraction revealed that the majority of steadystate Agn1-myc protein was bound to cells (Figure 2, compare lanes 2 and 5). Our data demonstrate that, unlike Agn2p, Agn1p shares a functional signal sequence with characterized MutA proteins of T. harzianum and Penicillium purpurogenum, both of which also are secreted (Fuglsang et al., 2000).
Figure 2.
Agn1p is secreted into the culture medium, whereas Agn2p is not. Along with wild-type cells (strain 972; lanes 1 and 4), cells of genotypes agn1-myc (strain ND003; lanes 2 and 5) or agn2-myc (strain ND061; lanes 3 and 6) were grown to midexponential phase in YEA medium. Equivalent amounts of cell-bound fractions were prepared by collecting cells, whereas culture medium fractions were obtained by precipitation on ice with 5% (wt/vol) trichloroacetic acid. Samples were resolved by 8% SDS-PAGE under reducing conditions and visualized by immunoblot analysis using an anti-myc mAb, 9E10. Probing a similar blot with antibodies directed to cytoplasmic protein α-tubulin showed protein in the cell-bound samples, but not in the culture medium samples, from all three strains (our unpublished data).
Deletion of agn1+ Causes Cells to Clump
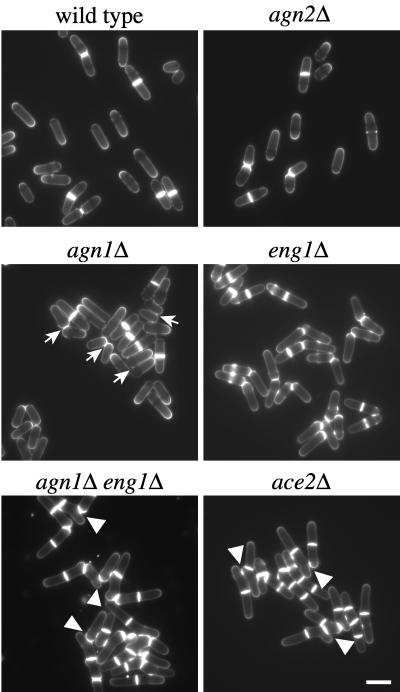
To study the cellular function of Agn1p, we deleted the complete agn1+ ORF from the genome of wild type, 972, and analyzed the phenotype of the resulting deletion mutant. agn1Δ cells were viable and displayed a typical cylindrical rod morphology (Figure 3, compare agn1Δ with wild type). However, rather than single cells, the agn1Δ mutant often formed small clumps of cells attached to each other via an area located near their tips (Figure 3, arrows). These data indicate that agn1Δ cells fail to separate effectively at the end of the cell cycle.
Figure 3.
agn1Δ cells clump as a result of inefficient cell separation. Along with wild-type cells (strain 972), cells of genotypes agn2Δ (strain ND028), agn1Δ (strain ND001), eng1Δ (strain ND005), agn1Δ eng1Δ (strain ND019), or ace2Δ (strain ND064) were grown in EMMA medium to midexponential phase and stained with calcofluor white to visualize septa. Note that agn1Δ cells remain attached via a small area near the base of their tips (arrows) and that branching is observed in the agn1Δ eng1Δ and ace2Δ strains (arrowheads). Bar, 10 μm.
Cell clumping has been observed not only with agn1Δ cells but also with cells deleted for eng1+ or ace2+ (Martín-Cuadrado et al., 2003). To compare these clumping phenotypes, we deleted the eng1+ and ace2+ ORFs individually and verified the phenotypes of the resulting strains (Figure 3). Although eng1Δ and ace2Δ cells, like agn1Δ cells, formed clumps, the extent of cell clumping seemed to be most severe for the ace2Δ mutant in light microscopy. To compare the extents of clumping more quantitatively, we set up a simple sedimentation assay (see MATERIALS AND METHODS). Wild-type cells hardly sedimented during a 30-min time period, whereas ace2Δ cells sedimented rapidly, thereby clearing the culture medium to a large extent (Table 2). agn1Δ and eng1Δ cells, on the other hand, sedimented with intermediate velocities, indicating that they each formed clumps that were generally smaller than those formed by the ace2Δ mutant (Table 2). Together, these data indicate that loss of agn1+ function causes S. pombe cells to clump to a similar extent as loss of eng1+ function.
Table 2.
Sedimentation analysis of mutants with a clumping phenotype
| Sedimentation timeb
|
|||
|---|---|---|---|
| Genotypea | Strain | 80% of initial OD595 | 50% of initial OD595 |
| Wild type | 972 | >30 | >30 |
| ace2Δ | ND064 | 6 ± 1 | 9 ± 1 |
| sep1Δ | ND102 | 5 ± 1 | 10 ± 2 |
| ace2Δsep1Δ | ND148 | 4 ± 1 | 6 ± 1 |
| agn1Δ | ND001 | 17 ± 2 | 21 ± 2 |
| eng1Δ | ND005 | 19 ± 1 | 22 ± 1 |
| agn1Δeng1Δ | ND019 | 11 ± 1 | 14 ± 1 |
| agn1Δ [pND02] (ON) | ND234 | 30 ± 5 | >30 |
| agn1Δ [pND02] (OFF) | ND234 | 19 ± 1 | 26 ± 1 |
| agn1Δ [pSE01] (OFF) | ND235 | 16 ± 1 | 20 ± 1 |
| agn2Δ | ND028 | >30 | >30 |
Complete genotypes are shown in Table 1
Sedimentation times (minutes) are the times required for the OD595 of the individual cultures to decrease to 80% or 50% of their initial values and are the mean ± SD of four individual experiments, except those of strains with genotypes wild type (n = 12), agn1Δ (n = 8), and ace2Δ (n = 8)
For a complementation analysis, we expressed the agn1-his gene from a multicopy plasmid in the agn1Δ strain. Induction of agn1-his resulted in a sedimentation index comparable with that of wild-type cells, whereas repression of agn1-his gene expression showed a clumping phenotype typical for the agn1Δ strain (Table 2, compare induction of pND02 with its repression). The observation that during agn1-his repression clumping was not completely identical to that of agn1Δ cells is explained by the fact that repression of the nmt1 promotor is not complete. Despite continuous overexpression of the agn1 ORF under the strong nmt1 promotor, cells showed a wild-type morphology and did not lyse, indicating that cells are resistant to agn1+ overexpression (our unpublished data). We confirmed this conclusion by demonstrating that cells overexpressing agn1+ are as resistant to an incubation with (1,3)-β-glucanase as wild-type cells, providing no evidence for a change in cell wall rigidity (our unpublished data). Together, these data show that Agn1p-his restores Agn1p function in agn1Δ cells, but that Agn1p-his, upon overexpression, is not cytolytic.
