Abstract
During untreated disease, HIV replication is concentrated within T follicular helper cells (TFH). Heightened permissiveness, the presence of highly infectious virions on follicular dendritic cells (FDC), low frequencies of virus-specific cytotoxic T lymphocytes (CTL) in B cell follicles, expansions in TFH, and TFH dysfunction all likely promote replication in TFH. Limited data suggest that memory TFH play a role in the latent or subclinical reservoir of HIV during antiretroviral therapy (ART), potentially for many of the same reasons. A better understanding of the role of memory TFH and FDC-bound virions in promoting recrudescent viremia in the setting of ART cessation is essential. Studies that target follicular virus reservoirs are needed to determine their role in HIV latency and to suggest successful cure strategies.
Keywords: HIV-1, T follicular helper cells, follicular dendritic cell, latency
Role of TFH in HIV Replication in Untreated Disease
T follicular helper cells (TFH) are a specialized subset of CD4+ helper T cells that express CXCR5, migrate into B cell follicles, and promote B cell maturation and antibody production during infections (reviewed in [1]). In untreated, asymptomatic HIV infection, TFH serve as the major site of HIV infection and replication (Figure 1) [2–5]. A median of 60–75% of HIV-producing cells are located within B cell follicles in lymph nodes from untreated, asymptomatically infected individuals [2, 4], and a CD4+ T cell located within a B cell follicle is 40 times more likely to be productively infected than a CD4+ T cell located outside of the follicle [4]. Similarly, in chronically SIV-infected rhesus macaques without simian AIDS (SAIDS), the majority of SIV-producing cells are located within follicles [6, 7]. Even after normalizing for differences in memory cells between the follicular and extrafollicular compartments, follicular CD4+ T cells are a median of 6.5 times more likely to be producing SIV RNA than extrafollicular CD4+ T cells [6]. Interestingly, in non-pathogenic SIV-infection in sooty mangabeys, a follicular concentration of virus replication is not observed [7], suggesting that productive TFH infection may be a critical driver of HIV and SIV immunopathogenesis.
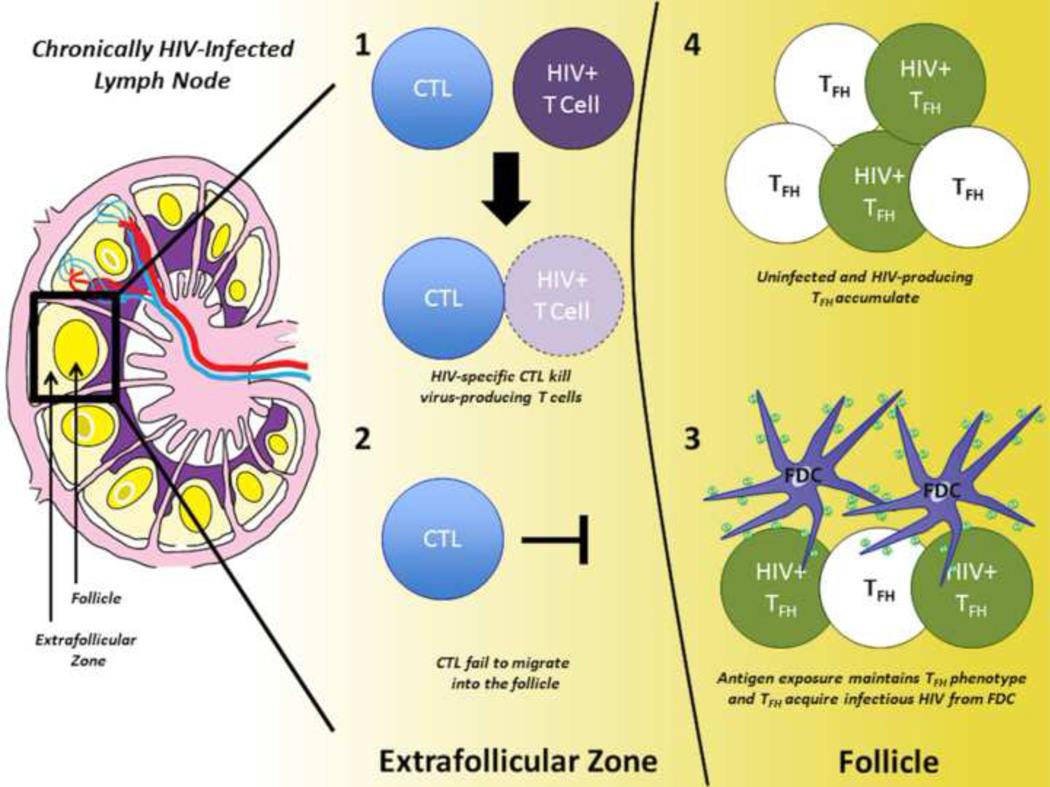
Figure 1. Model of TFH Accumulation in Chronic, Untreated HIV Infection.
