Abstract
Background:
Selective serotonin reuptake inhibitors (SSRIs) have been suggested to offer therapeutic benefit in patients with pulmonary arterial hypertension (PAH). We conducted two analyses to explore the association between SSRI use and PAH outcomes using the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry).
Methods:
First, new users (SSRI-naive patients who initiated treatment after enrollment, incident use analysis, n = 220) were matched (1:2) with non-SSRI users (nonusers, n = 440) by enrollment center, sex, date of most recent visit, age, and 6-min walk distance. Second, a cross-sectional design was used to compare nonusers (n = 2,463), high-affinity SSRI users (n = 430), and non-high-affinity SSRI users (n = 125) at enrollment. Mortality and a composite end point defined by events indicative of clinical worsening were evaluated.
Results:
New users had a higher risk of death (unadjusted hazard ratio [HR], 1.74; 95% CI, 1.19-2.54; P = .004) and were less likely to be free from the composite end point 2 years after enrollment vs nonusers (25.7% vs 43.2%, respectively; P < .001). Similarly, among prevalent SSRI users (patients with a history of SSRI use at enrollment), high-affinity SSRI users were less likely to be free from the composite end point vs nonusers (unadjusted HR, 1.20; 95% CI, 1.07-1.36; P = .003). In both analyses, differences in outcome were maintained after adjustment for clinical variables previously associated with PAH outcomes.
Conclusions:
In a large population of patients with PAH, incident SSRI use was associated with increased mortality and a greater risk of clinical worsening, although we could not adjust for all potential confounders.
The serotonin hypothesis of pulmonary arterial hypertension (PAH) emerged > 40 years ago and was reemphasized in the 1990s following the association of pulmonary hypertension (PH)1 with anorexic agents such as aminorex fumarate and fenfluramine.2‐5 Serotonin promotes pulmonary arterial smooth muscle cell and fibroblast proliferation, pulmonary arterial vasoconstriction, and local microthrombosis—all key pathogenic features in PAH. These effects of serotonin are mediated by interactions between serotonin and its transporter and receptors.6‐10 In particular, the serotonin transporter (SERT) plays a key role in the pathogenesis of experimental PH; in animal models, SERT overexpression predisposes to the development of PH, whereas pharmacologic blockade of SERT is protective.1,11‐14 In humans, a functional polymorphism in the SERT gene correlates with more severe PH associated with COPD.15
Selective serotonin reuptake inhibitors (SSRIs) act via blockade of SERT, resulting in an extracellular accumulation of serotonin and increased activation of serotonin receptors.16 SSRIs have been associated with both protection against and regression of PH in animal models, suggesting a possible role in the treatment of PAH in humans.1,14,17 Early observational studies have suggested therapeutic efficacy of SSRIs in patients with PAH.18,19 However, maternal SSRI use has been identified as a potential risk factor for the development of persistent PH of the newborn, raising the possibility that SSRI exposure may actually be harmful to human pulmonary vascular development.20 Furthermore, a more recent population-based study in Canada reported a positive association between SSRI use and PAH, which was ascribed to residual confounding.21
Considering these inconsistencies, we used the large, multicenter, observational, US-based, longitudinal registry of patients with group I PAH, the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry), to assess the association between SSRI use and outcomes in patients with PAH.
Materials and Methods
Patient Population
In the REVEAL Registry, PAH was defined as a mean pulmonary artery pressure > 25 mm Hg at rest or > 30 mm Hg with exercise, pulmonary capillary wedge pressure (PCWP) or left ventricular end-diastolic pressure ≤ 18 mm Hg, and pulmonary vascular resistance ≥ 240 dyne/s/cm5. For this analysis, we excluded patients with PCWP or left ventricular end-diastolic pressure > 15 mm Hg and aged ≤ 18 years, with the goal of focusing on adults with PAH. The registry design and baseline characteristics of the enrolled patients have been described previously.22,23
Incident and Prevalent Use Analysis
To detect an association between SSRI use and clinical outcomes, we applied two analytical approaches. In the first (incident use analysis), a nested case-control design was used to match REVEAL Registry patients reporting new SSRI use (new users, or those who started an SSRI after the initial REVEAL Registry enrollment visit) to non-SSRI users. New SSRI (n = 220) and non-SSRI users (n = 440) were matched by a 1:2 ratio by enrollment center, date of most recent visit, sex, age, and 6-min walk distance (6MWD) at the assessment corresponding to SSRI initiation. In the second approach (prevalent use analysis), a cross-sectional design was used. In this analysis, SSRI use at the time of enrollment served to classify patients into one of three groups: non-SSRI users (n = 2,463), high-affinity SSRI users (n = 430), and non-high-affinity SSRI users (other users; n = 125).
SSRI Classification
SSRIs were classified according to their affinity for SERT.24 High-affinity SSRIs (dissociation constant, Kd < 1 nmol) include paroxetine, escitalopram, sertraline, and fluoxetine.25,26 Other SSRIs were those with moderate and low affinity (eg, citalopram, duloxetine, nefazodone, and venlafaxine).
Outcome Measures
Two outcome variables were assessed: (1) mortality from enrollment and (2) a composite clinical end point. The composite end point consisted of major events (death, transplantation, or atrial septostomy), hospitalization, a 15% reduction in 6MWD, and/or worsened New York Heart Association (NYHA) functional class (FC).
Statistical Analyses
Categorical data are presented as percentages and were analyzed using the χ2 or Fisher exact test where appropriate. Continuous data are summarized as mean ± SD and were analyzed using the student t test. Kaplan-Meier estimates and Cox proportional hazards models were used for the outcome analyses. In the incident use analysis, results were adjusted by the most recent PAH group I diagnosis, NYHA FC, and depression at baseline. In the prevalent use analysis the model was adjusted using the REVEAL Registry multivariable prognostic equation.27 P < .05 was considered statistically significant.
Results
Patient Characteristics
Of the 3,523 subjects in the REVEAL Registry, 3,018 were included in this study (Fig 1). High-affinity SSRI users were more often women compared with nonusers (P < .001), and SSRI users overall were more likely to be white (Table 1). The geographic distribution of patients by enrollment center was similar across all study groups (all P > .05) (Table 1). Clinical subtypes of PAH were similarly distributed, with the exception of increased PAH associated with drugs and toxins among other SSRI users compared with non-SSRI users in the prevalent use analysis (P < .001). Among those reporting a history of drug or toxin exposure, anorexigens (fenfluramine and its derivatives) or amphetamines were the most common agents used (2.3%-7.2% and 2.1%-4.5%, respectively). New users were more likely to be diagnosed with connective tissue disease associated with PAH compared with matched nonusers (P = .034) (Table 1).