Agn1p Is an endo-(1,3)-α-Glucanase
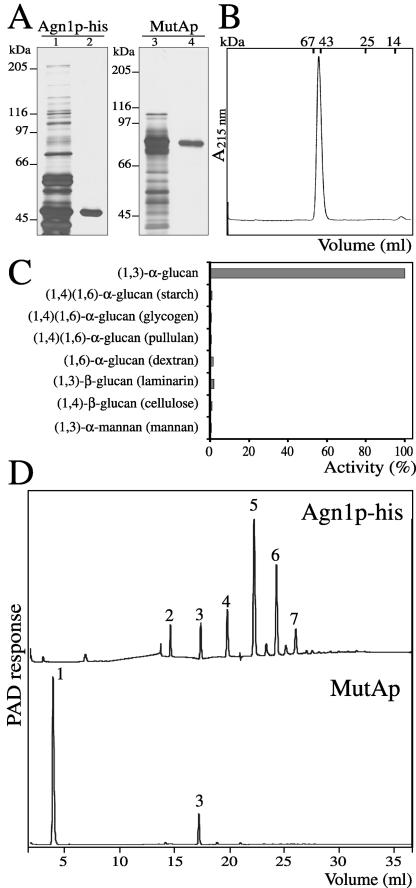
To characterize the role of Agn1p in cell separation, we studied its specific enzymatic activity. To obtain recombinant Agn1-his protein, we purified it from culture medium by immobilized metal-ion affinity chromatography and anion-exchange chromatography (Figure 4A, lanes 1 and 2), and assessed its purity as follows. First, SDS-PAGE followed by silver staining resolved only a single band with an apparent molecular mass of ∼47 kDa (Figure 4A, lane 2), in close agreement with its calculated molecular mass of 48.4 kDa. In size exclusion chromatography, Agn1p-his eluted as a single peak with an elution volume corresponding to a molecular mass of ∼50 kDa, indicating that it is a monomer (Figure 4B). Although mature Agn1p-his possesses one potential N-glycan attachment site at Asn-384, treatment with trifluoromethanesulfonic acid to eliminate glycosyl residues did not change its mobility in SDS-PAGE (our unpublished data), indicating that recombinant Agn1p-his was not glycosylated. Second, the 47-kDa protein band was subjected to peptide mass fingerprinting by matrix-assisted laser desorption ionization time-of-flight by using trypsin or V8 protease. All major nonprotease peaks in the obtained spectra were accounted for by Agn1p-his, providing no evidence for the presence of contaminating proteins or for posttranslational modification of Agn1-his protein. Electrospray mass spectrometry analysis of Agn1p-his yielded a major peak of molecular mass 45,980.1 ± 1.2 Da and a minor peak of mass 46,117.4 ± 1.8 Da. The major peak corresponds to an Agn1-his polypeptide without posttranslational modifications that starts, as predicted above, at Asp-21 and terminates with four His residues, indicating that two carboxy-terminal His residues were removed, presumably by proteases. The minor peak corresponds to an Agn1 polypeptide that terminates with five His residues. In conclusion, we purified Agn1-his protein to homogeneity and showed that its signal sequence was cleaved off to generate mature protein.
Figure 4.
Agn1p-his is an endo-(1,3)-α-glucanase producing predominantly (1,3)-α-glucan pentasaccharides. (A) Purification of Agn1p-his and T. harzianum MutAp. Agn1p-his was purified from concentrated culture supernatant of strain ND236 by immobilized nickel-nitrilotriacetic acid affinity chromatography (lane 1), followed by anion-exchange chromatography (lane 2). MutAp was purified from a commercial preparation (lane 3) by (1,3)-α-glucan adsorption chromatography (lane 4). Samples were resolved by 8% SDS-PAGE under reducing conditions and visualized by silver staining. (B) Agn1p-his is a monomeric protein. Size-exclusion chromatography of purified Agn1p-his on a Superdex 75 column. Elution volumes of molecular mass markers are indicated. (C) Agn1p-his specifically hydrolyzes (1,3)-α-glucan. Purified Agn1p-his was incubated with indicated substrates at a concentration of 4 mg/ml. To lower backgrounds, substrates were reduced, except for (1,4)-β-glucan (cellulose) and (1,4)(1,6)-α-glucan (starch). To solubilize the (1,3)-α-glucan and (1,4)-β-glucan substrates, they were carboxy-methylated. Data are a percentage of the amount of reducing ends released from reduced and carboxy-methylated (1,3)-α-glucan. (D) Agn1p-his produces mainly (1,3)-α-glucan pentasaccharides. HPAEC analysis with pulsed amperometric detection (PAD) of reaction products released after a 5-h incubation at 37°C from insoluble (1,3)-α-glucan by Agn1p-his (top) or MutAp (bottom). Numbers refer to the degree of polymerization.
