Abstract
Early in the age of modern medicine the consequences of vitamin A deficiency drew attention to the fundamental link between retinoid-dependent homeostatic regulation and malignant hyperproliferative diseases. The term retinoid includes a handful of endogenous and a large group of synthetic derivatives of vitamin A. These multifunctional lipid-soluble compounds directly regulate target genes of specific biological functions and critical signaling pathways to orchestrate complex functions from vision to development, metabolism and inflammation. Many of the retinoid activities on the cellular level have been well characterized and translated to the regulation of processes like differentiation and cell death, which play critical roles in the outcome of malignant transformation of tissues. In fact, retinoid-based differentiation therapy of acute promyelocytic leukemia was one of the first successful examples of molecularly targeted treatment strategies. The selectivity, high receptor binding affinity and the ability of retinoids to directly modulate gene expression programs present a distinct pharmacological opportunity for cancer treatment and prevention. However, to fully exploit their potential, the adverse effects of retinoids must be averted. In this review we provide an overview of the biology of retinoid (activated by nuclear retinoic acid receptors, RARs) and rexinoid (engaged by nuclear retinoid X receptors, RXRs) action concluded from a long line of preclinical studies, in relation to normal and transformed states of cells. We will also discuss the past and current uses of retinoids in the treatment of malignancies, the potential of rexinoids in the cancer prevention setting, both as single agents and in combinations.
Introduction and historic background
The term ‘retinoid’ refers to all natural and synthetic compounds that have structural or biological activities similar to retinol or vitamin A. Their versatile biological activities include pattern formation during embryogenesis, vision, energy homeostasis, metabolism, immune function and inflammation. The classical mechanism of retinoid action is a nuclear hormone receptor-mediated activation of the transcriptional machinery acting on select target genes. Counterintuitively, retinoid receptors exert extranuclear and non-transcriptional effects as well, for instance by inducing rapid and transient activation of kinase cascades. The sum of the transcriptional programs and non-genomic effects amount to a broad spectrum of biological events regulated by retinoids. While the exact molecular mechanism regulating complex processes may be elusive, the propensity to coordinately target multiple pathways and regulatory mechanisms may account for their vast therapeutic potential.
Early rodent experiments using a retinoid deficient diet showed dysplastic alterations of various epithelial tissues, and an inverse correlation has been established between serum retinoid levels and extent of dedifferentiation or metaplasias. Aberrant retinoid signaling mechanisms have been identified as causative factors of certain cancers, suggesting that retinoid pathways may be suitable targets to treat certain forms of malignant diseases. Therefore, the ability of retinoids to exert anticancer activity against epithelial tumors has been in the forefront of interest since the 1950s [1, 2], with investigations later extending to a variety of tissues [3]. At the same time it was proposed that retinoids might also be effective as a neoplastic prophylactic intervention [4, 5]. One of the earliest clinical observations in this area established that treatment with 13-cis-retinoic acid (13-cis-RA, isotretinoin) would delay the development of second primary head and neck squamous cell carcinomas, a major cause of mortality in patients after successful treatment of the primary tumor [6]. 13-cis-RA has also been reported to cause clinical resolution of upper aerodigestive tract premalignant lesions [7] [8]. Further on a host of clinical trials testing the cancer preventive potential of retinoids in various tissues were conducted to determine the best indications and modalities of retinoid use. These will be summarized in more detail in this review.
1. Basic molecular biology of retinoids
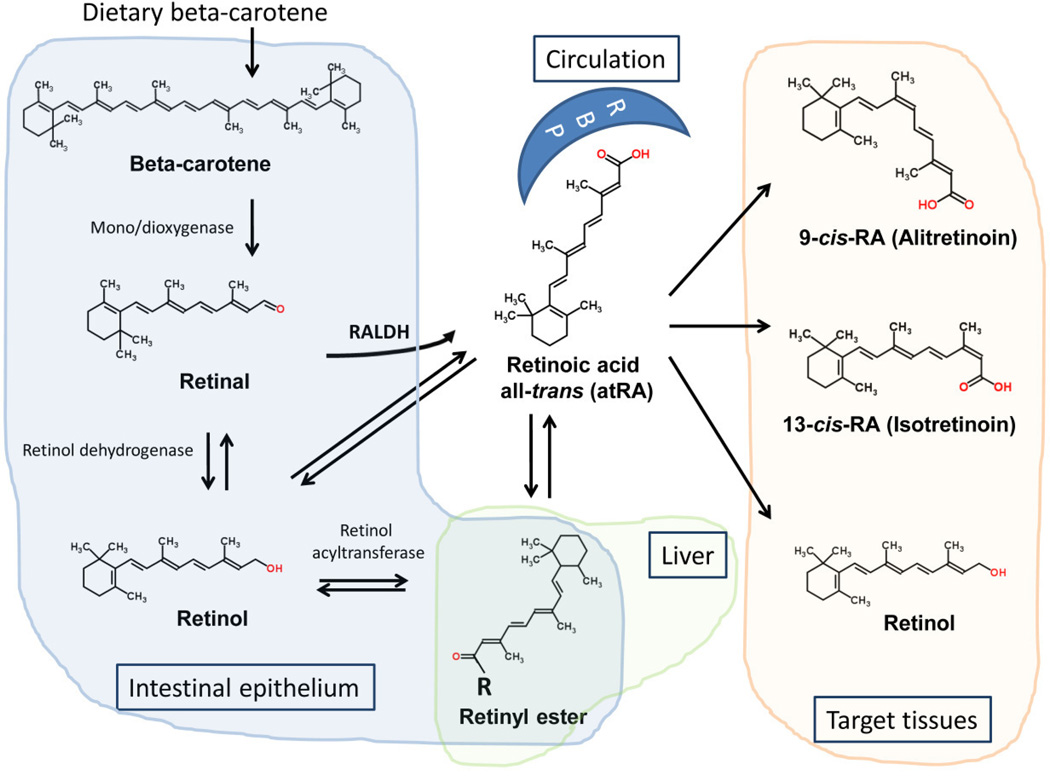
Retinol, the precursor of retinoic acid (RA), is generally acquired as provitamin A in the form of carotenoids, synthesized in leafy and yellow vegetables. Preformed vitamin A and all-trans retinyl esters are found in liver, kidney and butter fat. Retinyl esters are hydrolyzed in the intestinal lumen by retinyl ester hydrolases (REHs) to retinol. Other derivatives dissolve in dietary lipids and are absorbed in the upper intestinal tract, although a direct absorption pathway from bile also exists. The intestinal transcription factor ISX regulates critical steps of β-carotenoid uptake and retinol production [9]. Carotenoids are then processed to retinaldehyde and converted to retinol. Retinol can be re-esterified with palmitate or acetate by the lecithin-retinol acyltransferase (LRAT) enzyme in mucosal cells and secreted in chylomicrons into the lymphatic system and the circulation [10]. The liver captures retinoids from chylomicron remnants, where they undergo further conversion to retinol and other bioactive forms. A minor fraction is distributed to peripheral tissues bound to retinol binding proteins (RBPs) or albumin, but 90% of the stored vitamin A remains in the liver [11]. At the target tissues the retinaldehyde dehydrogenase enzymes (RALDHs) catalyze the rate-limiting step to local epithelial RA production (Figure 1) [12]. Through the activation of retinoid signaling RALDH isoform 1 was recently shown to regulate cellular respiration and adaptive thermogenesis in adipose tissue [13].
Figure 1. Pathways of retinoid metabolism and distribution throughout the body.
Vitamin A, derived from β-carotene, or as a provitamin from animal sources, is processed to retinol and either stored as retinyl esters or distributed to the peripheral tissues via retinol binding proteins. Target tissues create and use various isomers for specific signaling purposes.
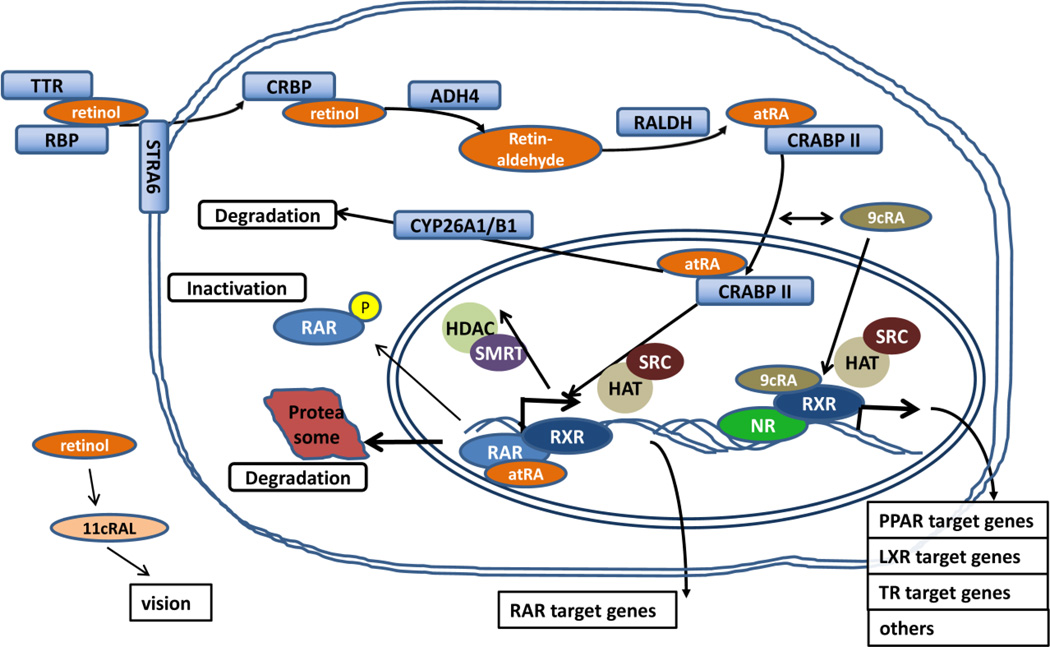
The lipophilic retinol can pass through the lipid bilayers of membranes, but the majority of retinoids are taken up by target cells via the RBP4 receptor Stra6 [14]. Once reaching the target tissues, retinol requires several enzymatic steps and proteins facilitating its transport to exert its full range of biological activities (Figure 2). In fact, with respect to retinoid-modulated transcription, cellular sensitivity to retinoic acid is primarily attributed to the subnanomolar affinity of its intracellular binding proteins (CRABP II and FABP5), delivering the ligand directly to its nuclear receptors. Retinol is reversibly transformed into isomers such as 9-cis-retinoic acid (9-cis-RA), 13-cis-RA or all-trans-retinoic acid (atRA), resulting in slightly different receptor binding properties and hence biological activities. In the eye retinol is transformed into 11-cis retinaldehyde (11cRAL) and is responsible for phototransduction through binding to the G-protein opsin. Other derivatives and chemically distinct synthetic compounds activating the same signaling pathways are also termed ‘retinoids’, with some exhibiting greater activity than the most common atRA. It is important to note, that the key steps responsible for setting physiologic retinol concentrations are frequently altered in tumors and the changes associated with carcinogenesis generally result in diminished retinoid signaling [15].
Figure 2. Molecular mechanisms of retinoid and rexinoid action in the target cell.
To be active, vitamin A requires multiple enzymatic steps and transport. Cellular retinol (ADH4) and retinaldehyde (RALDH) dehydrogenases oxidize retinol to retinaldehyde and further to all-trans-retinoic acid (atRA). High affinity binding to CRABP II delivers the ligands to activate transcription through their nuclear receptors. Following activation, atRA is degraded by the CYP26A1 system, while RAR is degraded in the proteasome or inactivated by phosphorylation.
For the purpose of storage, upon entering the target tissues, retinal is esterified to a fatty acid, providing the means of concentrating retinol within the cells [11]. The degradation of retinoids in the body is also highly regulated and is catalyzed by cytochrome P-450 dependent enzymes in a hydroxylation and a subsequent oxidation step. Oxidized metabolites of RA, primarily 4-oxo-RA, undergo conjugation with glucuronic acid and are secreted with the bile.
In 1931 the Noble Prize in chemistry was awarded to Paul Karrer for determining the structure of retinol, which he later successfully synthesized, and the compound soon became commercially available. 9-cis-RA was the first compound shown to interact with RXRs [16] and these ligand-bound RXR proteins were found to bind other nuclear hormone receptors [17]. While RXR-binding compounds sharing some structural similarity with classic retinoids are commonly referred to as ‘rexinoids’, chemically distinct molecules, like the long-chain omega-3 fatty acid docosahexaenoic acid (DHA), were identified as endogenous ligands of RXR and other nuclear receptors (i.e. PPARs) [18, 19].