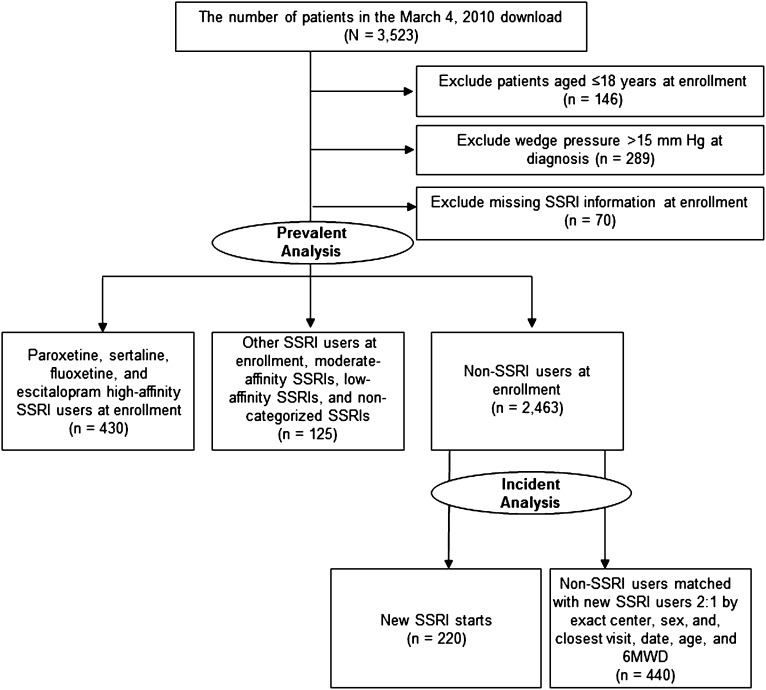
Figure 1.
STROBE diagram for the incident and prevalent use analysis. New users started SSRIs after enrollment in the REVEAL Registry and were matched with non-SSRI user control subjects. The prevalent use analysis comprised patients who started SSRI at time of enrollment. 6MWD = 6-min walk distance; SSRI = selective serotonin reuptake inhibitor.
Table 1.
—Demographic and Clinical Characteristics
| Characteristic | Incident Use Analysis, Incident SSRI Usea |
Prevalent Use Analysis, Prevalent SSRI Use |
||||||
| New SSRI Users (n = 220) | Matching Non- SSRI Users (n = 440) | P Value | HA SSRI Usersb (n = 430) | Other SSRI Usersc (n = 125) | All Non-SSRI Users (n = 2,463) | P Valued | P Valuee | |
| Age at baseline, y | ||||||||
| Mean ± SD | 50.7 ± 15.1 | 51.6 ± 14.8 | .43 | 54.0 ± 13.2 | 52.1 ± 12.6 | 52.3 ± 15.2 | .034 | .85 |
| Female | 183 (83.2) | 364 (82.7) | .88 | 373 (86.7) | 104 (83.2) | 1,902 (77.2) | < .001 | .12 |
| Race | ||||||||
| White | 166 (75.5) | 297 (67.5) | .081 | 351 (81.6) | 106 (84.8) | 1,721 (69.9) | < .001 | .003 |
| Black | 18 (8.2) | 65 (14.8) | … | 28 (6.5) | 12 (9.6) | 356 (14.5) | … | … |
| Hispanic | 22 (10.0) | 51 (11.6) | … | 36 (8.4) | 4 (3.2) | 218 (8.9) | … | … |
| Other | 14 (6.4) | 27 (6.1) | … | 15 (3.5) | 3 (2.4) | 168 (6.8) | … | … |
| Time from diagnosis to baseline, mo | ||||||||
| Median | 34.2 | 39.7 | .13 | 25.7 | 19.5 | 16.8 | < .001 | .80 |
| Geographic regionf | ||||||||
| Midwest | 45 (20.5) | 91 (20.8) | > .99 | 102 (23.7) | 31 (24.8) | 571 (23.2) | .96 | .19 |
| Northeast | 25 (11.4) | 50 (11.4) | … | 89 (20.7) | 32 (25.6) | 537 (21.8) | … | … |
| South | 75 (34.2) | 148 (33.8) | … | 126 (29.3) | 25 (20.0) | 709 (28.8) | … | … |
| West | 74 (33.8) | 149 (34.0) | … | 113 (26.3) | 37 (29.6) | 641 (26.1) | … | … |
| Clinical subtype | .74 | .74 | .02 | |||||
| Idiopathic PAH | 84 (38.7) | 199 (45.6) | .092 | 188 (43.7) | 51 (40.8) | 1,127 (45.8) | .43 | .28 |
| Familial PAH | 4 (1.8) | 12 (2.8) | .48 | 14 (3.3) | 2 (1.6) | 66 (2.7) | .50 | .46 |
| Associated with PAHg | .075 | .56 | .21 | |||||
| CHD | 23 (10.6) | 42 (9.6) | .70 | 37 (8.6) | 9 (7.2) | 245 (9.9) | .39 | .31 |
| CTD | 73 (33.6) | 112 (25.7) | .034 | 121 (28.1) | 32 (25.6) | 637 (25.9) | .32 | .95 |
| Drugs/toxinsh | 13 (6.0) | 26 (6.0) | .99 | 25 (5.8) | 16 (12.8) | 121 (4.9) | .43 | < .001 |
| HIV | 3 (1.4) | 7 (1.6) | > .99 | 11 (2.6) | 3 (2.4) | 40 (1.6) | .17 | .46 |
| PoPH | 11 (5.1) | 24 (5.5) | .82 | 20 (4.7) | 5 (4.0) | 149 (6.0) | .25 | .34 |
| Other | 5 (2.3) | 14 (3.2) | .52 | 12 (2.8) | 6 (4.8) | 65 (2.6) | .86 | .15 |
| Pulmonary venoocclusive disease | 1 (0.5) | 0 (0.0) | .33 | 2 (0.5) | 1 (0.8) | 12 (0.5) | > .99 | .48 |
| Comorbidities at baseline | ||||||||
| Hypertensioni | 97 (44.1) | 166 (37.7) | .12 | 198 (46.0) | 53 (42.4) | 959 (39.0) | .006 | .44 |
| Obesej | 78 (36.3) | 144 (33.6) | .49 | 162 (38.8) | 54 (46.6) | 753 (32.3) | .009 | .001 |
| CTDk | 79 (35.9) | 139 (31.6) | .27 | 138 (32.1) | 41 (32.8) | 707 (28.7) | .16 | .33 |
| Clinical depression | 83 (37.7) | 37 (8.4) | < .001 | 183 (42.6) | 55 (44.0) | 171 (6.9) | < .001 | < .001 |
| OADl | 60 (27.3) | 84 (19.1) | .016 | 110 (25.6) | 36 (28.8) | 536 (21.8) | .082 | .065 |
| Sleep apnea | 49 (23.4) | 101 (24.5) | .78 | 108 (26.3) | 41 (35.7) | 448 (19.4) | .001 | < .001 |
| Thyroid diseasem | 57 (25.9) | 114 (25.9) | > .99 | 121 (28.1) | 30 (24.0) | 504 (20.5) | < .001 | .34 |
| Diabetes | 29 (13.2) | 52 (11.8) | .61 | 49 (11.4) | 20 (16.0) | 299 (12.2) | .66 | .20 |
Data are presented as No. (%) unless noted otherwise. CHD = congenital heart disease, CTD = connective tissue disease; HA = high affinity; OAD = obstructive airway disease; PAH = pulmonary arterial hypertension; PoPH = portopulmonary hypertension; SSRI = selective serotonin reuptake inhibitor.
