Abstract
Background:
To evaluate variations in fibrin network patterns of the platelet-rich fibrin (PRF) in different age groups.
Materials and Methods:
Ninety-five patients were divided into three age groups: Group 1: (20–39 years); Group 2: (40–59 years); and Group 3: (60 years and above). PRF was prepared from blood samples of all patients and were subjected to cell block cytology method of histological analysis and slides were prepared to histologically assess the age-related changes in (i) fibrin network patterns in terms of density and (ii) entrapment of platelets and white blood cells (WBCs) within fibrin meshwork.
Results:
Two types of fibrin network pattern arrangements noticed: Dense and loose types in three age groups. However, there was a noticeable decrease in the dense type of fibrin network with progressing age and increase in the loose type of fibrin arrangement. Furthermore, variation in a number of platelets and WBCs entrapped within fibrin network in relation to age was noticed.
Conclusion:
From the current study it can be concluded that age can be considered as one of the influencing factors on quality of PRF in terms of fibrin network patterns and hence, platelet and WBCs entrapment within these fibrin networks.
Keywords: Age, fibrin network patterns, platelet-rich fibrin, platelets, white blood cell
INTRODUCTION
Periodontitis is an infectious disease that causes the destruction of the tooth attachment apparatus. Untreated periodontitis results in progressive attachment loss that may eventually lead to early tooth loss.[1] Hence, the aim of periodontal treatment is to arrest the disease progression and to achieve the ultimate goal of regeneration of the destroyed periodontal tissues. With an urge to achieve this very goal of regeneration through periodontal treatment, the use of various bone grafts and biomaterials such as platelet-rich plasma (PRP), platelet-rich fibrin (PRF) have been introduced, tried and tested for in conjunction with the conventional open flap debridement.
The greatest challenge in clinical research is the development of bioactive surgical additives, which help to regulate inflammation and increase the speed of healing process.[2] Fibrin glue was originally described in 1970 and is formed by polymerizing fibrinogen with thrombin and calcium.[3] However, the biochemical architecture of fibrin fibrillae obtained by this protocol is condensed tetramolecular or bilateral junctions. This type is least favorable to cytokine enmeshment and cellular migration.[4] PRP is an autologous modification of fibrin glue and was introduced for the 1st time by Marx et al.[5] It also is a concentration of growth factors proved to be actively secreted by activated platelets to initiate wound healing. PRP is more than just a platelet concentrate since it contains a small volume of plasma and hence, three proteins (fibrin, fibronectin, and vitronectin) which act as cell adhesion molecules for osteoconduction and epithelial migration. However, PRP results in tetramolecular or bilateral junctions constituted with strong thrombin concentrations, allows the thickening of fibrin polymers: This leads to a rigid network, not very favorable to cytokine enmeshment and cellular migration,[6] leading to cytokines which are small soluble molecules, released too quickly to be closely built in inside the fibrin matrix during polymerization.[2] Thus, PRF was first developed in France by Choukroun et al. in 2001. This second generation platelet concentrate eliminates the risk associated with the use of bovine thrombin. On the other hand, a slow physiological fibrin polymerization results in higher percentage of equilateral junctions, leading to a flexible network capable of cytokine entrapment and cellular migration.[7] The evolution in platelet concentrates is thus, to obtain a biomaterial, with a composition of high-density fibrin network wherein the platelet concentrates not only act as biomaterial but also the matrix itself has healing effects.[7] For regeneration, it is known that a blood clot is the center focus of initiating any soft tissue healing and bone regeneration. The natural blood clot is seen to contain 95% red blood cells (RBCs), 5% platelets, <1% white blood cells (WBCs) and numerous amounts of fibrin strands.[3] This natural blood clot is seen to contain only a small percentage of platelets which, however, are seen to have a central role to play in the process of regeneration. Hence, PRF was introduced and is based on a simple strategy of enhancing the healing capacity of a natural blood clot by supplementing the natural blood clot with increased platelet concentrations. Thus, a PRF blood clot contains platelet concentration (>97%), high enough to accelerate the soft and hard tissue healing. Considering the composition of PRF, it is seen to consist of a fibrin matrix polymerized in a tetramolecular structure, with the incorporation of platelets, leukocytes, cytokines, and circulating stem cells. The factors that affect fibrin formation and structure may be: Genetic factors, acquired factors like (abnormal concentration of thrombin and factor XIII in plasma, blood flow, platelet activation oxidative stress, hyperglycemia, hyper-homocysteinemia, medications, and cigarette smoking) and other parameters (such as microgravity, pH, temperature, reducing agents and concentration of chloride and calcium ions)[8] including the age of the patient. In relation to PRF various studies till date have been performed for a better understanding of the characteristic features of platelets, WBCs and their role in regeneration. However, the patterns and arrangement of fibrin networks within the PRF clot, their capacity to entrap platelets and WBCs and the impact of age of the patient on fibrin network patterns and arrangement have not been highlighted. Thus, this is study aims to evaluate the variations in the fibrin network patterns of the PRF clot, isolated from individuals of different age groups.
