Abstract
Citrullination is the post-translational conversion of an arginine residue within a protein to the non-coded amino acid citrulline1. This modification leads to the loss of a positive charge and reduction in hydrogen bonding ability. It is carried out by a small family of tissue-specific vertebrate enzymes called peptidylarginine deiminases (PADIs)2 and is associated with the development of diverse pathological states such as autoimmunity, cancer, neurodegenerative disorders, prion diseases and thrombosis2,3. Nonetheless, the physiological functions of citrullination remain ill-defined, though citrullination of core histones has been linked to transcriptional regulation and the DNA damage response4–8. PADI4 (or PAD4/PADV), the only PADI with a nuclear localization signal9, was previously shown to act in myeloid cells where it mediates profound chromatin decondensation during the innate immune response to infection10. Here we show that the expression and enzymatic activity of PADI4 are also induced under conditions of ground state pluripotency and during reprogramming. PADI4 is part of the pluripotency transcriptional network, binding to regulatory elements of key stem cell genes and activating their expression. Its inhibition lowers the percentage of pluripotent cells in the early mouse embryo and significantly reduces reprogramming efficiency. Using an unbiased proteomic approach we identify linker histone H1 variants, which are involved in the generation of compact chromatin11, as novel PADI4 substrates. Citrullination of a single arginine residue within the DNA binding site of H1 results in its displacement from chromatin and global chromatin decondensation. Together, these results uncover a role for citrullination in the regulation of pluripotency and provide new mechanistic insights into how citrullination regulates chromatin compaction.
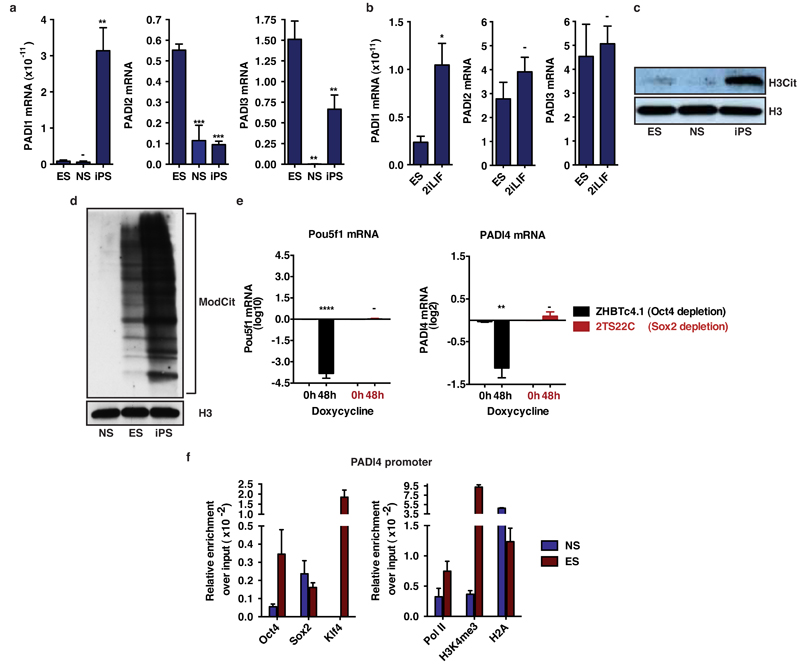
Pluripotent cells have the capacity to self-renew and differentiate into all somatic and germ cell lineages and, hence, possess immense therapeutic potential for a multitude of medical conditions. Their generation by reprogramming of differentiated somatic cells has been achieved by nuclear transfer, cell fusion and transduction of transcription factors, such as Oct4, Sox2, Klf4 and c-Myc12. Pluripotent cells have a distinctly open chromatin structure that is essential for unrestricted developmental potential13,14 and reprogramming involves an almost complete epigenetic resetting of somatic cells13. The ability of PADI4-mediated histone citrullination to induce chromatin decondensation in neutrophils10 prompted us to ask whether it can play a role in pluripotency, where chromatin decondensation is also necessary. To investigate this we first assessed the expression of Padi4 in the embryonic stem cell line ES Oct4-GIP (ES), the neural stem cell line NSO4G (NS) and in induced pluripotent stem (iPS) cells derived NSO4G (see Methods). Padi4 is expressed in pluripotent ES and iPS but not multipotent NS cells (Fig.1a). Culture of ES cells in 2i/LIF medium establishes a ground state of pluripotency15. This leads to the down-regulation of lineage specific markers and the up-regulation of pluripotency factors, as well as rapid induction of Padi4 (Fig. 1b). The pattern of Padi4 expression follows closely that of Nanog, an essential transcription factor for the transition to ground state pluripotency16(Fig 1a,b). While other PADIs are expressed in pluripotent cells, PADI4 is the only one whose expression clearly associates with naïve pluripotency (Extended Data Fig. 1a,b). Citrullination of histone H3 (H3Cit), a modification shown previously to be carried out specifically by PADI417, is detectable in ES and iPS cells (Extended Data Fig. 1c), indicating that PADI4 is also enzymatically active. H3 and global citrullination are undetectable in NS cells (Extended Data Fig. 1c,d).
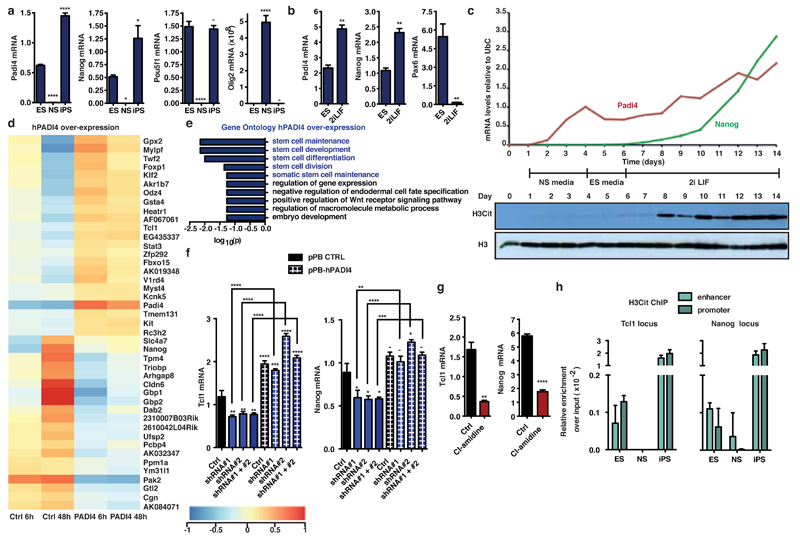
Figure 1: PADI4 expression and activity are features of pluripotent cells.
(a,b) qRT-PCR for Padi4 and Nanog expression in ES, NS and iPS cells (a), and in ES cells upon culture in 2i/LIF for one passage (b). Pou5f1, Olig2 and Pax6 are presented as controls. Expression normalized to Ubiquitin (UbC). Error bars: standard error of the mean of three biological replicates.
(c) qRT-PCR for Padi4 and Nanog expression and H3Cit immunoblot during the course of reprogramming (see also Extended Data Fig. 5a). Loading control: total histone H3. Representative of four experiments.
(d) Heat map of the genes regulated upon hPADI4 over-expression in mES cells, as determined by microarray analysis. Displayed values are normalized log intensities, minus the mean expression of the gene across the four samples. Hierarchical clustering based on correlation.
(e) Gene Ontology for Biological Process (GOBP) analysis of the above microarray dataset. P- value is corrected for multiple testing using Benjamini and Hochberg False Discovery Rate (FDR).
(f,g) qRT-PCR for Tcl1 and Nanog expression in mES cells after transient knock-down with Padi4 or control (Ctrl) shRNAs, and over-expression of human PADI4 or control vector (pPB CTRL) (f), and after treatment with 200µM Cl-amidine (g). Expression normalized to UbC. Error bars: standard error of the mean of three biological replicates.