HIV-specific CTL recognize and kill virus producing T cells (HIV+ T cell) in the extrafollicular zone (1), but are found in low numbers within the follicle due to low CXCR5 expression (2). Within the follicle, T follicular helper cells (TFH) receive both activation signals and infectious HIV from interactions with follicular dendritic cells (FDC) (3). TFH, including HIV-producing TFH (HIV+), accumulate within the follicle (4).
Mechanisms that promote HIV replication within TFH are not fully understood. TFH from human tonsils are highly permissive to both CCR5- and CXCR4-tropic HIV compared to other CD4+ T cell subsets ex vivo [8, 9]. The heightened permissivity of tonsillar TFH cannot be fully explained by differences in memory subsets, cellular activation, or chemokine co-receptor expression [9]. Importantly, in an HIV-model system using humanized mice, TFH rapidly accumulate in gut and female reproductive tract mucosal tissues and are the most permissive CD4+ T cell subset to HIV [10], suggesting that gut and vaginal TFH may play a key role in establishment of HIV infection as well as ongoing virus replication.
The presence of a follicular dendritic cell (FDC) network and large amounts of FDC-associated HIV virions adjacent to TFH within germinal centers (GC) [11–13] is one contributing factor predisposing TFH to high levels of HIV infection and replication. This extracellular burden of virions is bound to FDC via antibody through complement and FC receptors [14] and appears shortly after infection [15]. In chronic disease, the amount of viral RNA (vRNA) associated with FDC is 10- to 40-fold more than is found in lymphoid mononuclear cells [12]. FDC harbour archived virus from the host [16], and these virions are potently infectious to CD4+ T cells, even in the presence of neutralizing antibodies [13]. FDC further promote HIV replication within TFH by upregulating virus transcription through release of tumor necrosis factor-α (TNF-α) [8].
High concentrations of virus replication within TFH are further promoted by a paucity of virus-specific cytotoxic T lymphocytes (CTL) within B cell follicles in both HIV [4] and SIV infection [6, 17]. Few SIV-specific CTL express the follicular homing molecule CXCR5 in the absence of the extrafollicular retention molecule CCR7, which restricts most from entering the follicle [6]. SIV replication is widespread throughout lymphoid tissues and viral replication is not concentrated in the follicle during acute infection, prior to when the CTL response has evolved to control the virus [6]. Similarly, compartmentalization of virus replication in TFH is attenuated during AIDS, when the virus-specific CTL response is known to wane [6], and ablated following CD8 depletion in rhesus macaques [18]. Thus, other lymphoid cells besides TFH are capable of replicating HIV-1, but largely restricted from doing so during asymptomatic disease by the presence of CTL.
Remarkably, despite the fact that TFH are the major virus-producing cells in asymptomatic disease, they increase in number during early and mid-stages of chronic HIV [3, 19] and SIV infection [20]. It was shown in rhesus macaques that acute SIV infection results in the rapid formation of GC and an accumulation of TFH coinciding with high levels of p27 expression in the follicle [21]. Furthermore, in lymph nodes from chronically HIV-infected individuals, virus-specific TFH, particularly HIV Gag-specific TFH, are expanded [19]. Thus, at least part of TFH expansion is due to HIV antigen, as CD4+ T cells require antigen exposure for most cell division [22] and antigen persistence in the GC is required to sustain TFH phenotypes [23]. Within the GC, TFH are chronically exposed to HIV virions and antigens through interactions with B cells [24] and FDCs [11, 12]. Circulating TFH are decreased in untreated chronically-HIV infected individuals [25], suggesting that the GC offers a unique environment allowing for TFH expansion. It should be noted that in advanced infection, TFH expansions and GC morphology are lost and this is associated with the onset of SAIDS in rhesus macaques [26] and AIDS in humans [27].
