Abstract
Objective:
To evaluate the frequency of HIV-associated neurocognitive disorder (HAND) in HIV+ individuals and determine whether the frequency of HAND changed over 4 years of follow-up.
Methods:
The Multicenter AIDS Cohort Study (MACS) is a prospective study of gay/bisexual men. Beginning in 2007, all MACS participants received a full neuropsychological test battery and functional assessments every 2 years to allow for HAND classification.
Results:
The frequency of HAND for the 364 HIV+ individuals seen in 2007–2008 was 33% and for the 197 HIV+ individuals seen at all time periods during the 2007–2008, 2009–2010, and 2011–2012 periods were 25%, 25%, and 31%, respectively. The overall frequency of HAND increased from 2009–2010 to 2011–2012 (p = 0.048). Over the 4-year study, 77% of the 197 HIV+ individuals remained at their same stage, with 13% showing deterioration and 10% showing improvement in HAND stage. Hypercholesterolemia was associated with HAND progression. A diagnosis of asymptomatic neurocognitive impairment was associated with a 2-fold increased risk of symptomatic HAND compared to a diagnosis of normal cognition.
Conclusion:
HAND remains common in HIV+ individuals. However, for the majority of HIV+ individuals on combination antiretroviral therapy with systemic virologic suppression, the diagnosis of HAND is not a progressive condition over 4 years of follow-up. Future studies should evaluate longitudinal changes in HAND and specific neurocognitive domains over a longer time period.
Prior to the introduction of combination antiretroviral therapy (cART) in 1996, HIV-associated dementia (HAD) was a progressive neurocognitive disorder leading to death within months. In the cART era, the mean survival after HAD increased,1 and milder forms of cognitive impairment became more common.2 The syndrome of HIV-associated neurocognitive disorders (HAND) was defined in 20073 with 3 stages of cognitive impairment: (1) asymptomatic neurocognitive impairment (ANI), (2) minor neurocognitive disorder (MND), and (3) HAD. An important modification from prior staging criteria was the addition of the ANI category to describe HIV-infected (HIV+) individuals with mild cognitive impairment defined by formal neuropsychological testing but without any impairment in everyday functioning. This category emphasizes the mildest clinical phenotype of HIV-associated cognitive impairment in individuals on cART who frequently have suppressed systemic viral load. Using the revised criteria for HAND, the frequency of HAD has decreased dramatically, but the frequency of ANI and MND remain high among participants in the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort.2
The question arises whether HAND continues to be a progressive neurologic syndrome as it was in the pre-cART era, particularly given that the HIV+ population is living longer and subject to age-associated conditions, which themselves can lead to greater risk of cognitive impairment. The objectives of this study were to (1) evaluate the frequency of HAND in HIV+ individuals in the Multicenter AIDS Cohort Study (MACS) and (2) determine whether the frequency of HAND changed over a 4-year period of follow-up.
METHODS
Research participants.
The study was conducted within the MACS, a study of HIV infection among gay/bisexual men.4 Participants undergo an interview and clinical assessment including an evaluation of psychomotor speed performance every 6 months and a neuropsychological test battery administered to HIV+ individuals biannually. An instrumental activities of daily living (IADL) scale5 was also administered at each visit beginning in 2007, allowing for the classification of HAND.
HIV+ participants with confounding conditions likely to contribute to cognitive impairment were excluded from the analysis. These included any history of brain opportunistic infections, non-HIV-associated neurologic disease, history of major psychiatric disorder, current alcohol or substance dependence, collagen vascular disease, thyroid disease, chronic obstructive pulmonary disease, congestive heart failure, angina pectoris, myocardial infarction within the prior 6 months, hepatic failure, renal failure, daily use of systemic steroids, narcotic/opioid analgesics, or immunostimulant/immunosuppressive medications.
Standard protocol approvals, registrations, and patient consents.
An institutional review board/ethical standards committee approved the use of human subjects for this study at each site. Written informed consent was obtained from all participants.
HAND classification in the MACS.
