Abstract
Hypertension is a highly prevalent condition with numerous health risks, and the incidence of hypertension is greatest among older adults. Traditional discussions of hypertension have largely focused on the risks for cardiovascular disease and associated events. However, there are a number of collateral effects, including risks for dementia, physical disability, and falls/fractures which are increasingly garnering attention in the hypertension literature. Several key mechanisms – including inflammation, oxidative stress, and endothelial dysfunction – are common to biologic aging and hypertension development and appear to have key mechanistic roles in the development of the cardiovascular and collateral risks of late-life hypertension. The objective of the present review is to highlight the multi-dimensional risks of hypertension among older adults and discuss potential strategies for treatment and future areas of research for improving overall care for older adults with hypertension.
Keywords: Cardiovascular, Falls, Disability, Cognition, Blood Pressure, Antihypertensive
Graphical Abstract
“Our great struggle in medicine these days is not just with ignorance and uncertainty. It's also with complexity: how much you have to make sure you have in your head and think about. There are a thousand ways things can go wrong.”
Atul Gawande(Gawande, 2009)
1. Introduction
Life expectancy continues to increase in developed countries worldwide (Roberts, 2011), leading to ever-increasing representation of older adults (i.e. persons over 65 years of age) within the population. In fact, life expectancy worldwide has increased by 20 years since 1950. In the United States, the number of older adults is expected to double to approximately 80 million in the next three decades (Federal Interagency Forum on Aging-Related Statistics, 2009). Given the dramatically disproportionate utilization of health care resources by older adults – e.g. nearly three quarters of cardiovascular disease (CVD)-related expenditures (Hodgson and Cohen, 1999) – the maintenance of health and well-being among older adults is a critical scientific and public health priority (Institute of Medicine, 2008; National Institute on Aging, 2007).
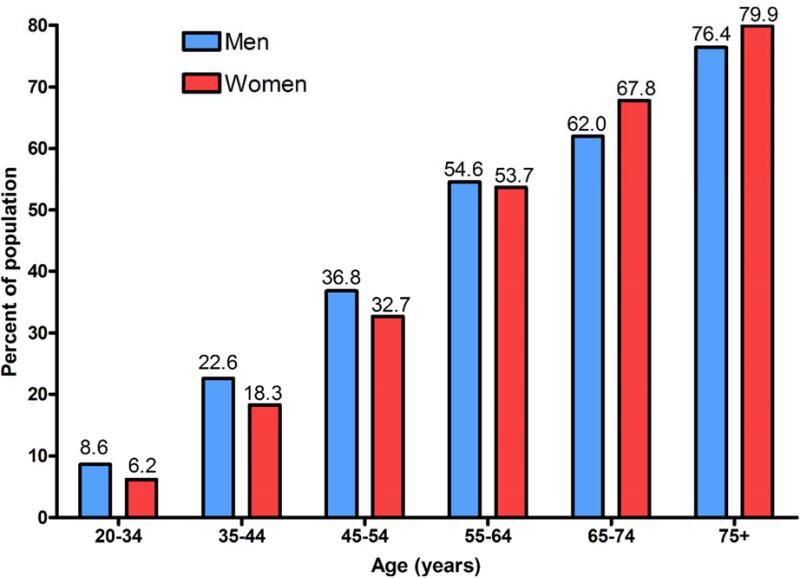
Among the potential targets for improving health among older adults, hypertension represents one of the most prevalent and potentially modifiable. Hypertension causes over 7 million premature deaths per year and contributes to 4.5% of the total disease burden worldwide (Bramlage and Hasford, 2009). Notably, older adults account for the bulk of hypertension-related morbidity and mortality – due largely to dramatically greater prevalence among the elderly (Mozaffarian et al., 2015). In fact, recent data from the National Health and Nutrition Examination Survey indicate that 70% of older adults have hypertension, compared to only 32% for adults aged 40-59 years (Figure 1) (Mozaffarian et al., 2015). Despite the well-documented pervasiveness of late-life hypertension among older adults, many challenges remain. A 2010 report from the Institute of Medicine (IOM) called hypertension a neglected disease that is often ignored by the general public and underappreciated by the medical community (Institute of Medicine, 2010). “Although hypertension is relatively easy to prevent, simple to diagnose, and relatively inexpensive to treat, it remains the second leading cause of death among Americans, and as such should rightly be called a neglected disease”, said David W. Fleming, MD, chair of the committee that prepared the report (Mitka, 2010). Proper screening and adherence to treatment guidelines was particularly emphasized for elderly patients.
Figure 1.
Prevalence of hypertension among adults by age and sex according to the National Health and Nutrition Examination Survey: 2007-2012. Re-created from Chart 9.1 in Mozaffarian et al.(Mozaffarian et al., 2015)
Since the release of the IOM report, a number of large clinical trials, systematic reviews, and meta-analyses have been published focusing on proper treatment for patients with hypertension. However, relatively few of these have focused on the treatment of hypertension among older adults (Goeres et al., 2014). This relative paucity of data in this area may be related to the common practice of excluding older adults from randomized controlled trials (RCTs) due to concerns about safety and/or confounding effects of co-morbid conditions (Pahor and Cesari, 2012; Van Spall et al., 2007). Indeed, numerous challenges exist in the treatment of hypertension among older adults – including and altered drug metabolism (Belmin et al., 2000), multiple concomitant medications and co-morbidities (Benetos et al., 2015), as well as increased blood pressure variability and orthostatic hypotension (Sera and McPherson, 2012) – that make it difficult to obtain definitive evidence of proper treatment guidelines. Still, it is these challenges which make it critical to conduct studies which will improve medical decision making related to the treatment of hypertension among older adults.
Furthermore, RCTs have primarily focused on cardiovascular effects of hypertension treatment despite well-documented links between late-life hypertension and other health outcomes relevant to older adults including physical function, bone health, and cognition. It is critical that we improve our understanding of how hypertension treatment influences such geriatric outcomes as they, like cardiovascular disease, dramatically influence risks for hospitalization, morbidity, and mortality (Ray et al., 1997; Sachs et al., 2011; Studenski et al., 2011). Decision making in treating hypertensive older adults is further convoluted by the fact that these varied health outcomes are not independent but often have similar etiologies and may interact to exacerbate the progression of one another. It is this complexity that makes it necessary to view geriatric hypertension decision making not from the perspective of a single outcome (e.g. CVD, dementia) but rather using a holistic approach that incorporates the individual characteristics of each patient and uses a broad lens to assess health. The objective of this review is to therefore synthesize hypertension literature from several fields into a framework which integrates these distinct but overlapping fields.
2. Common Mechanisms of Aging and Hypertension – The Vascular Health Triad
In humans, aging is a continual and progressive process that results in decreased physiologic function across all organ systems (Franceschi et al., 2008). These physiologic decrements result in an increased vulnerability to infection and disease which dramatically elevate mortality risk (Candore et al., 2006; Troen, 2003). In fact, compared to persons 25-44 years of age, mortality risk among older adults is elevated by 100-fold for stroke and chronic lung disease, roughly 90-fold for heart disease, pneumonia and influenza, and over 40-fold for cancer (Troen, 2003). As diverse as the etiologies of age-related diseases are, significant evidence implicates two interconnected mechanisms among the most common biologic contributors to age-related disease: 1) chronic, low-grade inflammation (Cevenini et al., 2010; Chung et al., 2009; Singh and Newman, 2011; Vasto et al., 2007) and 2) increased cellular oxidative stress (Chen et al., 2007; Harman, 1956; Valko et al., 2007; Yu and Yang, 1996).
Inflammation is a localized response to tissue injury or infection which aids in the repair of damaged tissue and/or destruction of the harmful agent. Classically characterized by pain, heat, redness, swelling, and loss of function – acute inflammation is typically resolved in relatively short order to promote the restoration of tissue function. However, during advanced age, the ability to resolve inflammation becomes impaired leading to sustained tissue infiltration of leukocytes and the chronic release of pro-inflammatory cytokines and chemokines (Sarkar and Fisher, 2006). As a result, the initial local event has long-term systemic consequences. Though factors such as obesity and insulin resistance, smoking, and changes in circulating sex hormone concentrations are associated with age-related inflammation (Krabbe et al., 2004), increases in inflammatory mediators are thought to derive most directly from decreases in the efficiency of the immune system (i.e. immuno-senescence) (Chung et al., 2009; Vasto et al., 2007). Immuno-senescence is characterized by thymus atrophy, reductions in neutrophil function, naïve T cell number, and the cytotoxic capacity of natural killer cells, and lowered B-cell antibody production in response to antigen (Hawkley and Cacioppo, 2004; Phillips et al., 2007). The most widely held belief regarding the cause of immune-senescence is that chronic antigen burden over the course of a lifetime exhausts a finite capacity of the immune system (Buford and Willoughby, 2008; De Martinis et al., 2007). As a result, we trade the long-term risk of chronic inflammation and disease for life-long protection against infection and injury (Wick et al., 2003).
Inflammation is the most consistently documented biological feature of aging, though it remains unknown whether inflammatory mediators directly cause adverse health outcomes. The inflammatory biomarkers most consistently associated with aging are elevated circulating concentrations of interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α) (Singh and Newman, 2011). Notably, these markers increase during aging even in the absence of acute infection (Ershler et al., 1993; Fagiolo et al., 1993; Wei et al., 1992) and have been associated with the prevalence of a wide-range of age-related co-morbidities – cardiovascular disease (Cesari et al., 2003; Cesari et al., 2003; Tracy et al., 1997), insulin resistance and diabetes (Bertoni et al., 2010; Pickup et al., 2000; Pradhan et al., 2001), osteoporosis (Ding et al., 2008; Khosla et al., 1994; Zheng et al., 1997), cognitive decline and dementia (Engelhart et al., 2004; Weaver et al., 2002; Yaffe et al., 2003), frailty and disability (Ershler and Keller, 2000; Ferrucci et al., 2002; Walston et al., 2002), and cancer (Aggarwal et al., 2006; Il'yasova et al., 2005; Lu et al., 2006) – as well as mortality (Harris et al., 1999; Newman et al., 2009; Roubenoff et al., 2003).
Though a conclusive causal relationship between inflammation and age-related conditions has not been firmly established, one potential supportive link is the contribution of inflammation to intracellular oxidative stress. In addition to producing pro-inflammatory cytokines, tissue-infiltrating leukocytes such as neutrophils and macrophages produce reactive oxygen species (ROS) [e.g. superoxide (O2–) and hydrogen peroxide (H2O2)] to kill pathogens (Crowley, 2014). Under homeostatic conditions, ROS and other free radicals (i.e. reactive nitrogen species; RNS) are necessary intracellular signaling molecules involved in ongoing reduction-oxidation (redox) reactions that occur as a part of normal metabolic processes (Brown and Griendling, 2009; Gillespie et al., 2009). However, chronic elevations in inflammatory mediators during late life contribute to a deleterious chronic overproduction of ROS (Dinh et al., 2014). Coupled with aged-related declines in nitric oxide (NO) production and bioavailability (Torregrossa et al., 2011), these increases in ROS formation contribute to an imbalance between the production and breakdown of ROS (i.e. oxidative stress) which leads to damage of cellular proteins and organelles. It is this chronic cellular damage and dysfunction that is thought to at least partially contribute to physiologic dysfunction and the development of age-related disease.
Notably, though chronic inflammation is a driver of oxidative stress, several other age-related changes – including mitochondrial dysfunction (Kujoth et al., 2005; Pang et al., 2008) and decrease in the efficiency of various endogenous antioxidant defense systems (Ji et al., 1998; Pinzani et al., 1997) – contribute to the development of oxidative stress in late life. Moreover, chronic oxidative stress also reflexively stimulates inflammation at least partially through the induction of the transcription factor nuclear factor kappa B (NF-kB) and the subsequent biosynthesis of prostaglandins via cyclooxygenase (COX) activity (Baek et al., 2001; Chung et al., 1999). Thus, though elevations in inflammation and oxidative stress stem at least partially from distinct age-related physiologic changes, they are also intricately linked as elevations in one induce concordant increases in the other (Kim et al., 2006; Sarkar and Fisher, 2006) – sometimes referred to as a “vicious cycle” (Chung et al., 2009).