Non-antigen-specific influences may promote expansion and persistence of TFH in HIV and SIV infection in early stages of disease as well. Alterations of cytokine production observed during HIV and SIV infection, such as decreased interleukin-2 (IL-2) [28] and increased IL-6 [20, 29] and interferon-γ (IFN-γ) [30] promote TFH expansions [1]. T follicular regulatory cells (TFR) are a subset of follicular cells that directly regulate numbers and function of TFH during HIV and SIV infection [31–34]. Two studies linked relative decreases of TFR to expansions of TFH in the SIV-infected rhesus macaque model [31, 32]. Increased cell survival may contribute to the persistence of TFH as well. CXCR5+ cells in healthy human tonsils express elevated levels of the anti-apoptotic protein Bcl-2, and in the context of ex vivo R5 infection, Bcl-2 is upregulated in productively infected cells [35]. Interestingly, the depletion of CD8 T cells in untreated SIV infection leads to similar levels of replication in lymphoid memory TFH and memory non-TFH, however the memory TFH contain higher levels of RNA and DNA copies up to 135 days after depletion and after CD8 recovery [18], suggesting possible preferential survival of TFH.
HIV infection not only leads to alterations in TFH numbers, but also changes in TFH function. TFH from lymph nodes of untreated individuals demonstrate expansions of HIV-specific IL-21+, INF-γ+, and TNF-α+ TFH compared to treated individuals [19]. Furthermore, B cells from untreated individuals demonstrate a skewed phenotype [19]. Hypergammaglobulinemia is a well described phenomenon in untreated HIV-infected individuals, and Bcl6 expression in TFH correlates with immunoglobulin G (IgG) levels [19]. Nevertheless, TFH from untreated HIV patients are unable to stimulate robust IgG production in vitro [36], suggesting significant impairments in TFH in the context of untreated infection, which is consistent with known clinical impairments in responses to vaccines. In vitro, impairments in the ability of TFH to stimulate IgG are reversed by supplementation with IL-21 or blockade of PD-1 ligation on TFH [36]. Circulating IL-21+ CD4+ T cells were shown to be transcriptionally equivalent to TFH in seronegative individuals and peripheral HIV-specific IL-21+ TFH in untreated HIV+ subjects were able to stimulate autologous CD8+ T cell activation and B cell class switching [37]. Expansions in TFR occur in the context of both HIV and SIV infection, and these cells impair the ability of TFH to secrete IL-21 and IL-4 [33], suggesting another potential mechanism for TFH dysfunction.
Follicular HIV Reservoirs During Treated Disease
A stable and inducible latent viral reservoir within memory CD4+ T cells is maintained in HIV-infected individuals despite years of antiretroviral therapy (ART) that renders plasma viremia undetectable [38–40]. Furthermore, low frequencies of vRNA+ lymphocytes persist in peripheral blood [41] and lymph nodes of treated individuals [42] undergoing successful long-term ART. Virus replication rebounds from multiple foci in secondary lymphoid tissues once ART is stopped in both HIV-infected humans and SIV-infected rhesus macaques [43, 44]. Characteristics of the viral reservoir that is the source of recrudescent viremia are incompletely understood, and this is a major barrier to the development of an HIV cure. Furthermore, whether virus replication is ongoing in the context of ART is a matter of controversy. The presence of vRNA+ cells during ART could be due to reactivation of latently infected cells or alternatively ongoing rounds of virus replication. Although drug concentrations have been reported to be suboptimal in the lymph node [45], curiously little evidence for virus evolution has been found in most studies in patients whose viral loads remained well suppressed [46–48]. Furthermore, ART-related resistance mutations do not develop after long term virologic suppression [47, 49], which one would expect to occur if indeed drug concentrations in lymph nodes were suboptimal. Indeed, founder variants are the main HIV strains that emerge from lymphatic tissues during treatment interruption [44], and increasing data suggest that homeostatic proliferation of viral DNA+ cells is the major factor underlying persistent viral DNA levels [48, 50].