(h) ChIP-qPCR for H3Cit on regulatory regions of Tcl1 and Nanog in mES, NS and iPS cells. Error bars: standard deviation of three technical qPCR replicates. Representative of three experiments.
Asterisks denote difference with ES cells (a) or media (b), Control (f, g) and between samples (f); - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001, by ANOVA (a,f) or t-test (b,g).
To determine the kinetics of PADI4 activation during the establishment of pluripotency, we examined RNA and protein samples collected daily during the course of reprogramming of NSO4G into iPS cells16. Padi4 is rapidly induced in NS cells after transduction of reprogramming factors but only becomes active to citrullinate H3 after introduction of 2i/LIF, closely following the onset of Nanog expression (Fig. 1c). These observations strongly suggested that PADI4 activity is associated with ground state pluripotency and prompted us to examine whether Padi4 is part of the pluripotency transcriptional network.
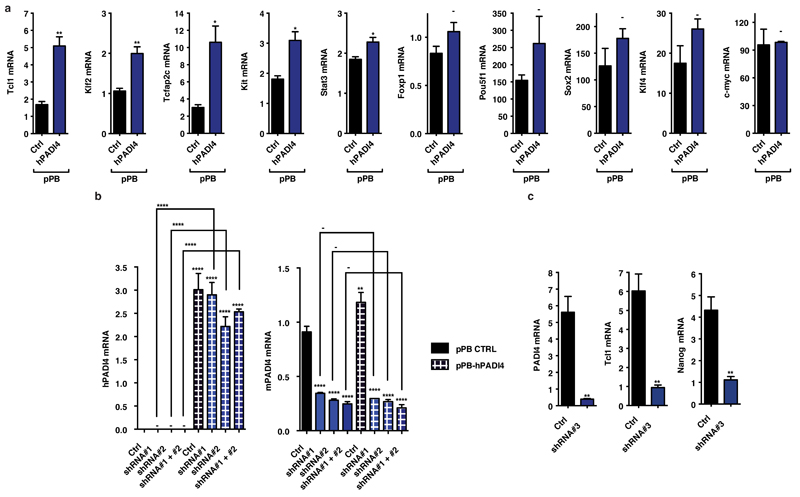
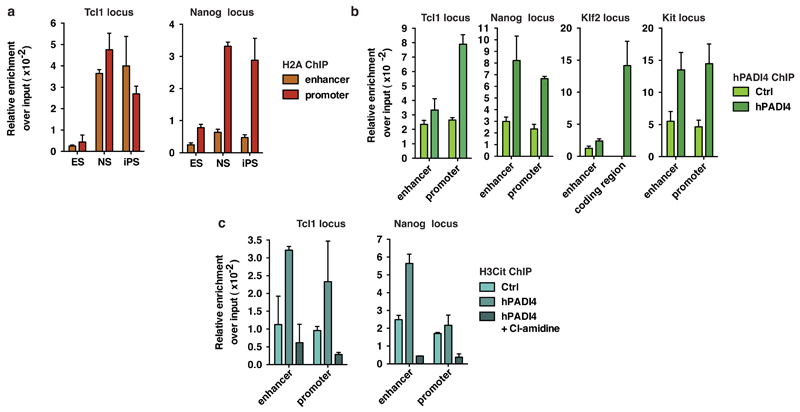
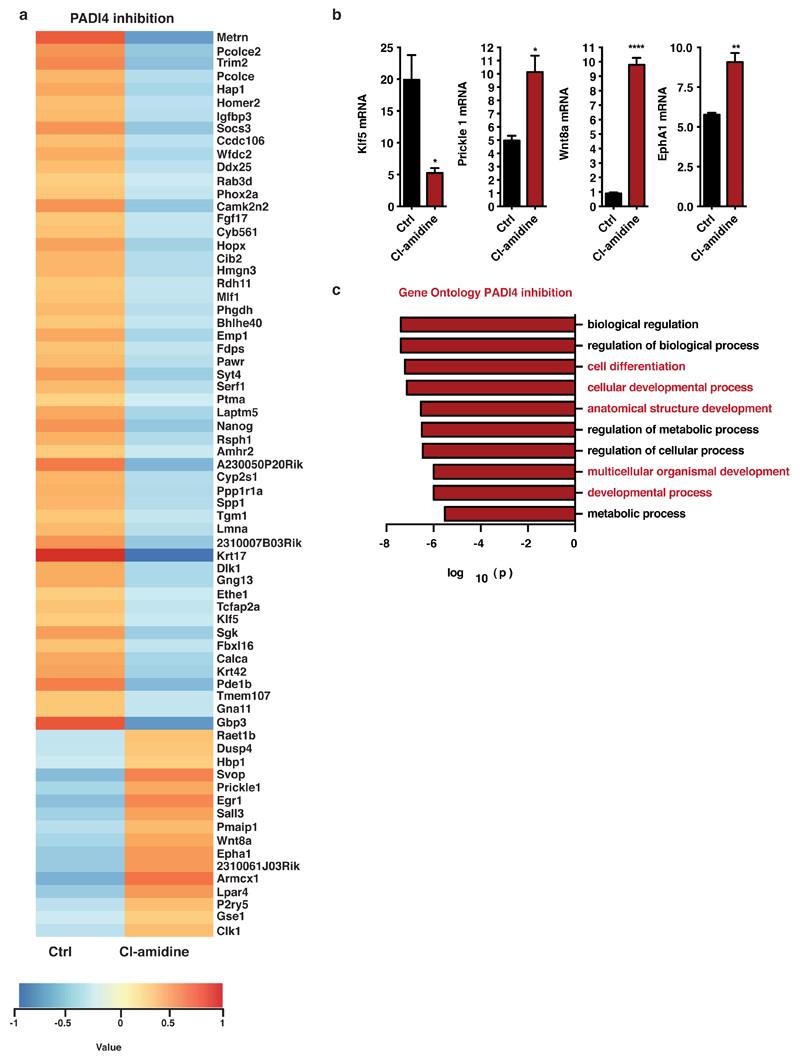
First, we asked whether the reprogramming factors regulate Padi4 expression, utilizing the ZHBTc4.1 and 2TS22C cell lines where Oct4 and Sox2, respectively, can be deleted acutely in response to doxycycline treatment (see Methods). Deletion of Oct4, but not Sox2, led to a decrease in Padi4 mRNA levels (Extended Data Fig. 1e). Furthermore, while Oct4 and Klf4 occupy the Padi4 promoter in ES, but not NS cells, Sox2 is bound in both cell types (Extended Data Fig. 1f). To understand the effects of PADI4 on transcriptional regulation in pluripotent cells, we analyzed the transcriptome of ES cells upon PADI4 over-expression and inhibition. Several key pluripotency genes are up-regulated in response to PADI4 over-expression (Fig. 1d, Extended Data Figure 2a and Supplementary Table 2), including Klf2, Tcl1, Tcfap2c, and Kit. Tcl1 was previously identified as the only regulator of self-renewal up-regulated in ground state pluripotency15 and over-expression of Tcl1 or Tcfap2c positively influence this process18. Gene Ontology (GO) analysis of this dataset indicates an enrichment of genes involved in stem cell development and maintenance (Fig. 1e). In addition, knockdown of Padi4 in mES cells leads to decreased expression of Tcl1 and Nanog, which is rescued by exogenous expression of RNAi-resistant human PADI4 (Fig. 1f, Extended Data Fig. 2b,c). These genes are under the control of PADI4 enzymatic activity since treatment with the chemical inhibitor Cl-amidine, which disrupts citrullination by PADI419, down-regulates their expression (Fig. 1g). Chromatin immunoprecipitation (ChIP) analysis indicated that H3Cit is present on regulatory regions of Tcl1 and Nanog in ES and iPS cells, but not NS cells (Fig. 1h and Extended Data Fig. 3a). Accordingly, exogenously expressed human PADI4 localizes to and is enzymatically active on these regions, as well as regulatory regions of Klf2 and Kit in ES cells (Extended Data Fig. 3b,c). In contrast to PADI4 over-expression, treatment of ES cells with Cl-amidine led to up-regulation of differentiation markers such as Prickle1, EphA1 and Wnt8a and down-regulation of pluripotency markers such as Klf5 (Extended Data Fig. 4a,b, and Supplementary Table 3), in addition to Nanog and Tcl1 (Fig. 1g). GO analysis of this dataset indicated enrichment in genes involved in cell differentiation (Extended Data Fig. 4c). Pou5f1 (Oct4), Klf4, Sox2 and c-Myc, were not affected by PADI4 modulation (Extended Data Fig. 2a and Supplementary Tables 2 and 3). Cumulatively, the above results place PADI4 within the pluripotency transcriptional network, suggesting that it acts downstream of some of the cardinal reprogramming factors to regulate a specific subset of pluripotency genes.
