Abstract
Background
Manual therapy (MT) and exercise have been extensively used to treat people with musculoskeletal conditions such as temporomandibular disorders (TMD). The evidence regarding their effectiveness provided by early systematic reviews is outdated.
Purpose
The aim of this study was to summarize evidence from and evaluate the methodological quality of randomized controlled trials that examined the effectiveness of MT and therapeutic exercise interventions compared with other active interventions or standard care for treatment of TMD.
Data Sources
Electronic data searches of 6 databases were performed, in addition to a manual search.
Study Selection
Randomized controlled trials involving adults with TMD that compared any type of MT intervention (eg, mobilization, manipulation) or exercise therapy with a placebo intervention, controlled comparison intervention, or standard care were included. The main outcomes of this systematic review were pain, range of motion, and oral function. Forty-eight studies met the inclusion criteria and were analyzed.
Data Extraction
Data were extracted in duplicate on specific study characteristics.
Data Synthesis
The overall evidence for this systematic review was considered low. The trials included in this review had unclear or high risk of bias. Thus, the evidence was generally downgraded based on assessments of risk of bias. Most of the effect sizes were low to moderate, with no clear indication of superiority of exercises versus other conservative treatments for TMD. However, MT alone or in combination with exercises at the jaw or cervical level showed promising effects.
Limitations
Quality of the evidence and heterogeneity of the studies were limitations of the study.
Conclusions
No high-quality evidence was found, indicating that there is great uncertainty about the effectiveness of exercise and MT for treatment of TMD.
Temporomandibular disorders (TMD) consist of a group of pathologies affecting the masticatory muscles, the temporomandibular joint, and related structures.1,2 Temporomandibular disorders constitute a major public health problem, as they are one of the main sources of chronic orofacial pain interfering with daily activities. These disorders also are commonly associated with other symptoms affecting the head and neck region, such as headache, ear-related symptoms, cervical spine dysfunction,3,4 and altered head and cervical posture.5–15
Physical therapy has been used for decades for treating craniomandibular disorders using thermal packs, vapocoolants, and transcutaneous electrical nerve stimulation (TENS).16 In 1997, Feine and Lund17 recognized that dentists valued physical therapy treatment for TMD, and a recent national survey in the United Kingdom showed that, despite limited evidence, 72% of respondents considered physical therapy to be an effective treatment option for TMD, with jaw exercise (79%), ultrasound (52%), manual therapy (MT) (48%), acupuncture (41%), and laser therapy (15%) as the most effective modalities for managing TMD.18 To date, evidence supports the use of conservative and reversible treatment approaches for TMD treatment, although a multidisciplinary health care approach may be required. Physical therapy is among the 10 most commonly used treatments for TMD,19 focused on decreasing neck and jaw pain, improving range of motion (ROM), and promoting exercise to maintain healthy function.
The goals of physical therapy in the treatment of TMD are to decrease pain, enable muscle relaxation, reduce muscular hyperactivity, and re-establish muscle function and joint mobility.20 Physical therapy treatment is reversible and noninvasive and provides self-care management in an environment to create patient responsibility for their own health. Physical therapy modalities include electrophysical modalities (ultrasound, microwave, laser), electroanalgesic modalities (TENS, interferential current, biofeedback), acupuncture, therapeutic exercise, and MT. Therapeutic exercise and MT are used to improve strength, coordination, and mobility and to reduce pain,21 and treatment may include and focus on poor posture, cervical muscle spasm or pain, and treatment for referred cervical origin orofacial pain (pain referred from upper levels of the cervical spine).22 The evidence for the effect of electrophysical modalities has been questioned.23
Manual therapy (including joint mobilization, manipulation, or treatment of the soft tissues) and therapeutic exercises in physical therapy treatments have been increasingly used by clinicians and researched due to positive outcomes in some conditions, especially for low back pain, neck pain, and related disorders.24 Manual therapy has been used to restore normal ROM, reduce local ischemia, stimulate proprioception, break fibrous adhesions, stimulate synovial fluid production, and reduce pain. In the area of orofacial pain, several systematic reviews have been conducted regarding physical therapy and specifically MT and exercise interventions for TMD.19,23,25 Most of these early systematic reviews highlighted the positive effects of exercises and MT to improve symptoms and function in people with TMD. However, 2 reviews19,23 were conducted 9 years previously and included few randomized controlled trials (RCTs). Research has expanded over the last few years, and new RCTs have been conducted, which implies that the information from earliest reviews is now outdated. Another recent systematic review25 combined pathologies of the upper extremity and TMD. That review included several types of designs and did not focus on RCTs, which are the best evidence when looking at interventions. In addition, based on a preliminary search performed by our team, it was realized that this review missed important RCTs in the area (included only 5 studies). In addition, none of these systematic reviews provided a meta-analysis of the trials. Therefore, the objectives of this systematic review were: (1) to summarize the evidence from and evaluate the methodological quality of RCTs that examined the effectiveness of MT and therapeutic exercise interventions in the management of TMD and (2) to determine the magnitude of the effect of these interventions to manage TMD.
Method
The reporting of this systematic review is based on the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.26 The aim of the PRISMA statement is to help authors improve the reporting of systematic reviews and meta-analyses. It consists of a 27-item checklist and 4-phase flow diagram. This systematic review was registered in PROSPERO (CRD42013005628).
Study Selection
Inclusion criteria for this review were as follows.
Participants.
This review was restricted to trials with participants meeting the following criteria: (1) diagnosis of TMD according to the research diagnostic criteria for temporomandibular disorders (RDC/TMD) established by Dworkin and LeResche27 or any clinical diagnosis involving signs and symptoms of TMD,28,29 (2) adult (>18 years of age), (3) musculoskeletal dysfunction, (4) pain impairment, (5) no previous surgery in the temporomandibular region, and (6) no other serious comorbid conditions (eg, fracture in region, cancer, neurological disease).
Studies.
This review targeted RCTs comparing any type of MT intervention (eg, mobilization, manipulation, soft tissue mobilization) or exercise therapy alone or in combination with other therapies with a placebo intervention, controlled comparison intervention, or standard care (ie, treatment that normally is offered).
Outcomes
The primary outcomes of interest for this systematic review were pain, ROM, and oral function. Oral function for this systematic review focused on limitations of daily activities of patients with TMD measured through different questionnaires. A secondary outcome of interest was pressure pain threshold (PPT).
The minimal clinically important difference for pain has been reported to range from 1.5 to 3.2 points.30–34 The smallest detectable difference of maximal mouth opening in healthy people has been reported to be 5 mm, indicating that an important change of at least 5 mm can be considered clinically relevant.35 Measurements of PPT have been shown to have good or excellent interrater and intrarater reliability.36–38 The minimal important difference for PPTs has been reported to be ≥1.10 kg/cm2/s.39,40 This systematic review was open to all time points: immediately posttreatment and short-term, intermediate-term, and long-term follow-up.
Data Sources and Searches
A bibliographic search of 6 electronic databases was conducted:
MEDLINE (database root [1966]–April 7, 2015),
EMBASE (database root [1988)]–April 7, 2015),
Cochrane Library and Best Evidence (database root [1991]–April 7, 2015),
ISI Web of Science (database root [1965]–April 7, 2015),
EBM reviews–Cochrane Central Register of Controlled Trials (database root [1991]–April 7, 2015), and
CINAHL (database root [1982]–April 7, 2015).
Key words and medical subject headings were identified with the assistance of a librarian who specialized in health science databases and experts in the orofacial pain field. No restrictions were made regarding the language of publication. A manual search of the references of selected studies was conducted as well (refer to the Appendix for an example of the search strategy).
Data Screening
Two independent investigators screened the titles of publications found in the databases and, if available, the abstract of the publication. The Early Review Organizing Software (EROS) (http://www.eros-systematic-review.org/) Web platform was used for screening the articles for inclusion. In order for papers to be included in the review, the paper had to meet all inclusion criteria of this systematic review on the rating form created in EROS software. Studies were analyzed with the available information. Authors were not contacted.
Disagreements between reviewers on inclusion were resolved by consensus. The kappa statistic was calculated using STATA software, version 12, (StataCorp LP, College Station, Texas) to determine the level of agreement between raters on trial inclusion before consensus. Criteria proposed by Byrt41 were used to interpret kappa values.
Data Extraction
The information of each study included in this review was extracted and entered into Excel or Microsoft Word (Microsoft Corp, Redmond, Washington) files. For each part of the review, data extraction was carried out independently by 2 reviewers. Data were extracted on study characteristics, including the design, type of TMD, type of interventions, main and secondary outcomes, and treatment estimates. Any disagreements on data extraction were resolved by consensus.
Quality Assessment (Risk of Bias)
Assessments of quality (risk of bias) were completed by 2 independent reviewers (any 2 members of the research team). For the assessment of RCTs, our team used a compiled set of items based on the 7 tools most commonly used to evaluate the risk of bias in complex physical therapy trials.42 In addition, the risk of bias tool was used with the main outcome of each study to make the assessments. We followed the guidelines established by the Cochrane Collaboration to perform assessments of risk of bias; however, we developed specific decision rules to make decisions as described elsewhere.43 For the overall assessment of risk of bias, a trial was considered at low risk of bias if it was rated as low risk in all individual domains, if the rating was unclear in at least one domain and the other domains were unclear or low, or if the overall assessment of risk of bias was unclear. Finally, an overall assessment of high risk of bias was considered if at least one domain was rated as high. These criteria have been used previously by our team and other authors.43,44
Any discrepancies in quality ratings were resolved by discussion. If consensus could not be reached, a third member of the review team with expertise in quality assessments (S.A-O.) acted as an arbitrator and made a final decision.