Substrate specificity of recombinant Agn1p-his was assayed with different glucan or mannan substrates. In the presence of (1,3)-α-glucan, we detected the release of digestion products by using a colorimetric assay (Figure 4C). No activity was detected using (1,3)-β-glucan, (1,3)-α-mannan, (1,4)-α-glucan, or (1,6)-α-glucan as substrates, demonstrating a specificity for (1,3)-α-glucosidic linkages (Figure 4C). Agn1p-his shows a broad pH optimum, with a maximum activity ranging from pH 3.5–5.5 (our unpublished data). Furthermore, 4-nitrophenyl-α-glucose, a synthetic compound that is cleaved only by glucanases with an exo-type mechanism of hydrolysis, was not hydrolyzed (our unpublished data). Also, Agn1p-his activity was not inhibited by 1-deoxynojirimycin, N-methyl-1-deoxynojirimycin, castanospermin, or d-glucono-1,5-lactone, all inhibitors of exo-type enzymes (our unpublished data).
The observation that S. pombe Agn1p and T. harzianum MutAp share a putative catalytic domain but not a Pro-Ser-Thr-rich linker or polysaccharide-binding domain (see above) prompted us to compare the enzyme characteristics of both (1,3)-α-glucanases. For this purpose, we purified MutA protein by (1,3)-α-glucan adsorption chromatography, yielding a single band in SDS-PAGE analysis that resolved at a molecular mass of ∼75 kDa (Figure 4A, lane 4), consistent with previous results (Fuglsang et al., 2000). To compare the reaction products of both enzymes, insoluble (1,3)-α-glucan purified from S. pombe was incubated with Agn1p-his or MutAp for 5 h. A high-performance anion-exchange chromatography profile of MutAp reaction products showed the presence of mostly glucose, with small amounts of (1,3)-α-glucan trisaccharide (Figure 4D, bottom). Importantly, the reaction product of Agn1p-his lacked glucose, but instead contained (1,3)-α-glucan oligosaccharides with a degree of polymerization of 2–7, with (1,3)-α-glucan pentasaccharide as the predominant reaction product (Figure 4D, top). Together, these results demonstrate that Agn1p is an endo-(1,3)-α-glucanase that releases predominantly (1,3)-α-glucan pentasaccharides.
Agn1p Is Involved in Hydrolysis of the Septum Edging but Not the Primary Septum
Given that Agn1p and Eng1p are both glucanases, we investigated whether their functions are redundant. First, we compared the cellular morphologies of mutants with single or double deletions for agn1+ and eng1+. Although the morphology of agn1Δ eng1Δ cells resembled that of eng1Δ cells, the double mutant often contained more cells per chain of cells (Figure 3). Frequently, these chains of cells contained branches (Figure 3, arrowheads), a morphology hardly ever observed with either single deletion mutant. Second, we compared the extent of clumping of the single and double mutants in our sedimentation assay. Interestingly, agn1Δ eng1Δ cells sedimented significantly faster than agn1Δ or eng1Δ cells (Table 2), indicating that clumps of the double mutant were on average significantly larger than those of either single mutant. Together, these data suggest that agn1+ and eng1+ fulfill nonoverlapping functions.
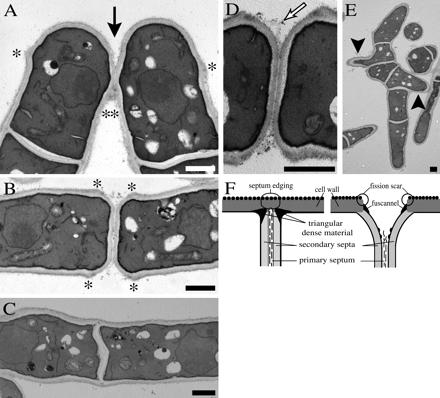
To gain insight into the precise roles of Agn1p and Eng1p in cell separation, we analyzed the ultrastructures of agn1Δ and eng1Δ cells. Electron microscopy analysis of agn1Δ cells showed that septum assembly (and thus cytokinesis) and dissolution of the primary septum progressed normally in this mutant (Figure 5A). These results were corroborated by microscopy analysis on agn1Δ cells stained with calcofluor white, a dye that specifically stains the primary septum and remnants thereof. Staining of the septa in agn1Δ cells was intense during cytokinesis, but diminished in intensity during cell separation (Figure 3). By contrast, a defect in dissolution of the septum edging was evident in agn1Δ cells (Figure 5A). The septum edging in agn1Δ cells was broken down only partially, causing daughter cells to remain attached via a small stretch of septum-edging material (Figure 5A, solid arrow).
Figure 5.
agn1Δ cells remain attached via remnants of the septum edging. Electron micrographs of thin sections from cells of genotypes: (A) agn1Δ (strain ND001). (B and D) eng1Δ (strain ND005). (C and E) ace2Δ (strain ND064). Cells were grown to midexponential phase in YEA medium. Solid arrow indicates locations of cell attachment, open arrow indicates remnants of the primary septum, solid arrowheads indicate branched outgrowths, and * indicates fission scars. Bar, 1 μm. (F) Schematic representation of the septum and septum edging immediately before (left) and during (right) cell separation. Adapted from Johnson et al. (1973).
The ultrastructure of the septal region in agn1Δ cells is clearly distinct from that in eng1Δ cells. In eng1Δ cells, we observed that the primary septum often fails to break down (Figure 5B), exposing primary-septum material to the extracellular space (Figure 5D, open arrow). This defect causes eng1Δ cells to remain attached via the primary septum, which is visualized clearly by staining with calcofluor white (Figure 3). The septum edging, on the other hand, seems to be broken down normally in eng1Δ cells (Figure 5B), resulting in invaginations at the septa and in exposure of fission scars (Figure 5B, asterisks). We conclude that eng1+ and agn1+ fulfill distinct roles in cell separation: eng1+ is involved in dissolution of the primary septum, whereas agn1+ is involved in dissolution of the septum edging.