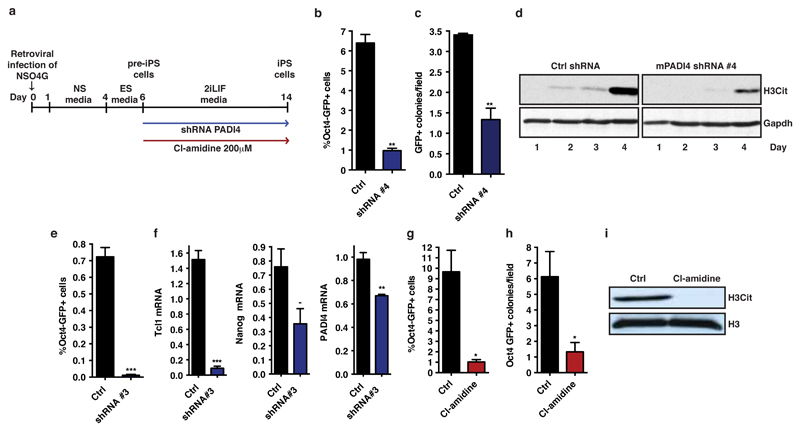
Prompted by the above observations, we investigated whether PADI4 is necessary for pluripotency, as assessed during reprogramming (Extended Data Fig. 5a) and in the early stages of embryo development. NSO4G cells express a GFP reporter under the control of the Oct4 regulatory sequences, which is activated upon acquisition of pluripotency16, allowing us to trace reprogrammed cells. Knock-down of Padi4 in NSO4G-derived pre-iPS cells impaired the ability of the cells to establish H3Cit upon switch to 2i/LIF medium and led to a clear reduction in reprogramming (Fig. 2a,b, Extended Data Fig. 5b-e and Extended Data time-lapse video). Consistent with this, levels of Tcl1 and Nanog were not elevated upon reprogramming to the same extent as in control cells (Fig. 2b and Extended Data Fig. 5f). Cl-amidine treatment led to a dramatic reduction of reprogramming efficiency and H3Cit (Fig. 2c and Extended Data Fig. 5g-i), suggesting that the catalytic activity of PADI4 is important for the induction of pluripotency.
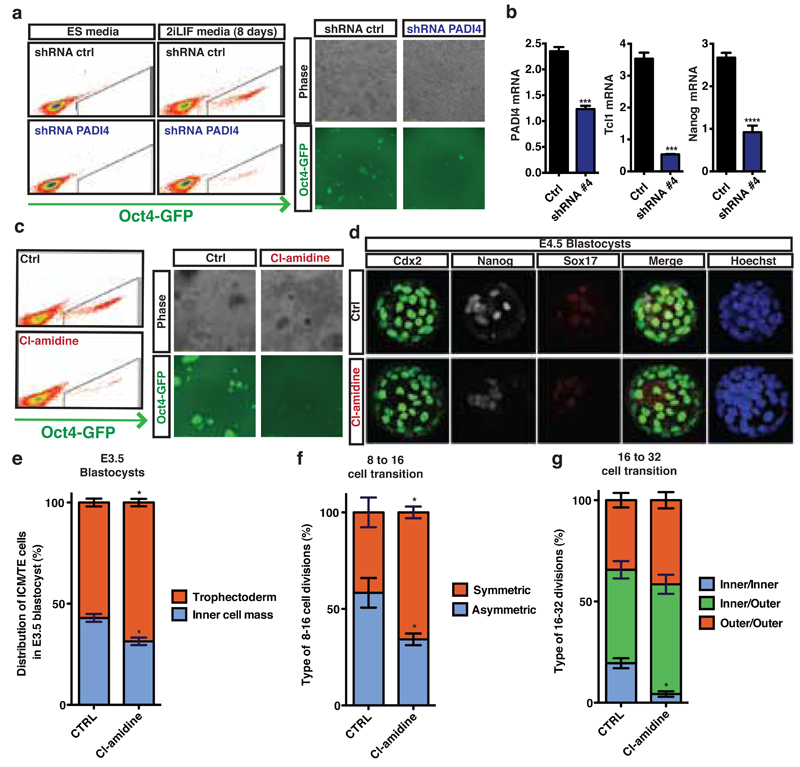
Figure 2: Citrullination and PADI4 regulate pluripotency during reprogramming and early embryo development.
(a) Flow cytometry analysis and phase contrast/fluorescence images for the assessment of Oct4-GFP reporter expression after reprogramming of pre-iPS cells stably expressing Padi4 and Ctrl shRNAs. Representative of four independent experiments. Time-lapse video in supplementary data online.
(b) qRT-PCR for expression of Tcl1, Nanog and Padi4 at the end of the above reprogramming assay. Error bars: standard error of the mean of triplicate samples.
(c) Flow cytometry analysis and phase contrast/fluorescence images for the assessment of Oct4-GFP reporter expression after reprogramming assay of pre-iPS cells treated with 200µM Cl-amidine. Representative of three independent experiments.
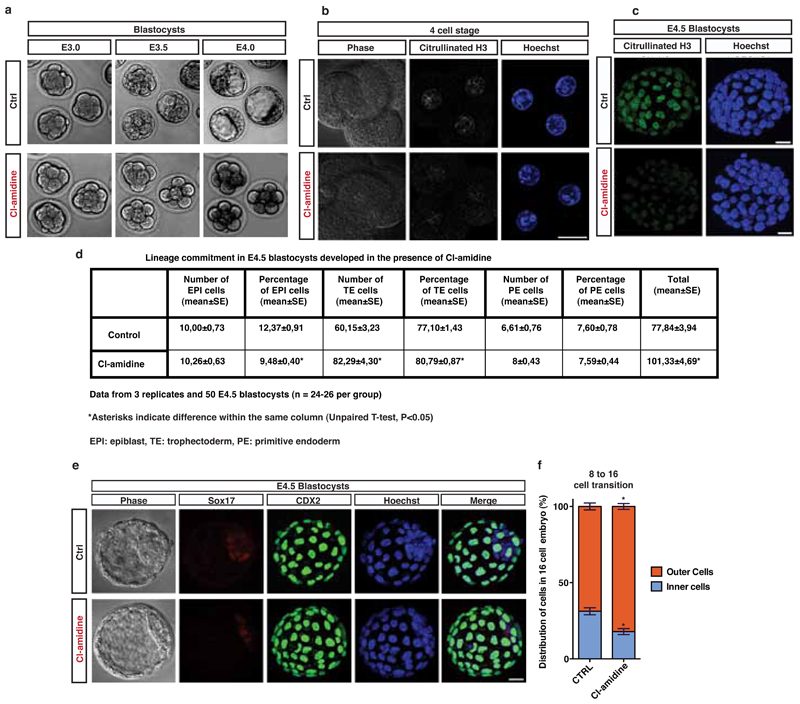
(d) E4.5 blastocysts from 2-cell stage embryos treated with 10µM Cl-amidine. SOX17 (primitive endoderm marker, red), Cdx2 (trophectoderm marker, green), Nanog (epiblast marker, white) and HOECHST 33342 (blue).
(e) Distribution of inner cell mass versus trophectoderm cells in E3.5 blastocyst treated as above.
