With the increase in average life span, the percentage of patients with neurological diseases is getting higher and higher.1 Thus, developing effective neuropharmaceuticals is critically important and urgent. Sadly, many promising candidates fail to show expected effects because of their inability to cross the blood–brain barrier (BBB), a dynamic interface that separates the central nervous system (CNS) from the circulation system.1 BBB maintains the homeostasis of CNS microenvironment and proper neurological functions by regulating the exchange of substances between the 2 systems.2 Perturbation of BBB has been found in many neurological disorders, including neurodegenerative diseases,3–6 trauma,7,8 brain tumors,9,10 and stroke.11–13 Restoring BBB integrity in pathological conditions to maintain brain homeostasis and opening BBB temporarily to allow efficient delivery of drugs to the CNS are potential therapeutic options for patients with these disorders. For these purposes, a lot of research has focused on the regulation of BBB permeability. Because of the complexity of the in vivo BBB, many simplified in vitro BBB models have been developed and studied, including the monolayer models, coculture models, dynamic models, and microfluidic BBB models. Because no in vitro BBB models can fully replicate the in vivo conditions, there is no perfect in vitro BBB model. Understanding the limitations of these in vitro BBB models would be critical to the design of experiments and interpretation of data.
There have been a large number of excellent reviews on in vitro BBB models in the literature. For example, Gumbleton and Audus14 reviewed immortalized cell lines and primary cells used in in vitro BBB models and suggested that an ideal model should have low permeability, possess endothelial-like morphology, express functional transporters, and be easy to construct. Deli et al15 summarized permeability data on in vitro BBB models in both normal and pathological conditions. They also reviewed the effects of various biological factors and pharmaceutical molecules on signaling transduction and BBB permeability.15 Additionally, Abbott et al16 recently published an in-depth review on in vitro culture models of the CNS barriers, including stem cell–based approaches and techniques used to characterize the BBB properties. Here in this review, we summarize the most widely used in vitro BBB models, including the newly developed nonhollow fiber-based microfluidic models, compare their strengths and weaknesses, and provide suggestions on model selection in BBB research and new-drug research and development (R&D).
Blood–Brain Barrier
The existence of a barrier between the CNS and the systemic circulation was first described by Paul Ehrlich in 188517 and Edwin Goldmann in 1913.18 The term BBB was first used by Stern and Gaultier in 1922.19 The BBB shields the brain from harmful substances in the blood and prevents the entrance of blood cells, but it allows the uptake of nutrients and hormones from blood (see below). The major BBB components include brain microvascular endothelial cells (BMECs), astrocytes, and pericytes.20 To discuss in vitro BBB models, we first briefly introduce the biological properties and functions of individual BBB components. A more detailed illustration of the BBB can be found in other references.1,21
Brain Microvascular Endothelial Cells
BMECs are a specialized type of endothelial cells. Structurally, BMECs have more mitochondria and less pinocytotic vesicles/fenestrations compared with peripheral endothelial cells.22–25 Functionally, BMECs form much tighter capillary endothelium than peripheral endothelial cells.26 The brain is basically not permeable to polar molecules, although it is estimated that capillaries in human brain have a length of ≈650 km and a surface area of ≈10 to 20 m.2,27–29 This tight barrier property can be attributed to the unique paracellular and intracellular transportation properties of BMECs.
In the interendothelial space, tight junctions (TJs) seal gaps between BMECs and limit paracellular permeability through the expression of tight junction proteins (TJPs), such as occludin, claudins, and zonula occludens (ZO-1, ZO-2, and ZO-3).25,30–33 Accumulating evidence shows that the levels of TJPs negatively correlate with paracellular permeability, and loss of TJP expression leads to paracellular leakage,25,31,33–36 suggesting that TJPs play a crucial role in the regulation of paracellular permeability. Another way to regulate BBB permeability is via vesicular transport.4,37–39 Two major mechanisms are used by BMECs to regulate intracellular transportation. First, small lipophilic molecules, such as oxygen and carbon dioxide, diffuse across BMECs freely.40 Second, some hydrophilic molecules are transported across BMECs via specific transporters and receptors. Depending on the subcellular distribution, 3 major types of transporters and receptors are found: (1) Bidirectional transporters and receptors expressed on both the luminal and abluminal sides of BMECs. These transporters and receptors usually function to facilitate nutrient transportation. For example, glucose transporter 1, mono-carboxylate transporter 1, L1 amino acid transporters, and y+cationic amino acid transporter transport glucose, lactate, and large neutral and cationic essential amino acids in and out of BMECs, respectively.41,42 (2) Unidirectional transporters and receptors expressed on both the luminal and abluminal sides of BMECs. This group of transporters and receptors shifts molecules either in or out of the brain/blood system. For example, transferrin receptor and insulin receptor mediate endocytosis of transferrin and insulin, respectively, leading to accumulation of these ligands in BMECs.43–45 (3) Transporters and receptors expressed on either the luminal or abluminal side of BMECs. These unevenly distributed transporters and receptors contribute to the polarity of BMECs and are involved in unidirectional transportation of substances. For instance, multidrug resistance-related protein 1 (also called ATP-binding cassette subfamily C member 1),46–50 P-glycoprotein (also called ATP-binding cassette subfamily B member 1),51,52 and breast cancer resistance protein (also called ATP-binding cassette subfamily G member 2)53–58 are predominantly expressed in the luminal side of BMECs. These transporters recognize a large spectrum of lipophilic substrates and pump them into capillary lumen to prevent their penetration across the BBB.59 Beside BMECs, these transporters have also been found in many types of tumor cells, including glioma,60 leukemia,61 esophageal carcinoma,62 and colon/lung/kidney cancer.63,64 Because many anticancer drugs are substrates of these transporters,59,65 the access of these drugs to CNS tissue or tumors is limited, making patients resistant to anticancer drug treatment, a phenomenon known as multidrug resistance.65 In contrast to tumor cells, lower expression of these transporters was found in blood vessels supplying CNS metastases.66 For example, P-glycoprotein levels in blood vessels around CNS metastases of melanoma and lung cancer were ≈5% and 40% of that in normal brain tissue, respectively.67 These data suggest that systematically delivered chemotherapy may reach CNS tumor cells, whereas it is hard to penetrate and kill them. Various inhibitors for these transporters, including verapamil, probenecid, and fumitremorgin C, have been used to increase drug delivery to brain tumors and improve the therapeutic efficacy.66 On the other hand, excitatory amino acid transporters 1 to 3 are found solely on the abluminal side of BMECs to efficiently remove the excitatory neurotransmitter glutamate from the brain.68 Low-density lipoprotein receptor-related protein 1, which is predominately expressed on the abluminal side of BMECs, facilitates the elimination of amyloid-β from the brain,69–71 although there is also evidence suggesting that lipoprotein receptor-related protein 1 may not contribute to the efflux of amyloid-β across the BBB.72,73 Expressed only on the abluminal side of BMECs, (Na+-K+)ATPase regulates ion homeostasis in the brain and thus proper neuronal and synaptic functions.42,58 The expression of these transporters and receptors is summarized in Figure 1. For a more complete list of transporters in BMECs, please see the other excellent reviews.57,58 Additionally, other mechanisms may also modulate substance transport in and out of BMECs. It has been shown that BMECs express high levels of γ-glutamyl transpeptidase and alkaline phosphatase, which modify many molecules to prevent their entrance into the brain.74,75
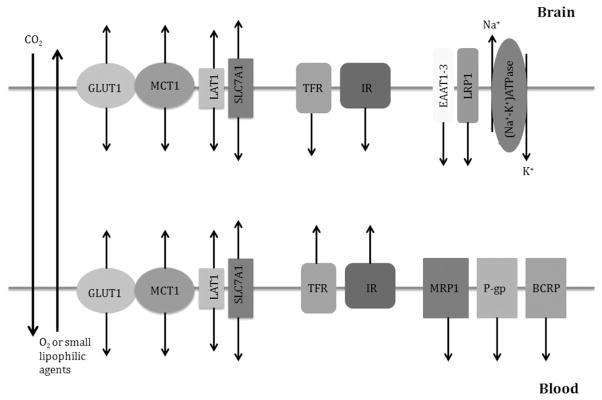
Figure 1.