Non-SSRI users were matched 2:1 with new SSRI users by enrollment center, sex, date of most recent visit, age, and 6-min walk distance.
HA SSRIs include paroxetine, sertraline, fluoxetine, and escitalopram.
Other SSRIs include moderate-affinity SSRIs, low-affinity SSRIs, and nonselective serotonin transporter inhibitors.
Comparison of all non-SSRI users and HA SSRI users.
Comparison of all non-SSRI users and other SSRI users.
The geographic region is categorized according to the census region and is determined by the state of the site.
Associated PAH subgroups are mutually exclusive according to the following hierarchy: CHD, CTD, PoPH, drugs/toxins, HIV, and other.
Drugs and toxins were subclassified (not mutually exclusive) as anorexigens, amphetamines, cocaine, and not identified. The number of patients within each subcategory is listed (respectively) by the analysis. Incident: new SSRI users (5, 6, 0, 2), non-SSRI users (14, 12, 0, 0). Prevalent: HA-SSRI (15, 9, 0, 1), other SSRI users (9, 6, 0, 1), non-SSRI users (62, 52, 6, 5).
Includes patients with hypertension and/or patients with reported use of β-blockers.
BMI ≥ 30 kg/m2.
CTD is collected on the comorbid condition form and includes scleroderma, CTD, lupus, rheumatoid arthritis, and other CTD.
Defined as obstructive lung disease, reactive airway disease, and COPD.
Includes patients with hyperthyroidism/hypothyroidism and/or patients with reported use of synthetic thyroid replacement treatment of hypothyroidism.
Comorbid Conditions
The prevalence of comorbid conditions was similar (with the exception of obstructive airway disease) between new users and nonusers in the incident use analysis (Table 1). In the prevalent use analysis, however, hypertension, obesity, sleep apnea, and thyroid disease were more frequent in high-affinity SSRI users compared with nonusers (all P < .05). Other SSRI users were more likely to have obesity or sleep apnea compared with nonusers (all P < .05). As expected, SSRI users had a higher proportion of patients with depression than nonusers in both analyses (37.7%-44.0% vs 6.9%-8.4%, respectively; P < .001), although depression was reported in only a minority of SSRI users.
Functional Status and Hemodynamics
In both analyses, SSRI users were more likely to have NYHA FC III/IV at enrollment than nonusers. New users and high-affinity SSRI users had a significantly shorter 6MWD vs matched nonusers or all nonusers (Table 2). There were no significant differences in hemodynamic measurements between new users and nonusers (incident use analysis). In the prevalent use analysis, high-affinity SSRI users had a slightly higher PCWP compared with nonusers (10.0 ± 4.2 vs 9.6 ± 3.8, P = .049). No differences were detected in mean right atrial pressure, cardiac index, or mixed venous oxygen saturation between SSRI users and nonusers in either analytic approach (Table 2).
Table 2.
—Functional and Hemodynamic Variables
| Characteristic | Incident Use Analysis, Incident SSRI Usea |
Prevalent Use Analysis, Prevalent SSRI Use |
||||||
| New SSRI Users (n = 220) | Matching Non- SSRI Users (n = 440) | P Value | HA SSRI Usersb (n = 430) | Other SSRI Usersc (n = 125) | All Non-SSRI Users (n = 2,463) | P Valued | P Valuee | |
| Baseline NYHA FC, No. (%) | ||||||||
| I | 5 (2.4) | 36 (8.6) | .001 | 12 (3.1) | 4 (3.5) | 173 (7.8) | .001 | .16 |
| II | 80 (37.7) | 182 (43.2) | … | 123 (31.9) | 34 (30.1) | 781 (35.4) | … | … |
| III | 108 (50.9) | 184 (43.7) | … | 217 (56.4) | 66 (58.4) | 1,106 (50.1) | … | … |
| IV | 19 (9.0) | 19 (4.5) | … | 33 (8.6) | 9 (8.0) | 149 (6.7) | … | … |
| Baseline 6MWD, m | ||||||||
| Median | 354.0 | 386.0 | .009 | 350.5 | 341.4 | 374.9 | .005 | .30 |
| Hemodynamics, Mean ± SD (No.) | ||||||||
| mPAP, mm Hg | 48.9 ± 14.7 (219) | 49.7 ± 14.2 (439) | .52 | 48.4 ± 13.9 (426) | 46.5 ± 14.1 (125) | 49.6 ± 14.2 (2,431) | .10 | .017 |
| PCWP, mm Hg | 9.5 ± 4.5 (218) | 10.0 ± 4.3 (436) | .12 | 10.0 ± 4.2 (424) | 9.9 ± 4.5 (124) | 9.6 ± 3.8 (2,404) | .049 | .32 |
| mRAP, mm Hg | 8.5 ± 5.2 (205) | 8.7 ± 5.2 (417) | .59 | 9.1 ± 5.2 (397) | 9.4 ± 5.2 (118) | 8.9 ± 5.5 (2,241) | .43 | .33 |
| PVRI, Wood units | 17.9 ± 10.0 (193) | 18.4 ± 10.9 (375) | .55 | 17.8 ± 10.6 (357) | 16.5 ± 9.3 (105) | 18.9 ± 10.6 (2,029) | .086 | .025 |
| Fick or thermodilution cardiac index, L/min m2 | 2.5 ± 0.8 (194) | 2.5 ± 0.9 (378) | .99 | 2.5 ± 0.8 (359) | 2.5 ± 0.7 (106) | 2.5 ± 0.9 (2,048) | .58 | .59 |
| Svo2, % | 64.3 ± 10.3 (149) | 64.2 ± 9.7 (310) | .96 | 64.0 ± 9.6 (269) | 63.3 ± 9.5 (84) | 63.9 ± 9.8 (1,491) | .93 | .56 |
For incident SSRI use, baseline is defined as the assessment during which new users initiate an SSRI and the assessment used to match non-SSRI users with the new SSRI users. 6MWD = 6-min walk distance; FC = functional class; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; NYHA = New York Heart Association; PVRI = pulmonary vascular resistance index; Svo2 = mixed venous oxygen saturation. See Table 1 legend for expansion of other abbreviation.