(f,g) Time-lapse analysis of embryos in 10µM Cl-amidine from 2-cell stage. Number of symmetric versus asymmetric divisions at the 8 to 16-cell transition (f) and type of divisions at the 16 to 32-cell transition (g). Error bars: standard error of the mean.
Statistical significance was determined by unpaired t-test (b), or Mann Whitney test upon non-normal distribution (e-g). Asterisks denote difference with Control; - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001.
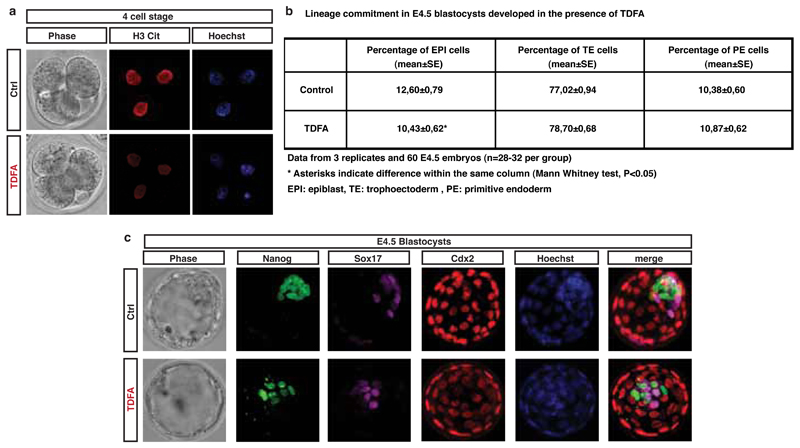
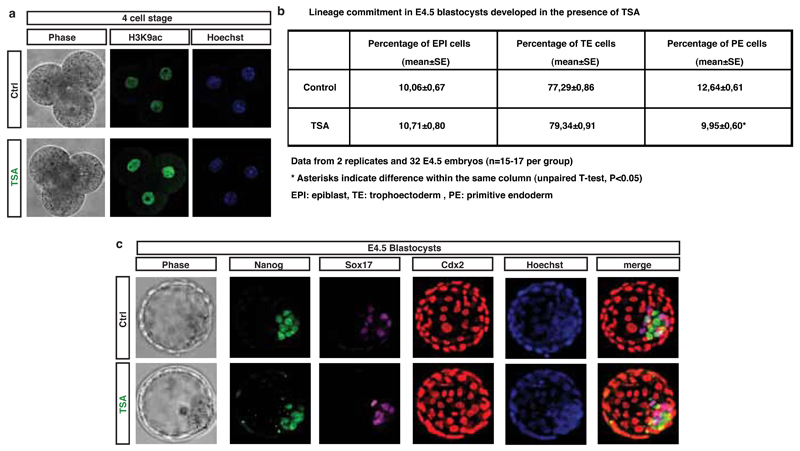
Padi4 expression and H3Cit are detected in the early embryo20,21 and Padi4-null mice are born in lower numbers than would be expected by Mendelian inheritance22, suggesting that PADI4 loss affects embryonic development. To assess the role of PADI4 in early development, we cultured mouse embryos in Cl-amidine-containing medium from the 2-cell stage and throughout pre-implantation development (see Methods and Extended Data Fig. 6a-c). Using 200µM Cl-amidine resulted in a complete developmental arrest of the embryos at the 8-cell stage (Extended Data Fig. 6a). We therefore used the maximum dose of Cl-amidine that reduced H3Cit (Extended Data Fig. S6b,c) but did not induce arrest (10µM). This led to a reduced percentage of pluripotent Nanog-positive epiblast cells and an increased percentage of differentiated trophectoderm cells at the blastocyst stage (Fig. 2d,e and Extended Data Fig. 6d,e). Time-course analyses of the cleavage patterns and cell fate decisions in early embryos showed that Cl-amidine increased the number of symmetric cell divisions at the expense of asymmetric divisions at the 8 to 16- and 16 to 32-cell transitions (Fig. 2f,g). This resulted in 16-cell stage embryos with fewer inner cells (destined for pluripotency) and greater numbers of outer cells (destined for differentiation into trophectoderm, reviewed in 23) (Extended Data Fig. 6f). Treatment with another PADI4 inhibitor, TDFA24, but not the HDAC inhibitor TSA, had similar effects (Extended Data Fig. 7, 8). These results indicate that PADI4 activity also promotes the maintenance of pluripotent cells in the early mouse embryo.
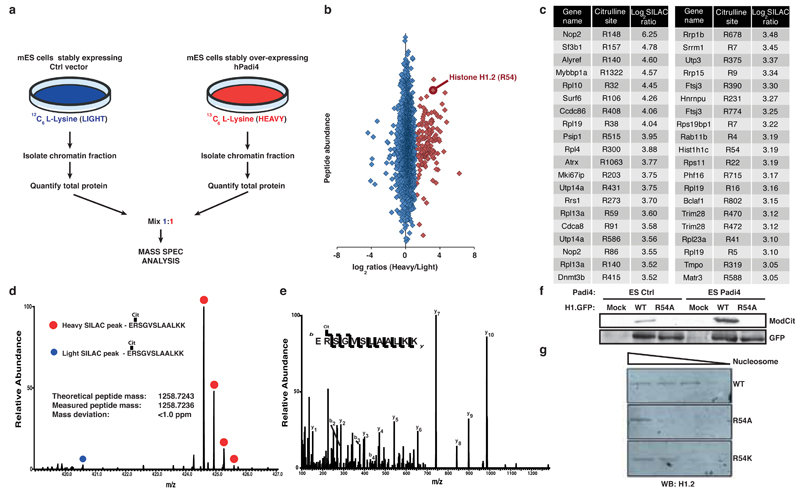
To elucidate the molecular mechanisms by which PADI4 regulates pluripotency, we aimed to identify PADI4 substrates in the chromatin fraction of mES cells using Stable Isotope Labeling of Amino acids in Cell culture (SILAC) (Fig. 3a and Extended Data Fig. 9a,b). Among the identified PADI4 substrates were Atrx, Dnmt3b, Trim28 and variants of linker histone H1 (Fig. 3b-e, Extended Data Fig. S9c-f, S10 and Supplementary Table 4), all of which can impact pluripotency. Histone H1 stabilizes the nucleosome and facilitates chromatin condensation, a state that is less permissive to processes that require access to the DNA, such as transcription11. The identified citrullinated H1 peptides correspond to, and are common between, variants H1.2, H1.3 and H1.4 (Fig. 3d,e), while an additional peptide corresponds to the same residue in H1.5 (Extended Data Fig. 10a,b). In ES cells, H1.2, H1.3 and H1.4 are required for chromatin compaction25, while their depletion leads to increased expression of pluripotency genes such as Nanog and stalls them in a self-renewal state with impaired differentiation capability26. Notably, H1 is more loosely bound to chromatin in ES cells than in differentiated cells14 and its genomic localization in cancer cells was shown to anti-correlate with that of PADI47.
Figure 3: PADI4 citrullinates Arg54 on linker histone H1 and affects its binding to nucleosomal DNA.
(a) Experimental strategy for screening for PADI4 citrullination substrates in the chromatin fraction of ES cells.
(b) Scatter plot representing the overall fold change for all identified citrullination sites. Red diamonds: PADI4-regulated citrullinations.
(c) Table representing the 40 most highly regulated PADI4 substrates, their individual citrullination sites and the log2 SILAC ratio. Complete dataset in Supplementary Table 4.
(d) Quantification of citrullination site R54 on H1.2 through differential regulation of the triply charged peptide ERSGVSLAALKK.
(e) Fragmentation spectra of the triply charged and heavy SILAC labeled LysC peptide ERSGVSLAALKK surrounding Arginine 54 of H1.2. The y and b series indicate fragments at amide bonds of the peptide.
(f) Citrullination immunoblot of wild-type and R54A mutant GFP-tagged H1.2 expressed and pulled-down from ES cells expressing PADI4 or control vector (Mock). Control for the efficiency of the pull-down: GFP.
