Abstract
Purpose
Both hepatitis B (HBV) and C viruses (HCV) are causes of hepatocellular carcinoma (HCC), but lifetime risk and sex difference remain unclear. This study aimed to assess the lifetime risk and sex difference of HCC among patients with chronic HBV and/or HCV.
Methods
A prospective cohort of 23,820 residents of Taiwan age 30 to 65 years were enrolled from 1991 to 1992, with 477 instances of HCC occurring subsequently. Serum samples collected at enrollment were tested for seromarkers and viral load of HBV and HCV. Newly developed HCC was ascertained through computerized data linkage with national cancer registry and death certification systems.
Results
The cumulative lifetime (age 30 to 75 years) incidences of HCC for men and women positive for both HBV surface antigen (HBsAg) and antibodies against HCV (anti-HCV) were 38.35% and 27.40%; for those positive for HBsAg only, 27.38% and 7.99%; for those positive for anti-HCV only, 23.73% and 16.71%; and for those positive for neither, 1.55% and 1.03%, respectively. There was a significant male predominance in incidence of HCC for chronic HBV carriers but not for chronic carriers of HCV or both. Multivariate adjusted hazard ratio of developing HCC decreased with age in HBsAg-seropositive men but increased with age in anti-HCV–seropositive women. Among dual-infected participants, there was an inverse association between HBV and HCV viral load. Risk of HCC increased significantly with increasing viral load of HBV and HCV.
Conclusion
There exists a suppressive effect of HCV on HBV viral load. Individual and combined effects of the two viruses on HCC vary with sex and age.
INTRODUCTION
There are 350 million and 170 million persons chronically infected with hepatitis B virus (HBV) and hepatitis C virus (HCV) in the world, respectively.1 Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of death resulting from cancer because of its poor prognosis.2 Chronic infections of HBV and HCV are well-documented etiologic factors for HCC. Both viruses have been classified as human carcinogens by the International Agency for Research on Cancer.3
Taiwan is a hyperendemic area of chronic HBV. Before a national HBV vaccination program was implemented in 1984, 15% to 20% of the general population of Taiwan was chronically infected with HBV.4 A majority of HBV surface antigen (HBsAg) –seropositive residents of Taiwan were infected with HBV perinatally before 3 years of age, whereas infection after 3 years of age rarely resulted in a chronic infection state.5 In contrast, the prevalence of HCV infection varies by region in Taiwan, ranging from 1.6% to 37%.6–8 Horizontal routes, especially iatrogenic contact with contaminated syringes or needles, are the major transmission route of HCV in Taiwan.9,10 Furthermore, the seroprevalence of antibodies against HCV (anti-HCV) is less than 1% for children younger than 12 years, and HCV infection mainly occurs in young adulthood.11 Hence, most patients with dual chronic infection of HBV and HCV in Taiwan can be assumed to be chronic HBV carriers superinfected by HCV. It has also been found that patients with HCV-associated HCC are older than those with HBV-associated HCC,12 and HCV leads to liver cirrhosis more often than HBV, which may also indicate different hepatocarcinogenic mechanisms between HBV and HCV.
There have been numerous reports on the combined effect of chronic HBV and HCV infection on HCC risk.13–24 Most involved case-series and case-control studies; to our knowledge, we reported the only community-based cohort study in men.23 Here we further analyze updated data for both men and women, with the estimation of cumulative lifetime (age 30 to 75 years) incidence of HCC. This community-based prospective cohort study aimed first, to estimate the lifetime risk of HCC for participants with chronic HBV and/or HCV infection by sex; second, to compare HCC risk associated with chronic HBV and/or HCV infection between men and women and between young and old carriers; and third, to assess the association with HCC risk for HBV and/or HCV viral load.
METHODS
Study Cohort
The enrollment of study participants has been described previously.25–28 Briefly, we recruited 23,820 residents from seven townships of Taiwan from 1991 to 1992. They provided written informed consent for the questionnaire interview, biospecimen collection, health examinations, and computerized data linkage of health status with national cancer registry and death certification profiles.
Data Collection and Blood Tests
At cohort entry, all participants were personally interviewed by well-trained research nurses using a structured questionnaire. A 10-mL peripheral blood sample was collected from each participant using a disposable vacuum syringe with needle. Blood samples were fractionated on collection day and stored in deep freezers (at −70°C) until use. The serum samples collected at cohort entry were tested for HBsAg by radioimmunoassay using commercial kits (Abbott Laboratories, North Chicago, IL), anti-HCV by enzyme immunoassay using a second-generation commercial kit (Abbott Laboratories), and ALT by a serum chemistry autoanalyzer (model 736, Hitachi, Tokyo, Japan). For those seropositive for HBsAg and anti-HCV, HBV DNA (copies/mL) and HCV RNA (IU/mL) were further measured by the Cobas Amplicor HBV monitor test kit and Cobas TaqMan HCV test v2.0 (Roche Diagnostics, Indianapolis, IN), respectively.
Ascertainment of HCC
At enrollment, any participant who had an elevated serum level of ALT (≥ 45 IU/L), AST (≥ 40 IU/L), or α-fetoprotein (≥ 20 ng/mL); serostatus of HBsAg or anti-HCV; or family history of cirrhosis or HCC among first-degree relatives was referred for abdominal ultrasonography and confirmatory diagnosis. None of the study participants were affected by HCC at enrollment based on health examination at cohort entry, questionnaire interview on personal history of HCC, and data linkage with the national cancer registry profile.