In addition to their role in the aging process, inflammation and oxidative stress have also each been associated with hypertension. Several studies have demonstrated higher plasma levels of CRP (Bautista et al., 2004; Stuveling et al., 2004; Sung et al., 2003), IL-6 (Bautista et al., 2005; Chae et al., 2001; Fernandez-Real et al., 2001), TNF-α (Bautista et al., 2005; Furumoto et al., 2002; Yu et al., 2010), and IL-1β (Dalekos et al., 1997; Zhao et al., 2004) in hypertensive patients compared to normotensive peers. The association between oxidative stress and hypertension is less substantiated but this could be partially due to challenges in measuring in vivo oxidative stress in humans. However, clinical and pre-clinical evidence also indicates that vascular O2– production and systemic oxidative stress are present prior to substantial elevations in blood pressure and may contribute to the transition from prehypertension to hypertension (Lacy et al., 1998; Lacy et al., 2000; Nabha et al., 2005). Animal studies have documented increased production of vascular, renal, cardiac, and neural production of O2– and H2O2 production in Ang II-induced hypertension(Briones and Touyz, 2010) and oxidative stress in salt-sensitive forms of hypertension (Callera et al., 2003; Lassegue and Clempus, 2003). Meanwhile, clinical evidence previously indicated elevations in nonspecific markers of oxidative injury in plasma and urine from middle-aged, hypertensive patients (Ward et al., 2004) and elevated O2– and H2O2 concentrations in vascular smooth muscle cells from patients with essential hypertension (Touyz and Schiffrin, 2001).
Notably, with evidence suggesting potential bi-directional relationship whereby these mechanisms both contribute to and are exacerbated by hypertension (Briones and Touyz, 2010; Dinh et al., 2014; Rubio-Ruiz et al., 2014; Wadley et al., 2013). For example, common secondary causes of hypertension such as obstructive sleep apnea, chronic kidney disease, and renal artery stenosis are all associated with inflammation and highly prevalent in the elderly (Rafey, 2009). These conditions are thought to stem at least partially from sympathetic nervous system (SNS) activation – a common feature of hypertension (Guyenet, 2006). Increased sympathetic drive to the kidneys causes the release of renin and subsequently raises blood pressure (Bunag et al., 1966). Such increases in SNS activity have been demonstrated in response to elevations in both central (Zhang et al., 2010) and systemic (Yu et al., 2010) inflammation. Meanwhile, most immune cells express catecholamine receptors (Nance and Sanders, 2007) – facilitating the reciprocal stimulation of inflammation by the SNS demonstrated in pre-clinical studies (Lob et al., 2010; Veelken et al., 2008).
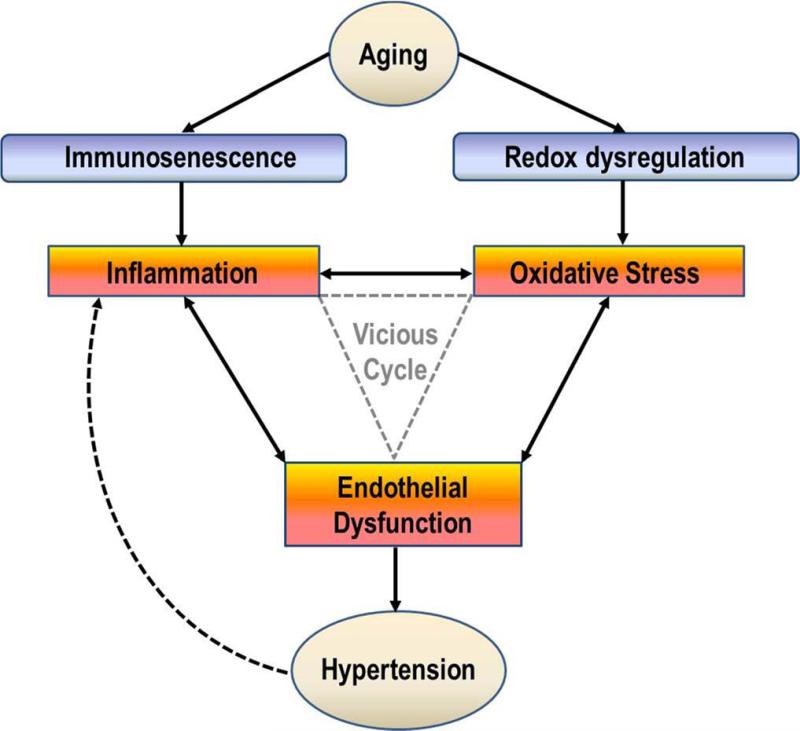
Presently, the strongest link between inflammation/oxidative stress and hypertension appears to be vascular dysfunction (Briones and Touyz, 2010; Dinh et al., 2014; Rubio-Ruiz et al., 2014; Wadley et al., 2013). In fact, the relationships among these three biological mechanisms have been termed the “Vascular Health Triad” (Figure 2) which has been implicated separately in both aging (Wadley et al., 2013) and hypertension (Dinh et al., 2014). Indeed, inflammation and oxidative stress have been consistently documented as contributors to endothelial dysfunction (Brinkley et al., 2009; Donato et al., 2007; Pierce et al., 2009; Rodriguez-Manas et al., 2009). For example, increases in free radical formation cause deterioration of the NO cascade, alter and activate prostaglandin metabolism, and promotes novel oxidative posttranslational protein modifications that interfere with vascular and cell signaling pathways (Rubio-Ruiz et al., 2014). Endothelial dysfunction develops in response these changes and contributes directly to increased systemic vascular resistance, and therefore increased blood pressure, due to an imbalance between vasodilatory and vasocontrictory substances (Chrissobolis et al., 2011). Thus, endothelial dysfunction contributes directly to the pathogenesis of hypertension and associated health effects. However, endothelial dysfunction also contributes to further exacerbating inflammation and oxidative stress, creating an even larger vicious cycle (Dinh et al., 2014; Wadley et al., 2013). Healthy endothelium exerts anti-inflammatory effects such as NO-dependent inhibition of leukocyte adhesion (Kubes et al., 1991). When NO is released it also causes smooth muscle relaxation and subsequent vasodilation (Chrissobolis et al., 2011). However, in response to aging and hypertension, the endothelium also releases other vasoactive substances such as endothelin-1 (ET-1), angiotensin II (Ang II), and COX-derived prostanoid and superoxide anions which contribute to impaired endothelium-dependent vasodilation (Rubio-Ruiz et al., 2014). Moreover, NO also tends to react with oxidants, particularly O2– to form the potent free radical peroxynitrite (ONOO–) which removes NO from the endothelium thereby reducing the vasodilatory capacity of the vessel (Squadrito and Pryor, 1995). Thus, because of chronic elevations in O2–, NO-dependent vasodilation is impaired – further exacerbating the vicious cycle.
Figure 2.
Simplified schematic illustrating common relationships of aging and hypertension with inflammation, oxidative stress, and vascular dysfunction – i.e. Vascular Health Triad. Modified from Figure 2 in Wadley et al.(Wadley et al., 2013) (Figure 2) and Figure 1 in Dinh et al.(Dinh et al., 2014)
3. Collateral Health Risks among Older Adults with Hypertension
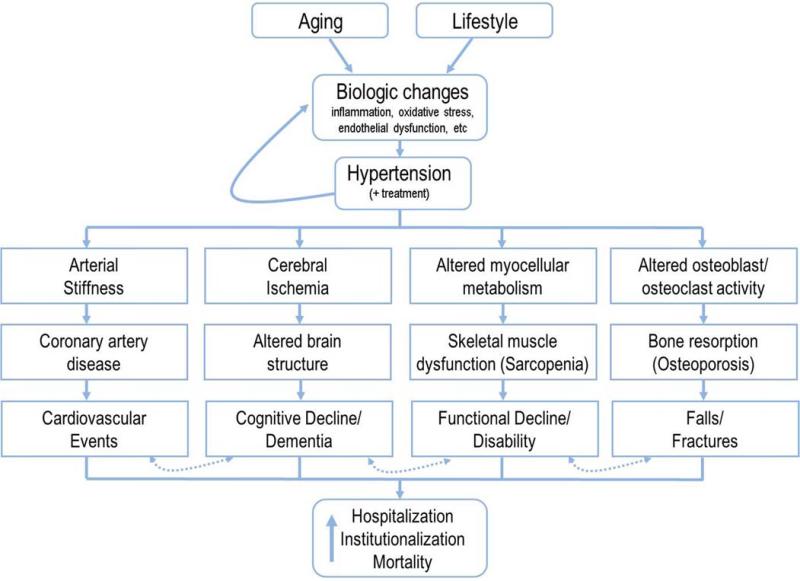
Traditional discussions of the Vascular Health Triad and hypertension have largely focused on the risks for cardiovascular disease and associated events. Indeed, it is well established that cardiovascular events are a critical risk for older adults with hypertension and reviews on this topic can be found elsewhere (Goeres et al., 2014; Rubio-Ruiz et al., 2014; Sun, 2015). However, there are a number of “collateral effects” (e.g. cognitive, musculoskeletal) which are increasingly garnering attention in the hypertension literature. These effects are particularly relevant to older adults given the independent effects of age on these outcomes. The following section outlines the evidence regarding the joint contributions of hypertension and aging to three such collateral effects including 1) declines in cognition and the development of dementia, 2) declining physical abilities (“functional decline”) and disablement, and 3) the incidence of falls and injurious fractures (Figure 3). It should be noted, however, that these collateral effects are not independent of one another as numerous cross-relationships exist. For example, cardiovascular burden is key predictor of both dementia (Duron and Hanon, 2008; Ihle-Hansen et al., 2015; Launer et al., 2000; Peters et al., 2013) and physical disability (Bootsma-van der Wiel et al., 2002; Hajjar et al., 2007; Iritani et al., 2014; Newman et al., 2006). Declining cognitive function is also strongly associated with both functional decline/disability (Carlson et al., 1999; Hajjar et al., 2009; Mielke et al., 2013; Watson et al., 2010) and the incidence of falls (Rubenstein, 2006; Segev-Jacubovski et al., 2011; Springer et al., 2006; Tinetti et al., 1988). Additionally, functional decline and falls share several etiological features and can reciprocally exacerbate risk for the other (Abellan van Kan et al., 2009; Gill et al., 2013; Tinetti et al., 1988; Tinetti and Williams, 1998). Thus, though we discuss each of the conditions separately here, the interdependence of these conditions highlights the tremendous health risks and importance of managing hypertension in the elderly.
Figure 3.
Simplified schematic of the multi-dimensional health risks for older adults with hypertension. Note the dashed arrows indicating the inter-related nature of health outcomes whereby the presence of one condition tends to increase risk of one or more of the others.
3.1 Cognitive Decline and Dementia
Among age-related conditions, perhaps none are more concerning or disheartening to both the individual and loved ones than the loss of memory and (in some cases) subsequent onset of dementia (Riley et al., 2014; Wikler et al., 2013). Dementia, of which Alzheimer's disease (AD) is the most common form, is a potentially debilitating condition which negatively impacts quality of life for both affected patients and caregivers and has tremendous associated healthcare costs (Boustani et al., 2007). Because of the increases in life expectancy, the number of people with dementia worldwide is expected to increase to over 80 million by the year 2040 (Duron and Hanon, 2008). Despite the tremendous public health burden expected from dementia incidence, these statistics underestimate the potential consequences of age-related declines in cognition. Mild cognitive impairment (MCI), an intermediate stage between normal cognitive function and dementia (Petersen et al., 2001), is about four-times as prevalent as dementia (DeCarli, 2003). MCI is typically associated with either memory impairments or deficits in cognitive and/or motor performance (Gauthier et al., 2006; Kluger et al., 2008; Petersen et al., 1999). Importantly, MCI is associated with an increased risk of dementia (DeCarli, 2003; Gauthier et al., 2006; Ward et al., 2012) and is a strong predictor of excess mortality (i.e. premature death)(Bennett et al., 2002; Contador et al., 2014; Sachs et al., 2011). Recognition of these consequences has led to a growing awareness of the need to treat MCI among older adults (Eshkoor et al., 2015; Langa and Levine, 2014).
