Abstract
The in vitro activities of the novel fungal Cyp51 inhibitor VT-1129 were evaluated against a large panel of Cryptococcus neoformans and Cryptococcus gattii isolates. VT-1129 demonstrated potent activities against both Cryptococcus species as demonstrated by low MIC50 and MIC90 values. For C. gattii, the in vitro potency was maintained against all genotypes. In addition, significantly lower geometric mean MICs were observed for VT-1129 than for fluconazole against C. neoformans, including isolates with reduced fluconazole susceptibility.
TEXT
Cryptococcosis remains a clinically significant invasive fungal infection and is associated with significant morbidity and mortality in immunocompromised patients (1–3). Cryptococcal infections are caused by members of the Cryptococcus neoformans and Cryptococcus gattii species complexes. The two most common manifestations of cryptococcosis are primary pulmonary infection and cryptococcal meningitis. Cryptococcal meningitis is a devastating disease estimated to cause >600,000 deaths worldwide annually (4). The treatment of cryptococcosis typically involves the use of fluconazole, either as the primary therapy for mild to moderate pulmonary involvement or as a consolidation and maintenance therapy following induction therapy with intravenous amphotericin B with or without flucytosine for cryptococcal meningoencephalitis or complicated lung disease (1). VT-1129 (Fig. 1) is a member of a new class of orally available fungal Cyp51 (lanosterol 14-α-demethylase) inhibitors that employ a tetrazole to bind to the active-site heme iron (compound 7c in reference 5). Compared to the approved azole class of antifungal drugs, which contain either a triazole or an imidazole, members of this novel group have greater selectivity for the fungal enzyme than for mammalian cytochrome P450 enzymes (5, 6). Specifically, VT-1129 has been shown to bind tightly to cryptococcal CYP51 recombinant proteins, displaying type II difference spectra essentially identical to those of the approved azole inhibitors, while weakly inhibiting key human CYP450 enzymes (e.g., 3A4, 2C9, and 2C19) (7). Our objective was to measure the in vitro activity of VT-1129 against cryptococcal isolates, including members of the C. neoformans and C. gattii species complexes. The potency of this investigational agent against C. neoformans was compared to that of fluconazole; we also included strains with reduced susceptibility to this azole in our study.
FIG 1.
Chemical structure of VT-1129.
VT-1129 powder was provided by Viamet Pharmaceuticals, Inc. (Durham, NC), and fluconazole powder was obtained from Pfizer (New York, NY). Stock solutions were prepared in dimethyl sulfoxide (DMSO) and were stored frozen at −70°C. Further dilutions were prepared in DMSO and RPMI broth. A total of 180 clinical C. neoformans isolates were used in this study, including 100 from HIV-positive patients in South Africa and 80 collected from institutions in North America (8). In addition, 321 isolates of Cryptococcus gattii from Africa, Australia, and North America were also used to evaluate the activity of VT-1129 against this species (9, 10). The molecular types of 300 of the C. gattii isolates were further identified by multilocus sequence typing (MLST) as previously described (9–11). The MLST data are available within the MLST database (http://mlst.mycologylab.org/defaultinfo.aspx?page=CG). Testing was performed by broth microdilution according to the Clinical and Laboratory Standards Institute M27-A3 reference standard (12). Testing was performed independently by the Mycotic Diseases Branch of the Centers for Disease Control and Prevention (CDC) and the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio (UTHSCSA). All VT-1129 MIC values were determined visually as the lowest drug concentrations at which there were 50% and 100% inhibitions of growth compared to the growth controls after 72 h of incubation at 35°C. For fluconazole, 50% inhibition of growth was the endpoint used. The fluconazole MICs for isolates tested at the CDC had been previously determined (8), while fluconazole and VT-1129 susceptibility testing procedures were performed concurrently at UTHSCSA. Quality control isolates Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6058 were included on each day of testing. The MIC range, modal MIC, MIC50, MIC90, and geometric mean (GM) MIC values were determined. The differences in GM MIC values were assessed for significance by a paired t test. P values of <0.05 were considered significant.