Newly developed HCC was ascertained by computerized data linkage of the national cancer registry and national death certification profiles in Taiwan for a period from January 1, 1984, to June 30, 2008. Medical record verification of all diagnoses of HCC was based on the following criteria: histopathologic examination, positive lesion detected by at least two different imaging techniques (abdominal ultrasonography, angiogram, or computed tomography), or imaging technique with a serum α-fetoprotein level greater than 400 ng/mL. Ascertainment of newly developed HCC was considered complete and accurate. More detailed information is provided in the Appendix (online only).
Statistical Analysis
For each participant, the period at risk for HCC was calculated from date of enrollment to date of diagnosis of newly developed HCC, date of death, or last date of linked data available from the national cancer registry (June 30, 2008), whichever came first. Analyses using follow-up time as the time scale with stratification of birth year and adjustment of age at enrollment showed similar results with those using age as the time scale. Cumulative lifetime (age 30 to 75 years old) incidence of HCC was estimated for men and women with chronic HBV and/or HCV infection. For further investigation of the age-varying effect of HBV and HCV, age was used as the primary time scale, with stratification of birth year and covariate adjustment of age at enrollment to fully account for the effects of both age and birth cohort throughout the study. The proportional hazards assumption was satisfied for each covariate in the final multivariate adjusted Cox model according to Schoenfeld residuals.
The hazard ratios (HRs) and 95% CIs for the groups seropositive for HBsAg only, anti-HCV only, and both were obtained with Cox proportional hazards models, using participants seronegative for both HBsAg and anti-HCV as the reference group. Multivariate adjusted HRs were derived from the Cox model, with stratification of birth year and sex and adjustment of age at enrollment (40 to 49, 50 to 59, and 60 to 65 years [reference group, 30 to 39 years]), cigarette smoking (yes or no), alcohol consumption (yes or no), and serum ALT level (≥ 45 and 15 to 44 IU/L [reference group, < 15 IU/L]). Multivariate adjusted cumulative incidences of HCC at age 75 years were estimated from the Cox model. To investigate the dose-response relationship of HBV and HCV viral load and HCC risk, the penalized spline was implemented in the Cox model. Age-varying effects (> 65 and ≤ 65 years) of the four HBV and/or HCV carrier groups on HCC were estimated by time-varying Cox model.
The viral load of HBV and HCV in participants with mono- and dual infection was compared by Wilcoxon rank sum test and multiple linear regression analysis (ordinary least squares). HBV and HCV viral loads were natural log transformed because of their skewness. Among participants with dual infection, the association between HBV and HCV viral load was further investigated by Spearman correlation, locally weighted scatterplot smoothing, and multivariate linear model regressing natural log of HBV viral load on spline or linear term of natural log of HCV viral load with multivariate adjustment.
RESULTS
The seroprevalence of HBsAg and anti-HCV among study participants by age at enrollment, sex, and serum ALT level is shown in Table 1. There were 4,149 (17.44%) and 1,313 participants (5.52%) seropositive for HBsAg and anti-HCV, respectively. The seroprevalence of HBsAg decreased with age (P < .001 for trend), whereas that of anti-HCV increased with age (P < .001 for trend). Men had a higher seroprevalence of HBsAg than women (P < .001), and women had a higher seroprevalence of anti-HCV than men (P < .001). Serum ALT levels were associated with chronic HBV and/or HCV (P < .001 for trend).
Table 1.
Prevalence of HBV and HCV Infection Status at Study Entry by Age, Sex, and ALT Level
| Variable | Total No. of Participants | HBsAg Seropositive, Anti-HCV Seronegative |
HBsAg Seronegative, Anti-HCV Seropositive |
HBsAg Seropositive, Anti-HCV Seropositive |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | P* | No. | % | P* | No. | % | P* | ||
| Age, years | < .001 | < .001 | < .001 | |||||||
| 30-39 | 6,820 | 1,297 | 19.0 | 186 | 2.7 | 40 | 0.6 | |||
| 40-49 | 6,240 | 1,091 | 17.5 | 243 | 3.9 | 54 | 0.9 | |||
| 50-59 | 7,295 | 1,142 | 15.7 | 444 | 6.1 | 85 | 1.2 | |||
| 60-65 | 3,430 | 401 | 11.7 | 222 | 6.5 | 39 | 1.1 | |||
| Sex | < .001 | < .001 | .79 | |||||||
| Women | 11,817 | 1,600 | 13.5 | 630 | 5.3 | 106 | 0.9 | |||
| Men | 11,968 | 2,331 | 19.5 | 465 | 3.9 | 112 | 0.9 | |||
| ALT, IU/L | < .001 | < .001 | < .001 | |||||||
| < 15 | 16,601 | 2,401 | 14.5 | 465 | 2.8 | 96 | 0.6 | |||
| 15-44 | 6,356 | 1,304 | 20.5 | 449 | 7.1 | 88 | 1.4 | |||
| ≥ 45 | 828 | 226 | 27.3 | 181 | 21.9 | 34 | 4.1 | |||
Abbreviations: anti-HCV, antibodies against HCV; HBsAg, HBV surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus.
P values calculated by Cochran-Armitage trend test for age at enrollment (30-39, 40-49, 50-59, and 60-65 years) and ALT level (< 15, 15-44, and ≥ 45 IU/L) and by Fisher exact test for sex.