The fact that TFH display anti-apoptotic properties, as discussed above, despite being the major virus-producing cell subset in untreated, asymptomatic HIV, suggests that they could harbour a significant amount of the latent viral DNA (vDNA) during treated disease. Indeed, in ART-suppressed SIV-infected rhesus macaques, the major vRNA+ cells in lymphoid tissues are TFH, as determined by cell sorting studies [18], although vDNA was not concentrated in this subset. Virus replication in these cells could represent reactivation of latently infected memory TFH. The existence of memory TFH in humans has only recently been recognized [1, 51], and they have not been extensively studied in the context of HIV or SIV infection. TFH transcription factors may promote establishment of HIV latency. The HIV long terminal repeat (LTR) contains binding sites for the master TFH transcription factor Bcl6 that allows Bcl6 to repress HIV transcription [52] and it has been speculated that Bcl6 supports HIV latency [53]. Memory TFH can be long-lived and primarily have a central memory phenotype, similar to the resting CD4+ T cell reservoir that harbors the majority of HIV DNA in treated individuals [50]. One recent study reported that the majority of HIV-1 DNA in peripheral blood was harboured by central memory TFH in subjects with sustained virologic suppression on ART [54]. Nevertheless, the precise phenotype of peripheral TFH is somewhat controversial [55] and until an accurate phenotype is determined, identifying the role of TFH in the latent HIV reservoir using peripheral blood will be challenging.
During ART treatment TFH numbers decrease relative to untreated disease, but remain elevated compared to healthy individuals [19]. Phenotypically, lymph node TFH from treated HIV+ subjects have lower activation rates as evidenced by decreased Bcl6 expression and fewer HIV-specific IL-21+ TFH, INF-γ+ TFH, and TNF-α+ TFH [19]. The expansion of TFH in both HIV+ untreated and treated subjects, compared to healthy controls, is also associated with an increased transitional B cell phenotype and lower memory B cell formation [19]. The number of functional circulating TFH is decreased in untreated HIV+ subjects, and TFH numbers and function slightly recover in treated HIV+ subjects [25]. However, TFH function is still incomplete and HIV+ subjects still have functional impairments [19] and poor vaccine responses [56, 57]. TFH function has also been found to be crucial for subsequent vaccine responses in treated HIV+ subjects, as responses to flu vaccination were directly dependent on the ability of TFH to proliferate, express ICOS, and produce IL-21 [58].
An alternative or additional explanation for the presence of HIV RNA+ cells in TFH in the context of suppressive ART is that they represent new infection from virus archived on FDC. Following 6 months of ART, FDC-bound virions decrease but are nonetheless detectable; the residual number of copies of HIV RNA present on FDC was estimated to be nearly 108 virions in an average individual [59]. How long FDC bound virions remain infectious is unknown. In a non-permissive mouse model, FDC-bound HIV remained infectious for the maximum period studied, which was 9 months [60]. Although antiretroviral drugs that block entry, reverse transcription, and integration prevent most infections, these may not be fully effective resulting in occasional virus integration and replication. Thus, FDC-bound HIV could potentially provide a low level of new infections that manifest as founder virus, due to the archived nature of FDC bound virions [16].
Eliminating Follicular HIV Reservoirs
To date only one person has been successfully cured of HIV infection after receiving a bone marrow transplant with donor cells that were genetically resistant to HIV infection [61]. Multiple other attempts to achieve a cure or reduce the HIV proviral load have not achieved success. Knowledge of the follicular reservoirs outlined here and the hurdles that they pose to achieving a cure could provide some insights into these failures, as well as redirect efforts to more successful approaches.