MATERIALS AND METHODS
The study protocol involved blood sample collection, PRF preparation and preparation of slides for histological analysis.
Patient selection and distribution
The study was performed at “The Oxford Dental College and Hospital,” Bengaluru. Patients participating in the study included those reporting for treatment to the Department of Periodontics and Department of Oral and Maxillofacial Pathology for complete blood count. The patients had a minimum of 20 teeth. The inclusion criterion for this study was patients with a normal platelet count for all age groups. The exclusion criteria included tobacco smoking and chewing habits, platelet and coagulation disorders, systemic diseases/conditions, medications affecting the blood, pregnant and lactating women. Ninety-five patients were divided into three age groups, i.e., 33 samples in Group 1 (20–39 years); 32 samples in Group 2 (40–59 years), and 30 samples in Group 3 (60 years and above) with random gender distribution, i.e. Group 1 (M/F = 20/13); Group 2 (M/F = 20/12); and Group 3 (M/F = 12/18).
Table 1 provides detailed information regarding a number of patients distributed within each of these two age groups.
Table 1.
Provides detailed information regarding number of patients distributed within each of these three age groups

Platelet-rich fibrin preparation
Intravenous blood 3–4 ml (by venipuncture the antecubital vein) was collected with a 5 ml disposable syringe and was immediately transferred to disposable vacuum test tube and centrifuged at 3000 rpm for 10 min in the centrifugation machine (REMI). Because of differential densities, it resulted in the separation of three basic fractions: A base of RBCs at the bottom, acellular plasma on the surface, and finally a PRF clot between the two [Figure 1].[9]
Figure 1.
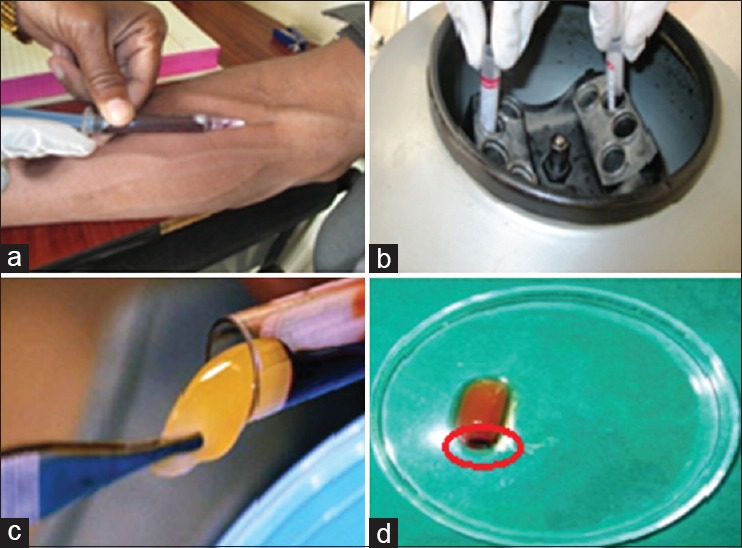
Platelet-rich fibrin preparation (a) blood collection; (b) centrifugation machine; (c) platelet-rich fibrin clot; (d) platelet-rich fibrin clot with red blood cell's layer
The PRF clot thus formed was obtained with the help of sterile tweezer and scissors by cutting it in such a manner as to preserve a small RBC layer since the platelets and WBCs are concentrated in an intermediate layer located between RBCs and the PRF clot.[10]
Platelet-rich fibrin clot slide preparation for histological analysis
The isolated PRF clots were prepared into slides by cell block cytology method. Cell block cytology method is the latest method of analyzing fine needle aspiration cytology fluids by cytopathologists. However, for this study the same cell block cytology method was applied to study the PRF clot [Figure 2].