Non-SSRI users were matched 2:1 with new SSRI users by enrollment center, sex, date of most recent visit, age, and 6MWD.
HA SSRIs include paroxetine, sertraline, fluoxetine, and escitalopram.
Other SSRIs include moderate-affinity SSRIs, low-affinity SSRIs, and non-selective serotonin transporter inhibitors.
Comparison of all non-SSRI users and HA SSRI users.
Comparison of all non-SSRI users and other SSRI users.
PAH-Specific and Other Medications at Enrollment
In the incident use analysis, new users were more likely than nonusers to be receiving an endothelin receptor antagonist (57.5% vs 47.9%, P = .020) (Table 3). No differences in the use of prostanoids, phosphodiesterase type 5 inhibitors, diuretics, warfarin, digoxin, thyroid hormone, or supplemental oxygen were detected. In the prevalent use analysis, high-affinity SSRI users were significantly more likely than nonusers to be treated with prostanoids, diuretics, oxygen, and thyroid hormone replacement.
Table 3.
—Most Recent PAH-Specific Medications and Concomitant Medications at Baseline by SSRI Use
| Characteristic, No. (%) | Incident Use Analysis, Incident SSRI Usea |
Prevalent Use Analysis, Prevalent SSRI Use |
||||||
| New SSRI Users (n = 220) | Matching Non- SSRI Users (n = 440) | P Value | HA SSRI Usersb (n = 430) | Other SSRI Usersc (n = 125) | All Non-SSRI Users (n = 2,463) | P Valued | P Valuee | |
| PAH-specific medications | ||||||||
| ERA | 126 (57.5) | 207 (47.9) | .020 | 192 (45.1) | 54 (43.2) | 1,003 (41.2) | .13 | .66 |
| PDE-5 inhibitor | 138 (63.0) | 244 (56.5) | .11 | 205 (48.1) | 52 (41.6) | 1,125 (46.2) | .46 | .31 |
| Any prostanoid | 109 (49.8) | 186 (43.1) | .10 | 190 (44.6) | 46 (36.8) | 894 (36.7) | .002 | .98 |
| Concomitant medications | ||||||||
| Diuretic | 152 (69.1) | 298 (67.7) | .72 | 317 (73.7) | 82 (65.6) | 1,617 (65.7) | .001 | > .99 |
| Warfarin | 110 (50.0) | 221 (50.2) | .96 | 215 (50.0) | 51 (40.8) | 1,211 (49.2) | .75 | .068 |
| Oxygen | 87 (39.5) | 161 (36.6) | .46 | 200 (46.5) | 57 (45.6) | 924 (37.5) | < .001 | .069 |
| Digoxin | 60 (27.3) | 126 (28.6) | .71 | 115 (26.7) | 32 (25.6) | 605 (24.6) | .33 | .79 |
| Thyroid replacement | 46 (20.9) | 95 (21.6) | .84 | 119 (27.7) | 24 (19.2) | 472 (19.2) | < .001 | > .99 |
For the new SSRI starts, “baseline” is defined at the assessment during which new users initiate an SSRI and the assessment used to match the non-SSRI users to the new SSRI users. For the enrollment SSRI usage, “baseline” is defined as study enrollment. “Most recent” is defined as the baseline value, if available; otherwise the last available value prior to and closest to the baseline assessment. Any prostanoid = IV epoprostenol, IV treprostinil, subcutaneous treprostinil, inhaled iloprost, inhaled treprostinil, and oral treprostinil; ERA = endothelin receptor antagonist, including bosentan, sitaxsentan, and ambrisentan; PDE-5 inhibitor = phosphodiesterase type 5 inhibitor, including sildenafil and tadalafil. See Table 1 legend for expansion of other abbreviations.
Matching non-SSRI users were matched 2:1 to new SSRI users by exact center and sex, and closest visit date, age, and 6MWD.
HA SSRI includes paroxetine, sertraline, fluoxetine, and escitalopram.
Other SSRI includes moderate-affinity SSRI, low-affinity SSRI, and noncategorized SSRI.
Comparing all non-SSRI users and HA SSRI users.
Comparing all non-SSRI users and other SSRI users.
SSRI Use
Patients in the REVEAL Registry used a total of 15 different SSRIs (Table 4). Among new users, escitalopram was used most frequently (n = 47, 21.4%), followed by citalopram (n = 46, 20.9%) and sertraline (n = 37, 16.8%). Among the 555 prevalent SSRI users, most (n = 430) were using a high-affinity SSRI, such as sertraline (n = 131, 30.5%) or fluoxetine (n = 109, 25.3%). A small group of patients was using multiple SSRIs: three patients in the incident and 16 in the prevalent use analysis.
Table 4.
—SSRI Use at Baseline
| Characteristic, No. (mean ± SD) | Incident Use, Analysis Incident SSRI Usea | Prevalent Use, Analysis Prevalent SSRI Use |
|
| New SSRI Users (n = 220) | HA SSRI Usersb (n = 430) | Other SSRI Usersc (n = 125) | |
| Escitalopram | 47 (21.4) | 101 (23.5) | 0 (0.0) |
| Sertraline | 37 (16.8) | 131 (30.5) | 0 (0.0) |
| Fluoxetine | 32 (14.5) | 109 (25.3) | 0 (0.0) |
| Paroxetine | 19 (8.6) | 91 (21.2) | 0 (0.0) |
| Citalopram | 46 (20.9) | 1 (0.2) | 67 (53.6) |
| Duloxetine | 17 (7.7) | 3 (0.7) | 26 (20.8) |
| Nefazodone | 1 (0.5) | 0 (0.0) | 3 (2.4) |
| Fluvoxamine | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Otherd | 24 (10.9) | 7 (1.6) | 32 (25.6) |
For the new SSRI starts, “baseline” is defined at the assessment during which new users initiate an SSRI, and the assessment used to match the non-SSRI users to the new SSRI users. SSRI use was not mutually exclusive, and more than one SSRI could be counted per subject. A total of 16 (incident use) and three (prevalent use) subjects reported multiple SSRI use. A total of 15 different SSRIs were reported used by REVEAL Registry subjects. See Table 1 legend for expansion of abbreviation.