Early attempts to activate and purge the viral reservoir were conducted using IL-2 [62] or CD3 activation [63], with the assumption that the viral reservoir would be purged from latently infected cells through HIV cytopathic effects or immune clearance. Patients receiving IL-2 in addition to ART had lower levels of replication competent HIV in peripheral blood CD4+ T cells [62] but upon interruption of ART plasma HIV levels quickly rebounded [64]. Thus, broad immune activation and treatment cessation proved unsuccessful in purging the HIV reservoir. More recent approaches to activate and purge the HIV reservoir have been directed at activating latent gene expression through the use of inhibitors of histone deacetylases (HDAC). This approach activates viral transcription without activating the cells. Clinical trials of several HDAC inhibitors have demonstrated increases in HIV RNA expression [65–67], however, none of these studies have revealed significant decreases in the latent resting CD4+ T cell reservoir [65–68]. One reason for this failure has been hypothesized to be a lack of an effector CTL response, as in vitro virus-specific CTL have been shown to reduce latently infected cells upon activation [69]. An alternative explanation is that virus-specific CTL are present, but do not enter follicles, as has been reported in untreated disease [4, 6]. Thus, strategies to boost numbers of CTL in B cell follicles, such as by transduction of the follicular homing molecule CXCR5 into HIV-specific CTL [70], could be an important component of HIV cure in combination with latency reversing agents. Although it is unknown if this approach would have deleterious effects, such as disruption of the GC reaction or alteration of antibody production, directing HIV-specific CTL into the follicles could eliminate HIV-infected cells within follicles.
The use of broadly neutralizing antibodies prophylactically after HIV exposure led to decreased establishment of HIV reservoirs in humanized mice and was effective in decreasing established reservoirs when combined with a combination of viral inducers [71]. Although the humanized mouse models are useful to study many aspects of HIV immunopathogenesis, most do not recapitulate key aspects of human lymphoid tissues. Specifically, they lack FDC and GC, and fail to develop IgG responses [72]. As noted above, FDC-bound virions are potently infectious to TFH, even in the presence of neutralizing antibodies [13]. Thus, it would be important to carefully examine this strategy in a more physiologically relevant model, such as the SIV-infected rhesus macaques, before proceeding to test it in humans.
Despite the successful bone marrow transplant of the “Berlin patient” [61], subsequent transplants have proven unsuccessful in preventing HIV rebound [73, 74], although HIV DNA was not detected in these individuals peripheral blood mononuclear cells (PBMC) prior to interruption of therapy. Viral reservoirs also persist in simian-human immunodeficiency virus (SHIV)-infected rhesus macaques following transplantation [75]. Although some have concluded that this failure is due to residual infection of host CD4+ T cells, it is difficult to imagine that any purging strategy will more effectively eliminate infected CD4+ T cells than a bone marrow transplant, particularly in cases of graft versus host disease, which occurred in some of these transplants. An alternative explanation is that the transplantation regimens failed to eliminate cell-free infectious virus on FDC. It would be important to evaluate this possibility particularly in animal models, and develop approaches that are more effective against FDC in the transplant setting. Furthermore, strategies to displace virions bound to FDC outside of the transplant setting should be evaluated to determine if they diminish the reservoir. Previous work has shown the effectiveness of complement and CD21 antibody-mediated displacement of virions from the surface of B cells from HIV-infected patients ex vivo [24]. An immunotoxin-conjugated agent might be effective against FDC-bound virions as well [76].
Concluding Remarks
TFH expand in number and are a major site of virus infection and replication in untreated, asymptomatic HIV infection as well as in treated disease. Multiple factors likely contribute to the concentration of virus replication in TFH including heightened permissiveness, the presence of highly infectious virions on FDC, low frequencies of virus-specific CTL in B cell follicles, expansions in numbers and impairments in function. Emerging data suggest that memory TFH constitute a significant fraction of the latent reservoir in treated disease, and this is an important area for future research (see Outstanding Questions). Whether interactions between FDC harbouring infectious virions and TFH further contribute to the latent HIV reservoir remains to be determined. Strategies aimed at targeting follicular reservoirs of HIV could provide important insights into these questions, and potentially a cure for HIV infection. It should be noted that although TFH may be a major cellular reservoir, other reservoirs have been described including the nasopharynx, lung, male and female genital tracts, as well as the brain [77, 78]. The relationship between follicular reservoirs and these sites, specifically whether additional therapies may be necessary to achieve a cure, remains to be determined and is an important question for future research.
Outstanding Questions.
What fraction of latently infected CD4+ T cells are memory TFH?
How long do infectious virions persist on FDC in the setting of ART?
Can removal of infectious complexes from FDC reduce the HIV reservoir and prevent viral rebound?
Will expression of CXCR5 by virus-specific CTL induce them to home to follicles and suppress virus replication and ultimately clear the latent HIV reservoir?
