Abstract
Background:
Remodeling targeted tissues for reception of tumor cells metastasizing from primary lesions is a consequence of communication between the tumor and the environment that governs metastasis. This study describes a novel approach that aims to disrupt the process of metastasis by interfering with this intense dialogue.
Methods:
Proteomics and adhesion assays identified exosomes purified from the ascitic fluid of ovarian cancer patients (n = 9) as intermediaries of tumor cell attachment. A novel tumor cell capture device was fabricated by embedding exosomes onto a 3D scaffold (metastatic trap [M-Trap]). Murine models of ovarian metastasis (n = 3 to 34 mice per group) were used to demonstrate the efficacy of M-Trap to capture metastatic cells disseminating in the peritoneal cavity. Kaplan-Meier survival curves were used to estimate cumulative survival probabilities. All statistical tests were two-sided.
Results:
The exosome-based M-Trap device promoted tumor cell adhesion with a nonpharmacological mode of action. M-Trap served as a preferential site for metastasis formation and completely remodeled the pattern of peritoneal metastasis in clinically relevant models of ovarian cancer. Most importantly, M-Trap demonstrated a statistically significant benefit in survival outcomes, with mean survival increasing from 117.5 to 198.8 days in the presence of M-Trap; removal of the device upon tumor cell capture further improved survival to a mean of 309.4 days (P < .001).
Conclusions:
A potent artificial premetastatic niche based on exosomes is an effective approach to impair the crosstalk between metastatic cells and their environment. In the clinical setting, the capacity to modulate the pattern of dissemination represents an opportunity to control the process of metastasis. In summary, M-Trap transforms a systemic, fatal disease into a focalized disease where proven therapeutic approaches such as surgery can extend survival.
Metastasis represents the most devastating event in oncology (1). Loco-regional and distant metastasis is associated with a contraindication to surgery and radiotherapy, with resistance to chemotherapy. Because of these factors, cancer metastasis is responsible for more than 90% of cancer related deaths. Homing and colonization of disseminating and circulating metastatic cells at appropriate conditioned sites is the result of an intense dialogue between primary tumors with their environment (2). A novel approach in oncology that disrupts the process of metastasis by interfering with this intense dialogue could transform a systemic, fatal disease into a focalized disease where current therapeutic approaches have proven efficacy.
Tissue-specific metastasis (3) and premetastatic niches (4) are concepts that are beginning to illustrate the active role of carcinomas in determining the most adequate sites to colonize. The concept of “premetastatic niches” refers to the conditioning of future sites of metastasis or “soil” in preparation for the reception of tumor cells (5). These niches represent a specialized microenvironment that facilitates and promotes the invasion, survival, and outgrowth of disseminated tumor cells (6). Recent findings in melanoma describe exosomes, a subset of microvesicles involved in the transfer of information as a mode of cell-cell communication, as a systemic factor critical to premetastatic niche formation (7,8). Exosomes act as mediators in the crosstalk and homing of metastatic tumor cells to the niche (9).
The impact of these primed sites for the implantation of metastatic cells is particularly pronounced for intraperitoneal metastases. Patients presenting with tumor cell dissemination on the peritoneal surfaces of the abdomen, such as gastrointestinal and gynecologic malignancies, face drastically worse prognosis (10,11). Among gynecologic malignancies, ovarian cancer is usually diagnosed at an advanced stage when tumors have spread in diffuse peritoneal lesions that impede surgical removal. The survival rate at five years in advanced ovarian cancer is only 25% (12). The peritoneal cavity is particularly receptive to metastasis because disseminating tumor cells attach to a single surface layer of mesothelial cells and the associated underlying extracellular matrix (ECM). The presence of ascites, an accumulation of protein-rich exudate in the peritoneal cavity, further promotes carcinomatosis and metastasis. Changes in the tumor microenvironment in ovarian cancer are reflected in this large volume peritoneal fluid, with exosomes and inflammatory mediators involved in cancer cell attachment (13).
To interfere with the communication between tumor cells and the host, an artificial premetastatic niche based on exosomes as key drivers of this crosstalk was created to compete with natural niches for the capture of metastatic tumor cells. Proof-of-concept in murine models of ovarian cancer intraperitoneal dissemination are presented: 1) characterization of exosomes as components within the ascitic fluid of ovarian cancer patients with the ability to communicate with tumor cells and modulate their attachment; 2) fabrication of a tumor cell capture device comprised of exosomes embedded on a 3D scaffold where metastatic tumor cells preferentially home (metastatic trap [M-Trap]); 3) demonstration that M-Trap completely remodels the peritoneal pattern of metastasis in clinically relevant ovarian cancer models; and 4) evaluation of the impact of M-Trap on the survival outcomes in the murine model of ovarian metastasis.
Methods
Exosome Purification From Ovarian Cancer Patients’ Ascites
Ascites fluid from advanced stage III/IV ovarian cancer patients (n = 9) was collected in sterile conditions at the Medical Oncology Department at the University Hospital of Santiago de Compostela (Spain) under fully informed consent and ethical approval by the Galician Ethical Committee (reference: 2014/309). Ascites samples were sequentially centrifuged (300g, 10 minutes; 800g, 15 minutes; 10.000g, 30 minutes) and filtered (0.22 μm) prior to exosome purification by ultracentrifugation as described (8). Briefly, cleared ascites samples were centrifuged at 100.000g for one hour, the pellet was resuspended in phosphate-buffered solution (PBS), and centrifuged at 100.000g for one hour. Exosomes were resuspended in PBS and stored at -80°C. Reproducibility among batches of exosomes was assessed by morphology/size (Zeta sizer device; Malvern instrument Ltd. Malvern, Worcestershire, UK), protein content by nanodrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, MA) and BCA assay (Thermo Fisher Scientific Inc., Wilmington, MA), and adhesion assays. Pretreatment of polystyrene wells with 50 μl of total cell-free ascites was performed overnight at 37ºC in humidified CO2 conditions before the PBS wash and cell seed. Ascites fractionation and mass spectrometry are described in detail in the Supplementary Methods (available online).
Cell Lines
Human ovarian cancer SKOV3, OV90, and TOV112 cells, human endometrial cancer Ishikawa cells, and human colorectal cancer HCT116 cells were purchased from ATCC and authenticated by short tandem repeat DNA fingerprint. Cells were transfected with the luciferase reporter pEGFPLuc by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer conditions, selected with Geneticin (InvivoGen, San Diego, CA), and maintained in McKoy’s complete medium (10% fetal bovine serum 2mM glutamine, 100U/mL penicillin and 100 ug/mL streptomycin).