Figure 2.
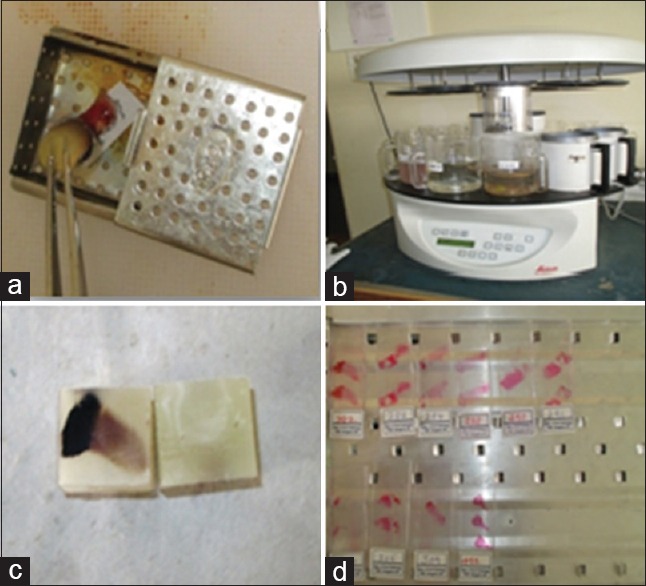
Platelet-rich fibrin clot slide preparation for histological analysis (a) fixing and labeling of platelet-rich fibrin clot; (b) tissue processing (c) embedding; (d) prepared slide
The procedural steps followed for obtaining the PRF clot slides by this method were as follows:
Step 1: Fixing – the isolated PRF clot, was transferred into a medium sized perforated stainless steel cassette inside which, a small chit containing the patient number was written with a lead pencil was placed, for the purpose of ease of identification. The cassettes containing the PRF clot were transferred into a container containing 10% formalin where they were fixed for 24 h. The aim of fixing is to preserve the biological tissues in life like state, thereby preventing autolysis or putrefaction.
Step 2: Tissue processing – after 24 h, from the 10% formalin containing container, the cassettes were transferred into a perforated stainless steel cylindrical container which were subjected to various steps such as dehydration, clearing, and infiltration of wax into the PRF clot as they passed through various processing solutions such as 10% formalin, 60%, 70%, 80%, 90%, and 100% isopropanol alcohol, xylene (two changes) and paraffin wax in an orderly manner. Tissue processing was performed for 16 h in an automated tissue processor (Leica). The aim of tissue processing is to remove water from tissues and replace with a medium that solidifies to allow thin sections to be cut.
Step 3: Embedding – leuchars blocks were used as molds for embedding tissue using paraffin wax. Embedding grants support to the tissue section for sectioning and production of a slide.
Step 4: Tissue sectioning – sectioning was done using Leica microtome and sections of 4 µm thickness are sliced.
Step 5: Dewaxing – sections were deparaffinized firstly by heating the slides for about 55°C and then immediately were dropped into xylene to eliminate wax. The purpose of dewaxing is to allow the tissues to be stained.
Step 6: Tissue staining – sections were stained using hematoxylin and eosin stain. Staining is employed to give contrast to the tissue as well as highlighting particular features of interest.
Step 7: Slide numbering – based on the order of patients maintained in the registers of the department of periodontics and oral pathology.
Step 8: Histological slide analysis - was carried out using compound microscope at 20x, 40x and 100x magnifications.