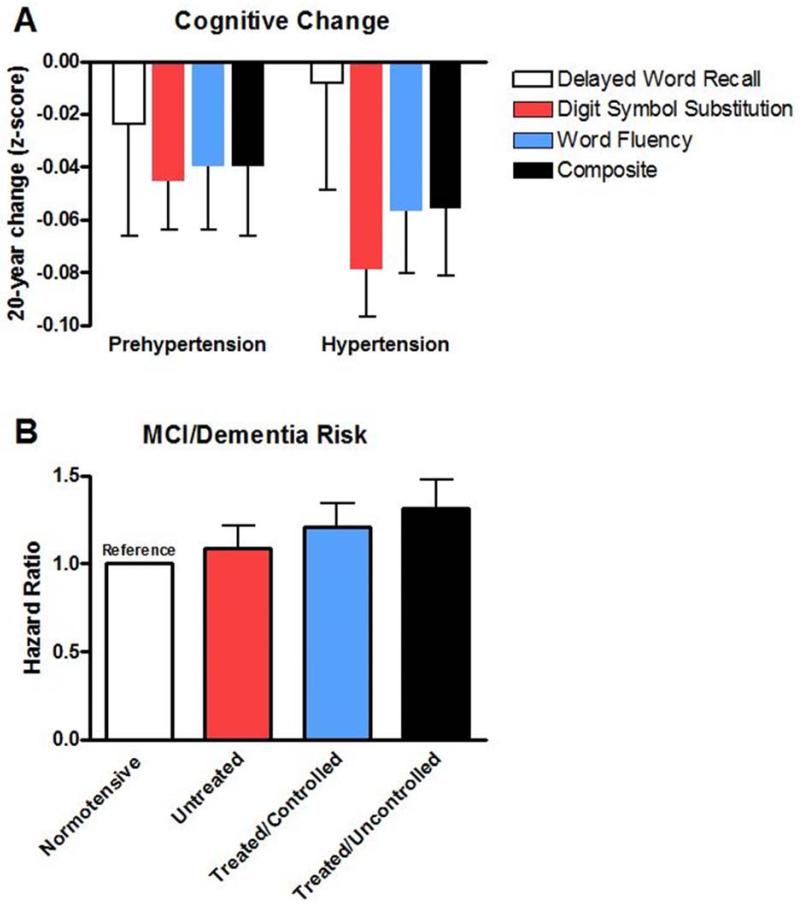
In recent years, hypertension has become increasingly recognized as an important risk factor for the development of MCI and dementia (Cherubini et al., 2010; DeCarli, 2015; Hanon and Forette, 2005; Power et al., 2011). Though cognition may be affected by hypertension through increased risk for stroke and subsequent post-stroke cognitive decline (Ihle-Hansen et al., 2015), hypertension appears to also have a distinct association with cognitive decline independent of stroke incidence. For example, Haring et al.(Haring et al., 2015) recently reported findings from over 6,000 women aged 65-79 indicating that hypertension was associated with an increased risk of MCI or probable dementia (HR = 1.20) and risk was highest among those with blood pressure ≥ 140/90 (HR = 1.30; Figure 4). Though the biologic source of such associations has not been completely refined, the relationship is thought by many to center around the development of “vascular dementia” whereby age- and hypertension-related changes in the cerebral vasculature impair cerebral perfusion and induce tissue damage – particularly in the white matter (Kim et al., 2015; McEvoy et al., 2015). Excellent reviews on this specific topic are available elsewhere (Cherubini et al., 2010; Ritter and Pillai, 2015; Venkat et al., 2015).
Figure 4.
Risks of cognitive decline and development of mild cognitive impairment (MCI)/dementia among hypertensive older adults. Panel A): Change in objective measures of cognition over 20 years among pre-hypertensive and hypertensive persons aged 48-67 years at baseline. Created from data in Table 2 of Gottesman et al.(Gottesman et al., 2014) (N = 13, 476) and indicate z-score changes relative to normotensive individuals. A z-score change of 0 indicates similar changes to normotensive individuals. Panel B): Risk of developing MCI or dementia over a mean follow-up of 9.1 years among 6,426 women aged 65-79 years. Created from data in Table 1 of Haring et al.(Haring et al., 2015). Hazard ratio (HR) indicates risk of experiencing MCI/dementia compared to normotensive individuals where each 0.1 increase in HR indicates a 10% increase in risk.
To date, mid-life hypertension appears to be even more strongly associated with the development of MCI/dementia than late-life hypertension (Freitag et al., 2006; Kivipelto et al., 2001; Launer et al., 1995; Launer et al., 2000). In a recent study from the Atherosclerosis Risk in Communities cohort, Gottesman et al.(Gottesman et al., 2014) reported findings more than 15,000 individuals (mean age of 56 years old at baseline) followed for 20 years indicating a modest but significant association between systolic blood pressure (SBP) at baseline and rate of cognitive decline (Figure 4). When adjusted for mortality, hypertension increased risk of cognitive decline by nearly 70%. Interestingly, this study reported greater cognitive declines with higher midlife blood pressure among Caucasians than African Americans. This observation is in contrast to recent reports from the National Health and Nutrition Examination Survey (NHANES) indicating a higher burden of cognitive limitations among hypertensive African Americans than Caucasians (Hajjar et al., 2015). These differences may be due to the fact that the latter study included a wider range of participants (20 years and older) and utilized subjective reports of cognitive limitations rather than objective measures of cognition. Future studies are likely needed to tease out potential racial differences in the effects of hypertension on age-related cognition and the development of MCI/dementia.
In recent years, appreciation has increased for the role of lifestyle-related factors relevant to the progression of hypertension (e.g. diet and exercise) in the prevention of onset and progression of dementia. For instance, a high-fat diet and commonly associated consequences (e.g. obesity, insulin resistance) are thought to contribute to dementia development largely through the processes of the Vascular Health Triad (Freeman et al., 2014) possibly resulting in disruption of the blood-brain barrier (Hsu and Kanoski, 2014). Other studies have proposed potential roles for intake of dietary sodium (Fiocco et al., 2012; Haring et al., 2015) and antioxidants (Crichton et al., 2013), though these links are yet to be firmly substantiated. Still, several studies have reported that diets commonly recommended for hypertensive individuals – including a Mediterranean-style diet and the Dietary Approach to Stop Hypertension (DASH) diet – are associated with improved cognition and potential reductions in the incidences of MCI and dementia (Alles et al., 2012; Singh et al., 2014; Wengreen et al., 2013). The lack of physical regular exercise has also been associated with increased risk for cognitive decline (Vassilaki et al., 2015), and though not definitive, several studies suggest that exercise may aid in the maintenance of cognitive function and prevention of dementia (Aarsland et al., 2010; Hamer and Chida, 2009; Hindin and Zelinski, 2012; Kelly et al., 2014). Thus, though much remains to be evaluated in this area, it appears as if lifestyle behaviors typically recommended for the treatment of hypertension hold promise for not only cardiovascular benefits but possibly cognitive benefits as well.
3.2 Functional Decline and Physical Disability
The maintenance of one's physical capabilities during older age is an essential part of healthy aging. The capacity to perform basic physical functions is a central aspect of health-related quality of life (Muszalik et al., 2011) and a key predictor of hospitalization, surgical outcomes, and mortality (Afilalo et al., 2010; Dumurgier et al., 2009; Penninx et al., 2000; Studenski et al., 2011). Declines in basic physical functions such as walking are also strong predictors of future cardiovascular events. In fact, declines in walking speed are associated with incident stroke (McGinn et al., 2008), adverse outcomes following cardiac surgery (Afilalo et al., 2010), as well as cardiovascular mortality (Dumurgier et al., 2009; Newman et al., 2006; Studenski et al., 2011). Accordingly, maintenance of independent functioning is a critical factor in preserving the health and well-being of older adults. In the U.S., nearly half of the 37.3 million persons aged ≥ 65 years report having one or more physical limitations in performing essential daily tasks (Seeman et al., 2010). The physical disablement of older adults is also a central contributor to rising healthcare costs, accounting for a significant portion of the U.S.'s annual $300 billion in disability-related medical costs (US Department of Health and Human Services, 2005). This burden is also expected to dramatically rise in coming years given rising healthcare costs and the fact that older adults represent the fastest growing segment of the population (Federal Interagency Forum on Aging-Related Statistics, 2009; U.S. Census Bureau, ).
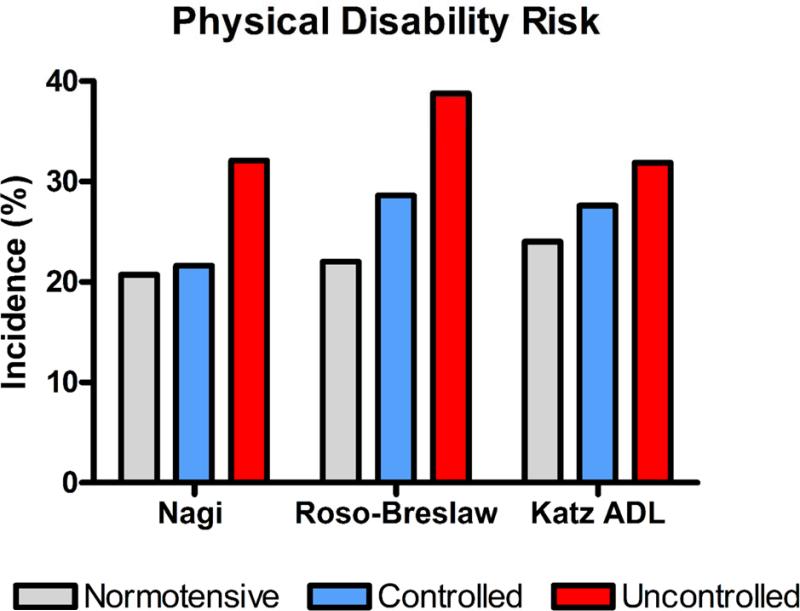
Compared to normotensive counterparts, older adults with hypertension experience accelerated declines in physical function and increased incidence of disability (Balzi et al., 2010; Dumurgier et al., 2010; Hajjar et al., 2007; Rosano et al., 2011). Among 999 older adults (68.5 ± SE = 0.2 years) enrolled in the Charleston Heart Study and evaluated between 1984 and 1993, higher systolic blood pressure was associated with increased risk of developing new disability.(Hajjar et al., 2007) According to three validated scales of disability, participants with hypertension had elevated risk of disablement (HR = 1.3) and those with uncontrolled hypertension were at greatest risk compared with normotensive participants (Figure 5). Similar results were observed among 897 older adults in the InCHIANTI study, an Italian population based study (Balzi et al., 2010). Over 3 years of follow up, hypertension was significantly associated (HR = 1.91) with worsening disability in the performance of activities of daily living (ADLs). Hypertension has also been strongly associated with accelerated declines in walking speed, a key indicator of functional status and recommended indicator of health and well-being among seniors (Abellan van Kan et al., 2009; Hall, 2006). Among 3604 older adults (65-85 years) enrolled in the Three-City study conducted in France, baseline walking speed was lower among hypertensive (1.51 ± 0.31 m/s) than non-hypertensive (1.59 ± 0.30 m/s) – a difference that is clinically meaningful based on established minimal clinically-meaningful difference of 0.05 m/s (Dumurgier et al., 2010). Moreover, during a mean follow-up of 7.0 ± 0.05 years, annual decline in walking speed was significantly greater among hypertensive (−2.30 ± 3.4 cm/s) than non-hypertensive (−1.87 ± 3.3 cm/s) individuals. Similar results were observed among 2,733 participants in the U.S.-based Cardiovascular Health Study as rates of decline in walking speed over 18 years were significantly greater among those with hypertension (~ Δ = 0.4 cm/s/yr) even after adjustment or numerous potentially confounding factors (Rosano et al., 2011).
Figure 5.
Risk of developing physical disability among older adults according to hypertension status, i.e. normotensive, controlled hypertension, or uncontrolled hypertension. Created from data in Table 3 of Hajjar et al.(Hajjar et al., 2007) Data reflect rates of disability based on three validated disability scales: the Nagi Scale, Rosow-Breslaw Scale, and Katz Activities of Daily Living (ADL) Scale.
Taken together, these data from large-scale cohort studies appear to indicate that hypertension is involved in accelerated functional decline and the development of physical disability. However, the mechanisms by which these changes occur are yet to be fully elucidated. Sarcopenia, the age-related decline in muscle mass and strength, may represent one possible mechanism through which hypertension accelerates functional declines – though this potential is presently speculative given the limited evidence in this area. Several studies from Asian populations have recently demonstrated a cross-sectional link between hypertension and sarcopenia (Han et al., 2014; Park et al., 2013; Wu et al., 2014), while a longitudinal cohort study in Finish individuals reported hypertension as a significant predictor of muscle strength decline over 22 years of follow-up (Stenholm et al., 2012). This potential acceleration of sarcopenia could result in response to hypertension-mediated increases in inflammation and oxidative stress (as discussed above) – key molecular contributors to the development and progression of sarcopenia (Buford et al., 2010; Marzetti et al., 2013; Roubenoff, 2003). Such changes may also be partly attributable to the common concomitant prevalence of insulin resistance and diabetes as these conditions are also associated with increased inflammation and oxidative stress (Liu et al., 2007; Monnier et al., 2006) as well as accelerated sarcopenia progression (O'Neill et al., 2010; Park et al., 2006; Sakkas et al., 2006).