Cell Adhesion Assay
1x105 human ovarian carcinoma SKOV3 cells labeled with calcein (4mM; Life Technologies, Thermo Fisher Scientific Inc., Waltham, MA) for 30 minutes were seeded onto 96 microtiter black polystyrene 96-well plates and allowed to adhere for 60 minutes. Upon supernatant removal and PBS wash, adhered cells were quantified in a luminometer (FLUOstar OPTIMA, BMG Labtech GMBH, Ortenberg, Germany). The human phospho-kinase assay is described in detail in the Supplementary Methods (available online).
M-TRAP Device
Exosomes (25 µl at 2 µg/µl) were embedded into 3D Insert scaffold (polystyrene polycaprolactone; 3D Biotech LLC, North Brunswick, NJ) and incubated overnight at 37ºC in humidified CO2 conditions. Alternatively, purified exosomes were labeled with 1mM Vibrant DiD cell-labeling solution (Molecular Probes, Life Technologies, Thermo Fisher Scientific Inc., Waltham, MA) following manufacturer’s instructions before decoration of 3D scaffolds. The M-Trap dynamic cell adhesion assay and electron microscopy methods are described in detail in the Supplementary Methods (available online).
Ovarian Cancer Mouse Model for Peritoneal Dissemination and Surgical M-Trap Implantation
Female SCID Beige mice (Barcelona Biomedical Research Park, Barcelona, Spain) aged eight weeks were injected intraperitoneally with 100 μL PBS containing 1x106 luciferase-expressing SKOV3 cells, and the pattern of peritoneal metastasis was analyzed one week later by bioluminescence in an in vivo image system Series Pre-clinical In Vivo Imaging System (Xenogen Corp., Caliper Life Sciences Inc., PerkinElmer), by peritoneal injection of 10 μL/g of mice D-luciferine substrate (P/N 122796, PerkinElmer, Waltham, MA), in the presence (n = 24) and absence (n = 34) of M-Trap devices. Additionally the pattern of metastasis was evaluated with the bare scaffold (n = 15), with Poly-Hema pretreated bare scaffold (n = 8), and with bare scaffold decorated with the adhesive tetraspanins CD9 (n = 3) and CD81 (n = 3). Alternatively, 1x105 luciferase-expressing SKOV3 cells embedded in matrigel were directly injected into one of the ovaries of mice to generate an orthotopic model of peritoneal dissemination (14) and evaluate the efficacy of M-Trap in the presence (n = 5) or absence (n = 5) of the device. M-Trap devices were surgically implanted one week before SKOV3 cell injection to avoid any impact from the inflammatory reaction to surgery. Mice were anesthetized with Ketamin (Ketolar, Pfizer, New York, NY) at 75mg/kg and Metomidin (Domtor, Orion Pharma Corp., Orionintie, Finland) as analgesic at 1mg/kg. M-TRAP was implanted after laparotomy at the inner wall of the peritoneum using surgical glue (Glubran2, GEM S.R.L. ref. G-ND-2, Italy) to fix the device to the mesothelium on the side opposite the pancreas (see Figure 3B). The wound was closed (Ethicon VICRIL suture; Johnson & Johnson, New Brunswick, NJ), and mice reverted from anesthesia with Atipemazol (1mg/kg; Antisedan, Orion pharma Corp.). Survival was assessed as indicated in Figure 5A, with in vivo bioluminescence imaging and monitoring of weight of mice as follow-up upon intraperitoneal injection of 2.5x106 luciferase-expressing SKOV3 cells in the presence (n = 7) or absence (n = 7) of the M-Trap device, with a third arm including M-Trap removal one month after SKOV3 cell injection (n = 4). The endpoint was defined as a drop maximum weight of 25%; mice were killed in a CO2 chamber and by cervical dislocation. Mice were housed and maintained under specific pathogen-free conditions and used in accordance with institutional guidelines approved by the Use Committee for Animal Care.
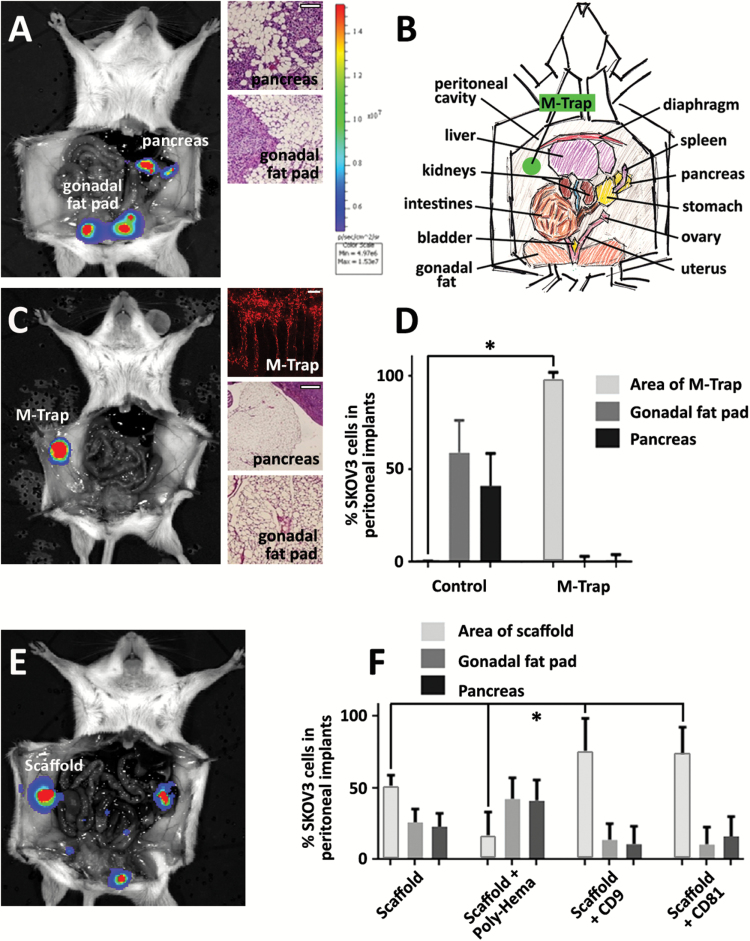
Figure 3.
