Abstract
Multiple familial meningiomas occur in rare genetic syndromes, particularly neurofibromatosis type 2. The association of meningiomas and cerebral cavernous malformations (CCMs) has been reported in few patients in the medical literature. The purpose of our study is to corroborate a preferential association of CCMs and multiple meningiomas in subjects harbouring mutations in the PDCD10 gene (also known as CCM3). Three members of an Italian family affected by seizures underwent conventional brain Magnetic Resonance Imaging (MRI) with gadolinium contrast agent including gradient echo (GRE) imaging. The three CCM-causative genes were sequenced by Sanger method. Literature data reporting patients with coexistence of CCMs and meningiomas were reviewed. MRI demonstrated dural-based meningioma-like lesions associated to multiple parenchymal CCMs in all affected individuals. A disease-causative mutation in the PDCD10 gene (p.Gln112PhefsX13) was identified. Based on neuroradiological and molecular data as well as on literature review, we outline a consistent association between PDCD10 mutations and a syndrome of CCMs with multiple meningiomas. This condition should be considered in the differential diagnosis of multiple/familial meningioma syndromes. In case of multiple/familial meningioma the use of appropriate MRI technique may include GRE and/or susceptibility-weighted imaging (SWI) to rule out CCM. By contrast, proper post-gadolinium scans may aid defining dural lesions in CCM patients and are indicated in PDCD10-mutated individuals.
Keywords: Magnetic resonance imaging, multiple meningioma, cerebral cavernous malformation, PDCD10.
Introduction
Meningiomas are the most common benign intracranial neoplasm1 accounting for up to 20% of all primary intracranial tumours1,2 with annual incidence 5.5 per 100,000 individuals.3 They arise from the arachnoid cap cells of meningeal coverings of the spinal cord and the brain.1 Meningiomas occur as multiple tumours in less than 10% of cases, either isolated or in combination with other features, e.g. neurofibromatosis type 2 (NF2).3
Cerebral cavernous malformations (CCMs; Online Mendelian Inheritance in Man (OMIM)#116860) represent approximately 15% of all cerebral vascular lesions affecting 0.1–0.8% of the general population, with a familial incidence of almost 20%.5,6 CCMs are asymptomatic in up to 40% of cases or may clinically present with a variety of neurological manifestations.6 Loss-of-function mutations in one of three genes (CCM1/KRIT1, CCM2/MGC4607 or CCM3/PDCD10) predispose to CCM and are detected in up to 94% of familial CCM cases and 60% of sporadic CCM cases.5,7 A phenotype consisting of CCM and dural lesions showing meningioma features at Magnetic Resonance Imaging (MRI) has been reported in a dozen patients.8–10 Histopathological analyses defined the dural lesions as meningiomas in some of them. In a family study, the penetrance of this meningioma-phenotype was lower than CCM-phenotype alone and interestingly all genetically tested individuals with CCM and meningioma were mutated in the PDCD10 gene.8
Here, we describe an Italian family in which three symptomatic individuals mutated in the PDCD10 gene harboured parenchymal CCM lesions associated to multiple dural lesions with meningioma features at MRI.
Materials and methods
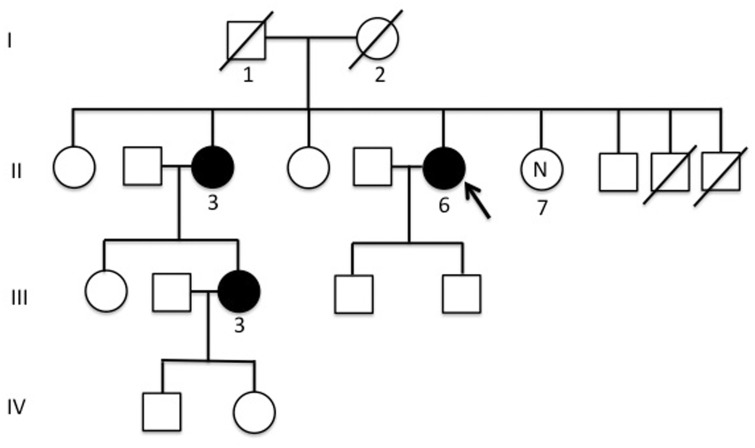
Three related individuals came to our observation in the course of the clinical-radiological assessment for seizure crises. The family pedigree is shown in Figure 1. The index case (II-6) presented with seizures at the age of 66 years. She had been suffering from recurrent headaches for 10 years previously. Her parents were non-consanguineous and her father (I-1), who suffered from recurrent headaches, died at the age of 84 years from femur fracture complications, while her mother (I-2) died at the age of 78 years from stroke. One of her sibling (II-3) presented at the age of 73 years with a first episode of seizure and had a daughter (III-3) affected by a severe form of epilepsy since she was 25 years old.