(g) Nucleosome pull-down assay using wild-type and R54-mutant H1.2. WB: Western Blot.
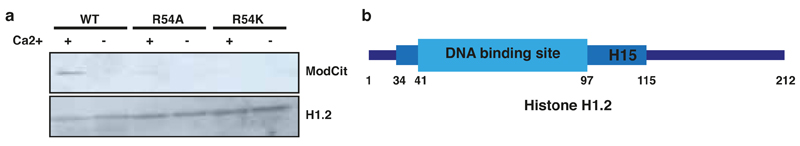
Mass spectrometric analysis accounted for all arginine residues within H1 but indicated that Arg54 (H1R54) is the only site citrullinated by PADI4 (Fig. 3e and Extended Data Fig. 9c). Indeed, we found that while H1.2 is citrullinated in ES cells (already by endogenous PADI4, and significantly increased upon PADI4 over-expression), it is refractory to modification when Arg54 is mutated (Fig. 3f). Similar results were obtained in in vitro citrullination assays (Extended Data Fig. 11a). H1R54 lies within the globular domain of H1 (Extended Data Fig. 11b), which is highly conserved among the linker histone family and is necessary for interaction with nucleosomal DNA27,28. To test whether H1R54 is necessary for binding of H1 to nucleosomes, we mutated and assessed it in nucleosome-binding assays. Figure 3g shows that an R54A mutant, which mimics the charge change that accompanies citrullination, is impaired for nucleosome binding. An R54K mutant, which retains the positive charge, is impaired to a lesser extend (Fig. 3g) suggesting that H1R54 is important for electrostatic interactions between H1.2 and the nucleosome.
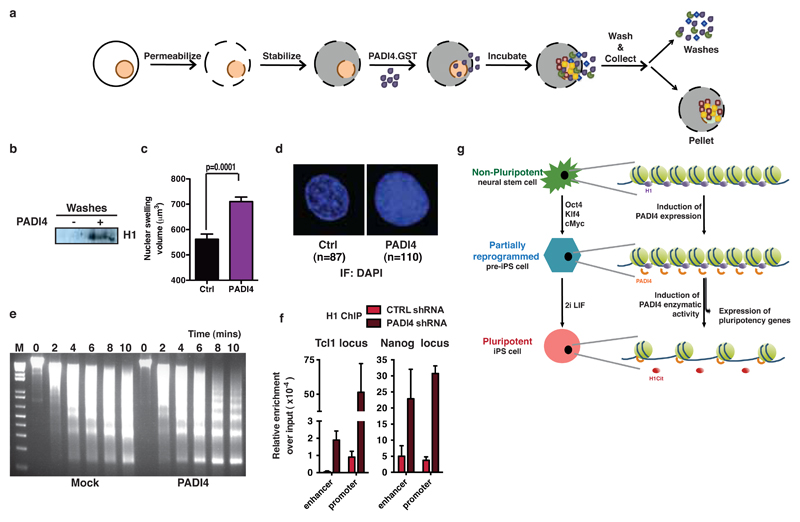
The above results open up the possibility that PADI4 may affect chromatin compaction in pluripotent cells. To test this hypothesis, we first assessed whether citrullination by ectopic PADI4 can lead to decondensation of differentiated cell chromatin. Recombinant PADI4 was added to permeabilized and stabilized differentiated C2C12 mouse myoblast nuclei (Fig. 4a). This protocol ensures stabilization of the nuclear component while allowing the free diffusion of non-chromatin bound nuclear proteins into the extra-nuclear fraction, and their collection by washing. Incubation with active PADI4 (Extended Data Fig. 12a,b), leads to the eviction of H1 from the chromatin and its diffusion out of the permeabilized nucleus (Fig. 4b). The evicted H1 is citrullinated on R54, as determined by mass spectrometry (Extended Data Figure 12c,d). Consistent with this, PADI4-treated cells showed evidence of decondensed chromatin, as determined by nuclear swelling, diffuse DAPI staining and increased sensitivity to micrococcal nuclease (Fig. 4c,d and Extended Data Figure 12e). Similar results were observed when PADI4 was over-expressed in C2C12 cells (Fig. 4e) or NS cells (data not shown). To monitor if PADI4 can affect H1 binding on pluripotent cell chromatin, we performed ChIP-qPCR analyses of H1.2 on the regulatory regions of Tcl1 and Nanog and found that it is stabilized upon Padi4 knockdown (Fig. 4f). The ability of PADI4 to disrupt the binding of H1 to nucleosomal DNA provides a novel mechanistic example of how citrullination regulates protein function and chromatin condensation.
Figure 4: PADI4 evicts histone H1 from chromatin and affects chromatin condensation.
(a) Schematic representation of treatment of C2C12 myoblast nuclei with recombinant PADI4.
(b) Immunoblot analysis of the wash fraction after the above treatment, for histone H1.2.
(c,d) Quantification of nuclear volume (c) and representative DAPI fluorescence (d) upon treatment of permeabilized C2C12 nuclei with recombinant PADI4. Error bars: standard error of the mean. Statistical significance determined by unpaired student t-test.
(e) Micrococcal nuclease digestion of C2C12 cells overexpressing an empty vector (Mock) or hPADI4.
(f) ChIP-qPCR for H1.2 on the regulatory regions of Tcl1 and Nanog in mES cells stably expressing Padi4 or Ctrl shRNA. Error bars: standard error of the mean of three technical qPCR replicates. Representative of two experiments.
(g) Proposed model for the role of PADI4 in the regulation of pluripotency.
The work described above identifies citrullination of chromatin components by PADI4 as a feature of pluripotency (Fig. 4g), in addition to its previously described role in the myeloid lineage. One of the reasons for the restricted expression pattern of PADI4 may be the requirement for an open chromatin state in these cell types. The selective expression characteristics and the inducible nature of the catalytic activity of PADI4, suggest that it is under tight spatial and temporal regulation, giving it a unique status among chromatin modifying enzymes. As such, inappropriate PADI4 activity may have deleterious consequences, which may explain its activation in cancers of varying origin during progression to malignancy29. Indeed, citrullination is a common feature of several unrelated diseases, suggesting that strict regulation is likely to be a requirement for the physiological function of all PADIs. During review of this manuscript, Coonrod and colleagues suggested that PADI2, thought to be mainly cytoplasmic, can also citrullinate histones and lead to transcriptional activation30. This opens the intriguing possibility that other PADIs may mediate nuclear events in specific contexts, including in pluripotent cells. Further research into the function and targets of PADIs is likely to shed light into the etiology of several pathologies.
Extended Data
Extended Data Figure 1:
(a) Transcript levels for Padi1, Padi2 and Padi3 in ES, NS and iPS cells, as assessed by qRT-PCR. Padi6 was undetectable in all three cell types. Expression normalized to endogenous levels of Ubiquitin (UbC). Error bars represent the standard error of the mean of three biological replicates.
(b) Transcript levels of Padi1, Padi2 and Padi3 in ES cells upon switch to 2i containing medium for one passage, as assessed by qRT-PCR. Padi6 was undetectable in both conditions. Expression normalized to UbC. Error bars represent the standard error of the mean of three biological replicates.
(c) Immunoblot analysis of H3Cit levels in ES, NS and iPS cells. Total H3 is presented as loading control.
(d) Immunoblot analysis of total citrullination the levels in ES, NS and iPS cells, using an antibody against Modified Citrulline (ModCit) which recognizes peptidylcitrulline irrespective of amino acid sequence. Total H3 is presented as loading control.
(e) ZHBTc4.1 and 2TS22C ES cell lines were treated with 1µg/ml doxycycline for 48 hours, resulting in depletion of Oct4 or Sox2 (data not shown). Padi4 mRNA was significantly reduced upon Oct4, but not Sox2 knockdown, as assessed by qPCR. Error bars represent standard error of the mean of four biological replicates.