Data Analysis and Synthesis
Data analysis was performed based on type of intervention (ie, exercise, mobilization, and manipulation), TMD diagnosis (myogenous TMD, arthrogenous TMD, mixed TMD), and type of outcome (eg, pain intensity, range of mouth opening [ROM], oral function (oral-related quality of life]). For analysis of continuous outcome data, we used the mean difference (MD) and the standardized mean difference (SMD) with 95% confidence interval (95% CI) to pool data. Heterogeneity was evaluated statistically using the I2 statistic. The MD and SMD were defined according to the Cochrane Collaboration,45 as follows:
MD is a standard statistic that measures the absolute difference between the mean value in 2 groups in a clinical trial. It estimates the amount by which the experimental intervention changes the outcome on average compared with the control. It can be used as a summary statistic in meta-analysis when outcome measurements in all studies are made on the same scale.
The SMD is used as a summary statistic in meta-analysis when the studies assess the same outcome but measure it in a variety of ways (ie, use different psychometric scales). In this circumstance, it is necessary to standardize the results of the studies to a uniform scale before they can be combined. The standardized mean difference expresses the size of the intervention effect in each study relative to the variability observed in that study.
We decided to pool studies based on TMD diagnosis, intervention provided, and outcome. We grouped studies that had the same diagnosis (myogenous, arthrogenous, or mixed), similar intervention of interest (ie, MT, exercises), and the same underlying outcome. Thus, we created groups of studies that were similar in terms of these characteristics and pooled them. In the presence of clinical heterogeneity in the study population or intervention, the DerSimonian and Laird random-effects model of pooling was used based on the assumption of the presence of interstudy variability to provide a more conservative estimate of the true effect.46,47
Cohen's criteria were used to interpret values of effect sizes found for our pooled estimates.48 Cohen described 0.2, 0.5, and 0.8 as small, moderate, and large effect sizes, respectively.48 Review Manager (RevMan) version 5.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2008) was used to summarize the effects (ie, pooled MD values) and construct forest plots for all comparisons.
Subgroup and sensitivity analysis.
In order to investigate and accommodate heterogeneity (clinical heterogeneity in the study population or intervention) as explained above, a random-effects model was used across all the comparisons. Furthermore, in order to explain the heterogeneity in terms of study-level covariates, we could have attempted a meta-regression model. However, because of the small number of studies (<10) for comparison, this analysis was not possible. We attempted to perform sensitivity analyses when possible.
We did not perform sensitivity analyses based on quality because the risk of bias of the analyzed studies was either unclear or high, with no study being classified as low risk. These factors precluded sensitivity analyses by different levels of biases. Therefore, the pooled data should be interpreted carefully.
Data synthesis.
The quality of the body of the evidence was assessed using the GRADE approach.49 The evidence was classified as high, moderate, low, and very low, as described by Guyatt et al.49 Domains that may decrease the quality of the evidence are: (1) the study design, (2) risk of bias, (3) inconsistency of results, (4) indirectness (not generalizable), (5) imprecision (insufficient data), and (6) other factors (eg, reporting bias).
Role of the Funding Source
Dr Armijo-Olivo is supported by the Canadian Institutes of Health Research (CIHR) through a full-time Banting fellowship, by the Alberta Innovates Health Solution through an incentive award, by the STIHR Training Program of Knowledge Translation (KT) Canada, and by the Music and Motion Fellowship from the Faculty of Rehabilitation Medicine of the University of Alberta. The funding bodies had no input in the design, collection, analysis, or interpretation of data; writing of the manuscript; or the decision to submit the manuscript for publication.
Results
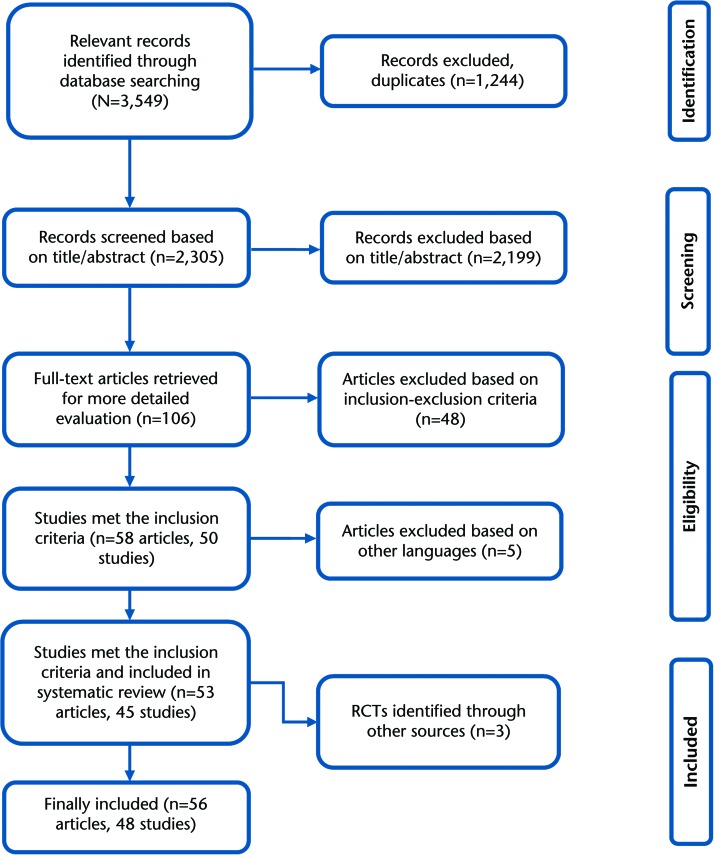
The search of the literature resulted in a total of 3,549 published articles. Of the 3,549 published articles, 106 were considered to be potentially relevant. Independent review (in duplicate) of these 106 articles led to the inclusion of 58 articles representing 50 studies (some studies reported data from the same population in 2 manuscripts). There were 5 articles in other languages50–54 that were not possible to translate by our study team and were not included in the final analysis. Thus, 45 studies were included for this review from the search of the databases. In addition, 3 studies55–57 were obtained through a manual search. Therefore, a total of 48 studies were included in the final analysis (Fig. 1). The agreement between reviewers to select the articles for this review was kappa=0.98 (95% CI=0.977, 0.99). According to Byrt's criteria,41 the agreement between reviewers was excellent. Details of included studies are provided in eTable 1.
Figure 1.
Flowchart of trial selection based on PRISMA guidelines.
Diagnosis
There was considerable diversity in the clinical presentations and diagnoses of participants with TMD among the included studies (eTab. 1). Fourteen of the studies examined the effectiveness of the exercise or MT interventions in muscular TMD (myogenous TMD), 14 studies examined the effectiveness in patients with articular TMD (arthrogenous TMD), and 19 studies examined the effectiveness in patients with mixed diagnoses of TMD (including both myogenous and arthrogenous TMD).28,29 One study looked at both myogenous and arthrogenous TMD.58 Twenty-one of the studies57–76 used the RDC/TMD established by Dworkin and LeResche27 to classify the patients as having TMD. The remaining 27 studies used their own diagnostic criteria, based on signs and symptoms of the patients.
Methodological Quality Assessment
The results of the critical appraisal of the selected studies are presented in eTable 2. Only 6 studies accomplished more than 60% of the items listed in eTable 2.57,66,67,76–78 Most of the studies did not accomplish items with important methodological indicators of risk of bias, such as randomization, allocation concealment, blinding, and intention to treat (ITT). For example, study flaws regarding patient selection were mainly related to description and appropriateness of the randomization procedure and concealment of allocation, with only 20 (41.6%) and 4 (8.3%) of the studies meeting these criteria, respectively. As expected, items related to blinding were not achieved by the majority of the studies. Only 3 of the studies used a double-blinded design and could blind participants. These studies used a placebo arm, which is hard to obtain in these types of interventions. In addition, only 12 (25%) of the studies used blinded assessment of outcomes, and none of the studies blinded the therapist. Thus, blinding was the area that was the hardest section to be met by the analyzed studies. When analyzing issues regarding intervention, we found that although it is expected that interventions would be well described to be reproducible, only 64.6% (n=30) of the studies described the main intervention to be tested. In addition, most studies failed to control for cointerventions. Only 6 studies met this item.
Testing participants' adherence to intervention and having adequate adherence was another issue that was not met by many studies (only 11 and 7 studies, respectively). Furthermore, adverse effects were reported on only 10 of the studies, but there was no specific description of such events when they occurred in all of the studies.
Despite the fact that the adequate handling of dropouts is considered an important method used to prevent bias in data analysis, only 17 of the analyzed studies included information regarding the reasons of withdrawals and dropouts, and only 16 studies used intention-to-treat analysis. The outcome measures were not described well in terms of validity, reliability, or responsiveness. Only a few studies reported these items (11, 17, and 3 studies, respectively). Moreover, the authors did not report intrarater or interrater reliability of the assessors who performed outcome measurements. Regarding statistical issues, it was uncertain if sample size was adequate in 30 of the studies, and only 18 studies reported an evaluation of the clinical significance of their results. Risk of bias assessments using the risk of bias tool determined that none of the studies was considered as low risk of bias. Most of them were classified as either unclear (58.4%) or high risk of bias (41.6%).