Capture of metastatic tumor cells by metastatic trap (M-Trap) technology in an in vivo model of ovarian cancer dissemination. A) Representative bioluminescent distribution of metastasis at pancreatic fat and gonadal fat pad as natural pattern of dissemination one week after intraperitoneal injection of SKOV3 cells, stably transfected with luciferase gene reporter. Representative haematoxylin-eosin sections of pancreatic fat and gonadal fat pad revealing the presence of tumor infiltrates (right panels; scale bar for HE images = 100 μm). B) Schematic peritoneal anatomy of mice indicating M-TRAP implantation at the inner wall of the peritoneum. C) Representative distribution of peritoneal metastasis in the presence of M-Trap, one week after intraperitoneal injection of SKOV3 cells (left panel). Maximum projection of confocal microscopy images from M-Trap device after death and surgical removal, showing fluorescent DiD-labeled SKOV3 cells aligned along the fibers of M-Trap (upper right panel; scale bar = 100 μm). Representative haematoxylin-eosin sections confirming the absence of tumor implants at the natural sites of metastasis (middle and lower right panels; scale bar for HE images = 100 μm). D) Distribution of SKOV3 cell implants represented as percentage of total biolumiscence signal per mouse quantified with the in vivo image system, in the presence (M-Trap; n = 24) or absence (control; n = 34) of M-Trap device. Error bars represent standard deviation (P < .0001, Student′s t test). E) Representative bioluminescence pattern of SKOV3 cell implants in the presence of an empty scaffold, resulting in a partial metastasis remodeling effect. F) Distribution of SKOV3 cell implants under modulated adhesion properties: empty 3D scaffold (n = 15); empty 3D scaffold pretreated with Poly-Hema (P < .0001; n = 8); empty 3D scaffold decorated with CD9 (P = .003; n = 3); or CD81. Error bars represent standard deviation (P =.004, one-way analysis of variance; n = 3). M-TRAP = metastatic trap.
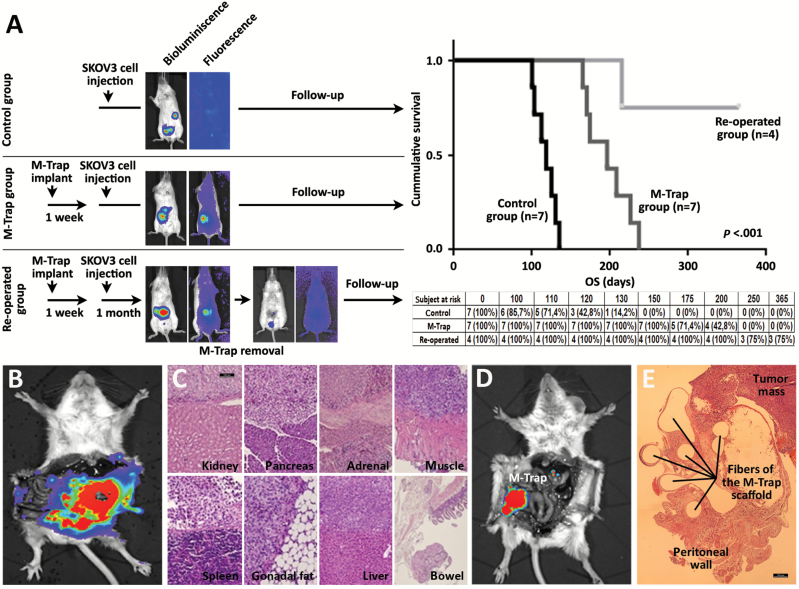
Figure 5.
Preclinical trial with metastatic trap (M-Trap) technology demonstrates a benefit in survival. A) Schema with the three arms of the study: 1) control group: mice intraperitoneally injected with 2.5x106 luciferase-expressing SKOV3 cells and follow-up (n = 7; upper panel); 2) M-Trap group: mice surgically implanted with M-Trap device one week before SKOV3 cells intraperitoneal injection and follow-up (n = 7; middle panel); 3) re-operated group: mice implanted with M-Trap device one week before SKOV3 cell intraperitoneal injection and surgically removed one month after injection, and follow-up (n = 4; lower panel). In vivo images showing SKOV3 cells (bioluminescence signal) and M-Trap device (fluorescence signal) confirmed the eradication of peritoneal disease upon surgical removal of M-Trap. Follow-up was performed with in vivo image system until endpoint, defined as a drop in 25% maximum weight. Kaplan-Meier survival curves show the cumulative survival probabilities for the three groups in the study, with subject at risk and survival proportions (re-operated group with three out of four mice still alive at one year follow-up; mean survival for control group was 117.5 days (95% confidence interval [CI] = 107.6 to 127.4); M-Trap group was 198.8 days (95% CI = 170.4 to 209.2); re-operated group was 309.4 days (95% CI = 249.4 to 369.4); P <.001, two-sided log-rank test). B) Extensive peritoneal carcinomatosis shown by bioluminescence in control group at endpoint. C) Representative immunohistological haematoxylin-eosin section of tissues targeted of metastasis at endpoint in control group. Scale bar = 100 µm. D) Bioluminiscence examination of M-Trap group at endpoint. Disseminated peritoneal carcinomatosis in control group has been transformed into a focalized disease, with a tumor mass growing within the device. E) Immunohistological haematoxylin-eosin section of M-Trap device at endpoint showing the tumor mass growing within M-Trap scaffold implanted at the inner wall of the peritoneum. Scale bar = 100 µm. M-TRAP = metastatic trap.
Histology Analysis
Tissue samples were immersion fixed in 10% buffered formalin for 24 hours, dehydrated in ethanol series, embedded in paraffin, sectioned (4 μm thick), mounted on microslides, and, after drying, dewaxing, and rehydratation, tissues were stained with haematoxylin-eosin and examined using an Olympus BX51 microscope equipped with an Olympus DP70 camera (Olympus, Tokyo, Japan).
Immunoblot
Immuno blot analysis was performed using 40 μg of total protein extracted from exosomes purified from ovarian cancer patients’ ascitic fluid and incubated overnight with mouse monoclonal antibodies directed against human CD9 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and CD81 (1:1000; Santa Cruz Biotechnology). A secondary antimouse monoclonal antibody (1:1000; Santa Cruz Biotechnology) was used to visualize proteins using an AmershamTM ECLTM immuno blotting analysis system (GE Healthcare, Amersham Biotechnology, UK).