Figure 1.
Pedigree of the reported family with cerebral cavernous malformations (CCMs) and multiple meningiomas recurring in multiple affected subjects. Males and females are represented as squares and circles, respectively. The arrow indicates the index case. The filled circles represent affected individuals. The circle labelled ‘N’ indicates the healthy relative who did not carry the familial mutation.
The three symptomatic subjects (II-3, II-6 and III-3) underwent MRI examination in the course of their clinical assessment. All of them, and an unaffected relative (II-7), agreed with informed consent for genetic testing of CCM genes. The molecular analysis of DNA extracted from blood lymphocytes was performed as previously described8 and included direct sequencing of KRIT1, MGC4607 and PDCD10 genes and the sequencing for the identified PDCD10 gene mutation.
Results
All subjects showed multiple dural masses with meningioma features, presenting as multiple masses or diffuse dural thickening, and multiple intra-axial lesions, some with central areas of mixed signal on T1-weighted images other with low signal on gradient echo (GRE), consistent with CCMs (Figures 2–4). According to Zabramski classification,11 different types of CCM were observed in the same subjects. MR images were reviewed by three experienced neuroradiologists, who reached final interpretation (meningiomas and CCMs) by consensus.
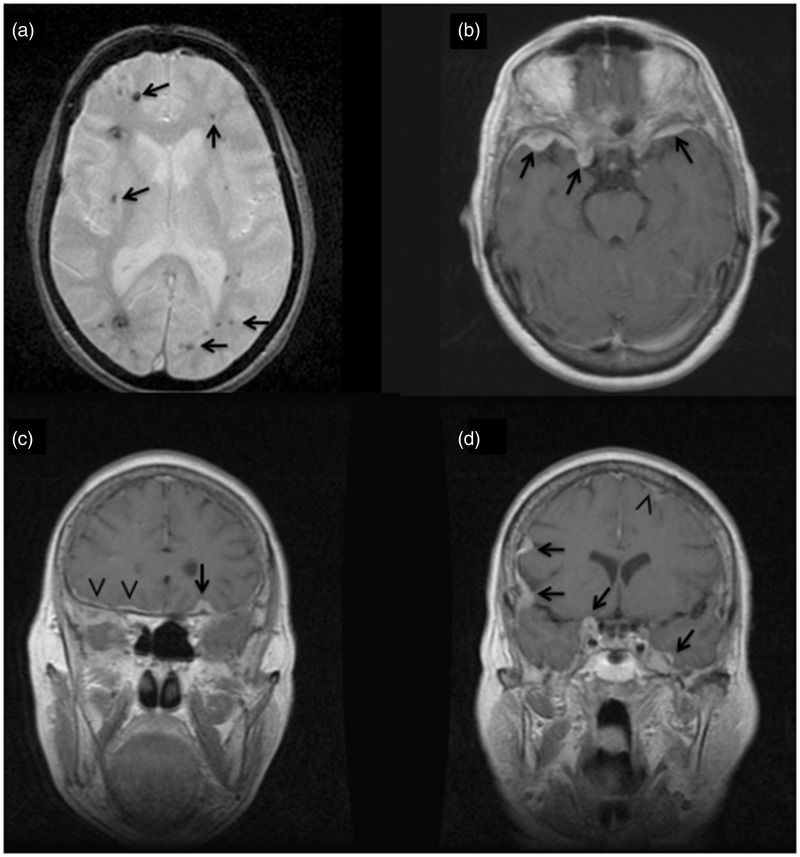
Figure 2.