(f) ChIP-qPCR for Oct4, Sox2, Klf4, RNA polymerase II (PolII), H3K4me3 and H2A on the promoter of Padi4 in mES and NS cells. For each cell condition, the signal is presented as fold enrichment over Input and after subtracting background signal from the beads. Error bars represent the standard deviation of three technical qPCR replicates.
Asterisks denote difference with ES cells (a) or media (b), and 0h time point (e); - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001 by ANOVA (a) or t-test (b,e).
Extended Data Figure 2:
(a) Validation of selected targets from the PADI4 over-expression microarray dataset by qRT-PCR. Expression of Pou5f1, Sox2, Klf4 and c-Myc is not affected by PADI4 over-expression. Expression normalised to UbC. Error bars presented as standard error of the mean of three biological replicates.
(b) Transcript levels of mouse Padi4 and human PADI4 in mES cells after transient knock-down with Padi4 or control (Ctrl) shRNA, and over-expression of human PADI4 or control vector (pPB CTRL), as assessed by qRT-PCR. Expression normalized to UbC. Error bars represent the standard error of the mean of three biological replicates.
(c) Transcript levels of mouse Padi4, Tcl1 and Nanog in mES cell clones stably expressing Padi4 or control (Ctrl) shRNA, as assessed by qRT-PCR. Expression normalized to UbC. Error bars represent the standard error of the mean of three biological replicates.
Asterisks denote difference with Ctrl (a,b,c) and between samples (b); - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001 by ANOVA (b) or t-test (a,c).
Extended Data Figure 3:
(a) Representative ChIP-qPCR for H2A on regulatory regions of Tcl1 and Nanog in mES, NS and iPS cells (corresponding to Fig. 1h). For each cell condition, the signal is presented as fold enrichment over Input and after subtracting background signal from the beads. Error bars represent the standard deviation of three technical qPCR replicates.
(b) ChIP-qPCR for hPADI4 on regulatory regions of Tcl1, Nanog, Klf2 and Kit, which are up-regulated by hPADI4 over-expression, in mES cells stably expressing hPADI4. For each cell condition, the signal is presented as fold enrichment over Input and after subtracting background signal from the beads. Error bars represent the standard deviation of three technical qPCR replicates.
(c) Representative ChIP-qPCR for H3Cit on regulatory regions of Tcl1 and Nanog in mES cells stably expressing hPADI4 and treated with 200μM Cl-amidine for 48h. For each cell condition, the signal is presented as fold enrichment over Input and after subtracting background signal from the beads. Error bars represent the standard deviation of three technical qPCR replicates.
Extended Data Figure 4:
(a) Heat map of the top 70 genes that showed differential expression after PADI4 inhibition in ES cells by with 200μM Cl-amidine for 48h, as determined by microarray analysis. Displayed values are normalized log intensities, minus the mean expression of the gene across the two samples. Hierarchical clustering based on correlation.
(b) Validation of selected targets from the above microarray dataset by qRT-PCR. Expression normalised to UbC. Error bars presented as standard error of the mean of three biological replicates. Asterisks denote difference with Ctrl; - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001 by t-test.
(c) Gene Ontology for Biological Process (GOBP) analysis for the most regulated gene categories within the microarray dataset of Cl-amidine treatment in mES cells. P-value is corrected for multiple testing using Benjamini and Hochberg FDR.
Extended Data Figure 5:
(a) Scheme of reprogramming of neural stem cells to pluripotent state. NSO4G cells were retrovirally transduced with Oct4, Klf4 and c-Myc. After 6 days, partially reprogrammed pre-iPS cells arose. For shRNA experiments, pre-iPS cells were stably transfected with Ctrl or PADI4 shRNA and then full reprogramming was performed in the presence of 2iLIF media for 8 days. For PADI4 enzymatic inhibition, pre-iPS cells were immediately changed to 2iLIF media in the presence of the inhibitor Cl-amidine for 8 days.
(b) Quantification of flow cytometry analysis for the assessment of Oct4-GFP reporter expression in a reprogramming assay using pre-iPS cells stably expressing Padi4 shRNA #4 and Ctrl shRNA. Error bars represent standard error of the mean of triplicate samples within a representative from four reprogramming experiments.
(c) Quantification of Oct4-GFP positive colonies in the reprogramming assay where pre-iPS cells were Padi4 shRNA #4 versus control (see Fig. 2a), after time-lapse image acquisition with Biostation CT. Error bars represent standard error of the mean of triplicate samples within a representative reprogramming experiment. Time-lapse video in supplementary data online.
(d) Immunoblot analysis of H3Cit in pre-iPS cells treated with 2i, after Padi4 knock-down (PADI4 shRNA #4) versus control cells (Ctrl shRNA). 2i-containing medium was added on day 2. Total histone H3 presented as loading control.
(e) Quantification of flow cytometry analysis for the assessment of Oct4-GFP reporter expression in a reprogramming assay using pre-iPS cells stably expressing Padi4 shRNA #3 and Ctrl shRNA. Error bars represent standard error of the mean of triplicate samples.
(f) qRT-PCR analysis for the expression of Tcl, Nanog and Padi4 mRNAs at the end of the above reprogramming assay (e). Error bars represent standard error of the mean of triplicate samples.
(g) Quantification of flow cytometry analysis for the assessment of Oct4-GFP reporter expression in a reprogramming assay were treated with 200μM Cl-amidine. Error bars represent standard error of the mean of triplicate samples within a representative from three reprogramming experiment.
(h) Quantification of Oct4-GFP positive colonies in the reprogramming assay where pre-iPS cells were treated with 200μM Cl-amidine (see Fig. 2c) after time-lapse image acquisition with Biostation CT. Error bars represent standard error of the mean of triplicate samples within a representative reprogramming experiment.
(i) Immunoblot analysis for the presence of H3Cit at the end of the above reprogramming assay (g). Total histone H3 presented as loading control.
Asterisks denote difference with Control; - not significant, * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001 by t-test
Extended Data Figure 6:
(a) Embryos at 2-cell stage were treated with 200µM Cl-amidine and snapshots were taken at E3.0, E3.5 and E4.0. 200μM Cl-amidine embryos arrested at 8-cell stage, while Control embryos continued development to form blastocysts. Phase contrast images are shown.
(b) Embryos at 2-cell stage were treated with 10µM Cl-amidine for 12 hours, fixed and stained for H3Cit at the 4-cell stage. Phase contrast, H3Cit (white) and HOECHST 33342 (blue) images are shown. Bar represents 20µm.
(c) Embryos at E3.5 were treated with 10µM Cl-amidine for 24 hours, fixed and stained for H3Cit at E4.5. H3Cit (green) and HOECHST 33342 (blue) images are shown. Bar represents 20µm.
(d) Table with quantifications of lineage commitment in E4.5 blastocysts treated with 10µM Cl-amidine from the 2-cell stage. Asterisks denote difference with Control, unpaired t-test, * = p<0.05. n=3 (50 embryos).
(e) Embryos were cultured in medium supplemented with 10µM Cl-amidine from 2-cell stage and through preimplantation development. E4.5 blastocysts were fixed and stained for SOX17 (primitive endoderm marker, red), Cdx2 (trophectoderm marker, green) and HOECHST 33342 (blue). Bar represents 20µm.
(f) Time-lapse analysis of distribution of inner and outer cells at the 8 to 16-cell transition, upon culturing of embryos with medium containing 10µM Cl-amidine from 2-cell stage. Error bars represent standard error of the mean. Statistical significance was determined by unpaired t-test or Mann Whitney test upon non-normal distribution. Asterisks denote difference with Control; * P≤0.05.
Extended Data Figure 7:
(a) Embryos at 2-cell stage were treated with 100µM TDFA for 12 hours and fixed and stained for H3Cit at 4-cell stage. H3Cit and HOECHST 33342 images are shown.
(b) Table representing the percentage of cells committed to each embryonic lineage in E4.5 blastocysts upon treatment of embryos at 2-cell stage with 100µM TDFA. Bars represent mean percentage (±SEM). Asterisks denote difference with Control, Mann-Whitney test, * = p<0.05. n=3 (60 embryos).