Matching non-SSRI users were matched 2:1 to new SSRI users by exact center and sex, and closest visit date, age, and 6MWD.
HA SSRIs include paroxetine, sertraline, fluoxetine, and escitalopram.
Other SSRI users reported use of non-HA SSRIs including moderate-affinity, low-affinity, and noncategorized SSRIs.
Other SSRIs reported were amitriptyline, bupropion, desvenlafaxine, doxepin, mirtazapine, trazodone, and venlafaxine.
Unadjusted Outcomes: Mortality and Composite End Point
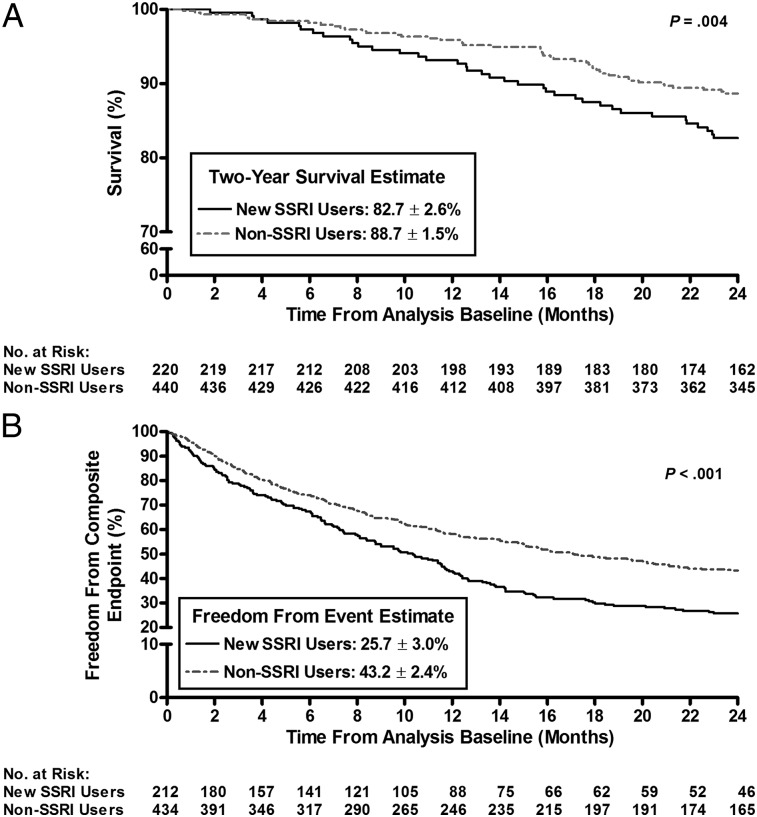
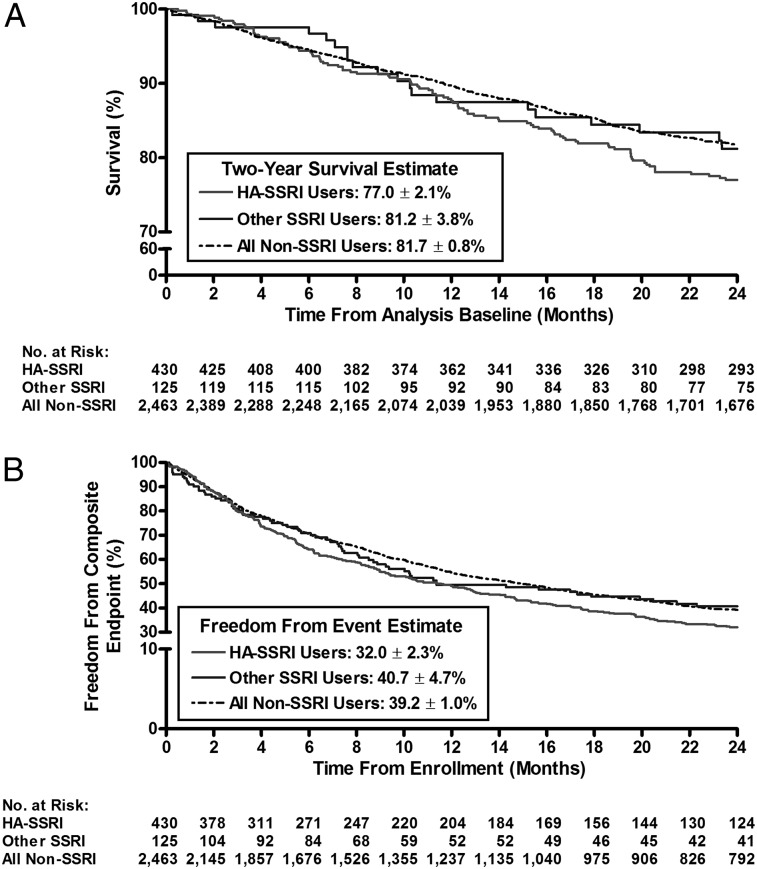
Kaplan-Meier survival estimates revealed that new users had significantly worse survival and were less likely to be free from the composite end point compared with matched nonusers at 2 years after enrollment (82.7% ± 2.6% vs 88.7% ± 1.5%, respectively, P = .004; and 25.7% ± 3.0% vs 43.2% ± 2.4%, P < .001) (Figs 2A, 2B). In an unadjusted Cox proportional hazard model, new users demonstrated a significantly increased risk of death (hazard ratio [HR], 1.74; 95% CI, 1.19-2.54; P < .004) and a greater risk of clinical worsening (HR, 1.57; 95% CI, 1.30-1.91; P < .001) compared with matched control subjects. In the prevalent use analysis, irrespective of SSRI affinity, there were no differences in 2-year survival from the time of enrollment between nonusers and SSRI users (Fig 3A); however, the unadjusted Cox proportional hazard model showed that high-affinity SSRI users had a worse freedom from the composite end point compared with nonusers (HR, 1.20; 95% CI, 1.07-1.36; P = .003) (data not shown).
Figure 2.
A, Kaplan-Meier estimates of 2-year survival in new SSRI users vs non-SSRI users (incident use analysis). New users had a worse survival compared with matched non-SSRI users (82.7% ± 2.6% vs 88.7% ± 1.5%, respectively; P = .004). B, Kaplan-Meier estimates of 2-y freedom from the composite end point in new SSRI users vs non-SSRI users (incident use analysis). Fewer new SSRI users remained free from the composite end point vs matched nonusers (25.7% ± 3.0% vs 43.2% ± 2.4%, respectively; P < .001). The composite end point included major events (death, transplantation, or atrial septostomy), hospitalization, a 15% reduction in 6MWD, and/or worsened New York Heart Association functional class. See Figure 1 legend for expansion of abbreviations.