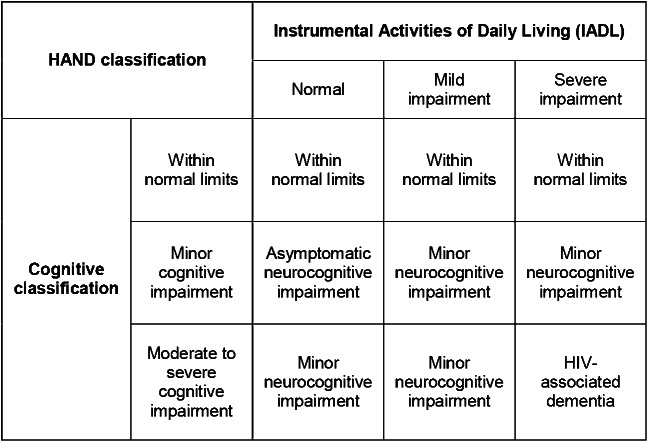
Diagnoses of HAND were operationalized using criteria outlined previously.3,6 The HAND classification system requires (1) cognitive testing, (2) an index of activities of daily living, and (3) medical history and testing to rule out alternative diagnoses.
Cognitive testing: 6 cognitive domains were defined using measures from the MACS neuropsychological test battery: (1) executive function (Trail-Making Part B, Stroop Interference); (2) speed of information processing (Symbol Digit Modalities Test, Stroop Color Naming); (3) attention and working memory (2 measures of one-back reaction time using the CalCAP Reaction Time program); (4) learning (Rey Auditory Verbal Learning Test—sum of Trials 1 through 5, Rey Complex Figure Immediate Recall); (5) memory (Rey Auditory Verbal Learning Test Delayed Recall, Rey Complex Figure Delayed Recall); and (6) motor (most impaired hand on the Grooved Pegboard). A T score was derived for each component test adjusting for age, years of education, ethnicity (Caucasian vs other), and number of times the test had been administered. For each functional domain, a summary T score was derived by averaging the 2 T scores or, in the case of the motor domain, using the lowest T score on either the dominant or nondominant hand of the Grooved Pegboard. If only one test in a domain was completed,6 the T score for that test was used. Following the procedures described previously, if the T score for one of the tests was ≥40 and one was <40, then the domain was assigned the lowest obtained T score plus 5. A global index of cognitive functioning was derived for individuals who had T scores for at least 4 of the 6 cognitive domains. Using the 2007 criteria,3 cognitive scores were assigned as follows: (1) within normal limits if one or fewer domains had T scores 1 SD or more below the mean; (2) minor cognitive impairment if 2 or more domains had T scores 1 SD or more below the mean, and the individual did not meet criteria for the more severe category 3 that follows; and (3) moderate to severe cognitive impairment if 2 or more domains had T scores 2 SDs or more below the mean, or one domain had a T score 2.5 SDs or more below the mean.
Using this classification model, we ran Monte Carlo analyses7,8 applying these criteria to 100,000 random datasets constrained by assumptions of normal distributions for each neuropsychological test and intercorrelations based on MACS data from the HIV-negative cohort. We found that, in a random set of individuals in the general population, 78% would be classified as within normal limits, 20% would be classified as minor cognitive impairment, and 3% would be classified as moderate to severe cognitive impairment. These numbers represent baseline frequencies for these classifications that we might reasonably expect in a population of similar uninfected gay/bisexual men.
Activities of daily living: The MACS uses an adaptation of Lawton and Brody's5 IADL scale. A global classification of normal, mild impairment, or severe impairment for IADLs was made if at least 14 of the 16 questions on the IADL questionnaire were completed. Severe impairment was defined as major decline on 2 or more questions or major or minor declines on 4 or more questions. Mild impairment was defined as minor decline on 2 or more questions or major or minor declines on 2 or more questions (but not on enough questions to qualify for severe impairment). Individuals who did not meet criteria for major or minor impairment were classified as normal.
Medical comorbidity: The MACS collects self-reported medical information from participants every 6 months. A history of vascular risk factors such as hypertension, diabetes, or hypercholesterolemia was defined by a subject self-reporting these conditions. Serious medical conditions are verified through medical charts. After removing study participants with medical conditions that might confound HAND diagnoses, each individual was assigned a HAND diagnosis (see above exclusion criteria) (within normal limits, ANI, MND, or HAD) (figure 1).
Figure 1. HIV-associated neurocognitive disorders (HAND) algorithm used in the Multicenter AIDS Cohort Study.