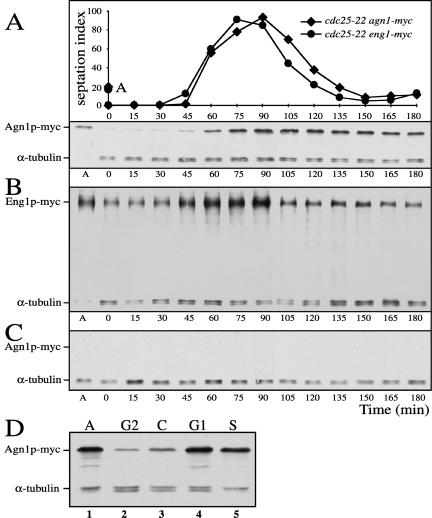
Agn1p Protein Expression Oscillates during the Cell Cycle
The observation that Agn1p acts during cell separation prompted us to examine whether Agn1 protein expression was up-regulated during this stage of the cell cycle. To this end, we arrested temperature-sensitive (ts) cdc25-22 agn1-myc cells at the G2-M transition and then released them synchronously into the cell cycle, while monitoring cell synchrony by septation-index analysis and Agn1-myc protein levels by immunoblot analysis. Agn1-myc protein levels oscillated considerably as cells traversed the first cell cycle, peaking concomitantly with septation and cell separation (Figure 6A). During the following cell cycle, the oscillation was less dramatic, because Agn1-myc protein is a stable protein with a half-life of >2 h (our unpublished data), and its levels did not decrease to their initial levels observed at arrest. In an arrest-release experiment on cdc25-22 eng1-myc cells, Eng1-myc protein levels oscillated in a manner similar to the oscillation of Agn1-myc protein levels (Figure 6, compare B and A). This oscillation in Eng1 protein levels is in good agreement with the oscillation reported for eng1+ transcript levels (Martín-Cuadrado et al., 2003).
Figure 6.
(A–C) Periodic expression of Agn1p-myc resembles that of Eng1p-myc and depends on ace2+. Cells of genotypes cdc25-22 agn1-myc (strain ND049) (A), cdc25-22 eng1-myc (strain ND051) (B), or cdc25-22 ace2Δ agn1-myc (strain ND111) (C) were arrested at G2-M transition and then released synchronously. Cells were collected at indicated times after start of the release. Degrees of synchrony are indicated by septation index. A indicates asynchronous controls. Quantitation of the blots by normalization to the α-tubulin loading control confirmed the apparent peak in Agn1p levels at about the time of septation and cell separation both in the experiment shown and in a second experiment in which the synchronous population was followed into the second cell cycle (our unpublished data). Note that upon ace2+ deletion Agn1p-myc is not expressed at any stage of the cell cycle. (D) Agn1-myc protein expression is up-regulated during G1 and S phases. Cells of genotypes cdc25-22 agn1-myc (strain ND049; lane 2), cps1-191 agn1-myc (strain ND154; lane 3), and cdc10-129 agn1-myc (strain ND054; lane 4) were grown in YEA medium to midexponential phase at 28°C and then arrested at G2 phase, cytokinesis (C phase), or G1 phase, respectively, by a shift to 36°C for 4 h. For an arrest at S phase, exponentially growing cells of genotype agn1-myc (ND003) were incubated with hydroxyurea at a concentration of 11 mM at 28°C for 4 h (lane 5). Along with a total lysate of asynchronous cells (indicated as A, lane 1), cell lysates were resolved by 8% SDS-PAGE under reducing conditions and visualized by immunoblot analysis by using an anti-myc mAb, 9E10. α-Tubulin served as a loading control.
To compare more precisely Agn1 protein levels at specific stages of the cell cycle, we used, along with the cdc25ts mutant to block entry into mitosis, a cps1ts mutant to block septum assembly and a cdc10ts mutant to block in late G1 phase. Also, we treated agn1-myc cells with hydroxyurea for arrest in S phase. Immunoblot analysis showed that during the G2 and C phases Agn1-myc protein levels are low, whereas during the G1 and S phases they increased (Figure 6D). These data not only confirm the results of the cdc25-22 arrest-release experiment, they also narrow down the broad period of septation and cell separation to the G1 and S phases. In summary, we conclude that Agn1 and Eng1 protein levels oscillate during the cell cycle and that Agn1 protein levels peak during G1 and S phases.
agn1+ and eng1+ Expression Depend on Transcription Factors Sep1p and Ace2p
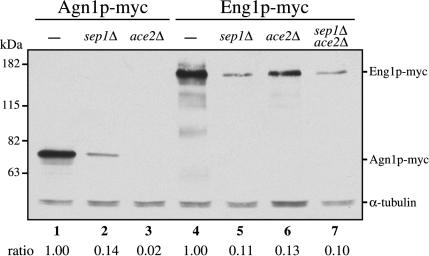
To investigate whether transcription factors Sep1p and Ace2p are involved in regulating Agn1 protein levels, we crossed agn1-myc into ace2Δ and sep1Δ backgrounds. Immunoblot analysis showed that the Agn1-myc protein level had decreased dramatically in both these mutants (Figure 7, compare lanes 2 and 3 to lane 1). To exclude the possibility that the observed lack of Agn1-myc protein expression was the result of a change in the frequency of cells in G1 and S phases, we performed an arrest-release experiment on cdc25-22 agn1-myc cells deleted for ace2+. At no stage of the cell cycle were we able to detect Agn1-myc protein in the ace2Δ background (Figure 6, compare A and C). These data show that ace2+ and sep1+ each are required for Agn1 protein expression.
Figure 7.
Agn1-myc protein expression depends on ace2+ and sep1+. Cells of genotypes agn1-myc (strain ND003; lane 1), agn1-myc sep1Δ (strain ND140; lane 2), agn1-myc ace2Δ (strain ND132; lane 3), eng1-myc (strain ND007; lane 4), eng1-myc sep1Δ (strain ND144; lane 5), eng1-myc ace2Δ (strain ND136; lane 6), or eng1-myc sep1Δ ace2Δ (strain ND218; lane 7) were grown in YEA medium to midexponential phase. Cell lysates were resolved by 8% SDS-PAGE under reducing conditions and visualized by immunoblot analysis by using an anti-myc mAb, 9E10. α-Tubulin served as a loading control. Ratios indicate normalized Agn1p-myc or Eng1p-myc levels corrected for α-tubulin loading controls.