What is the relationship between follicular HIV reservoirs and other HIV reservoirs throughout the body?
Trends.
T follicular helper cells (TFH) are the major HIV-producing cells in untreated disease.
Heightened TFH permissivity, follicular dendritic cells (FDC) virion retention, cytotoxic T lymphocytes (CTL) exclusion from the follicle, expansion of the TFH subset, and TFH dysfunction all contribute to HIV replication in TFH.
Limited data suggest that follicular reservoirs of HIV, including latently infected memory TFH and FDC-bound virions, exist in treated disease.
Strategies that target follicular reservoirs of HIV could be used to dissect out their role in the latent reservoir and HIV persistence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkvord JM, et al. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Research & Human Retroviruses. 2005;21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 3.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connick E, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. The Journal of Immunology. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 5.Hufert FT, et al. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. Aids. 1997;11:849–857. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Connick E, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. The Journal of Immunology. 2014;193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacker TC, et al. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. Journal of virology. 2009;83:150–158. doi: 10.1128/JVI.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler SL, P MN, Folkvord JM, Arends T, Miller SM, Miles B, Meditz AL, McCarter M, Levy DN, Connick E. Germinal Center T Follicular Helper Cells (GC Tfh) are Highly Permissive to HIV-1 and Alter Their Phenotype During Virus Replication. Journal of Immunology. 2016 doi: 10.4049/jimmunol.1502174. www.jimmunol.org/cgi/doi/10.4049/jimmunol.1502174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allam A, et al. TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Scientific reports. 2015;5:e10443. doi: 10.1038/srep10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel H, et al. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. The American journal of pathology. 1992;140:15. [PMC free article] [PubMed] [Google Scholar]
- 12.Haase AT, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 13.Heath SL, et al. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 14.van Nierop K, de Groot C. Seminars in immunology. Elsevier; 2002. Human follicular dendritic cells: function, origin and development; pp. 251–257. [DOI] [PubMed] [Google Scholar]
- 15.Schacker T, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. Journal of Infectious Diseases. 2000;181:354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 16.Keele BF, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. Journal of virology. 2008;82:5548–5561. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tjernlund A, et al. In situ detection of Gag-specific CD8+ cells in the GI tract of SIV infected Rhesus macaques. Retrovirology. 2010;7:12. doi: 10.1186/1742-4690-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukazawa Y, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nature medicine. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122:3271. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation. 2012;122:3281. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong JJ, et al. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. The Journal of Immunology. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi YS, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. The Journal of Immunology. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumjohann D, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Moir S, et al. B cells of HIV-1–infected patients bind virions through CD21–complement interactions and transmit infectious virus to activated T cells. The Journal of experimental medicine. 2000;192:637–646. doi: 10.1084/jem.192.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boswell KL, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS pathogens. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, et al. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. The Journal of Immunology. 2015;195:4351–4357. doi: 10.4049/jimmunol.1501273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 28.Fan J, et al. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. The Journal of Immunology. 1993;151:5031–5040. [PubMed] [Google Scholar]
- 29.Nakajima K, et al. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. The Journal of Immunology. 1989;142:531–536. [PubMed] [Google Scholar]
- 30.Harari A, et al. Analysis of HIV-1–and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood. 2002;100:1381–1387. doi: 10.1182/blood-2001-11-0080. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury A, et al. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus–infected rhesus macaques may contribute to accumulation of TFH in chronic infection. The Journal of Immunology. 2015;195:3237–3247. doi: 10.4049/jimmunol.1402701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackburn MJ, et al. Regulatory and helper follicular T cells and antibody avidity to simian immunodeficiency virus glycoprotein 120. The Journal of Immunology. 2015;195:3227–3236. doi: 10.4049/jimmunol.1402699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles B, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nature communications. 2015;6:8608. doi: 10.1038/ncomms9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colineau L, et al. HIV-Infected Spleens Present Altered Follicular Helper T Cell (Tfh) Subsets and Skewed B Cell Maturation. PloS one. 2015;10:e0140978. doi: 10.1371/journal.pone.0140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas MK, et al. Distinct Patterns of Bcl-2 Expression Occur in R5-and X4-Tropic HIV-1-Producing Lymphoid Tissue Cells Infected Ex Vivo. AIDS research and human retroviruses. 2015;31:298–304. doi: 10.1089/aid.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cubas RA, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz Bruce T, et al. Circulating HIV-Specific Interleukin-21+CD4+ T Cells Represent Peripheral Tfh Cells with Antigen-Dependent Helper Functions. Immunity. 