Major transporters and receptors in brain microvascular endothelial cells (BMECs). Oxygen, carbon dioxide, and mall lipophilic agents diffuse across blood–brain barrier freely. Glucose transporter (GLUT1), lactate transporter (MCT1), essential amino acid transporters (LAT1 and SLC7A1) are expressed on both luminal and abluminal sides of BMECs, and transport nutrients bidirectionally. Transferrin receptor (TFR) and insulin receptor (IR) are also expressed on both sides of BMECs, and mediate endocytosis of transferrin and insulin, respectively, leading to accumulation of these molecules in BMECs. Multidrug resistance-related protein 1 (MRP1), P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP) are predominantly expressed on the luminal side of BMECs and regulate drug efflux. excitatory amino acid transporters 1 to 3 (EAAT1–3) and lipoprotein receptor-related protein 1 (LRP1) are solely expressed on the abluminal side of BMECs and remove glutamate and β-amyloid from the brain, respectively. (Na+-K+)ATPase is expressed only on the abluminal side of BMECs, and controls ion homeostasis in the brain.
Astrocytes
Using chimeric grafting experiments, Stewart and Wiley76 in 1981 elegantly showed that CNS tissue induces the unique properties of BMECs mentioned above. It has been suggested that astrocytes, the most abundant glial cells in the brain, whose endfeet cover ≥99% of the vascular surface,26,77–83 contribute to the unique properties of BMECs and thus integrity of the BBB. In agreement with this hypothesis, adding astrocytes to BMECs significantly increased the transendothelial electric resistance (TEER) and decreased their permeability to various molecules in vitro.34,84–87 Additionally, astrocyte numbers have been positively correlated with the BBB integrity in vivo,88 suggesting that astrocytes do contribute to the integrity of the BBB.
Pericytes
A group of perivascular cells, first described by Rouget in 1873,89 was named pericytes by Zimmermann in 1923.90 Sandwiched in between BMECs and astrocytic endfeet,91 pericytes are embedded in the endothelial basement membrane.21,92 Pericytes cover capillaries in the brain and the degree of coverage negatively correlates with capillary permeability.92 Pericytes have been shown to enhance TEER in the BMEC–astrocyte coculture BBB model.93,94 Additionally, pericytes upregulate P-glycoprotein functional activity and regulate TJ permeability in endothelial cells.95–97 Recently, 3 groups concomitantly reported a key role of pericytes in BBB maturation/regulation in vivo. Using mice lacking pericytes, Daneman et al98 elegantly demonstrated that pericyte numbers positively correlate with BBB tightness. Consistent with this report, Armulik et al99 and Bell et al4 found that loss of pericytes leads to BBB breakdown in vivo. These data strongly suggest that pericytes actively regulate BBB integrity and should be included in in vitro BBB models to better mimic the in vivo conditions. It should be noted that pericyte differentiation status affects their function in BBB permeability. Smooth muscle actin-α-high pericytes have been shown to decrease BBB integrity, whereas smooth muscle actin-α-low pericytes stabilize BBB integrity.100 Therefore, the differentiation status of pericytes should be taken into consideration when they are used in in vitro BBB models.
Other Cellular Components
Besides the cells mentioned above, other components, such as neurons and microglia, may also contribute to the integrity of the BBB. For example, neurons may regulate BBB permeability indirectly by modulating BMECs and astrocytes.2,101–107 It has been shown that neurons decrease sucrose leakage across BBB in vitro,108 probably via regulating the localization of occludin.109,110 Neurons are also able to induce BBB properties on BMECs.111,112 Like neurons, microglia are speculated to modulate BBB integrity because of their close relations with the neurovascular unit.113 How microglia modulates BBB permeability, however, remains controversial. There is evidence showing that microglial activation promotes the restoration of a disrupted BBB.114 Microglia-released tumor necrosis factor-α, however, has been reported to impair BBB integrity, probably via tumor necrosis factor-α receptor-mediated cytotoxicity.115 These controversial results could be explained by the complexity of microglial biology. In physiological conditions, microglia exist in the brain in the resting state with a ramified morphology.116 On injury, they quickly change their morphology/gene expression profiles and become activated.117 Early after injury, microglia take the M1 proinflammatory phenotype, characterized by the secretion of tumor necrosis factor-α and nitric oxide, to prevent the spread of injury.118,119 With the progress of disease, microglia transit to the M2 phenotype, characterized by the secretion of interleukin-10 and transforming growth factor-β, to clean up cell debris and promote recovery.118,119 Because the functions of microglia depend on their phenotypes and the transition between these phenotypes is differentially regulated in different types of insults,117–119 the roles of microglia in BBB permeability may vary depending on injury types and time after injuries.
In Vitro BBB Models
To facilitate cerebrovascular research and expedite the R&D of novel drugs for various neurological diseases, many in vitro BBB models have been developed. However, because none of these in vitro models fully replicates the in vivo conditions, there is no perfect in vitro BBB model. Thus, cautions should be taken when one chooses in vitro BBB models and interprets the data. Here we summarize the most widely used in vitro BBB models, including the recently developed microfluidic BBB models, and analyze their advantages and disadvantages. Based on whether shear stress is replicated, these in vitro BBB models are divided into 2 categories: static and dynamic models.
Static Models
Static models do not replicate the shear stress generated by the flow of blood in in vivo conditions. According to the number of cell types involved, static BBB models are further categorized into monolayer and coculture models.
Monolayer Models
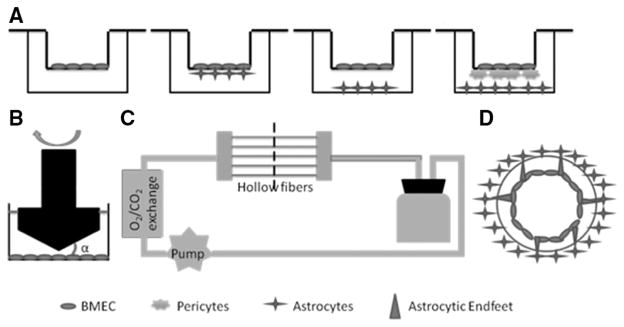
A monolayer of endothelial cells grown in the Transwell insert (Figure 2A) is used as a simple in vitro BBB model. The insert mimics the blood (luminal) side, whereas the well in which the insert sits mimics the parenchymal (abluminal) side. The microporous membrane support (0.4 μm) allows the exchange of small molecules and cell-secreted growth factors but prevents the migration of cells between the 2 compartments. To mimic the unique properties of BMECs (see Brain Microvascular Endothelial Cell section of this article), primary or low passage BMECs are used. Accumulating evidence shows that primary or low passage BMECs retain many of the biochemical and morphological properties that characterize the BBB in vivo, such as the presence of TJs and polarized expression of transporters, receptors, and enzymes.1 Two big challenges, however, exist in isolating and culturing primary BMECs: the low yield and contamination by mural cells. Because the brain vasculature accounts only for 0.1% (v/v) of the brain, a large number of rodents are usually needed to generate enough BMECs.120 Therefore, larger species, including bovine, porcine, and nonhuman primate, are used to increase the yield of BMECs. The problems with larger species, however, are that these animals are usually nontransgenic and there are no or few respective antibodies available for these species. For these reasons, rodents are still the most widely used animals in BBB research. Recently, temporary application of puromycin in the culture medium has been shown to dramatically improve the purity of primary BMECs.34,121,122 This methodological breakthrough enables the use of BMECs at high purity and significantly advances BBB research.
Figure 2.
In vitro blood–brain barrier (BBB) models. A, Brain microvascular endothelial cell (BMEC) monolayer model (left), BMEC–astrocyte coculture models (middle 2), and BMEC–pericyte–astrocyte triple-culture model (right). B, Cone-plate apparatus. α is the cone angle. C, Dynamic in vitro BBB model. D, Cross-section of one hollow fiber.