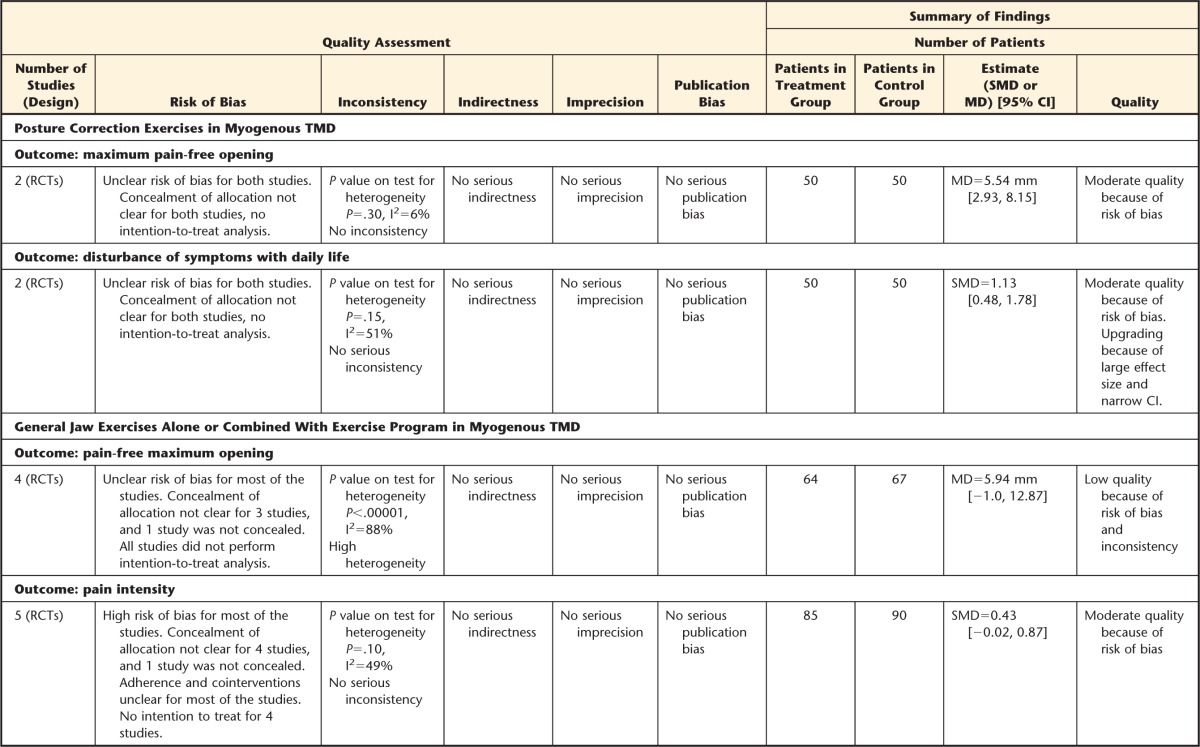
Effectiveness of Intervention by TMD Diagnoses: Posture Correction Exercises in Myogenous TMD
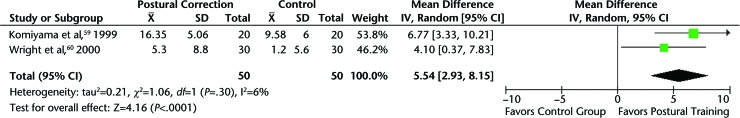
Two studies59,60 evaluated the effectiveness of posture correction exercises for patients with myofascial pain. Both studies showed positive results of postural exercises for improving symptoms of muscular TMD. When pooling the data for these 2 studies, which had similar interventions, diagnoses, and outcomes, maximum pain-free mouth opening significantly increased in patients receiving postural training compared with a control group. The MD in maximum pain-free mouth opening was 5.54 mm (95% CI=2.93, 8.15) (Fig. 2), which was clinically significant in favor of postural training.35 Furthermore, patients treated with postural training had significantly fewer symptoms and disturbance with daily living compared with a control group. The SMD in symptoms and disturbance of symptoms with daily life was 1.13 (95% CI=0.48, 1.78), indicating a large, clinically significant effect size for this pooled outcome.
Figure 2.
Maximum pain-free opening: postural training versus control group in patients with myogenous temporomandibular disorders. CI=confidence interval, IV=inverse variance.
General Jaw Exercises Alone or Combined With Neck Exercise Program in Myogenous TMD
Eight studies56–58,62,63,66,79,80 looked at the effect of exercises alone or combined with other therapies for myogenous TMD. The results of these studies were equivocal. Five of them did not find significant differences between a general physical therapy exercise program targeted to the jaw56,62,79,80 or jaw and neck57 compared with a control or other active treatments, such as biofeedback, TENS, use of the TheraBite Jaw Motion Rehabilitation System (ATOS Medical AB, Hörby, Sweden), or oral splint therapy. However, 3 studies58,63,66 showed better outcomes, especially on pain and ROM, compared with control groups.
Data were pooled from studies that had similar outcomes and diagnoses and compared an exercise program and other forms of therapy, such as education,62,66 or splint therapy.56,58 Data from these studies56,58,62,66 indicated that there was a trend to favor exercise therapy for pain-free maximum mouth opening and pain intensity compared with a control group. The MD for pain-free maximum mouth opening was 5.94 mm (95% CI=−1.0, 12.87), which is considered clinically relevant.35 The SMD for pain intensity pooling 5 studies57,58,62,63,66 was 0.43 (95% CI=−0.02, 0.87), with a moderate effect size according to Cohen's guidelines.48 When performing sensitivity analyses, grouping studies comparing exercise therapy and education,62,66 a nonsignificant effect was found on pain-free maximum mouth opening (1.92 mm; 95% CI=−0.57, 4.41). However, when comparing exercises and splint therapy,56,58 a statistically and clinically meaningful effect was found (12.31 mm; 95% CI=7.73, 16.89).
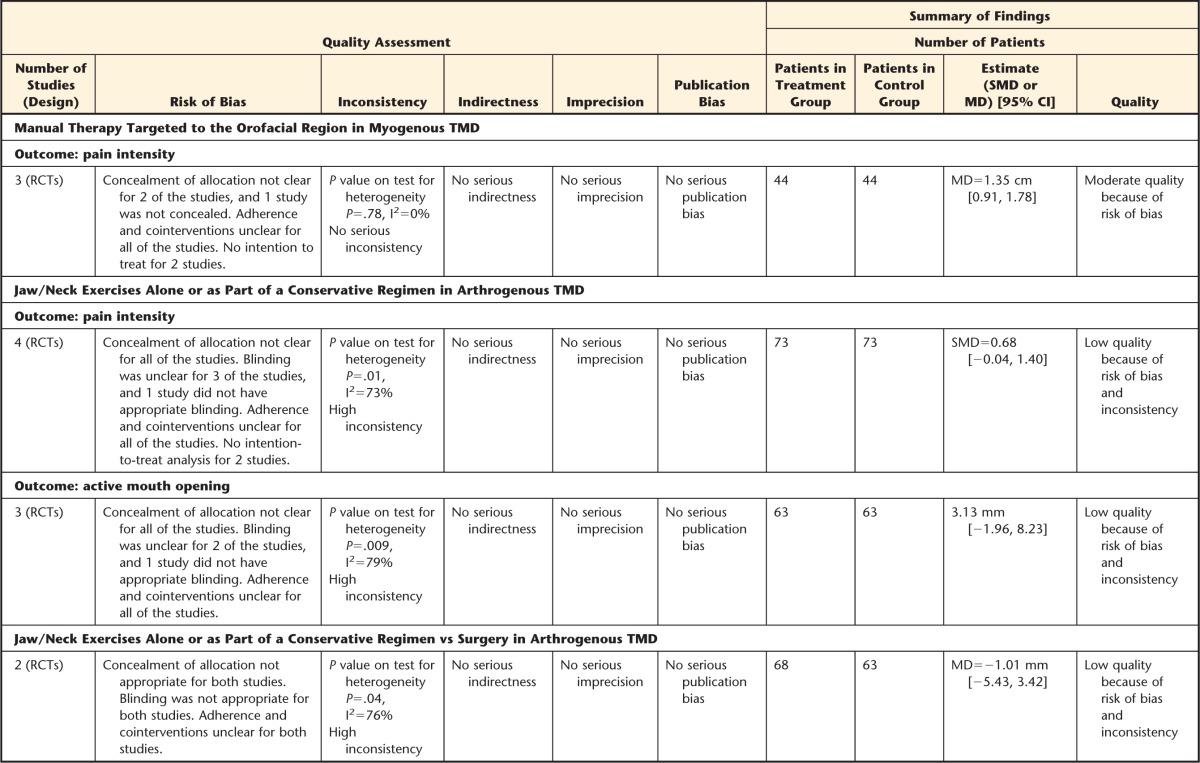
Manual Therapy Targeted to the Orofacial Region in Myogenous TMD
Four studies64,65,77,81 looked at MT techniques, such as facial manipulation versus botulinum toxin81 or intraoral myofascial therapy versus waiting list, and self-care education and exercises for people with myogenous TMD.64,65,77 The results of these studies support the use of MT to treat myogenous TMD, as people treated with all of these approaches had improved mouth opening and reduced jaw pain from baseline. Although the results for the intraoral myofascial therapy and exercise groups were superior to the results for the waiting-list control group, there was no statistically significant difference between them. In addition, facial manipulation had an equivalent effect as botulinum toxin. However, at 3 months after treatment, facial manipulation was slightly superior in reducing subjective pain perception, and botulinum toxin injections were slightly superior in increasing ROM. When pooling the results from 3 of these studies based on similar outcomes and diagnoses and comparing similar interventions regarding MT,64,65,81 we found that MT significantly reduced pain at 4 to 6 weeks of treatment compared with botulinum toxin or waiting list, approaching a clinically relevant value. The MD for pain intensity was 1.35 cm (95% CI=0.91, 1.78). When pooling the studies that considered the comparison of MT versus a waiting list only, similar results were obtained (1.31 cm; 95% CI=0.86, 1.76).