Index case II-6 axial gradient echo image (a) shows multifocal ‘black dots’ (arrows) characteristic of Zabramski type IV cerebral cavernous malformations (CCMs) (punctate microhaemorrage); axial (b) and coronal (c), (d) T1-weighted post-gadolinium images demonstrate multiple strongly enhanced extra-dural based masses (arrows) and diffuse dural thickness (arrowheads).
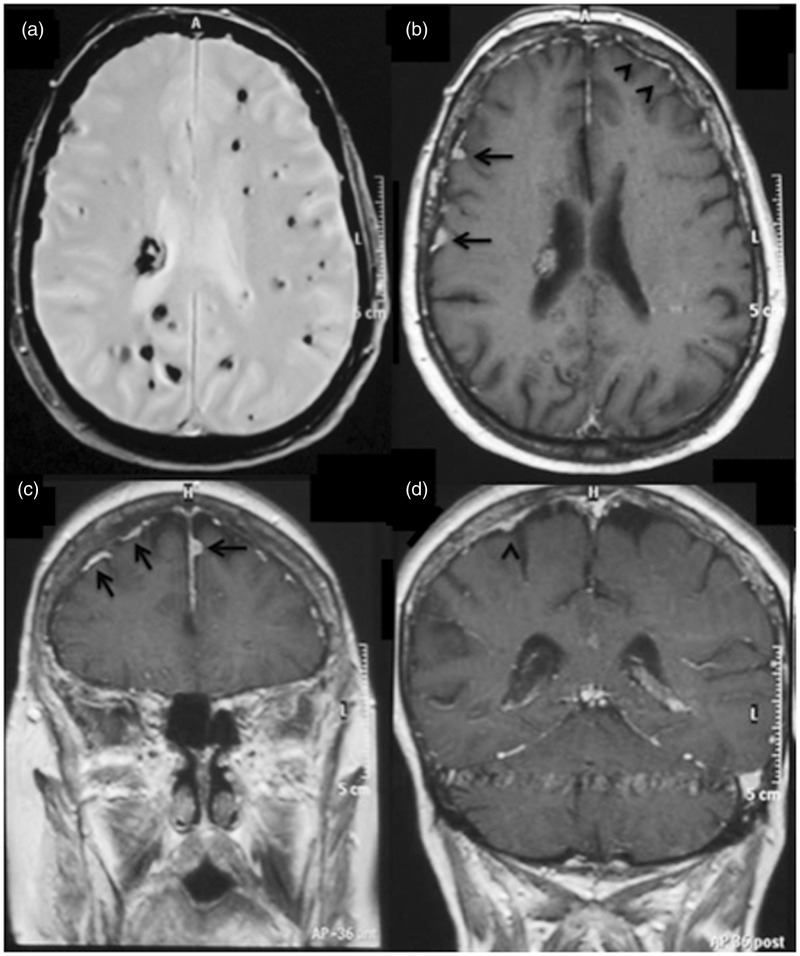
Figure 3.
Patient II-3 axial gradient echo image (a) shows multiple foci of hypointesity, consistent with multiple cavernomas; axial (b) and coronal (c), (d) GD T1-WI demonstrate multiple dural lesions with intense enhancement and slight dural thickness (arrows).