Human cells may be needed when human-specific transporters/receptors or immunologic issues are involved. Primary human cells, however, are not usually available because of ethical reasons. To circumvent this issue, many immortalized human cell lines, such as human cerebral microvascular endothelial cell line (hCMEC/D3)123 and immortalized human cerebral endothelial cells,124 have been generated. One big benefit of the immortalized cell lines is their significantly enhanced ability to proliferate in culture. Because of this advantage, immortalized brain endothelial cell lines from rodents, such as rat brain endothelial cell line, mouse brain endothelial cell line, and mouse cerebral endothelial cell line, have also been generated. These immortalized cell lines, however, unlike primary or low passage BMECs, lose some of the unique properties that characterize the in vivo BBB.125–129 It has been shown that some of the BBB-specific transporters and enzymes are expressed at much lower levels in the immortalized cell lines. For example, the expression of TJPs, including occludin and claudin-5, in hCMEC/ D3 cells is lower than that in primary cerebral endothelial cells.130 RBE4 and hCMEC/D3 cells have been found to have less glucose transporter 1 compared with brain endothelium in situ.130–132 Additionally, γ-glutamyl transpeptidase and alkaline phosphatase activity is dramatically reduced in RBE4 cells and other rat brain endothelial cell lines, compared with isolated rat brain capillaries.133,134 Because of the above-mentioned limitations, these immortalized cell lines are not able to generate a tight monolayer and thus have inadequate barrier function. To overcome this problem, reagents that modulate BBB properties, such as cAMP and glucocorticoids, have been used to improve the tightness of the endothelial monolayer.135–140
An alternative way to generate human BMECs is through human pluripotent stem cells (hPSC). Although previous studies showed that hPSC-generated endothelial cells do not have organ-specific properties,141–143 Lippmann et al144 successfully differentiated hPSC into endothelial cells with unique BBB properties. These hPSC-derived endothelial cells expressed TJPs, various nutrient receptors, amino acid and peptide transporters, and efflux transporters.144
It should be noted that the monolayer model only has 1 major cell type (BMECs) and lacks the communication among different cell types. Accumulating evidence shows that the BBB properties are dictated by the microenvironment in the brain rather than intrinsic to BMECs, and cell–cell communications play a critical role in the maintenance of these properties.76,145–147 Thus, this oversimplified monolayer model is not ideal for the study of BBB integrity. Because of the intrinsic simplicity,148 however, monolayer models have been widely used in signaling pathway and transporter kinetic studies, binding affinity measurement, and high-throughput screening.
Coculture Models
To better mimic the anatomic structure of the in vivo BBB, BMECs are cocultured with other cells that directly contribute to the barrier properties of BBB.4,26,77–83,98,99 Based on the evidence that interaction between BMECs and astrocytes increases the expression of transporters and TJs in BMECs, induces the formation of cell polarity in BMECs, and promotes a phenotype more closely mimicking the in vivo BBB,26,149–158 BMECs are cocultured with astrocytes to improve their barrier properties. In this coculture model, BMECs are seeded in the Transwell insert and astrocytes are grown either on the underside of the Transwell insert or at the bottom of the well in which the insert sits (Figure 2A). Consistent with previous reports, higher TEER and lower permeability to molecular tracers are observed in this BMEC–astrocyte coculture model.135,159–161 Cells of different origins have been used in this model. For example, mouse BMECs cocultured with astrocytes generated a TEER of 200 to 300 Ω·cm2,162 whereas bovine BMECs cocultured with astrocytes produced a higher TEER around 500 to 600 Ω·cm2.159–161 Additionally, a TEER of 900 Ω·cm2 was achieved when porcine BMECs were used.163 When hPSC-derived endothelial cells were cocultured with rat astrocytes, the TEER reached 860 Ω·cm2.144 The different TEER values and thus the barrier properties of BMECs are probably because of more efficient BMEC isolation/purification methods rather than species difference, given that equally high TEER values (600–1000 Ω·cm2) were observed in the BMEC–astrocyte coculture model independent of species.16,164–166 However, to answer the question clearly whether there is a species difference in BMEC tightness, comparative studies using the same methods and conditions within the same laboratory are needed. It should be noted that the TEER values obtained from the BMEC–astrocyte coculture model are still lower than that in in vivo conditions, which is estimated to be more than 1000 Ω·cm2.14,167 suggesting that other players also contribute to the integrity of BBB.
The BMEC–astrocyte coculture model has been widely used to study stroke, the leading cause of disability and the third most common cause of death in the United States.168 Hypoxia and hypoglycemia, 2 pathological changes in stroke, can be easily mimicked in vitro by lowering oxygen and glucose levels.169 This in vitro stroke model has been applied to investigate endothelial pathophysiology,30,170 endothelial barrier permeability,171,172 cell–cell communication,173 and free radicals174 during stroke. Additionally, it has also been used in large-scale screening for compounds that have protective function in hypoxia-reoxygenation induced injury.175 The advantages of using this model to study stroke include169 (1) the severity of stroke can be easily controlled by regulating oxygen and glucose levels, (2) reperfusion-induced injury can be replicated by reoxygenation, (3) the contribution of hypoxia/hypoglycemia alone or together can be investigated, (4) the contribution of each cell type can be easily identified, (5) the molecular mechanism underlying stroke can be dissected out because of the intrinsic simplicity of this system, and (6) it allows large-scale screening of compounds that have therapeutic potentials for stroke. One major disadvantage of this in vitro model is that it lacks shear stress (see Dynamic Models section of this article for details).
Because of the key role of pericytes in BBB regulation,4,98,99 a BMEC–pericyte coculture model has also been developed. The cells are grown in Transwell insert as described above. Pericytes dramatically enhanced the TEER in this coculture model, compared with the monolayer model.176 In addition, multidrug resistance-related protein 6 expression and matrix metalloproteinase secretion in BMECs were increased in this model.177,178 This model is particularly useful in the study of BEMC–pericyte interaction/communication. Although pericytes enhance BMEC tightness, astrocytes are absent in this BMEC–pericyte coculture model, suggesting that this model may be further improved by incorporating astrocytes.
To replicate the anatomic structure of the in vivo BBB fully, a BMEC–pericyte–astrocyte triple coculture system was built and characterized. In this model, BMECs are plated in the Transwell insert with pericytes on the underside of the insert and astrocytes at the bottom of the well (Figure 2A). The direct contact of BMECs and pericytes mimics the anatomic structure of neurovascular unit in vivo. Although direct cell–cell communication between astrocytes and BMECs/ pericytes is missing in this triple-culture model, indirect effect of astrocytes in BBB regulation via secreted soluble factors is included. As expected, this triple coculture model has significantly higher TEER and lower permeability,94,179 indicating that both pericytes and astrocytes contribute to the integrity of BBB. These data suggest that the BMEC–pericyte–astrocyte triple coculture model is a more reliable in vitro BBB model. Therefore, this model has been widely used in many BBB permeability studies.180–185 Like the BMEC–astrocyte coculture model, this triple-culture model can also be used as an ischemia-reperfusion model for pathophysiological studies or large-scale screenings.
In addition to the above-mentioned cell types, neurons and other cells have also been cultured with BMECs. It has been shown that neurons induce the expression of BBB-related enzymes and occludin in BMECs.111,112 Neurons also significantly decreased the leakage of sucrose in the BMEC–astrocyte coculture model.108–110 Additionally, macrophages when cocultured with BMECs significantly increased the TEER.186 Recently, stem cells and stem cell-derived cells have been used in in vitro BBB models. For example, embryonic neural progenitor cells have been shown to induce BBB properties in BMECs.187–189 Neural progenitor cell-derived cell mixtures, including neurons, astrocytes, oligodendrocytes, and proliferating neural progenitors, have also been shown to induce the BBB phenotype in rat BMECs.190
The coculture models incorporate important players other than BMECs and thus better mirror the anatomic structure of the in vivo BBB. These coculture models generate a tighter barrier and are ideal for permeability studies, cell–cell interaction, and lead compound identification/optimization in new-drug R&D. The most significant disadvantage of these coculture models is the lack of shear stress, which plays a critical role in the induction and maintenance of the BBB phenotype.86,191 Therefore, it is advisable to validate the results generated from the static models in dynamic models and in vivo.