Notably, altered function of the renin-angiotensin system (RAS) is a common identifying characteristic of both insulin resistance and hypertension (Henriksen and Prasannarong, 2013). Thus, RAS activity may be a key mechanistic contributor to functional decline in this population. Moreover, in addition to effects in mitigating hypertension, several antihypertensive drugs are also thought to have pleiotropic physiologic effects that extend beyond lowering blood pressure (Sica, 2011; Zoccali and Mallamaci, 2014). For instance, prior work has demonstrated the effects of angiotensin converting enzyme (ACE) inhibitors in endocrine signaling, regulation to tissue-specific oxidative stress and inflammation, and in the regulation of body composition (Carter et al., 2011; Giovannini et al., 2010; Marzetti et al., 2012). Epidemiological studies have also suggested that use of ACE inhibitors may indeed slow functional decline among seniors (Gambassi et al., 2000; Onder et al., 2002), though subsequent evidence suggests that these benefits may only exist when combined with physical exercise (Buford et al., 2012; Carter et al., 2012). However, future research is needed to fully elucidate the true role of ACE inhibitors and other antihypertensive medications in functional decline of hypertensive older adults.
3.3 Falls and Fractures
Among older adults, falls are the leading cause of both fatal and non-fatal injuries (Centers for Disease Control and Prevention). In fact, about 30% of community-living older persons fall each year and 20-30% of those who fall suffer moderate to severe injuries such as lacerations, fractures, or head trauma (Centers for Disease Control and Prevention, 2008; Nevitt et al., 1991; O'Loughlin et al., 1993; Sattin et al., 1990; Tinetti et al., 1995). In 2010, over 2.3 million nonfatal fall injuries were treated in U.S. emergency departments and over 662,000 of these patients were hospitalized (Centers for Disease Control and Prevention) – contributing to an estimated $28.2 billion annual healthcare burden related to falls (Centers for Disease Control and Prevention). The clinical burden on the patient is also quite significant as falls, particularly those causing injury, are independently associated with a subsequent decline in the ability to carry out important daily functions including bathing, dressing, shopping and housekeeping (Tinetti and Williams, 1997; Tinetti and Williams, 1998). Injurious falls also significantly increase risk of long-term nursing home admission (Gill et al., 2013; Tinetti and Williams, 1997; Tinetti and Williams, 1998), further adding to the tremendous importance fall prevention among older adults.
Hypertension – or more commonly the use of antihypertensive drugs to control hypertension – has been commonly proposed as a risk factor for falls and fractures. The acute administration of antihypertensive medications can produce significant hypotension in older adults which may threaten cerebral perfusion thereby causing dizziness, syncope, and falls (Pepersack et al., 2013; Rutan et al., 1992; Shannon et al., 1986; Slavachevsky et al., 2000). This mechanism may explain recent findings indicating from hundreds of thousands of Canadian older adults indicating that initiation of antihypertensive treatment significantly increases risk of both falls (Incident Risk Ratio [IRR] = 1.69] and hip fractures [IRR = 1.43] within the first 45 days of treatment (Butt et al., 2012; Butt et al., 2013). To our knowledge these are the strongest data indicating the risks of early antihypertensive treatment on falls and fractures in the elderly. However, these negative effects on cerebral perfusion are thought to be short-term and wane after prolonged drug administration (Pepersack et al., 2013; Rutan et al., 1992; Shannon et al., 1986; Slavachevsky et al., 2000). As a result, Lai and Laio reiterated the importance of a key principle for medication prescription for older adults: “start low and go slow” (Lai and Liao, 2013).
Rather than hypotension and syncope, the longer-term risk for falls and fractures among older adults may be more related to the development of osteoporosis (El-Bikai et al., 2015; Vestergaard et al., 2009). Prior studies have demonstrated that bone loss is positively associated with both systolic and diastolic blood pressure (Metz et al., 1999; Rejnmark et al., 2006). Persons with hypertension have increased urinary calcium excretion and increased mobilization of calcium from the bones (Cappuccio et al., 1999; Metz et al., 1999; Perez-Castrillon et al., 2005). These changes are thought to occur either as a result of increased blood volume (as sodium intake is also positively associated with calcium excretion (Cappuccio et al., 1999)) or as a result of a disorder within the kidney tubule (Ilic et al., 2013). Diminished intake of vitamins D and K, which are each important to calcium absorption (Ilic et al., 2013), may also play a role. In either case, decreased serum calcium stimulates parathyroid hormone which further increases bone turnover (Perez-Castrillon et al., 2005). Meanwhile, angiotensin II activity has also been reported to contribute to osteoporosis by activating osteoclasts while impairing osteoblast activity (Asaba et al., 2009; Li et al., 2014; Shimizu et al., 2008). These findings are thus in line with two studies indicating the benefits of angiotensin receptor II antagonists (ARBs) and ACE inhibitors in the preservation of bone mass among mice and rats (Shimizu et al., 2009; Zhao et al., 2014).
However, such a finding of a benefit to these drugs remains inconclusive among humans. In fact, the only firm conclusion regarding the effect of individual drug classes appears to be the contraindication of loop diuretics as they increase risks of both orthostatic hypotension and osteoporosis (Arnold and Shibao, 2013; Ghosh and Majumdar, 2014; Ilic et al., 2013). Moreover, the overall association of antihypertensive use with older adults’ risk of falls and fractures remains hotly debated. Numerous studies exist to both support (Butt et al., 2012; Butt et al., 2013; Choi et al., 2015; Tinetti et al., 2014) and refute (Gribbin et al., 2010; Lipsitz et al., 2015; Margolis et al., 2014; Peters et al., 2010; Thorell et al., 2014; Wong et al., 2013; Zang, 2013) the notion that antihypertensive use increases the risk of falls and fractures among older adults (Table 1). Numerous issues, including disparities between individuals included in observational studies versus clinical trials, treatment dose and duration, and patient age at time of medication initiation remain to be fully investigated to potentially reconcile the differences between these studies.
Table 1.
Selected studies of antihypertensive treatment and fall/fracture risk among hypertensive older adults
| Study | Study type | Sample size | Study Dates | Participants | Outcome | Results | Overall Effect on Fall/Fracture Risk |
|---|---|---|---|---|---|---|---|
| Gribbin et al. 2010(Gribbin et al., 2010) | Retrospective, matched case-control study | Cases = 9,682 Controls = 52,100 |
2003-2006 | British patients ≥ 60 yr: Cases = those with first recorded fall | Falls | Increased risk with thiazide diuretic prescription (aOR = 1.28) Decreased risk with BB prescription (aOR = 0.90) No other classes significantly associated |
↔ |
| Peters et al. 2010(Peters et al., 2010) | Randomized controlled trial | N = 3,845 | 2000-2007 (mean follow-up = 2.1 yr) | Hypertensive older adults 80+ yr (Multi-national) | Fracture | Treatment with a thiazide diuretic and ACEi did not increase and trended toward decreasing fracture risk (HR = 0.69) | ↔ |
| Butt et al. 2012(Butt et al., 2012) | Population-based, self-controlled case series | N = 310,591 | 2000-2009 | Community-dwelling, hypertensive Canadian elderly: 80.8 ± 7.3 yr | Fracture | 43% increased risk of hip fracture in first 45 days of treatment. Results consistent across drug classes but highest risk with ACEi (IRR = 1.54) and BB (IRR = 2.08) use. |
↑ |
| Butt et al. 2013(Butt et al., 2013) | Population-based, self-controlled case series | N = 543, 572 | 2000-2009 | Community-dwelling, hypertensive Canadian elderly: 80.8 ± 7.3 yr | Falls | 94% increased risk of fall during first 14 days of treatment; 69% increased risk in first 45 days. Generally consistent across drug classes. |
↑ |
| Wong et al. 2013(Wong et al., 2013) | Prospective cohort study | N = 520 | 2008-2009; 1-yr follow-up | Community-dwelling older adults from Sydney (AUS): 79.4 ± 4.4 yr | Falls | Antihypertensive drugs overall not associated with falls, though trend toward risk reduction (aOR = 0.69) Reduced risk of falls with ARB use (aOR = 0.68) |
↔ |
| Zang et al. 2013(Zang, 2013) | Systematic review and meta-analysis | 62 articles | 1975-2012 | Cohort and case-control studies: patient age 60+ years | Fall injuries | No significant association between antihypertensive use and fall injuries | ↔ |
| Thorell et al. 2014(Thorell et al., 2014) | Retrospective cohort study | N = 38, 407 | 2006-2007 | Swedish older adults (75+ year) | Fracture | No class of antihypertensive drug was associated with increased risk of hip fracture | ↔ |
| Tinetti et al. 2014(Tinetti et al., 2014) | Retrospective nationwide medical claim study | N = 4, 961 | 2004-2007 | Community-dwelling, hypertensive American elderly: 80.2 ± 6.8 yr | Serious fall injuries | Increased risk with both moderate (aHR = 1.40) and high (aHR = 1.28) levels of AHT use. No specific class associated with increased risk. |
↑ |
| Margolis et al. 2014(Margolis et al., 2014) | Randomized controlled trial | N = 3,099 | 2006+ (mean follow-up = 4.9 years) | Hypertensive adults (Mean = 62 years) with Type 2 diabetes (USA/Canada) | Falls/fractures | Intensive BP control not significantly different from standard control for risk of falls (RR = 0.84) and non-spine fractures (HR = 0.79) | ↔ |
| Choi et al. 2015(Choi et al., 2015) | Retrospective nationwide medical claim study | N = 528, 522 | 2007-2011 | Middle- and older-aged (50+ year), hypertensive Koreans | Fracture | Increased risk with all classes expect ARBs Highest fracture rate among AB (aHR = 2.26) and ACEi (aHR = 1.68) | ↑ |
| Lipsitz et al. 2015(Lipsitz et al., 2015) | Prospective observational study | N = 598 | Dates NR: 1-yr follow-up | Hypertensive older adults from Boston, MA (USA): 78.4 ± 5.4 yr | Falls | Overall AHT use not significantly associated with falls. CCB use associated with reduced risk of falls (aOR = 0.62). ACEi use associated with reduced risk of injurious falls (aOR = 0.62). |
↔ |
Abbreviations: aOR= adjusted odds ratio; HR = hazard ratio; IRR = incidence rate ratio; RR = relative risk; AB = alpha-blocker; BB = beta-blocker; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor antagonist; NR = not reported
4. Future Directions – Potential Points of Intervention
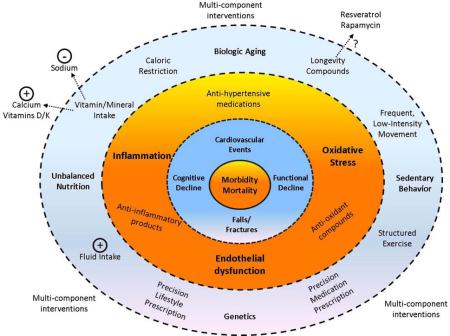
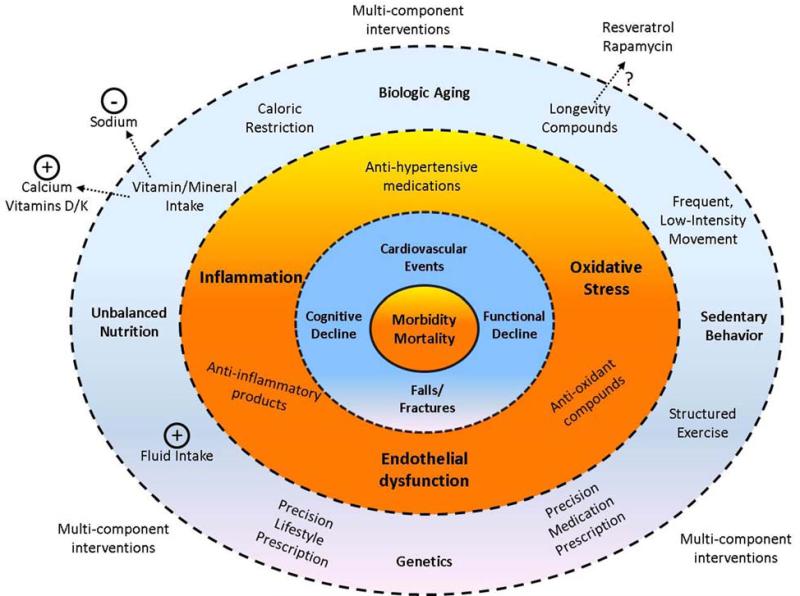
Given the multi-dimensional health risks of hypertension in late life, treatment strategies to fully mitigate each of these risks among hypertensive older adults may be quite diverse (Figure 6). Development of such a treatment approach may also require different considerations for older adults than for younger populations (Lee et al., 2013; Schwartz, 2015). Moreover, rather than simply treating blood pressure, therapies designed to treat the underlying biologic mechanisms (i.e. inflammation, oxidative stress, endothelial dysfunction) may aid in the prevention of adverse clinical outcomes among this population. Notably, because of the underlying contributions of aging to changes in these biologic functions, interventions thought to promote longevity and/or healthy aging may also have utility for older adults with hypertension. A number of potential “longevity interventions” have been investigated to date, with varying degrees of promise (de Cabo et al., 2014). Though more study is certainly needed related to the use of these interventions in the context of aging per se, future studies might consider hypertension as a potential model to investigate their potential utility for promoting healthy aging.