Not only did Agn1-myc protein levels decrease considerably in both the sep1Δ and ace2Δ backgrounds but also Eng1-myc protein levels (Figure 7, compare lanes 5 and 6 to lane 4). Simultaneous deletion of sep1+ and ace2+ did not further decrease Eng1-myc protein levels, suggesting that their effects are not additive (Figure 7, lane 7). This observation was substantiated by the finding that the ace2Δ and sep1Δ single and double mutants formed clumps to a similar extent, although the double mutant sedimented a little faster than either single mutant (Table 2).
To study the effect of a simultaneous loss of agn1+ and eng1+ expression in the ace2Δ mutant (see above), we studied its ultrastructure. We observed that in ace2Δ cells both the septum edging and the primary septum remained intact (Figure 5C). Also, in this mutant, apical growth was redirected toward the side of the septum, causing branching (Figure 5E, solid arrowheads). This phenotype was also observed for the agn1Δ eng1Δ double mutant (Figure 3, arrowheads). From these experiments we conclude that both Sep1p and Ace2p are required for periodic expression of both Agn1 and Eng1 proteins.
Agn2p Is Essential for Endolysis of the Ascus Wall during Sporulation
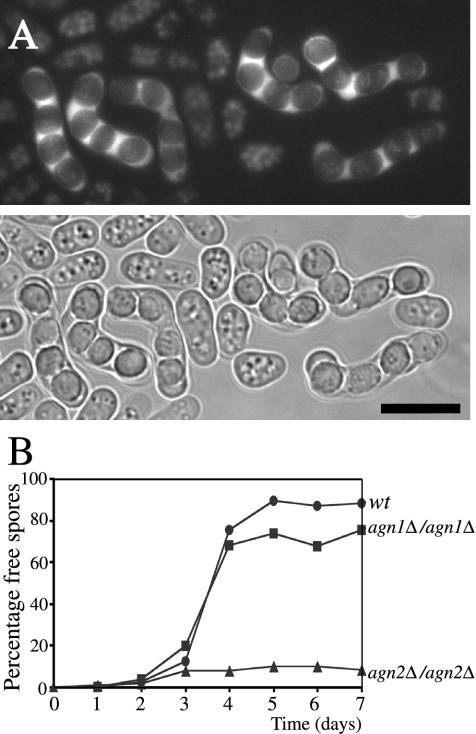
To investigate whether the cellular function of agn2+ is similar to that of agn1+, we deleted the agn2+ ORF and analyzed the resulting deletion phenotype. agn2Δ cells were viable and grew in YEA culture medium at 28°C with a generation time similar to that of wild-type cells (our unpublished data). agn2Δ cells also displayed a cylindrical rod morphology indistinguishable from that of wild-type cells (Figure 3), and they did not form clumps (Table 2). Furthermore, northern blot analysis indicated that agn2 transcript levels did not fluctuate during the cell cycle (our unpublished data). We conclude that, unlike agn1+, agn2+ does not seem to have a role during vegetative growth.
agn2+ expression is up-regulated temporarily during sporulation (Mata et al., 2002). To localize Agn2p in sporulating cells, we crossed haploid agn2-gfp cells with haploid wild-type cells of opposite mating type and analyzed Agn2p-GFP fluorescence in living mating products. Although Agn2p-GFP fluorescence in the haploid agn2-gfp cells was weak and diffuse, it was more intense in sporulating diploid cells. Agn2p-GFP was excluded from ascospores, but instead localized to the residual cytoplasm within the ascus wall (Figure 8A), known as the epiplasm (Yoo et al., 1973). To assess Agn2p function, we analyzed agn2Δ cells in a simple mating assay. No differences in conjugation and sporulation efficiency were observed with agn2Δ cells compared with wild-type cells (our unpublished data). To determine whether agn2+ plays a role in the subsequent step of endolysis of the ascus wall, during which the ascospores are released, we transferred agn2Δ/agn2Δ diploid cells to sporulation medium. Like the majority of matching wild-type diploids, the majority of agn2Δ/agn2Δ cells developed into asci containing four ascospores, called tetrads, within 48 h after start of induction, confirming that sporulation was not affected by agn2+ deletion (our unpublished data). However, whereas the ascus walls lysed in a majority of wild-type tetrads, releasing individual ascospores within the following 48 h, the ascus walls remained intact in a majority of agn2Δ/agn2Δ tetrads, even after 5 d after tetrad formation (Figure 8B). Small numbers of free ascospores were observed, but they represented only ∼10% of the tetrads. We conclude from these experiments that Agn2p is involved in endolysis of the ascus wall.
Figure 8.
Agn2p localizes to the cytoplasm of the ascus and contributes to hydrolysis of the ascus wall. (A) Haploid cells of genotype agn2-gfp (strain ND172) and matching wild-type cells (strain FYC15) were allowed to mate on a sporulation plate and resulting zygotic asci were examined using fluorescence microscopy. Agn2p-GFP fluorescence is excluded from ascospores, but instead localizes to the cytoplasm of the ascus, the epiplasm. Bar, 10 μm (B). Along with wild-type diploid cells (strain ND080), diploid cells of genotype agn1Δ/agn1Δ (strain ND084) and agn2Δ/agn2Δ (strain ND088) were grown to exponential phase in EMM medium containing 2% ammonium sulfate to inhibit sporulation. Subsequently, sporulation was induced synchronously by a shift to EMM medium containing 0.5% sodium glutamate, a poor nitrogen source. At the indicated time intervals, percentages of free ascospores were determined. Note that in the absence of agn2+ the ascus wall fails to undergo endolysis.