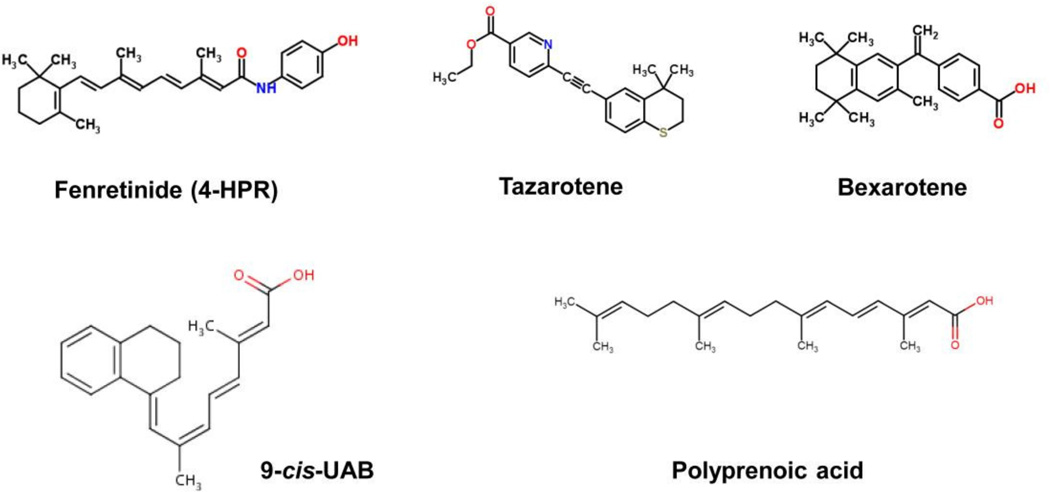
The list of endogenous and natural retinoids grew by the addition of synthetic derivatives (Figure 3). First generation retinoids were nonaromatic molecules and included atRA (Tretinone), 13-cis-RA (Isotretinoin) and 9-cis-RA (Alitretinoin). Second generation compounds, such as etretinate and acitretin, were based on a monoaromatic structure and the third generation agents, such as adapalene, tazorotene and bexarotene, are polyaromatic. The number of synthetic retinoids developed to date is in the thousands.
Figure 3. Synthetic retinoids and rexinoids tested or used for cancer prevention.
The structure of synthetic retinoids used in preclinical and clinical cancer prevention studies are shown.
Receptor-mediated retinoid signaling
The diversity of the functional effectors of vitamin A signaling is a function of the active metabolites, their receptors and dimerization partners, potential co-regulators and response element polymorphisms. The biological activity of natural retinoids is primarily mediated by selective ligand-activated transcription factors of the nuclear hormone receptor family [20]. The receptors that bind naturally occurring classical retinoids, RARs have three isoforms, α (NR1B1), β (NR1B2) and γ(NR1B3), as well as several splice variants arising from alternative promoters. Another group of retinoid receptors, RXRs, whose ligands were unknown at the time of their discovery, is also comprised of three isoforms. RXR-RAR heterodimers act as ligand-dependent transcriptional regulators by binding to specific DNA-response elements found in the promoter regions of target genes.
Phylogenically and structurally related, RARs and RXRs are both characterized by similar, highly conserved modular domain structures. These contain C-terminal ligand binding domains (LBDs) and central DNA-binding domains (DBDs) that include two zinc-finger modules and two α-helices, as well as variable N-terminal regions. The N-terminal domain that contains naturally disordered protein structures and whose role therefore has initially been elusive, was implicated in a role of providing the flexibility needed for critical posttranslational modification events by kinases and ubiquitin-ligases [21]. Nevertheless, the functions of RARs are not limited to the regulation of cognate target genes. The assembly of co-regulatory protein complexes is directed by the C-terminal ligand-binding domain of RARs. In addition, RARs can act as co-repressors for other gene pathways.
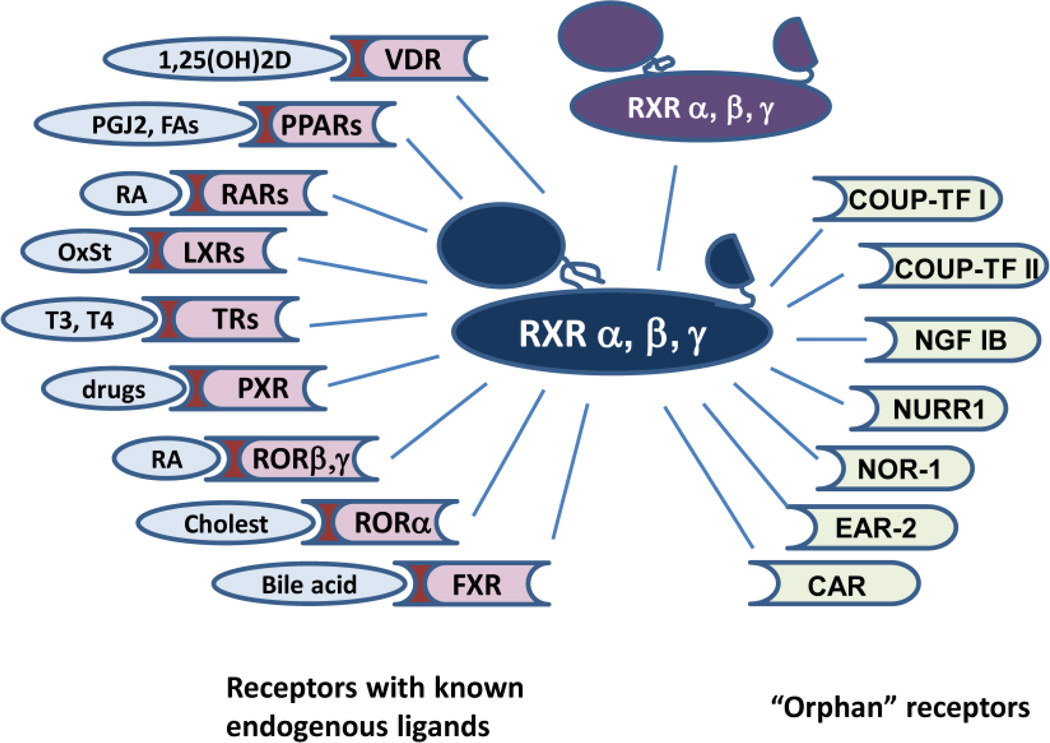
RARs generally dimerize with an RXR as a partner to be activated by a ligand. This is an unequal relationship, where RAR agonists can autonomously activate transcription, while a full response to rexinoids requires binding of an RAR agonist. This is commonly referred to as a ‘non-permissive’ nuclear receptor interaction. In contrast, RXR ligands are able to act on their own through an RXR homodimer, or a variety of partners (PPARs, LXRs, THRs, FXR, etc.). The latter partnering allows RXRs to activate transcription in the absence of a rexinoid. Therefore these are called “permissive” heterodimers (Figure 4). Similar to most type II nuclear receptors, retinoid receptors are retained in the nucleus regardless of their ligand binding status. Bound to regulatory elements of their target genes as RXR/RAR heterodimers, in the absence of ligands, they interact with the co-repressors NCoR, its genetic paralog SMRT, Sin3 or others. These transcriptional repressor complexes, in turn, recruit histone deacetylases that lead to the repression of target gene transcription [22]. Binding of an agonist triggers the repositioning of the H-12 C-terminal helix which results in the dissociation of inhibitory co-regulators and recruitment of cofactor complexes such as CBP/p300, SRCs or CARM1. Co-activators share a consensus α-helical LXXLL motif that fits into the hydrophobic cleft of the receptor LBD that modify local chromatin structure and activate the basal transcription machinery.
Figure 4. Nuclear Hormone Receptor Partners of RXR with or without known ligands.
RXR partners with RAR to form the dimeric retinoid receptor, and also with many other partners that bind ligands (Vitamin D receptor, PPAR receptor, thyroid hormone receptor (TR), and other partners shown on the left side of this figure). RXR also binds partners that have no known ligand designated “orphan receptors” (such as COUP-TF I and II, NGF1B, and nurr 1 and others shown on the right side of the figure).
While in normal tissues retinoid target genes are readily activated by an agonist, in tumors epigenetic events often interfere with normal retinoid response. Critical elements of the regulatory regions are methylated, which prevents the synthesis of gene specific messenger RNA. A prime example is RARβ, in itself a retinoid-regulated gene, and a key regulator of antineoplastic pathways induced by retinoids. The silencing of RARβ during epithelial carcinogenesis is a prominent reason for retinoid resistance and limited efficacy of retinoid action [23].
The majority of retinoids interact with the ligand binding domains of some or all of the RARs, and some RAR-selective retinoids will bind RXRs, but with a very low affinity. The significance of RXRs is more pronounced in their essential role for the efficient binding to their genomic response elements of the subfamily 1 members of the nuclear hormone receptor superfamily. The latter are generally considered potential RXR partners (Figure 4). When forming dimers with RAR, RXRs are not able to respond to RXR-selective ligands, unless RAR is liganded. This phenomenon is known as RXR subordination. Yet, RXRs are not silent partners in RXR-RAR heterodimers, as simultaneous ligand binding of both receptors results in synergistic transcriptional activation [24]. In contrast, some other receptors (i.e. PPARs, LXRs, FXR) allow dimer activation by an RXR agonist, often replicating the phenotypic response to their own ligands. Rexinoids may modulate gene expression through biochemically detectable dimers of two RXRs; however activation of a target gene as a result of RXR homodimer activation is difficult to show. Rexinoids exert anti-proliferative, proapoptotic and differentiating biological effects on epithelial cells in vitro, and have also been shown to inhibit cancer cell migration, invasiveness and angiogenesis [25]. Naturally occurring ligands of RXRs include 9-cis-RA, docosahexaenoic acid and phytanic acid, and the list extends over a line of synthetic agonists with improved side-effect profiles or heterodimer-selective properties.
Retinoid receptor partners
The transcriptional outcome upon ligand activation is generally specific for the partner receptor and results in the modulation of a transcriptional program commensurate with well-defined biologic responses. For example, vitamin D receptor activation induces genes responsible for maintaining calcium homeostasis and bone formation, LXR agonists activate cholesterol transport mechanisms to protect cells from cholesterol overload, and FXR regulates the enterohepatic circulation of bile acids. Recent in vitro data indicate that retinoid signaling could also be mediated by other nuclear hormone receptors, such as COUP-TF II (NR2F2) [26], RORβ (NR1F2) or TR2 [27], although the relevance of these interactions in vivo remains to be shown.
Molecular mechanisms that modulate retinoid activity. The make-up of a nuclear receptor dimer not only determines which genes it can regulate but also determines which of the many co-regulatory molecules it may bind. The large number of possible complexes formed by dimers and co-regulators lends a level of plasticity almost unparalleled by other therapeutic targets. Furthermore, isotype-selective agonists may dictate differential responses by isotype-restricted co-repressor recruitment [28]. An additional mechanism to modulate retinoid-dependent cell signaling pathways or diminish retinoid signaling has been described in various cancer cells. Altered localization of RXRalpha was identified as a mechanism that provides the means for rapid changes in hepatoma cell response to LPS, as well as a survival strategy for breast cancer cells, which undergo apoptosis upon nucleoplasmic overexpression of RXR [29, 30]. In hepatocellular carcinoma elevated ERK activity phosphorylates RXRalpha on serine 260 and threonine 82, impairing its ubiquitination and inhibiting transcription of its target genes by a dominant-negative mechanism [31]. Phosphorylation events on RAR or co-repressors have also been reported to restore transcriptional competence to RXR and promote differentiation in leukemia cells. This raises the possibility of exploiting mechanisms that modulate heterodimer non-permissivity as a therapeutic option [32].
The magnitude and duration of the hormone response is regulated by the ubiquitin-dependent degradation of RARs and RXRs in the proteasome. In addition, retinoid receptors integrate a series of signaling pathways by means of phosphorylation events which cooperate with retinoids for the control of target gene expression. The highly conserved ligand binding domain (LBD) contains several phosphorylation sites used by the kinases PKA and MSK1, while the N-terminal domain contains a proline-rich motif that serves as substrate for CDKs and MAPKs. The complexity in the transcriptional responses produced by these multiple levels of regulation presents a unique therapeutic opportunity. Although the induced molecular changes overlap, RXR selective agonists differ in their anti-tumor effects from retinoids both in the preclinical and clinical settings.