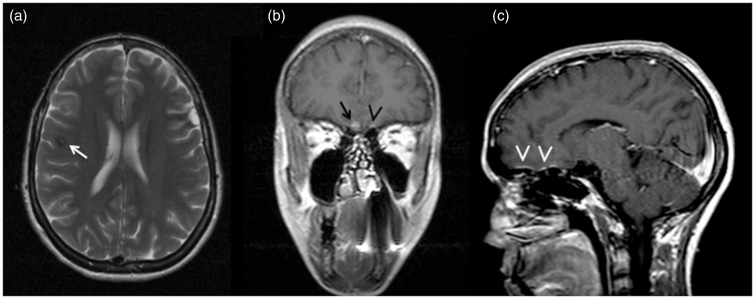
Figure 4.
Patient III-3 axial T2-weighted image (a) shows a subcortical hypointense lesion (arrow) with central hyperintense spot due to bloods in different stages and hypointense hemosiderin rim likely consistent with a cerebral cavernous malformation (CCM) (gradient echo (GRE) images not available); coronal (b) and sagittal (c) T1-weighted post-gadolinium image demonstrate fronto-basal extra-axial hyperintense lesion consistent with meningioma (arrow) and contralateral subtle dural thickness (arrowheads).
A mutation in the PDCD10 gene was detected in all affected individuals (II-3, II-6 and III-3). This mutation consisted in a deletion of four base-pairs in exon 7 (c.334_337del) that results in a frame-shift leading to a premature stop codon (p.Gln112PhefsX13). The mutation was absent in the healthy relative (II-7).
Discussion
Multiple meningiomas occur in 1–10% of patients in different series.12 NF2 is the most common genetic condition displaying multiple meningiomas, which have also been described in other genetic syndromes, including schwannomatosis, Werner and Proteus syndromes. At present, it is unclear whether these represent true or casual association. In addition, familial multiple meningiomas showing autosomal dominant inheritance not linked to the NF2 locus has been exceptionally described.3
This is the first report describing multiple individuals from the same family with both CCMs and dural lesions, following autosomal dominant inheritance. We reviewed all previously 12 reported sporadic patients with CCMs and meningiomas from the literature and, surprisingly, all genetically tested patients harboured mutations in the PDCD10 gene, as observed in our family.8–10 Notably, the penetrance of this CCM ‘meningioma-phenotype’ in our family is higher than in previously reported families. The mutation we identified was previously described by Spiegler et al. in an individual presenting with familial CCMs but in whom the presence of dural lesions were not investigated.7 We cannot exclude that dural lesions may have been underdiagnosed in previously reported CCM families, hence the importance of a detailed neuroradiologic examination, especially in PDCD10-mutated individuals.
No histopathologic definition of the dural lesions of our patients is available as none of them underwent surgery or biopsy. However, all MRI findings were highly suggestive of meningiomas. Indeed, the dural masses of our patients presented homogenous enhancement and the typical ‘dural tails’ after gadolinium injection. Moreover, a thickened dura (isolated or close to the masses) was almost always observed, frequently featuring the ‘en plaque’ meningiomas. Importantly, among PDCD10-mutated patients presenting multiple parenchymal CCMs associated with dural enhancing lesions (multiples in all cases except one), some have been examined and confirmed as meningioma.8–10 Although different type of lesions might be found within the dura mater, according to the neuroradiological pattern as well as to the evidence from the literature, we consider that the most likely diagnosis of the dural lesions herein reported is meningioma.
Our data support the association of CCMs and meningioma representing a distinct syndromic entity linked to CCM3/PDCD10. Although rare, this phenotype is clinically relevant because CCMs are high-risk lesions with clinical manifestations in approximately 60% of cases, namely recurrent headaches, seizures, progressive focal neurological deficits and haemorrhagic stroke, depending on the localisation, size and number of the lesions and the occurrence of bleeding.11,13 Meningiomas are commonly encountered in neuroradiological practice, but they are routinely diagnosed through imaging techniques that are not highly sensitive for CCMs so that latter lesions may in some cases be under diagnosed in meningioma patients. In particular, GRE sequences have been shown to reveal up to three times the number of CCMs compared to routine T1 and T2-weighted imaging MRI sequences.14 Susceptibility-weighted imaging (SWI) sequences seem to have an even higher sensitivity for multiple/familial CCMs as compared to classical GRE imaging.15 Aside from the unsolved discussion on which technique is the best imaging tool, we suggest the investigation of CCM lesions, performing appropriate MRI sequences (either GRE or SWI), whenever multiple and/or familial meningioma-like lesions are observed. Conversely, when dural masses are suspected in CCMs, post gadolinium injection scans may help defining the meningioma-phenotype, especially in PDCD10-mutated patients.