Seromarkers of HBV and HCV
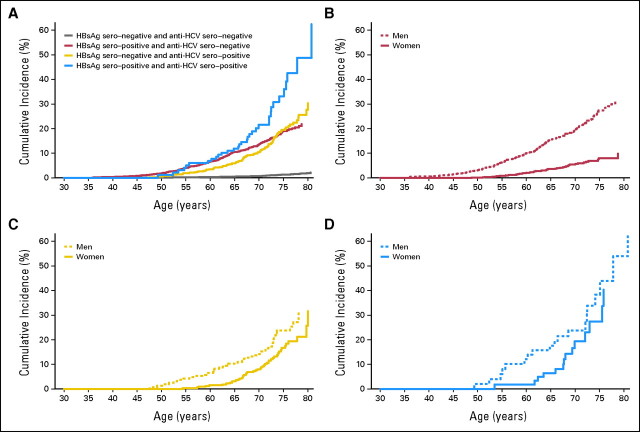
As listed in Table 2, the cumulative lifetime (age 30 to 75 years) incidences of HCC for men and women positive for both HBsAg and anti-HCV were 38.35% and 27.40%; for those positive for HBsAg only, 27.38% and 7.99%; for those positive for anti-HCV only, 23.73% and 16.71%; and for those positive for neither, 1.55% and 1.03%, respectively. The corresponding HCC incidence rates per 100,000 person-years were 1130.75, 593.31, 683.99, and 40.26, respectively, in men and 875.28, 164.98, 492.62, and 22.35, respectively, in women. In the Cox model, multivariate adjusted HRs (95% confidence interval) of developing hepatocellular carcinoma were 19.5 (95% CI, 12.9 to 29.4), 12.9 (95% CI, 10.2 to 16.5), 10.3 (95% CI, 7.6 to 13.9), and 1.0 (reference group), respectively, after adjustment for age, sex, habits of cigarette smoking and alcohol drinking, and serum ALT levels. With adequate adjustment, results with age or follow-up time as the time scale were similar, as shown in Table 2. The HR of cigarette smoking (yes v no) was 1.20 (95% CI, 0.96 to 1.51; P = .12), and that of alcohol consumption (yes v no) was 1.45 (95% CI, 1.13 to 1.86; P = .0035). As shown in Table 2 and Figure 1, HCC risk was significantly higher in those with dual infection of HBV and HCV than in those with monoinfection (P = .030 and .0019, respectively), especially in women (P = .0016 and .049, respectively). However, the interactive effect on HCC of HBV and HCV was significant away from the multiplicative interaction (P < .001). There was a significant male predominance in incidence of HCC for chronic HBV carriers (P = .0044), but no sex difference in HCC risk was found for chronic carriers of HCV, both HBV and HCV, or neither, as shown in Table 2 and Figure 1.
Table 2.
Independent and Combined Effects of HBV and HCV Infections on Development of HCC
| Effect | HBsAg Seronegative, Anti-HCV Seronegative | HBsAg Seropositive, Anti-HCV Seronegative | HBsAg Seronegative, Anti-HCV Seropositive | HBsAg Seropositive, Anti-HCV Seropositive | P* |
|---|---|---|---|---|---|
| No. of patients with HCC | |||||
| Women | 35 of 9,481 | 43 of 1,600 | 49 of 630 | 14 of 106 | |
| Men | 58 of 9,060 | 212 of 2,331 | 47 of 465 | 19 of 112 | |
| Cumulative incidence at age 75 years, % | |||||
| Women | 1.03 | 7.99 | 16.71 | 27.40 | |
| 95% CI | 0.70 to 1.50 | 5.69 to 11.22 | 12.16 to 22.97 | 14.97 to 50.16 | |
| Men | 1.55 | 27.38 | 23.73 | 38.35 | |
| 95% CI | 1.16 to 2.07 | 23.45 to 31.96 | 17.40 to 32.37 | 22.92 to 64.17 | |
| HCC incidence rate, per 105 person-years | |||||
| Women | 22.35 | 164.98 | 492.62 | 875.28 | < .001 |
| 95% CI | 16.05 to 31.13 | 122.36 to 222.46 | 372.31 to 651.79 | 518.38 to 1,477.90 | |
| Men | 40.26 | 593.31 | 683.99 | 1,130.75 | .039 |
| 95% CI | 31.12 to 52.07 | 518.58 to 678.80 | 513.91 to 910.36 | 721.25 to 1,772.76 | |
| Crude HR† | |||||
| Women | Referent | 7.68 | 17.10 | 31.20 | < .001 |
| 95% CI | 4.93 to 11.95 | 11.11 to 26.31 | 16.82 to 57.86 | ||
| Men | 1.63 | 17.32 | 14.46 | 26.19 | .092 |
| 95% CI | 1.08 to 2.46 | 12.99 to 23.09 | 9.87 to 21.19 | 15.63 to 43.88 | |
| Adjusted HR I‡ | |||||
| Women | Referent | 7.04 | 10.32 | 18.55 | .0067 |
| 95% CI | 4.51 to 11.00 | 6.64 to 16.06 | 9.92 to 34.72 | ||
| Men | 1.15 | 15.10 | 9.25 | 16.98 | .010 |
| 95% CI | 0.74 to 1.79 | 11.29 to 20.19 | 6.23 to 13.74 | 10.01 to 28.82 | |
| Adjusted HR II† | |||||
| Women | Referent | 7.18 | 10.57 | 19.26 | .0056 |
| 95% CI | 4.60 to 11.22 | 6.80 to 16.43 | 10.30 to 36.02 | ||
| Men | 1.15 | 15.52 | 9.36 | 18.79 | .0056 |
| 95% CI | 0.74 to 1.79 | 11.61 to 20.76 | 6.30 to 13.91 | 11.10 to 31.83 | |
| P§ | .52 | .0044 | .68 | .95 |
Abbreviations: anti-HCV, antibodies against HCV; HBsAg, HBV surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio.