(c) Embryos at 2-cell stage were treated with 100µM TDFA and fixed at embryonic day E4.5. Phase contrast, Nanog (green), Sox17 (purple), Cdx2 (red) and HOECHST 33342 (blue) images are shown.
Extended Data Figure 8:
(a) Embryos at 2-cell stage were treated with 10nM TSA for 12 hours, and fixed and stained for H3K9ac at 4-cell stage. H3K9ac and HOECHST 33342 images are shown.
(b) Table representing the percentage of cells committed to each embryonic lineage in E4.5 blastocysts upon treatment of embryos at 2-cell stage with 10nM TSA. Bars represent mean percentage (±SEM). Asterisks denote difference with Control, unpaired t-test, * = p<0.05. n=2 (32 embryos).
(c) Embryos at 2-cell stage were treated with 10nM TSA and fixed at embryonic day E4.5. Phase contrast, Nanog (green), Sox17 (purple), Cdx2 (red) and HOECHST 33342 (blue) images are shown.
Extended Data Figure 9:
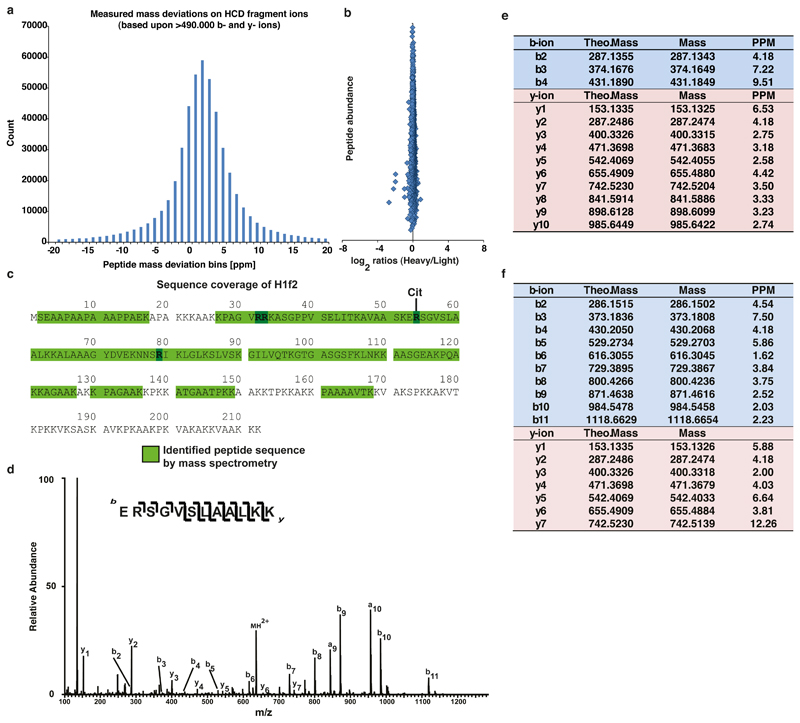
(a) Histogram demonstrating the mass accuracies of all fragment ion masses used for identifying citrullinated peptides in our HCD MS/MS spectra. >490.000 y- and b-ion masses are depicted. The average absolute mass accuracy for all of these fragment ions is 3.97 ppm.
(b) Scatter plot representing SILAC ratios in ES cells cultured in 13C6 L-Lysine (HEAVY) and LIGHT medium separately, to assess extend and quality of SILAC labeling. No significant outliers are observed, indicating equal labeling.
(c) Peptide coverage of histone H1 by LC-MS analysis. Detected peptides are highlighted in light green and cover >60% of H1. While all arginine residues of Histone H1 (highlighted in dark green) were accounted for by the analysis, Arg54 was the only one found citrullinated.
(d) Fragmentation spectra of the unmodified LysC peptide ERSGVSLAALKK surrounding Arginine 54 of H1.2 (unmodified counterpart of citrullinated peptide depicted in Fig. 3d). The y and b series indicate fragments at amide bonds of the peptide.
(e) Fragment ion table (expected and observed masses for detected y and b ions) for the identified H1R54 citrullination of peptide ERSGVSLAALKK on histone H1.2 (as shown in Fig. 3d). All measured fragment ions were detected with mass accuracies <10ppm, unambiguously identifying that the detected peptide sequence harbors a citrullination at position R54.
(f) Theoretical and measured b- and y-ion fragment masses for the corresponding unmodified and heavy SILAC labeled H1.2 peptide, as presented in (d) above.
Extended Data Figure 10:
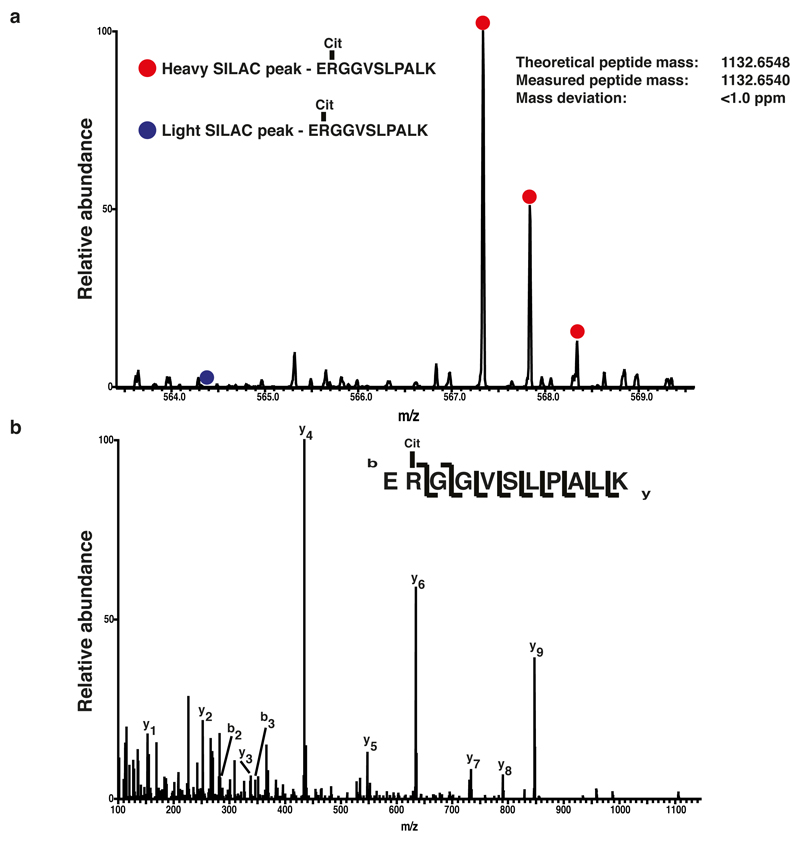
(a) MS spectrum of Histone H1.5 in a SILAC proteomic screen for identification of PADI4 substrates. Linker histone H1.5 is deiminated by PADI4, as identified by a highly increased SILAC ratio of the heavy labeled identified peptide (marked by a red dot).
(b) Fragmentation spectra of the doubly charged LysC peptide ERGGVSLPALK surrounding Arginine 54 of H1.5. The y and b series indicate fragments at amide bonds of the peptide, unambiguously verifying the citrullinated peptide.
Extended Data Figure 11:
(a) Mutation of R54 renders histone H1.2 refractory to deimination. Immunoblot analysis of recombinant histone H1.2 using an antibody that detects all deimination events (ModCit). Wild type and R54-mutant H1.2 were treated with recombinant PADI4, in the presence of activating calcium. Only wild-type H1.2 can be deiminated, indicating that R54 is the only substrate of PADI4 in H1.2. No-calcium reactions presented as negative controls. Total H1.2 presented as loading control.
(b) Schematic representation of the position of R54 within the globular domain linker histone H1.2.