Statistical Analysis
All experiments were repeated at least three times, and data are represented as mean values with 95% confidence intervals. The Student’s t test was used to compare the differences between two groups, and analysis of variance was used to compare the differences among multiple groups. Kaplan-Meier survival curves were used to estimate cumulative survival probabilities using IBM SPSS statistics. Overall P value was calculated using a log-rank test. Statistical significance was defined as a P value less than .05. All statistical tests were two-sided.
Results
Exosomes as Key Components in Ovarian Cancer Communication
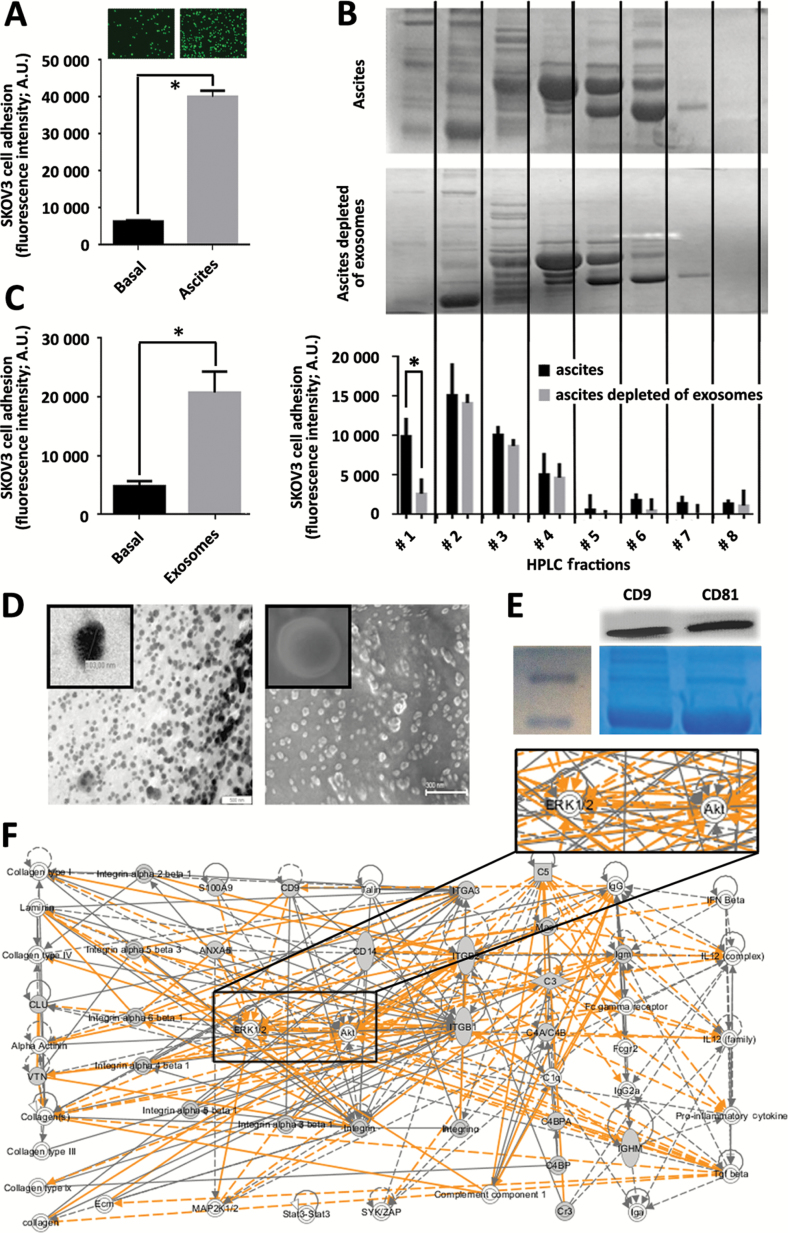
We evaluated the role of ovarian cancer ascites and its exosomal component in the crosstalk between metastatic tumor cells and the environment and in the promotion of tumor cell attachment to the peritoneum. Cellular-free ascitic fluid from advanced ovarian cancer patients efficiently promoted in vitro adhesion of fluorescence-labeled ovarian cancer SKOV3 cells (6.43-fold, P = .0012, Student’s t test) (Figure 1A). High-Performance Liquid Chromatography (HPLC) fractionation of the ascitic fluid (upper panel in Figure 1B) showed adhesive components concentrated in the first fractions corresponding to 670-44kDa proteins (black bars in histogram of Figure 1B). The depletion of multivesicular bodies including exosomes from ascites by ultracentrifugation (middle panel in Figure 1B) resulted in a statistically significantly 0.21-fold reduced adhesive potential of SKOV3 cells mediated by the first HPLC fraction of ascites (P = .0035, Student’s t test) (gray bars in histogram of Figure 1B). Conversely, exosomes purified from ascites of ovarian cancer patients exhibited the capacity to promote in vitro adhesion of SKOV3 cells compared to the untreated control (4.34-fold, P < .001, Student’s t test) (Figure 1C). Purified exosomes were characterized in terms of morphological and biomarker features. Exosomes were shown to be a homogeneous population of vesicles approximately 100nm in diameter by Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM) (Figure 1D), with the presence of CD9/CD81 exosome markers (Figure 1E). Mass spectrometry was used to further characterize the proteome of exosomes purified from ovarian cancer ascites (Supplementary Table 1, available online). One hundred eighty-three proteins were identified, and comprehensive pathway and network analysis was employed to accurately interpret the biological significance of these proteins. This analysis indicated that the principal biological role of exosomes is to mediate cell communication and interaction with the environment (Figure 1F). Phospho-kinase assay and adhesion impairment with specific inhibitors further highlighted the ERK1/2 and Akt pathways as the underlying mechanism in exosome-mediated ovarian tumor cell attachment (Supplementary Figure 1, available online). Collectively, these findings demonstrate the important role of ascites-derived exosomes in ovarian cancer cell communication with the environment. As critical intermediaries of tumor cell attachment, exosomes offer the potential to function as a specific modulator of metastatic tumor cell attachment and homing in ovarian cancer.
Figure 1.