P values were obtained with tests for homogeneity of effects among those with HBsAg-seropositive and anti-HCV–seronegative, HBsAg-seronegative and anti-HCV–seropositive, and HBsAg-seropositive and anti-HCV–seropositive statuses in crude incidence rates of HCC, crude HRs, and HRs adjusted for age at enrollment, cigarette smoking status, alcohol consumption, and ALT and stratified by birth year and sex.
Cox model with age as time scale (in crude and adjusted HR II), adjusted for age at enrollment (40-49, 50-59, and 60-65 years, with those age 30-39 years as reference group), cigarette smoking (yes v no), alcohol drinking (yes v no), and ALT level (15-44 and ≥ 45 IU/L, with those with ALT < 15 IU/L as reference group) and stratified by birth year (adjusted HR II).
Stratified Cox model with follow-up time as time scale, adjusted for age at enrollment (40-49, 50-59, and 60-65 years, with those age 30-39 years as reference group), cigarette smoking (yes v no), alcohol drinking (yes v no), and ALT level (15-44 and ≥ 45 IU/L, with those with ALT < 15 IU/L as reference group) and stratified by birth year and sex.
P values were obtained with tests for difference of HRs between men and women in multivariate adjusted Cox model with age as time scale, stratification of birth year, and adjustment of covariates as mentioned.
Fig 1.
Multivariate adjusted cumulative incidence of hepatocellular carcinoma by serostatus of hepatitis B virus surface antigen (HBsAg) and antibodies against hepatitis C virus (anti-HCV). Cumulative incidence was estimated from multivariate adjusted Cox model with age as time scale, stratified by birth year and sex and adjusted for age at enrollment, cigarette smoking, alcohol consumption, and serum ALT level. (A) All patients (N = 23,785); (B) HBsAg-seropositive and anti-HCV–seronegative patients (n = 3,931); (C) HBsAg-seronegative and anti-HCV–seropositive patients (n = 1,095); (D) HBsAg- and anti-HCV–seropositive patients (n = 218).
The HR of developing HCC associated with serostatus of HBsAg and/or anti-HCV also varied with age, as shown in Table 3 and Figure 1. The multivariate adjusted HR of developing HCC significantly decreased with age in HBsAg-seropositive men (from 20.14 to 10.29; P = .0092) and significantly increased with age in anti-HCV–seropositive women (from 5.13 to 15.01; P = .0074). Because the age-specific HCC HR was derived by comparing noncarriers with those in the reference group of the same age, whose HCC risk also increased with age, the HBV-associated HCC HR might thus be lower.
Table 3.
Independent and Combined Age-Varying Effects (age ≤ 65 and > 65 years) of HBV and HCV Infections on Development of HCC
| Characteristic | HBsAg Seropositive, Anti-HCV Seronegative |
HBsAg Seronegative, Anti-HCV Seropositive |
HBsAg Seropositive, Anti-HCV Seropositive |
P† | |||
|---|---|---|---|---|---|---|---|
| HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | ||
| Men | |||||||
| Age, years | |||||||
| ≤ 65 | 20.14 | 13.85 to 29.30 | 9.56 | 5.48 to 16.66 | 19.07 | 9.05 to 40.18 | .0077 |
| > 65 | 10.29 | 6.85 to 15.45 | 9.17 | 5.51 to 15.25 | 18.81 | 9.28 to 38.10 | .15 |
| P‡ | .0092 | .91 | .98 | ||||
| Women | |||||||
| Age, years | |||||||
| ≤ 65 | 7.82 | 4.51 to 13.55 | 5.13 | 2.52 to 10.47 | 11.11 | 3.85 to 32.09 | .34 |
| > 65 | 6.58 | 3.57 to 12.14 | 15.01 | 9.04 to 24.92 | 27.29 | 13.06 to 57.05 | .001 |
| P‡ | .65 | .0074 | .15 | ||||
Abbreviations: anti-HCV, antibodies against HCV; HBsAg, HBV surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Multivariate adjusted HRs; stratified Cox model with age as time scale, stratified by birth year and adjusted for age at enrollment (40-49, 50-59, and 60-65 years, with those age 30-39 years as reference group), sex, cigarette smoking (yes v no), alcohol consumption (yes v no), ALT level (15-44 and ≥ 45 IU/L, with those with ALT < 15 IU/L as reference group), effects of which were assumed constant across different ages and sexes, with noncarriers (men and women) as reference group.
P values were calculated to test difference of effect estimates across three virus-carrying statuses among patients age ≤ 65 or > 65 years.
P values were calculated to test difference between effect estimates of age ≤ 65 and > 65 years.
Before age 65 years, the HRs for dual infection are subadditive of those for single infection, especially in men. After age 65 years, the HR for dual infection was consistently higher than that for single infection of HBV and HCV in all participants (22.38 v 8.94 and 12.34; P < .001 and = .023, respectively), men (18.81 v 10.29 and 9.17; P = .084 and .059, respectively), and women (27.29 v 6.58 and 15.01; P < .001 and = .096, respectively). However, the interactive effects on HCC of HBV and HCV were still significantly different from the multiplicative interaction.