Dynamic Models
Shear stress, generated by the flow of blood in physiological conditions, affects transporter and TJ expression as well as endothelial barrier function.191 It has been shown that incorporation of flow significantly increases the expression of ZO-1 and decreases the permeability of human BMEC monolayer.86 Thus, dynamic BBB models, which include shear stress, have been developed. There are 3 major types of dynamic BBB models: the cone-plate apparatus, dynamic in vitro BBB model, and microfluidic in vitro BBB models.
Cone-Plate Apparatus
The cone and plate viscometer was first used to build shear force.192 In this model, a rotating cone generates shear force, which is transmitted to the endothelial monolayer via the medium (Figure 2B). The angular velocity and the cone angle determine the shear stress it generates. Because the shear stress is not evenly distributed along the radius of the plates, the endothelial monolayer receives different shear stress depending on its location in the plates. In addition, other cell components of the BBB, such as astrocytes and pericytes, are not included in this model, which limits its application in BBB research and diminishes the reliability and significance of data it generates.
Dynamic In Vitro BBB Model
To incorporate both shear stress and other cell types, microporous hollow fibers are used (Figure 2C).193–196 In this model, BMECs and astrocytes are plated in the inner (luminal) and outer (abluminal) sides of the porous hollow fibers, respectively (Figure 2D). The culture medium is then pumped into the system via a variable-speed pump to generate shear stress comparable with that seen in physiological conditions in vivo (5–23 dynes/cm2).197,198 To maintain the stable microenvironment, a gas-permeable tubing system is used for the exchange of O2 and CO2. Using bovine aortic endothelial cells and C6 glioma cell line, a TEER value of ≈600 Ω·cm2 was achieved in this dynamic model.199 Further side-by-side comparative studies showed that this dynamic model generated a TEER >10× higher than that in the Transwell coculture model and that its permeability to sucrose and phenytoin was, respectively, 10× and 5× less than that in the coculture system,200 suggesting a tighter barrier function of this model. Cucullo et al196 also compared the barrier function of this model using different endothelial cells and found that brain endothelial cells (human BMECs and hCMEC/D3) generated a much higher TEER (1200 Ω·cm2) and less permeability to sucrose or phenytoin than endothelial cells of peripheral origin (human umbilical vein endothelial cells), rationalizing the use of brain endothelial cells to construct in vitro BBB models. Additionally, the expression of transporters, ion channels, and efflux proteins was dramatically induced in BMECs in this model.199,201,202 Altogether, these data suggest that this dynamic model better mimics the in vivo BBB by replicating its anatomic and physiological properties.86,167,194,196,199,200
This dynamic in vitro BBB model has been used to study the pathophysiology of various CNS diseases, including ischemia-reperfusion-induced injury and epilepsy.167,196,202 The molecular events during ischemia include flow disturbance (loss of shear stress) and induction of inflammatory response (release of reactive oxygen species and cytokines by white blood cells).203 These changes have been replicated in the dynamic BBB model by flow cessation and reperfusion in the presence of white blood cells.204 It has been shown that flow cessation for 1 hour in this system induces a biphasic opening of the BBB167,196 and ibuprofen pretreatment partially prevents BBB failure and decreases the duration and degree of BBB breakdown,196 suggesting that the inflammation immediately after ischemic stroke is one of the major causes of BBB failure. In another study, endothelial cells and astrocytes from normal or drug-resistant epileptic human brain tissue were cultured in this dynamic system.202 The permeability to phenytoin, a substrate for P-glycoprotein, was significantly reduced when endothelial cells from epileptic brain were used, although TEER and permeability to sucrose were not affected.202 This effect was independent of the origin of astrocytes and could be reversed by P-glycoprotein blocker XR9576,202 suggesting that the drug-resistant BBB phenotype in epileptic patients can be replicated in this dynamic BBB model.
Recently, a revised model with hollow fibers with trans-mural microholes of 2 to 4 μm has been developed to allow transmigration/trafficking studies.167 Extravasation of white blood cells was observed when the BBB is breached by flow cessation and reperfusion.167 It should be noted that the space between BMECs and astrocytes (the thickness of the hollow fiber) is 150 μm167 compared with the immediate contact of these cell types in vivo. This extremely thick layer may affect the extravasation of immune cells from the luminal side to the abluminal side. Thus, cautions should be taken when interpreting data generated from this dynamic in vitro BBB model. In addition, this dynamic in vitro BBB model also has three other disadvantages. First, it does not allow direct visualization of the endothelial morphology in the luminal compartment. Although cells can be characterized after trypsin treatment, the harvesting process may change the morphology and physiological characteristics of the cells. Second, the cell number (>1×106) and technical skills required to build this model are relatively high. Third, the time required to reach steady-state TEER is longer (usually 9–12 days)167,199 compared with that in coculture models (usually 3–4 days). These shortcomings prevent the use of this dynamic in vitro BBB model in large-scale screens. This model, however, may be useful in lead compound validation/optimization in new-drug R&D.
Microfluidic-Based BBB Models
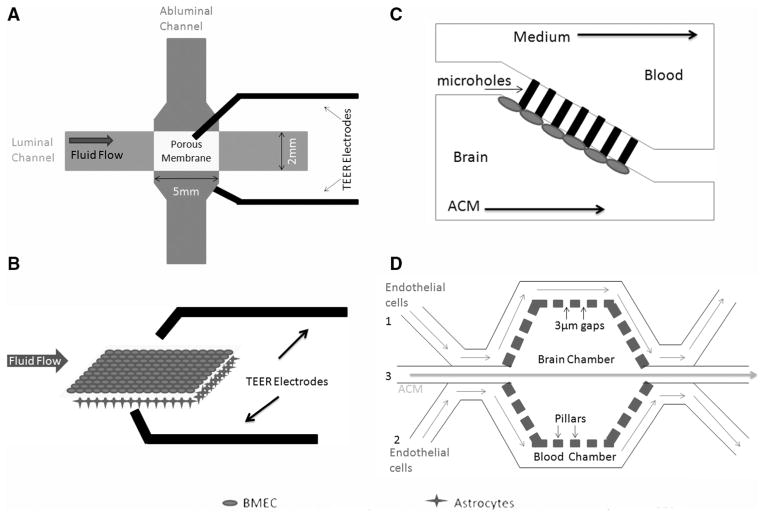
To address the limitations of the dynamic in vitro BBB model, microfluidic device-based in vitro BBB models have been developed.205,206 The microfluidic BBB (μBBB) model is one of the first such models. It contains 2 perpendicularly crossing channels, which allow dynamic flow and generate shear stress; a polycarbonate porous membrane at the intersection of these channels, which enables coculture of BMECs and astrocytes; and multiple built-in Ag/AgCl electrodes for TEER measurement205,206 (Figure 3A). These channels have a height of 200 μm and widths of 2 mm (luminal) and 5 mm (abluminal). In this model, BMECs and astrocytes are seeded on the luminal and abluminal sides of the membrane, respectively (Figure 3B). A pump and a gas-permeable tubing system are used to generate shear stress and allow O2–CO2 exchange, respectively. Under a shear stress of 0.023 dynes/cm2 (much lower than that in physiological conditions), this μBBB model significantly improved the TEER of bEND.3 cells to 140 Ω·cm2 after 3 days of culture, in contrast to 15 Ω·cm2 in the static model.205,206 When cocultured with astrocytes, the TEER was further increased to ≥250 Ω·cm2.205,206 Additionally, the permeability increased on histamine exposure, followed by recovery, suggesting the stability of this model.205,206 This μBBB model was further improved by replacing the oxidation sensitive AgCl electrodes with inert platinum ones and decreasing the cross-sectional area.207 These modifications allow accurate measurement of TEER and reduce the amount of cells needed. Without shear stress, hCMEC/D3 cells reached a TEER of ≈40 Ω·cm2 within 3 days of culture in this improved μBBB model.207 With only 18 hours of physiological shear stress (5.8 dynes/cm2), the TEER was increased to 120 Ω·cm2.207 Tumor necrosis factor-α, a known inflammatory cytokine, dramatically decreased the TEER to 12 Ω·cm2.207 These data suggest that this improved model is able to respond to mechanical and biochemical stimuli and thus can be used in permeability studies. The different TEER values in these studies may be because of different cell types (bEND.3 versus hCMEC/D3 and monolayer versus coculture), shear stress (0.023 versus 5.8 dynes/cm2), and time (3 days versus 18 hours).