Exosomes as components of ascitic fluid involved in cell communication. A) Short-term calcein-labeled SKOV3 cell adhesion assay performed under Basal (nontreated) and Ascites (ascites-pretreated) conditions. Upper panels illustrate representative fluorescence images of adhered cells, while quantification of fluorescence intensity is represented in the histogram. Data are means from three independent experiments. Error bars represent standard deviation (P = .0012, two-sided Student’s t test). B) Representative electrophoresis gel performed under denaturant conditions showing protein distribution upon HPLC fractionation of total cell-free ascites (upper panel) and ascites depleted of exosomes (middle panel) by ultracentrifugation (see the Supplementary Methods, available online). SKOV3 cell adhesion assay performed upon pretreatment with each HPLC fraction demonstrated a statistically significant reduction in adhesion ability of ascites after depletion of exosomes (data are means from three independent experiments). Error bars represent standard deviation (P = .0035, Student’s t test; lower panel). C) Adhesion assay performed as in panel (A) under basal (nontreated) and exosomes (pretreated with 50 μg of purified exosomes), demonstrated statistically significant adhesion mediated by purified exosomes (data are means from three independent experiments). Error bars represent standard deviation (P < .001, Student’s t test). D) Electron microscopy images of exosomes purified from ascites of ovarian cancer patients (see the Supplementary Methods, available online): SEM, left panel; scale bar = 500nm; representative electron microscopy, right panel; scale bar = 300nm; inserts showing a 10-fold magnification of a representative vesicle. E) Immuno blot of 40 μg of total protein extracted from exosomes purified from the ascitic fluid of ovarian cancer patients, demonstrated human CD9 and CD81 as markers of exosomes (upper panel) (see the Supplementary Methods, available online). Coomassie staining of total extract is shown as loading control (lower panel). F) Ingenuity pathway analysis (IPA) upon proteome analysis by Maldi TOF/TOF of exosomes purified from ascitic fluid of ovarian cancer patients (see the Supplementary Methods, available online). Comprehensive protein network analysis pointed to cell adhesion as a main biological process associated with exosome proteome, characterized by integrins and extracellular matrix proteins and a central role for the ERK 1/2 and Akt pathways (insert).
Design and Characterization of M-Trap Device
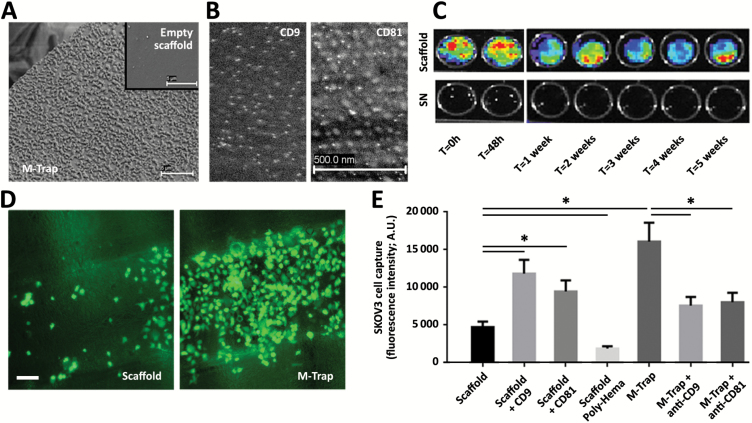
A novel tumor cell capture device (M-Trap) was constructed by embedding exosomes purified from ovarian cancer patient ascites onto 3D-Polystyrene/Polycaprolactone inserts. Electron microscopy showed exosomes attached to the fibers of the 3D scaffold (Figure 2A, compared with the empty scaffold without exosomes, shown in the insert). Immunogold staining with antibodies against two membrane proteins used as markers of exosomes, tetraspanins CD9 (left panel in Figure 2B) and CD81 (right panel in Figure 2B), confirmed the adherence of the exosomes to the fibers of the scaffolds.
Figure 2.
Metastatic trap (M-Trap) design and characterization of mode of action. A) Exosomes purified from ascites of ovarian cancer patients (25 µL at 2 µg/µL) were embedded into the 3D scaffold; representative electron microscopy (TEM) of a fiber of the 3D nanomesh scaffold with adhered exosomes, in comparison with the surface of a fiber of the scaffold in the absence of exosomes (insert; scale bar = 5 μm). B) Representative electron microscopy (TEM) images showing immunogold staining of exosomes adhered to M-Trap device, revealed by TEM as vesicles containing CD9 (left panel) and CD81 (right panel) colloidal gold particles (scale bar = 500nm for both panels) (see the Supplementary Methods, available online). C) Substrate-bound nonpharmacological mode of action of M-Trap as demonstrated by release experiments. DiD-labeled exosomes (25 µl at 2 µg/µl) were immersed in phosphate-buffered solution, and supernatants and devices (scaffold) were collected at indicated times and fluorescent imaged (in vivo image system). D) Representative fluorescent images of SKOV3 cells adhered to a fiber of the 3D scaffold in the presence (M-Trap; right panel) or not (Scaffold; left panel) of exosomes under dynamic orbital rotation conditions (scale bar = 100 μm) (see the Supplementary Methods, available online). E) Luminometer quantification of calcein-labeled SKOV3 cells captured under dynamic conditions to bare 3D scaffolds (scaffold); bare 3D scaffolds decorated with tetraspanins CD9 (scaffold + CD9) or CD81 (scaffold + CD81); bare 3D scaffold pretreated with Poly-Hema (scaffold Poly-Hema); M-Trap device (M-Trap); M-Trap device pretreated with blocking antibodies against CD9 (M-Trap + anti-CD9); and CD81 (M-Trap + anti-CD81) (see the Supplementary Methods, available online). Modulation of cell adhesion resulted in a gradual capacity to capture SKOV3 cells with a maximal effect by M-Trap (data are means from three independent experiments). Error bars represent standard deviation (P = .001, M-Trap versus bare scaffold, two-sided Student’s t test). SN = supernatant.
Release of exosomes from the 3D scaffold was assessed to verify the stability of the M-Trap exosome coating. No substantial in vitro release of exosomes decorated with the fluorescent marker DiD was observed for up to five weeks (Figure 2C). Concordantly, mass spectrometry analysis with nano-HPLC (Supplementary Table 2, available online) and short-term adhesion experiments (Supplementary Figure 2A, available online) also demonstrated that there was no substantial release of exosomes from the M-Trap device and also no sustained release of protein components from the exosomes to the environment, indicative of the nonpharmacological mode of action of the device.