Figure 6.
Conceptual figure illustrating the potential points of intervention for the multi-dimensional risks of hypertension among older adults. Inner rings indicate the potential health risks of late-life hypertension; middle ring indicates mechanistic risk factors exacerbated by hypertension (bold) and potential interventions to combat these risks (non-bold); outer ring indicates concomitant risk factors which contribute to both hypertension and associated health outcomes (bold) as well as potential interventions to combat these risks (non-bold).
Treatment strategies for late-life hypertension may also need to increasingly encompass lifestyle-related factors. For instance, the benefits of exercise and physical activity are well known and include contributions to improved cardiovascular fitness, bone health, as well as physical and cognitive functions (Fiuza-Luces et al., 2013). Yet despite the well-known benefits of exercise and the fact that a clinical recommendation doubles the likelihood of meeting exercise guidelines, nearly one in five hypertensive patients do not receive such a recommendation from their healthcare provider (Mu et al., 2015). Moreover, many healthcare teams do not involve consultation with a dietician despite the fact that diet plays an important role in maintaining blood pressure and maintaining overall health. These areas may represent opportunities for improvement through the creation of more comprehensive healthcare teams.
Increasing evidence also indicates that consideration of a hypertensive patient's genetic profile could improve care. In 2007, the U.S. Congress passed the Genomic and Personalized Medicine Act with the intent of advancing this new field of medicine (Obama, 2007). The importance of this area was later reiterated by the Institute of Medicine (Institute of Medicine, 2009) and the NIH Director (Hamburg and Collins, 2010). In light of this mandate, scientists have increasingly elucidated genetic factors which influence patients’ responsiveness to antihypertensive and other cardiovascular medications (Cavallari et al., 2011; Cooper et al., 2008; Hindorff et al., 2008; Johnson et al., 2009; McDonough et al., 2013; Shuldiner et al., 2009; Thompson et al., 2005). Though the application of findings from pharmacogenomics studies in hypertension are yet to be widely implemented clinically, this possibility may exist in the not too distant future as such program have already launched within academic healthcare settings (Johnson et al., 2013; Shuldiner et al., 2013; Weitzel et al., 2014). Interestingly, genetics may also play a vital role in the efficacy of lifestyle-based interventions (e.g. diet and exercise) as well. For instance, a number of studies have reported that extreme variability exists in individual responses to exercise even under well-controlled, experimental conditions (Bouchard, 1995; Bouchard and Rankinen, 2001; Kohrt et al., 1991) and genetics appears to play an important role in determining the extent of these differences (An et al., 2003; Bouchard et al., 2011; Buford et al., 2014; Giaccaglia et al., 2008; Rankinen et al., 2012; Rice et al., 2002; Thompson et al., 2004). Similarly, several large-scale clinical trials (e.g. LOOK AHEAD, DASH, and VISP) have also identified genetic factors which contribute to determining responsiveness to dietary interventions (Hsu et al., 2011; McCaffery et al., 2012; Svetkey et al., 2011). Accordingly, though extensive research remains to be conducted in this area, genetic profiling may at some point aid in the prescription of both pharmaceutical and lifestyle-based interventions for the treatment of hypertension, even in late-life.
Despite the potential utility of each of the aforementioned approaches, the most promising interventions for treating the multiple risks of late-life hypertension may be those which incorporate multiple components. Increasing evidence indicates that clinicians and scientists should focus not only on drug-drug interactions (Hines and Murphy, 2011) but may also consider other potential interactions between treatments. For instance, exercise and prescription medications exhibit a variety of biologic interactions whereby one intervention can be critical to the proper prescription of the other (Lenz et al., 2004). In one direction, exercise has the ability to alter drug pharmacokinetics by altering hemodynamic and metabolic parameters (Lenz et al., 2004; Lenz, 2011). Conversely, many drug therapies have the ability to impact exercise performance – e.g. the attenuated heart rate response and decreased cellular oxygen intake induced by β-blockers (Kaiser et al., 1985; Tesch, 1985) or skeletal muscle weakness associated with statin use (Baer and Wortmann, 2007; Thompson et al., 2003). While these treatments may have negative interactions that must be considered, they may also prove to be beneficial as well – as evidenced by the earlier reference to potentially beneficial effects of antihypertensive medications and physical exercise on physical function (Buford et al., 2012). Thus, it will be important to continue to develop our collective understanding of interactions between not only medications and exercise but other combinations of interventions as well.
Finally, as with genetics, an increased consideration of biologic sex in intervention development may contribute to improved health outcomes among hypertensive older adults. As depicted in Figure 1, rates of hypertension among younger women are much lower than age-matched men but dramatically increase in late middle-age (Kearney et al., 2005; Wiinberg et al., 1995). This age-related increase in the prevalence of hypertension among women is attributable at least partially to the rapid decrease in sex hormones (e.g. estrogen) accompanying menopause (Maranon and Reckelhoff, 2013; Maric-Bilkan et al., 2014; Staessen et al., 1997). While the influence of menopause on increases in blood pressure is well-recognized, the mechanisms which underlie this change are still being elucidated. Presently, sex differences in several physiologic mechanisms including sympathetic nerve activity, cardiovascular hemodynamics, and immunomodulation/inflammation have been implicated as potential causal factors (Hart et al., 2012; Hay, 2016; Sandberg et al., 2015). Moreover, sex differences are also known to influence a variety of age-related physiologic changes in several tissues (e.g. brain, skeletal muscle, bone) which are relevant to collateral health outcomes among hypertensive older adults (Hay, 2016; Pendergrass et al., 2008; Puntus et al., 2011). Continued investigation is needed to fully elucidate the mechanisms which influence differential rates of hypertension among older adults and to determine if sex influences rates of dementia, physical disability, and falls/fractures among this population. Once these questions are answered, it may be necessary to develop differential treatment strategies for older men and women with hypertension. At minimum, sex may be at least more carefully considered as a relevant variable in the clinical decision making process for this population.
5. Conclusion
In conclusion, hypertension is a highly prevalent condition that dramatically rises in incidence with increasing age. Several key mechanisms, represented by the Vascular Health Triad, influence the development of cardiovascular and other “collateral” risks among older adults. Targeting these mechanisms, in addition to blood pressure control, may represent important points of intervention for preventing adverse health events in this growing population. Future research is needed to identify efficacious methods to prevent the variety of potential health events posed by hypertension in late life. The use of multi-component interventions and individual health characteristics appears to hold tremendous promise for substantially advancing treatment options according to the principles of precision medicine. Yet further research is still needed to evaluate the influence of relevant biologic characteristics – including genetics and biologic sex – on the efficacy of potential interventions. I look forward to witnessing continued advances in this area to hopefully reduce the number of older adults with hypertension and the overall health burden among those with the condition.
Highlights.
Hypertension is a highly prevalent condition with numerous health risks, and the incidence of hypertension is greatest among older adults.
In addition to cardiovascular risk, there are a number of collateral risks of hypertension among older adults including dementia, physical disability, and falls/fractures.
Several key mechanisms – including inflammation, oxidative stress, and endothelial dysfunction – are common to biologic aging and hypertension development and appear to have key mechanistic roles in the development of the cardiovascular and collateral risks of late-life hypertension.
This review highlights the multi-dimensional risks of hypertension among older adults and discuss potential strategies for treatment and future areas of research for improving overall care for older adults with hypertension.
Acknowledgments
I would like to acknowledge the litany of relevant literature related to this topic which I was unable to cite within the present review due to the breadth of scope. Scientists too numerous to count are to be commended for their important contributions to each of the aforementioned sub-fields related to hypertension and aging. Readers are also encouraged to consult the numerous excellent works cited here for extended details on any specific topic covered within the present review. This work was supported by grants from the American Heart Association (13SDG17080033) and National Institutes of Health (2P30AG028740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Sardahaee FS, Anderssen S, Ballard C, Alzheimer's Society Systematic Review group. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment.Health. 2010;14:386–395. doi: 10.1080/13607860903586136. [DOI] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J.Nutr.Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J.Am.Coll.Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem.Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Alles B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr.Res.Rev. 2012;25:207–222. doi: 10.1017/S0954422412000133. [DOI] [PubMed] [Google Scholar]
- An P, Borecki IB, Rankinen T, Perusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Evidence of major genes for exercise heart rate and blood pressure at baseline and in response to 20 weeks of endurance training: the HERITAGE family study. Int.J.Sports Med. 2003;24:492–498. doi: 10.1055/s-2003-42011. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr.Hypertens.Rep. 2013;15:304–312. doi: 10.1007/s11906-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaba Y, Ito M, Fumoto T, Watanabe K, Fukuhara R, Takeshita S, Nimura Y, Ishida J, Fukamizu A, Ikeda K. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J.Bone Miner.Res. 2009;24:241–250. doi: 10.1359/jbmr.081006. [DOI] [PubMed] [Google Scholar]
- Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS, Yoo MA, Yu BP, Chung HY. Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J.Gerontol.A Biol.Sci.Med.Sci. 2001;56:B426–31. doi: 10.1093/gerona/56.10.b426. [DOI] [PubMed] [Google Scholar]
- Baer AN, Wortmann RL. Myotoxicity associated with lipid-lowering drugs. Curr.Opin.Rheumatol. 2007;19:67–73. doi: 10.1097/BOR.0b013e328010c559. [DOI] [PubMed] [Google Scholar]
- Balzi D, Lauretani F, Barchielli A, Ferrucci L, Bandinelli S, Buiatti E, Milaneschi Y, Guralnik JM. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39:92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista LE, Atwood JE, O'Malley PG, Taylor AJ. Association between C-reactive protein and hypertension in healthy middle-aged men and women. Coron.Artery Dis. 2004;15:331–336. doi: 10.1097/00019501-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J.Hum.Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- Belmin J, Abderrhamane M, Medjahed S, Sibony-Prat J, Bruhat A, Bojic N, Marquet T. Variability of blood pressure response to orthostatism and reproducibility of the diagnosis of orthostatic hypotension in elderly subjects. J.Gerontol.A Biol.Sci.Med.Sci. 2000;55:M667–71. doi: 10.1093/gerona/55.11.m667. [DOI] [PubMed] [Google Scholar]