Overall, VT-1129 demonstrated potent activity against C. neoformans with MIC values ranging between ≤0.015 and 2 μg/ml at the 50% inhibition of growth endpoint and between ≤0.015 and 4 μg/ml at the 100% endpoint (Table 1). VT-1129 was also significantly more potent than fluconazole against C. neoformans, as evident by the lower MIC range and MIC50 and MIC90 values. In addition, the VT-1129 GM MICs at both the 50% and 100% inhibition endpoints (0.0271 and 0.2052 μg/ml, respectively) were significantly lower than that of fluconazole (2.280 μg/ml; P < 0.001). The MIC distributions for VT-1129 and fluconazole against the C. neoformans isolates are shown in Fig. 2A and B. Against the 31 isolates with elevated fluconazole MICs (≥8 μg/ml based on the MIC90 value for this azole), VT-1129 maintained potent activity. With the 50% inhibition endpoint, the VT-1129 MIC50, MIC90, and GM MIC values were 0.030, 0.125, and 0.0506 μg/ml, respectively, while at the 100% inhibition endpoint, these values were 0.5, 1, and 0.3818 μg/ml. The in vitro potency observed with VT-1129 against C. neoformans clinical isolates was also consistent between the two laboratories with similar MIC50, MIC90, and GM MIC values. In fact, the modal MIC and MIC50 values using the 50% inhibition endpoint were both ≤0.015 μg/ml for each laboratory. Because many of the VT-1129 MICs were below the lowest concentration tested, another group of 54 clinical isolates, with no overlap with the first group, was tested at UTHSCSA using a lower concentration range (0.0005 to 0.25 μg/ml). As shown in Table 1, the modal MIC, MIC50, and GM MIC values were lower with the 50% inhibition endpoint at this lower concentration range than at the higher concentration range (0.015 to 8 μg/ml).
TABLE 1.
VT-1129 and fluconazole MICs against Cryptococcus neoformans isolatesa
| Parameter | MIC for agent (endpoint) |
UTHSCSA MIC over lower concn range of VT-1129 (n = 54) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative (n = 180) |
CDC (n = 100) |
UTHSCSA (n = 80) |
||||||||||
| VT-1129 (50%) | VT-1129 (100%) | FLU (50%) | VT-1129 (50%) | VT-1129 (100%) | FLU (50%) | VT-1129 (50%) | VT-1129 (100%) | FLU (50%) | VT-1129 (50%) | VT-1129 (100%) | FLU (50%) | |
| Range | ≤0.015–2 | ≤0.015–4 | 0.25–>64 | ≤0.015–0.125 | ≤0.015–2 | 0.5–16 | ≤0.015–2 | ≤0.015–4 | 0.25–>64 | 0.004–>0.25 | 0.015–>0.25 | ≤0.125–>64 |
| Mode | ≤0.015 | 0.25 | 2 | ≤0.015 | 0.5 | 4 | ≤0.015 | 0.125 | 2 | 0.008 | 0.25 | 2 |
| MIC50 | ≤0.015 | 0.25 | 2 | ≤0.015 | 0.25 | 2 | ≤0.015 | 0.125 | 2 | 0.008 | 0.25 | 2 |
| MIC90 | 0.06 | 0.5 | 8 | 0.06 | 0.5 | 8 | 0.06 | 0.5 | 8 | 0.06 | >0.25 | 8 |
| GM MICb | 0.0271 | 0.2052 | 2.280 | 0.0264 | 0.2997 | 2.250 | 0.0281 | 0.1278 | 2.317 | 0.0126 | 0.1519 | 2.488 |
All MICs were measured after 72 h of incubation at 35°C. VT-1129 MICs were read at 50% and 100% inhibition of growth compared to growth control. Fluconazole (FLU) MICs were measured at 50% inhibition of growth. Fluconazole MICs for CDC isolates are historical values.
GM MIC, geometric mean MIC.
FIG 2.