Manual Therapy Mobilization of the Cervical Spine and Myogenous TMD
A recent RCT conducted by La Touche et al67 testing a more specific approach directed to the cervical spine to treat patients with cervico-craniofacial pain of myofascial origin was performed. This preliminary study showed that mobilizations targeted to the cervical spine drastically decreased pain intensity and pain sensitivity (via PPT evaluation) in patients with cervico-craniofacial pain of myofascial origin immediately after the application of the technique compared with placebo treatment. The effect sizes found in this study for pain intensity (28.75 points; 95% CI=21.65, 35.85) and PPT (1.12 kg/cm2; 95% CI=0.96, 1.29) were considered clinically relevant.
Jaw and Neck Exercises Alone or as Part of a Conservative Regimen in Arthrogenous TMD
Eight studies58,61,68,82–86 that examined patients with arthrogenous TMD focused on jaw and neck exercises alone or combined with other therapies, such as medications, surgery, or self-care recommendations. Six studies58,61,68,84–86 focused on exercise therapy alone,85,86 exercise therapy combined with conventional treatment,61 or the combination of jaw exercises with TheraBite58 or myofunctional therapy.68 The remaining 2 studies82,83 looked at the effectiveness of surgery (arthrocentesis or arthroscopy) combined with conservative treatment including exercises for the jaw versus jaw exercises alone.
Although the results were mixed, most of the studies favored the use of exercises alone or as part of a general regimen to treat people with arthrogenous TMD, including disk displacements with or without reduction.58,68,84–86 However, one study61 did not find that exercises were superior to a control group involving general physical therapy treatment.
Data were pooled from studies with similar outcomes and diagnoses that compared an exercise program with other forms of therapy, such as education61 or splint therapy,58 or with a control group.68,86 When pooling the results of the studies investigating the effectiveness of exercise alone or in combination with other conservative therapies on pain intensity,58,61,68,86 we found that there was no statistically significant difference in pain between exercise and control groups. Nevertheless, there was a trend to favor the exercise group compared with the control group. The SMD for pain intensity was 0.68 (95% CI=−0.04, 1.40), with a moderate effect size according to Cohen's guidelines.48 When pooling was focused on those studies including only a control group,68,86 similar results were found, although the SMD increased (SMD=1.11; 95% CI=−0.73, 2.94). Regarding active mouth opening, a nonsignificant effect was found between general jaw exercises and education, splint therapy, or a control group when pooling 3 studies.58,61,86 The MD for active mouth opening was 3.13 mm (95% CI=−1.96, 8.23). A trend favoring exercises was observed based on the 95% CI values.
When pooling the studies82,83 that looked at exercises plus arthrocentesis or arthroscopy versus conservative therapy including exercises alone on active mouth opening at 6 months, we found no differences between these approaches. The MD was −1.01 mm (95% CI=−5.43, 3.42), implying that conservative treatment plus exercises is appropriate to treat disk displacement without reduction or when patients have restricted mandibular movement. The results indicate noninvasive procedures as a first line of treatment.
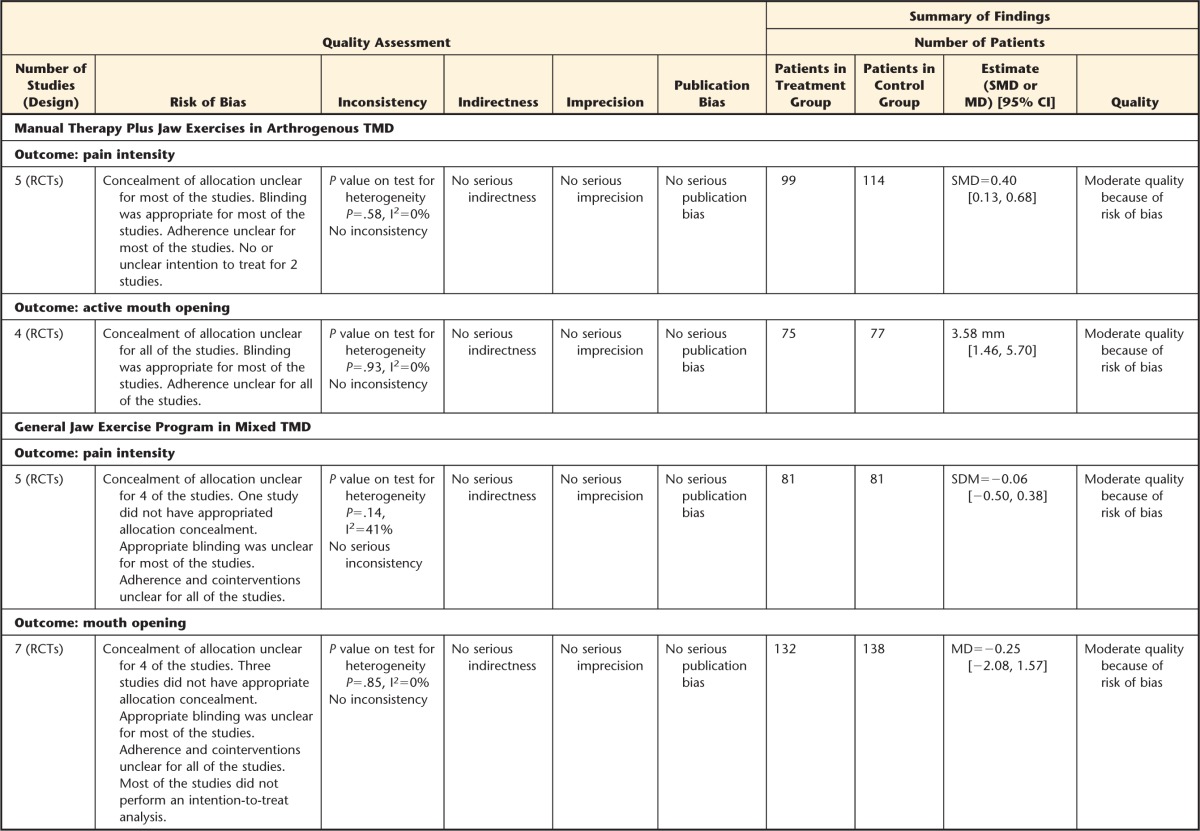
Manual Therapy Plus Jaw Exercises in Arthrogenous TMD
Seven studies69,70,78,87–91 looked at the combined effect of MT plus jaw exercises for people with arthrogenous TMD. Three studies69,87,88 compared MT and exercises versus splint therapy, 1 study89,90 compared MT and exercise with self-care and advice regarding prognosis, and 2 studies78,91 used medication as a comparison. In addition, one study70 compared anesthetic blockage of the auriculotemporal nerve and MT and exercises in addition to blockage of the auriculotemporal nerve.
In general, we found that MT plus exercises reduced symptoms and increased ROM for patients with arthrogenous TMD, particularly for those with reduced ROM due to disk displacements without reduction (“closed lock”). Five of these studies69,78,87,88,91 favored the use of MT in conjunction with exercises compared with splints69,87,88 or with medications91 or other nonconservative treatments for arthrogenous TMD, such as arthroscopy and arthroplasty.78
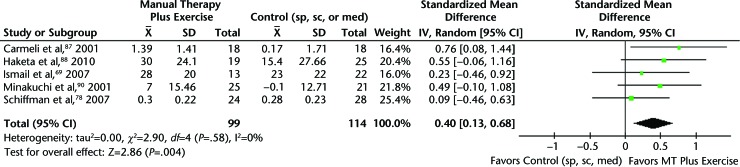
When pooling the results from the studies with homogeneous interventions and similar outcomes, diagnoses, and available data,69,78,87,88,90 we found that pain was significantly reduced in patients receiving MT combined with exercises compared with splint therapy, self-care, or medications. The SMD for pain intensity at 4 weeks to 3 months was 0.40 (95% CI=0.13, 0.68), with a moderate effect size according to Cohen's guidelines48 (Fig. 3).
Figure 3.
Pain intensity at 4 weeks to 3 months: manual therapy plus excercises versus control group in patients with arthrogenous temporomandibular disorders. CI=confidence interval, IV=inverse variance, sp=splint, sc=standard care, med=medications.
When looking at active mouth opening,69,87,88,90 we found that MT plus exercises significantly increased active mouth opening compared with splint therapy, self-care, or medications. The MD for active mouth opening at 4 weeks to 3 months was 3.58 mm (95% CI=1.46, 5.70).
General Jaw Exercise Program in Mixed TMD
Eleven studies55,71–74,92–99 looked at exercises alone or as part of a general conservative therapeutic regimen to treat patients with mixed TMD. In general, exercises for mixed TMD compared with control groups had better results for decreasing pain and improving function and pain sensitivity of the masticatory muscles.55,71,96,97 However, compared with other forms of active treatments, such as splints, a global postural re-education program, or acupuncture,72–74,95,95,98,99 no significant differences between these treatments were found.