Extra-nuclear retinoid signaling
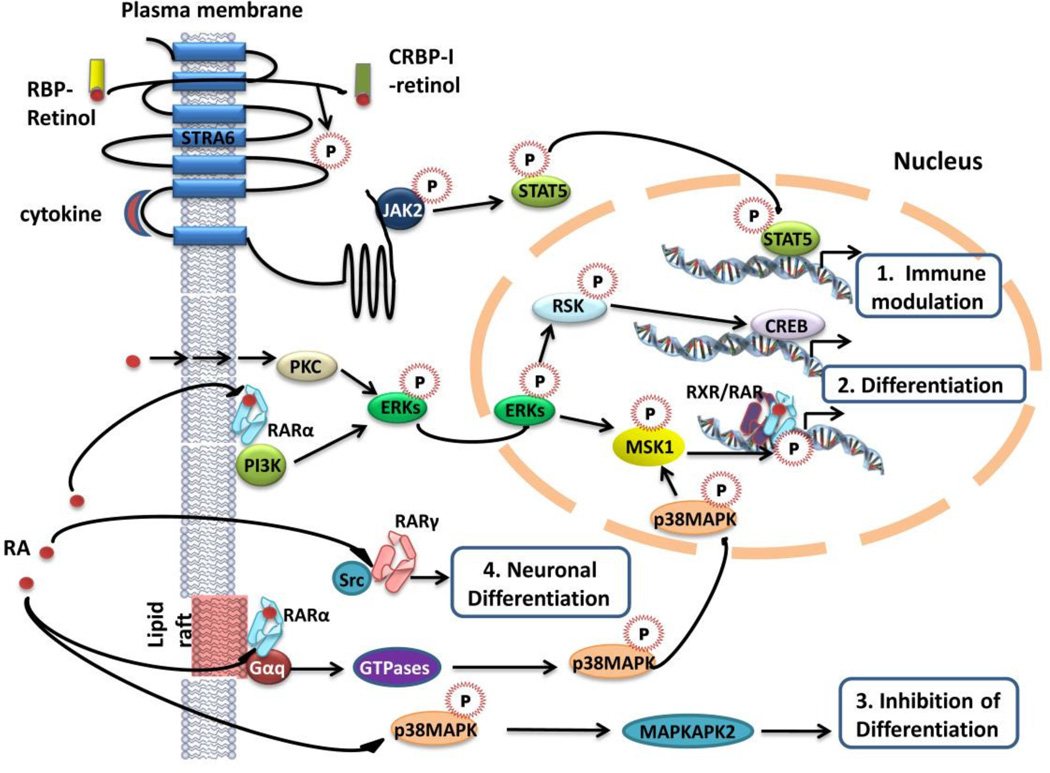
In the classical sense, RA is a prototype of hormones that activate nuclear receptor transcription factors that directly regulate gene expression. However, various studies from the past decade demonstrated that several kinase cascades may be activated just minutes after addition of retinol. As these events are transient, the possibility of a transcription-dependent phenomenon can be ruled out. Numerous studies suggested that retinol potentially exerts non-genomic activities through different types of interactions with signaling kinases (Figure 5) [33]. For example, retinol can induce cytokine-dependent activation of JAK2 and subsequently the STAT5 transcription factor, while being exchanged from extracellular RBP to intracellular CRBP-I through Stra6 [34], creating a close link between retinoid homeostasis and immune modulation. Stable association was shown between the regulatory p85 subunit of PI3K and membrane-localized RARalpha in neuroblastoma cells independent of the presence of RA, shifting to an RAR association with the p110 catalytic subunit upon RA liganding [35]. PI3K activation then results in sequential phosphorylation of downstream MAPKs and ribosomal S6 kinases, and is critical for RA-dependent differentiation. Retinol may also induce differentiation in a receptor-independent manner in bronchial epithelial cells by CREB-dependent transcriptional regulation. This mechanism is mediated by protein kinase C through the activation of Erk1/2 and the p90 S6 kinase and is not dependent on retinoid receptors [36]. Extranuclear RARα localized to lipid rafts was shown to trigger the phosphorylation of Rac1, p38 MAPKs and MSK1 through activation of Gαq upon ligand binding (Figure 5) [37]. Activation of MSK1 phosphorylates RARs and ultimately triggers their recruitment to response elements of select target genes, such as Cyp26A1 and RARbeta2 [38]. Nevertheless, RA-induced p38 activation has also been associated with negative regulation of cell differentiation and growth inhibition [39]. In neurons, RA was shown to play a role in neuritogenic differentiation through direct interaction with cytoplasmic c-Src [40]. Cytosolic translocation of RXR has been reported in hepatocytes, where it was implicated in the suppression of the inflammatory response to LPS administration [29]. Another intriguing novel function of RAR was proposed by Chen et al. in a study that localized RARalpha to the dendrites of neurons in association with mRNA granules and revealed its involvement in the regulation of translation [41]. However, many of these new mechanisms were identified in vitro and their relevance to human health and pharmacology is not sufficiently understood yet.
Figure 5. Extranuclear and non-transcriptional effects of retinoic acid (RA).
Pathways involving extranuclear biological activity of RA may result in various outcomes. 1. Cytokine-dependent retinol uptake via Stra6 Involves STAT5 activation and immune regulation. 2. Differentiation through CREB-dependent induction of retinoid target genes was reported to be triggered by receptor-independent activation of protein kinase C (PKC), the Erk1/2 MAPK (ERK) and the p90S6 kinase (RSK) by RA, or the interaction of membrane-associated PI3K and RAR-alpha. Lipid raft-bound RAR-alpha activating Gαq upon agonist stimulation leads to phosphorylation and nuclear translocation of p38MAPKs, activating MSK1 and inducing differentiation. 3. Activation of p38 by RA may also inhibit cell differentiation via downstream MAPKAPK2 activation, 4. Neuronal differentiation through the direct interaction of cytosolic RAR-gamma with Src.
2. Medical use of retinoids and rexinoids
Insufficient levels of vitamin A cause defects in vision, including night blindness, abnormal formation of keratin in mucosal membranes and altered bone remodeling. Today, the classic form of vitamin A deficiency due to malnutrition is rare. However, since it is an essential nutrient, total vitamin A deprivation can ultimately result in death.
Retinoids have been used successfully for the treatment of skin disorders, in particular acne, since the late 1940’s. In oncology, retinoids were first applied therapeutically and later as preventive agents. However, with increasing understanding of their molecular action new therapeutic indications were proposed. These include premalignant conditions, chronic obstructive pulmonary disease and more recently Alzheimer’s and Parkinson’s disease. This section will briefly summarize the current and proposed uses of retinoids and mechanistic insight for their clinical utility, for oncologic and non-cancer related applications.
2.1. Retinoids in Dermatology
Commercially retinoids have made the greatest impact in the cosmetics arena, and they are frequently used in creams and ointments to alleviate skin aging. Oral RA is efficacious in keratinizing disorders, but is considered too toxic; therefore, the pharmaceutical industry undertook the synthesis of retinoid derivatives to generate safer alternatives. Minor structural changes, such as forming cis-isomers, resulted in a new class of oral retinoids with strikingly different pharmacological properties. This was a milestone in dermatologic drug discovery. For instance, the isomer 13-cis-retinoic acid demonstrated impressive efficacy in acne and could cure severe acne conglobata [42]. In addition to systemic administration, several retinoids are also available for topical use (Table 1).
Table 1.
Retinoids Tested for Therapy of Benign Conditions
| Test Indications | Name (trade name) | Target Receptor | Phase | Subjects | Ref |
|---|---|---|---|---|---|
| Acne | Isotretinoin (13-cis-RA, Accutane) | RAR-gamma, RXR (metabolites) |
II | 180 | [160] |
| Tazarotene (AGN190168, Tazorac) | RAR-beta, RAR-gamma |
IV, III | 744 | [161] [162] |
|
| Tretinoin, gel | RAR-alpha, RAR-gamma |
III | 1537 | [163] | |
| Adapalene, gel | III | 900 | [164] | ||
| COPD, emphysema | Tretinoin (all-trans retinoic acid, Avita) | RAR-alpha, RAR-gamma |
II | 300 non-smoking |
[151] |
| Isotretinoin (13-cis-RA, Accutane) | RAR- gamma, RXR | II | 148 | [46] [151] |
|
| Palovarotene (R-667), (REPAIR trial) | RAR- gamma | II | 262 | [48] | |
| Cosmetic use, anti-aging | Motretinide (MDI 403) | RAR-alpha | - | - | [165] |
| Lichen, macular amyloidosis | Tretinoin tocoferil (Tocoretinate) | RAR-alpha | I | 10 | [43] |
| Photodamage | MDI-301 | RAR-alpha | ex vivo | - | [166] [167] |
| Psoriasis | Tazarotene (AGN190168, Tazorac) | RAR-beta, RAR-gamma |
IV, III | 744 | [162] |
| Skin ulcers | Tretinoin tocoferil (Tocoretinate) | RAR-alpha |
The major skin diseases benefiting from retinoids include acne, prosiarsis, disorders of keratinization, as well as precancerous lesions and non-melanoma skin cancer. For the treatment of acne vulgaris retinoids represented a breakthrough in the 1980s. Isotretinoin, the cis isomer of RA, normalizes epidermal differentiation, depresses sebum secretion and reduces inflammation, making long-lasting remission possible. Psoriasis treatment often involves immunosuppressive agents such as cyclosporine, tacrolimus and methotrexate and biologic agents targeting T-cells or tumor necrosis factor-α. Therefore, retinoids play an important role in cases where immunosuppression is not an acceptable side-effect, i.e. in children, patients at high risk of cancer or HIV carriers. Furthermore, the use of retinoids allows a dose reduction of phototherapy with UVB or PUVA, when used in combination. In plaque psoriasis acitretin and etretinate monotherapy may result in substantial improvement in 50% of the cases. In erythrodermic and pustular psoriasis these retinoids represent first-line therapy. Complete clearance requires the combination of retinoids with topical steroids, vitamin D derivatives or phototherapy. Three retinoids are currently approved for systemic therapy, isotretinoin (as Accutane, since 1982) for severe recalcitrant nodular acne, etretinate (Tegison, 1986) for severe recalcitrant psoriasis and acitretin (Soriatane, 1996) for severe psoriasis. Tretinoin tocoferil, the alpha-tocopherol ester of atRA, has been successfully used to treat lichen and macular amyloidosis and skin ulcers [43] (see Table 1).
The prevention of cutaneous malignant diseases was among the first chemopreventive applications of retinoids. Premalignant skin lesions, including actinic keratosis and HPV-induced tumors are frequently treated with retinoids [44]. Patients at high-risk for skin cancer because of genetic disposition (xeroderma pigmentosum), organ transplantation or history of multiple tumors, may be treated with etretinate or acitretin for 6 months [45].
Chronic obstructive pulmonary disease (COPD) and emphysema
Endogeous retinoids play a critical role in the pre- and postnatal development of the lungs. Indeed, the spatial and temporal distribution of retinaldehyde dehydrogenases, enzymes responsible for local RA production define the regions and periods of embryonal lung development [12]. Low retinol levels have been associated with bronchopulmonary dysplasia, a chronic lung condition arising from premature birth. Lung hypoplasia has also been associated with impaired cellular retinol uptake observed in clinical cases of Stra6 mutation [14]. Nevertheless, the importance of retinoid signaling extends to adult life and healthy pulmonary function. An inverse relationship between serum retinol levels and lung function was established in epidemiological studies conducted in smokers with COPD. Therefore, it is an intriguing notion that retinoids might trigger alveolar regeneration in the adult lung after tissue damage has occurred. In spite of the expectations, the Feasibility of Retinoids for the Treatment of Emphysema (FORTE) study using atRA or 13-cis-RA in a 6-month trial with a 3-month crossover period failed to detect significant improvements in CT densitometry score, gas transfer or quality of life [46]. Preclinical models of dexamethasone-impaired alveologenesis and airspace enlargement were used to identify the retinoid receptor subtypes critical for mediating improvement in lung function. 13-cis-RA, retinol, or RXR agonists did not, but 4-oxo RA and RARγ selective agents were able to induce regeneration [47]. Thus, the safety and efficacy of palovarotene (R667), an oral γ-selective retinoid agonist were tested in a randomized, double-blind, placebo-controlled study administering drug for 1 year to 262 patients with emphysema or severe a(1)-antitrypsin deficiency (Table 1) [48]. While none of the differences reached statistical significance, most functional lung parameters changed favorably in the retinoid-treated group versus the placebo group, and pavarotene was generally well tolerated.
Neurologic diseases
There is a growing body of literature that suggests that pharmacological targeting of certain nuclear receptors, particularly RXRs, LXRs and PPARs, is beneficial in neurodegenerative diseases [49, 50]. Genetic studies revealed that markers close to four of six retinoid receptor genes and genes encoding three of the four known retinol-binding proteins had linkage to Alzheimer’s disease (AD). More importantly, Cramer and coworkers showed that oral administration of bexarotene in a mouse model of AD resulted in the clearance of soluble β-amyloid (Abeta) in an apoE-dependent manner, and this was associated with a reversal of cognitive, social, and olfactory deficits [51]. Some subsequent attempts to independently reproduce the original report yielded controversial results, possibly due to the use of different formulations of bexarotene. However, in a similar study PPARgamma and RXRalpha agonists additively enhanced the microglial uptake of Abeta and improved spatial memory [50]. A phase 1b and a phase 2 study are underway to examine the effect of bexarotene on Abeta production and clearance.
In 2013 Parkinson’s disease was added to the list of conditions that could potentially benefit from rexinoids. Screening for small molecules that interact with the orphan nuclear receptor Nurr1 complexes, McFarland et al. showed that bexarotene can activate Nurr1, thereby promoting growth and survival of dopaminergic neurons and preventing motor deficit [52]. While Nurr1 has no known natural agonists, by forming heterodimers with RXR, rexinoids may indirectly modulate Nurr 1 action [53]. Interestingly, these effects were observed when administering markedly lower than the typical clinical doses of bexarotene.
Successful clinical use of retinoids as cancer treatment
Retinoids were shown to promote differentiation and cell death of cancer cells in a number of experimental systems [54, 55]. Some of the most successful therapeutic uses of retinoids are due to their differentiation-inducing effects. The most relevant therapeutic trials and applications of retinoids are summarized in Table 2.
Table 2.