- Benetos A, Labat C, Rossignol P, Fay R, Rolland Y, Valbusa F, Salvi P, Zamboni M, Manckoundia P, Hanon O, Gautier S. Treatment With Multiple Blood Pressure Medications, Achieved Blood Pressure, and Mortality in Older Nursing Home Residents: The PARTAGE Study. JAMA Intern.Med. 2015;175:989–995. doi: 10.1001/jamainternmed.2014.8012. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2010;33:804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma-van der Wiel A, Gussekloo J, De Craen AJ, Van Exel E, Bloem BR, Westendorp RG. Common chronic diseases and general impairments as determinants of walking disability in the oldest-old population. J.Am.Geriatr.Soc. 2002;50:1405–1410. doi: 10.1046/j.1532-5415.2002.50363.x. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Individual differences in the response to regular exercise. Int.J.Obes.Relat.Metab.Disord. 1995;19(Suppl 4):S5–8. [PubMed] [Google Scholar]
- Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med.Sci.Sports Exerc. 2001;33:S446–51. doi: 10.1097/00005768-200106001-00013. discussion S452-3. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal O(2) uptake response to standardized exercise training programs. J.Appl.Physiol. 2011;110:1160–1170. doi: 10.1152/japplphysiol.00973.2010. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustani M, Sachs G, Callahan CM. Can primary care meet the biopsychosocial needs of older adults with dementia? J.Gen.Intern.Med. 2007;22:1625–1627. doi: 10.1007/s11606-007-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlage P, Hasford J. Blood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment--a review. Cardiovasc.Diabetol. 2009;8:18–2840-8-18. doi: 10.1186/1475-2840-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr.Hypertens.Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic.Biol.Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Willoughby DS. Impact of DHEA(S) and cortisol on immune function in aging: a brief review. Appl.Physiol.Nutr.Metab. 2008;33:429–433. doi: 10.1139/H08-013. [DOI] [PubMed] [Google Scholar]
- Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res.Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Manini TM, Hsu FC, Cesari M, Anton SD, Nayfield S, Stafford RS, Church TS, Pahor M, Carter CS. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J.Am.Geriatr.Soc. 2012;60:1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Hsu FC, Brinkley TE, Carter CS, Church TS, Dodson JA, Goodpaster BH, McGrae McDermott M, Nicklas BJ, Yank V, Johnson JA, Pahor M. Genetic Influence on Exercise-Induced Changes in Physical Function among Mobility-Limited Older Adults. Physiol.Genomics. 2014 doi: 10.1152/physiolgenomics.00169.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunag RD, Page IH, McCubbin JW. Neural stimulation of release of renin. Circ.Res. 1966;19:851–858. doi: 10.1161/01.res.19.4.851. [DOI] [PubMed] [Google Scholar]
- Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch.Intern.Med. 2012;172:1739–1744. doi: 10.1001/2013.jamainternmed.469. [DOI] [PubMed] [Google Scholar]
- Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of falls on initiation of antihypertensive drugs in the elderly. Osteoporos.Int. 2013;24:2649–2657. doi: 10.1007/s00198-013-2369-7. [DOI] [PubMed] [Google Scholar]
- Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- Candore G, Colonna-Romano G, Balistreri CR, Di Carlo D, Grimaldi MP, Listi F, Nuzzo D, Vasto S, Lio D, Caruso C. Biology of longevity: role of the innate immune system. Rejuvenation Res. 2006;9:143–148. doi: 10.1089/rej.2006.9.143. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J.Gerontol.B Psychol.Sci.Soc.Sci. 1999;54:S262–70. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- Carter CS, Giovannini S, Seo DO, DuPree J, Morgan D, Chung HY, Lees H, Daniels M, Hubbard GB, Lee S, Ikeno Y, Foster TC, Buford TW, Marzetti E. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 x Brown Norway rats. Age (Dordr) 2011;33:167–183. doi: 10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Marzetti E, Leeuwenburgh C, Manini T, Foster TC, Groban L, Scarpace PJ, Morgan D. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J.Gerontol.A Biol.Sci.Med.Sci. 2012;67:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari LH, Momary KM, Patel SR, Shapiro NL, Nutescu E, Viana MA. Pharmacogenomics of warfarin dose requirements in Hispanics. Blood Cells Mol.Dis. 2011;46:147–150. doi: 10.1016/j.bcmd.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Web-based injury statistics query and reporting system (WISQARS) 2015 [Google Scholar]
- Centers for Disease Control and Prevention Cost of falls among older adults. 2015 [Google Scholar]
- Centers for Disease Control and Prevention Self-reported falls and fall-related injuries among persons aged > or =65 years--United States, 2006. MMWR Morb.Mortal.Wkly.Rep. 2008;57:225–229. [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am.J.Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr.Pharm.Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Lowenthal DT, Paran E, Mecocci P, Williams LS, Senin U. Hypertension and cognitive function in the elderly. Dis.Mon. 2010;56:106–147. doi: 10.1016/j.disamonth.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Park C, Lee YK, Ha YC, Jang S, Shin CS. Risk of fractures in subjects with antihypertensive medications: A nationwide claim study. Int.J.Cardiol. 2015;184:62–67. doi: 10.1016/j.ijcard.2015.01.072. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front.Biosci.(Landmark Ed) 2011;16:1733–1745. doi: 10.2741/3816. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Shim KH, Kim KW. Dietary modulation of prostanoid synthesis in the aging process: role of cyclooxygenase-2. Mech.Ageing Dev. 1999;111:97–106. doi: 10.1016/s0047-6374(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res.Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contador I, Bermejo-Pareja F, Mitchell AJ, Trincado R, Villarejo A, Sanchez-Ferro A, Benito-Leon J. Cause of death in mild cognitive impairment: a prospective study (NEDICES). Eur.J.Neurol. 2014;21:253–e9. doi: 10.1111/ene.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI, Ritchie MD, Stein CM, Roden DM, Smith JD, Veenstra DL, Rettie AE, Rieder MJ. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia--a systematic review. Plant Foods Hum.Nutr. 2013;68:279–292. doi: 10.1007/s11130-013-0370-0. [DOI] [PubMed] [Google Scholar]
- Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid.Redox Signal. 2014;20:102–120. doi: 10.1089/ars.2013.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J.Lab.Clin.Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Apoptosis remodeling in immunosenescence: implications for strategies to delay ageing. Curr.Med.Chem. 2007;14:1389–1397. doi: 10.2174/092986707780831122. [DOI] [PubMed] [Google Scholar]
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- DeCarli C. Blood pressure control and cognitive performance: something to think about with aging. JAMA. 2015;313:1963–1964. doi: 10.1001/jama.2015.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J.Clin.Endocrinol.Metab. 2008;93:1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed.Res.Int. 2014;2014:406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ.Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumurgier J, Elbaz A, Dufouil C, Tavernier B, Tzourio C. Hypertension and lower walking speed in the elderly: the Three-City study. J.Hypertens. 2010;28:1506–1514. doi: 10.1097/HJH.0b013e328338bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron E, Hanon O. Hypertension, cognitive decline and dementia. Arch.Cardiovasc.Dis. 2008;101:181–189. doi: 10.1016/s1875-2136(08)71801-1. [DOI] [PubMed] [Google Scholar]
- El-Bikai R, Tahir MR, Tremblay J, Joffres M, Seda O, Sedova L, Awadalla P, Laberge C, Knoppers BM, Dumas P, Gaudet D, Ste-Marie LG, Hamet P. Association of age-dependent height and bone mineral density decline with increased arterial stiffness and rate of fractures in hypertensive individuals. J.Hypertens. 2015;33:727–35. doi: 10.1097/HJH.0000000000000475. discussion 735. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch.Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu.Rev.Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Eshkoor SA, Hamid TA, Mun CY, Ng CK. Mild cognitive impairment and its management in older people. Clin.Interv.Aging. 2015;10:687–693. doi: 10.2147/CIA.S73922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur.J.Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. [October 14, 2009];Statistical Data of Older Americans. 2009 [Google Scholar]
- Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J.Clin.Endocrinol.Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J.Am.Geriatr.Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- Fiocco AJ, Shatenstein B, Ferland G, Payette H, Belleville S, Kergoat MJ, Morais JA, Greenwood CE. Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol.Aging. 2012;33:829, e21–829, e28. doi: 10.1016/j.neurobiolaging.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the Real Polypill. Physiology (Bethesda) 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Motta L, Motta M, Malaguarnera M, Capri M, Vasto S, Candore G, Caruso C, IMUSCE. The extreme longevity: the state of the art in Italy. Exp.Gerontol. 2008;43:45–52. doi: 10.1016/j.exger.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr.Neurosci. 2014;17:241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- Furumoto T, Saito N, Dong J, Mikami T, Fujii S, Kitabatake A. Association of cardiovascular risk factors and endothelial dysfunction in japanese hypertensive patients: implications for early atherosclerosis. Hypertens.Res. 2002;25:475–480. doi: 10.1291/hypres.25.475. [DOI] [PubMed] [Google Scholar]
- Gambassi G, Lapane KL, Sgadari A, Carbonin P, Gatsonis C, Lipsitz LA, Mor V, Bernabei R. Effects of angiotensin-converting enzyme inhibitors and digoxin on health outcomes of very old patients with heart failure. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. Arch.Intern.Med. 2000;160:53–60. doi: 10.1001/archinte.160.1.53. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B, International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Gawande A. The Checklist Manifesto: How to Get Things Right. 2009:224. [Google Scholar]
- Ghosh M, Majumdar SR. Antihypertensive medications, bone mineral density, and fractures: a review of old cardiac drugs that provides new insights into osteoporosis. Endocrine. 2014;46:397–405. doi: 10.1007/s12020-014-0167-4. [DOI] [PubMed] [Google Scholar]
- Giaccaglia V, Nicklas B, Kritchevsky S, Mychalecky J, Messier S, Bleecker E, Pahor M. Interaction between angiotensin converting enzyme insertion/deletion genotype and exercise training on knee extensor strength in older individuals. Int.J.Sports Med. 2008;29:40–44. doi: 10.1055/s-2007-964842. [DOI] [PubMed] [Google Scholar]
- Gill TM, Murphy TE, Gahbauer EA, Allore HG. Association of injurious falls with disability outcomes and nursing home admissions in community-living older persons. Am.J.Epidemiol. 2013;178:418–425. doi: 10.1093/aje/kws554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie MN, Pastukh V, Ruchko MV. Oxidative DNA modifications in hypoxic signaling. Ann.N.Y.Acad.Sci. 2009;1177:140–150. doi: 10.1111/j.1749-6632.2009.05036.x. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J.Nutr.Health Aging. 2010;14:457–460. doi: 10.1007/s12603-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeres LM, Williams CD, Eckstrom E, Lee DS. Pharmacotherapy for hypertension in older adults: a systematic review. Drugs Aging. 2014;31:897–910. doi: 10.1007/s40266-014-0219-8. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbin J, Hubbard R, Gladman JR, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: population-based case-control study. Age Ageing. 2010;39:592–597. doi: 10.1093/ageing/afq092. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat.Rev.Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Lackland DT, Cupples LA, Lipsitz LA. Association between concurrent and remote blood pressure and disability in older adults. Hypertension. 2007;50:1026–1032. doi: 10.1161/HYPERTENSIONAHA.107.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Yang F, Sorond F, Jones RN, Milberg W, Cupples LA, Lipsitz LA. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:994–1001. doi: 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Wharton W, Mack WJ, Levey AI, Goldstein FC. Racial Disparity in Cognitive and Functional Disability in Hypertension and All-Cause Mortality. Am.J.Hypertens. 2015 doi: 10.1093/ajh/hpv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WJ. Update in geriatrics. Ann.Intern.Med. 2006;145:538–543. doi: 10.7326/0003-4819-145-7-200610030-00012. [DOI] [PubMed] [Google Scholar]