RESULTS
The stained sections of PRF clot, in all the three age groups were assessed for (i) dense fibrin network pattern (ii) loose fibrin network pattern (iii) the entrapment pattern of platelets and WBCs within the dense and loose type of fibrin networks.
The histologic sections of all age groups, in general, showed an outermost layer of RBCs, followed by the dense fibrin network layer with maximum number of platelet and WBCs entrapped and the inner layers as moving away from RBC layer were seen to be dominated by loose fibrin network pattern with reduced entrapment of platelets and WBCs.
The results of the study reveal that patient in 20 years age group showed both dense as well as the loose type of fibrin network pattern, with the domination of dense type of fibrin network pattern than which can be described as packeting like the pattern of fibrin network. Thus, the dense fibrin network pattern was seen to have a homogenous distribution throughout the PRF clot. Furthermore, the associated entrapment of platelets and WBCs is in the form of aggregates within the dense fibrin network [Figure 3a].
Figure 3.
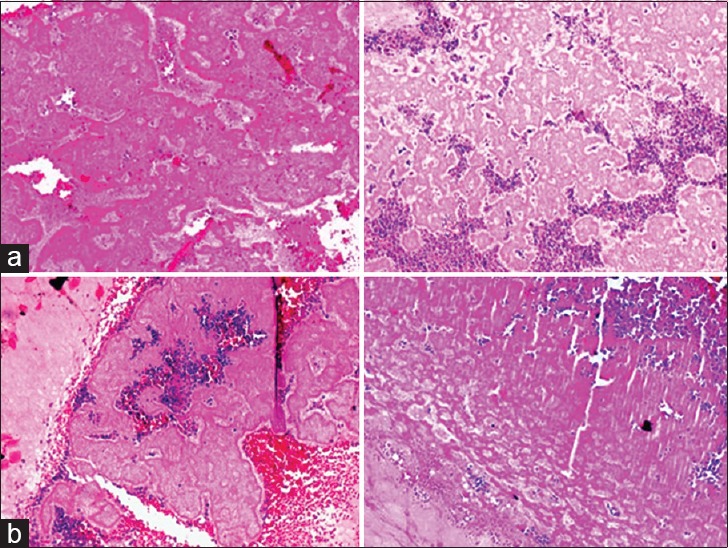
Histologic presentation of fibrin network pattern in platelet rich fibrin clot in age groups: (a) Age group 20 years: Blue areas – platelet and white blood cell aggregates, pink areas – dense fibrin network predominantly, packeting like pattern and (b) age group 30 years: Red areas – red blood cell, blue areas – platelets and white blood cell aggregates, dark pink areas – dense fibrin network pattern, light pink areas – loose fibrin network pattern
However, patients from 30 years age group showed both dense as well as the loose type of fibrin network pattern, with the distribution of dense pattern of fibrin network being dominant throughout the PRF clot. Fibrin network pattern was less homogenous as compared to 20 years age range. Furthermore, the entrapment of platelets was seen to be maximum with the dense type of fibrin network as compared to the loose pattern of fibrin network arrangement [Figure 3b].
Patients in the late 30 years and 40 years age group showed almost an equal distribution of both dense and loose type of fibrin networks throughout the PRF clot. A distinct feature noticed in this age group is that within the dense fibrin network pattern there were areas of loosely arranged fibers present. Thus, this age range can be considered as the “transition period.” Also, the entrapment of platelets and WBCs showed a layered arrangement within the dense type of fibrin network and scattered type of distribution of platelets and WBCs in the loose type of fibrin network [Figure 4].
Figure 4.