Figure 3.
Microfluidic-based in vitro blood–brain barrier (BBB) models. A, Microfluidic BBB (μBBB) model. B, Three-dimensional view of the porous membrane at the intersection of the flow channels in the μBBB model. C, Diagram of the microfluidic device containing micro-holes. D, Structure of the synthetic microvasculature model of the BBB model. ACM indicates astrocyte-conditioned medium; BMEC, brain microvascular endothelial cell; and TEER, transendothelial electric resistance.
Another microfluidic-based BBB model originally designed to study the BBB permeability of drugs involves a microhole structure.208 This model is composed of 2 horizontally aligned chambers connected by a microhole structure (Figure 3C).208 Endothelial cells suspended in medium will be infused in the brain chamber and trapped in the microholes because of pressure gradient. By using human umbilical vein endothelial cells and astrocyte-conditioned medium with a shear stress of 0.28 to 8.91 dynes/cm2, Yeon et al208 successfully demonstrated that the permeability of this system to fluorescein isothiocyanate-dextran inversely correlated with its size. Additionally, by comparing the permeability of 5 well-known drugs in this system with their in vivo data, this group further validated the reliability of this model, suggesting its application in predicting the CNS permeability of new compounds.208 The TEER measurement in this system, however, has not been reported. Two major concerns of this device are (1) it lacks cell–cell contact, a key feature of the in vivo BBB and (2) it does not replicate the dimensions of microvasculature in vivo. Recently, this microfluidic device has been modified and a new microfluidic-based synthetic microvasculature model of the BBB (SyM-BBB) has been developed.209 This SyM-BBB model contains 2 microchannels separated by microfabricated pillars with 3-μm gaps (Figure 3D).209 The pillars separated by 3-μm gaps mimic the porous membrane in the μBBB model. Endothelial cells will be infused to the blood chamber via ports 1 and 2, whereas astrocyte-conditioned medium or astrocytes can be infused to the brain chamber from port 3. The flow speed of medium in these chambers determines the shear stress. This design better mimics the in vivo microcirculatory system by including the diverging and converging bifurcations.209 It has been reported that astrocyte-conditioned medium significantly increases the expression of TJP in RBE4 cells and decreases their permeability to fluorescein isothiocyanate-dextran in this model.209 TEER values were not available because of the lack of electrodes in the system at this stage. Additionally, efflux activity assay revealed a functional P-glycoprotein efflux system,209 suggesting that the Sym-BBB model can be used in drug discovery and BBB permeability studies. Further work should focus on including other cell types (astrocytes or pericytes) and integrating electrodes for TEER measurement.
Compared with the dynamic in vitro BBB model, these microfluidic in vitro BBB models have many advantages. First, because the thickness of the membrane or pillars is <10 μm,205,206 they allow transmigration/trafficking studies in a condition that better replicates the in vivo BBB structure. Second, nondestructive microscopy is possible because of the transparency of the materials.205,206 Third, it takes less time (3–4 days) to reach steady-state TEER.205,206 Fourth, they only require a small amount of cells and are less demanding in terms of technical skills. These microfluidic models, on the other hand, also have some shortcomings. For example, TEER value is not high enough (250–300 Ω·cm2) in these models. This may be because of the short time in culture (3–4 days). Future work should examine whether the TEER continues to increase at later time points. Additionally, such as the dynamic in vitro BBB model, these microfluidic-based models can only incorporate 2 cell types, given that the membrane and pillars (hollow fibers in the dynamic in vitro BBB model) have only 2 sides. Although there are only limited publications about these microfluidic-based BBB models to date (probably because they are relatively new), they show promising results as dynamic in vitro BBB models.205,206 With the accumulation of research data, these microfluidic models may be used to aid neurovascular research and new-drug R&D in the future because of its small size, short time to reach steady-state TEER, low-to-moderate technical skill requirement, and low cost. The advantages and disadvantages of these in vitro BBB models are summarized in the Table.
Table.
Comparison Among Different In Vitro BBB Models
| Other Cell Types | Sheer Stress | No. of Animals Needed | Time to Steady TEER | Steady TEER Value | Gap Between Different Cell Types | Suitable for Migration Assays | Technical Requirements | Cost | Nondestructive Microscopy | |
|---|---|---|---|---|---|---|---|---|---|---|
| Monolayer models | No | No | Moderate | 3–4 d | Low to moderate | N/A | Yes | Low | Low | Yes |
| Coculture models | Yes | No | High | 3–4 d | Moderate to high | <10 μm | Yes | Moderate | Low to moderate | Yes |
| Cone-plate apparatus | No | Yes | Moderate | 3–4 d | N/A | N/A | No | Low to moderate | Low | No |
| Dynamic in vitro BBB model | Yes | Yes | Very high | 9–12 d | High | >150 μm | No | High | High | No |
| Microfluidic-based models | Yes | Yes | Low | 3–4 d | Moderate to high | <10 μm | Yes | Moderate | Low | Yes |
BBB indicates blood–brain barrier; and TEER, transendothelial electric resistance.
How to Select the Appropriate Models?
Choosing appropriate in vitro models not only saves time and money, but also enables accurate interpretation of the data. One of the most important criteria in model selection is the purpose of study. If the aim is to investigate the permeability of the BBB, it is advisable to use the coculture models and dynamic in vitro BBB model if possible. Some multiculture models are commercially available now, which significantly reduces the difficulty and time in model preparation, but increases the cost. If cell trafficking/migration is involved, microfluidic BBB model is the first choice because of the thin space between BMECs and astrocytes (<10 μm) and incorporation of shear stress. If the purpose is to explore signaling pathways, study transporter kinetics, or measure binding affinities, the use of monolayer model is very helpful because of its intrinsic simplicity. The results, however, should be validated in more sophisticated dynamic models and in vivo, before a conclusion is drawn. It is also recommended that the results in human cells be validated (if nonhuman cells are used), given that species differences may exist.
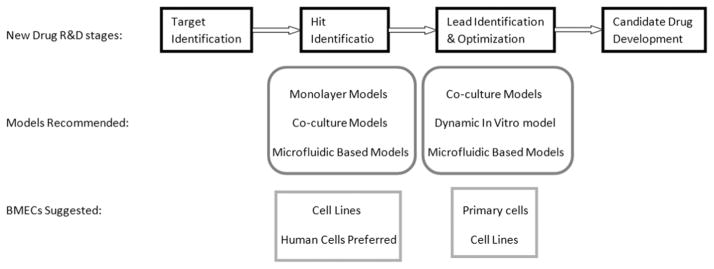
In vitro BBB models are extensively used in the first few stages of new-drug R&D processes, including target identification, hit identification, and lead identification/optimization.1 Once a target (kinases, receptors, etc) is identified, high-throughput screening is used to identify potential drug candidates. At this stage, a very large number of compounds need to be screened, demanding an easy and fast in vitro BBB model. The monolayer models and coculture models, which take 3 to 4 days to reach steady-state TEER and are relatively easy to construct, can be used. It has been shown that, among different immortalized cell lines, the P-glycoprotein transfected Madin–Darby canine kidney cells give the best separation of passively distributed and effluxed compounds and the best correlation between in vitro and in vivo data in permeability assays.210 Recently, a comparative study showed that the permeability of the P-glycoprotein transfected Madin–Darby canine kidney cells and vinblastine-treated Caco-2 cells alone was similar to that of the BMEC–pericyte–astrocyte triple coculture model.180 These data suggest that the P-glycoprotein transfected Madin–Darby canine kidney cells and vinblastine-treated Caco-2 cells may be used in high-throughput screening for potential candidate identification. Because these cells are not from the brain vasculature, validation using BMECs in more sophisticated models is usually necessary. In the lead identification/optimization stage, identified compounds are validated, and structure–activity relationships and toxicological profile are investigated. Sensitive in vitro models that replicate the majority of the in vivo conditions are required. Such models include the static coculture system and dynamic models. Because of the high technical skill requirement and long culturing time (9–12 days) of the dynamic in vitro BBB model, the newly developed microfluidic-based BBB models are an alternative choice. It is advisable to use primary cells rather than immortalized cell lines at this stage to better replicate the biological properties of the BMECs in vivo. Validation using human cells is highly recommended to avoid species difference-caused failure in the subsequent R&D stages. The suggestions on the selection of in vitro BBB models at different R&D stages are summarized in Figure 4.