44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Chun T-W, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 40.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 41.DeMaster LK, et al. A subset of CD4/CD8 double negative T cells expresses HIV proteins in patients on ART. Journal of virology. 2015 doi: 10.1128/JVI.01913-15. JVI. 01913-01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. New England Journal of Medicine. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 43.Horiike M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423:107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Rothenberger MK, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences. 2015;112:E1126–E1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher CV, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proceedings of the National Academy of Sciences. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Günthard HF, et al. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. Journal of Virology. 1998;72:2422–2428. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nettles RE, et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 48.von Stockenstrom S, et al. Longitudinal genetic characterization reveals that cell proliferation maintains persistent HIV-1 during effective HIV therapy. Journal of Infectious Diseases. 2015;212:596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermankova M, et al. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA. 2001;286:196–207. doi: 10.1001/jama.286.2.196. [DOI] [PubMed] [Google Scholar]
- 50.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature medicine. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Frontiers in immunology. 2015;6:16. doi: 10.3389/fimmu.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baron BW, et al. BCL6 can repress transcription from the human immunodeficiency virus type I promoter/enhancer region. Genes, Chromosomes and Cancer. 1997;19:14–21. [PubMed] [Google Scholar]
- 53.Kaczmarek K, et al. T cell transcription factors and their impact on HIV expression. Virology: research and treatment. 2013;2013:41. doi: 10.4137/VRT.S12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pallikkuth S, et al. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir Within Central Memory CD4 T Cells in Peripheral Blood from chronic HIV infected individuals on cART. Journal of virology. 2015 doi: 10.1128/JVI.02883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chahroudi A, Silvestri G. HIV and Tfh Cells: Circulating New Ideas to Identify and Protect. Immunity. 2016;44:16–18. doi: 10.1016/j.immuni.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Barradas MC, et al. Quantitative and qualitative antibody responses to immunization with the pneumococcal polysaccharide vaccine in HIV-Infected patients after initiation of antiretroviral treatment: results from a randomized clinical trial. Journal of Infectious Diseases. 2014;211:1703–1711. doi: 10.1093/infdis/jiu819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sepako E, et al. Incomplete recovery of pneumococcal CD4 T cell immunity after initiation of antiretroviral therapy in HIV-infected malawian adults. PloS one. 2014;9:e100640. doi: 10.1371/journal.pone.0100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pallikkuth S, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavert W, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 60.Smith BA, et al. Persistence of infectious HIV on follicular dendritic cells. The Journal of Immunology. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 61.Hütter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. New England Journal of Medicine. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 62.Chun T-W, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nature medicine. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 63.Prins JM, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. Aids. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 64.Davey RT, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proceedings of the National Academy of Sciences. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Archin N, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen TA, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The Lancet HIV. 2014;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 67.Elliott JH, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Routy J, et al. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV medicine. 2012;13:291–296. doi: 10.1111/j.1468-1293.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 69.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skinner P, Connick E. Overcoming the immune privilege of B cell follicles to cure HIV-1 infection. J. Hum. Virol. Retrovirol. 2014;1:00001–00003. [Google Scholar]
- 71.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karpel ME, et al. BLT humanized mice as a small animal model of HIV infection. Current opinion in virology. 2015;13:75–80. doi: 10.1016/j.coviro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henrich TJ, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Annals of internal medicine. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cillo AR, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. Journal of acquired immune deficiency syndromes (1999) 2013;63:438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mavigner M, et al. Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant. PLoS Pathog. 2014;10:e1004406. doi: 10.1371/journal.ppat.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denton AE, et al. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proceedings of the National Academy of Sciences. 2014;111:12139–12144. doi: 10.1073/pnas.1412910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santangelo PJ, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nature methods. 2015;12:427–432. doi: 10.1038/nmeth.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hellmuth J, et al. CNS reservoirs for HIV: implications for eradication. Journal of virus eradication. 2015;1:67. doi: 10.1016/S2055-6640(20)30489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]