Viral Loads of HBV and HCV
As shown in Figure 2A, serum HBV DNA levels were significantly lower in those with dual infection than in those with HBV monoinfection (geometric mean ± standard deviation: 5,258.1 ± 48.5 v 16,737.7 ± 62.9 copies/mL; P < .001), especially in men (4,684.3 ± 45.8 v 20,422.4 ± 64.1 copies/mL; P < .001). Furthermore, there was a significant inverse association between serum HBV DNA and HCV RNA levels (Spearman correlation = −0.27; P < .001) among participants with dual infection (Fig 2B), which was also more significant in men (Spearman correlation = −0.28; P < .001).
Fig 2.
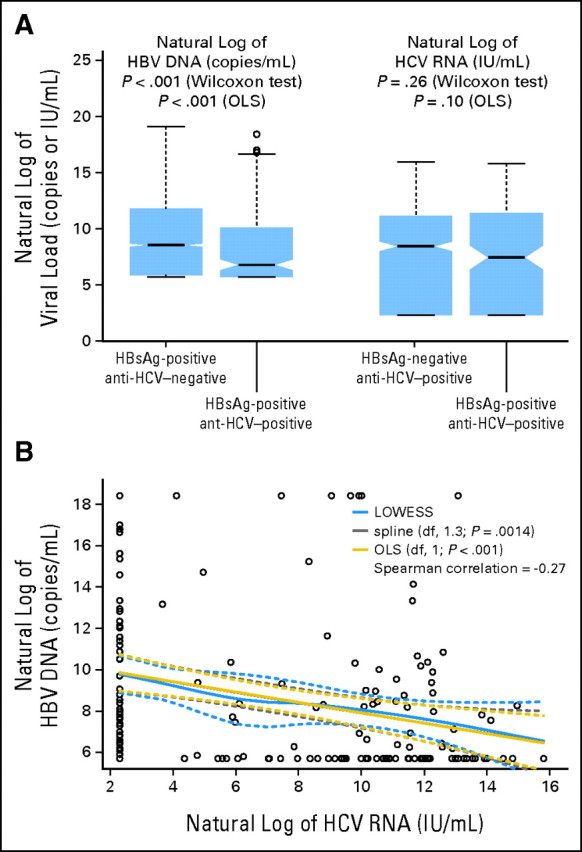
Association between hepatitis B virus (HBV) and hepatitis C virus (HCV) viral load. (A) Viral load compared between groups with mono- and dual infection (both men and women); (B) additional investigation of association between two viral loads. Anti-HCV, antibodies against HCV; df, degree of freedom; HBsAg, serostatus of HBV surface antigen; LOWESS, locally weighted scatterplot smoothing; OLS, ordinary least squares.
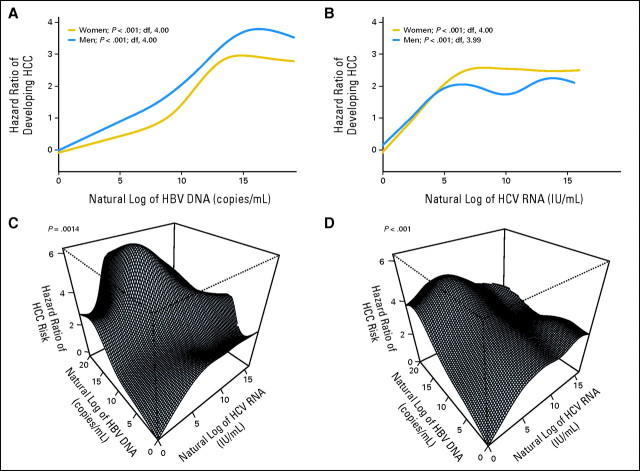
Both HBV and HCV viral loads (in natural log scale) were associated with increasing HCC HR (in natural log scale) and then reached a plateau (Figs 3A, 3B). In women, superimposed infection by HCV with moderate viral load increased HCC risk more dramatically (Fig 3C). In men, HCC risk of HBV carriers superimposed by HCV infection increased along with the increase of HCV viral load, reached a plateau when HBV viral load was low to moderate, and then started to decline when HBV viral load was high (Fig 3D). The convexity of dose-response surfaces in men indicated strong submultiplicative interaction (P < .001). The apex of the surface (highest risk of HCC) was located in the combination of high HBV viral load and moderate to low HCV viral load.
Fig 3.
Independent and combined effects of hepatitis B virus (HBV) and hepatitis C virus (HCV) viral load on hepatocellular carcinoma (HCC). Dose-response relationship of HBV and/or HCV viral load against risk of HCC were estimated for (A, B) marginal and (C, D) combined effects using penalized spline in Cox model. df, degree of freedom.
DISCUSSION
The decreasing seroprevalence of HBsAg with age observed in this study is consistent with previous findings in Taiwan.29,30 It may be the result of the following: first, patients seropositive for HBsAg had an increased mortality compared with those who were seronegative; second, spontaneous negative seroconversion of HBsAg accumulated with age; and third, older birth cohorts had a lower infection rate at birth or in early childhood than younger cohorts.31 The decrease in HBsAg seroprevalence with increasing age also suggests that almost all chronic HBV carriers in this study were infected in infancy or early childhood. The increasing seroprevalence of anti-HCV with age found in this study is also similar to previous findings in Taiwan.9,32 This may be attributable to the increased cumulative risk of HCV infection with age and the long-term seropositivity of anti-HCV after infection.
In this study, we first documented the cumulative lifetime (age 30 to 75 years) risk of developing HCC for men and women chronically infected with HBV and/or HCV. Lifetime risk estimates are important for the delineation of the natural history of chronic HBV and HCV and for the evaluation of the cost effectiveness of antiviral treatments for both diseases. By the age of 75 years, one quarter of patients or more affected by chronic viral hepatitis will be affected by HCC. Thus, it is essential to treat chronic HBV and HCV and monitor HCC development regularly. For the age group of 65 years or older, men and women with dual infection had a significantly higher HCC risk than those with single infection. More intensive clinical management of patients with dual infection of HBV and HCV is recommended.