When pooling the results of studies with available data and similar interventions and outcomes,55,71,73,93,94 we found that exercises in the form of general jaw exercises plus conventional treatment or with the addition of an oral device94 were not superior to other treatment modalities, such as splint therapy, global re-education posture, splint plus counseling, acupuncture, or standard conservative care, in improving pain intensity. The SMD for pain intensity was −0.06 (95% CI=−0.50, 0.38), with a very small effect size according to Cohen's guidelines.48
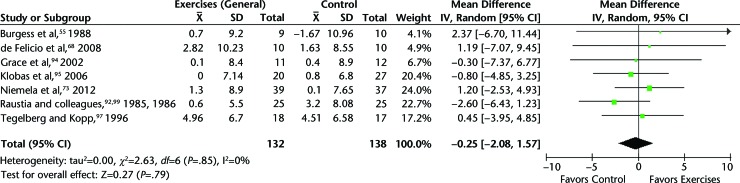
When pooling results for mouth opening,55,71,73,92,94,95,97 nonsignificant differences were obtained between general jaw exercises and splint therapy, global re-education posture, splint plus counseling, or standard conservative care. The MD for mouth opening was −0.25 mm (95% CI=−2.08, 1.57) (Fig. 4).
Figure 4.
Mouth opening: general jaw excercises versus splint therapy, global re-education posture, splint plus counseling, or standard conservative care in patients with mixed temporomandibular disorders. CI=confidence interval, IV=inverse variance.
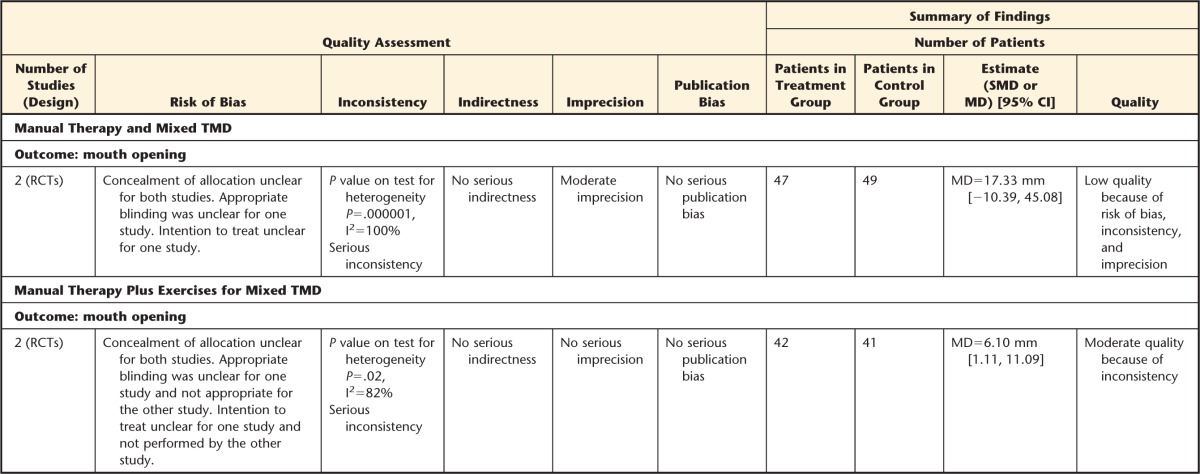
Manual Therapy and Mixed TMD
Six studies76,100–104 looked at MT alone, such as mobilization of atlantoaxial joint,101,103 mobilization at the level of cervical spine,102 manipulation of the upper thoracic spine (D1),76 massage to masticatory muscles,104 or mobilizations at the level of TMJ joint,100 for treating patients with mixed TMD. Results were mixed. The studies by Mansilla-Ferragud et al101 and Otano and Legal103 showed positive results at improving mouth ROM and increasing PPT in the orofacial region when comparing mobilization of the atlantoaxial joint versus placebo. However, no statistical differences were found between MT targeted to the jaw and jaw exercises plus splint therapy,100 between cervical chiropractic adjustment and cervical trigger point therapy,102 upper thoracic manipulation and placebo,76 or masticatory muscle massage and splint104 for improving symptoms of patients with mixed TMD.
When pooling data from the 2 studies that looked at similar manual techniques, TMD diagnoses, and outcomes,101,103 although there was no significant difference between mobilization of atlantoaxial joint or control group receiving no mobilization, the MD in mouth opening between control and MT groups was 17.33 mm (95% CI=−10.39, 45.06). This difference can be considered as a clinically relevant improvement in mouth opening favoring MT treatment.
Manual Therapy Plus Exercises for Mixed TMD
Two studies75,105,106 investigated the effect of MT combined with exercises in people with mixed TMD. Tuncer et al75 looked at the specific effect of orofacial and cervical MT combined with stretching techniques for the masticatory and neck muscles compared with exercises for the jaw and neck alone and education (home physical therapy). Von Piekartz and Ludtke106 compared the effect of orofacial physical therapy and neck exercises and MT techniques targeted to both orofacial and cervical regions plus home exercises compared with treatment targeted to the cervical spine only in people with mixed TMD. Pooling the results of the 2 studies75,105,106 with similar interventions, outcomes, and diagnoses, we found that MT targeted to the orofacial region or in combination with cervical treatment was better than home exercises for the jaw and neck alone or treatment to cervical spine alone for improving mouth opening. The mouth opening between control and MT groups was 6.10 mm (95% CI=1.11, 11.09) favoring MT groups. This difference was clinically relevant.35
Adverse Events
Adverse effects were reported in only 10 of the 48 included trials. Eight of the trials57,65,67,74,75,77,87,88 reported no adverse events with the treatments. Nascimento et al70 reported some adverse events due to the anesthetic blockages procedure. In that study, 29.4% of the patients (66/224) had temporary facial nerve paralysis, 0.44% (1/224) had hematoma, and 2.23% (5/224) had positive aspirations. Niemela et al73 reported that pain on TMJ palpation increased significantly in the splint group compared with the control group. No adverse events regarding exercise therapy or MT treatments were reported among the trials included.
Data Synthesis
The overall quality of evidence for most comparisons was low to moderate according to the GRADE approach.49 The trials included in this review had unclear or high risk of bias. Thus, the evidence was generally downgraded for 3 reasons: (1) risk of bias, 2) level of heterogeneity (inconsistency), and (3) some imprecision surrounding the effect estimate. Details of GRADE assessment of the included studies are displayed in the Table. From the 14 analyses performed, most of the evidence was considered moderate (9 analyses). The rest of the evidence was considered low. Thus, we can say that the total evidence was considered low.
Table.
GRADE Evidence Profilea
SMD=standardized mean difference, MD=mean difference, CI=confidence interval, RCT=randomized controlled trial.
Discussion
Main Results
Although the quality of the evidence is mostly uncertain and low, the results of our systematic review showed positive results when using postural exercises and jaw exercises to treat both myogenous and arthrogenous TMD disorders. Manual therapy alone or in combination with exercises shows promising effects. Manual therapy targeted to the cervical spine decreased pain and increased mouth ROM in patients with myogenous TMD. Exercises did not show superiority over other treatments for treating mixed TMD. A general exercise program was effective compared with arthrocentesis or arthrography for treatment of arthrogenous TMD, with conservative treatments as a first line of treatment. There remain limited RCTs of high quality that have investigated the effectiveness of MT and exercises to treat TMD.
Effect of Exercise for Treating TMD
Exercise programs are advocated for treating people with musculoskeletal disorders. Therapeutic exercises are prescribed to address TMD. Passive and active stretching of muscles are performed to increase mouth ROM and reduce pain. Postural exercises are helpful.21 The results of our systematic review are consistent with previous reviews,19,23 showing positive effects when using exercises to treat myogenous and arthrogenous TMD. In particular, interventions including exercises to correct head and neck posture and active and passive oral exercises can be effective for reducing musculoskeletal pain and improving oromotor function.59,60 However, most of these exercise programs were part of a general conservative treatment regimen including other therapies and did not provide clear information regarding dosage, frequency, or adherence, so the isolated effect of exercise to treat TMD and the optimal regimen are uncertain at this time. General aerobic exercises have been shown to improve muscle strength, flexibility, and functional capacity and could induce analgesia.107 Further research is needed to investigate the usefulness of aerobic exercise and focused muscular training, especially exercises targeted to cervical muscles in people with TMD.
Effect of Manual Therapy for Treating TMD
Manual therapy has been used to restore normal ROM, reduce local ischemia, stimulate proprioception, break fibrous adhesions, stimulate synovial fluid production, and reduce pain. Based on the results of this systematic review, MT shows promising results for treatment of myogenous, arthrogenous, and mixed TMD, although the evidence is limited and low. A combination of MT for the orofacial region plus MT of the cervical spine was more effective than home exercises or treatment to cervical spine alone in people with mixed TMD. Research, to date, suggests that a mixed therapy involving MT techniques and exercises improves patient outcome. Other systematic reviews have shown similar results.24
Mobilization of the cervical spine resulted in decreases in pain intensity and pain sensitivity (via PPT evaluation) in patients with myogenous TMD that exceed suggested values for minimum clinically important differences for pain and treatment of the cervical spine. Manual therapy techniques such as mobilization of the cervical spine could have an influence on orofacial pain and movement in the jaw through the connections of these 2 systems in the trigeminocervical nucleus.108
Methodological Elements and Overall Quality of the Evidence Affecting Observed Effect
The overall rating of the evidence for this review was low. This finding was due mainly to the risk of bias of the analyzed studies. The methodological biases common to the included studies could have an impact on results. Selection bias could have existed, as only 20 trials reported appropriate randomization and only 4 reported concealment of allocation.