Figure 3.
A, Kaplan-Meier estimates of 2-year survival in HA-SSRI, other SSRI, and non-SSRI users (prevalent use analysis). Similar survival was observed in the HA-SSRI group vs non-SSRI users (77.0% ± 2.1% vs 81.7% ± 0.8%, respectively; P = .13) and in other SSRI users vs non-SSRI users (81.2% ± 3.8% vs 81.7% ± 0.8%, respectively; P = .96). B, Kaplan-Meier estimates of 2-y freedom from the composite end point in HA-SSRI, other SSRI, and non-SSRI users (prevalent use analysis). Fewer HA-SSRI users compared with non-SSRI users remained free from the composite end point (32.0% ± 2.3% vs 39.2% ± 1.0%, respectively; P = .003). Other SSRI users and non-SSRI users reached the composite end point at a similar rate (40.7% ± 4.7% vs 39.2% ± 1.0%, respectively; P = .87). The composite end point included major events (death, transplantation, or atrial septostomy), hospitalization, a 15% reduction in 6MWD, and/or worsened New York Heart Association functional class. HA = high affinity. See Figure 1 legend for expansion of other abbreviations.
Adjusted Outcomes
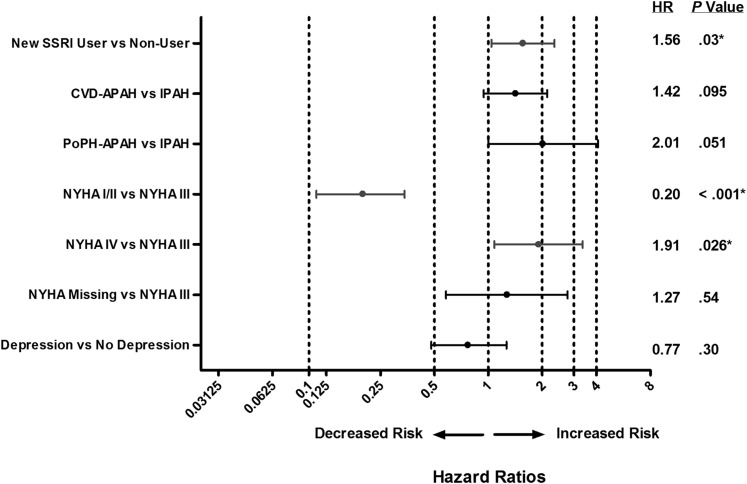
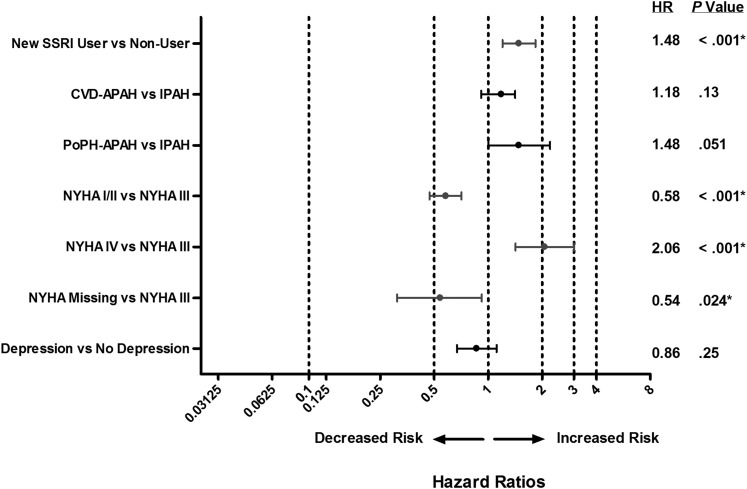
After adjustment for group I subtype, NYHA FC, and depression at baseline in the incident use analysis, new SSRI use was associated with an increased risk of death (HR, 1.56; P = .030) and a greater risk of achieving the composite end point (HR, 1.48; P < .001) compared with matched control subjects (Figs 4, 5). In the prevalent use analysis, after adjustment for terms comprising the REVEAL Registry prognostic equation,27 SSRI use was not associated with mortality (P > .05), and high-affinity SSRI use was associated with a greater risk of achieving the composite end point compared with nonusers (HR, 1.18; P = .008; data not shown). Depression was not associated with mortality or clinical worsening in the incident use analysis (Figs 4, 5).
Figure 4.
Forest plot of adjusted HRs of survival showing decreased and increased risk of death in new SSRI users vs non-SSRI users (incident use analysis). APAH = associated with pulmonary arterial hypertension; CTD = connective tissue disease; FC = functional class; HR = hazard ratio; IPAH = idiopathic pulmonary arterial hypertension; NYHA = New York Heart Association; PoPH = portopulmonary hypertension. See Figure 1 legend for expansion of other abbreviation.
Figure 5.
Forest plot of adjusted HRs of composite end points showing decreased and increased risk of clinical worsening in new SSRI users vs non-SSRI users (incident use analysis). The composite end point included major events (death, transplantation, or atrial septostomy), hospitalization, a 15% reduction in 6MWD, and/or worsened NYHA FC. See Figure 1 and 4 legends for expansion of abbreviations.
Discussion
In this large, contemporary cohort of patients with PAH, our incident use analysis showed that SSRI use was associated with a greater risk of death and clinical worsening during a 2-year follow-up period from enrollment. In the prevalent use analysis, high-affinity SSRI users had a similar survival but accelerated clinical worsening compared with nonusers. These findings were maintained despite adjustment for potentially confounding variables.
Our data also demonstrate that patients who were using an SSRI had a worse NYHA FC and a shorter 6MWD at baseline compared with nonusers. In addition, a larger proportion of SSRI users were receiving PAH-specific medications, including endothelin receptor antagonists and prostanoids, compared with nonusers. These data suggest that SSRI users had more severe PAH than nonusers. Alternatively, the functional capacity of these patients could have been affected by the presence of depression, consistent with the results of a previous study indicating a trend toward worse baseline 6MWD and a higher FC in patients with symptoms suggestive of major depression.28 However, our data demonstrate that pulmonary hemodynamics were comparable between SSRI users and nonusers in the incident use analysis. In the prevalent use analysis, PCWP was only slightly higher among high-affinity SSRI users compared with nonusers, and this difference is not considered clinically relevant. Discrepancies between hemodynamics and other indicators of severity raise the possibility that other factors like depression, obesity, or SSRI use may supplant the predictive value of baseline pulmonary hemodynamics.