In this study, the interactive effect of HBV and HCV on HCC ranged from sub- to superadditive but was much smaller than that with multiplicative interaction. Many studies have aimed to assess the combined effect of chronic HBV and HCV infections on development of HCC.13–24 Statistically, the interactive effect (ie, relative risks for HBV single infection, HCV single infection, and dual infection) has varied from above multiplicative (24.6, 4.0, and infinity, respectively)20 to superadditive (20.4, 23.6, and 135.0, respectively,21 and 15.6, 8.1, and 35.7, respectively24), additive (14.0, 27.1, and 40.1, respectively),17 and subadditive (53.4, 32.3, and 46.2, respectively,22 and 38.5, 21.5, and 30.4, respectively23). The discrepancy among studies may have resulted from the following reasons: first, the number of controls with dual infection was too small to derive an accurate estimation of the interactive effect in previous studies, which might have led to a wide range of estimates for interactive effect; second, most previous studies were based on cross-sectional observations, in which reverse causation might be of concern (eg, nosocomial infection of HCV33 may occur in patients with severe chronic HBV because of complicated liver disease and long hospitalization); and third, there were different distributions of age, sex, ethnicity, and prevalence of chronic HBV and HCV infections in different studies.
The finding that dual infection was associated with a subadditive combined effect before age 65 years (Table 3) and later onset of HCC than single infection of HBV (Fig 1) may indicate an antagonistic effect between HBV and HCV on the development of HCC at the beginning of superimposed HCV infection. The antagonism between HCV and HBV is also consistent with the inverse association between HBV and HCV viral loads (Figs 2A, 2B) and compatible with that the submultiplicative interactive effect of viral load on HCC. Thus, we conclude that HCC risk with dual infection is higher than that with single infection, but the magnitude is submultiplicative or even subadditive in men and in the younger population.
The suppression of HBV replication by HCV was first described in chimpanzee studies34 and case-series studies.35,36 The suppression of expression and replication of HBV by the core protein of HCV have also been reported in in vitro and in vivo studies.37,38 In humans, it was reported that seroclearance of HBsAg was higher in patients with dual infection than in those with HBV infection alone.35 The higher HCC risk with dual infection may result from the chronic necroinflammation of the liver through repeated attacks of the host immune system against both HBV and HCV.31,39 Thus, the malignant transformation of hepatocytes occurs as a result of continuous cell death and reactive proliferation. In addition to the common mechanism, HBV DNA can integrate into human genome and may initiate hepatocarcinogenesis,31 which enables HBV to transform hepatocytes even in the absence of chronic inflammation. It may also be reflected by our finding that HBV-associated HCC involves earlier onset.
Our analyses revealed that the risk of HBV-associated HCC was higher in men than in women, and the risk of HCV-associated HCC tended to increase when women became postmenopausal. Both findings imply the role of sex hormones in the virus-associated hepatocarcinogenesis. It has been reported that elevated serum testosterone is associated with development of HCC,40 and hepatic androgen receptor increases HBV-induced hepatocarcinogenesis in mice.41 In women, the ratio of estradiol to testosterone has been shown to be significantly lower in patients with HCC.42 The role of sex hormone imbalance in hepatocarcinogenesis may also explain the later onset of HCC in women, mostly after menopause. However, it remains unclear what role sex hormones play in the different patterns of HBV and HCV interactive effects on HCC.
This study has the advantages of a prospective community-based design, large sample size, and long-term follow-up. It has the limitation of unknown age at viral infection, especially for HCV. However, we may assume that chronic HBV infection occurred before the age of 3 years, and chronic HCV infection occurred mainly in late childhood and early adulthood.5,11 Another limitation is competing cause of death in the analysis of age-dependent HCC risk. We may not exclude the possibility that the decrease in HCC risk results from the high mortality of other diseases. In the analysis of liver-related disease mortality and overall mortality, women with chronic HCV infection seemed to have higher mortality resulting from liver-related diseases than men. But the dose-response relationships with viral load were similar in HCC incidence, HCC mortality, and liver-related disease mortality.
In conclusion, there is an inverse association between HBV and HCV viral load, and the effect of dual infection on HCC is higher than that of monoinfection, although in a submultiplicative pattern. Risk of HBV-associated HCC decreases with age in men, and HCV-associated HCC risk increases with age in women. More intensive clinical management is recommended for patients with dual HBV and HCV infection, especially for men of all ages and menopausal women.
Acknowledgment
Other members of the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer–Hepatitis B and C Virus Study Group: National Taiwan University Hospital: C.Y. Hsieh, H.S. Lee, P.M. Yang, C.H. Chen, J.D. Chen, S.P. Huang, C.F. Jan; National Taiwan University: T.H.H. Chen; National Defense Medical Center: C.A. Sun; Taipei City Psychiatric Center: M.H. Wu; Tzu Chi University: L.Y. Wang, S.Y. Chen; Shin Kong Wu Ho-Su Memorial Hospital: K.E. Chu; Huhsi Health Center, Penghu County: S.C. Ho, T.G. Lu; Provincial Penghu Hospital: W.P. Wu, T.Y. Ou; Sanchi Health Center, Taipei County: C.G. Lin; Provincial Chutung Hospital: K.C. Shih; Provincial Potzu Hospital: W.S. Chung, C. Li; Kaohsu Health Center, Pingtung County: C.C. Chen; Paihsa Health Center, Penghu County: W.C. How.