Another important bias was the lack of blinding, especially of the patients and assessors. Only 12 studies used blinded assessment of clinician-assessed outcomes such as mouth opening. However, we also were interested in pain, which is a subjective outcome and dependent on the patient's report. It is likely that lack of blinding could have affected the results of these studies. However, because of the nature of the interventions investigated, blinding would not be possible in many of them. There is empirical evidence showing that trials without appropriate randomization, concealment of allocation, and blinding tend to report an inaccurate treatment effect compared with trials that include these features.109 Thus, the results of this systematic review should be interpreted with caution, especially in trials with subjective self-reported outcomes.
Other potential biases that could potentially have affected observed effects were inappropriate handling of withdrawals and dropouts (only 16 trials used ITT analysis). Effect sizes from trials that excluded participants in their analysis or that used a modified ITT protocol tended to be more beneficial than those from trials without exclusions, demonstrating that the ITT principle is important to preserve the benefits of randomization and keep unbiased estimates when the objective of the trial is to investigate effectiveness.110
Studies did not report interventions in sufficient detail to be reproducible. In addition, they did not control for cointerventions and did not have adequate adherence to treatment. These issues are of importance for this study, as it is unclear if the effects on selected outcomes were due to the effect of exercise, MT, or other cointerventions. In addition, it is unclear if the participants received enough dosage of treatments, as adequate adherence was accomplished by only a very small proportion of studies (15.2%). Adherence testing should be systematically studied in future studies with exercise prescriptions.
The present study used a compilation of items from all of the scales used in the reviewed physical therapy literature in addition to the risk of bias tool. Our recent analysis of health scales used to evaluate methodological quality determined that none of these scales are adequate for use alone.44,111 Therefore, we decided to use all of the scales, using a compilation of their items, to provide a comprehensive and sensitive evaluation of the quality of individual trials. Research investigating methodological predictors for determining trial quality in physical therapy is needed.
Limitations
The findings of this review are specific to TMD (nonsurgical) and to exercise and MT. As with any systematic review, there is the potential for selection bias, yet our group used a comprehensive search strategy and included databases as well as manual search. There was a small proportion of studies in other languages that our team could not translate. However, we believe that most of the representative studies were included in the final analysis of this systematic review. In addition, it has been reported that language-restricted meta-analyses only minimally overestimate treatment effects (∼2% on average) compared with language-inclusive meta-analyses.112 Therefore, language-restricted meta-analyses do not appear to lead to biased estimates of intervention effectiveness.112,113
The heterogeneity among studies, particularly with respect to TMD diagnosis, study intervention, and chosen control or comparison intervention was a challenge. Many studies included the use of exercises or MT as part of a general treatment program, which made the evaluation of these treatments in isolation difficult. Moreover, different diagnostic criteria for TMD were used. Only 21 out of 48 studies included a diagnostic tool that had been demonstrated as being valid, reliable, and reproducible to diagnose TMD. Thus, diagnoses used for the analyzed studies might not be appropriate. Despite this lack of standardized diagnosis, the study populations in all trials appeared to be representative of patients seen in clinical practice. We encourage clinicians and researchers using the new diagnostic criteria for TMD (RDC/TMD) in future studies to allow consistent diagnoses according to the same criteria, taxonomy, and nomenclature to avoid confusion and misunderstanding.114
Research Implications
No high-quality evidence was found, indicating that there is great uncertainty about the effectiveness of exercise and manual MT for TMD. There is a clear need for well-designed RCTs examining exercise and MT interventions for TMD. Specifically, it is necessary that trials be performed isolating the type of exercise and manual technique that is under testing to allow understanding the effectiveness of this type of treatment. In addition, details of exercise, dosage, and frequency as well as details on manual techniques should be reported to create reproducible results. High-quality trials with larger sample sizes are needed.
Clinical Implications
Although the overall level of evidence is low, exercises and MT are safe and simple interventions that could potentially be beneficial for patients with TMD. Active and passive exercise for the jaw, postural exercises, and neck exercises appear to have favorable effects for patients with TMD. Manual therapy alone or in combination with exercises shows promising effects. Exercises did not show clear superiority over other conservative treatments for TMD.
Supplementary Material
Appendix.
Appendix.
Search Strategy Example: Ovid MEDLINE in Process and Other Nonindexed Citations and Ovid MEDLINE, 1946–Present
Footnotes
Dr Armijo-Olivo, Dr Pitance, and Dr Michelotti provided concept/idea/research design. All authors provided writing, data collection, data analysis, and consultation (including review of manuscript before submission).
Dr Armijo-Olivo is supported by the Canadian Institutes of Health Research (CIHR) through a full-time Banting fellowship, by the Alberta Innovates Health Solution through an incentive award, by the STIHR Training Program of Knowledge Translation (KT) Canada, and by the Music and Motion Fellowship from the Faculty of Rehabilitation Medicine of the University of Alberta.
References
- 1. McNeill C. Epidemiology. In: McNeill C, ed. Temporomandibular Disorders: Guidelines for Classification, Assessment, and Management. 2nd ed Chicago, IL: Quintessence Publishing Co; 1993:19–22. [Google Scholar]
- 2. Di Fabio RP. Physical therapy for patients with TMD: a descriptive study of treatment, disability, and health status. J Orofac Pain. 1998;12:124–135. [PubMed] [Google Scholar]
- 3. Gremillion HA. The prevalence and etiology of temporomandibular disorders and orofacial pain. Tex Dent J. 2000;117:30–39. [PubMed] [Google Scholar]
- 4. de Wijer A, de Leeuw JR, Steenks MH, Bosman F. Temporomandibular and cervical spine disorders: self-reported signs and symptoms. Spine (Phila Pa 1976). 1996;21:1638–1646. [DOI] [PubMed] [Google Scholar]
- 5. Kritsineli M, Shim YS. Malocclusion, body posture, and temporomandibular disorder in children with primary and mixed dentition. J Clin Pediatr Dent. 1992;16:86–93. [PubMed] [Google Scholar]
- 6. Lee WY, Okeson JP, Lindroth J. The relationship between forward head posture and temporomandibular disorders. J Orofac Pain. 1995;9:161–167. [PubMed] [Google Scholar]
- 7. Mannheimer JS, Rosenthal RM. Acute and chronic postural abnormalities as related to craniofacial pain and temporomandibular disorders. Dent Clin North Am. 1991;35:185–208. [PubMed] [Google Scholar]
- 8. Nicolakis P, Nicolakis M, Piehslinger E, et al. Relationship between craniomandibular disorders and poor posture. Cranio. 2000;18:106–112. [DOI] [PubMed] [Google Scholar]
- 9. Solow B, Sandham A. Cranio-cervical posture: a factor in the development and function of the dentofacial structures. Eur J Orthod. 2002;24:447–456. [DOI] [PubMed] [Google Scholar]
- 10. Visscher CM, De Boer W, Lobbezoo F, et al. Is there a relationship between head posture and craniomandibular pain? J Oral Rehabil. 2002;29:1030–1036. [DOI] [PubMed] [Google Scholar]
- 11. Fuentes R, Freesmeyer W, Henriquez J. Influence of body posture in the prevalence of craniomandibular dysfunction [article in Spanish]. Rev Med Chil. 1999;127:1079–1085. [PubMed] [Google Scholar]
- 12. Darlow LA, Pesco J, Greenberg MS. The relationship of posture to myofascial pain dysfunction syndrome. J Am Dent Assoc. 1987;114:73–75. [DOI] [PubMed] [Google Scholar]
- 13. Armijo S, Frugone R, Wahl F, Gaete J. Clinic and teleradiographic alterations in patients with anterior disc displacement with reduction. Kinesiologia. 2001;64:82–87. [Google Scholar]
- 14. Braun BL. Postural differences between asymptomatic men and women and craniofacial pain patients. Arch Phys Med Rehabil. 1991;72:653–656. [PubMed] [Google Scholar]
- 15. Sonnesen L, Bakke M, Solow B. Temporomandibular disorders in relation to craniofacial dimensions, head posture and bite force in children selected for orthodontic treatment. Eur J Orthod. 2001;23:179–192. [DOI] [PubMed] [Google Scholar]
- 16. Glass EG, Glaros AG, McGlynn FD. Myofascial pain dysfunction: treatments used by ADA members. Cranio. 1993;11:25–29. [DOI] [PubMed] [Google Scholar]
- 17. Feine JS, Lund JP. An assessment of the efficacy of physical therapy and physical modalities for the control of chronic musculoskeletal pain. Pain. 1997;71:5–23. [DOI] [PubMed] [Google Scholar]