DISCUSSION
In this report, we have identified Agn1p as an endo-(1,3)-α-glucanase that is involved in the dissolution of cell wall material that surrounds the septum, the septum edging (Figure 5F). Deletion of agn1+ abrogates the ability of S. pombe cells to fully separate as free cells and results in mutant cells that remain attached to each other via remnants of the septum edging. However, agn1+ deletion does not affect hydrolysis of the primary septum, based on the normal dissolution of primary-septum material stained by calcofluor white (Figure 3) and on the ultrastructure of agn1Δ cells (Figure 5A). These observations are consistent with the presence of (1,3)-α-glucan in the septum edging and with its absence from the primary septum. By contrast, deletion of eng1+ does not affect dissolution of the septum edging, based on the observed invaginations at the site of the septum in eng1Δ cells, but rather abrogates dissolution of the primary septum (Figure 5B). The roles of Agn1p and Eng1p in cell separation seem functionally distinct in that the agn1Δ eng1Δ double mutant clumps more extensively that either single mutant (Table 2). Furthermore, the ace2Δ mutant, which expresses decreased levels of both Eng1p and Agn1p (Figure 7), displays a hyphal morphology characterized by intact primary septa and intact septum edgings (Figure 5C). We conclude that the dissolution of both the primary septum and the septum edging are facilitated by hydrolases but that these processes involve dedicated hydrolases Eng1p and Agn1p.
A series of distinct steps is required for successful delivery of cell-separation hydrolases to their target destination at the right stage of the cell cycle, including cell cycle-regulated synthesis, targeted secretion, and localized hydrolysis. For the first step in cell separation, transcription factors are involved. We show that expression of Agn1p and Eng1p in S. pombe requires transcription factors Sep1p and Ace2p. In S. cerevisiae, two homologues of Sep1p (ScFkh1p and ScFkh2p) regulate the cell cycle oscillation of an entire gene cluster that includes ScAce2p, the S. cerevisiae homolog of Ace2p (Zhu et al., 2000). ScAce2p, in turn, directly regulates cell cycle-dependent expression of cell-separation hydrolases ScEng1p and ScCts1p (Dohrmann et al., 1992; Baladrón et al., 2002). The peaks in ScENG1 and ScCTS1 transcript levels during G1 phase in S. cerevisiae (Dohrmann et al., 1992; Spellman et al., 1998) overlap with the peak in Agn1 protein levels during G1 and S phases in S. pombe (Figure 6D), suggesting that agn1+ expression may be directly controlled by Ace2p. During targeted secretion, the second step in cell separation, hydrolases are transported to the septal region via the secretory pathway. We hypothesize that secretory vesicles containing cell-separation hydrolases, such as Agn1p and Eng1p, may be targeted to the septum via the exocyst complex, a multiprotein complex present in yeasts (but also in humans) (Hsu et al., 1998). The exocyst complex might be involved in recruiting secretion vesicles to specific regions of the plasma membrane (Hsu et al., 1999), and the cell-separation defect observed in exocyst mutants in S. pombe (Wang et al., 2002) is consistent with this hypothesis. Also certain septins, filament-forming proteins that form a ring structure near the septal region, together with the septin-organizing protein Mid2p, may be involved in localizing secretion of cell-separation hydrolases to the septal region, because their gene deletion phenotypes show similar cell-separation defects (Berlin et al., 2003, Tasto et al., 2003). Localized hydrolysis, the third and final step in cell separation, follows targeted secretion of cell-separation hydrolases. Oulevey et al. (1970) observed a triangular region of dense material in the extracellular space between the septum and the septum edging opposite the intracellular exocyst ring (Figure 5F). It seems not unreasonable to suggest that this region may contain secreted cell-separation hydrolases that diffuse toward the septum and the septum edging. Indeed, one of these hydrolases, Eng1p, localizes to a ring structure overlaying the primary septum (Martín-Cuadrado et al., 2003).
Cell separation was not inhibited completely in any of the mutants studied here, not in the agn1Δ and eng1Δ single and double mutants, nor in the ace2Δ and sep1Δ single and double mutants. Our observation that cells of the agn1Δ eng1Δ double mutant can separate (Figure 3 and Table 2) suggests that additional factors may contribute to cell separation in S. pombe. One possibility is physical breakage of septum or septum edging by mechanical forces caused by agitation. More likely, however, is the presence of additional cell-separation hydrolases besides Agn1p and Eng1p. Perhaps one (or more) of the three putative exo-(1,3)-β-glucanases encoded by the S. pombe genome are involved (Martín-Cuadrado et al., 2003). Furthermore, our finding that cells of the ace2Δ sep1Δ double mutant can separate, albeit to the lowest extent of all mutants studied here (Table 2), may indicate that one (or more) additional transcription factors are involved in regulating expression of cell-separation hydrolases. Indeed, even after deletion of ace2+ and sep1+, low levels of Eng1p remain detectable (Figure 7).
Overexpression of endo-(1,3)-α-glucanase Agn1p did not compromise the structural integrity of S. pombe cells and was not cytolytic, even though we overexpressed this hydrolase continuously during the entire cell cycle. This observation may be explained as follows. First, it is possible that Agn1p did not reach concentrations high enough to weaken the cell wall effectively. Second, effective impairment of cell wall (1,3)-α-glucan may be avoided by the enzyme's endo-catalytic mode of action, cleaving intrachain glycosidic linkages, rather than an exo-catalytic mode, which would have cleaved glycosidic linkages at one of the ends of the polysaccharide. Perhaps, accumulation of internal breaks may be less disruptive to the three-dimensional structure, and thus to the rigidity, of (1,3)-α-glucan than accumulation of successive cleavages from one end. Third, it is conceivable that its simple domain structure prevents Agn1p from compromising structural integrity. Sequence comparisons with T. harzianum MutAp (Figure 1) and Penicillium purpurogenum MutAp (Fuglsang et al., 2000) show that Agn1p lacks a carboxy-terminal polysaccharide-binding domain together with a Pro-Ser-Thr-rich linker. The presence of a polysaccharide-binding domain is common among hydrolases, such as cellulases, xylanases, chitinases, and amylases (Gilkes et al., 1991). Because T. harzianum and P. purpurogenum are parasitic fungi, they secrete (1,3)-α-glucanases to degrade (1,3)-α-glucan of host fungi, producing glucose, which can be taken up as a carbon source. Our analyses on Agn1p show (Figure 4C) that a polysaccharide-binding domain is not essential for hydrolysis of (1,3)-α-glucan. Nonetheless, the absence of a polysaccharide-binding domain may diminish the efficiency of hydrolysis by Agn1p or may limit its access to (1,3)-α-glucan, thereby preventing cytolysis.