Extended Data Figure 12:
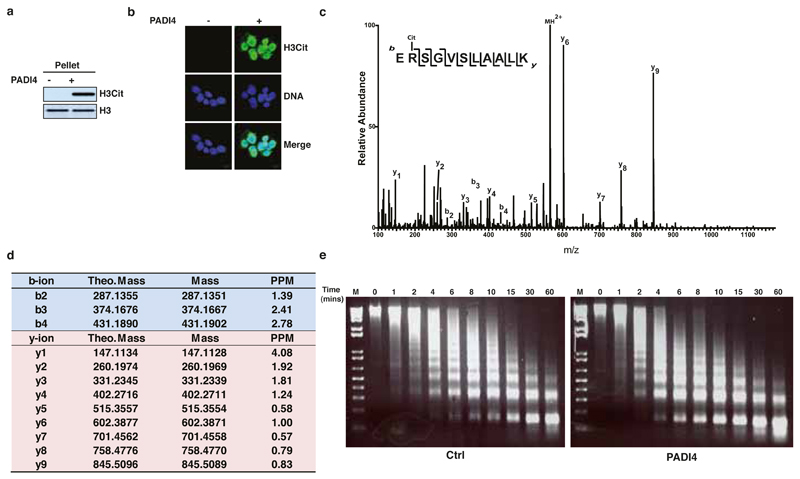
(a) Immunoblot analysis of the “Pellet” fraction of C2C12 permeabilised cells treated with recombinant PADI4. Presence of H3Cit species indicates PADI4 activity. Total H3 is presented as a control for equal use of starting material in the two experimental conditions.
(b) Immunofluorescence analysis of C2C12 nuclei after treatment with recombinant PADI4. Presence of H3Cit species indicates PADI4 activity. DNA is visualised by staining with DAPI.
(c) Fragmentation spectra of the citrullination site R54 on the evicted H1.2 peptide ERSGVSLAALK (corresponding to Fig. 4b). The evicted Histone H1 is citrulinated at R54.
(d) Theoretical and measured b- and y-ion fragment masses for the citrullinated H1.2 peptide (peptide sequence ERSGVSLAALK) evicted after treatment of C2C12 cells with recombinant hPADI4 (corresponding to Fig. 4b).
(e) Micrococcal nuclease digestion of C2C12 nuclei after treatment with recombinant PADI4, as described in Fig. 4a. M= size marker.
Supplemental Information
Complete experimental methods and references in Methods file.
Primer sequences in Supplementary Table S1.
Complete microarray data for PADI4 over-expression in ES cells in Supplementary Table S2.
Complete microarray data for PADI4 inhibition in ES cells in Supplementary Table S3.
Complete Mass Spec dataset in Supplementary Table S4.
Time-lapse video of reprogramming experiments in Supplementary video file.
Acknlowledgements
This work was funded by programme grants from Cancer Research UK (T.K.) and EMBL (P.B., R.L.). R.P.H-S and JBG are supported by the Medical Research Council [G1001690] and the Wellcome Trust. G.C.-B. was funded by EMBO (Long-Term Post-Doctoral Fellowship), European Union (FP7 Marie Curie Intra-European Fellowship for Career Development) and Swedish Research Council. M.A.C was funded by an EMBO Long-Term Post-Doctoral Fellowship and a Human Frontier Science Programme Long-Term Post-Doctoral Fellowship. C.S.O was supported by FAPESP (Foundation for Research Support of the State of São Paulo) and mouse embryo work was supported by the Wellcome Trust programme grant to M.Z.G. M.L.N was partly supported by the Novo Nordisk Foundation Center for Protein Research, the Lundbeck Foundation, and by and the European Commission’s 7th Framework Programme HEALTH-F7-2010-242129/SYBOSS. We would like to thank Sri Lestari, Alistair Cook and Cynthia Hill for technical assistance; Paul Thompson for kindly providing the TDFA compound; GSK Epinova for Cl-amidine; Till Bartke for the kind gift of histone octamers and help with nucleosome pull-down assays; Andrew Finch for help with FPLC chromatography; Agnieszka Jedrusik for help with embryo work; Rachael Walker at the Flow Cytometry Core Facility at Wellcome Trust Centre for Stem Cell Research, University of Cambridge and Thor Theunissen for help with the flow cytometry; and members of the Kouzarides laboratory for critical discussions of the work. 2TS22C cells were kindly provided by Dr. Hitoshi Niwa, at the RIKEN Center for Developmental Biology, Kobe, Japan. The ChIP grade H1.2 antibody was a generous gift from Prof. Arthur Skoultchi, Albert Einstein School of Medicine, New York, USA.
References
- 1.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays: news and reviews in molecular, cellular and developmental biology. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinod K, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagiwara T, Nakashima K, Hirano H, Senshu T, Yamada M. Deimination of arginine residues in nucleophosmin/B23 and histones in HL-60 granulocytes. Biochemical and biophysical research communications. 2002;290:979–983. doi: 10.1006/bbrc.2001.6303. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbert GL, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, et al. Genome-wide analysis reveals PADI4 cooperates with Elk-1 to activate c-Fos expression in breast cancer cells. PLoS genetics. 2011;7:e1002112. doi: 10.1371/journal.pgen.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanikawa C, et al. Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nature communications. 2012;3:676. doi: 10.1038/ncomms1676. [DOI] [PubMed] [Google Scholar]
- 9.Asaga H, Nakashima K, Senshu T, Ishigami A, Yamada M. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. Journal of leukocyte biology. 2001;70:46–51. [PubMed] [Google Scholar]
- 10.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 11.Buttinelli M, Panetta G, Rhodes D, Travers A. The role of histone H1 in chromatin condensation and transcriptional repression. Genetica. 1999;106:117–124. doi: 10.1023/a:1003745315540. [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaspar-Maia A, Alajem A, Meshorer E, Ramalho-Santos M. Open chromatin in pluripotency and reprogramming. Nature reviews. Molecular cell biology. 2011;12:36–47. doi: 10.1038/nrm3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meshorer E, et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks H, et al. The Transcriptional and Epigenomic Foundations of Ground State Pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theunissen TW, et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol. 2011;21:65–71. doi: 10.1016/j.cub.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Annals of the rheumatic diseases. 2012;71:92–98. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polo JM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmajosyula M, Miyake M. Localization and expression of peptidylarginine deiminase 4 (PAD4) in mammalian oocytes and preimplantation embryos. Zygote. 2011:1–11. doi: 10.1017/S0967199411000633. [DOI] [PubMed] [Google Scholar]
- 21.Kan R, et al. Potential role for PADI-mediated histone citrullination in preimplantation development. BMC developmental biology. 2012;12:19. doi: 10.1186/1471-213X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zernicka-Goetz M, Morris SA, Bruce AW. Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet. 2009;10:467–477. doi: 10.1038/nrg2564. [DOI] [PubMed] [Google Scholar]
- 24.Jones JE, et al. Synthesis and screening of a haloacetamidine containing library to identify PAD4 selective inhibitors. ACS chemical biology. 2012;7:160–165. doi: 10.1021/cb200258q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y, et al. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005;123:1199–1212. doi: 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. Histone h1 depletion impairs embryonic stem cell differentiation. PLoS genetics. 2012;8:e1002691. doi: 10.1371/journal.pgen.1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goytisolo FA, et al. Identification of two DNA-binding sites on the globular domain of histone H5. EMBO J. 1996;15:3421–3429. [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DT, Izard T, Misteli T. Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo. Nat Struct Mol Biol. 2006;13:250–255. doi: 10.1038/nsmb1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang X, et al. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC cancer. 2009;9:40. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc Natl Acad Sci U S A. 2012;109:13331–13336. doi: 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete experimental methods and references in Methods file.
Primer sequences in Supplementary Table S1.
Complete microarray data for PADI4 over-expression in ES cells in Supplementary Table S2.
Complete microarray data for PADI4 inhibition in ES cells in Supplementary Table S3.
Complete Mass Spec dataset in Supplementary Table S4.
Time-lapse video of reprogramming experiments in Supplementary video file.