- 18. Rashid A, Matthews NS, Cowgill H. Physiotherapy in the management of disorders of the temporomandibular joint; perceived effectiveness and access to services: a national United Kingdom survey. Br J Oral Maxillofac Surg. 2013;51:52–57. [DOI] [PubMed] [Google Scholar]
- 19. Medlicott MS, Harris SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. 2006;86:955–973. [PubMed] [Google Scholar]
- 20. Kogawa EM, Kato MT, Santos CN, Conti PC. Evaluation of the efficacy of low-level laser therapy (LLLT) and the microelectric neurostimulation (MENS) in the treatment of myogenic temporomandibular disorders: a randomized clinical trial. J Appl Oral Sci. 2005;13:280–285. [DOI] [PubMed] [Google Scholar]
- 21. Rocabado M. The importance of soft tissue mechanics in stability and instability of the cervical spine: a functional diagnosis for treatment planning. Cranio. 1987;5:130–138. [DOI] [PubMed] [Google Scholar]
- 22. Armijo-Olivo S, Magee DJ, Parfitt M, et al. The association between the cervical spine, the stomatognathic system, and craniofacial pain: a critical review. J Orofac Pain. 2006;20:271–287. [PubMed] [Google Scholar]
- 23. McNeely M, Armijo Olivo S, Magee D. A systematic review of physical therapy intervention for temporomandibular disorders. Phys Ther. 2006;86:710–720. [PubMed] [Google Scholar]
- 24. Miller J, Gross A, D'Sylva J, et al. Manual therapy and exercise for neck pain: a systematic review. Man Ther. 2010;15:334–354. [PubMed] [Google Scholar]
- 25. Brantingham JW, Cassa TK, Bonnefin D, et al. Manipulative and multimodal therapy for upper extremity and temporomandibular disorders: a systematic review. J Manipulative Physiol Ther. 2013;36:143–201. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 27. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 28. Kraus S. Temporomandibular disorders, head and orofacial pain: cervical spine considerations. Dent Clin North Am. 2007;51:161–193, vii. [DOI] [PubMed] [Google Scholar]
- 29. Okeson JP. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Chicago, IL: Quintessence Publishing Co; 1996. [Google Scholar]
- 30. Kovacs FM, Abraira V, Royuela A, et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine (Phila Pa 1976). 2007;32:2915–2920. [DOI] [PubMed] [Google Scholar]
- 31. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 32. van der Roer N, Ostelo RW, Bekkering GE, et al. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine (Phila Pa 1976). 2006;31:578–582. [DOI] [PubMed] [Google Scholar]
- 33. Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 35. Kropmans TJ, Dijkstra RU, Stegenga B, et al. Smallest detectable difference in outcome variables related to painful restriction of the temporomandibular joint. J Dent Res. 1999;78:784–789. [DOI] [PubMed] [Google Scholar]
- 36. Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther. 1998;78:160–169. [DOI] [PubMed] [Google Scholar]
- 37. Ylinen J, Takala EP, Kautiainen H, et al. Effect of long-term neck muscle training on pressure pain threshold: a randomized controlled trial. Eur J Pain. 2005;9:673–681. [DOI] [PubMed] [Google Scholar]
- 38. Cathcart S, Pritchard D. Reliability of pain threshold measurement in young adults. J Headache Pain. 2006;7:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chesterton LS, Foster NE, Wright CC, et al. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106:73–80. [DOI] [PubMed] [Google Scholar]
- 40. Fuentes CJ, Armijo-Olivo S, Magee DJ, Gross DP. A preliminary investigation into the effects of active interferential current therapy and placebo on pressure pain sensitivity: a random crossover placebo controlled study. Physiotherapy. 2011;97:291–301. [DOI] [PubMed] [Google Scholar]
- 41. Byrt T. How good is that agreement? Epidemiology. 1996;7:561. [DOI] [PubMed] [Google Scholar]
- 42. Armijo-Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–175. [DOI] [PubMed] [Google Scholar]
- 43. Armijo-Olivo S, Ospina M, da Costa BR, et al. Poor reliability between Cochrane reviewers and blinded external reviewers when applying the Cochrane risk of bias tool in physical therapy trials. PloS One. 2014;9:e96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hartling L, Hamm MP, Milne A, et al. Testing the risk of bias tool showed low reliability between individual reviewers and across consensus assessments of reviewer pairs. J Clin Epidemiol. 2012;66:973–981. [DOI] [PubMed] [Google Scholar]
- 45. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 4.2.6. In: Higgins J, Green S, eds. The Cochrane Library. Issue 4 Chichester, United Kingdom: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 46. Berard A, Bravo G. Combining studies using effect sizes and quality scores: application to bone loss in postmenopausal women. J Clin Epidemiol. 1998;51:801–807. [DOI] [PubMed] [Google Scholar]
- 47. Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. Available at: http://www.cochrane-handbook.org. Updated March 2011 Accessed June 24, 2015.
- 48. Cohen J. The concepts of power analysis. In: Cohen J, ed. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Academic Press Inc; 1988:1–17. [Google Scholar]
- 49. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fink M, Ismail F, Hessling K, et al. The use of physcial therapy for the treatment of craniomandibular disorders: a prospective, randomised clinical trial [article in German]. Manuelle Medizin Issue. 2007;4:255–260. [Google Scholar]
- 51. Lalabonova KH, Bakyrdzhiev A. Physical and medicinal treatment of TMJ arthrosis [article in Russian]. Stomatologiia (Mosk). 2008;87:50–51. [PubMed] [Google Scholar]
- 52. Petz R, Muska K. Therapy of restricted jaw movements [article in German]. Z Arztl Fortbild (Jena). 1974;68:841–844. [PubMed] [Google Scholar]
- 53. van der Glas HW, Buchner R, van Grootel RJ. Vergelijking tussen behandelingsvormen bij myogene temporomandibulaire dysfunctie [Comparison of treatment options for myogenous temporomandibular dysfunction]. Ned Tijdschr Tandheelkd. 2000;107:505–512. [PubMed] [Google Scholar]
- 54. Xue WH, Ding M, Su XC, et al. Clinical observation on warming needle moxibustion plus exercise for treatment of temporomandibular joint dysfunction syndrome [article in Chinese]. Zhongguo Zhen Jiu. 2007;27:322–324. [PubMed] [Google Scholar]
- 55. Burgess JA, Sommers EE, Truelove EL, Dworkin SF. Short-term effect of two therapeutic methods on myofascial pain and dysfunction of the masticatory system. J Prosthetic Dent. 1988;60:606–610. [DOI] [PubMed] [Google Scholar]
- 56. Magnusson T, Syren M. Therapeutic jaw exercises and interocclusal appliance therapy: a comparison between two common treatments of temporomandibular disorders. Swed Dent J. 1999;23:27–37. [PubMed] [Google Scholar]
- 57. Mulet M, Decker KL, Look JO, et al. A randomized clinical trial assessing the efficacy of adding 6 × 6 exercises to self-care for the treatment of masticatory myofascial pain. J Orofac Pain. 2007;21:318–328. [PubMed] [Google Scholar]
- 58. Maloney GE, Mehta N, Forgione AG, et al. Effect of a passive jaw motion device on pain and range of motion in TMD patients not responding to flat plane intraoral appliances. Cranio. 2002;20:55–65. [DOI] [PubMed] [Google Scholar]
- 59. Komiyama O, Kawara M, Arai M, et al. Posture correction as part of behavioural therapy in treatment of myofascial pain with limited opening. J Oral Rehabil. 1999;26:428–435. [DOI] [PubMed] [Google Scholar]
- 60. Wright EF, Domenech MA, Fischer JR., Jr Usefulness of posture training for patients with temporomandibular disorders. J Am Dent Assoc. 2000;131:202–210. [DOI] [PubMed] [Google Scholar]
- 61. Craane B, Dijkstra PU, Stappaerts K, De Laat A. Randomized controlled trial on physical therapy for TMJ closed lock. J Dent Res. 2012;91:364–369. [DOI] [PubMed] [Google Scholar]
- 62. Craane B, Dijkstra PU, Stappaerts K, De Laat A. One-year evaluation of the effect of physical therapy for masticatory muscle pain: a randomized controlled trial. Eur J Pain. 2012;16:737–747. [DOI] [PubMed] [Google Scholar]
- 63. Gavish A, Winocur E, Astandzelov-Nachmias T, Gazit E. Effect of controlled masticatory exercise on pain and muscle performance in myofascial pain patients: a pilot study. Cranio. 2006;24:184–190. [DOI] [PubMed] [Google Scholar]
- 64. Kalamir A, Graham PL, Vitiello AL, et al. Intra-oral myofascial therapy versus education and self-care in the treatment of chronic, myogenous temporomandibular disorder: a randomised, clinical trial. Chiropr Man Therap. 2013;21:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kalamir A, Pollard H, Vitiello A, Bonello R. Intra-oral myofascial therapy for chronic myogenous temporomandibular disorders: a randomized, controlled pilot study. J Man Manip Ther. 2010;18:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Michelotti A, Steenks MH, Farella M, et al. The additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: short-term results of a randomized clinical trial [erratum in: J Orofac Pain. 2006;20:106]. J Orofac Pain. 2004;18:114–125. [PubMed] [Google Scholar]
- 67. La Touche R, Paris-Alemany A, Mannheimer JS, et al. Does mobilization of the upper cervical spine affect pain sensitivity and autonomic nervous system function in patients with cervico-craniofacial pain: a randomized-controlled trial. Clin J Pain. 2013;29:205–215. [DOI] [PubMed] [Google Scholar]
- 68. de Felicio CM, Melchior Mde O, Ferreira CL, Da Silva MA. Otologic symptoms of temporomandibular disorder and effect of orofacial myofunctional therapy. Cranio. 2008;26:118–125. [DOI] [PubMed] [Google Scholar]
- 69. Ismail F, Demling A, Hessling K, et al. Short-term efficacy of physical therapy compared to splint therapy in treatment of arthrogenous TMD. J Oral Rehabil. 2007;34:807–813. [DOI] [PubMed] [Google Scholar]