The paralog of Agn1p in S. pombe, Agn2p, lacks a signal sequence for entry into the secretory pathway (Figure 1), fails to be secreted into the culture medium (Figure 2), and localizes to the cytoplasm of the ascus, the epiplasm (Figure 8A). To our knowledge, Agn2p is the first member of glycoside hydrolase family 71 that localizes, at least initially, to the cytoplasm. DNA-microarray analysis showed that agn2+ is up-regulated 180-fold during sporulation (Mata et al., 2002). agn2Δ/agn2Δ diploid cells induced to sporulate were able to generate four ascospores per ascus, which is typical for S. pombe, but then failed to undergo effective endolysis of the ascus wall. As a consequence, few ascospores were released as free entities and most remained enclosed within the ascus wall, even after a prolonged incubation (Figure 8B). We speculate that in wild-type asci the ascus membrane, which encloses the four ascospores, may disintegrate after formation of the ascospores, bringing proteins of the epiplasm including Agn2p into direct contact with the ascus wall. Given that the ascus wall is derived from the cell wall of the diploid cell that underwent sporulation, it contains (1,3)-α-glucan, which, after disintegration of the ascus membrane, may be broken down from within by hydrolases, such as Agn2p. The S. pombe genome encodes not only endo-(1,3)-α-glucanases in two forms, one with and the other without a signal sequence, but also endo-(1,3)-β-glucanases. Whereas Eng1p contains a signal sequence, its paralog in S. pombe, Eng2p, lacks such a sequence. Like Agn2p, Eng2p is temporarily up-regulated during sporulation (Mata et al., 2002), suggesting that it may also be involved in endolysis of the ascus wall. Homologues of these two forms of endo-(1,3)-β-glucanases also have been identified in S. cerevisiae and in C. albicans (Baladrón et al., 2002).
Characterization of the roles of the Agn proteins in S. pombe has provided new insights into our understanding of the cellular functions of (1,3)-α-glucanases. Not only S. pombe but also medically important dimorphic fungi, Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitidis, and Coccidioides immitis, contain substantial amounts of cell wall α-glucan in their pathogenic yeast form (Hogan et al., 1996), which must be hydrolyzed locally during cell separation to allow efficient dispersal in the infected organism. Furthermore, cell wall α-glucan is abundantly present in the human fungal pathogen Cryptococcus neoformans (James et al., 1990), for which not only immunocompromised patients but also healthy individuals are at risk due to its ubiquitous environmental presence (Stephen et al., 2002). Interestingly, the C. neoformans genome encodes four homologues of the S. pombe endo-(1,3)-α-glucanases, allowing future investigations into potential roles of these enzymes for virulence.
Acknowledgments
We thank H. Dekker and JW. Back for performing electrospray (tandem) mass spectometry; P. Nurse, R. Dahr, and M. Balasubramanian for providing S. pombe strains; C. de Vries for providing the anti-myc-antibody; J.R. Pringle for providing kanMX6 plasmids; L. de Koning for initial experiments on agn2+; Profs. J.P. Kamerling and J.F.G. Vliegenthart (Bijvoet Center, Utrecht University) for general discussions; and members of our medical yeast group, in particular B. Distel and R. Benne, for helpful discussions and critically reading the manuscript. This work was supported by the Netherlands Organization for Scientific Research (NWO-CW 99006).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-04-0319. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0319.
References
- Akada, R., Kawahata, M., and Nishizawa, Y. (2000). Elevated temperature greatly improves transformation of fresh and frozen competent cells in yeast. Biotechniques 28, 854-856. [PubMed] [Google Scholar]
- Bähler, J., Wu, J.-Q., Longtine, M.S., Shah, N.G., McKenzie, A. 3rd, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. [DOI] [PubMed] [Google Scholar]
- Baladrón, V., Ufano, S., Dueñas, E., Martín-Cuadrado, A.B., del Rey, F., and Vázquez de Aldana, C.R. (2002). Eng1p, an endo-1,3-β-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot. Cell 1, 774-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, A., Paoletti, A., and Chang, F. (2003). Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160, 1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib, E., Roh, D.-H., Schmidt, M., Crotti, L.B., and Varma, A. (2001). The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276, 19679-19682. [DOI] [PubMed] [Google Scholar]
- Chang, F. (2001). Studies in fission yeast on mechanisms of cell division site placement. Cell Struct. Funct. 26, 539-544. [DOI] [PubMed] [Google Scholar]
- Dohrmann, P.R., Butler, G., Tamai, K., Dorland, S., Greene, J.R., Thiele, D.J., and Stillman, D.J. (1992). Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 6, 93-104. [DOI] [PubMed] [Google Scholar]
- Evan, G.I., Lewis, G.K., Ramsay, G., and Bishop, J.M. (1985). Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang, C.C., Berka, R.M., Wahleithner, J.A., Kauppinen, S., Shuster, J.R., Rasmussen, G., Halkier, T., Dalbøge, H., and Henrissat, B. (2000). Biochemical analysis of recombinant fungal mutanases. A new family of α-1,3-glucanases with novel carbohydrate-binding domains. J. Biol. Chem. 275, 2009-2018. [DOI] [PubMed] [Google Scholar]
- Gilkes, N.R., Henrissat, B., Kilburn, D.G., Miller, R.C., Jr., and Warren, R.A.J. (1991). Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol. Rev. 55, 303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldener, U., Heck, S., Fielder, T., Beinhauer, J., and Hegemann, J.H. (1996). A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach, F., Klis, F.M., Van Den Ende, H., Van Donselaar, E., Peters, P.J., and Klausner, R.D. (1998). Identification of a putative α-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 95, 9161-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, L.H., Klein, B.S., and Levitz, S.M. (1996). Virulence factors of medically important fungi. Clin. Microbiol. Rev. 9, 469-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, S.-C., Hazuka, C.D., Foletti, D.L., and Scheller, R.H. (1999). Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol. 9, 150-153. [DOI] [PubMed] [Google Scholar]