Retinoids Tested for Therapy of Malignant Disease
| Test Indications | Name (trade name) | Target Receptor | Phase | Subjects | Ref |
|---|---|---|---|---|---|
| APL | Tretinoin (all-trans retinoic acid, Avita) | RAR-alpha, RAR-gamma | II, III | 156 | [62], [58] |
| Basal cell skin cancer | Tazarotene (AGN190168, Tazorac) | RAR-beta, RAR-gamma | II | 36 | [168] |
| CTCL | Tazarotene (AGN190168, Tazorac) | RAR-beta, RAR-gamma | II | 36 | [168] |
| Bexarotene (LGD1069, Targretin) | RAR-alpha | II | 66 | [169] | |
| Hepatocellular carcinoma | TAC-101 | RAR-alpha | I/II | 29 | [170], [171] |
| Peretinoin (NIK-333; acyclic retinoid) | RARs | II/III | 392 | [149], [172], [173] | |
| High-grade glioma | Isotretinoin (13-cis-RA, Accutane) | RAR-gamma, RXR | II | 23 | [174], [175] |
| Kaposi’s sarcoma | Alitretinoin (9-cis-RA, Pancretin) | RAR-alpha, RAR-beta, RAR-gamma, RXR-alpha, RXR-beta, RXR-gamma |
II, III | 66 | [64], [63] |
| Metastatic breast cancer | Tretinoin (all-trans retinoic acid, Avita) | RAR-alpha, RAR-gamma | I/II | 26 | [176] |
| Tretinoin (+/− tamoxifen +/− alpha-INF2) | RAR-alpha, RAR-gamma, ER-alpha | II | 55 | [74] | |
| Retinyl palmitate (plua beta-IFN and tamoxifen) | RARs | II | 65 | [76] | |
| Isotretinoin (13-cis-RA, Accutane) | RAR-gamma, RXR (metabolites) |
II | 99 | [75] | |
| Bexarotene (LGD1069, Targretin) | RXR-alpha | II | 145 | [77] | |
| All-trans retinoic acid | II | 17 | [73] | ||
| Metastatic renal carcinoma | Isotretinoin (13-cis-RA, Accutane) (in combination with IFN-alpha-2a) |
RAR-gamma, RXR | III | 341 | [65] |
| Myelomonocytic leukemia | Fenretinide (4-HPR) | RAR-alpha, RAR-gamma, RXR-alpha | II | 15 | [177] |
| Tretinoin tocoferil (Tocoretinate) | RAR-alpha, PML/RAR | [152] | |||
| Neuroblastoma | Isotretinoin (13-cis-RA, Accutane) | RAR-gamma, RXR (metabolites) | II | 379 | [71] |
| 4-HPR oral powderized lipid complex | RAR-alpha, RAR-gamma, RXR-alpha | II | 107 | [72] | |
| NSCLC | Tretinoin (all-trans retinoic acid, Avita) | RAR-alpha, RAR-gamma | II | 107 | [78] |
| Bexarotene (LGD1069, Targretin) | RXR-alpha | III | 623 | [80] | |
| NSCLC (K-Ras mutation) | Bexarotene (LGD1069, Targretin) | RXR-alpha | II | 40 | [82], [113] |
| Prostate cancer | Tretinoin (all-trans retinoic acid, Avita) | RAR-alpha, RAR-gamma | I/II | 17 | [126] |
| Isotretinoin (13-cis-RA, Accutane) (in combination with alpha-IFN2a) |
RAR-gamma, RXR | I, II | 70 | [178], [179] | |
| Squamous cell carcinoma | Etretinate | RARs | II | 316 | [180] |
Acute promyelocytic leukemia (APL): The best characterized example of retinoid receptor malfunction in human disease is linked to acute promyelocyte leukemia (APL), in which reciprocal chromosomal t(15,17) translocation between the genes of RARα and the promyelocyte leukemia protein (PML) lead to the alteration of signaling through both proteins. The resulting PML/RXRα fusion protein causes transcriptional repression and is the single causative factor in the etiology of APL. Preclinical investigations revealed that atRA induces terminal differentiation in the leukemic cells by promoting the degradation of the fusion protein through the action of the UBE1L ubiquitin-activating enzyme [56]. Recent studies identified the ubiquitin-specific protease UBP43, an antagonist of UBE1L, as an antineoplastic target whose inhibition destabilizes the t(15;17) PML/RARα fusion protein and induces apoptosis of APL cells [57]. The causative role of a single molecular defect paved the way for differentiation-based therapy and led to successful clinical application of atRA and 13-cis-RA for the treatment of APL (Table 2) [58]. Thus, the addition of atRA to the standard, anthracycline-based therapy increases the odds of long-term survival to 80%, compared to the chemotherapy alone which is curative in only 40% of the cases [58]. The 12 year follow-up of the 379 APL patients enrolled in the First North American Intergroup Study has shown 80% complete responses (CR) in the patients randomized to atRA-treatment for one year after chemotherapy, versus observation [59]. Notably, the differentiating effect of atRA is synergistically enhanced by arsenic trioxide, a compound targeting the PML component of the oncoprotein [60], which achieves remission in the rare cases of relapse after atRA and anthracycline therapy [61]. A recent phase III trial carried out in low-intermediate risk APL patients demonstrated better two-year event-free (97% vs. 86%) and overall survival rates with the combination of atRA with arsenic trioxide than with chemotherapy [62].
The use of retinoids in APL patients fundamentally changed the course of the disease for most patients, and it is bound to transform our approach to cancer treatment in more than one way. First, from a disease with a generally poor prognosis APL was transformed into the most prognostically favorable subtype of acute myeloid leukemia. In addition, the identification of this molecular defect led to the development of one of the first targeted therapies and provided the rationale for personalized medicine. Moreover, the possibility of achieving optimal efficacy without the use of chemotherapeutic drugs marks a novel path in curative therapy that emphasizes improved standard of care.
Kaposi’s sarcoma: 9-cis-RA (alitretinoin) is successfully used in the treatment of refractory cutaneous Kaposi’s sarcoma. Topical treatment with alitretinoin in a randomized, vehicle-controlled phase III trial was associated with a 30% higher response rate with mild to moderate adverse events over 12 weeks (Table 2) [63]. In AIDS-related Kaposi’s sarcoma cases with increased IL-6 levels durable objective response to alitretinoin was reported with improved rates and times to progression [64].
Squamous cell skin cancer: Following positive responses in the initial phase I/II studies with 13-cis-RA administered in combination with interferon-α2a, two out of three phase II/III trials also demonstrated increased progression-free survival in advanced skin, cervical and renal cancers [65]. A phase III randomized trial did not confirm the results, as no prevention of tumor recurrence or second primary tumors were detected, possibly due to a lack of appropriate statistical power [66]. Nonrandomized trials of 13-cis-RA in combination with interferon-α2a and chemotherapy for the treatment of advanced head and neck cancers showed promising results, and are awaiting confirmation by randomized studies [67].
Cutaneous T-cell lymphoma (CTCL): Natural and synthetic analogs of RA have been used for years both as monotherapy and in combinations for the treatment of CTCL [45]. The RXR-selective retinoid bexarotene was found to be active against all stages of the disease. Bexarotene showed a 45–54% response rate in a small phase 2 trial [68] and a 60% response rate in a retrospective study of long-term CTCL patients, earning FDA approval for the treatment of CTCL refractory to at least one previous therapy [68, 69].
Neuroblastoma: More than a decade ago it was suggested that retinoids may be beneficial in the treatment of neuroblastoma [70]. The greatest challenge in the treatment of neuroblastomas is its high recurrence rate. Recently a randomized phase III study established that intermittent dosing of 13-cis-RA significantly improved survival (Table 2) [71]. A phase I trial of fenretinide delivered in an oral powderized lipid complex (LXS) was reported to have anti-tumor activity in patients with refractory neuroblastoma, reaching higher plasma levels yet reduced toxicity as compared to conventional pills [72].
Breast cancer: In the only single agent clinical trial with atRA, this agent failed to achieve the primary response endpoint in patients with metastatic breast cancer [73]. Thus, subsequent studies investigated retinoid efficacy in combination with tamoxifen or interferons [74]. Isotretinoin, alitretinoin and retinyl palmitate were also tested as therapies in late-stage post-menopausal breast cancer patients (Table 2). No potentiation in the effect of tamoxifen or a difference in survival was detected [75], although a clinical response was seen in 55% of the patients treated with retinyl palmitate [76]. Clinical benefit with bexarotene was documented in 20% of patients with hormone-refractory metastatic breast cancers, with lower toxicity than seen with retinoids [77].
Lung cancer: Preclinical findings of anti-proliferative and pro-apoptotic activity in squamous cell cancers inspired a randomized phase II trial in advanced non-small cell lung cancer (NSCLC). The addition of atRA to chemotherapy yielded a benefit of increased response rates and progression-free survival [78]. Furthermore, a phase I/II first-line metastatic study of bexarotene combined with vinorelbine and cisplatin demonstrated a marked extension in median survival [79]. Unfortunately, these results were not confirmed in subsequent phase III trials of bexarotene plus cisplatin and vinorelbine, as progression-free or overall survival of NSCLC patients receiving combined treatment including bexarotene was indistinguishable from the placebo group [80] (Table 2). The Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial marked a paradigm shift in the design of clinical studies by achieving personalized therapy through adaptive randomization of patients [81]. With one of the four treatment arms incorporating bexarotene in combination with the tyrosine kinase inhibitor erlotinib, the BATTLE trial also revealed further detail on the applicability of bexarotene in lung cancer. Erlotinib plus bexarotene produced a 50% overall 8-week disease control rate (DCR) in pretreated lung cancer patients, and were particularly effective in the groups positive for EGFR mutations and RXRα/cyclinD1 protein expression.
Related clinical results were obtained and reported in a phase II trial conducted in chemotherapy-refractory KRAS mutation-positive NSCLCs [82]. But in addition to showing major clinical responses induced by the combination of bexarotene and erlotinib in advanced NSCLC, these studies defined cyclin D1 as a predictive biomarker, which enables oncologists to make personalized therapy decisions [83]. Cyclin D1, frequently overexpressed in lung cancer, plays a significant role in premalignant lung carcinogenesis, and is therefore a relevant target. Retinoid receptor selective agonists in human bronchial epithelial cells induced cyclin D1 degradation through RARbeta and RXR-dependent ubiquitination [84]. It has been proposed that dual targeting of cyclin D1 will result in increased efficacy, and overcome limitations of retinoid treatment in patients carrying polymorphisms in the cyclin D1 gene that make cyclin D1 resistant to degradation.
Challenges in the Medical Use of Retinoids
Pharmacodynamic considerations: Retinoid bioavailability is a crucial factor for the prediction of retinoid effectiveness and toxicity, particularly in light of the multiple enzymatic steps involved in both the creation and the degradation of active metabolites. Retinoid pharmacokinetics is further complicated by the differential lipid solubility of these agents. For example, etretinate is 50 times more lipophilic than acitretin and is released slowly from adipose tissues, where it is stored [85]. Thus, etretinate levels are detectable in serum for 2–3 years, while acitretin, isotretinoin and its metabolites reach normal levels two weeks after discontinuation of treatment. In vivo, pharmacodynamic mechanisms exert marked pharmacokinetic effects on locally available, intratumoral drug levels and have been shown to be a major determinant of the biological response to treatment [83].
Retinoid toxicity: Systemic retinoid toxicity in adults is similar to hypervitaminosis A, manifested in skin dryness, conjunctivitis and hair loss. The cutaneous and metabolic side-effects of retinoid treatment are dose-dependent and reversible. A minor fraction of APL patients receiving atRA (14–16%) develop retinoic acid syndrome (RAS), encompassing dyspnea, fever, pulmonary hemorrhage and respiratory failure. While these potentially lethal complications can be successfully treated by high-dose steroids, identifying patients who will develop RAS is critical. High initial and increasing white blood cell counts and CD13 expression on leukemic cells indicate predisposition to RAS [86].
Yet, in general, retinoids belong to the group of often well-tolerated pharmacologic agents. Many patients have been treated with retinoids, particularly etretinate or isotretinoin, continuously for as many as 15 years without developing signs of chronic toxicity [87]. However, because retinoids play an important role in the differentiation of embryonic tissue and organ formation, exposure of the fetus to therapeutic doses of retinoids is associated with teratogenic side effects. Thus, systemic use of these agents in patients of child-bearing age requires contraception for the duration of sustained retinoid levels in the body.
In contrast to retinoids, RXR-selective ligands have reduced teratogenicity and cutaneous toxicity. The most frequent adverse effects associated with rexinoid use are hyperlipidemia including triglycerides and cholesterol, the suppression of thyroid function and osteoporosis. Hyperlipidemia is treated with dose adjustment and lipid lowering drugs [88]. Individuals requiring life-long treatment should be subject to close monitoring. In comparison, long-term treatment with rexinoids is associated with a lower level of adverse effects than retinoids.
Retinoid resistance: Malignant transformation of epithelial cells may profoundly change retinol metabolism and contribute to the development of retinoid resistance [15]. Cellular retinoid uptake is regulated by LRAT, the enzyme that esterifies retinol to retinyl esters, and the RBP receptor Stra6. LRAT expression is suppressed in many human tissues undergoing malignant transformation, including breast, prostate, skin, kidney or bladder, resulting in low intracellular retinyl ester levels [89]. Stra6 mutations are associated with developmental errors [90]. Breast and prostate cancers also express aberrantly low levels of ALDH1a2, the enzyme forming RA from retinaldehyde [91]. ALDH1a2 has therefore been proposed as a tumor suppressor [92]. Mechanisms of retinoid clearance may also be affected in cancer cells, mainly through the over-expression of CYP26A1, the enzyme that oxidizes RA to more polar metabolites [93].
3. The use of retinoids for cancer prevention
The original concept of cancer prevention using specific compounds traces back to the early 20th century when Wolbach first reported on his rat study with “tissue changes following deprivation of fat-soluble A vitamin” and the reverse changes that follow, when the rats are restored to an adequate vitamin A diet [94]. Epidemiological data indicate that vitamin A deficiency is associated with higher susceptibility to chemical carcinogens [95, 96]. Few preclinical studies are designed to determine which stage of carcinogenesis is most effectively inhibited by retinoids [97]; yet this question needs to be elucidated. On the molecular level, the potential of retinoids to prevent cancers is based on the notion that specific early molecular alterations required for cancer progression, but not essential in later stages of tumorigenicity, may be effectively targeted by pharmacologic interventions. Accordingly, in experimental models retinoids appear more successful in blocking pre-neoplastic transformation or early phase neoplasia than reversing cancer. Although vitamin A derivatives have proved to be curative or useful as adjuvants in select overt cancers in humans, clinically applied retinoids are most effective in the treatment of precancerous lesions, such as leukoplakias, cervical dysplasia, and actinic keratosis [98] [44, 99].