- 70. Nascimento MM, Vasconcelos BC, Porto GG, et al. Physical therapy and anesthetic blockage for treating temporomandibular disorders: a clinical trial. Med Oral Patol Oral Cir Bucal. 2013;18:e81–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Felicio CM, de Oliveira MM, da Silva MA. Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio. 2010;28:249–259. [DOI] [PubMed] [Google Scholar]
- 72. Ficnar T, Middelberg C, Rademacher B, et al. Evaluation of the effectiveness of a semi-finished occlusal appliance: a randomized, controlled clinical trial. Head Face Med. 2013;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Niemela K, Korpela M, Raustia A, et al. Efficacy of stabilisation splint treatment on temporomandibular disorders. J Oral Rehabil. 2012;39:799–804. [DOI] [PubMed] [Google Scholar]
- 74. Truelove E, Huggins KH, Mancl L, Dworkin SF. The efficacy of traditional, low-cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. J Am Dent Assoc. 2006;137:1099–1107. [DOI] [PubMed] [Google Scholar]
- 75. Tuncer AB, Ergun N, Tuncer AH, Karahan S. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: a randomized controlled trial. J Bodyw Mov Ther. 2013;17:302–308. [DOI] [PubMed] [Google Scholar]
- 76. Packer AC, Pires PF, Dibai-Filho AV, Rodrigues-Bigaton D. Effects of upper thoracic manipulation on pressure pain sensitivity in women with temporomandibular disorder: a randomized, double-blind, clinical trial. Am J Phys Med Rehabil. 2014;93:160–168. [DOI] [PubMed] [Google Scholar]
- 77. Kalamir A, Bonello R, Graham P, et al. Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized controlled trial. J Manipulative Physiol Ther. 2012;35:26–37. [DOI] [PubMed] [Google Scholar]
- 78. Schiffman EL, Look JO, Hodges JS, et al. Randomized effectiveness study of four therapeutic strategies for TMJ closed lock [erratum in: J Dent Res. 2013;92:98]. J Dent Res. 2007;86:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Crockett DJ, Foreman ME, Alden L, Blasberg B. A comparison of treatment modes in the management of myofascial pain dysfunction syndrome. Biofeedback Self Regul. 1986;11:279–291. [DOI] [PubMed] [Google Scholar]
- 80. Kraaijenga S, van der Molen L, van Tinteren H, et al. Treatment of myogenic temporomandibular disorder: a prospective randomized clinical trial, comparing a mechanical stretching device (TheraBite®) with standard physical therapy exercise. Cranio. 2014;32:208–216. [DOI] [PubMed] [Google Scholar]
- 81. Guarda-Nardini L, Stecco A, Stecco C, et al. Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio. 2012;30:95–102. [DOI] [PubMed] [Google Scholar]
- 82. Diracoglu D, Bayraktar Saral I, Keklik B, et al. Arthrocentesis vs conventional methods in the pain and functional status of the patients with temporomandibular disc displacement without reduction. Pain Practice. 2009;9:47. [DOI] [PubMed] [Google Scholar]
- 83. Stegenga B, De Bont LG, Dijkstra PU, Boering G. Short-term outcome of arthroscopic surgery of temporomandibular joint osteoarthrosis and internal derangement: a randomized controlled clinical trial. Br J Oral Maxillofac Surg. 1993;31:3–14. [DOI] [PubMed] [Google Scholar]
- 84. Yoda T, Sakamoto I, Imai H, et al. A randomized controlled trial of therapeutic exercise for clicking due to disk anterior displacement with reduction in the temporomandibular joint. Cranio. 2003;21:10–16. [DOI] [PubMed] [Google Scholar]
- 85. Yoshida H, Sakata T, Hayashi T, et al. Evaluation of mandibular condylar movement exercise for patients with internal derangement of the temporomandibular joint on initial presentation. Br J Oral Maxillofac Surg. 2011;49:310–313. [DOI] [PubMed] [Google Scholar]
- 86. Yuasa H, Kurita K; Treatment Group on Temporomandibular Disorders. Randomized clinical trial of primary treatment for temporomandibular joint disk displacement without reduction and without osseous changes: a combination of NSAIDs and mouth-opening exercise versus no treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:671–675. [DOI] [PubMed] [Google Scholar]
- 87. Carmeli E, Sheklow SL, Bloomenfeld I. Comparative study of repositioning splint therapy and passive manual range of motion techniques for anterior displaced temporomandibular discs with unstable excursive reduction. Physiotherapy. 2001;87:26–36. [Google Scholar]
- 88. Haketa T, Kino K, Sugisaki M, et al. Randomized clinical trial of treatment for TMJ disc displacement. J Dent Res. 2010;89:1259–1263. [DOI] [PubMed] [Google Scholar]
- 89. Minakuchi H, Kuboki T, Maekawa K, et al. Self-reported remission, difficulty, and satisfaction with nonsurgical therapy used to treat anterior disc displacement without reduction. Oral Surg Oral Med Oral Pathol Oral Rad Endod. 2004;98:435–440. [DOI] [PubMed] [Google Scholar]
- 90. Minakuchi H, Kuboki T, Matsuka Y, et al. Randomized controlled evaluation of non-surgical treatments for temporomandibular joint anterior disk displacement without reduction. J Dent Res. 2001;80:924–928. [DOI] [PubMed] [Google Scholar]
- 91. Yoshida H, Fukumura Y, Suzuki S, et al. Simple manipulation therapy for temporomandibular joint internal derangement with closed lock. Asian J Oral Maxillofac Surg. 2005;17:256–260. [Google Scholar]
- 92. Raustia AM, Pohjola RT, Virtanen KK. Acupuncture compared with stomatognathic treatment for TMJ dysfunction, part I: a randomized study. J Prosthet Dent. 1985;54:581–585. [DOI] [PubMed] [Google Scholar]
- 93. Maluf S, Moreno BG, Osvaldo C, Pasqual Marques A. A comparison of two muscular stretching modalities on pain in women with myogenous temporomandibular disorders. Pain Pract. 2009;9:49. [Google Scholar]
- 94. Grace EG, Sarlani E, Reid B. The use of an oral exercise device in the treatment of muscular TMD [erratum in: Cranio. 2003;21:A–5]. Cranio. 2002;20:204–208. [DOI] [PubMed] [Google Scholar]
- 95. Klobas L, Axelsson S, Tegelberg A. Effect of therapeutic jaw exercise on temporomandibular disorders in individuals with chronic whiplash-associated disorders. Acta Odontol Scand. 2006;64:341–347. [DOI] [PubMed] [Google Scholar]
- 96. Tegelberg A, Kopp S. Short-term effect of physical training on temporomandibular joint disorder in individuals with rheumatoid arthritis and ankylosing spondylitis. Acta Odontol Scand. 1988;46:49–56. [DOI] [PubMed] [Google Scholar]
- 97. Tegelberg A, Kopp S. A 3-year follow-up of temporomandibular disorders in rheumatoid arthritis and ankylosing spondylitis. Acta Odontol Scand. 1996;54:14–18. [DOI] [PubMed] [Google Scholar]
- 98. Tavera AT, Montoya MC, Calderón EF, et al. Approaching temporomandibular disorders from a new direction: a randomized controlled clinical trial of the TMDes ear system. Cranio. 2012;30:172–182. [DOI] [PubMed] [Google Scholar]
- 99. Raustia AM, Pohjola RT. Acupuncture compared with stomatognathic treatment for TMJ dysfunction, part III: effect of treatment on mobility. J Prosthet Dent. 1986;56:616–623. [DOI] [PubMed] [Google Scholar]
- 100. Cuccia AM, Caradonna C, Annunziata V, Caradonna D. Osteopathic manual therapy versus conventional conservative therapy in the treatment of temporomandibular disorders: a randomized controlled trial. J Bodyw Mov Ther. 2010;14:179–184. [DOI] [PubMed] [Google Scholar]
- 101. Mansilla-Ferragud P, Bosca Gandia JJ. Efecto de la manipulacion de la charnela occipito-atlo-axoidea en la apertura de la boca [Effect of upper cervical spine manipulation on mouth opening]. Osteopatia Cientifica. 2008;3:45–51. [Google Scholar]
- 102. O'Reilly A, Pollard H. TMJ pain and chiropractic adjustment: a pilot study. Chiropractic J Aust. 1996;26:4. [Google Scholar]
- 103. Otano L, Legal L. Modificaciones radiologicas del espacio entre el occipucio y el cuerpo del atlas tras una manipulacion global (OAA) de Fryette [Radiological changes in the atlanto-occipital space after Fryette global manipulation (OAA)]. Osteopatia Cientifica. 2010;5:38–46. [Google Scholar]
- 104. Gomes CA, Politti F, Andrade DV, et al. Effects of massage therapy and occlusal splint therapy on mandibular range of motion in individuals with temporomandibular disorder: a randomized clinical trial. J Manipulative Physiol Ther. 2014;37:164–169. [DOI] [PubMed] [Google Scholar]
- 105. von Piekartz H, Hall T. Orofacial manual therapy improves cervical movement impairment associated with headache and features of temporomandibular dysfunction: a randomized controlled trial. Man Ther. 2013;18:345–350. [DOI] [PubMed] [Google Scholar]
- 106. von Piekartz H, Ludtke K. Effect of treatment of temporomandibular disorders (TMD) in patients with cervicogenic headache: a single-blind, randomized controlled study. Cranio. 2011;29:43–56. [DOI] [PubMed] [Google Scholar]
- 107. Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010;90:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci. 1999;26(suppl 3):S7–S11. [DOI] [PubMed] [Google Scholar]
- 109. Savovic J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med. 2012;157:429–438. [DOI] [PubMed] [Google Scholar]
- 110. Nuesch E, Trelle S, Reichenbach S, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Armijo-Olivo S, Fuentes CJ, Ospina M, et al. Inconsistency in the items included in tools used in general health research and physical therapy to evaluate the methodological quality of randomized controlled trials: a descriptive analysis. BMC Med Res Method. 2013;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7:1–90. [DOI] [PubMed] [Google Scholar]
- 113. Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol. 2000;53:964–972. [DOI] [PubMed] [Google Scholar]
- 114. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.