The efficacy of SKOV3 cell attachment to M-Trap in a dynamic in vitro environment was assessed by subjecting M-Trap devices to orbital movement designed to mimic the natural flow of peritoneal fluid within the abdominal cavity (15). In the absence of an active pharmacological chemotaxis attraction of metastatic SKOV3 cells, the adhesive capacity of the M-Trap technology behaves as the principal mode of action to capture tumor cells in this dynamic orbital assay. The M-Trap device showed a 3.4-fold enhanced SKOV3 cell attachment compared with bare scaffold (P = .001), while decoration of the bare scaffold with CD9 improved adhesion (2.5-fold, P = .003), and conversely pretreatment of M-Trap with blocking antibody against CD9 reduced its SKOV3 cell capture efficacy (0.47-fold, P = .005) (Figure 2, D-E; Supplementary Figure 2, B-G, available online). In summary, these findings characterize M-Trap as a device comprised of exosomes stably adhered to the fibers of a scaffold with substrate-bound exosomes promoting tumor cell attachment via a nonpharmacological, adhesive mode of action.
Efficacy of M-Trap Technology to Capture Metastatic Tumor Cells in an In Vivo Model of Ovarian Cancer Dissemination
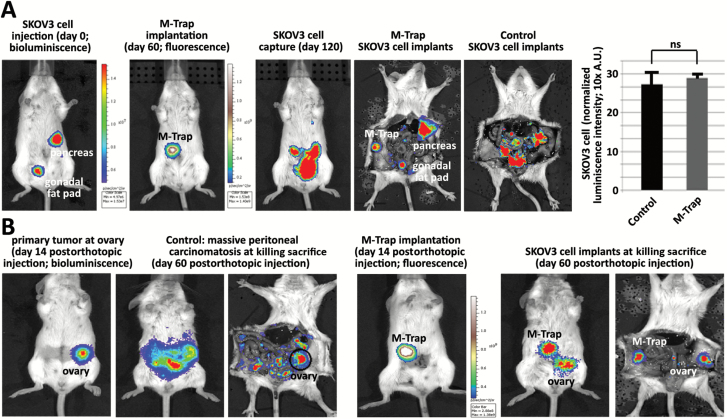
An in vivo murine model of ovarian cancer peritoneal dissemination and natural metastasis was created by intraperitoneally injecting 1x106 SKOV3 cells stably expressing the luciferase reporter gene (16). One week later, the pattern of natural peritoneal dissemination was evaluated by bioluminescence. Results showed the pancreas and gonadal fat pad as preferential sites of tumor cell attachment and homing (left panel in Figure 3A). Haematoxylin-eosin histological examination further confirmed the presence of tumor implants in the pancreas and gonadal fat pad (right panels in Figure 3A). Remarkably, when an M-Trap device was surgically implanted at the inner wall of the peritoneum opposite to the pancreas and the gonadal fat pad (scheme in Figure 3B), the pattern of dissemination of SKOV3 metastatic ovarian tumor cells was completely remodeled, with eradication of the regular sites of metastasis and the focalization of metastasis in a new, unique focus within the M-Trap device (left panel in Figure 3C). Confocal microscopy of the M-Trap device immediately upon removal from the peritoneum of the killed mice showed DiD-labeled SKOV3 cells captured by M-Trap, decorating the fibers of the 3D scaffold (upper right panel in Figure 3C). Histological examination of the pancreas and gonadal fat pad confirmed the absence of tumor implants at these natural sites of metastasis (middle and lower right panels in Figure 3C). Moreover, quantification of the bioluminescence signal in a broad series of control mice (0.16 mean % of signal at area of M-Trap implantation, 95% CI = 0.05 to 0.37, n = 34) and M-Trap mice (98.36 mean % of signal, 95% CI = 96.82 to 99.89, n = 24) confirmed the capacity of M-Trap to capture SKOV3 tumor cells disseminating within the peritoneal cavity and to completely remodel the pattern of metastasis in a murine model of ovarian cancer dissemination (P < .0001, Student’s Test) (Figure 3D). Similar to in vitro data, an empty scaffold without exosomes presented an intermediate pattern of metastasis with the presence of SKOV3 implants within the natural sites of metastasis (pancreas and gonadal fat pad) with an additional focus of SKOV3 cells within the empty scaffold (48.04 mean % of signal at area of implantation, 95% CI = 42.75 to 53.33, n = 15) (Figure 3, E-F). Moreover, when the adhesive capacity of the scaffold was limited by pretreatment with Poly-Hema, a reduced ability to capture metastatic SKOV3 cells was observed (11.71 mean % of signal at area of implantation, 95% CI = 3.01 to 20.40, P < .0001, one-way analysis of variance [ANOVA], n = 8) (Figure 3F). Conversely, when the adhesive capacity of the 3D scaffold was enhanced by decoration with tetraspanin CD9, an increased ability to capture metastatic SKOV3 cells was seen (79.76 mean % of signal at area of implantation, 95% CI = 61.65 to 97.87, P = .003, one-way ANOVA, n = 3) (Figure 3F). Similar results were obtained with CD81 (P = .004, n = 3) (Figure 3F). These results indicate that M-Trap tumor cell capture efficacy is a function of the adhesive capacity of the scaffold coating for the attachment and homing of metastatic cells, with the exosome-based M-Trap device showing the optimal adhesive capacity as evidence of a complete remodeling of the pattern of metastasis.
Similar results were obtained with clinically representative primary cultures isolated from ascites of ovarian cancer patients, cell lines of papillary serous and endometrioid ovarian cancer histologies (OV90 and TOV112 cell lines, respectively), and colorectal and endometrial cancer derived cell lines (n = 3) (Supplementary Figure 3, available online). These results support the broad applicability of M-Trap to a range of gynecologic and gastrointestinal cancers exhibiting peritoneal metastasis.