Histologic presentation of fibrin network pattern in platelet rich fibrin clot in the age group of the late 30s and 40 years. Red areas – red blood cells, blue areas – platelets and white blood cell (layered arrangement), dark pink – dense fibrin network with interspersed loose fibrin network, light pink – loose fibrin network
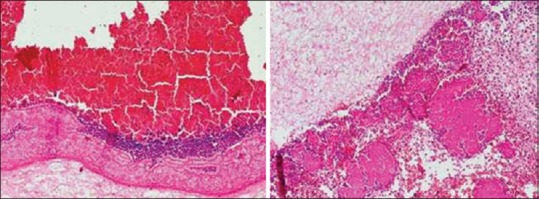
Patients in the 50 age group showed both dense and loose type of fibrin network pattern, with the dominant pattern being the loose fibrin network distributed throughout the PRF clot. The dense fibrin network arrangement was more as a concentrated band like the arrangement with a comparative reduction in its quantity. Also, the entrapment of platelets and WBCs was seen in dense fibrin network patterns with a marked reduction in the entrapment capacity of the loose fibrin network and hence, reduction in platelet and WBCs number [Figure 5a].
Figure 5.
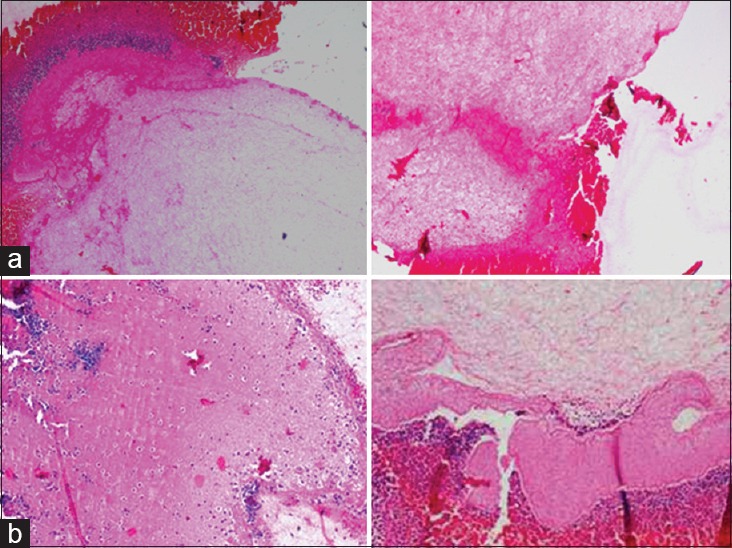
Histologic presentation of fibrin network pattern in platelet rich fibrin clot in (a) age group 50 years: Red area – red blood cells, blue area – platelets and white blood cells, dark pink area – dense fibrin network, light pink area – loose fibrin network (scanty) and (b) age group 60 and above: Red area – red blood cells, blue area – platelets and white blood cells, blue area – platelets and white blood cells, dark pink area – dense fibrin network, light pink area – loose fibrin network
Patients in the age group of 60 and above also showed both dense and loose fibrin network patterns of arrangement. However, the dense fibrin network pattern though was not reduced in quantity, however, showed less dense fibrin network arrangement when compared to the younger and 50 years age groups and showed scattered arrangement of platelets and WBCs. The loose fibrin network pattern arrangement is scanty in nature with very minimal entrapment of platelet and WBC entrapment [Figure 5b].
Comparing the 50 age group with 60 and above age group, though no major differences are noted with the dense fibrin network pattern in both the groups, notable variation is noted with the loose fibrin network pattern arrangement, i.e., it being more scanty in the 60 and above age group.
DISCUSSION
Till date extensive research has been carried out to study the functions and properties platelets and WBCs within the PRF clot and how they can affect the treatment outcome. However, this is one of its kind study which tries to shift the focus toward a better understanding of the fibrin network in terms of its arrangement, distribution and hence, the important role it can play in influencing the functions and activity of the platelets.
The study results thus obtained the state that, as the age of the individual progress, the fibrin network patterning becomes more loose and irregular in arrangement with each fibrin becoming thinner in nature and also the entrapment of the platelets and WBCs within the fibrin network is progressively reduced.
In the current research, we could observe the following:
The variations in the platelet – WBC distribution within the PRF clot: The highest platelet/leukocyte density is found in the 1st mm of the yellow clot, just after the red clot. The platelet/leukocyte distribution becomes increasingly scarce as they move away from the red clot. These results correlated to some extent with a study by Patel et al.[11]
Dense type of fibrin pattern – mainly in the areas associated closely with the sites of platelet distribution and close to the RBC layer and the density of fibrin network reduced in terms of quantity and quality in older patients as compared to younger patients.