Appendix
Ascertainment of Hepatocellular Carcinoma
In Taiwan, all deaths are registered mandatorily through a nationwide system of offices called the Household Registration Offices. These registered records are subsequently double-checked annually by registration officers; when available, autopsy data are used to verify the diagnoses. The death certificates are then coded by the National Department of Health; these records became computerized in 1971. The Taiwan National Cancer Registry System was established by the Department of Health in 1979. Since that time, instances of all neoplasms that have been coded according to the International Classification of Diseases for Oncology–Field Trial coding system from each hospital with more than 50 beds have been reported to the registry. The information registered includes patient name, national identification number, sex, age, date of diagnosis, location of tumors, and histologic data. These data are checked for quality assurance and duplications using unique national identification number.
The data linkage of these two datasets was performed using the unique national identification number assigned to each resident in Taiwan at birth. The linked data were validated further by comparing birth dates and names in the datasets. Even though enrollment started in 1991, we performed data linkage from 1984 to further confirm that all participants were free of hepatocellular carcinoma (HCC) before enrollment. Medical records of patients identified as having HCC were then reviewed by gastroenterologists using a standard case abstraction form. Clinical information in the case abstraction form was used to confirm HCC according to established criteria: histopathologic examination; positive lesion detected by at least two different imaging techniques (abdominal ultrasonography, angiogram, or computed tomography); or one imaging technique with a serum α-fetoprotein level greater than 400 ng/mL.
Footnotes
Written on behalf of the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer–Hepatitis B and C Virus Study Group.
Supported in part by Grant No. AI463059 from Bristol-Myers Squibb (C.-J.C.) and by the Department of Health, Executive Yuan, Taiwan.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jun Su, Bristol-Myers Squibb (C); Uchenna H. Iloeje, Bristol-Myers Squibb (C) Consultant or Advisory Role: None Stock Ownership: Jun Su, Bristol-Myers Squibb; Uchenna H. Iloeje, Bristol-Myers Squibb Honoraria: None Research Funding: Chien-Jen Chen, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Yen-Tsung Huang, Chin-Lan Jen, Hwai-I Yang, Jun Su, Sheng-Nan Lu, Uchenna H. Iloeje, Chien-Jen Chen
Financial support: Hwai-I Yang, Jun Su, Chien-Jen Chen
Administrative support: Hwai-I Yang, Chien-Jen Chen
Provision of study materials or patients: Chin-Lan Jen, Hwai-I Yang, Chien-Jen Chen
Collection and assembly of data: Chin-Lan Jen, Hwai-I Yang, Mei-Hsuan Lee, Chien-Jen Chen
Data analysis and interpretation: Yen-Tsung Huang, Chin-Lan Jen, Hwai-I Yang, Sheng-Nan Lu, Uchenna H. Iloeje, Chien-Jen Chen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Hepatitis viruses. IARC Monogr Eval Carcinog Risks Hum. 1994;59:1–255. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15(suppl):E3–E6. doi: 10.1046/j.1440-1746.2000.02124.x. [DOI] [PubMed] [Google Scholar]
- 5.Hsu HY, Chang MH, Chen DS, et al. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: A study just before mass hepatitis B vaccination program in Taiwan. J Med Virol. 1986;18:301–307. doi: 10.1002/jmv.1890180402. [DOI] [PubMed] [Google Scholar]
- 6.Wu JS, Lu CF, Chou WH, et al. High prevalence of hepatitis C virus infection in aborigines in Taiwan. Jpn J Med Sci Biol. 1992;45:165–174. doi: 10.7883/yoken1952.45.165. [DOI] [PubMed] [Google Scholar]
- 7.Lu SN, Chue PY, Chen IL, et al. Incidence of hepatitis C infection in a hepatitis C endemic township in southern Taiwan. Kaohsiung J Med Sci. 1997;13:605–608. [PubMed] [Google Scholar]
- 8.Sun CA, Chen HC, Lu CF, et al. Transmission of hepatitis C virus in Taiwan: Prevalence and risk factors based on a nationwide survey. J Med Virol. 1999;59:290–296. [PubMed] [Google Scholar]
- 9.Lee SD, Chan CY, Wang YJ, et al. Seroepidemiology of hepatitis C virus infection in Taiwan. Hepatology. 1991;13:830–833. [PubMed] [Google Scholar]
- 10.Sun CA, Chen HC, Lu SN, et al. Persistent hyperendemicity of hepatitis C virus infection in Taiwan: The important role of iatrogenic risk factors. J Med Virol. 2001;65:30–34. [PubMed] [Google Scholar]
- 11.Lu SN, Chen HC, Tang CM, et al. Prevalence and manifestations of hepatitis C seropositivity in children in an endemic area. Pediatr Infect Dis J. 1998;17:142–145. doi: 10.1097/00006454-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Lee SD, Lee FY, Wu JC, et al. The prevalence of anti-hepatitis C virus among Chinese patients with hepatocellular carcinoma. Cancer. 1992;69:342–345. doi: 10.1002/1097-0142(19920115)69:2<342::aid-cncr2820690211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Tzonou A, Trichopoulos D, Kaklamani E, et al. Epidemiologic assessment of interactions of hepatitis-C virus with seromarkers of hepatitis-B and -D viruses, cirrhosis and tobacco smoking in hepatocellular carcinoma. Int J Cancer. 1991;49:377–380. doi: 10.1002/ijc.2910490311. [DOI] [PubMed] [Google Scholar]