Data analysis.
The frequency of each HAND stage and total frequency of HAND (ANI, MND, and HAD) for the entire HIV+ cohort was evaluated over a 6-year longitudinal period examining data from 2007–2008, 2009–2010, and 2011–2012. We estimated the proportion of HIV+ patients in each HAND classification for each time period. The distribution of HAND classification was compared across time periods. The likelihood of increasing cognitive severity was modeled using logistic regression. Stability of HAND classification was calculated as stable (no change in HAND severity), worsened (increase in HAND severity), and improved (decrease in HAND severity) between each of the following time periods: 2007–2008 to 2009–2010, 2007–2008 to 2011–2012, and 2009–2010 to 2011–2012.
To evaluate the role that risk factors play in HAND stage progression, individuals were characterized with respect to age (by decade and stratified around 50 years of age), education, HIV duration, CD4 cell count and nadir, plasma HIV RNA (count and detectability), type of cART, site of evaluation, hepatitis C coinfection, and diagnosis of hypertension, diabetes, or hypercholesterolemia. Likelihood of HAND stage progression was modeled using a logistic regression analysis. Odds ratio (OR) and 95% confidence interval (CI) were calculated for each risk factor.
RESULTS
A total of 364 HIV+ individuals had HAND classifications in the 2007–2008 period. A total of 197 of them were classified during all 3 time periods (2007–2008, 2009–2010, and 2011–2012). The characteristics for all 364 HIV+ individuals at the baseline visit, the 197 HIV+ individuals seen at all 3 visits, and the 167 HIV+ individuals seen at some but not all of these visits are described in the table. HIV+ individuals who did not attend all 3 periods were older than HIV+ individuals who attended all 3 visits (p = 0.002). The overall frequency of HAND for the 364 HIV+ individuals was 33%, and each HAND stage was distributed as follows: ANI 14%, MND 14%, HAD 5%. HIV+ individuals who did not attend all 3 time periods had an increased frequency of HAND in 2007–2008 (41%) compared to HIV+ individuals who did attend all 3 time periods (25%) (p = 0.004).
Table.
Demographics of HIV+ individuals at baseline visit (2007–2008)
The overall frequency of all HAND classifications (including ANI, MND, and HAD) for the 197 HIV+ individuals seen at all 3 times during the 2007–2008 period, 2009–2010 period, and 2011–2012 period were 25%, 25%, and 31%. The increase between 2009–2010 and 2011–2012 was significant (p = 0.048). The frequencies of each HAND stage for 197 HIV+ individuals at each 2-year period are shown in figure 2. The frequency of ANI increased from 12% in 2007–2008, and 8% in 2009–2010, to 19% in 2011–2012 (2007–2008 to 2011–2012 [p = 0.14]; 2009–2010 to 2011–2012 [p = 0.016]). There was no change in the frequency of either MND or HAD across these time periods. The frequency of HAD was low at all 3 time periods (2007–2008, 2009–2010, and 2011–2012 HAD frequency 2%, 3%, and 2%, respectively).
Figure 2. Frequency of HIV-associated neurocognitive disorders stages during each 2-year period.
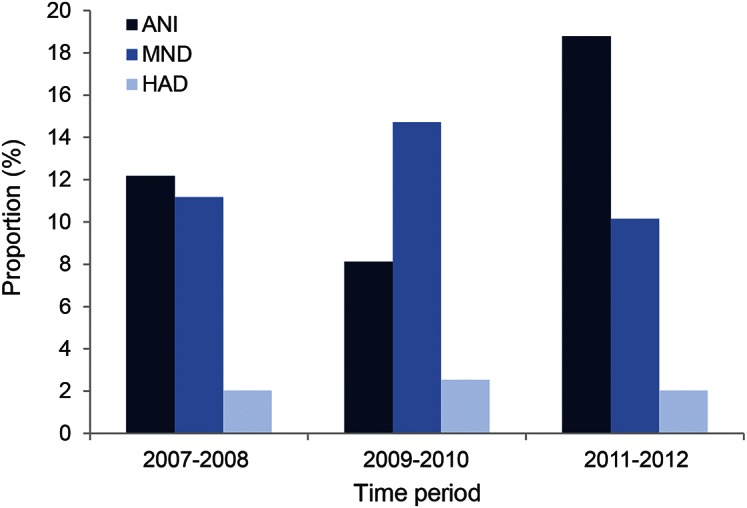
ANI = asymptomatic neurocognitive impairment; HAD = HIV dementia; MND = mild neurocognitive disorder.