Loose type of fibrin pattern – in relation with area away from the platelet distribution. This study thus, shows denser fibrin network and a larger amount of WBC and platelet entrapment in younger individuals as compared to the older individuals. From the study, it can also be stated that there are significant intergroup differences in the patterns of fibrin network arrangement and their ability to entrap the platelets and WBCs observed, as the age progresses, i.e., from 20 to 55 years and above.
However, in the age group 55 and above, dense and loose fibrin network arrangement pattern did not show major differences.
The study included only healthy patients with no habits like tobacco and alcohol consumption. Yet there were intragroup variations noted, which could be because of nutritional status, the gender of the patients, the influence of which was not assessed in this study.
These results show that the fibrin network density and their entrapment capacity of platelet and WBCs share a positive correlation. This clearly points out at the fact that, platelets and fibrin are interdependent in carrying out the process of wound healing and that their interaction is the key to successful wound healing and hence, regeneration. This interdependency can be explained on the basis of the functions and role of platelets and fibrin network in natural mechanism of wound healing that follows at the site of vascular injury.
Platelets accumulate in significant numbers at the site of vascular injury, when subendothelium becomes exposed to the bloodstream.[12,13] Fibrillar collagen is a major thrombogenic component of subendothelium, and it triggers platelet thrombus formation in flowing human blood.[14,15] The formation of a platelet thrombus is dependent on the platelet glycoprotein IIb-IIIa, which binds to its bivalent ligand fibrinogen and thereby cross-links the platelets.[16] In addition, activated platelets participate in coagulationby providing assembly sites for the factor VIIIa (F.VIIIa)/F.LXa and F. Va/F. Xa complexes.[17,18,19] This results in the generation of thrombin on the platelet surface and the formation of a fibrin network. Thus, this step throws light on a very important function of platelets, i.e., “procoagulant activity” which the collagen adherent platelets exhibit and hence mediate the fibrin network formation at the site of injury.[20]
Thus, correlating the interactions between platelets and fibrin network in the natural process of wound healing, with that of biochemical analysis of the PRF composition indicates that, the fibrin network density and distribution is directly proportional to the distribution of platelets in association with them.
Now that the fibrin formation is initiated by the platelets, this formed fibrin continues to form in networks at the site of injury and in turn entraps the platelets and secures them to stay at the site for a prolonged time and continue with the function of growth factor release an essential step in wound healing process. Thus, fibrin network supports the platelets to efficiently carry out their functions by acting as a scaffold. Hence, it would not be wrong to say that, the binding of fibrin (ogen) to hemostasis proteins and platelets as well as to several different cells such as endothelial cells, smooth muscle cells, fibroblasts, leukocytes, and keratinocytes is indispensable during the process of wound repair.
Laurens et al. stated that the outcome of wound healing depends largely on the fibrin structure, such as the thickness of the fibers, the number of branch points, the porosity, and the permeability.[21] Second, wound healing also depends upon the platelet concentration and their functions. Hence, focusing on the fibrin network functions in wound healing and their interaction with platelets and WBCs become very important for us to know in detail, the factors which can influence the fibrin network structure. Thus, this is the first study that attempts explore the influence of one such factor called age, on the fibrin network arrangement and the resultant variations and hence, their influence on the interactions between platelets and fibrin networks.
Dohan et al. showed the potential benefits of using PRF in periodontal regeneration is due to the cytokines which are present in platelet concentrates and the fine, flexible fibrin network which is more elastic in nature than PRP favoring cytokine entrapment and cellular migration,[22] thus highlighting the importance of fibrin network in periodontal regeneration.
This article shows how age can influence the fibrin network patterns and hence, platelet – WBC entrapment which in turn could influence the cytokine entrapment. Therefore, it can be stated that the age of the patient can be one of the influential factors in determining the PRF clot efficacy obtained from subjects of different age groups and hence, can be an important predictor of how much success to expect in terms of regeneration when treating subjects of different age groups.