Figure 4.
Suggestions on model selection at early stages of new-drug research and development (R&D). During hit identification stage, simple models, including the monolayer and coculture models, are recommended. microfluidic-based dynamic models may also be used given their incorporation of shear stress and low cost. Because of the large-scale nature of this stage, immortalized endothelial cell lines, especially human cells, should be used. When it comes to the lead identification and optimization stage, the number of compounds is dramatically reduced. Thus, sophisticated sensitive models that better replicate the in vivo blood–brain barrier (BBB) conditions are strongly recommended, such as the coculture models and the dynamic in vitro BBB model. Microfluidic-based dynamic models may also be used at this stage. It is advised to use primary cells at this stage, although cell lines may also be used. BMEC indicates brain micro-vascular endothelial cell.
Conclusions
In vitro BBB models are of key importance to our understanding of the BBB functions in physiological and pathological conditions and the R&D of new drugs for various neurological disorders. Although many in vitro BBB models have been developed and used in a variety of studies, none of them can fully replicate the in vivo conditions. Knowing the advantages and disadvantages of these models and choosing the suitable models enable accurate interpretation of the data and significantly facilitate the R&D of new drugs for many neurological diseases. In this review, we summarize the widely used and newly developed in vitro BBB models, compare their strengths and weaknesses, and provide suggestions for model selection.
Acknowledgments
Drs He and Yao searched the literature and drafted the article. Drs Tsirka and Cao gave suggestions and edited the article. All authors read and approved the final article.
Sources of Funding
This work was partially supported by the National Natural Science Foundation of China granted to Dr Cao.
Footnotes
Disclosures
None.
References
- 1.Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 2.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 7.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos MC, Saadoun S, Woodrow CJ, Davies DC, Costa-Martins P, Moss RF, et al. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol Appl Neurobiol. 2001;27:384–395. doi: 10.1046/j.0305-1846.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 13.Benchenane K, López-Atalaya JP, Fernández-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J Pharm Sci. 2001;90:1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 15.Deli MA, Abrahám CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott NJ, Dolman DEM, Yusof SR, Reichel A. Drug Delivery to the Brain. New York, NY: Springer; 2014. In vitro models of CNS barriers; pp. 163–197. [Google Scholar]
- 17.Ehrlich P. Das Sauerstoff-Bedürfniss des Organismus; Eine Farbenanalytische Studie. Berlin: Hirschwald; 1885. [Google Scholar]
- 18.Goldmann EE. Vitalfarbung am Zentral-Nervensystem. Abhandlungen Preussischen Akademie der Wissenschaften Physikalisch Mathematisch Klasse I. Berlin: Springer; 1913. pp. 1–60. [Google Scholar]
- 19.Stern L, Gantier R. Les rapport entre le liquide céphalorachdien et les éléments nerveux de l’axe cérébrospinal. Arch Int Physiol. 1922;17:391–408. [Google Scholar]
- 20.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 23.Sedlakova R, Shivers RR, Del Maestro RF. Ultrastructure of the blood-brain barrier in the rabbit. J Submicrosc Cytol Pathol. 1999;31:149–161. [PubMed] [Google Scholar]
- 24.Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 25.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardridge WM, Triguero D, Yang J, Cancilla PA. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J Pharmacol Exp Ther. 1990;253:884–891. [PubMed] [Google Scholar]
- 28.Pardridge WM. Peptide Drug Delivery to the Brain. New York: Raven Press; 1991. [Google Scholar]
- 29.Bradbury MWB. The Concept of a Blood-Brain Barrier. Chichester, England: Wiley; 1979. [Google Scholar]
- 30.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 31.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain Res Brain Res Rev. 2003;42:221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 32.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 33.Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, et al. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/s0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 34.Yao Y, Tsirka SE. Truncation of monocyte chemoattractant protein 1 by plasmin promotes blood-brain barrier disruption. J Cell Sci. 2011;124(pt 9):1486–1495. doi: 10.1242/jcs.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y, Tsirka SE. Mouse MCP1 C-terminus inhibits human MCP1-induced chemotaxis and BBB compromise. J Neurochem. 2011;118:215–223. doi: 10.1111/j.1471-4159.2011.07319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacAulay N, Zeuthen T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience. 2010;168:941–956. doi: 10.1016/j.neuroscience.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(suppl 4):95–104. [PubMed] [Google Scholar]
- 40.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Redzic Z. Molecular biology of the blood-brain and the blood-cerebro-spinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Banks WA. Blood-brain barrier as a regulatory interface. Forum Nutr. 2010;63:102–110. doi: 10.1159/000264398. [DOI] [PubMed] [Google Scholar]
- 46.ElAli A, Hermann DM. ATP-binding cassette transporters and their roles in protecting the brain. Neuroscientist. 2011;17:423–436. doi: 10.1177/1073858410391270. [DOI] [PubMed] [Google Scholar]
- 47.Abuznait AH, Kaddoumi A. Role of ABC transporters in the pathogenesis of Alzheimer’s disease. ACS Chem Neurosci. 2012;3:820–831. doi: 10.1021/cn300077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nies AT, Jedlitschky G, König J, Herold-Mende C, Steiner HH, Schmitt HP, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 49.Soontornmalai A, Vlaming ML, Fritschy JM. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience. 2006;138:159–169. doi: 10.1016/j.neuroscience.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 50.van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005;46:1569–1580. doi: 10.1111/j.1528-1167.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 51.Beaulieu E, Demeule M, Ghitescu L, Béliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenblätter T, Hüwel S, Galla HJ. Characterisation of the brain multi-drug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221–231. doi: 10.1016/s0006-8993(03)02401-6. [DOI] [PubMed] [Google Scholar]
- 54.von Wedel-Parlow M, Wölte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- 55.Lemmen J, Tozakidis IE, Galla HJ. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res. 2013;1491:1–13. doi: 10.1016/j.brainres.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 56.Lemmen J, Tozakidis IE, Bele P, Galla HJ. Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation. Brain Res. 2013;1501:68–80. doi: 10.1016/j.brainres.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 57.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 58.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersson U, Malmer B, Bergenheim AT, Brännström T, Henriksson R. Heterogeneity in the expression of markers for drug resistance in brain tumors. Clin Neuropathol. 2004;23:21–27. [PubMed] [Google Scholar]
- 61.Dorr R, Karanes C, Spier C, Grogan T, Greer J, Moore J, et al. Phase I/ II study of the P-glycoprotein modulator PSC 833 in patients with acute myeloid leukemia. J Clin Oncol. 2001;19:1589–1599. doi: 10.1200/JCO.2001.19.6.1589. [DOI] [PubMed] [Google Scholar]
- 62.Nooter K, Westerman AM, Flens MJ, Zaman GJ, Scheper RJ, van Wingerden KE, et al. Expression of the multidrug resistance-associated protein (MRP) gene in human cancers. Clin Cancer Res. 1995;1:1301–1310. [PubMed] [Google Scholar]
- 63.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein LJ, Galski H, Fojo A, Willingham M, Lai SL, Gazdar A, et al. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989;81:116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- 65.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 66.Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 67.Régina A, Demeule M, Laplante A, Jodoin J, Dagenais C, Berthelet F, et al. Multidrug resistance in brain tumors: roles of the blood-brain barrier. Cancer Metastasis Rev. 2001;20:13–25. doi: 10.1023/a:1013104423154. [DOI] [PubMed] [Google Scholar]
- 68.O’Kane RL, Martínez-López I, DeJoseph MR, Viña JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–31895. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 69.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gosselet F, Saint-Pol J, Candela P, Fenart L. Amyloid-β peptides, Alzheimer’s disease and the blood-brain barrier. Curr Alzheimer Res. 2013;10:1015–1033. doi: 10.2174/15672050113106660174. [DOI] [PubMed] [Google Scholar]
- 73.Ito S, Ueno T, Ohtsuki S, Terasaki T. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: involvement of an LRP-1-independent pathway. J Neurochem. 2010;113:1356–1363. doi: 10.1111/j.1471-4159.2010.06708.x. [DOI] [PubMed] [Google Scholar]
- 74.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 75.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 77.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- 79.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 81.Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- 82.DeBault LE, Cancilla PA. Gamma-glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science. 1980;207:653–655. doi: 10.1126/science.6101511. [DOI] [PubMed] [Google Scholar]