- Hamburg MA, Collins FS. The path to personalized medicine. N.Engl.J.Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol.Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, Lee WC, Park YG, Kim MK, Park YM. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008-2010. PLoS One. 2014;9:e86902. doi: 10.1371/journal.pone.0086902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon O, Forette F. Treatment of hypertension and prevention of dementia. Alzheimers Dement. 2005;1:30–37. doi: 10.1016/j.jalz.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, Rossouw JE, Wassertheil-Smoller S. Hypertension, Dietary Sodium, and Cognitive Decline: Results From the Women's Health Initiative Memory Study. Am.J.Hypertens. 2015 doi: 10.1093/ajh/hpv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J.Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am.J.Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J.Physiol. 2012;590:2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav.Immun. 2004;18:114–119. doi: 10.1016/j.bbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin.Sci.(Lond) 2016;130:9–18. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Prasannarong M. The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol.Cell.Endocrinol. 2013;378:15–22. doi: 10.1016/j.mce.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: a meta-analysis. J.Am.Geriatr.Soc. 2012;60:136–141. doi: 10.1111/j.1532-5415.2011.03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Lemaitre RN, Smith NL, Bis JC, Marciante KD, Rice KM, Lumley T, Enquobahrie DA, Li G, Heckbert SR, Psaty BM. Common genetic variation in six lipid-related and statin-related genes, statin use and risk of incident nonfatal myocardial infarction and stroke. Pharmacogenet Genomics. 2008;18:677–682. doi: 10.1097/FPC.0b013e3283033528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: a review. Am.J.Geriatr.Pharmacother. 2011;9:364–377. doi: 10.1016/j.amjopharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hodgson TA, Cohen AJ. Medical care expenditures for selected circulatory diseases: opportunities for reducing national health expenditures. Med.Care. 1999;37:994–1012. doi: 10.1097/00005650-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Sides EG, Mychaleckyj JC, Worrall BB, Elias GA, Liu Y, Chen WM, Coull BM, Toole JF, Rich SS, Furie KL, Sale MM. Transcobalamin 2 variant associated with poststroke homocysteine modifies recurrent stroke risk. Neurology. 2011;77:1543–1550. doi: 10.1212/WNL.0b013e318233b1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front.Aging Neurosci. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle-Hansen H, Thommessen B, Fagerland MW, Oksengard AR, Wyller TB, Engedal K, Fure B. Blood pressure control to prevent decline in cognition after stroke. Vasc.Health.Risk Manag. 2015;11:311–316. doi: 10.2147/VHRM.S82839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif.Tissue Int. 2013;92:217–227. doi: 10.1007/s00223-012-9671-9. [DOI] [PubMed] [Google Scholar]
- Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol.Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. 2008:1–2-300. [PubMed] [Google Scholar]
- Institute of Medicine. <br />Initial National Priorities for Comparative Effectiveness Research. 2009:252. [Google Scholar]
- Institute of Medicine. A population-based policy and systems change approach to prevent and control hypertension. 2010 [PubMed] [Google Scholar]
- Iritani O, Koizumi Y, Hamazaki Y, Yano H, Morita T, Himeno T, Okuno T, Okuro M, Iwai K, Morimoto S. Association between blood pressure and disability-free survival among community-dwelling elderly patients receiving antihypertensive treatment. Hypertens.Res. 2014 doi: 10.1038/hr.2014.67. [DOI] [PubMed] [Google Scholar]
- Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann.N.Y.Acad.Sci. 1998;854:102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Boerwinkle E, Zineh I, Chapman AB, Bailey K, Cooper-DeHoff RM, Gums J, Curry RW, Gong Y, Beitelshees AL, Schwartz G, Turner ST. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am.Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14:723–726. doi: 10.2217/pgs.13.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P, Tesch PA, Thorsson A, Karlsson J, Kaijser L. Skeletal muscle glycolysis during submaximal exercise following acute beta-adrenergic blockade in man. Acta Physiol.Scand. 1985;123:285–291. doi: 10.1111/j.1748-1716.1985.tb07589.x. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res.Rev. 2014;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Khosla S, Peterson JM, Egan K, Jones JD, Riggs BL. Circulating cytokine levels in osteoporotic and normal women. J.Clin.Endocrinol.Metab. 1994;79:707–711. doi: 10.1210/jcem.79.3.8077350. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kwon HK, Lee JM, Kim YJ, Kim HJ, Jung NY, Kim ST, Lee KH, Na DL, Seo SW. White matter microstructural changes in pure Alzheimer's disease and subcortical vascular dementia. Eur.J.Neurol. 2015;22:709–716. doi: 10.1111/ene.12645. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, Wagner A, Jr, Wagner D, Scheurich S. Clinical features of MCI: motor changes. Int.Psychogeriatr. 2008;20:32–39. doi: 10.1017/S1041610207006461. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH, 3rd, Holloszy JO. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J.Appl.Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp.Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc.Natl.Acad.Sci.U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lacy F, O'Connor DT, Schmid-Schonbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J.Hypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- Lai SW, Liao KF. Risk of hip fracture and antihypertensive drugs in older people. JAMA Intern.Med. 2013;173:934. doi: 10.1001/jamainternmed.2013.386. [DOI] [PubMed] [Google Scholar]
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2003;285:R277–97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- Launer LJ, Oudkerk M, Nilsson LG, Alperovitch A, Berger K, Breteler MM, Fuhrer R, Giampaoli S, Nissinen A, Pajak A, Sans S, Schmidt R, Hofman A. CASCADE: a European collaborative study on vascular determinants of brain lesions. Study design and objectives. Neuroepidemiology. 2000;19:113–120. doi: 10.1159/000026246. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol.Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310:2609–2610. doi: 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz TL, Lenz NJ, Faulkner MA. Potential interactions between exercise and drug therapy. Sports Med. 2004;34:293–306. doi: 10.2165/00007256-200434050-00002. [DOI] [PubMed] [Google Scholar]
- Lenz TL. The effects of high physical activity on pharmacokinetic drug interactions. Expert Opin.Drug Metab.Toxicol. 2011;7:257–266. doi: 10.1517/17425255.2011.553190. [DOI] [PubMed] [Google Scholar]
- Li Y, Shen G, Yu C, Li G, Shen J, Gong J, Xu Y. Angiotensin II induces mitochondrial oxidative stress and mtDNA damage in osteoblasts by inhibiting SIRT1-FoxO3a-MnSOD pathway. Biochem.Biophys.Res.Commun. 2014;455:113–118. doi: 10.1016/j.bbrc.2014.10.123. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Habtemariam D, Gagnon M, Iloputaife I, Sorond F, Tchalla AE, Dantoine TF, Travison TG. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension. 2015;66:183–189. doi: 10.1161/HYPERTENSIONAHA.115.05513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tinker L, Song Y, Rifai N, Bonds DE, Cook NR, Heiss G, Howard BV, Hotamisligil GS, Hu FB, Kuller LH, Manson JE. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch.Intern.Med. 2007;167:1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–83. doi: 10.1161/HYPERTENSIONAHA.109.142646. 6p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol.Cancer.Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin.Sci.(Lond) 2013;125:311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Palermo L, Vittinghoff E, Evans GW, Atkinson HH, Hamilton BP, Josse RG, O'Connor PJ, Simmons DL, Tiktin M, Schwartz AV. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J.Gen.Intern.Med. 2014;29:1599–1606. doi: 10.1007/s11606-014-2961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric-Bilkan C, Gilbert EL, Ryan MJ. Impact of ovarian function on cardiovascular health in women: focus on hypertension. Int.J.Womens Health. 2014;6:131–139. doi: 10.2147/IJWH.S38084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Dupree J, Lees HA, Giovannini S, Seo DO, Buford TW, Sweet K, Morgan D, Strehler KY, Diz D, Borst SE, Moningka N, Krotova K, Carter CS. Late-life enalapril administration induces nitric oxide-dependent and independent metabolic adaptations in the rat skeletal muscle. Age (Dordr) 2012 doi: 10.1007/s11357-012-9428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int.J.Biochem.Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Peter I, Huggins GS, Raynor HA, Delahanty LM, Cheskin LJ, Balasubramanyam A, Wagenknecht LE, Wing RR, Genetic Subgroup of Look AHEAD, Look AHEAD Research Group. Obesity susceptibility loci and dietary intake in the Look AHEAD Trial. Am.J.Clin.Nutr. 2012;95:1477–1486. doi: 10.3945/ajcn.111.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CW, Gillis NK, Alsultan A, Chang SW, Kawaguchi-Suzuki M, Lang JE, Shahin MH, Buford TW, El Rouby NM, Sa AC, Langaee TY, Gums JG, Chapman AB, Cooper-Dehoff RM, Turner ST, Gong Y, Johnson JA. Atenolol Induced HDL-C Change in the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) Study. PLoS One. 2013;8:e76984. doi: 10.1371/journal.pone.0076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Eyler LT, Franz CE, Hagler DJ, Jr, Lyons MJ, Panizzon MS, Rinker DA, Dale AM, Kremen WS. Hypertension-Related Alterations in White Matter Microstructure Detectable in Middle Age. Hypertension. 2015;66:317–323. doi: 10.1161/HYPERTENSIONAHA.115.05336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn AP, Kaplan RC, Verghese J, Rosenbaum DM, Psaty BM, Baird AE, Lynch JK, Wolf PA, Kooperberg C, Larson JC, Wassertheil-Smoller S. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke. 2008;39:1233–1239. doi: 10.1161/STROKEAHA.107.500850. [DOI] [PubMed] [Google Scholar]
- Metz JA, Morris CD, Roberts LA, McClung MR, McCarron DA. Blood pressure and calcium intake are related to bone density in adult males. Br.J.Nutr. 1999;81:383–388. [PubMed] [Google Scholar]
- Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, Pankratz VS, Geda YE, Machulda MM, Ivnik RJ, Knopman DS, Boeve BF, Rocca WA, Petersen RC. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J.Gerontol.A Biol.Sci.Med.Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. IOM urges more attention by physicians, public on “neglected” hypertension. JAMA. 2010;303:1354–1355. doi: 10.1001/jama.2010.358. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Mu L, Cohen AJ, Mukamal KJ. Prevalence and predictors of resistance and aerobic exercise among hypertensive adults in the United States. J.Hum.Hypertens. 2015;29:394–395. doi: 10.1038/jhh.2014.104. [DOI] [PubMed] [Google Scholar]
- Muszalik M, Dijkstra A, Kedziora-Kornatowska K, Zielinska-Wieczkowska H, Kornatowski T. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL). Arch.Gerontol.Geriatr. 2011;52:e204–9. doi: 10.1016/j.archger.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Nabha L, Garbern JC, Buller CL, Charpie JR. Vascular oxidative stress precedes high blood pressure in spontaneously hypertensive rats. Clin.Exp.Hypertens. 2005;27:71–82. doi: 10.1081/ceh-200044267. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav.Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Aging. Living Long & Well in the 21st Century: Strategic Directions for Research on Aging. 2007 http://www.nia.nih.gov/sites/default/files/strategic_plan108.pdf.
- Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J.Gerontol. 1991;46:M164–70. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Psaty BM, Harris TB, Robbins JA, Burke GL, Kuller LH, Lumley T. Total and cause-specific mortality in the cardiovascular health study. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obama B. Genomics and Personalized Medicine Act of 2007. 2007 [PubMed] [Google Scholar]
- O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am.J.Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- O'Neill ED, Wilding JP, Kahn CR, Van Remmen H, McArdle A, Jackson MJ, Close GL. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age (Dordr) 2010;32:209–222. doi: 10.1007/s11357-009-9125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Cesari M. Study Design: Randomized Controlled Trials. 2012:27–28-47. [Google Scholar]
- Pang CY, Ma YS, Wei YU. MtDNA mutations, functional decline and turnover of mitochondria in aging. Front.Biosci. 2008;13:3661–3675. doi: 10.2741/2957. [DOI] [PubMed] [Google Scholar]
- Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY, Park J, Shin KJ, Kim D, Moon YS. Sarcopenic obesity as an independent risk factor of hypertension. J.Am.Soc.Hypertens. 2013;7:420–425. doi: 10.1016/j.jash.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am.J.Physiol.Heart Circ.Physiol. 2008;295:H10–20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J.Gerontol.A Biol.Sci.Med.Sci. 2000;55:M691–7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- Pepersack T, Gilles C, Petrovic M, Spinnewine A, Baeyens H, Beyer I, Boland B, Dalleur O, De Lepeleire J, Even-Adin D, Van Nes MC, Samalea-Suarez A, Somers A, Working Group Clinical Pharmacology, Pharmacotherapy and Pharmaceutical Care, Belgian Society for Gerontology and Geriatrics. Prevalence of orthostatic hypotension and relationship with drug use amongst older patients. Acta Clin.Belg. 2013;68:107–112. doi: 10.2143/ACB.3215. [DOI] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Justo I, Sanz-Cantalapiedra A, Pueyo C, Hernandez G, Deunas A. Effect of the antihypertensive treatment on the bone mineral density and osteoporotic fracture. Curr Hypertens Rev. 2005;1:61–62-66. [Google Scholar]
- Peters R, Beckett N, Burch L, de Vernejoul MC, Liu L, Duggan J, Swift C, Gil-Extremera B, Fletcher A, Bulpitt C. The effect of treatment based on a diuretic (indapamide) +/− ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET). Age Ageing. 2010;39:609–616. doi: 10.1093/ageing/afq071. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Fagard R, Thijs L, Wang JG, Forette F, Pereira L, Fletcher A, Bulpitt C. Increased pulse pressure linked to dementia: further results from the Hypertension in the Very Elderly Trial - HYVET. J.Hypertens. 2013;31:1868–1875. doi: 10.1097/HJH.0b013e3283622cc6. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch.Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch.Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Burns VE, Lord JM. Stress and exercise: Getting the balance right for aging immunity. Exerc.Sport Sci.Rev. 2007;35:35–39. doi: 10.1097/jes.0b013e31802d7008. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani P, Petruzzi E, Orlando C, Stefanescu A, Antonini MF, Serio M, Pazzagli M. Reduced serum antioxidant capacity in healthy centenarians. Clin.Chem. 1997;43:855–856. [PubMed] [Google Scholar]
- Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Puntus T, Schneider B, Meran J, Peterlik M, Kudlacek S. Influence of age and gender on associations of body mass index with bone mineral density, bone turnover markers and circulating calcium-regulating and bone-active sex hormones. Bone. 2011;49:824–829. doi: 10.1016/j.bone.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Rafey MA. Resistant hypertension in the elderly. Clin.Geriatr.Med. 2009;25:289–301. doi: 10.1016/j.cger.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Sung YJ, Sarzynski MA, Rice TK, Rao DC, Bouchard C. Heritability of submaximal exercise heart rate response to exercise training is accounted for by nine SNPs. J.Appl.Physiol. 2012;112:892–897. doi: 10.1152/japplphysiol.01287.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J.Bone Miner.Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- Rejnmark L, Vestergaard P, Mosekilde L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J.Hypertens. 2006;24:581–589. doi: 10.1097/01.hjh.0000203845.26690.cb. [DOI] [PubMed] [Google Scholar]
- Rice T, An P, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Heritability of HR and BP response to exercise training in the HERITAGE Family Study. Med.Sci.Sports Exerc. 2002;34:972–979. doi: 10.1097/00005768-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Riley RJ, Burgener S, Buckwalter KC. Anxiety and stigma in dementia: a threat to aging in place. Nurs.Clin.North Am. 2014;49:213–231. doi: 10.1016/j.cnur.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A, Pillai JA. Treatment of Vascular Cognitive Impairment. Curr.Treat.Options Neurol. 2015;17:367–015-0367-0. doi: 10.1007/s11940-015-0367-0. [DOI] [PubMed] [Google Scholar]
- Roberts L. 9 Billion? Science. 2011;333:540–543. doi: 10.1126/science.333.6042.540. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Longstreth WT, Jr, Boudreau R, Taylor CA, Du Y, Kuller LH, Newman AB. High Blood Pressure Accelerates Gait Slowing in Well-Functioning Older Adults over 18-Years of Follow-Up. J.Am.Geriatr.Soc. 2011;59:390–397. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr.Opin.Clin.Nutr.Metab.Care. 2003;6:295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am.J.Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- Rubio-Ruiz ME, Perez-Torres I, Soto ME, Pastelin G, Guarner-Lans V. Aging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res.Rev. 2014;18:132–147. doi: 10.1016/j.arr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- Sachs GA, Carter R, Holtz LR, Smith F, Stump TE, Tu W, Callahan CM. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann.Intern.Med. 2011;155:300–308. doi: 10.7326/0003-4819-155-5-201109060-00007. [DOI] [PubMed] [Google Scholar]
- Sakkas GK, Kent-Braun JA, Doyle JW, Shubert T, Gordon P, Johansen KL. Effect of diabetes mellitus on muscle size and strength in patients receiving dialysis therapy. Am.J.Kidney Dis. 2006;47:862–869. doi: 10.1053/j.ajkd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell.Immunol. 2015;294:95–101. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Sattin RW, Lambert Huber DA, DeVito CA, Rodriguez JG, Ros A, Bacchelli S, Stevens JA, Waxweiler RJ. The incidence of fall injury events among the elderly in a defined population. Am.J.Epidemiol. 1990;131:1028–1037. doi: 10.1093/oxfordjournals.aje.a115594. [DOI] [PubMed] [Google Scholar]
- Schwartz JB. Primary prevention: do the very elderly require a different approach? Trends Cardiovasc.Med. 2015;25:228–239. doi: 10.1016/j.tcm.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988-1994 and 1999-2004. Am.J.Public Health. 2010;100:100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev.Neurother. 2011;11:1057–1075. doi: 10.1586/ern.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera LC, McPherson ML. Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin.Geriatr.Med. 2012;28:273–286. doi: 10.1016/j.cger.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Shannon RP, Wei JY, Rosa RM, Epstein FH, Rowe JW. The effect of age and sodium depletion on cardiovascular response to orthostasis. Hypertension. 1986;8:438–443. doi: 10.1161/01.hyp.8.5.438. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, Tomita T, Yoshikawa H, Ogihara T, Morishita R. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008;22:2465–2475. doi: 10.1096/fj.07-098954. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Nakagami H, Osako MK, Nakagami F, Kunugiza Y, Tomita T, Yoshikawa H, Rakugi H, Ogihara T, Morishita R. Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive rats. Hypertens.Res. 2009;32:786–790. doi: 10.1038/hr.2009.99. [DOI] [PubMed] [Google Scholar]
- Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner AR, Relling MV, Peterson JF, Hicks K, Freimuth RR, Sadee W, Pereira NL, Roden DM, Johnson JA, Klein TE. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Overcoming Challenges of Real-World Implementation. Clin.Pharmacol.Ther. 2013 doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica D. Are there pleiotropic effects of antihypertensive medications or is it all about the blood pressure in the patient with diabetes and hypertension? J.Clin.Hypertens.(Greenwich) 2011;13:301–304. doi: 10.1111/j.1751-7176.2011.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO. Association of mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J.Alzheimers Dis. 2014;39:271–282. doi: 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res.Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavachevsky I, Rachmani R, Levi Z, Brosh D, Lidar M, Ravid M. Effect of enalapril and nifedipine on orthostatic hypotension in older hypertensive patients. J.Am.Geriatr.Soc. 2000;48:807–810. doi: 10.1111/j.1532-5415.2000.tb04757.x. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov.Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Squadrito GL, Pryor WA. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem.Biol.Interact. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-u. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J.Hum.Hypertens. 1997;11:507–514. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliovaara M, Impivaara O, Koskinen S. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J.Am.Geriatr.Soc. 2012;60:77–85. doi: 10.1111/j.1532-5415.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuveling EM, Bakker SJ, Hillege HL, Burgerhof JG, de Jong PE, Gans RO, de Zeeuw D, PREVEND Study Group. C-reactive protein modifies the relationship between blood pressure and microalbuminuria. Hypertension. 2004;43:791–796. doi: 10.1161/01.HYP.0000120125.08867.42. [DOI] [PubMed] [Google Scholar]
- Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am.J.Hypertens. 2003;16:429–433. doi: 10.1016/s0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Harris EL, Martin E, Vollmer WM, Meltesen GT, Ricchiuti V, Williams G, Appel LJ, Bray GA, Moore TJ, Winn MP, Conlin PR. Modulation of the BP response to diet by genes in the renin-angiotensin system and the adrenergic nervous system. Am.J.Hypertens. 2011;24:209–217. doi: 10.1038/ajh.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch PA. Exercise performance and beta-blockade. Sports Med. 1985;2:389–412. doi: 10.2165/00007256-198502060-00002. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, Banerjee P, Milos PM, Myrand SP, Paulauskis J, Milad MA, Sasiela WJ. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics J. 2005;5:352–358. doi: 10.1038/sj.tpj.6500328. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Moyna N, Seip R, Price T, Clarkson P, Angelopoulos T, Gordon P, Pescatello L, Visich P, Zoeller R, Devaney JM, Gordish H, Bilbie S, Hoffman EP. Functional polymorphisms associated with human muscle size and strength. Med.Sci.Sports Exerc. 2004;36:1132–1139. doi: 10.1249/01.mss.0000132274.26612.23. [DOI] [PubMed] [Google Scholar]
- Thorell K, Ranstad K, Midlov P, Borgquist L, Halling A. Is use of fall risk-increasing drugs in an elderly population associated with an increased risk of hip fracture, after adjustment for multimorbidity level: a cohort study. BMC Geriatr. 2014;14:131–2318-14-131. doi: 10.1186/1471-2318-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N.Engl.J.Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J.Am.Geriatr.Soc. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N.Engl.J.Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J.Gerontol.A Biol.Sci.Med.Sci. 1998;53:M112–9. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern.Med. 2014;174:588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa AC, Aranke M, Bryan NS. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J.Geriatr.Cardiol. 2011;8:230–242. doi: 10.3724/SP.J.1263.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J.Hypertens. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler.Thromb.Vasc.Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- Troen BR. The biology of aging. Mt.Sinai J.Med. 2003;70:3–22. [PubMed] [Google Scholar]
- U.S. Census Bureau U.S. Census Data. Oct;14:2009. [Google Scholar]
- US Department of Health and Human Services. The Surgeon General's call to action to improve the health and wellness of persons with disabilities. 2005 [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int.J.Biochem.Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- Vassilaki M, Cha RH, Aakre JA, Therneau TM, Geda YE, Mielke MM, Knopman DS, Petersen RC, Roberts RO. Mortality in mild cognitive impairment varies by subtype, sex, and lifestyle factors: the Mayo Clinic Study of Aging. J.Alzheimers Dis. 2015;45:1237–1245. doi: 10.3233/JAD-143078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech.Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J.Am.Soc.Nephrol. 2008;19:1371–1378. doi: 10.1681/ASN.2007050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp.Neurol. 2015 doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L. Hypertension is a risk factor for fractures. Calcif.Tissue Int. 2009;84:103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- Wadley AJ, Veldhuijzen van Zanten JJ, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age (Dordr) 2013;35:705–718. doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch.Intern.Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD. Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic.Biol.Med. 2004;36:226–232. doi: 10.1016/j.freeradbiomed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Watson NL, Rosano C, Boudreau RM, Simonsick EM, Ferrucci L, Sutton-Tyrrell K, Hardy SE, Atkinson HH, Yaffe K, Satterfield S, Harris TB, Newman AB, Health ABC Study. Executive function, memory, and gait speed decline in well-functioning older adults. J.Gerontol.A Biol.Sci.Med.Sci. 2010;65:1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Weitzel KW, Elsey AR, Langaee TY, Burkley B, Nessl DR, Obeng AO, Staley BJ, Dong HJ, Allan RW, Liu JF, Cooper-Dehoff RM, Anderson RD, Conlon M, Clare-Salzler MJ, Nelson DR, Johnson JA. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am.J.Med.Genet.C.Semin.Med.Genet. 2014;166C:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am.J.Clin.Nutr. 2013;98:1263–1271. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G, Berger P, Jansen-Durr P, Grubeck-Loebenstein B. A Darwinian-evolutionary concept of age-related diseases. Exp.Gerontol. 2003;38:13–25. doi: 10.1016/s0531-5565(02)00161-4. [DOI] [PubMed] [Google Scholar]
- Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am.J.Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimers Res.Ther. 2013;5:43. doi: 10.1186/alzrt206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Lord SR, Sturnieks DL, Delbaere K, Trollor JN, Close JC. Angiotensin system-blocking medications are associated with fewer falls over 12 months in community-dwelling older people. J.Am.Geriatr.Soc. 2013;61:776–781. doi: 10.1111/jgs.12205. [DOI] [PubMed] [Google Scholar]
- Wu CH, Chen KT, Hou MT, Chang YF, Chang CS, Liu PY, Wu SJ, Chiu CJ, Jou IM, Chen CY. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: the Tianliao Old People study 04. Geriatr.Gerontol.Int. 2014;14(Suppl 1):69–75. doi: 10.1111/ggi.12233. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yu BP, Yang R. Critical evaluation of the free radical theory of aging. A proposal for the oxidative stress hypothesis. Ann.N.Y.Acad.Sci. 1996;786:1–11. doi: 10.1111/j.1749-6632.1996.tb39047.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Yang Z, Yu M. Correlation of tumor necrosis factor alpha and interleukin 6 with hypertensive renal damage. Ren.Fail. 2010;32:475–479. doi: 10.3109/08860221003664280. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang G. Antihypertensive drugs and the risk of fall injuries: a systematic review and meta-analysis. J.Int.Med.Res. 2013;41:1408–1417. doi: 10.1177/0300060513497562. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J.Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Li Q, Liu L, Xu Z, Xiao J. Simvastatin reduces interleukin-1beta secretion by peripheral blood mononuclear cells in patients with essential hypertension. Clin.Chim.Acta. 2004;344:195–200. doi: 10.1016/j.cccn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wang JX, Feng YF, Wu ZX, Zhang Y, Shi L, Tan QC, Yan YB, Lei W. Systemic treatment with telmisartan improves femur fracture healing in mice. PLoS One. 2014;9:e92085. doi: 10.1371/journal.pone.0092085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, Franchimont N, Geenen V, Albert A, Reginster JY. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/s0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F. Pleiotropic effects of angiotensin II blockers in hemodialysis patients: myth or reality? Kidney Int. 2014;86:469–471. doi: 10.1038/ki.2014.155. [DOI] [PubMed] [Google Scholar]