Frequency of HAND stage transitions in individual HIV+ participants.
Between 2007–2008 and 2009–2010, 70% of the HIV+ individuals remained at their same HAND stage, with 15% showing a deterioration in HAND stage (e.g., ANI to MND or dementia), and 15% showing an improvement in HAND stage (e.g., MND to ANI). From 2007–2008 to 2011–2012, 77% of the HIV+ individuals remained at their same HAND stage, whereas 13% showed a deterioration in HAND stage and 10% showed an improvement in HAND stage. From 2009–2010 to 2011–2012, 71% of the HIV+ individuals remained at their same HAND stage, while 15% showed a worsening in HAND stage and 14% showed an improvement in HAND stage.
Frequency of HAND stratified by age.
For all 364 HIV+ individuals evaluated for HAND in 2007–2008, the overall frequencies of HAND when stratified by each decade of life in HIV+ individuals in the age ranges of 20–29 years (n = 16), 30–39 years (n = 48), 40–49 years (n = 150), 50–59 years (n = 122), and 60–69 years (n = 26) were 31%, 31%, 36%, 29%, and 27%, respectively, suggesting no increased frequency of HAND with each advancing decade. However, the overall frequencies of HAD when stratified by each decade of life increased from 0% (20–29 years) and 2% (30–39 years) to 5% (40–49 years), 6% (50–59 years), and 8% (60–69 years), respectively.
At the 2007–2008 time periods, 41% of HIV+ individuals were <50 years of age, and 59% of HIV+ individuals were ≥50 years of age. The overall frequencies of all HAND classification for HIV+ individuals <50 years of age during the 2007–2008 period, 2009–2010 period, and 2011–2012 period were 34%, 31%, and 31%, respectively. The overall frequencies of HAND for HIV+ individuals ≥50 years of age during the 2007–2008 period were 28%, 27%, and 28%, respectively. There was no significant change in the overall frequency of HAND over this 4-year period for either HIV+ individuals <50 years of age or for HIV+ individuals ≥50 years of age. There was also no difference in the frequency of ANI, MND, or HAD across the 3 time periods for either HIV+ individuals <50 years of age or HIV+ individuals ≥50 years of age.
Risk factors for HAND stage progression.
Demographic risk factors for HAND stage progression from either 2007–2008 to 2009–2010 or from 2007–2008 to 2011–2012 were examined. A diagnosis of hypercholesterolemia was associated with an increased risk for worsening HAND stage (OR 2.8 [CI 1.3–5.9] [p = 0.01]). A total of 32% of HIV+ individuals with a history of hypercholesterolemia were on statin therapy. There was no association of age, education (8th grade or less vs above 8th grade), duration of HIV infection, CD4 cell count, nadir CD4, quantitated plasma HIV RNA, detectability of plasma HIV RNA, type of cART (protease inhibitor vs non-nucleoside reverse transcriptase inhibitor–based cART), site of evaluation, hepatitis C coinfection, or diagnosis of hypertension or diabetes and risk of HAND stage progression.
DISCUSSION
The results from this study suggest that HAND is common in HIV+ individuals, mostly on cART with effective systemic virologic suppression. The frequency of HAND was 25%–33% from 2007 to 2012. This frequency of HAND is somewhat lower than the estimates for HAND in other large cohort studies that report a HAND prevalence of 47% excluding severely confounded cases.9 Our study excluded additional potentially contributing confounders for cognitive impairment and that could account for the lower prevalence rate of HAND. Demographic differences between the MACS and CHARTER cohorts (e.g., the MACS cohort has a higher mean education and the CHARTER cohort includes both men and women) could also account for these differences.