To study the fibrin networks, the changes in their efficacy their ability to entrap the platelets and WBCs we performed the cell block cytology method which has been tried for the 1st time to study PRF till date as per our knowledge. The advantages being its cost effectiveness, easy availability, and less complicated procedure.
This method was performed using hematoxylin and eosin stain, proved to be satisfactory in identifying the variations in the fibrin network pattern and distribution with age and also the variations in platelet – WBCs concentrations entrapped within them. However, drawbacks noticed were, inability to correctly appreciate the changes in the individual fibrin strand morphology and thickness, difficulty in identifying and separating out the platelets and WBCs from each other represented by hematoxylin staining and exact counting of the platelets entrapped within the fibrin meshwork.
Thus, considering the very evident changes noticed with the fibrin network patterns in various age groups in this study, these results can be taken into account for clinical application, in estimating the outcome in terms of regeneration to be expected when treating intrabony defects using PRF in different age groups.
From this study, it can be stated that, the two major constituents of PRF, i.e., the platelets and fibrin network are interdependent thus, clearly showing that this interaction between them is the basis for the various properties of the PRF clot formed. The interdependency is that platelets actively participate in the initiation of coagulation cascade and then formed fibrin network pattern in turn entraps the platelets and WBCs and help them perform their functions for a sustained period of time.
Also, this study concludes that age can be one of the factors playing a significant role in altering fibrin network patterns and hence, its interaction with platelets thus, influencing the quality of the PRF clot.
However, further studies focusing on finding out the influence of nutrition and gender in association with age on the fibrin network pattern and their platelet – WBC entrapment capacity could be beneficial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000. 2009;51:208–19. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 4.van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426–37. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 6.Shobha P. Platelet concentrates: Past, present and future. Oral Surg. 2011;10:45–9. doi: 10.1007/s12663-011-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–67. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 8.Nunes CR, Roedersheimer MT, Simske SJ, Luttges MW. Effect of microgravity, temperature, and concentration on fibrin and collagen assembly. Microgravity Sci Technol. 1995;8:125–30. [PubMed] [Google Scholar]
- 9.Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodontol. 2011;38:925–32. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 10.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 11.Patel J, Deshpande N, Shah M, Dave D, Shah C, Shah S. PRF-from self to self. Res Rev J Dent Sci. 2013;1:30–4. [Google Scholar]
- 12.Baumgartner HR. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973;5:167–79. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- 13.Turitto VT, Baumgartner HR. Initial deposition of platelets and fibrin on vascular surfaces in flowing blood. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Textbook of Hemostasis and Thrombosis. 3rd ed. Philadelphia, PA: Lippincott; 1994. p. 805. [Google Scholar]
- 14.Baumgartner HR. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977;37:1–16. [PubMed] [Google Scholar]
- 15.Houdijk WP, Sakariassen KS, Nievelstein PF, Sixma JJ. Role of factor VIII-von willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J Clin Invest. 1985;75:531–40. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71:831–43. [PubMed] [Google Scholar]
- 17.Walsh PN. Platelet-coagulant protein interactions. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Textbook of Hemostasis and Thrombosis. 3rd ed. Philadelphia, PA: Lippincott; 1994. p. 629. [Google Scholar]
- 18.Hemker HC, van Rijn JL, Rosing J, van Dieijen G, Bevers EM, Zwaal RF. Platelet membrane involvement in blood coagulation. Blood Cells. 1983;9:303–17. [PubMed] [Google Scholar]
- 19.Mann KG, Krishnaswamy S, Lawson JH. Surface-dependent hemostasis. Semin Hematol. 1992;29:213–26. [PubMed] [Google Scholar]
- 20.Kirchhofer D, Tschopp TB, Steiner B, Baumgartner HR. Role of collagen-adherent platelets in mediating fibrin formation in flowing whole blood. Blood. 1995;86:3815–22. [PubMed] [Google Scholar]
- 21.Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–9. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 22.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]