Our findings are inconsistent with several previous reports. In a single-center, retrospective study of 84 patients with PAH, Kawut and colleagues18 found that 13 patients with PAH who were exposed to high-affinity SSRIs showed a tendency toward a reduced risk of death (HR, 0.53; 95% CI, 0.07-3.9; P = .53). More recently, using a combined retrospective and prospective database from Chicago that dates back to the 1980s, Shah and colleagues19 reported that SSRI use conferred a survival advantage in both group I PAH and non-group I PH patients (HR, 0.35; 95% CI, 0.14-0.87; P = .023). Interestingly, among incident SSRI users in that cohort, a nonsignificant trend toward improved survival was observed (P = .089). Compared with these studies, the present analysis provides greater statistical power because of the larger patient population, as well as a contemporary therapeutic background upon which to examine the association between PAH and SSRI use. For example, approximately one-half of the patients using SSRIs in the REVEAL Registry were also taking prostanoids, compared with none in the Chicago cohort.19
Our findings are concordant with a retrospective, population-based study examining the relationship between SSRIs and PAH in the Canadian health-care system.20 In that study, 460 patients with PAH (defined as patients who received one or more prescriptions for a PAH-specific medication) were matched with 4,536 control subjects, and SSRI use was associated with an increased risk of PAH requiring pharmacologic treatment (adjusted OR, 1.55). The authors concluded that SSRIs are not protective against PAH and speculated that the association between SSRIs and PAH was likely the result of residual confounders, such as depression and anxiety.21
Studies of the REVEAL Registry have shown that depression is common in patients with PAH, significantly exceeding the prevalence in the general population (25% vs 6.7%, respectively).22 In addition, a previous study using a validated tool to assess the prevalence of depressive symptoms in PAH found that 15% and 55% of patients with PAH have symptoms suggestive of major depressive disorder and depression, respectively.28 We were surprised to find that clinical depression was reported in only approximately 40% of SSRI users in the present study, raising the possibility that depression was underreported. Furthermore, SSRI use was more prevalent in white than in nonwhite women, which is consistent with patterns of SSRI use in non-PAH populations in the United States.29
Although it was not associated with mortality or a worse outcome in the incident use analysis, depression may have counteracted favorable effects associated with SSRIs. Depression is a well-established risk factor for heart failure and the progression of established atherosclerotic disease30,31 related to behaviors that impact health, particularly physical inactivity.32 Considering that patients with PAH with depression have higher brain natriuretic peptide levels and a trend toward shorter 6MWDs than patients with PAH without depression, the potential negative impact of depression on functional status and other outcomes in PAH could be substantial. In addition, depression may contribute directly to the pathogenesis and progression of PAH. Both depression and PAH are characterized by increased levels of proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, which could reflect a mechanistic link between these two disease processes.33‐35 These findings are provocative, but whether there is a causal relationship between depression and the progression of PAH cannot be determined based on the present results. Alternatively, alleviation of depression could have a beneficial effect on outcomes in PAH that is independent of any impact on pathogenic mechanisms; however, we were not able to evaluate any effect of SSRIs on depression in the present study.
Several aspects of our study limit our ability to definitively explore the relationship between PAH, SSRI use, depression, and serotonin biology. First, a longitudinal observational cohort study precludes conclusions about causality; we are limited to demonstrating associations. Also, SSRI-user groups had a greater number of comorbidities compared with nonusers, raising the concern that adjustments for residual (cryptic) group confounders were inadequate. In particular, the possibility of underreporting of depression would impede our ability to adjust for this potentially deleterious confounder. In addition, we were unable to incorporate into our model longitudinal changes in BMI; however, we acknowledge that weight gain could impact both functional status and outcomes in these patients.36 Finally, considering the posited contribution of excessive serotonin to the pathogenesis of PH, it would be interesting to examine potential interactions between SSRIs and other serotonergic drugs or exposures, such as anorexigens. Although an association was observed between anorexigen exposure and PAH in other SSRI users in the prevalent analysis, an evaluation of this interaction was precluded by the infrequency of exposures and lack of detail regarding the exposure intensity. Despite these limitations, the much larger size of the REVEAL Registry database, its established validity as a resource for defining contemporary PAH epidemiology, and our ability to identify a sizable control population strengthen the present observations compared with previous cohorts.
In conclusion, among patients enrolled in the REVEAL Registry, incident SSRI use was associated with greater mortality and worse clinical outcomes, and prevalent SSRI use was associated with similar outcomes compared with nonusers. These data contradict prior cohort studies demonstrating an association between improved survival and SSRI use. Furthermore, SSRI users in the present analysis had significantly worse NYHA FC and 6MWD at enrollment and higher rates of depression, which indicates a possible role for depression or other confounders, such as obesity, in the course of PAH. Further prospective, randomized, and observational studies with more detailed analyses of PAH-associated depression and obesity will be necessary to better delineate these relationships.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Hill is the guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Sadoughi: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Dr Roberts: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Dr Preston: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Ms Lai: contributed to study conception and design, acquisition and analysis of data, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Ms McCollister: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Dr Farber: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Dr Hill: contributed to study conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Sadoughi has received grant support from Empire Clinical Research Investigator Program. Dr Roberts receives grant support from the National Institutes of Health [Grant K23HL089812]. Dr Preston serves as a consultant to Actelion Pharmaceuticals US Inc; Aires Pharmaceuticals, Inc; Bayer AG; Gilead; Novartis Corporation; Pfizer, Inc; and United Therapeutics Corporation. Dr Preston also participates in speaking activities and/or advisory committees for Actelion Pharmaceuticals US Inc, Gilead, and United Therapeutics Corporation. Ms Lai is employed by ICON Late Phase & Outcomes Research, a company that receives research support from Actelion Pharmaceuticals US Inc and other pharmaceutical companies. Ms McCollister serves as a consultant to Actelion Pharmaceuticals US Inc; Gilead; Bayer AG; Novartis Corporation; Ikaria, Inc; and United Therapeutics Corporation. Dr Farber serves as a consultant and is on the speaker’s bureau for Actelion Pharmaceuticals US Inc. Dr Farber has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion Pharmaceuticals US Inc. Dr Hill receives grants from Actelion Pharmaceuticals US Inc; Bayer AG; Gilead; Novartis Corporation; Pfizer, Inc; and United Therapeutics Corporation. Relevant to Dr Hill’s honoraria, all industries listed provided research grants for multicenter or investigator-initiated trials, with all money going to Tufts Research Administration and no direct payments to the author.