Efficacy of M-Trap Device in Sustained Peritoneal Dissemination and Orthotopic Models
The efficacy of M-Trap was evaluated in a clinically relevant model to assess the ability of the device to capture a sustained release of ovarian tumor cells metastasizing from primary lesions within the peritoneal cavity. To create this model, luciferase-expressing SKOV3 cells were intraperitoneally injected to generate primary lesions at the pancreas and gonadal fat pad (Figure 4A). The primary lesions were allowed to progress prior to implantation of an M-Trap device two months after tumor cell injection (Figure 4A). At four months after tumor cell injection, SKOV3 cells released into the peritoneal cavity from the primary lesions at the pancreas and gonadal fat pad were captured by M-Trap technology (Figure 4A). At death, the pattern of metastasis in this sustained release model was distributed at the two sites of primary implantation at the pancreas and gonadal fat pad, plus a unique focus of metastasis implantation within the M-Trap device (n = 5), compared with the peritoneal carcinomatosis observed in control mice in the absence of the M-Trap device (n = 5) (Figure 4A). Quantification of bioluminescence at death demonstrated a similar overall tumor burden in the control and M-Trap groups, but a complete remodeling of the pattern of metastasis (histogram in Figure 4A).
Figure 4.
Efficiency of metastatic trap (M-Trap) technology in clinically relevant models of sustained ovarian peritoneal metastasis. A) A first scenario of sustained release of tumor cells into the peritoneal cavity was achieved by intraperitoneal injection of SKOV3 cells generating implants at natural sites of metastasis (pancreas and gonadal fat pad; first left panel). M-Trap device decorated with DiD-fluorescent exosomes was surgically implanted 60 days after SKOV3 cell injection (second panel). SKOV3 cells sustainably released by the pancreas and gonadal fat pad lesions into the peritoneal cavity were captured by M-Trap device 120 days after SKOV3 cell injection (third panel). Peritoneal pattern of SKOV3 cell implants upon death revealed the presence of primary lesions at gonadal fat pad and pancreatic fat. An additional and focalized implant within M-Trap device resulting from the efficient capture of SKOV3 cells metastasizing into the peritoneal cavity from the primary lesions during the four months of the experiment (fourth panel; n = 5) was found in comparison with the natural pattern of massive peritoneal carcinomatosis at four months shown in control mice in the absence of M-Trap device (fifth panel; n = 5). Quantification of global peritoneal bioluminescence signal in control and M-Trap groups revealed no statistical difference in tumor burden (histogram showing similar global peritoneal SKOV3 cell signal for control (mean = 27.2, 95% confidence interval [CI] = 22.1 to 36.9) and M-Trap (mean 28.3, 95% CI = 20.1 to 39.6) groups. Error bars represent standard deviation). B) Second scenario of sustained tumor cell release into the peritoneum was achieved by orthotopic injection of SKOV3 cells (first left panel), resulting in a massive peritoneal dissemination after two months, as evidenced both in vivo (second panel) and upon death (third panel; n = 5). Implantation of M-Trap device with DiD-fluorescent labeled exosomes two weeks after generation of orthotopic tumors (fourth panel) resulted in an efficient capture of tumor cells sustainably disseminating from the primary tumor into the peritoneal cavity, as evidenced both by in vivo imaging showing a focalized disease (fifth panel) and a completely remodeled pattern of dissemination with two lesions at ovary and M-Trap device upon death (sixth panel; n = 5). M-TRAP = metastatic trap.
M-Trap efficacy was further demonstrated in an orthotopic model created by direct injection of SKOV3 cells into the ovary (Figure 4B). Orthotopic tumors were allowed to progress for two weeks prior to implantation of an M-Trap device decorated with fluorescent exosomes (Figure 4B). In contrast to the massive peritoneal carcinomatosis present in control mice in the absence of M-Trap (n = 5) (Figure 4B), use of M-Trap resulted in a focalized disease at the primary ovary lesion and within the M-Trap device, as a result of the capture of metastatic cells sustainably released from the ovarian tumor (n = 5) (Figure 4B).
Finally, the ability of M-Trap to capture tumor cells disseminating throughout the peritoneal cavity in sequential injections of SKOV3 cells (Supplementary Figure 4, available online) further validated the efficacy of M-Trap in another clinically relevant model of sustained ovarian metastasis. From all these results we conclude that M-Trap acts as a potent artificial premetastatic niche that preferentially captures metastatic cells disseminating in the peritoneum in competition with natural sites of homing and metastasis.
Impact of M-Trap on Survival in the Ovarian Metastasis Model
The impact of M-Trap technology on survival outcomes was assessed in the murine model of ovarian cancer peritoneal dissemination. This preclinical trial included three arms simulating the following scenarios: 1) the natural pattern of massive peritoneal dissemination in ovarian cancer (control group; n = 7); 2) the transformation of massive peritoneal dissemination into a focalized disease by the M-Trap technology (M-Trap group; n = 7); and 3) the surgical removal of M-Trap upon focalization of the metastatic disease (re-operated group; n = 4) (see scheme in Figure 5A).
Kaplan-Meyer survival curves illustrated that in absence of M-Trap the control group reached the study endpoint at four months after intraperitoneal injection of 2.5x106 SKOV3 cells (mean survival = 117.5 days, 95% CI = 107.6 to 127.4) (Figure 5A) because of massive peritoneal carcinomatosis (Figure 5, B and C). The control group was designed to mimic the clinical scenario today in advanced ovarian cancer. Remarkably, focalization of the disease by the M-Trap technology resulted in a tumor mass contained within the device, thereby preventing further dissemination (Figure 5D) and resulting in a statistically significant cumulative survival (mean survival = 198.8 days, 95% CI = 170.4 to 209.2, P < .001, log-rank Test) (Figure 5A). Of note, the M-Trap group exhibited 100% survival at five months when all mice included in the control group reached the endpoint of the study (Figure 5A). Histological evaluation of the explanted M-Trap device showed an infiltrative component of fibroblasts associated with a foreign body reaction (17) (Figure 5E). Finally, surgical removal of M-Trap upon focalization of the disease resulted in the eradication of large metastasis with a residual luciferase signal at the site of M-Trap implantation (re-operated group) (Figure 5A). This scenario represents the expected clinical use of the M-Trap technology and led to an even more marked survival benefit, with three out of four mice alive one year after SKOV3 tumor cell injection (mean survival = 309.4, 95% CI = 249.4 to 369.4) (Figure 5A). These results clearly demonstrate the substantial survival benefits attributable to M-Trap. In summary, M-Trap technology is a compelling approach, disrupting the process of metastasis that can effectively transform a systemic disease into a focalized disease where proven therapeutic approaches such as surgery can extend survival.