- 83.Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood-brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neuhaus J, Risau W, Wolburg H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in trans-filter coculture. Ann N Y Acad Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 85.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen-Kashi Malina K, Cooper I, Teichberg VI. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 2009;1284:12–21. doi: 10.1016/j.brainres.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 88.Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, et al. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45:325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 89.Rouget C. Memoire sur les developpement, la structure et les proprietes physiologiques des capillaires sanguins et lymphatiques. Archs Physiol Norm Pathol. 1873;5:603–663. [Google Scholar]
- 90.Zimmermann KW. Der Feinere Bau der Blutcappillaren. München: Bergmann; 1923. [Google Scholar]
- 91.Sá-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 92.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Dente CJ, Steffes CP, Speyer C, Tyburski JG. Pericytes augment the capillary barrier in in vitro cocultures. J Surg Res. 2001;97:85–91. doi: 10.1006/jsre.2001.6117. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 96.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Pericytes and their role in microvasculature homeostasis. J Surg Res. 2006;135:305–311. doi: 10.1016/j.jss.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 100.Thanabalasundaram G, Schneidewind J, Pieper C, Galla HJ. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol. 2011;43:1284–1293. doi: 10.1016/j.biocel.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Ben-Menachem E, Johansson BB, Svensson TH. Increased vulnerability of the blood-brain barrier to acute hypertension following depletion of brain noradrenaline. J Neural Transm. 1982;53:159–167. doi: 10.1007/BF01243407. [DOI] [PubMed] [Google Scholar]
- 102.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab. 1997;17:894–904. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 103.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 104.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with antero-gradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92:163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 106.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–171. [PubMed] [Google Scholar]
- 107.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 108.Schiera G, Sala S, Gallo A, Raffa MP, Pitarresi GL, Savettieri G, et al. Permeability properties of a three-cell type in vitro model of blood-brain barrier. J Cell Mol Med. 2005;9:373–379. doi: 10.1111/j.1582-4934.2005.tb00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D’Agostino S, et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- 110.Schiera G, Bono E, Raffa MP, Gallo A, Pitarresi GL, Di Liegro I, et al. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. J Cell Mol Med. 2003;7:165–170. doi: 10.1111/j.1582-4934.2003.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 112.Cestelli A, Catania C, D’Agostino S, Di Liegro I, Licata L, Schiera G, et al. Functional feature of a novel model of blood brain barrier: studies on permeation of test compounds. J Control Release. 2001;76:139–147. doi: 10.1016/s0168-3659(01)00431-x. [DOI] [PubMed] [Google Scholar]
- 113.Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 114.Willis CL. Glia-induced reversible disruption of blood-brain barrier integrity and neuropathological response of the neurovascular unit. Toxicol Pathol. 2011;39:172–185. doi: 10.1177/0192623310385830. [DOI] [PubMed] [Google Scholar]
- 115.Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, et al. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci. 2010;112:251–254. doi: 10.1254/jphs.09292sc. [DOI] [PubMed] [Google Scholar]
- 116.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 117.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 118.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lippmann ES, Al-Ahmad A, Palecek SP, Shusta EV. Modeling the blood-brain barrier using stem cell sources. Fluids Barriers CNS. 2013;10:2. doi: 10.1186/2045-8118-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perrière N, Demeuse P, Garcia E, Regina A, Debray M, Andreux JP, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- 122.Perrière N, Yousif S, Cazaubon S, Chaverot N, Bourasset F, Cisternino S, et al. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Res. 2007;1150:1–13. doi: 10.1016/j.brainres.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 123.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muruganandam A, Herx LM, Monette R, Durkin JP, Stanimirovic DB. Development of immortalized human cerebromicrovascular endothelial cell line as an in vitro model of the human blood-brain barrier. FASEB J. 1997;11:1187–1197. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 125.Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell Mol Neurobiol. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain micro-vascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- 127.Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre JM, et al. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994;159:101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- 128.Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway KM, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 129.Kannan R, Chakrabarti R, Tang D, Kim KJ, Kaplowitz N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res. 2000;852:374–382. doi: 10.1016/s0006-8993(99)02184-8. [DOI] [PubMed] [Google Scholar]
- 130.Urich E, Lazic SE, Molnos J, Wells I, Freskgård PO. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One. 2012;7:e38149. doi: 10.1371/journal.pone.0038149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Régina A, Morchoisne S, Borson ND, McCall AL, Drewes LR, Roux F. Factor(s) released by glucose-deprived astrocytes enhance glucose transporter expression and activity in rat brain endothelial cells. Biochim Biophys Acta. 2001;1540:233–242. doi: 10.1016/s0167-4889(01)00133-1. [DOI] [PubMed] [Google Scholar]
- 132.Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, et al. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- 133.el Hafny B, Bourre JM, Roux F. Synergistic stimulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities by retinoic acid and astroglial factors in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1996;167:451–460. doi: 10.1002/(SICI)1097-4652(199606)167:3<451::AID-JCP9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 134.Hosoya KI, Takashima T, Tetsuka K, Nagura T, Ohtsuki S, Takanaga H, et al. mRna expression and transport characterization of conditionally immortalized rat brain capillary endothelial cell lines; a new in vitro BBB model for drug targeting. J Drug Target. 2000;8:357–370. doi: 10.3109/10611860008997912. [DOI] [PubMed] [Google Scholar]
- 135.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Donnell ME, Martinez A, Sun D. Cerebral microvascular endothelial cell Na-K-Cl cotransport: regulation by astrocyte-conditioned medium. Am J Physiol. 1995;268(3 pt 1):C747–C754. doi: 10.1152/ajpcell.1995.268.3.C747. [DOI] [PubMed] [Google Scholar]
- 137.Hurst RD, Fritz IB. Properties of an immortalised vascular endothelial/ glioma cell co-culture model of the blood-brain barrier. J Cell Physiol. 1996;167:81–88. doi: 10.1002/(SICI)1097-4652(199604)167:1<81::AID-JCP9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 138.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;247:312–315. [PubMed] [Google Scholar]
- 139.Franke H, Galla HJ, Beuckmann CT. An improved low-permeability in vitro-model of the blood-brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999;818:65–71. doi: 10.1016/s0006-8993(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 140.Boveri M, Berezowski V, Price A, Slupek S, Lenfant AM, Benaud C, et al. Induction of blood-brain barrier properties in cultured brain capillary endothelial cells: comparison between primary glial cells and C6 cell line. Glia. 2005;51:187–198. doi: 10.1002/glia.20189. [DOI] [PubMed] [Google Scholar]
- 141.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 146.Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W, et al. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- 147.Toimela T, Mäenpää H, Mannerström M, Tähti H. Development of an in vitro blood-brain barrier model-cytotoxicity of mercury and aluminum. Toxicol Appl Pharmacol. 2004;195:73–82. doi: 10.1016/j.taap.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Berezowski V, Landry C, Lundquist S, Dehouck L, Cecchelli R, Dehouck MP, et al. Transport screening of drug cocktails through an in vitro blood-brain barrier: is it a good strategy for increasing the throughput of the discovery pipeline? Pharm Res. 2004;21:756–760. doi: 10.1023/b:pham.0000026424.78528.11. [DOI] [PubMed] [Google Scholar]