Further work describing CCM patients with multiple meningiomas are needed to clarify the prevalence of this likely underestimated condition. More importantly, since CCMs are high-risk lesions early recognition of this phenotype is warranted to plan appropriate management and care.
Acknowledgements
Author contributions were as follows. Francesco Garaci: manuscript writing, data analysis, data management; Luisa Marsili: manuscript writing, data analysis, data management; Florence Riant: manuscript editing, data management; Simone Marziali: data management; Michaelle Cécillon: data analysis; Roberto Pasquarelli: data acquisition; Federica Sangiuolo:manuscript editing; Roberto Floris: data management; Giuseppe Novelli: manuscript editing; Elisabeth Tournier-Lasserve: manuscript editing, data management; Francesco Brancati: manuscript writing, project development, data analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006; 5: 1045–1054. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM. Brain tumors. N Engl J Med 2001; 11; 344: 114–123. [DOI] [PubMed] [Google Scholar]
- 3.Shen Y, Nunes F, Stemmer-Rachamimov A, et al. Genomic profiling distinguishes familial multiple and sporadic multiple meningiomas. BMC Med Genomics 2009; 2: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet 2004; 8; 363: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 5.Haasdijk RA, Cheng C, Maat-Kievit AJ, et al. Cerebral cavernous malformations: From molecular pathogenesis to genetic counselling and clinical management. European J Hum Genet 2012; 20: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra S, Lin D, Recinos PF, et al. Cavernous malformations: Natural history, diagnosis and treatment. Nat Rev Neurol 2009; 5: 659–670. [DOI] [PubMed] [Google Scholar]
- 7.Spiegler S, Najm J, Liu J, et al. High mutation detection rates in cerebral cavernous malformation upon stringent inclusion criteria: One-third of probands are minors. Mol Genet Genomic Med 2014; 2: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labauge P, Fontaine B, Neau JP, et al. Multiple dural lesions mimicking meningiomas in patients with CCM3/PDCD10 mutations. Neurology 2009; 9; 72: 2044–2046. [DOI] [PubMed] [Google Scholar]
- 9.Riant F, Bergametti F, Fournier HD, et al. CCM3 mutations are associated with early-onset cerebral hemorrhage and multiple meningiomas. Mol Syndromol 2013; 4: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder W, Najm J, Spiegler S, et al. Predictive genetic testing of at-risk relatives requires analysis of all CCM genes after identification of an unclassified CCM1 variant in an individual affected with cerebral cavernous malformations. Neurosurg Rev 2014; 37: 161–165. [DOI] [PubMed] [Google Scholar]
- 11.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: Results of an ongoing study. J Neurosurg 1994; 80: 422–432. [DOI] [PubMed] [Google Scholar]
- 12.Marosi C, Hassler M, Roessler K, et al. Meningioma. Crit Rev Oncol Hematol 2008; 67: 153–171. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shahi Salman R, Hall JM, Horne MA, et al. Scottish Audit of Intracranial Vascular Malformations (SAIVMs) collaborators. Untreated clinical course of cerebral cavernous malformations: A prospective, population-based cohort study. Lancet Neurol 2012; 11: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehnhardt FG, von Smekal U, Ruckriem B, et al. Value of gradient-echo magnetic resonance imaging in the diagnosis of familial cerebral cavernous malformation. Arch Neurol 2005; 62: 653–658. [DOI] [PubMed] [Google Scholar]
- 15.de Champfleur NM, Langlois C, Ankenbrandt WJ, et al. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery 2011; 68: 641–647. [DOI] [PubMed] [Google Scholar]