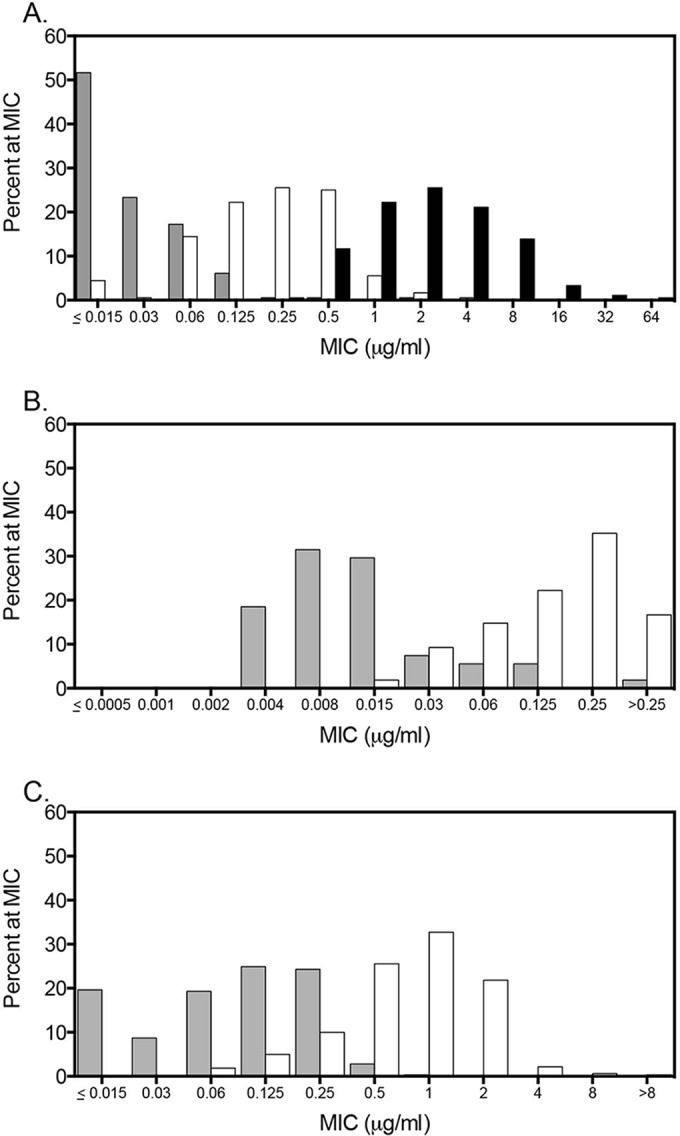
MIC distributions of VT-1129 and fluconazole against Cryptococcus neoformans and C. gattii isolates. (A) Percentage of isolates at different VT-1129 and fluconazole MIC levels against 180 C. neoformans isolates. (B) Percentage of isolates at different MIC levels for VT-1129 at the lower concentration range tested against 54 C. neoformans isolates. (C) Percentage of isolates at different VT-1129 MIC levels against 321 C. gattii isolates. VT-1129 MICs using the 50% inhibition of growth endpoint are shown by the gray bars and 100% inhibition by the white bars, and fluconazole MICs at 50% inhibition are shown by the black bars.
Potent in vitro activity was also observed for VT-1129 against C. gattii with MIC values ranging between ≤0.015 and 1 μg/ml at the 50% inhibition of growth endpoint and between 0.06 and >8 μg/ml at the 100% endpoint (Table 2 and Fig. 2C). Similar to the results observed against C. neoformans, the potency of VT-1129 against C. gattii was observed by both laboratories, although the values were somewhat higher for the isolates tested at the CDC. This may be a reflection of the larger number of strains that were tested by this laboratory (n = 300) than were tested at UTHSCSA (n = 21). Previous studies have shown that C. gattii antifungal susceptibility patterns vary according to the molecular type of the isolate, with VGII isolates generally having higher azole MIC values. This was largely true for VT-1129 as well. At 50% inhibition, the MIC50, MIC90, and GM MIC values for VGIV isolates were the lowest (0.015, 0.06, and 0.0253 μg/ml, respectively) followed by those for the VGI isolates (0.03, 0.125, and 0.0432 μg/ml, respectively) (Table 3). The VGII isolates and VGIII isolates had the highest MIC50, MIC90, and GM MIC values, but they were only 1 to 3 dilutions higher than those of the VGI isolates. Similar results were also observed when the 100% growth inhibition endpoint was used.
TABLE 2.
VT-1129 MICs against Cryptococcus gattii isolatesa
| Parameter | MIC for agent (endpoint) |
|||||
|---|---|---|---|---|---|---|
| Cumulative (n = 321) |
CDC (n = 300) |
UTHSCSA (n = 21) |
||||
| VT-1129 (50%) | VT-1129 (100%) | VT-1129 (50%) | VT-1129 (100%) | VT-1129 (50%) | VT-1129 (100%) | |
| Range | ≤0.015–1 | 0.06–>8 | ≤0.015–1 | 0.06–>8 | ≤0.015–0.25 | 0.06–1 |
| Mode | 0.125 | 1 | 0.25 | 1 | 0.125 | 0.25 |
| MIC50 | 0.125 | 1 | 0.125 | 1 | 0.06 | 0.25 |
| MIC90 | 0.25 | 2 | 0.25 | 2 | 0.125 | 0.5 |
| GM MICb | 0.0782 | 0.7573 | 0.0804 | 0.8112 | 0.0534 | 0.2842 |
All MICs were measured after 72 h of incubation at 35°C and read at 50% and 100% inhibition of growth compared to growth control.