Decreased proliferation is considered one of the best biomarkers of a cancer preventive effect. Many transcriptional target genes of retinoids help explain this correlation, as several inhibitors of cell cycle progression are direct targets of retinoids. The upregulation of RARβ2 by atRA and acyclic retinoids is a key factor in the inhibitory effect of RA on cell proliferation [100], as it induces the expression of p21CIP1 and p27Kip1, and inhibits the proteasome-dependent proteolysis of p27Kip1. Furthermore, RA stimulates the ubiquitin-mediated degradation of cyclin D1, thereby suppressing cyclin-dependent kinase (CDK) activities, interfering with retinoblastoma (Rb) phosphorylation and cyclin E expression which ultimately leads to the transition of cells into S-phase [101, 102]. Retinoids exhibit an ability to move cells towards more differentiated states [103]. However, in many cases these changes do not account for the net suppression of tumor formation in various tissues treated with retinoids. Retinoids and especially rexinoids have a major impact on cellular metabolism, harnessing the opportunity to differentially affect transformed versus normal epithelial cells [104–106]. Retinoids effectively modulate inflammatory pathways critical for carcinogenesis, which thus may offer viable targets for chemoprevention [107–109]. Furthermore, increasing evidence points to the reprogramming of stromal cells, fibroblasts, adipocytes, and immune cells by retinoids as potentially important factors in modulating tumorigenesis in a number of tissues [110, 111]. Some of the new approaches to retinoid-induced preventive activity may target the stromal environment or be based on immunomodulatory effects.
Preclinical investigations on the cancer preventive potential of retinoids. Although little epidemiologic data exist regarding the potential of vitamin A or carotenoids to reduce breast cancer risk, a wealth of in vitro studies have indicated which cellular pathways result in anti-proliferative and pro-differentiation mechanisms in the breast, as well as in other organs [112] [113].
The ability of retinoids to prevent cancer has been demonstrated in a number of animal models. Retinyl acetate and the synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) have been used to prevent mammary carcinogenesis in rats exposed to chemical carcinogens [114]. The naturally occurring 9-cis-RA was also found to suppress mammary tumors alone or in combination with low-dose tamoxifen [115, 116]. To avert the toxicity associated with 9-cis-RA, synthetic RXR-selective analogues such as bexarotene (Targretin, LGD1069) and LGD100268 were designed and tested. Both bexarotene and LGD100268 have proved superior to 9-cis-RA in preventing estrogen receptor (ER)-negative breast cancer in transgenic mice [117], either as single agents, or in combination with anti-estrogens [118, 119]. Potent chemopreventive efficacy of bexarotene was also demonstrated in combination with COX-2 inhibitors [120]. Interestingly, not only celecoxib, but bexarotene as well was found to reduce aromatase activity in the breast. Estrogen is a major driver in many breast cancers and stimulates cell proliferation, while RA has growth inhibitory effects in most cells. Hua et al. showed that RAR and estrogen receptor (ER)-α regulate their target genes in an antagonistic manner, with ER-α upregulating and RAR inhibiting proliferation and survival. This genomic antagonism between estrogen and retinoid signaling was demonstrated in a comprehensive study of genomic nuclear receptor binding and gene expression, which revealed a 39% overlap in genomic regions potentially binding both ER-α and RARs [121]. Understanding the crosstalk mechanisms between estrogen and retinoid signaling may open new opportunities for cancer preventive interventions.
Intestinal polyp formation in mice mutated in the adenomatous polyposis coli (APC) gene, Apc (Min/+) mice, was suppressed by dietary administration of bexarotene by up to 60% in male and 37% in female mice [122]. Intestinal tumors arising in spite of bexarotene dosing showed decreased proliferation markers and inflammatory cytokines. Chemoprevention studies in C57BL/6J-Apc(Min/+) mice demonstrated that suppression of tumor growth and prolonged survival could also be achieved by short-term and intermittent treatment by retinyl acetate and TRAIL (TNF-related apoptosis inducing ligand), due to apoptosis induced specifically in intestinal polyps [123].
Rexinoids were effective in A/J mouse models of lung carcinogenesis induced by K-ras or p53 dominant negative mutations, in monotherapy or in combination with triterpenoids [124, 125]. In both cases cancer preventive activities were associated with reduced cell proliferation and decreased expression of tumor growth-related biomarkers, i.e. cyclins D1 and E. Although preclinical data have suggested a potential role for retinoids against prostate cancer formation, these outcomes in animals have not translated into clinical efficacy [92] [126].
The successes and failures of the available retinoids have sparked further research to develop better anticancer agents with improved efficacy and side-effect profiles. Acyclic retinoids were found to be effective specifically for the prevention of hepatocellular carcinomas [127]. Long-term administration of acyclic retinoids significantly inhibited hepatic adenomas through inhibition of Ras and phosphorylation of ERK, and increased expression of RARβ and p21 [128]. The synthetic rexinoid analog, 9-cis UAB30 has been tested as a breast cancer prevention agent. It exhibits similar pharmacokinetic parameters to 9-cis-RA, but lacks the lipid inducing propensity of other RXR agonists [129].
Clinical investigations on the cancer preventive potential of retinoids: One of the first reports on molecular-targeted chemoprevention research demonstrated that an adjuvant high dose of isotretinoin (13-cis-RA) prevented second primary tumors in patients with curatively treated head and neck cancer [6]. Preclinical and epidemiologic data soon inspired clinical testing of the cancer preventive effects of retinoids in various target tissues.
Several retinoids have shown benefit in the prevention of skin cancers (Table 3). A sustained cancer preventive effect was demonstrated in patients with basal cell nevus syndrome treated with tazarotene, a topical retinoid, even 5 months after topical treatment was stopped; in addition, preexisting microscopic basal cell carcinomas were inhibited [168]. Furthermore, isotretinoin was shown to reverse suppression of RARβ in premalignant oral lesions [24]. Clinical efficacy in recurring and retinoid-resistant leukoplakia was achieved at a low dose of fenretinide [98, 130].
Table 3.
Completed randomized retinoid chemoprevention trials
| Treatment | Phase | Subjects | Risk group | Endpoint | Outcome | Ref |
|---|---|---|---|---|---|---|
| Etretinate | 2 | 316 | Squamous cell carcinoma |
Second primary in head and neck area |
No significant difference in overall survival (OS) | [180] |
| Fenretinide | 3 | 1739 | Breast cancer | Second primary breast cancer at 14.6 years |
Risk reduction in premenopausal women (HR=0.62) |
[144] |
| Fenretinide (200 mg/day) | 3 | 153 | Oral leukoplakia | Recurrence of leukoplakia |
Prevention of recurrence and new leukoplakias |
[98] |
| Fenretinide (200 mg/day) | 2 | 35 | Retinoid-resistant Oral leukoplakia |
1. Clinical response of oral intraepithelial neoplasia (IEN) 2. Toxicity, biomarkers |
34% partial response; Increase in apoptosis |
[130] |
| Fenretinide plus Tamoxifen | 3 | 1432 | Breast cancer | Contralateral breast cancer; toxicity |
Prevention of contralateral disease in premenopausal women |
[181] |
| Isotretinoin | 2 | 75 | Lung cancer, high risk Prior cancer |
Histologic progression by bronchial biopsy |
Non-significant change in histology; Reduced risk of treatment failure Fewer second primary tumors |
[182] [6] |
| Isotretinoin (100-50 mg/m2/day) | 2 | 103 | Head and neck squamous cell carcinoma (HNSCC) |
Prevention of second primary tumors |
No difference in recurrence of original tumors | [183] |
| Isotretinoin (2 mg/kg/day) | 2 | 44 | Oral leukoplakia | Recurrence | Delay in recurrence | [8] |
| Isotretinoin (0.5 mg/kg/day) or beta-carotene | 3 | 70 | Oral leukoplakia | Invasive carcinoma, toxicity |
92% response rate; Low-dose isotretinoin more active than beta- carotene |
[7] |
| Isotretinoin (low-dose: 30 mg/day) | 3 | 1190 | HNSCC | 1. Rate of 2nd primary tumors 2. Smoking-associated cancers |
No reduction in 2nd primary tumors | [131] |
| 9cUAB30 | 1 | 14 | Breast, high risk | Pharmacokinetics | Favorable toxicity | [154] |
|
Retinyl palmitate with or without beta-carotene versus 13-cis-RA |
3 | 162 | Oral premalignant lesions (OPL) |
OPL at 3 months | No benefit over 13-cis-RA | [184] |
| Peretinoin (Acyclic retinoid) | 2 | 89 | Hepatocellular carcinoma (HCC) |
Incidence of recurrent or new hepatoma |
Significant reduction | [185] [147] |
| Peretinoin (Polyprenoic acid) | 2/3 | 401 | Hepatitis C related HCC |
Incidence of recurrent or new hepatoma |
Improved survival with stable liver function | [149] |
| Tazarotene | 3 | 34 | Basal cell carcinoma (BCC) high-risk patients |
Incidence of BCC at 3 years; efficacy for chemotherapy |
Response in 6% of patients | [168] |
| Tretinoin (0.1% topical) | 3 | 1131 | Keratinocyte carcinoma (KC) |
Time to development of new BCC or invasive squamous cell carcinoma (SCC) |
No reduction in the risk of KC | [44] |
Clinical trials using isotretinoin to prevent recurrence or second primary head and neck squamous cell carcinomas (HNSCC) have yielded mixed results. In the initial phase II study isotretinoin treatment was associated with a significant reduction in second primary head and neck cancers or recurrence [6]. However, in a phase III study using a larger cohort (enrollment 1190) no significant delay in incidence of second primary cancers or recurrence with isotretinoin treatment was confimed [131, 132]. In order to facilitate prediction of favorable responses to isotretinoin as a cancer preventive agent, genetic polymorphisms in critical cancer signaling pathways were interrogated. Inclusion of several of these genetic loci improved the discriminatory ability of the risk/response prediction model to almost 80% as compared to approximately 66% for a model based solely on clinical and epidemiologic characteristics, suggesting the use of genetic markers as a promising route to identify the best responders to isotretinoin treatment [133].
Fenretinide (N-(4-hydroxyphenyl)retinamide, 4-HPR) was proposed for the treatment and chemoprevention of retinoblastoma based on preclinical studies, since it was shown to induce cell death in neuroectodermal tumors through oxidative stress and tumor cell ceramide levels [70]. Fenretinide also inhibited tumor growth in a xenograft model of Y79 neuroblastoma cells by inhibiting angiogenesis, making it potentially useful as an agent for early clinical intervention [134]. Clinical trials evaluating the combination of retinoids with ceramide modulators or anti-disialoganglioside antibodies were proposed for the treatment of high-risk neuroblastoma [135].
Epidemiologic data on vitamin A and β-carotene provided the primary rationale for the largest lung cancer prevention trials, because preclinical in vivo data supporting these agents were limited. A window-of-opportunity presurgical trial in non-small cell lung cancer demonstrated that critical biomarkers, including cyclin D1, Ki67 and phosphorylated EGFR were favorably modulated by bexarotene [83]. Treatment of former smokers for 3 months with 9-cis-RA led to a significant increase in RAR-β expression in the bronchial epithelium as compared with placebo [136]. However, in spite of promising prior biomarker data, the clinical trials of RAR agonists for lung cancer prevention were largely unsuccessful. Overall, beta-carotene proved ineffective in both the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) and the Carotene and Retinol Efficacy Trial (CARET). However, in former smokers beta-carotene slightly decreased lung cancer incidence (RR ~0.8), while increasing the chance of developing tumors in active smokers. In contrast to RAR agonists, bexarotene showed marked disease stabilization in the follow-up of the initial phase I trial in heavily pre-treated patients with advanced lung cancer [113]. A phase III study comparing the effectiveness of chemotherapy with or without bexarotene in the treatment of NSCLC found that the development of high-grade hypertriglyceridemia correlated with improved survival [137]. However, in unstratified patient populations, the addition of bexarotene to first-line treatment of NSCLC failed to show a benefit over standard chemotherapy [80]. These and many preclinical observations suggest a possible functional correlation between lipogenic activity and tumorigenesis, which remains to be assessed. Several groups have put forward the idea of inhalation as a more effective means of retinoid delivery with potentially reduced side effects. A pilot study showed that inhaled mid-dose 13-cis-RA upregulated the expression of all RARs and reduced carcinogen-induced tumor multiplicity by 67–88% [138]. In a small cell lung cancer (SCLC) model of lung-specific Rb1/p53 knock-out mice the combination of bexarotene with budesonide was shown to suppress tumor incidence and load [139]. Administration of aerosolized bexarotene in a rodent model not only inhibited lung tumorigenesis, but did so without elevating serum cholesterol or triglyceride levels [140].