Unlike the pre-cART era, when HIV-associated cognitive impairment was a progressive condition within months, the diagnosis of MND and HAD is not progressive over a 4-year period in individuals with systemic virologic suppression for the majority (70%) of HIV+ individuals. These results are consistent with a prior study in the MACS cohort showing longitudinally preserved psychomotor speed performance in long-term asymptomatic HIV+ individuals with controlled HIV viremia over a 5-year period.10 An increase in the frequency of ANI was noted in 2011–2012. It remains to be determined whether this increased frequency of ANI persists in subsequent years, and whether this increased frequency is due to HIV itself or age-related comorbid conditions unrelated to HIV infection.
A study in the CHARTER cohort found that a diagnosis of ANI was associated with an increased risk for progression to MND or HAD compared to HIV+ individuals with normal cognition.11 In our study, progression from ANI to either a MND or HAD stage was seen in 7/24 (29%) cases in HIV+ individuals over 4 years of follow-up. In contrast, 14/24 (58%) HIV+ individuals remained at ANI over 4 years, whereas 3/24 (13%) HIV+ individuals improved from ANI to normal cognition. Also, 18/147 (12%) HIV+ individuals with normal cognition progressed to MND or HAD. Thus, a diagnosis of ANI was associated with a 2-fold increased risk of symptomatic HAND (MND or HAD) compared to a diagnosis of normal cognition in HIV+ individuals, which is similar to the results in the CHARTER cohort. The results from the MACS suggest that the majority of HIV+ individuals with ANI stay at the same HAND stage over a 4-year time period and that progression to a more severe HAND stage is only slightly more common than improvement from ANI to normal cognition.
Another longitudinal study from the CHARTER cohort12 examining neurocognitive decline found that 61% of participants in CHARTER remained stable, 23% declined, and 17% improved over a mean time period of 35 months. These proportions are similar to the 77% of HIV+ individuals who remained stable, 13% who declined, and 10% who showed improvement in HAND stage over 4 years in our study.
Age was associated with increased risk of HAD, but age was not associated with an increased risk for all stages of HAND, and age was not associated with an increased risk of progression in HAND stage. These results suggest that age-related factors may play an important role in advanced stages of HAND (HAD) but may have less of a role in milder stages of HAND such as ANI. However, the proportion of HIV+ individuals ≥60 years of age was small. Further studies are needed in cohorts with more HIV+ individuals ≥60 years of age to evaluate the impact of age on HAND.
Even though HAND appears to be a stable clinical diagnosis for the majority of HIV+ individuals in this study (77%), there could still be ongoing CNS damage. Indeed, neuroimaging studies within the MACS suggest that gray matter and white matter atrophy,13 as well as subcortical atrophy in the caudate and putamen, occur in HIV+ individuals with well-controlled immune status and systemic viral replication.14 Other studies using CSF markers of inflammation such as neopterin15 and markers of active axonal injury16 also suggest ongoing CNS injury in HIV+ patients on cART. Thus, a clinical diagnosis of HAND may not be as sensitive as neuroimaging or CSF markers of CNS injury.
In a subset of individuals, approximately 13% showed clinical progression to more severe HAND. Risk factors for deterioration in clinical stage included hypercholesterolemia, suggesting that cerebrovascular disease could be contributing to some of the cognitive impairment seen in these HIV+ individuals. Genetic factors, variability in test measurements, or concomitant age-related contributors to CNS injury could lead to cognitive deterioration in these HIV+ individuals.
Several limitations should be noted. The MACS included gay/bisexual men and our results for HAND prevalence may not be applicable to other demographic groups of HIV+ individuals, especially women. Functional assessments (i.e., IADLs scale) were not performed in the MACS prior to 2007, so a diagnosis of HAND could not be applied prior to that time. Another limitation is that functional assessments other than the IADL scale are not available in the MACS to define functional impairment in the algorithm. Many of the MACS participants have seen the full neuropsychological test battery on multiple occasions. Thus, practice effects could have reduced the overall rate of prevalence of impairment or rate of neurocognitive progression. In addition, survivorship bias and loss to follow-up, which may be increased in HIV+ individuals with cognitive impairment, may result in an underestimation of the prevalence of HAND in the MACS.17 Indeed, HIV+ individuals who did not attend all 3 time periods had an increased frequency of HAND in 2007–2008 compared to HIV+ individuals who did attend all 3 periods, and these HIV+ individuals may have been the most likely individuals to deteriorate in their HAND stage. In addition, although an attempt was made to exclude HIV+ individuals with contributing confounding conditions, cognitive impairment in the HAND cases could have been from comorbid conditions such as hypertension or diabetes.