GM MIC, geometric mean MIC.
TABLE 3.
VT-1129 MICs against Cryptococcus gattii isolates by molecular typea
| Endpoint | MIC (μg/ml) |
|
|---|---|---|
| 50% | 100% | |
| VGI (n = 45) | ||
| Range | ≤0.015–0.5 | 0.125–8 |
| Mode | 0.015 | 0.25 |
| MIC50 | 0.03 | 0.5 |
| MIC90 | 0.125 | 1 |
| GM MICb | 0.0432 | 0.4019 |
| VGII (n = 7) | ||
| Range | 0.06–0.25 | 0.5–2 |
| Mode | 0.25 | 0.5–1 |
| MIC50 | 0.25 | 1 |
| MIC90 | 0.25 | 1 |
| GM MIC | 0.1506 | 0.8203 |
| VGIIa (n = 104) | ||
| Range | 0.06–0.5 | 0.5–2 |
| Mode | 0.125 | 1 |
| MIC50 | 0.125 | 1 |
| MIC90 | 0.25–2 | 2 |
| GM MIC | 0.1332 | 0.9477 |
| VGIIb (n = 20) | ||
| Range | 0.06–0.5 | 0.5–2 |
| Mode | 0.125–0.25 | 2 |
| MIC50 | 0.125 | 2 |
| MIC90 | 0.25 | 2 |
| GM MIC | 0.1761 | 1.319 |
| VGIIc (n = 32) | ||
| Range | 0.06–1 | 0.5–4 |
| Mode | 0.25 | 2 |
| MIC50 | 0.25 | 2 |
| MIC90 | 0.25 | 2 |
| GM MIC | 0.2187 | 1.874 |
| VGIII (n = 20) | ||
| Range | ≤0.015–0.25 | 0.25–2 |
| Mode | 0.125 | 1 |
| MIC50 | 0.125 | 1 |
| MIC90 | 0.25 | 1 |
| GM MIC | 0.1111 | 0.8123 |
| VGIV (n = 72) | ||
| Range | ≤0.015–0.25 | 0.125->8 |
| Mode | 0.015 | 0.5 |
| MIC50 | 0.015 | 0.5 |
| MIC90 | 0.06 | 2 |
| GM MIC | 0.0253 | 0.6019 |
All MICs were measured after 72 h of incubation at 35°C. VT-1129 MICs were read at 50% and 100% inhibition of growth compared to growth control.
GM MIC, geometric mean MIC.
In summary, VT-1129 demonstrated excellent in vitro activity against Cryptococcus species, including members of the C. neoformans and C. gattii species complexes. This activity was also maintained against isolates with reduced susceptibility to fluconazole. Although VT-1129 was not directly compared to antifungals other than fluconazole, its activities against Cryptococcus species were similar to those previously reported for amphotericin B and the extended spectrum azoles, posaconazole, voriconazole, and isavuconazole (13–18). These data suggest that VT-1129 may be a promising agent against Cryptococcus species. VT-1129 phase 1 studies are now under way to establish the safety and pharmacokinetic profile necessary for phase 2 studies in patients with cryptococcal meningitis.
ACKNOWLEDGMENTS
N.P.W. has received research support from Astellas, bioMérieux, Dow, F2G, Merck, Merz, Revolution Medicines, and Viamet and has served on advisory boards for Merck, Astellas, Toyama, and Viamet. T.F.P. has received research grants to UT Health Science Center San Antonio from Astellas, Merck, and Revolution Medicines and has served as a consultant for Astellas, Gilead, Merck, Pfizer, Revolution Medicines, Scynexis, Toyama, Viamet, and Vical. E.P.G., S.R.B., W.J.H., and R.J.S. are employees of Viamet Pharmaceuticals, Inc. The other authors declare no conflicts of interest.