Discussion
Ovarian cancer accounts for 4% of all cancers in women and is the leading cause of death from gynecologic malignancies. Despite therapy, 70% of patients relapse within two years with incurable disease (10). Metastatic progression of ovarian cancer is unique, as metastasis that causes death spreads loco-regionally throughout the peritoneal cavity. Malignant cells are shed off of the primary tumor and are carried by the intraperitoneal ascitic fluid in the form of multicellular clusters, or spheroids, that will either remain unattached or implant onto the organs and tissues of the peritoneal cavity. These disseminating cells anchor in submesothelial extracellular matrix and establish metastases (18). Primary ovarian cancer cells attach more efficiently to extracellular matrix than to mesothelial cells (19). M-Trap is a novel approach that targets cancer cell interactions within the peritoneum via the creation of an artificial niche that mimics the extracellular matrix. Proof-of-concept in a murine model of ovarian cancer peritoneal dissemination demonstrates that M-Trap effectively disrupts the natural course of peritoneal metastasis. The compartmentalized nature of ovarian cancer, its immunogenicity, abundance of inflammatory cells, aggressiveness, and the accessibility of its microenvironment make it an ideal disease for new microenvironmental interventions and offer promising opportunities for innovative clinical strategies (20).
The mode of action of M-Trap indicates that it competes with the natural sites of peritoneal implantation by offering a privileged environment for the attachment of tumor cells metastasizing within the peritoneal cavity. In addition, the natural flow of peritoneal fluid that exists within the abdominal cavity provides a route for the transcoelomic dissemination of detached tumor cells (15) and improves the efficiency of M-Trap in the capture of ovarian metastasis. Malignant ascites that accompanies advanced and recurrent disease and contributes to ovarian peritoneal metastasis also offers opportunities for translational research and innovative therapies (21). Exosomes derived from ascites are major components of the communication between tumor cells and their environment and, as such, optimal candidates for the functionalization of 3D scaffolds and the creation of the M-Trap device. In light of the growing clinical interest in the potential use of exosomes as disease biomarkers or delivery vehicles, a new clinical application of exosomes as the substrate-bound interlocutor for the capture of tumor cells may offer a promising new therapeutic option for advanced ovarian cancer patients.
Beyond ovarian cancer, M-Trap is broadly applicable to other cancers that metastasize within the abdominal cavity. Gynecologic malignancies such as endometrial cancer as well as gastrointestinal tumors, including colorectal, gastric, and pancreatic carcinomas, also disseminate intraperitoneally, in addition to their main hematological and lymphatic routes of metastasis. For example, invasive colorectal tumors can shed cells directly into the peritoneal cavity, or accidental perforation of the intestinal wall during surgery can introduce cancer cells from tumors that were previously not exposed to the peritoneal space. Irrespective of the means of introduction, peritoneal involvement drastically worsens the prognosis for these patients, in large part because of the rapid progression of peritoneal metastasis in comparison with hematological metastasis and the inefficacy of surgery against widespread peritoneal metastasis (22).
The impact of M-Trap technology on the evolution of metastatic disease can be summarized by a complete remodeling of the pattern of peritoneal metastasis and the transformation of a disseminated disease into a focalized disease. With this transformation, M-Trap enables gynecologists and oncologists to employ therapeutic approaches such as surgery and/or radio/chemotherapy that offer proven efficacy with primary carcinomas. Both intraperitoneal injection and sustained released models support the efficacy of M-Trap technology to capture tumor cells disseminating in the peritoneal cavity; nevertheless, these models represent a limited scenario to translate this technology into the clinics. Another limitation is that these preclinical studies have been conducted in immunocompromised mice, with no impact of the fibrotic reaction on the efficacy of M-Trap to capture metastatic cells. However, it is possible that other components of the immune system may enhance the tumor cell capture abilities of M-Trap. Finally, the potential use of nontumoral exosomes should be explored.
In conclusion, M-Trap represents a disruptive technology that complements current surgical and chemotherapeutic approaches in advanced ovarian cancer, with broad applicability to other gynecological and gastrointestinal malignancies with a peritoneal dissemination pattern.
Funding
This work was supported by Fundacion Pedro Barrie de la Maza (Fondo de Ciencia). Authors declare no conflict of interest.
Supplementary Material
We gratefully thank the patients for their willingness to participate in the study and Patricia Viaño (Health Research Institute of Santiago) for her technical support sample processing and histology analysis.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5):646–674. [DOI] [PubMed] [Google Scholar]
- 2. Sleeman JP, Christofori G, Fodde R, et al. Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol. 2012;22 (3):174–186. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9 (4):274–284. [DOI] [PubMed] [Google Scholar]
- 4. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9 (4):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21 (2):139–146. [DOI] [PubMed] [Google Scholar]
- 6. Sleeman JP. The metastatic niche and stromal progression. Cancer Metastasis Rev. 2012;31 (3–4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16 (4):415–421. [DOI] [PubMed] [Google Scholar]
- 8. Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18 (6):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zoccoli A, Iuliani M, Pantano F, et al. Premetastatic niche: ready for new therapeutic interventions? Expert Opin Ther Targets. 2012;16(Suppl 2):S119–S129. [DOI] [PubMed] [Google Scholar]
- 10. Colombo N, Van Gorp T, Parma G, et al. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60 (2):159–179. [DOI] [PubMed] [Google Scholar]
- 11. Ihemelandu CU, Shen P, Stewart JH, et al. Management of peritoneal carcinomatosis from colorectal cancer. Semin Oncol. 2011;38 (4):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64 (1):9–29. [DOI] [PubMed] [Google Scholar]
- 13. Sodek KL, Murphy KJ, Brown TJ, et al. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31 (1–2):397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helland O, Popa M, Vintermyr OK, et al. First in-mouse development and application of a surgically relevant xenograft model of ovarian carcinoma. PLoS One. 2014;9 (3):e89527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7 (11):925–934. [DOI] [PubMed] [Google Scholar]
- 16. Steinkamp MP, Winner KK, Davies S, et al. Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models. Front Oncol. 2013;3:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20 (2):86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93 (1):170–181. [DOI] [PubMed] [Google Scholar]
- 19. Niedbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160 (2):499–513. [DOI] [PubMed] [Google Scholar]
- 20. Scarlett UK, Conejo-Garcia JR. Modulating the tumor immune microenvironment as an ovarian cancer treatment strategy. Expert Rev Obstet Gynecol. 2012;7 (5):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13 (4):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243 (2):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.