- 14.Kaklamani E, Trichopoulos D, Tzonou A, et al. Hepatitis B and C viruses and their interaction in the origin of hepatocellular carcinoma. JAMA. 1991;265:1974–1976. [PubMed] [Google Scholar]
- 15.Simonetti RG, Camma C, Fiorello F, et al. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis: A case-control study. Ann Intern Med. 1992;116:97–102. doi: 10.7326/0003-4819-116-2-97. [DOI] [PubMed] [Google Scholar]
- 16.Chuang WL, Chang WY, Lu SN, et al. The role of hepatitis B and C viruses in hepatocellular carcinoma in a hepatitis B endemic area: A case-control study. Cancer. 1992;69:2052–2054. doi: 10.1002/1097-0142(19920415)69:8<2052::aid-cncr2820690808>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Chuang WL, Chang WY, Lu SN, et al. The role of hepatitis C virus in chronic hepatitis B virus infection. Gastroenterol Jpn. 1993;28(suppl 5):23–27. doi: 10.1007/BF02989199. [DOI] [PubMed] [Google Scholar]
- 18.Tsai JF, Chang WY, Jeng JE, et al. Effects of hepatitis C and B viruses infection on the development of hepatocellular carcinoma. J Med Virol. 1994;44:92–95. doi: 10.1002/jmv.1890440117. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Yu MW, Lu CF, et al. A nested case-control study on association between hepatitis C virus antibodies and primary liver cancer in a cohort of 9,775 men in Taiwan. J Med Virol. 1994;43:276–280. doi: 10.1002/jmv.1890430315. [DOI] [PubMed] [Google Scholar]
- 20.Sun CA, Farzadegan H, You SL, et al. Mutual confounding and interactive effects between hepatitis C and hepatitis B viral infections in hepatocellular carcinogenesis: A population-based case-control study in Taiwan. Cancer Epidemiol Biomarkers Prev. 1996;5:173–178. [PubMed] [Google Scholar]
- 21.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuper HE, Tzonou A, Kaklamani E, et al. Hepatitis B and C viruses in the etiology of hepatocellular carcinoma: A study in Greece using third-generation assays. Cancer Causes Control. 2000;11:171–175. doi: 10.1023/a:1008951901148. [DOI] [PubMed] [Google Scholar]
- 23.Sun CA, Wu DM, Lin CC, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: A prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674–682. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Zhu L, Liu S, et al. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 27.Iloeje UH, Yang HI, Jen CL, et al. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol. 2007;5:921–931. doi: 10.1016/j.cgh.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: Long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 29.Chung DC, Ko YC, Chen CJ, et al. Seroepidemiological studies on hepatitis B and D viruses infection among five ethnic groups in southern Taiwan. J Med Virol. 1988;26:411–418. doi: 10.1002/jmv.1890260408. [DOI] [PubMed] [Google Scholar]
- 30.Chang MH, Hsu HY, Hsu HC, et al. The significance of spontaneous hepatitis B e antigen seroconversion in childhood: With special emphasis on the clearance of hepatitis B e antigen before 3 years of age. Hepatology. 1995;22:1387–1392. [PubMed] [Google Scholar]
- 31.You SL, Yang HI, Chen CJ. Seropositivity of hepatitis B e antigen and hepatocellular carcinoma. Ann Med. 2004;36:215–224. doi: 10.1080/07853890310021580. [DOI] [PubMed] [Google Scholar]
- 32.Sheu JC, Wang JT, Wang TH, et al. Prevalence of hepatitis C viral infection in a community in Taiwan: Detection by synthetic peptide-based assay and polymerase chain reaction. J Hepatol. 1993;17:192–198. doi: 10.1016/s0168-8278(05)80037-6. [DOI] [PubMed] [Google Scholar]
- 33.Forns X, Martínez-Bauer E, Feliu A, et al. Nosocomial transmission of HCV in the liver unit of a tertiary care center. Hepatology. 2005;41:115–122. doi: 10.1002/hep.20515. [DOI] [PubMed] [Google Scholar]
- 34.Tsiquaye KN, Portmann B, Tovey G, et al. Non-A, non-B hepatitis in persistent carriers of hepatitis B virus. J Med Virol. 1983;11:179–189. doi: 10.1002/jmv.1890110302. [DOI] [PubMed] [Google Scholar]
- 35.Liaw YF. Role of hepatitis C virus in dual and triple hepatitis virus infection. Hepatology. 1995;22:1101–1108. doi: 10.1016/0270-9139(95)90615-0. [DOI] [PubMed] [Google Scholar]
- 36.Koike K, Yasuda K, Yotsuyanagi H, et al. Dominant replication of either virus in dual infection with hepatitis viruses B and C. J Med Virol. 1995;45:236–239. doi: 10.1002/jmv.1890450222. [DOI] [PubMed] [Google Scholar]
- 37.Shih CM, Lo SJ, Miyamura T, et al. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schüttler CG, Fiedler N, Schmidt K, et al. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J Hepatol. 2002;37:855–862. doi: 10.1016/s0168-8278(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 39.Anzola M. Hepatocellular carcinoma: Role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383–393. doi: 10.1111/j.1365-2893.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 40.Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993;53:790–794. [PubMed] [Google Scholar]
- 41.Wu MH, Ma WL, Hsu CL, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32–35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farinati F, De Maria N, Marafin C, et al. Hepatocellular carcinoma in alcoholic cirrhosis: Is sex hormone imbalance a pathogenetic factor? Eur J Gastroenterol Hepatol. 1995;7:145–150. [PubMed] [Google Scholar]