- Hsu, S.-C., Hazuka, C.D., Roth, R., Foletti, D.L., Heuser, J., and Scheller, R.H. (1998). Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 20, 1111-1122. [DOI] [PubMed] [Google Scholar]
- Humbel, B.M., Konomi, M., Takagi, T., Kamasawa, N., Ishijima, S.A., and Osumi, M. (2001). In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18, 433-444. [DOI] [PubMed] [Google Scholar]
- James, P.G., Cherniak, R., Jones, R.G., Stortz, C.A., and Reiss, E. (1990). Cell-wall glucans of Cryptococcus neoformans Cap 67. Carbohydr. Res. 198, 23-38. [DOI] [PubMed] [Google Scholar]
- Johnson, B.F., Yoo, B.Y., and Calleja, G.B. (1973). Cell division in yeasts: movement of organelles associated with cell plate growth of Schizosaccharomyces pombe. J. Bacteriol. 115, 358-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiho, T., Yoshida, I., Nagai, K., Ukai, S., and Hara, C. (1989). (1→3)-α-D-glucan from an alkaline extract of Agrocybe cylindracea, and antitumor activity of its O-(carboxymethyl)ated derivatives. Carbohydr. Res. 189, 273-279. [DOI] [PubMed] [Google Scholar]
- Klis, F.M., Mol, P., Hellingwerf, K., and Brul, S. (2002). Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26, 239-256. [DOI] [PubMed] [Google Scholar]
- Koerkamp, M.G., Rep, M., Bussemaker, H.J., Hardy, G.P., Mul, A., Piekarska, K., Szigyarto, C.A., De Mattos, J.M., and Tabak, H.F. (2002). Dissection of transient oxidative stress response in Saccharomyces cerevisiae by using DNA microarrays. Mol. Biol. Cell 13, 2783-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, K., Kubota, Y., Tanimoto, T., and Okada, Y. (1989). High-performance anion-exchange chromatography of homogeneous D-gluco-oligosaccharides and -polysaccharides (polymerization degree ≥50) with pulsed amperometric detection. J. Chromatogr. 464, 365-373. [DOI] [PubMed] [Google Scholar]
- Kuranda, M.J., and Robbins, P.W. (1991). Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266, 19758-19767. [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105-132. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., Utzig, S., and Simanis, V. (1999). Controlling septation in fission yeast: finding the middle, and timing it right. Curr. Genet. 35, 571-584. [DOI] [PubMed] [Google Scholar]
- Lever, M. (1972). A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47, 273-279. [DOI] [PubMed] [Google Scholar]
- Liu, J., Wang, H., McCollum, D., and Balasubramanian, M.K. (1999). Drc1p/Cps1p, a 1,3-β-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 153, 1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cuadrado, A.B., Dueñas, E., Sipiczki, M., Vázquez de Aldana, C.R., and del Rey, F. (2003). The endo-β-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 116, 1689-1698. [DOI] [PubMed] [Google Scholar]
- Mata, J., Lyne, R., Burns, G., and Bähler, J. (2002). The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32, 143-147. [DOI] [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127-130. [DOI] [PubMed] [Google Scholar]
- McCollum, D., and Gould, K.L. (2001). Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11, 89-95. [DOI] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795-823. [DOI] [PubMed] [Google Scholar]
- Nielsen, H., Engelbrecht, J., Brunak, S., and von Heijne, G. (1997). Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10, 1-6. [DOI] [PubMed] [Google Scholar]
- Oulevey, N., Deshusses, J., and Turian, G. (1970). Étude de la zone septale de Schizosaccharomyces pombe en division à ses étapes succesives. Protoplasma 70, 217-224. [Google Scholar]
- Reynolds, E.S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribár, B., Bánrévi, A., and Sipiczki, M. (1997). sep1+ encodes a transcription-factor homologue of the HNF-3/forkhead DNA-binding-domain family in Schizosaccharomyces pombe. Gene 202, 1-5. [DOI] [PubMed] [Google Scholar]
- Roh, D.H., Bowers, B., Schmidt, M., and Cabib, E. (2002). The septation apparatus, an autonomous system in budding yeast. Mol. Biol. Cell 13, 2747-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Sipiczki, M., Grallert, B., and Miklos, I. (1993). Mycelial and syncytial growth in Schizosaccharomyces pombe induced by novel septation mutations. J. Cell Sci. 104, 485-493. [DOI] [PubMed] [Google Scholar]
- Spellman, P.T., Sherlock, G., Zhang, M.Q., Iyer, V.R., Anders, K., Eisen, M.B., Brown, P.O., Botstein, D., and Futcher, B. (1998). Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9, 3273-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, C., Lester, S., Black, W., Fyfe, M., and Raverty, S. (2002). Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 43, 792-794. [PMC free article] [PubMed] [Google Scholar]
- Takasaki, S., and Kobata, A. (1978). Microdetermination of sugar composition by radioisotope labeling. Methods Enzymol. 50, 50-54. [DOI] [PubMed] [Google Scholar]
- Tasto, J.J., Morrell, J.L., and Gould, K.L. (2003). An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Tang, X., Liu, J., Trautmann, S., Balasundaram, D., McCollum, D., and Balasubramanian, M.K. (2002). The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 515-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, V., et al. (2002). The genome sequence of Schizosaccharomyces pombe. Nature 415, 871-880. [DOI] [PubMed] [Google Scholar]
- Yoo, B.Y., Calleja, G.B., and Johnson, B.F. (1973). Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch. Mikrobiol. 91, 1-10. [DOI] [PubMed] [Google Scholar]
- Zhu, G., Spellman, P.T., Volpe, T., Brown, P.O., Botstein, D., Davis, T.N., and Futcher, B. (2000). Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature 406, 90-94. [DOI] [PubMed] [Google Scholar]