- 149.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 150.Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood-brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 151.Cancilla PA, DeBault LE. Neutral amino acid transport properties of cerebral endothelial cells in vitro. J Neuropathol Exp Neurol. 1983;42:191–199. doi: 10.1097/00005072-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 152.Cucullo L, Marchi N, Marroni M, Fazio V, Namura S, Janigro D. Blood-brain barrier damage induces release of alpha2-macroglobulin. Mol Cell Proteomics. 2003;2:234–241. doi: 10.1074/mcp.M200077-MCP200. [DOI] [PubMed] [Google Scholar]
- 153.Bendayan R, Lee G, Bendayan M. Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc Res Tech. 2002;57:365–380. doi: 10.1002/jemt.10090. [DOI] [PubMed] [Google Scholar]
- 154.Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood-brain barrier: in vitro models. News Physiol Sci. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- 155.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 156.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, et al. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 157.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980;192:17–28. doi: 10.1016/0006-8993(80)91004-5. [DOI] [PubMed] [Google Scholar]
- 158.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 159.Zysk G, Schneider-Wald BK, Hwang JH, Bejo L, Kim KS, Mitchell TJ, et al. Pneumolysin is the main inducer of cytotoxicity to brain micro-vascular endothelial cells caused by Streptococcus pneumoniae. Infect Immun. 2001;69:845–852. doi: 10.1128/IAI.69.2.845-852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raub TJ. Signal transduction and glial cell modulation of cultured brain microvessel endothelial cell tight junctions. Am J Physiol. 1996;271(2 Pt 1):C495–C503. doi: 10.1152/ajpcell.1996.271.2.C495. [DOI] [PubMed] [Google Scholar]
- 161.Dehouck MP, Méresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 162.Hutamekalin P, Farkas AE, Orbók A, Wilhelm I, Nagyoszi P, Veszelka S, et al. Effect of nicotine and polyaromtic hydrocarbons on cerebral endothelial cells. Cell Biol Int. 2008;32:198–209. doi: 10.1016/j.cellbi.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 163.Smith M, Omidi Y, Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J Drug Target. 2007;15:253–268. doi: 10.1080/10611860701288539. [DOI] [PubMed] [Google Scholar]
- 164.Patabendige A, Skinner RA, Morgan L, Abbott NJ. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res. 2013;1521:16–30. doi: 10.1016/j.brainres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Helms HC, Madelung R, Waagepetersen HS, Nielsen CU, Brodin B. In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia. 2012;60:882–893. doi: 10.1002/glia.22321. [DOI] [PubMed] [Google Scholar]
- 166.Abbott NJ, Dolman DE, Drndarski S, Fredriksson SM. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol. 2012;814:415–430. doi: 10.1007/978-1-61779-452-0_28. [DOI] [PubMed] [Google Scholar]
- 167.Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 169.Yang L, Shah KK, Abbruscato TJ. An in vitro model of ischemic stroke. Methods Mol Biol. 2012;814:451–466. doi: 10.1007/978-1-61779-452-0_30. [DOI] [PubMed] [Google Scholar]
- 170.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 171.Paulson JR, Roder KE, McAfee G, Allen DD, Van der Schyf CJ, Abbruscato TJ. Tobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na,K,2Cl-cotransporter during hypoxia-reoxygenation. J Pharmacol Exp Ther. 2006;316:248–254. doi: 10.1124/jpet.105.090738. [DOI] [PubMed] [Google Scholar]
- 172.Yang T, Roder KE, Abbruscato TJ. Evaluation of bEnd5 cell line as an in vitro model for the blood-brain barrier under normal and hypoxic/aglycemic conditions. J Pharm Sci. 2007;96:3196–3213. doi: 10.1002/jps.21002. [DOI] [PubMed] [Google Scholar]
- 173.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276(4 pt 1):C812–C820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 174.Schroeter ML, Mertsch K, Giese H, Müller S, Sporbert A, Hickel B, et al. Astrocytes enhance radical defence in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 1999;449:241–244. doi: 10.1016/s0014-5793(99)00451-2. [DOI] [PubMed] [Google Scholar]
- 175.Szoleczky P, Módis K, Nagy N, Dóri Tóth Z, DeWitt D, Szabó C, et al. Identification of agents that reduce renal hypoxia-reoxygenation injury using cell-based screening: purine nucleosides are alternative energy sources in LLC-PK1 cells during hypoxia. Arch Biochem Biophys. 2012;517:53–70. doi: 10.1016/j.abb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept. 2004;123:77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 177.Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- 178.Zozulya A, Weidenfeller C, Galla HJ. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res. 2008;1189:1–11. doi: 10.1016/j.brainres.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 179.Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hellinger E, Veszelka S, Tóth AE, Walter F, Kittel A, Bakk ML, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm. 2012;82:340–351. doi: 10.1016/j.ejpb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 181.Imamura S, Tabuchi M, Kushida H, Nishi A, Kanno H, Yamaguchi T, et al. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol. 2011;31:787–793. doi: 10.1007/s10571-011-9676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ceruti S, Colombo L, Magni G, Viganò F, Boccazzi M, Deli MA, et al. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem Int. 2011;59:259–271. doi: 10.1016/j.neuint.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 183.Veszelka S, Toth AE, Walter FR, Datki Z, Mozes E, Fulop L, et al. Docosahexaenoic acid reduces amyloid-beta induced toxicity in cells of the neurovascular unit. J Alzheimers Dis. 2013;36:487–501. doi: 10.3233/JAD-120163. [DOI] [PubMed] [Google Scholar]
- 184.Toyoda K, Tanaka K, Nakagawa S, Thuy DH, Ujifuku K, Kamada K, et al. Initial contact of glioblastoma cells with existing normal brain endothelial cells strengthen the barrier function via fibroblast growth factor 2 secretion: a new in vitro blood-brain barrier model. Cell Mol Neurobiol. 2013;33:489–501. doi: 10.1007/s10571-013-9913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lukic-Panin V, Deguchi K, Yamashita T, Shang J, Zhang X, Tian F, et al. Free radical scavenger edaravone administration protects against tissue plasminogen activator induced oxidative stress and blood brain barrier damage. Curr Neurovasc Res. 2010;7:319–329. doi: 10.2174/156720210793180747. [DOI] [PubMed] [Google Scholar]
- 186.Zenker D, Begley D, Bratzke H, Rübsamen-Waigmann H, von Briesen H. Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells. J Physiol. 2003;551(pt 3):1023–1032. doi: 10.1113/jphysiol.2003.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J Neurochem. 2007;101:555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 189.Lim JC, Wolpaw AJ, Caldwell MA, Hladky SB, Barrand MA. Neural precursor cell influences on blood-brain barrier characteristics in rat brain endothelial cells. Brain Res. 2007;1159:67–76. doi: 10.1016/j.brainres.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 190.Lippmann ES, Weidenfeller C, Svendsen CN, Shusta EV. Blood-brain barrier modeling with co-cultured neural progenitor cell-derived astrocytes and neurons. J Neurochem. 2011;119:507–520. doi: 10.1111/j.1471-4159.2011.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982;53:1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 193.Stanness KA, Guatteo E, Janigro D. A dynamic model of the blood-brain barrier “in vitro”. Neurotoxicology. 1996;17:481–496. [PubMed] [Google Scholar]
- 194.Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, et al. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771:329–342. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 195.Janigro D, Leaman SM, Stanness KA. Dynamic modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm Sci Technolo Today. 1999;2:7–12. doi: 10.1016/s1461-5347(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 196.Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, et al. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 197.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 198.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 199.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, et al. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 200.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: a permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 201.McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, et al. Mechanisms of glucose transport at the blood-brain barrier: an in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- 202.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 203.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 204.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, et al. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 205.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 206.Booth R, Kim H. International Conference on Miniaturized Systems for Chemistry and Life Sciences. Red Hook, NY: Curran Associates, Inc; 2011. A multi-layered microfluidic device for in vitro blood-brain barrier permeability studies; pp. 1388–1390. [Google Scholar]
- 207.Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 208.Yeon JH, Na D, Choi K, Ryu SW, Choi C, Park JK. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices. 2012;14:1141–1148. doi: 10.1007/s10544-012-9680-5. [DOI] [PubMed] [Google Scholar]
- 209.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, et al. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, et al. In vitro models for the blood-brain barrier. Toxicol In Vitro. 2005;19:299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]