Our study evaluated the temporal progression of HAND over a 4-year period. Future studies will need to evaluate longitudinal changes in HAND over a longer period of time to characterize the potential contribution of comorbid conditions as well as to determine whether markers of CNS injury observed in imaging studies are associated with changes in the clinical characteristics of HAND. Future studies also should evaluate performance and rates of progression in specific neurocognitive domains.
Supplementary Material
ACKNOWLEDGMENT
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI035042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Cynthia Munro, Michael W. Plankey, Ned Sacktor, Ola Selnes, James Sheppard, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O'Gorman, David Ostrow, Frank Palella, Ann Ragin; Los Angeles (U01-AI35040): University of California, UCLA School of Public Health and Medicine: Roger Detels (PI), Otoniel Martinez-Maza (Co-PI) Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-A135041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; and the Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Alvaro Munoz (Co-PI), Alison Abraham, Keri Athoff, Christopher Cox, Jennifer Deal, Gypsyamber D'Sousa, Priya Duggal, Janet Schollenberger, Eric C. Seaberg, Sol Su, and Pamela Surkan. The authors thank Sandra Reynolds for acquisition of study data and Marie Sonderman for administrative assistance.
GLOSSARY
- ANI
asymptomatic neurocognitive impairment
- cART
combination antiretroviral therapy
- CHARTER
CNS HIV Anti-Retroviral Therapy Effects Research
- CI
confidence interval
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorders
- IADL
instrumental activities of daily living
- MACS
Multicenter AIDS Cohort Study
- MND
minor neurocognitive disorder
- OR
odds ratio
AUTHOR CONTRIBUTIONS
N. Sacktor was responsible for the conceptualization and design of the study, including analysis and interpretation of data, acquisition of study data, and drafted the manuscript. R.L. Skolasky assisted with analysis and interpretation of study data and revising the manuscript. E. Seaberg assisted with analysis and interpretation of study data and revising the manuscript. C. Munro assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. J. Becker assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. E. Martin assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. A. Ragin assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. A. Levine assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. E. Miller assisted with conceptualization and design of the study, acquisition of study data, and revising the manuscript. N. Sacktor, R.L. Skolasky, E. Seaberg, C Munro, J. Becker, E. Martin, A. Ragin, and A. Levine report no disclosures. E. Miller is the author of the CALCAP reaction time program used in this study and has a financial interest in the program.
STUDY FUNDING
The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) with additional cofunding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung and Blood Institute (NHLBI) and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Web site is located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH. The study was supported by the NIH (AI035042, MH075673, and NS081196).
DISCLOSURE
N. Sacktor, R. Skolasky, E. Seaberg, C. Munro, J. Becker, E. Martin, A. Ragin, and A. Levine report no disclosures relevant to the manuscript. E. Miller is the author of the reaction time software used in this study (CalCAP) and has a financial interest in the software. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 2003;17:1539–1545. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001;56:257–260. [DOI] [PubMed] [Google Scholar]
- 5.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 6.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004;26:759–778. [DOI] [PubMed] [Google Scholar]
- 7.Berg BA, Hsu HP. Metropolis simulations of Met-Enkephalin with solvent-accessible area parametrizations. Phys Rev E Stat Nonlin Soft Matter Phys 2004;69:026703. [DOI] [PubMed] [Google Scholar]
- 8.Fishman GS. Monte Carlo: Concepts, Algorithms, and Applications. New York: Springer; 1995. [Google Scholar]
- 9.Heaton RK, Franklin DR, Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015;60:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole MA, Margolick JB, Cox C, et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology 2007;69:2213–2220. [DOI] [PubMed] [Google Scholar]
- 11.Grant I, Franklin DR, Jr, Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014;82:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011;5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JT, Maruca V, Kingsley LA, et al. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology 2012;54:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 2014;9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker JT, Kingsley LA, Molsberry S, et al. Cohort profile: recruitment cohorts in the neuropsychological substudy of the multicenter AIDS cohort study. Int J Epidemiol Epub 2014 Apr 4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.