Several lines of preclinical evidence suggested that retinoids and rexinoids have strong potential to inhibit breast cancer formation in both ER-positive and ER-negative mouse models [117, 118]. Repeated serum measurements of carotenoids and tocopherols over a period of six years identified an inverse correlation with breast cancer risk in postmenopausal women [141]. Due to the minimal efficacy of first generation retinoids documented in the treatment trials, and because fenretinide selectively accumulates in mammary tissue, chemoprevention studies for breast cancer focused on fenretinide [142]. The first large randomized studies showed the ability of fenretinide to reduce the incidence of second breast cancers in premenopausal women (Table 3) [143]. Strikingly, the protective effect continued to be manifest for up to 10 years after cessation of the treatment. In addition to breast cancer, fenretinide also reduced the incidence of ovarian cancers in BRCA1 and 2 mutation carriers [144]. Bexarotene was tested in women at high risk of breast cancer in a multicenter biomarker trial for breast chemoprevention. While overall significant changes in proliferative and apoptotic markers could not be shown, cyclin D1 was suppressed by bexarotene in mammary biopsy tissues from pre-menopausal subjects [145].
Acyclic retinoids (ACRs) are a unique class of retinoids developed specifically for the purpose of chemoprevention, and successfully tested in hepatocellular carcinoma (HCC) patients for their ability to prevent second primary tumors. A double-blind, placebo-controlled clinical trial conducted in Japan using peretinoin following chemotherapy demonstrated significantly reduced recurrence in HCC patients [146]. Administration of ACR over one year improved recurrence-free survival, as well as overall survival after a follow-up of over 3 years [147]. Based on the results of a phase I pharmacokinetic study 300–600 mg/day were considered safe and used in a subsequent phase II/III randomized, double-blind, placebo-controlled study of peretinoin in patients with hepatitis C-related HCC, to determine benefits in recurrence-free survival (RFS) as a primary outcome [148]. Although the primary comparison with placebo was not significant (p=0.434), improved survival was assumed in patients with stable liver function and treated with the higher dose peretinoin [149]. The hazard ratios for RFS in the high dose vs. placebo group were markedly low (0.27) two years after randomization.
Strategies to minimize retinoid toxicity
Beneath the wealth of pharmacological benefits, toxicity represents the main limitation to the chronic use of retinoids. Therefore, it is imperative to work out viable strategies to minimize toxicity without compromising therapeutic effectiveness. One successful approach includes administering high, intermittent doses of a retinoid, which may be more effective than continuous low-dose therapy, as exemplified by 13-cis-RA to improve event-free survival of neuroblastoma patients [150]. Muindi et al. determined in pharmacokinetic measurements of the FORTE study that intermittent therapy with high-dose atRA would result in the greatest atRA exposure [151]. Other efforts to minimize retinoid-related adverse effects encompass modifications to the basic retinoid structure. For example, the alpha-tocopheryl ester of atRA, tretinoin tocoferil, is 150 times less toxic than the parent compound and lacks teratogenicity [152]. A synthetic analogue of 9-cis-retinoic acid, 9cUAB30, primarily binds RXR, yet lacks the hypertriglyceridemic effect of most rexinoids [153]. The first pharmacokinetic studies aim to determine the optimal dose and side-effects of this drug [154]. However, new drug development based on structure-function relationship is expensive and very time-consuming.
Alternatively, the use of combinations harbors great potential with respect to both increasing efficacy, as well as limiting toxicity. Combinations synergistic for the cancer preventive effect or its surrogate are expected to retain efficacy even at low doses of the agents, where drug-specific side-effects are reduced. Synergistic interactions may result from affecting the same or related pathways, or may involve unrelated pathways.
A unique mechanism of cooperation (or synergy) was shown between vitamin K2 and both acyclic retinoids or 9-cis-RA, offering a perspective for a potential chemopreventive combination. It was proposed that vitamin K2 prevents the phosphorylation and accumulation of inactive RXRα by inhibiting Ras and extracellular signal-regulated kinase activation, allowing retinoids to exert their cancer preventive effect in hepatic cells [155]. ACRs themselves may be able to restore impaired receptor function through inhibition of RXR phosphorylation. Another approach is to examine potential enhancing interactions among the multitude of partnering nuclear hormone receptors. This way anti-proliferative and anti-tumor cooperation was demonstrated between RXRs and PPARs in premalignant mammary epithelial cells, as well as in vivo [156, 157].
Targeting the same pathways by two agents may accentuate an overlapping spectrum of adverse effects. Therefore, current preclinical studies take an unbiased approach to identify synergistic combinations in vitro in phenotypic compound screens of premalignant precursor cells or more recently using computational methods. Such combinations might consist of a component that exhibits proven cancer preventive characteristics and a second agent selected from a pool of compounds based on its ability to enhance the effect of the primary chemopreventive agent. Candidate combinations are subsequently tested in mechanistic studies and animal models, with a goal of being translated into clinical settings. The inclusion of agents that are already FDA-approved and clinically tested, albeit with a different scope of indications, will require “drug repurposing”, but might be the fastest, and most efficient ways to arrive at clinically testable, low-toxicity alternatives for cancer prevention [158]. One novel prospect for a breast cancer preventive combination includes the rexinoid bexarotene and a non-selective beta-adrenergic inhibitor, carvedilol. Identified in high-throughput compound screens of premalignant breast epithelial cells for combinations synergistically anti-proliferative over a wide dose-range, low doses of these two agents are being tested in transgenic mouse models of breast cancer to establish their preventive capacity (I.U. unpublished observation). Beyond the immediate clinical applicability of the two FDA-approved agents, this and similar investigations will likely reveal potentially exploitable novel interactions of adrenergic signaling pathways and rexinoid-dependent anti-cancer activity.
Summary - The future of retinoids in the prevention of cancer
The medical use of retinoids extends over more than half a century and marks some of the successes in pharmacology. Topical treatments are valuable in conditions associated with differentiation defects and the application of Tazarotene was decisive for the curative outcome of APL therapy. However, apart from leukemias, skin cancers and neuroblastoma, among other examples, currently retinoids play a more limited role in cancer treatment and prevention than would be expected based on their favorable pharmacologic properties.
Retinoids and rexinoids are ligands recognized by specific receptors which directly interact with the genome and regulate concerted programs of gene expression. These programs elicit coordinated physiologic changes that can antagonize malignant transformation, promote differentiation, cause apoptosis, insulin sensitivity, oxidative metabolism, and immune modulation, or suppress inflammation. The large number of possible complexes of nuclear receptor dimers and co-regulators formed in response to ligand binding confers tissue-selective modulation of cell function that collectively exert antineoplastic effects.
Although both retinoids and rexinoids possess a spectrum of pleiotropic effects, it is unlikely that retinoid monotherapy will be a preventive strategy for most cancers. However, as multifunctional agents, retinoids are especially well-suited to combination therapies [159]. From a long line of studies it appears that to best exploit the potential of retinoid pharmacology optimal combinations with other agents will have to be identified and the issues of resistance and toxicity addressed systematically in preclinical models and relevant clinical trials. There is a need to identify validated biomarkers in order to predict retinoid response in the target tissue as clinical data demonstrate that retinoid action is often dependent on specific cellular contexts and epigenetic modifications. Such biomarkers may be derived from preclinical studies or from patient material from window-of-opportunity trials. To take advantage of the full potential of retinoids such biomarkers will have to be used to better define clinically-responsive populations.
Useful knowledge can be gained from retrospective studies asking pharmacogenomic questions, by linking specific genetic loci or single nucleotide polymorphims to therapeutic efficacy [133]. Ample preclinical and ex vivo data are available about the downstream transcriptional responses of target organs that should allow the monitoring of the target-specific activity after retinoid treatment. These changes in the expression of retinoid target genes would also be important for bioavailability assessment.
From the prevention aspect of clinical use, rexinoids seem to outweigh retinoid agonists in their activities. While exhibiting modest activity against existing tumors (with the exception of CTCL), rexinoids are far more effective preventive agents than are retinoids in preclinical models. Furthermore, long-term treatment with rexinoids is associated with significantly fewer adverse effects than retinoids. Therefore, rexinoids should be clinically tested for their ability to suppress tumor formation in organ sites where preclinical and biomarker studies have demonstrated efficacy. Future hypothesis-driven clinical trials are needed to take full advantage in the clinic of the promise of retinoid and rexinoid pharmacology.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) and National Cancer Institute (NCI) grants R01-CA087546 (E.D.), R01-CA190722 (E.D.), R01-CA062275 (E.D.),.R01-078480 (P.H.B.), R03 CA180550 (I.P.U.), the University of Texas MD Anderson CCSG grant P30 CA16672, the Norman Brinker Award for Research Excellence (P.H.B.), the John Charles Cain Distinguished Chair Award (P.H.B.), the Duncan Family Institute Seed Funding Research Program (I.P.U.), and a UT-STARS award (E.D.). E.D. is an American Cancer Society Professor supported by a generous gift from the F.M. Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI statement: Dr. Uray has no conflicts of interest.
COI statement: Dr. Dmitrovsky has no conflicts of interest.
COI statement: Dr. Brown is on the Scientific Advisory Board of Susan G. Komen for the Cure.
References
- 1.Ries J, Blasiu AP. [Vitamin A therapy of carcinomata] Munch Med Wochenschr. 1952;94(40):2033–2036. [PubMed] [Google Scholar]
- 2.Hyams MN, Gallaher PD. Vitamin A therapy in the treatment of vulvar leucoplakia. Am J Obstet Gynecol. 1950;59(6):1346–1354. doi: 10.1016/0002-9378(50)90302-4. [DOI] [PubMed] [Google Scholar]
- 3.Seifter E, et al. Inhibitory action of vitamin A on a murine sarcoma. Life Sci. 1973;13(7):945–952. doi: 10.1016/0024-3205(73)90084-2. [DOI] [PubMed] [Google Scholar]
- 4.Bollag W. Vitamin A AND VITAMIN A acid in the prophylaxis and therapy of epithelial tumours. Int Z Vitaminforsch. 1970;40(3):299–314. [PubMed] [Google Scholar]
- 5.Sporn MB, et al. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35(6):1332–1338. [PubMed] [Google Scholar]
- 6.Hong WK, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 7.Lippman SM, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328(1):15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 8.Hong WK, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315(24):1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 9.Lobo GP, et al. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta, beta-carotene absorption and vitamin A production. FASEB J. 2010;24(6):1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauvant P, et al. Amounts and types of fatty acids in meals affect the pattern of retinoids secreted in human chylomicrons after a high-dose preformed vitamin A intake. Metabolism. 2003;52(4):514–519. doi: 10.1053/meta.2003.50082. [DOI] [PubMed] [Google Scholar]
- 11.Wongsiriroj N, et al. Genetic dissection of retinoid esterification and accumulation in the liver and adipose tissue. J Lipid Res. 2014;55(1):104–114. doi: 10.1194/jlr.M043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hind M, Corcoran J, Maden M. Alveolar proliferation, retinoid synthesizing enzymes, and endogenous retinoids in the postnatal mouse lung. Different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol. 2002;26(1):67–73. doi: 10.1165/ajrcmb.26.1.4575. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer FW, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18(6):918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol. 2011;288:1–41. doi: 10.1016/B978-0-12-386041-5.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 16.Heyman RA, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 17.Kliewer SA, et al. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Urquiza AM, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290(5499):2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 19.Lengqvist J, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3(7):692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Germain P, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58(4):712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 21.Bour G, Lalevee S, Rochette-Egly C. Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol. 2007;17(6):302–309. doi: 10.1016/j.tcb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6(7):542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 23.Wan H, et al. Overexpression of retinoic acid receptor beta in head and neck squamous cell carcinoma cells increases their sensitivity to retinoid-induced suppression of squamous differentiation by retinoids. Cancer Res. 1999;59(14):3518–3526. [PubMed] [Google Scholar]
- 24.Lotan R, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332(21):1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 25.Yen WC, et al. A selective retinoid X receptor agonist bexarotene (LGD1069, targretin) inhibits angiogenesis and metastasis in solid tumours. Br J Cancer. 2006;94(5):654–660. doi: 10.1038/sj.bjc.6602995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruse SW, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6(9):e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stehlin-Gaon C, et al. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol. 2003;10(10):820–825. doi: 10.1038/nsb979. [DOI] [PubMed] [Google Scholar]
- 28.Farboud B, et al. Isotype-restricted corepressor recruitment: a constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol Cell Biol. 2003;23(8):2844–2858. doi: 10.1128/MCB.23.8.2844-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghose R, et al. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2(1):4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, et al. Altered localization of retinoid X receptor alpha coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells. Mol Cell Biol. 2004;24(9):3972–3982. doi: 10.1128/MCB.24.9.3972-3982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi S, et al. Phosphorylation of retinoid X receptor suppresses its ubiquitination in human hepatocellular carcinoma. Hepatology. 2002;35(2):332–340. doi: 10.1053/jhep.2002.31164. [DOI] [PubMed] [Google Scholar]
- 32.Altucci L, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65(19):8754–8765. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- 33.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 34.Berry DC, et al. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Biol. 2012;32(15):3164–3175. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masia S, et al. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21(10):2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal S, et al. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17(2):566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piskunov A, Rochette-Egly C. A retinoic acid receptor RARalpha pool present in membrane lipid rafts forms complexes with G protein alphaQ to activate p38MAPK. Oncogene. 2012;31(28):3333–3345. doi: 10.1038/onc.2011.499. [DOI] [PubMed] [Google Scholar]
- 38.Bruck N, et al. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28(1):34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alsayed Y, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J Biol Chem. 2001;276(6):4012–4019. doi: 10.1074/jbc.M007431200. [DOI] [PubMed] [Google Scholar]
- 40.Dey N, et al. CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol Cell Biol. 2007;27(11):4179–4197. doi: 10.1128/MCB.01352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283(30):20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peck GL, et al. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300(7):329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- 43.Terao M, et al. Clinical effect of tocoretinate on lichen and macular amyloidosis. J Dermatol. 2011;38(2):179–184. doi: 10.1111/j.1346-8138.2010.00962.x. [DOI] [PubMed] [Google Scholar]
- 44.Weinstock MA, et al. Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): a veterans affairs randomized chemoprevention trial. J Invest Dermatol. 2012;132(6):1583–1590. doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Duvic M. Retinoids: therapeutic applications and mechanisms of action in cutaneous T-cell lymphoma. Dermatol Ther. 2003;16(4):322–330. doi: 10.1111/j.1396-0296.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- 46.Roth MD, et al. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130(5):1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 47.Hind M, et al. Retinoid induction of alveolar regeneration: from mice to man? Thorax. 2009;64(5):451–457. doi: 10.1136/thx.2008.105437. [DOI] [PubMed] [Google Scholar]
- 48.Stolk J, et al. Randomised controlled trial for emphysema with a selective agonist of the gamma-type retinoic acid receptor. Eur Respir J. 2012;40(2):306–312. doi: 10.1183/09031936.00161911. [DOI] [PubMed] [Google Scholar]
- 49.Dai YB, et al. Liver X receptor beta protects dopaminergic neurons in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109(32):13112–13117. doi: 10.1073/pnas.1210833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamanaka M, et al. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32(48):17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cramer PE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFarland K, et al. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson's disease. ACS Chem Neurosci. 2013;4(11):1430–1438. doi: 10.1021/cn400100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aarnisalo P, et al. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J Biol Chem. 2002;277(38):35118–35123. doi: 10.1074/jbc.M201707200. [DOI] [PubMed] [Google Scholar]
- 54.Idres N, et al. Granulocytic differentiation of human NB4 promyelocytic leukemia cells induced by all-trans retinoic acid metabolites. Cancer Res. 2001;61(2):700–705. [PubMed] [Google Scholar]
- 55.Nagy L, et al. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995;15(7):3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitareewan S, et al. UBE1L is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(6):3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo Y, et al. Blockade of the ubiquitin protease UBP43 destabilizes transcription factor PML/RARalpha and inhibits the growth of acute promyelocytic leukemia. Cancer Res. 2010;70(23):9875–9885. doi: 10.1158/0008-5472.CAN-10-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrie K, Zelent A, Waxman S. Differentiation therapy of acute myeloid leukemia: past, present and future. Curr Opin Hematol. 2009;16(2):84–91. doi: 10.1097/MOH.0b013e3283257aee. [DOI] [PubMed] [Google Scholar]
- 59.Douer D, et al. All-trans retinoic acid and late relapses in acute promyelocytic leukemia: very long-term follow-up of the North American Intergroup Study I0129. Leuk Res. 2013;37(7):795–801. doi: 10.1016/j.leukres.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gianni M, et al. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998;91(11):4300–4310. [PubMed] [Google Scholar]
- 61.Grimwade D, et al. Acute promyelocytic leukemia: a paradigm for differentiation therapy. Cancer Treat Res. 2010;145:219–235. doi: 10.1007/978-0-387-69259-3_13. [DOI] [PubMed] [Google Scholar]
- 62.Lo-Coco F, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 63.Bodsworth NJ, et al. Phase III vehicle-controlled, multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS-related Kaposi's sarcoma. Am J Clin Dermatol. 2001;2(2):77–87. doi: 10.2165/00128071-200102020-00004. [DOI] [PubMed] [Google Scholar]
- 64.Miles SA, et al. Antitumor activity of oral 9-cis-retinoic acid in HIV-associated Kaposi’s sarcoma. AIDS. 2002;16(3):421–429. doi: 10.1097/00002030-200202150-00014. [DOI] [PubMed] [Google Scholar]
- 65.Atzpodien J, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a Prospectively Randomized Trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) J Clin Oncol. 2004;22(7):1188–1194. doi: 10.1200/JCO.2004.06.155. [DOI] [PubMed] [Google Scholar]
- 66.Brewster AM, et al. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alfa for patients with aggressive skin squamous cell carcinoma. J Clin Oncol. 2007;25(15):1974–1978. doi: 10.1200/JCO.2006.05.9873. [DOI] [PubMed] [Google Scholar]
- 67.Recchia F, et al. Ifosfamide, cisplatin, and 13-Cis retinoic acid for patients with advanced or recurrent squamous cell carcinoma of the head and neck: a phase I-II study. Cancer. 2001;92(4):814–821. doi: 10.1002/1097-0142(20010815)92:4<814::aid-cncr1387>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 68.Straus DJ, et al. Results of a phase II trial of oral bexarotene (Targretin) combined with interferon alfa-2b (Intron-A) for patients with cutaneous T-cell lymphoma. Cancer. 2007;109(9):1799–1803. doi: 10.1002/cncr.22596. [DOI] [PubMed] [Google Scholar]
- 69.Quereux G, et al. Bexarotene in cutaneous T-cell lymphoma: third retrospective study of long-term cohort and review of the literature. Expert Opin Pharmacother. 2013;14(13):1711–1721. doi: 10.1517/14656566.2013.810718. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds CP, et al. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197(1–2):185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 71.Matthay KK, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol. 2009;27(7):1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurer BJ, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60(11):1801–1808. doi: 10.1002/pbc.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutton LM, et al. Pharmacokinetics and clinical impact of all-trans retinoic acid in metastatic breast cancer: a phase II trial. Cancer Chemother Pharmacol. 1997;40(4):335–341. doi: 10.1007/s002800050666. [DOI] [PubMed] [Google Scholar]
- 74.Toma S, et al. Biological activity of all-trans-retinoic acid with and without tamoxifen and alpha-interferon 2a in breast cancer patients. Int J Oncol. 2000;17(5):991–1000. doi: 10.3892/ijo.17.5.991. [DOI] [PubMed] [Google Scholar]
- 75.Chiesa MD, et al. Tamoxifen vs Tamoxifen plus 13-cis-retinoic acid vs Tamoxifen plus Interferon alpha-2a as first-line endocrine treatments in advanced breast cancer: updated results of a phase II, prospective, randomised multicentre trial. Acta Biomed. 2007;78(3):204–209. [PubMed] [Google Scholar]
- 76.Recchia F, et al. Beta-interferon, retinoids and tamoxifen in metastatic breast cancer: long-term follow-up of a phase II study. Oncol Rep. 2009;21(4):1011–1016. doi: 10.3892/or_00000317. [DOI] [PubMed] [Google Scholar]
- 77.Esteva FJ, et al. Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J Clin Oncol. 2003;21(6):999–1006. doi: 10.1200/JCO.2003.05.068. [DOI] [PubMed] [Google Scholar]
- 78.Arrieta O, et al. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(21):3463–3471. doi: 10.1200/JCO.2009.26.6452. [DOI] [PubMed] [Google Scholar]
- 79.Khuri FR, et al. Multi-institutional phase I/II trial of oral bexarotene in combination with cisplatin and vinorelbine in previously untreated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2001;19(10):2626–2637. doi: 10.1200/JCO.2001.19.10.2626. [DOI] [PubMed] [Google Scholar]
- 80.Ramlau R, et al. Randomized phase III trial comparing bexarotene (L1069-49)/cisplatin/vinorelbine with cisplatin/vinorelbine in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT I. J Clin Oncol. 2008;26(11):1886–1892. doi: 10.1200/JCO.2007.12.2614. [DOI] [PubMed] [Google Scholar]
- 81.Kim ES, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dragnev KH, et al. Bexarotene plus erlotinib suppress lung carcinogenesis independent of KRAS mutations in two clinical trials and transgenic models. Cancer Prev Res (Phila) 2011;4(6):818–828. doi: 10.1158/1940-6207.CAPR-10-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dragnev KH, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res. 2007;13(6):1794–1800. doi: 10.1158/1078-0432.CCR-06-1836. [DOI] [PubMed] [Google Scholar]
- 84.Dragnev KH, et al. Cyclin proteolysis as a retinoid cancer prevention mechanism. Ann N Y Acad Sci. 2001;952:13–22. doi: 10.1111/j.1749-6632.2001.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 85.Eisenhardt EU, Bickel MH. Kinetics of tissue distribution and elimination of retinoid drugs in the rat. II. Etretinate. Drug Metab Dispos. 1994;22(1):31–35. [PubMed] [Google Scholar]
- 86.Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33(4):331–338. doi: 10.1111/j.1365-2710.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 87.Vahlquist A. Long-term safety of retinoid therapy. J Am Acad Dermatol. 1992;27(6 Pt 2):S29–S33. doi: 10.1016/s0190-9622(08)80257-5. [DOI] [PubMed] [Google Scholar]
- 88.Assaf C, et al. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: an expert opinion. Br J Dermatol. 2006;155(2):261–266. doi: 10.1111/j.1365-2133.2006.07329.x. [DOI] [PubMed] [Google Scholar]
- 89.O’Byrne SM, et al. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280(42):35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Segel R, et al. Pulmonary hypoplasia-diaphragmatic hernia-anophthalmia-cardiac defect (PDAC) syndrome due to STRA6 mutations--what are the minimal criteria? Am J Med Genet A. 2009;149A(11):2457–2463. doi: 10.1002/ajmg.a.33038. [DOI] [PubMed] [Google Scholar]
- 91.Mira YLR, et al. Retinol conversion to retinoic acid is impaired in breast cancer cell lines relative to normal cells. J Cell Physiol. 2000;185(2):302–309. doi: 10.1002/1097-4652(200011)185:2<302::AID-JCP15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 92.Kim H, et al. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65(18):8118–8124. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- 93.Osanai M, Sawada N, Lee GH. Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene. 2010;29(8):1135–1144. doi: 10.1038/onc.2009.414. [DOI] [PubMed] [Google Scholar]
- 94.Wolbach SB, Howe PR. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J Exp Med. 1925;42(6):753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Narisawa T, et al. Effect of vitamin A deficiency on rat colon carcinogenesis by N-methyl-N’-nitro-N-nitrosoguanidine. Cancer Res. 1976;36(4):1379–1383. [PubMed] [Google Scholar]
- 96.Nettesheim ML. Williams, The influence of vitamin A on the susceptibility of the rat lung to 3-methylcholanthrene. Int J Cancer. 1976;17(3):351–357. doi: 10.1002/ijc.2910170311. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, et al. The Rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. 2007;13(20):6224–6231. doi: 10.1158/1078-0432.CCR-06-2681. [DOI] [PubMed] [Google Scholar]
- 98.Costa A, et al. Prospects of chemoprevention of human cancers with the synthetic retinoid fenretinide. Cancer Res. 1994;54(7 Suppl):2032s–2037s. [PubMed] [Google Scholar]
- 99.Han J, et al. Evaluation of N-4-(hydroxycarbophenyl) retinamide as a cancer prevention agent and as a cancer chemotherapeutic agent. In Vivo. 1990;4(3):153–160. [PubMed] [Google Scholar]
- 100.Faria TN, et al. The targeted disruption of both alleles of RARbeta(2) in F9 cells results in the loss of retinoic acid-associated growth arrest. J Biol Chem. 1999;274(38):26783–26788. doi: 10.1074/jbc.274.38.26783. [DOI] [PubMed] [Google Scholar]
- 101.Ma Y, et al. Retinoid targeting of different D-type cyclins through distinct chemopreventive mechanisms. Cancer Res. 2005;65(14):6476–6483. doi: 10.1158/0008-5472.CAN-05-0370. [DOI] [PubMed] [Google Scholar]
- 102.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 103.Arisi MF, et al. All trans-retinoic acid (ATRA) induces re-differentiation of early transformed breast epithelial cells. Int J Oncol. 2014;44(6):1831–1842. doi: 10.3892/ijo.2014.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 105.Currie E, et al. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18(2):153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Altucci L, et al. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6(10):793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 107.Papi A, et al. PPARgamma and RXR Ligands Disrupt the Inflammatory Cross-talk in the Hypoxic Breast Cancer Stem Cells Niche. J Cell Physiol. 2014 doi: 10.1002/jcp.24601. [DOI] [PubMed] [Google Scholar]
- 108.Subbaramaiah K, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2(4):356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Disis ML, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prev Res (Phila) 2013;6(12):1273–1282. doi: 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DeFilippis RA, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2(9):826–839. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dumont N, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15(3):249–262. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garattini E, et al. Retinoids and breast cancer: from basic studies to the clinic and back again. Cancer Treat Rev. 2014;40(6):739–749. doi: 10.1016/j.ctrv.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Dragnev KH, et al. Nonclassical retinoids and lung carcinogenesis. Clin Lung Cancer. 2005;6(4):237–244. doi: 10.3816/CLC.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 114.Moon RC, et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39(4):1339–1346. [PubMed] [Google Scholar]
- 115.Wu K, et al. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res. 2000;6(9):3696–3704. [PubMed] [Google Scholar]
- 116.Anzano MA, et al. Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen. Cancer Res. 1994;54(17):4614–4617. [PubMed] [Google Scholar]
- 117.Wu K, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62(22):6376–6380. [PubMed] [Google Scholar]
- 118.Liby K, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12(19):5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 119.Mazumdar A, et al. The combination of tamoxifen and the rexinoid LG100268 prevents ER-positive and ER-negative mammary tumors in p53-null mammary gland mice. Cancer Prev Res (Phila) 2012;5(10):1195–1202. doi: 10.1158/1940-6207.CAPR-11-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown PH, et al. Combination chemoprevention of HER2/neu-induced breast cancer using a cyclooxygenase-2 inhibitor and a retinoid X receptor-selective retinoid. Cancer Prev Res (Phila) 2008;1(3):208–214. doi: 10.1158/1940-6207.CAPR-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hua S, Kittler R, White K. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137(7):1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janakiram NB, et al. Chemopreventive effects of RXR-selective rexinoid bexarotene on intestinal neoplasia of Apc(Min/+) mice. Neoplasia. 2012;14(2):159–168. doi: 10.1593/neo.111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464(7291):1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pereira MA, et al. Prevention of mouse lung tumors by targretin. Int J Cancer. 2006;118(9):2359–2362. doi: 10.1002/ijc.21618. [DOI] [PubMed] [Google Scholar]
- 125.Liby K, et al. The rexinoid LG100268 and the synthetic triterpenoid CDDO-methyl amide are more potent than erlotinib for prevention of mouse lung carcinogenesis. Mol Cancer Ther. 2008;7(5):1251–1257. doi: 10.1158/1535-7163.MCT-08-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trump DL, et al. A phase II trial of all-trans-retinoic acid in hormone-refractory prostate cancer: a clinical trial with detailed pharmacokinetic analysis. Cancer Chemother Pharmacol. 1997;39(4):349–356. doi: 10.1007/s002800050582. [DOI] [PubMed] [Google Scholar]
- 127.Kagawa M, et al. An acyclic retinoid, NIK-333, inhibits N-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of TGF-alpha expression and cell proliferation. Carcinogenesis. 2004;25(6):979–985. doi: 10.1093/carcin/bgh093. [DOI] [PubMed] [Google Scholar]
- 128.Shimizu M, et al. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- +(db)/+Lepr(db) mice. Cancer Prev Res (Phila) 2011;4(1):128–136. doi: 10.1158/1940-6207.CAPR-10-0163. [DOI] [PubMed] [Google Scholar]
- 129.Kapetanovic IM, et al. Murine oncogenicity and pharmacokinetics studies of 9-cis-UAB30, an RXR agonist, for breast cancer chemoprevention. Int J Toxicol. 2010;29(2):157–164. doi: 10.1177/1091581809360070. [DOI] [PubMed] [Google Scholar]
- 130.Lippman SM, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12(10):3109–3114. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 131.Khuri FR, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98(7):441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 132.Perry CF, et al. Chemoprevention of head and neck cancer with retinoids: a negative result. Arch Otolaryngol Head Neck Surg. 2005;131(3):198–203. doi: 10.1001/archotol.131.3.198. [DOI] [PubMed] [Google Scholar]
- 133.Hildebrandt MA, et al. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict head and neck cancer patient second primary tumor/recurrence risk and response to retinoid chemoprevention. Clin Cancer Res. 2012;18(13):3705–3713. doi: 10.1158/1078-0432.CCR-11-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tosetti F, et al. N-(4-hydroxyphenyl)retinamide inhibits retinoblastoma growth through reactive oxygen species-mediated cell death. Mol Pharmacol. 2003;63(3):565–573. doi: 10.1124/mol.63.3.565. [DOI] [PubMed] [Google Scholar]
- 135.Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma--the role of monoclonal antibodies. Ann Pharmacother. 2013;47(2):210–218. doi: 10.1345/aph.1R353. [DOI] [PubMed] [Google Scholar]
- 136.Kurie JM, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-beta expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95(3):206–214. doi: 10.1093/jnci/95.3.206. [DOI] [PubMed] [Google Scholar]
- 137.Blumenschein GR, Jr, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT II. J Clin Oncol. 2008;26(11):1879–1885. doi: 10.1200/JCO.2007.12.2689. [DOI] [PubMed] [Google Scholar]
- 138.Dahl AR, et al. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: a pilot study. Clin Cancer Res. 2000;6(8):3015–3024. [PubMed] [Google Scholar]
- 139.Wang Y, et al. Preventive effects of bexarotene and budesonide in a genetically engineered mouse model of small cell lung cancer. Cancer Prev Res (Phila) 2009;2(12):1059–1064. doi: 10.1158/1940-6207.CAPR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Q, et al. Aerosolized bexarotene inhibits lung tumorigenesis without increasing plasma triglyceride and cholesterol levels in mice. Cancer Prev Res (Phila) 2011;4(2):270–276. doi: 10.1158/1940-6207.CAPR-10-0246. [DOI] [PubMed] [Google Scholar]
- 141.Kabat GC, et al. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am J Clin Nutr. 2009;90(1):162–169. doi: 10.3945/ajcn.2009.27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cazzaniga M, et al. Fenretinide (4-HPR): a preventive chance for women at genetic and familial risk? J Biomed Biotechnol. 2012;2012:172897. doi: 10.1155/2012/172897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Veronesi U, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91(21):1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 144.Veronesi U, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17(7):1065–1071. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- 145.Brown PH, et al. Abstract CN04-04: Phase II trial of bexarotene in women at high risk of breast cancer: Comparison of protein and RNA biomarkers. Cancer Prev Res (Phila) 2008;1(7 Suppl) CN04-04. [Google Scholar]
- 146.Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340(13):1046–1047. doi: 10.1056/NEJM199904013401315. [DOI] [PubMed] [Google Scholar]
- 147.Takai K, et al. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Updated analysis of the long-term follow-up data. Intervirology. 2005;48(1):39–45. doi: 10.1159/000082093. [DOI] [PubMed] [Google Scholar]
- 148.Okita K, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okita K, et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Veal G, Rowbotham S, Boddy A. Pharmacokinetics and pharmacogenetics of 13-cis-retinoic acid in the treatment of neuroblastoma. Therapie. 2007;62(2):91–93. doi: 10.2515/therapie:2007020. [DOI] [PubMed] [Google Scholar]
- 151.Muindi JR, et al. Pharmacokinetics and metabolism of all-trans- and 13-cis-retinoic acid in pulmonary emphysema patients. J Clin Pharmacol. 2008;48(1):96–107. doi: 10.1177/0091270007309701. [DOI] [PubMed] [Google Scholar]
- 152.Makishima M, Honma Y. Tretinoin tocoferil as a possible differentiation-inducing agent against myelomonocytic leukemia. Leuk Lymphoma. 1997;26(1–2):43–48. doi: 10.3109/10428199709109156. [DOI] [PubMed] [Google Scholar]
- 153.Vedell PT, et al. Effects on gene expression in rat liver after administration of RXR agonists: UAB30, 4-methyl-UAB30, and Targretin (Bexarotene) Mol Pharmacol. 2013;83(3):698–708. doi: 10.1124/mol.112.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kolesar JM, et al. A pilot, first-in-human, pharmacokinetic study of 9cUAB30 in healthy volunteers. Cancer Prev Res (Phila) 2010;3(12):1565–1570. doi: 10.1158/1940-6207.CAPR-10-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kanamori T, et al. Synergistic growth inhibition by acyclic retinoid and vitamin K2 in human hepatocellular carcinoma cells. Cancer Sci. 2007;98(3):431–437. doi: 10.1111/j.1349-7006.2006.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uray IP, et al. Cancer-preventive rexinoid modulates neutral lipid contents of mammary epithelial cells through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Mol Pharmacol. 2012;81(2):228–238. doi: 10.1124/mol.111.072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mehta RG, et al. A ligand of peroxisome proliferator-activated receptor gamma, retinoids, and prevention of preneoplastic mammary lesions. J Natl Cancer Inst. 2000;92(5):418–423. doi: 10.1093/jnci/92.5.418. [DOI] [PubMed] [Google Scholar]
- 158.Anighoro A, Bajorath J, Rastelli G. Polypharmacology: Challenges and Opportunities in Drug Discovery. J Med Chem. 2014 doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 159.Kim ES, Lee JJ, Wistuba II. Cotargeting cyclin D1 starts a new chapter in lung cancer prevention and therapy. Cancer Prev Res (Phila) 2011;4(6):779–782. doi: 10.1158/1940-6207.CAPR-11-0143. [DOI] [PubMed] [Google Scholar]
- 160.Blasiak RC, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149(12):1392–1398. doi: 10.1001/jamadermatol.2013.6746. [DOI] [PubMed] [Google Scholar]
- 161.Feldman SR, Werner CP, Alio Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12(4):438–446. [PubMed] [Google Scholar]
- 162.Del Rosso JQ, Tanghetti E. A status report on topical tazarotene in the management of acne vulgaris. J Drugs Dermatol. 2013;12(3):s53–s58. [PubMed] [Google Scholar]
- 163.Webster G, et al. A combined analysis of 2 randomized clinical studies of tretinoin gel 0.05% for the treatment of acne. Cutis. 2009;83(3):146–154. [PubMed] [Google Scholar]
- 164.Millikan LE. Pivotal clinical trials of adapalene in the treatment of acne. J Eur Acad Dermatol Venereol. 2001;15(Suppl 3):19–22. doi: 10.1046/j.0926-9959.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 165.Zouboulis CC. Retinoids--which dermatological indications will benefit in the near future? Skin Pharmacol Appl Skin Physiol. 2001;14(5):303–315. doi: 10.1159/000056361. [DOI] [PubMed] [Google Scholar]
- 166.Varani J, Fay K, Perone P. MDI 301, a non-irritating retinoid, induces changes in human skin that underlie repair. Arch Dermatol Res. 2007;298(9):439–448. doi: 10.1007/s00403-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zeng W, et al. Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes. J Diabetes Complications. 2011;25(6):398–404. doi: 10.1016/j.jdiacomp.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tang JY, et al. Tazarotene: randomized, double-blind, vehicle-controlled, and open-label concurrent trials for basal cell carcinoma prevention and therapy in patients with basal cell nevus syndrome. Cancer Prev Res (Phila) 2014;7(3):292–299. doi: 10.1158/1940-6207.CAPR-13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abbott RA, et al. Bexarotene therapy for mycosis fungoides and Sezary syndrome. Br J Dermatol. 2009;160(6):1299–1307. doi: 10.1111/j.1365-2133.2009.09037.x. [DOI] [PubMed] [Google Scholar]
- 170.Rizvi NA, et al. Initial clinical trial of oral TAC-101, a novel retinoic acid receptor-alpha selective retinoid, in patients with advanced cancer. J Clin Oncol. 2002;20(16):3522–3532. doi: 10.1200/JCO.2002.02.090. [DOI] [PubMed] [Google Scholar]
- 171.Higginbotham KB, et al. A phase I/II trial of TAC-101, an oral synthetic retinoid, in patients with advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134(12):1325–1335. doi: 10.1007/s00432-008-0406-2. [DOI] [PubMed] [Google Scholar]
- 172.Okusaka T, et al. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol Res. 2011;41(6):542–552. doi: 10.1111/j.1872-034X.2011.00800.x. [DOI] [PubMed] [Google Scholar]
- 173.Okusaka T, et al. Phase I study of TAC-101, an oral synthetic retinoid, in Japanese patients with advanced hepatocellular carcinoma. Cancer Sci. 2012;103(8):1524–1530. doi: 10.1111/j.1349-7006.2012.02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wismeth C, et al. Maintenance therapy with 13-cis retinoid acid in high-grade glioma at complete response after first-line multimodal therapy--a phase-II study. J Neurooncol. 2004;68(1):79–86. doi: 10.1023/b:neon.0000024748.26608.2f. [DOI] [PubMed] [Google Scholar]
- 175.Yung WK, et al. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res. 1996;2(12):1931–1935. [PubMed] [Google Scholar]
- 176.Budd GT, et al. Phase I/II trial of all-trans retinoic acid and tamoxifen in patients with advanced breast cancer. Clin Cancer Res. 1998;4(3):635–642. [PubMed] [Google Scholar]
- 177.Garewal HS, et al. Phase II trial of fenretinide [N-(4-hydroxyphenyl) retinamide] in myelodysplasia: possible retinoid-induced disease acceleration. Leuk Res. 1989;13(4):339–343. doi: 10.1016/0145-2126(89)90071-4. [DOI] [PubMed] [Google Scholar]
- 178.Thalasila A, et al. A phase I trial of weekly paclitaxel, 13- cis-retinoic acid, and interferon alpha in patients with prostate cancer and other advanced malignancies. Cancer Chemother Pharmacol. 2003;52(2):119–124. doi: 10.1007/s00280-003-0644-6. [DOI] [PubMed] [Google Scholar]
- 179.DiPaola RS, et al. A randomized phase II trial of mitoxantrone, estramustine and vinorelbine or bcl-2 modulation with 13-cis retinoic acid, interferon and paclitaxel in patients with metastatic castrate-resistant prostate cancer: ECOG 3899. J Transl Med. 2010;8:20. doi: 10.1186/1479-5876-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bolla M, et al. Prevention of second primary tumours with etretinate in squamous cell carcinoma of the oral cavity and oropharynx. Results of a multicentric double-blind randomised study. Eur J Cancer. 1994;30A(6):767–772. doi: 10.1016/0959-8049(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 181.Camerini T, et al. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19(6):1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- 182.Kelly K, et al. A randomized phase II chemoprevention trial of 13-CIS retinoic acid with or without alpha tocopherol or observation in subjects at high risk for lung cancer. Cancer Prev Res (Phila) 2009;2(5):440–449. doi: 10.1158/1940-6207.CAPR-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Benner SE, et al. Toxicity of isotretinoin in a chemoprevention trial to prevent second primary tumors following head and neck cancer. J Natl Cancer Inst. 1994;86(23):1799–1801. doi: 10.1093/jnci/86.23.1799-a. [DOI] [PubMed] [Google Scholar]
- 184.Papadimitrakopoulou VA, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. 2009;27(4):599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muto Y, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study GrouN Engl J Med. 1996;334(24):1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]