Funding Statement
This project was funded by the National Institutes of Health National Institute of Allergy and Infectious Diseases under contract no. N01-AI-25475 (Thomas F. Patterson), the National Institutes of Health, National Center for Advancing Translational Sciences, Therapeutics for Rare and Neglected Diseases (TRND) Program (Shawn R. Lockhart), and Viamet Pharmaceuticals, Inc. (Nathan P. Wiederhold). VT-1129 powder was provided by Viamet Pharmaceuticals, Inc.
REFERENCES
- 1.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by The Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chayakulkeeree M, Perfect JR. 2006. Cryptococcosis. Infect Dis Clin North Am 20:507−544, v−vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Li SS, Mody CH. 2010. Cryptococcus. Proc Am Thorac Soc 7:186−196. doi: 10.1513/pats.200907-063AL. [DOI] [PubMed] [Google Scholar]
- 4.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 5.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 6.Warrilow AG, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvey EP, Hoekstra WJ, Schotzinger RJ, Fothergill AW, Wiederhold NP, Warrilow AGS, Kelly DE, Kelly SL. 2015. VT-1129 binds potently and selectively to recombinant cryptococcal CYP51 consistent with its potent in vitro anti-cryptococcal activity, poster M-848. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA. [Google Scholar]
- 8.Govender NP, Patel J, van Wyk M, Chiller TM, Lockhart SR. 2011. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates obtained through population-based surveillance in South Africa in 2002−2003 and 2007−2008. Antimicrob Agents Chemother 55:2606–2611. doi: 10.1128/AAC.00048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart SR, Iqbal N, Bolden CB, DeBess EE, Marsden-Haug N, Worhle R, Thakur R, Harris JR. 2012. Epidemiologic cutoff values for triazole drugs in Cryptococcus gattii: correlation of molecular type and in vitro susceptibility. Diagn Microbiol Infect Dis 73:144–148. doi: 10.1016/j.diagmicrobio.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal N, DeBess EE, Wohrle R, Sun B, Nett RJ, Ahlquist AM, Chiller T, Lockhart SR. 2010. Correlation of genotype and in vitro susceptibilities of Cryptococcus gattii strains from the Pacific Northwest of the United States. J Clin Microbiol 48:539–544. doi: 10.1128/JCM.01505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, Esposto MC, Fisher M, Gilgado F, Hagen F, Kaocharoen S, Litvintseva AP, Mitchell TG, Simwami SP, Trilles L, Viviani MA, Kwon-Chung J. 2009. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol 47:561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Thompson GR III, Wiederhold NP, Fothergill AW, Vallor AC, Wickes BL, Patterson TF. 2009. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother 53:309–311. doi: 10.1128/AAC.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yildiran ST, Fothergill AW, Sutton DA, Rinaldi MG. 2002. In vitro susceptibilities of cerebrospinal fluid isolates of Cryptococcus neoformans collected during a ten-year period against fluconazole, voriconazole and posaconazole (SCH56592). Mycoses 45:378–383. doi: 10.1046/j.1439-0507.2002.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Trilles L, Fernandez-Torres B, dos Santos Lazera M, Wanke B, Guarro J. 2004. In vitro antifungal susceptibility of Cryptococcus gattii. J Clin Microbiol 42:4815–4817. doi: 10.1128/JCM.42.10.4815-4817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson GR III, Fothergill AW, Wiederhold NP, Vallor AC, Wickes BL, Patterson TF. 2008. Evaluation of Etest method for determining isavuconazole MICs against Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother 52:2959–2961. doi: 10.1128/AAC.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinea J, Hagen F, Pelaez T, Boekhout T, Tahoune H, Torres-Narbona M, Bouza E. 2010. Antifungal susceptibility, serotyping, and genotyping of clinical Cryptococcus neoformans isolates collected during 18 years in a single institution in Madrid, Spain. Med Mycol 48:942–948. doi: 10.3109/13693781003690067. [DOI] [PubMed] [Google Scholar]
- 18.Hagen F, Illnait-Zaragozi MT, Bartlett KH, Swinne D, Geertsen E, Klaassen CH, Boekhout T, Meis JF. 2010. In vitro antifungal susceptibilities and amplified fragment length polymorphism genotyping of a worldwide collection of 350 clinical, veterinary, and environmental Cryptococcus gattii isolates. Antimicrob Agents Chemother 54:5139–5145. doi: 10.1128/AAC.00746-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