Role of sponsors: Actelion Pharmaceuticals US Inc provided financial support for the REVEAL Registry database as well as biostatistical support, but it had no role in data analysis or preparation of the manuscript.
Other contributions: Assistance in manuscript development was provided by Scarlett Geunes-Boyer, PhD, of inScience Communications, Springer Healthcare. Dave P. Miller, MS, and Michelle P. Turner, MS, from ICON Late Phase & Outcomes Research provided statistical consulting support. This support was funded by Actelion Pharmaceuticals US, Inc. We thank the Principal Investigators and their Study Coordinators for their participation in REVEAL Registry (e-Appendix 1 (351.1KB, pdf) ).
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- 6MWD
6-minute walk distance
- FC
functional class
- HR
hazard ratio
- NYHA
New York Heart Association
- PAH
pulmonary arterial hypertension
- PCWP
pulmonary capillary wedge pressure
- PH
pulmonary hypertension
- REVEAL Registry
Registry to Evaluate Early and Long-term PAH Disease Management
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Drs Sadoughi and Roberts contributed equally to the preparation of the manuscript.
Funding/Support: Funding and support for the REVEAL Registry was provided by CoTherix, Inc, and its affiliate Actelion Pharmaceuticals US, Inc. Medical writing support was provided by Scarlett Geunes-Boyer, PhD, of inScience Communications, Springer Science+Business Media, and funding was provided by Actelion Pharmaceuticals US, Inc.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Marcos E, Adnot S, Pham MH, et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2003;168(4):487-493. [DOI] [PubMed] [Google Scholar]
- 2.Kay JM, Crawford N, Heath D. Blood 5-hydroxytryptamine in rats with pulmonary hypertension produced by ingestion of Crotalaria spectabilis seeds. Experientia. 1968;24(11):1149-1150. [DOI] [PubMed] [Google Scholar]
- 3.Abenhaim L, Moride Y, Brenot F, et al. ; International Primary Pulmonary Hypertension Study Group. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335(9):609-616. [DOI] [PubMed] [Google Scholar]
- 4.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation. 1999;100(8):869-875. [DOI] [PubMed] [Google Scholar]
- 5.Hervé P, Launay JM, Scrobohaci ML, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99(3):249-254. [DOI] [PubMed] [Google Scholar]
- 6.MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131(2):161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272(5 pt 1):L795-L806. [DOI] [PubMed] [Google Scholar]
- 8.Welsh DJ, Harnett M, MacLean M, Peacock AJ. Proliferation and signaling in fibroblasts: role of 5-hydroxytryptamine2A receptor and transporter. Am J Respir Crit Care Med. 2004;170(3):252-259. [DOI] [PubMed] [Google Scholar]
- 9.Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol. 2008;155(4):455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon TJ, Hood JS, Nossaman BD, Kadowitz PJ. Analysis of responses to serotonin in the pulmonary vascular bed of the cat. J Appl Physiol. 1993;75(1):93-102. [DOI] [PubMed] [Google Scholar]
- 11.Morecroft I, Loughlin L, Nilsen M, et al. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313(2):539-548. [DOI] [PubMed] [Google Scholar]
- 12.Eddahibi S, Hanoun N, Lanfumey L, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105(11):1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddahibi S, Humbert M, Fadel E, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108(8):1141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guignabert C, Raffestin B, Benferhat R, et al. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005;111(21):2812-2819. [DOI] [PubMed] [Google Scholar]
- 15.Eddahibi S, Chaouat A, Morrell N, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108(15):1839-1844. [DOI] [PubMed] [Google Scholar]
- 16.Slattery DA, Hudson AL, Nutt DJ. Invited review: the evolution of antidepressant mechanisms. Fundam Clin Pharmacol. 2004;18(1):1-21. [DOI] [PubMed] [Google Scholar]
- 17.Eddahibi S, Fabre V, Boni C, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res. 1999;84(3):329-336. [DOI] [PubMed] [Google Scholar]
- 18.Kawut SM, Horn EM, Berekashvili KK, et al. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2006;19(5):370-374. [DOI] [PubMed] [Google Scholar]
- 19.Shah SJ, Gomberg-Maitland M, Thenappan T, Rich S. Selective serotonin reuptake inhibitors and the incidence and outcome of pulmonary hypertension. Chest. 2009;136(3):694-700. [DOI] [PubMed] [Google Scholar]
- 20.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587. [DOI] [PubMed] [Google Scholar]
- 21.Dhalla IA, Juurlink DN, Gomes T, Granton JT, Zheng H, Mamdani MM. Selective serotonin reuptake inhibitors and pulmonary arterial hypertension: a case-control study. Chest. 2012;141(2):348-353. [DOI] [PubMed] [Google Scholar]
- 22.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376-387. [DOI] [PubMed] [Google Scholar]
- 23.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923-931. [DOI] [PubMed] [Google Scholar]
- 24.Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108(1):32-36. [DOI] [PubMed] [Google Scholar]
- 25.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2-3):249-258. [DOI] [PubMed] [Google Scholar]
- 26.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5):345-350. [DOI] [PubMed] [Google Scholar]
- 27.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172. [DOI] [PubMed] [Google Scholar]
- 28.McCollister DH, Beutz M, McLaughlin V, et al. Depressive symptoms in pulmonary arterial hypertension: prevalence and association with functional status. Psychosomatics 2010;51(4):339-339.e8. [DOI] [PubMed] [Google Scholar]
- 29.Sclar DA, Robison LM, Skaer TL. Ethnicity/race and the diagnosis of depression and use of antidepressants by adults in the United States. Int Clin Psychopharmacol. 2008;23(2):106-109. [DOI] [PubMed] [Google Scholar]
- 30.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161(14):1725-1730. [DOI] [PubMed] [Google Scholar]
- 31.Wellenius GA, Mukamal KJ, Kulshreshtha A, Asonganyi S, Mittleman MA. Depressive symptoms and the risk of atherosclerotic progression among patients with coronary artery bypass grafts. Circulation. 2008;117(18):2313-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446-457. [DOI] [PubMed] [Google Scholar]
- 34.Conboy L, Varea E, Castro JE, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry. 2011;16(5):533-547. [DOI] [PubMed] [Google Scholar]
- 35.Hassoun PM, Mouthon L, Barberà JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(suppl 1):S10-S19. [DOI] [PubMed] [Google Scholar]
- 36.Sussman N, Ginsberg DL, Bikoff J. Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry. 2001;62(4):256-260. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement