Abstract
Purpose
Liver allograft antibody-mediated rejection (AMR) studies have lagged behind parallel efforts in kidney and heart because of a comparative inherent hepatic resistance to AMR. Three developments, however, have increased interest: 1) solid phase antibody testing enabled more precise antibody characterization; 2) increased expectations for long-term, morbidity-free survival; and 3) immunosuppression minimization trials.
Recent Findings
Two overlapping liver allograft AMR phenotypic expressions are beginning to emerge: acute and chronic AMR. Acute AMR usually occurs within the several weeks after transplantation and characterized clinically by DSA persistence, allograft dysfunction, thrombocytopenia, and hypocomplementemia. Acute AMR appears histopathologically similar to acute AMR in other organs: diffuse microvascular endothelial cell hypertrophy, C4d deposits, neutrophilic, eosinophilic, and macrophage-mediated microvasculitis/capillaritis, along with liver-specific ductular reaction, centrilobular hepatocyte swelling and hepatocanalicular cholestasis often combined with T cell-mediated rejection (TCMR). Chronic AMR is less well-defined, but strongly linked to serum class II DSA and associated with late-onset acute TCMR, fibrosis, chronic rejection and decreased survival. Unlike acute AMR, chronic AMR is a slowly evolving insult with a number of potential manifestations, but most commonly appears as low-grade lymphoplasmacytic portal and perivenular inflammation accompanied by unusual fibrosis patterns and variable microvascular C4d deposition; capillaritis is more difficult to identify than in acute AMR.
Summary
More precise DSA characterization, increasing expectations for long-term survival, and immunosuppression weaning precipitated a re-emergence of liver allograft AMR interest. Pathophysiological similarities exist between heart, kidney, and liver allografts, but liver-specific considerations may prove critical to our ultimate understanding of all solid organ AMR.
Keywords: acute AMR, chronic AMR, CDC: complement-dependent cytotoxicity, orthotopic liver transplant OLTx, C4d, donor-specific antibodies (DSA), T cell-mediated rejection (TCMR)
INTRODUCTION
The study of liver allograft AMR can be roughly divided into “early” years defined by crude cell-based assays used to detect donor-specific antibodies (DSA) and the current era, defined by more sophisticated solid phase assays.
Insights from the “Early Years”
Relatively crude cell-based donors-specific antibody (DSA) detection assays began the study of liver allograft antibody-mediated rejection (AMR): 8 – 15% recipients showed positive results (>50% lysis)[1-3]. It was quickly recognized that human liver allografts: a) were less sensitive than kidney allografts to acute adverse consequences of pre-formed DSA[4]; and b) could protect subsequent kidney and heart allografts from the same donor from AMR in most, but not all, sensitized experimental animals[5,6] and humans[7,8]: acute kidney allograft AMR was not seen in all sensitized recipients, but lower patient and graft survival (~40%) did not influence practice.
Most adequately powered studies revealed increased acute AMR and T cell-mediated rejection (TCMR) risk with or without graft failure when livers were transplanted into crossmatch-positive recipients[3,9-12]. AMR with or without TCMR was often treatable[1] and short-term penalties for ignoring crossmatch results were not felt to be clinically significant enough to institute routine HLA typing or DSA testing at most centers. Liver allografts, however, were not exempt from acute AMR: relative hepatic resistance could be overcome, in experimental animals[13,14] and humans[1,10,15], in very highly sensitized recipients.
Cataloging liver HLA class I and II antigen expression in normal liver was accomplished by immunostaining in peripheral liver tissue specimens[16-28]: all reported diffuse and strong class I HLA expression on all cell types, except hepatocytes where expression was weaker. HLA class II expression was largely restricted to portal, perivenular, and subcapsular dendritic cells, and Kupffer cells in normal livers with DQ demonstrating the weakest expression. Portal vein branch endothelia class II expression was consistently negative, but portal capillary, sinusoidal and central vein endothelia varied from negative to focally positive[16-28]. Since few studies specifically addressed class II expression in portal capillary/peribiliary plexus, lymphatic capillaries, inlet venules, and the peribiliary plexus of large extra-hepatic bile ducts; more work is needed on this topic.
All solid organ allografts, especially livers with co-existent pathology, show dynamic HLA expression, especially class II, which is regulated by class II transactivator [29,30]. Upregulation occurs following exposure to environmental/inflammatory stimuli (esp. γ-interferon) resulting in strong DR>DP>DQ expression in all endothelial cell compartments, biliary epithelium, and hepatocytes with co-existent liver pathology, especially TCMR[16-28].
Practical consequences of dynamic HLA expression include: 1) variable liver class II target antigen distribution versus comparatively constant heart and kidney microvascular endothelial expression (Figure 1A-D); 2) spectral downstream immunological effector mechanisms likely depend on endothelial cell antigen-antibody complex density[31-33]; and 3) precipitation and/or accentuation of potential adverse DSA consequences by co-existent pathology[34]. All these consideration likely affect underlying pathophysiological mechanisms and histopathology. Therefore, 4) a potential exists to improve outcomes with non-antibody-directed therapeutic interventions that down-regulate class II expression [35,36].
Figure 1.
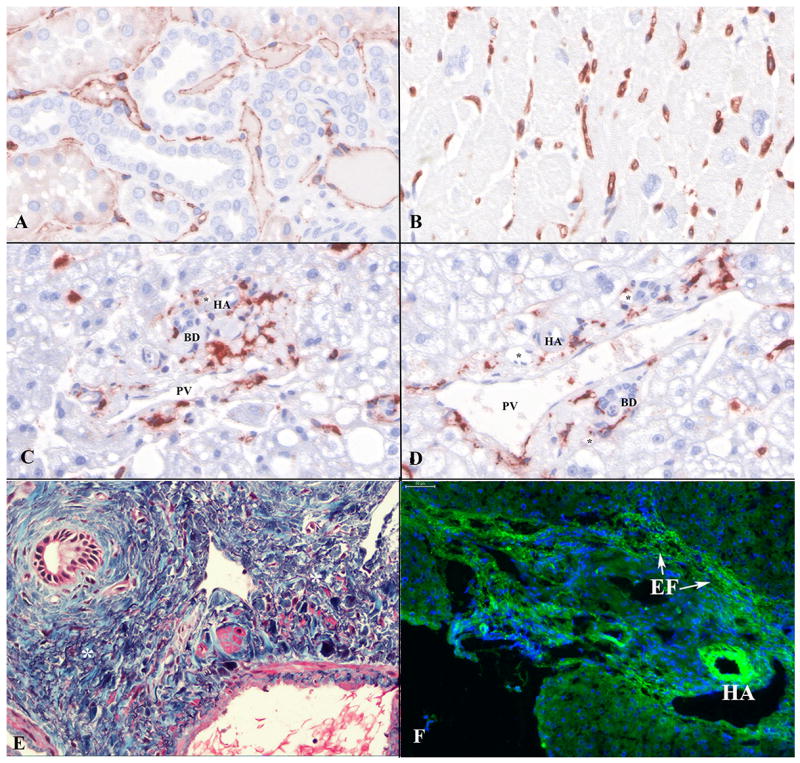
Typical HLA class II antigen (DR, DP, and DQ; monoclonal antibody: M0775, clone CR3/43, Dako) staining results on a tissue microarray that included normal human (A) kidney; (B) heart; and (C and D) representative portal tracts of liver. Note strong and diffuse class II expression in kidney peritubular capillaries (A) and heart interstitial capillaries (B), but absence of portal tract microvascular capillary staining (C and D). HLA class II+ cells in the liver portal tracts (C and D) are portal-based dendritic cellsIt is very difficult, if not impossible, to distinguish among portal peribiliary plexus capillaries; lymphatic capillaries; and inlet venules. (E) Elastic trichrome stain highlighting multiple squiggly elastic tissue fibers (*) scattered amongst blue-staining type 1 collagen bundles within the portal tracts; similar fibers are seen in the perivenular connective tissue surrounding larger hepatic veins (20X). (F) C4d on frozen tissue liver showing background/non-specific staining of the hepatic artery (HA) elastic lamina and wall and elastic tissue fibers (EF) within portal tracts (arrows; 20X). Abbreviations: BD: bile duct; EF: elastic fibers; HA: hepatic artery branch; PV: portal vein branch; *: portal capillary.
Extremely sensitized (>1: 540 CDC) recipients can experience rapid allograft failure caused by frank acute AMR: microvascular injury, thrombosis, and hemorrhagic necrosis[37]. A less fulminant acute AMR presentation is more commonly seen as graft dysfunction, thrombocytopenia, DSA persistence after post-transplant (more common with class II), appearance of circulating immune complexes[1,2,38], and histopathological changes described below. Lower-level sensitization usually resulted in rapid DSA disappearance and either no injury or transient antibody-mediated damage often misrepresented as “preservation injury”[1,2].
Understanding Why Liver Allografts Differed from Kidney and Heart Allografts
Early observations prompted studies that identified liver allograft AMR resistance mechanisms, all of which are likely contributory: 1) Kupffer cells could clear antibodies, activated complement, platelet aggregates, and immune complexes (formed by soluble donor class I HLA antigens that bound class I DSA)[5,6,39-41]. Supporting evidence includes: a) increased susceptibility to AMR and decreased protection of sequentially-placed extra-hepatic allografts in recipients of Kupffer cell-depleted liver allografts[5,6,40]; and b) delay or prevention of acute heart allograft AMR in sensitized recipients by gene therapy that delivers soluble donor class I antigens, similar to liver allografts[42]. 2) Variable hepatic [43] versus strong and constitutive kidney[44] and heart[45] microvascular class II expression provide less class II DSA targets. 3) Large liver size facilitates antigen-antibody complex dilution across the vast endothelial cell surface; potentially explaining increased AMR susceptibility in reduced-size allografts[46,47]. 4) Liver sinusoidal endothelial cells express Fc receptors[48] and lack a typical basement membrane; they are also normally lined by macrophages (Kupffer cells). All of these factors potentially influence antibody-endothelial interactions. 6) The liver’s regenerative capacity and ability to heal either without fibrosis or reverse fibrosis[49].
Current DSA Testing Era
Solid phase DSA testing defined the current era, during which prior observations were validated and extended [50]: pre-transplant CDC+-causing antibodies are encountered in ~10-15% of recipients with a female and autoimmune predilection[51-56]. Data linking the two eras show the ~96% of cell-based CDC- recipients also lacked DSA; however, >50% of isolated class I or II DSA+ patients were CDC-[51].
When DSA+ (defined as MFI≥5000) recipients underwent orthotopic liver transplantation (OLTx), the vast majority of lower MFI class I DSA (<10,000 MFI) disappeared without short-term overt liver allograft damage, but C4d deposits were detected in some highly sensitized recipients early after OLTx and long term consequences, if any, are unknown[51,56]. Regardless, preformed DSA did not adversely influence short-term survival in the vast majority of low to moderately sensitized recipients[51-56]. In contrast to class I, 1/3 of patients with high-MFI class II DSA (≥10,000) experienced persistence[51] with an increased risk of early TCMR, and perhaps, mixed TCMR and acute AMR[51]. A tiny fraction (<5%) of highly sensitized (DSA+) recipients have sufficient DSA (usually multiple class I and II usually in high MFI/titers)to cause clinically and histopathologically significant liver injury[50,55,57], which also depends on the baseline immunosuppressive regimen[55,56,58].
Precise DSA characterization helped clarify mechanisms underlying the “partial protection” liver allografts afford sequentially-placed kidney allografts from the same donor: low-level class I DSA rarely causes problems, but class II DSA resulted in kidney, and less-likely liver, allograft acute AMR[59,60]. It is tempting to speculate that less efficient class II DSA clearing is attributable to lower density class II expression and secretion.
De novo DSA develops in ~8 - 15% of liver allograft recipients[61,62], the vast majority directed at HLA class II, preferentially DQ[61,62]. Risk factors for de novo DSA include cyclosporine versus tacrolimus use, low levels of immunosuppression, young age, low Model for End-Stage Liver Disease (MELD) score[61], and previous transplants[62]. Multivariate analyses show that de novo DSA is associated with decreased patient and allograft survival[61] and fibrosis development[62]. IgG3 subclass testing may help facilitate identification of preformed and de novo DSAs associated with the highest risk of allograft damage[63,64]. Late onset acute AMR has been reported in suboptimally immunosuppressed de novo DSA+ individuals[62].
Plasma cell hepatitis (PCH) [a.k.a. de novo autoimmune hepatitis (AIH)], is an uncommon (~3-5% of recipients) cause of late (usually >1 year) graft dysfunction that resembles native liver AIH. More prevalent and severe bile duct damage, IgG4+ plasma cell-bias, and aggressive central perivenulitis in PCH, compared to standard AIH, suggests that this entity represents an overlap between auto- and alloimmunity[65]. TCMR and steroid-dependence are PCH risk factors in pediatric recipients[66]. Plasma cell-rich infiltrates and aggressive perivenular necro-inflammatory activity correlate with serological evidence of autoimmunity[67].
Atypical liver/kidney microsomal autoantibodies directed against the cytosolic enzyme glutathione-S-transferase T1 (GSST1 have been associated with PCH[68-70] in null GSTT1 genotype recipients of GSTT1+ donor livers; others have not seen this association[68-70]. A variety of other autoantibodies detected in the setting of PCH include cytokeratin 8/18 auto-antibodies[71] and atypical LKM antibodies directed at isoforms of carbonic anhydrase III, subunit β1 of proteasome, and members of different glutathione S-transferase (GST) families[72]. Angiotensin II Type-1 receptor DSA impairs renal allograft outcomes[73], and possibly contributes to fibrosis in combination with HLA DSA in liver allografts[74].
PATHOPHYSIOLOGY
The pathophysiological consequences of DSA in kidney and heart transplantation has been expertly reviewed[31,32,75-78]. Antibody binding to allograft microvascular (interstitial/peritubular capillary) endothelia can result in[31,32,75-78]: 1) damage via complement fixation, activation, and direct membrane damage (efficiency: IgG3 > IgG1 > IgG2 > IgG4); 2) stimulation of endothelial cell chemotactic and pro-coagulant factors, and upregulation of adhesion molecules; 3) complement-independent antibody-dependent cell cytotoxicity (ADCC) mediated by macrophages, neutrophils, and NK cells; and 4) proliferation or signaling pathway activation[31-33] or 5) have little, or potentially protective, effects via upregulation of anti-apoptotic molecules and complement regulatory proteins and Tregitope formation[31,32,75-83]. The fifth alternative is controversial and not necessarily stable.
If microvascular endothelial cell damage is severe enough to cause leukocyte margination and endothelial cell denudation, the following can occur: platelet-fibrin microthrombi, capillary dilatation, and rarefaction[84-88]. This triggers a proliferative repair response involving remaining capillary endothelia and myofibroblasts[88-90], which consequently assume irregular shapes and replacement fibrosis begins. Gradual microvascular atresia results in microvascular shunting, localized hypoxia, fibrosis and dysfunction.
The pathophysiology of liver allograft AMR is expected to be the same as kidney and heart allograft AMR, but liver-specific considerations include: timing of the antibody response[38,91,92], density and distribution of target antigens[38,91,92], level of complement activation, potential dual role of Kupffer cells, amount of soluble antigen release, importance of co-existent pathology, and stimulation of endothelial and stellate cells cytoprotective and proliferative signaling. Organ-specific “auto-antibodies” may also contribute to the injury[31,77,78,93].
Similar to kidney allografts, two generic categories of AMR are emerging in OLTx: 1) acute AMR, which usually occurs during the first month after transplantation in recipients with high-titer or high MFI, despite serial dilution, class I DSA[1,50,57,94,95]. It can also appear later in some recipients with de novo DSA[94]. 2) Chronic AMR usually manifests as low-grade chronic portal and perivenular lymphoplasmacytic inflammation and fibrosis with or without inflammation, which often shows an otherwise peculiar perivenular and sinusoidal distribution; and progression to otherwise typical chronic rejection.
Liver allograft acute AMR mechanisms are similar to AMR in other organs: obvious cases show evidence of microvascular endothelia DSA binding and complement activation (C4deposition) that trigger endothelial cell injury/responses, localized platelet-fibrin microthrombi, and “micro-vasculitis”/capillaritis, as described above. However, currently, acute AMR occurs only in 5% of DSA+ (8–15% of all recipients) recipients, or about 1%, of all OLTx recipients. Therefore, in the absence of testing and thoughtful consideration, it is often missed.
Pathophysiologic mechanisms and histopathological findings in putative chronic AMR are less well-defined, but it is clearly a slowly evolving process, similar to kidney allografts[78]. Potential pathways of injury might not be easily linked to DSA[78] because of: 1) fluctuations in antibody production and ephemeral nature of C4d deposits; 2) non-complement-dependent effector mechanisms; 3) participation of non-HLA auto- or allo-antibodies[74]; 4) current low incidence (<5%) of traditional “chronic” liver allograft rejection[96]; 5) triggering of non-capillaritis effector mechanisms in target cell populations, such as stellate cells; and 6) paucity of properly preserved specimens to evaluate capillary basement membrane alterations[78].
ACUTE ANTIBODY - MEDIATED REJECTION
Histopathological manifestations of DSA in human liver allografts is discussed from a temporal perspective, starting with post-reperfusion biopsies and early post-transplantation, followed by a discussion of C4d staining and interpretation, and finally potential manifestations of chronic AMR.
Histopathology
Post-reperfusion biopsies from patients with high titer/high MFI despite serial dilutions DSA show platelet aggregates in portal and/or central veins [1,97]. Diffuse portal microvasculature C4d staining[50,56], portal microvascular endothelial hypertrophy and cytoplasmic eosinophilia, occasionally resulting in “hobnailing” appear within days after OLTx in those who develop acute AMR[50,57]. Accompanying features include “micro-vasculitis” involving portal vein branches, inlet venules, portal capillaries, peribiliary plexus capillaries, and occasionally central veins. Endothelial inflammation is often mediated by eosinophils, macrophages, and neutrophils[50,57] and accompanied by a ductular reaction, spotty acidophilic necrosis of hepatocytes, centrilobular hepatocellular swelling and hepatocanalicular cholestasis; focal bile duct necrosis; and arterial changes strongly suggestive of arterial vasospasm[1]. Superimposed TCMR is almost universally present[1].
Inflammatory/necrotizing arteritis is rare, but is diagnostic of acute AMR when seen in conjunction with diffuse C4d deposits and DSA. Some histopathological changes resemble preservation/reperfusion injury and obstructive cholangiopathy, but marked portal microvascular endothelial cell hypertrophy and cytoplasmic eosinophilia and “microvasculitis”, especially when involving central veins, distinguish acute AMR. Regardless, stringent diagnostic criteria are needed to establish an AMR diagnosis with certainty: 1) histopathological changes consistent with AMR; 2) exclusion of other insults causing a similar injury pattern; 3) serum DSA; and 4) strong and diffuse complement (C4d) deposition[50,55,57,95,98,99], defined as strong portal vein and capillary and usually periportal sinusoidal endothelial staining involving a majority of portal tracts.
The “signature” histopathological lesion of acute AMR in kidney and heart allografts, “capillaritis”, is defined by endothelial cell hypertrophy, margination of leukocytes [macrophages, neutrophils, and lymphocytes (e.g. NK cells)], and dilatation with or without capillary disruption, depending on injury severity[77]. Capillaritis is usually accompanied by C4d staining and observed regardless of the time after heart or kidney transplantation. Similar changes are seen in liver acute AMR, but capillaritis can be difficult to recognize more than several weeks post-OLTx.
There are likely several key reasons for these inter-organ differences: 1) Distinguishing among typical portal capillaries, lymphatic capillaries, and inlet venules is extremely difficult[100]; made even more difficult in inflamed livers because small portal vessels are: a) obscured by co-existent inflammation; b) intermixed among normal elastic fibers that non-specifically stain for C4d, especially on frozen tissue (Figure 1E-F); and c) are gradually destroyed during chronic AMR[101-103]. Endothelial stains (e.g. CD34, CD31, D2-40) can help facilitate identification. 2) Lower class II expression might affect downstream effector mechanisms and instead of “capillaritis”, DSA might trigger endothelial cell phenotype changes and/or activation of pathways that promote or retard cellular inflammation, coagulation, apoptosis and complement deposition[31,32,75-78,80-83]. 3) Direct activation of stellate cells is possible given the strong empiric association between DSA and non-inflammatory fibrosis[62,104,105].
Interpreting of Liver Allograft Immune and C4d Staining
Classic immune deposits (e.g. IgG, C3, C4) are ephemeral in acute AMR, even in frozen tissue[1,95,106]. In severe cases, deposits of IgG, and/or IgM, C3, C4, and C4d can be diffusely detected in frozen sections along the sinusoids and in perihilar arteries, portal veins, and peribiliary plexus if tissue samples are obtained early in acute AMR development [1,95,106]. Recognition that C4d can: a) persist for several days; b) be detected in formalin-fixed paraffin-embedded (FFPE) tissues; and c) correlate with circulating DSA improved the diagnostic accuracy of liver allograft AMR[99,107].
If liver AMR is suspected, saving frozen tissue for immunofluorescence (IF) C4d staining may improve diagnostic accuracy. No studies directly compared frozen and immunoperoxidase staining on formalin-fixed, paraffin-embedded C4d staining for liver allografts. The first author’s experience can be summarized as: IF is more sensitive than immunoperoxidase, but more difficult to interpret because of high background/non-specific staining and difficulty in discerning the underlying architecture, especially the portal microvasculature. Sinusoidal C4d labeling is more common in frozen samples. Immunoperoxidase staining technique affects sensitivity: pressure cooker and high pH (pH = 9.0 vs. 6.0) antigen retrieval yield more sensitive results, but background staining can emerge leading to interpretational problems [107-115].
Normal liver allograft biopsies are usually negative for endothelial cell C4d staining, but background/nonspecific C4d labeling can be seen in: arterial elastic lamina; portal and perivenular elastic fibers; necrotic and steatotic hepatocytes, and areas of sinusoidal fibrosis (unpublished observation)(Figure 1E-F). Portal vein and capillary, sinusoidal, central vein, and arterial endothelium, lymphoid nodules, and periductal and portal stromal cell C4d staining has been described in native pediatric livers with hepatitis B (HBV) and C (HCV), and AIH[116] and in allografts when other insults are thought to be the primary cause of allograft dysfunction (e.g. biliary obstruction[107], recurrent HBV[108] or HCV[111], and plasma cell hepatitis (de novo AIH))[117]. Endothelial C4d deposits in rejection-unrelated allograft disorders, however, are reportedly less widespread and intense than in severe ACR or acute AMR[56,94,99,109,118,119].
Caveats aside, portal venous and capillary, arterial, and sinusoidal endothelial C4d staining has been significantly associated with CDC+ and DSA+ recipients more often than their negative controls[113]; in those with isolated AMR[95,115]; and associated with macrophage and plasma cell infiltrates[109], micro-vasculitis[1,50], and TCMR[55,98,107-115], which, in some studies, was directly proportional to Banff grade[107-115]. Portal microvascular and sinusoidal endothelial cell C4d staining appears to be most specific for acute AMR, but “portal C4d stromal” staining has also been described in ABO-incompatible AMR[110], ACR[113] and CR[98,120].
Since C4d deposition depends on the presence of target antigen and microvascular endothelial cell HLA class II expression can vary, the pattern of positive C4d staining can be contextual. For example, central perivenular form of TCMR can locally upregulate HLA class II leading to preferential C4d deposition in central vein and perivenular sinusoidal endothelium.
CHRONIC ANTIBODY-MEDIATED INJURY
Candidate histopathological lesions of chronic AMR are emerging primarily from: 1) long-term follow-up of pediatric liver allograft recipients; 2) suboptimally immunosuppressed recipients; and 3) immunosuppression weaning studies. Since most original pediatric diseases do not recur following transplantation recognizing potential chronic AMR changes has been less challenging than in adults where disease recurrence is common. This is especially true if putative changes differ substantially from otherwise typical TCMR, hepatitis, or biliary stricturing/obstruction.
DSA appearance in kidney allografts has been associated with tolerance loss[121], and de novo DSA not uncommonly develops in pediatric OLTx recipients weaned from immunosuppression[104,122]. However, protocol follow-up biopsy reports in patients after sustained lowering or withdrawal of immunosuppression (IS) are limited[123-126]. Regardless, not all patients that develop DSA after weaning experience significant liver inflammation, TCMR, fibrosis, architectural deterioration, or changes in sinusoidal endothelial (CD34- to CD34+) or stellate cell phenotype (SMA− to SMA+) within five years[122].
Pre-weaning biopsy findings associated with successful weaning include: 1) less prevalent microvascular C4d deposits[127] and portal lymphocytic inflammation[127,128]; 2) fewer CD3+ and CD8+ lymphocytes, but more lobular CD45RO+ lymphocytes[128]; and 3) less stainable hepatocyte iron[129]. It is tempting to speculate that the absence of co-existent pathology before weaning contributes somewhat to the stability observed after weaning when DSA appears.
European multicenter adult trials[126,129] showed DSA did not predispose to rejection or cause a particular pattern of injury before or after weaning. However, these studies were hampered by: 1) limited DSA evaluation (based on ELISA screening) that might have missed class II DSAs; and 2) allowance of HCV fibrosis progression pre- and post-weaning.
Japanese weaning trials, conducted primarily in pediatric recipients, showed increased periportal and perivenular fibrosis after weaning, with or without co-existent lymphocytic inflammation[104,130,131]. Histopathological changes observed were either directly or indirectly attributed to IS minimization[104,130,131] and associated with DSA, especially class II DSA, endothelial and stromal C4d, and CD20+ perivenular infiltrates[104]. Peribiliary plexus capillary and sinusoidal endothelial cell HLA-DR upregulation was spatially linked with nearby inflammation, which was most prominent in patients with co-existent TCMR with or without perivenular fibrosis[105]. Re-institution or increasing immunosuppression decreased C4d deposits and stabilized or reversed perivenular fibrosis[131]. It is tempting to speculate that the lymphocytic portal and perivenular inflammation in HCV- patients and tissue C4d deposits in DSA+ recipients might be related to each other and represent subclinical combined chronic AMR/TCMR that manifests biochemically after IS lowering[43,124].
A spectrum of liver allograft injury (non-inflammatory fibrosis, low-grade inflammation, biliary strictures, nodular regenerative hyperplasia, etc.) is suspected in chronic AMR[50]. Whether antibodies are contributing to obliterative arteriopathy not detected in peripheral needle biopsy or other, non-obvious pathology is uncertain. Except for obliterative arteriopathy, many candidate lesions are also caused by technical complications[70,123,124]. Determining the relative contribution of each insult will be challenging and require liver biopsy adequacy as defined by AASLD[132]/Banff Working[124] guidelines for liver biopsy adequacy: 2 passes with a 16 gauge needle >20 mm and >11 portal tracts to monitor fibrosis[133].
CLINICAL IMPLICATIONS
The immediate and long-term clinical implications of peri-transplant HLA and DSA testing and acute AMR are becoming clear, but chronic AMR, while a work in progress is in need of further study.
Peri-Transplant DSA
Since DSA pre-OLTx can impact early post-OLTx outcomes, more programs are testing; albeit in a minority of standard risk primary liver-only allograft recipients. High-risk patients (e.g. re-transplant candidates and dual organ recipients) should all have HLA testing, ideally before OLTx, to facilitate immunosuppression optimization and, in rare cases, unacceptable antigen listing. Sometimes flow crossmatch is the only information available, but ideally, centers further risk-stratify positive patients with single antigen bead testing. Highest risk recipients for acute AMR have preformed class I DSA in high-titer (or MFI >15,000 despite serial dilutions)[50], but risk is exacerbated by key factors including recipient illness (MELD score), and poor donor quality (Donor Risk Index (DRI)) (Figure 2). Lower level sensitization (MFI≥5000) in high MELD scores recipients or in recipients of suboptimal organs can increase the risk of allograft loss when class I DSA is present pre-OLTx.
Figure 2.
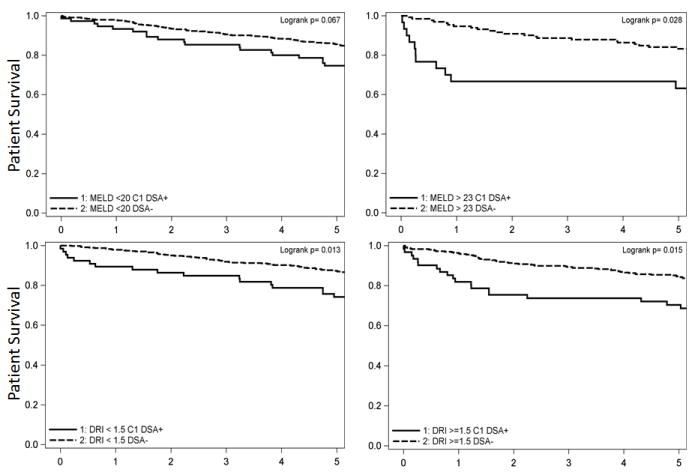
Preformed class I DSA (MFI≥5000) disproportionately affects patients transplanted with a high calculated Model for End-Stage Liver Disease (MELD) score and those who receive lower quality organs [Donor Risk Index(DRI) >1.5] when assessed in a cohort of 1270 patients from Baylor University Medical Center in Dallas from 1/00-4/09.
When acute AMR is diagnosed, most patients have already failed steroid recycle and T cell-depleting-antibody therapy for steroid-resistant rejection and receive B-cell or plasma cell depletion with either Rituximab or a proteasome inhibitor, such as Bortezomib, with or without plasmapheresis and/or IVIG[134,135]. Since patients are already severely immunosuppressed, strict criteria are needed to establish an acute AMR diagnosis emphasizing specificity over sensitivity[50,55,57]. Early diagnosis is key to treatment success since delay usually results in irrevocable allograft damage and possible development of niche resident plasma cells that facilitate ongoing chronic AMR. Absent overt acute AMR, preformed DSA can also be associated with TCMR[1,38,51,58,98]. Fortunately, induction immunosuppression in patients with preformed class II DSA has been shown to markedly decrease this risk[51].
Managing Chronic DSA
Chronic DSA is almost always directed against class II, but DSA should never be interpreted in isolation: when discovered correlation with biopsy findings is needed. If pathology (inflammation and fibrosis) is present, the following should be considered: 1) optimize immunosuppression to facilitate/ensure compliance; 2) utilize a tacrolimus-based immunosuppression regimen, whenever possible; 3) increase the intensity of immunosuppression unless non-compliance is strongly suspected, and 4) treat/cure any concomitant disease that may be contributing to chronic allograft inflammation, such as chronic viral hepatitis B or C. Outside this clinical algorithm the best treatment for chronic AMR remains unknown. Clinical studies can only begin after diagnostic criteria for chronic AMR are created and endpoints for trials are agreed upon.
CONCLUSIONS & FUTURE DIRECTIONS
Understanding the proper perspective of DSA in OLTx is impossible without donor and recipient HLA tissue typing and DSA monitoring. Ideal monitoring includes routine pre-transplant tissue typing and DSA determination followed by protocol periodic chronic DSA testing, although the cost-effective interval has yet to be determined. Frozen tissue samples for C4d staining at least when DSA-associated injury is suspected, or perhaps routinely, is desired. Standardization of C4d staining techniques for FFPE is underway. Raising pathologist’s awareness of typical lesions, enhancing their detection by tissue staining for CD31/34, HLA class II, and CD68 or CD163, and endothelial and stellate phenotypes using multiplex labeling makes the interpretation easier and enables quantification[136]. Distinguishing histopathological changes caused by technical complications (e.g. biliary strictures) from purely immunological insults; interactions between the two (e.g. via class II upregulation) will also be important.
KEY POINTS.
Liver allografts are relatively resistant to, but not totally spared from, antibody-mediated rejection (AMR).
Normal liver microvascular endothelia express high levels of HLA class I, but lower class II antigen than normal peritubular kidney or interstitial heart capillaries; however, liver microvascular HLA class II is induced by co-existent pathology (e.g. recurrent HCV, AIH, or TCMR).
Rare (~1% of all recipients) very highly sensitized recipients can develop acute AMR early after transplantation.
De novo DSA, usually class II (esp. DQ), appears in ~8-15% recipients and has been linked with chronic AMR in some, but not all circumstances.
Few studies have rigorously addressed chronic liver allograft AMR, but emerging literature links DSA to indolent low-grade chronic inflammation and slowly progressive fibrosis.
Acknowledgments
none
Financial support and sponsorship: Immune Tolerance Network 1UM1AI109565-01; U34 DK083031; NIH, 1U01AI104336; Thomas E. Starzl Professorship Endowment (AJD); Fisher Scientific (JGO); NIH, 1U01AI104336-01 (AZ)
Abbreviations
- AMR
antibody-mediated rejection
- DSA
donor-specific antibodies
- MFI
mean fluorescence intensity
- OLTx
orthotopic liver transplantation
- TCMR
T cell-mediated rejection
Footnotes
Conflicts of interest: none
References
- 1.Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, Murase N, Bronsther O, Manez R, Fung JJ, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16:671–681. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manez R, Kelly RH, Kobayashi M, Takaya S, Bronsther O, Kramer D, Duquesnoy RJ, Iwaki Y, Fung JJ, Starzl TE, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995;21:1345–1352. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle HR, Marino IR, Morelli F, Doria C, Aldrighetti L, McMichael J, Martell J, Gayowski T, Starzl TE. Assessing risk in liver transplantation. Special reference to the significance of a positive cytotoxic crossmatch. Ann Surg. 1996;224:168–177. doi: 10.1097/00000658-199608000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres GA, Ansell ID, Halgrimson CG, Hsu KC, Porter KA, Starzl TE, Accinni L, Calne RY, Herbertson BM, Penn I, et al. Immunopathological studies of orthotopic human liver allografts. Lancet. 1972;1:275–280. doi: 10.1016/s0140-6736(72)90288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gugenheim J, Amorosa L, Gigou M, Fabiani B, Rouger P, Gane P, Reynes M, Bismuth H. Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats. Transplantation. 1990;50:309–313. doi: 10.1097/00007890-199008000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Gugenheim J, Charpentier B, Gigou M, Cuomo O, Calise F, Amorosa L, Astarcioglu I, Trias i Folch M, Martin B, Bismuth H. Delayed rejection of heart allografts after extracorporeal donor-specific liver hemoperfusion. Role of Kupffer cells. Transplantation. 1988;45:628–632. doi: 10.1097/00007890-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Saidman SL, Duquesnoy RJ, Demetris AJ, McCauley J, Ramos H, Mazariegos G, Shapiro R, Starzl TE, Fung JJ. Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies. Transpl Immunol. 1994;2:61–67. doi: 10.1016/0966-3274(94)90080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid A, Moore SB, Wiesner RH, DeGoey SR, Nielson A, Krom RA. Evidence that the liver does not always protect the kidney from hyperacute rejection in combined liver-kidney transplantation across a positive lymphocyte crossmatch. Transplantation. 1990;50:331–334. [PubMed] [Google Scholar]
- 9.Karruppan S, Ericzon BG, Moller E. Relevance of a positive crossmatch in liver transplantation. Transplant Int. 1991;4:18–25. doi: 10.1007/BF00335511. [DOI] [PubMed] [Google Scholar]
- 10.Takaya S, Bronsther O, Iwaki Y, Nakamura K, Abu-Elmagd K, Yagihashi A, Demetris AJ, Kobayashi M, Todo S, Tzakis AG, et al. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53:400–406. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikaein A, Backman L, Jennings L, Levy MF, Goldstein R, Gonwa T, Stone MJ, Klintmalm G. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. Transplantation. 1994;58:786–792. [PubMed] [Google Scholar]
- 12.Bathgate AJ, McColl M, Garden OJ, Forsythe JL, Madhavan KK, Hayes PC. The effect of a positive T-lymphocytotoxic crossmatch on hepatic allograft survival and rejection. Liver Transpl Surg. 1998;4:280–284. doi: 10.1002/lt.500040411. [DOI] [PubMed] [Google Scholar]
- 13.Knechtle SJ, Kolbeck P, Tsuchimoto S, Sanfilippo F, Bollinger RR. Hyperacute rejection of liver transplants in rats. Current Surgery. 1986;43:303–305. [PubMed] [Google Scholar]
- 14.Knechtle SJ, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Hepatic transplantation into sensitized recipients. Demonstration of hyperacute rejection. Transplantation. 1987;43:8–12. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Takaya S, Duquesnoy R, Iwaki Y, Demetris J, Yagihashi A, Bronsther O, Iwatsuki S, Starzl TE. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991;23:396–399. [PMC free article] [PubMed] [Google Scholar]
- 16.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984;38:293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Lautenschlager I, Taskinen E, Inkinen K, Lehto VP, Virtanen I, Hayry P. Distribution of the major histocompatibility complex antigens on different cellular components of human liver. Cellular Immunology. 1984;85:191–200. doi: 10.1016/0008-8749(84)90289-2. [DOI] [PubMed] [Google Scholar]
- 19.Demetris AJ, Lasky S, Thiel DHV, Starzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts: An immunocytochemical and clinicopathologic analysis of twenty failed grafts. Transplantation. 1985;40:504–509. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballardini G, Bianchi FB, Mirakian R, Fallani M, Pisi E, Bottazzo GF. HLA-A,B,C, HLA-D/DR and HLA-D/DQ expression on unfixed liver biopsy sections from patients with chronic liver disease. Clinical & Experimental Immunology. 1987;70:35–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Barbatis C, Kelly P, Greveson J, Heryet A, McGee JO. Immunocytochemical analysis of HLA class II (DR) antigens in liver disease in man. Journal of Clinical Pathology. 1987;40:879–884. doi: 10.1136/jcp.40.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinhoff G, Wonigeit K, Pichlmayr R. Analysis of sequential changes in major histocompatibility complex expression in human liver grafts after transplantation. Transplantation. 1988;45:394–401. doi: 10.1097/00007890-198802000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Gouw AS, Huitema S, Grond J, Slooff MJ, Klompmaker IJ, Gips CH, Poppema S. Early induction of MHC antigens in human liver grafts. An immunohistologic study. Am J Pathol. 1988;133:82–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Steinhoff G, Wonigeit K, Schafers HJ, Haverich A. Sequential analysis of monomorphic and polymorphic major histocompatibility complex antigen expression in human heart allograft biopsy specimens. The Journal of heart transplantation. 1989;8:360–370. [PubMed] [Google Scholar]
- 25.Steinhoff G. Major histocompatibility complex antigens in human liver transplants. J Hepatol. 1990;11:9–15. doi: 10.1016/0168-8278(90)90264-r. [DOI] [PubMed] [Google Scholar]
- 26.Hubscher SG, Adams DH, Elias E. Changes in the expression of major histocompatibility complex class II antigens in liver allograft rejection. Journal of Pathology. 1990;162:165–171. doi: 10.1002/path.1711620210. [DOI] [PubMed] [Google Scholar]
- 27.Terada T, Nakanuma Y, Hoso M, Obata H. Expression of HLA-DR antigen on hepatic vascular endothelial cells in idiopathic portal hypertension. Clin Exp Immunol. 1991;84:303–307. [PMC free article] [PubMed] [Google Scholar]
- 28.Terada T, Nakanuma Y, Obata H. HLA-DR expression on the microvasculature of portal tracts in idiopathic portal hypertension. Immunohistochemical characteristics and relation to portal phlebosclerosis. Arch Pathol Lab Med. 1991;115:993–997. [PubMed] [Google Scholar]
- 29.Devaiah BN, Singer DS. CIITA and Its Dual Roles in MHC Gene Transcription. Front Immunol. 2013;4:476. doi: 10.3389/fimmu.2013.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisapia L, Pozzo GD, Barba P, Citro A, Harris PE, Maffei A. Contrasting effects of IFNalpha on MHC class II expression in professional vs. nonprofessional APCs: Role of CIITA type IV promoter. Results Immunol. 2012;2:174–183. doi: 10.1016/j.rinim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19:33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenzuela NM, Reed EF. Antibodies in transplantation: the effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Biol. 2013;1034:41–70. doi: 10.1007/978-1-62703-493-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela NM, Mulder A, Reed EF. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. J Immunol. 2013;190:6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Leary JG, Kaneku H, Jennings L, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific alloantibodies are associated with fibrosis progression after liver transplantation in hepatitis C virus-infected patients. Liver Transpl. 2014;20:655–663. doi: 10.1002/lt.23854. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Qin H, Benveniste EN. The IFN-gamma-induced transcriptional program of the CIITA gene is inhibited by statins. Eur J Immunol. 2008;38:2325–2336. doi: 10.1002/eji.200838189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nandan D, Reiner NE. TGF-beta attenuates the class II transactivator and reveals an accessory pathway of IFN-gamma action. J Immunol. 1997;158:1095–1101. [PubMed] [Google Scholar]
- 37.Starzl TE, Demetris AJ, Todo S. Evidence of hyperacute rejection of human liver grafts: The case of the canary kidneys. Clin Transpl. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 38.Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, Todo S, Iwatsuki S, Takaya S, Fung JJ, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. [Review] Seminars in Liver Disease. 1992;12:51–59. doi: 10.1055/s-2007-1007376. [DOI] [PubMed] [Google Scholar]
- 39.Gugenheim J, Houssin D, Tamisier D, Franco D, Martin E, Lang P, Bismuth H. Spontaneous long-term survival of liver allografts in inbred rats. Influence of hepatectomy of the recipient’s own liver. Transplantation. 1981;32:445–450. [PubMed] [Google Scholar]
- 40.Gugenheim J, Le Thai B, Rouger P, Gigou M, Gane P, Vial MC, Charpentier B, Reynes M, Bismuth H. Relationship between the liver and lymphocytotoxic alloantibodies in inbred rats. Specific absorption by nonparenchymal liver cells. Transplantation. 1988;45:474–478. doi: 10.1097/00007890-198802000-00046. [DOI] [PubMed] [Google Scholar]
- 41.Astarcioglu I, Gugenheim J, Crafa F, Saint PMC, Reynes M. Hyperacute rejection of liver allografts in sensitized rats: role of nonparenchymal liver cells. Journal of Surgical Research. 1995;58:182–188. doi: 10.1006/jsre.1995.1028. [DOI] [PubMed] [Google Scholar]
- 42.Geissler EK, Graeb C, Tange S, Guba M, Jauch KW, Scherer MN. Effective use of donor MHC class I gene therapy in organ transplantation: prevention of antibody-mediated hyperacute heart allograft rejection in highly sensitized rat recipients. Hum Gene Ther. 2000;11:459–469. doi: 10.1089/10430340050015923. [DOI] [PubMed] [Google Scholar]
- 43.Demetris AJ, Isse K. Tissue biopsy monitoring of operational tolerance in liver allograft recipients. Curr Opin Organ Transplant. 2013;18:345–353. doi: 10.1097/MOT.0b013e3283615d48. [DOI] [PubMed] [Google Scholar]
- 44.Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14:1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 45.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 46.Astarcioglu I, Cursio R, Reynes M, Gugenheim J. Increased risk of antibody-mediated rejection of reduced-size liver allografts. J Surg Res. 1999;87:258–262. doi: 10.1006/jsre.1999.5734. [DOI] [PubMed] [Google Scholar]
- 47.Shiraishi M, Csete ME, Yasunaga C, Drazan KE, Jurim O, Cramer DV, Busuttil RW, Shaked A. Regeneration-induced accelerated rejection in reduced-size liver grafts. Transplantation. 1994;57:336–340. doi: 10.1097/00007890-199402150-00004. [DOI] [PubMed] [Google Scholar]
- 48.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2014 doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Xu J, Brenner DA, Kisseleva T. Reversibility of Liver Fibrosis and Inactivation of Fibrogenic Myofibroblasts. Curr Pathobiol Rep. 2013;1:209–214. doi: 10.1007/s40139-013-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Leary JG, Kaneku H, Demetris AJ, Marr JD, Shiller SM, Susskind BM, Tillery GW, Terasaki PI, Klintmalm GB. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014;20:218–227. doi: 10.1002/lt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Leary JG, Kaneku H, Jennings LW, Banuelos N, Susskind BM, Terasaki PI, Klintmalm GB. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013;19:973–980. doi: 10.1002/lt.23687. [DOI] [PubMed] [Google Scholar]
- 52.Al-Sibae MR, Koffron AJ, Raofi V. Does a positive pretransplant crossmatch affect long-term outcome in liver transplantation? Transplantation. 2011;91:261–262. doi: 10.1097/TP.0b013e318204758c. [DOI] [PubMed] [Google Scholar]
- 53.Chan KM, Lee CS, Wu TJ, Lee CF, Chen TC, Lee WC. Clinical perspective of acute humoral rejection after blood type-compatible liver transplantation. Transplantation. 2011;91:e29–30. doi: 10.1097/TP.0b013e318208138c. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz R, Tomiyama K, Campsen J, Goldstein RM, Levy MF, McKenna GJ, Onaca N, Susskind B, Tillery GW, Klintmalm GB. Implications of a positive crossmatch in liver transplantation: a 20-year review. Liver Transpl. 2012;18:455–460. doi: 10.1002/lt.22474. [DOI] [PubMed] [Google Scholar]
- 55.Lunz J, Ruppert KM, Cajaiba MM, Isse K, Bentlejewski CA, Minervini M, Nalesnik MA, Randhawa P, Rubin E, Sasatomi E, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:171–182. doi: 10.1111/j.1600-6143.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 56.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, Heimbach JK. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 57.O’Leary JG, Michelle Shiller S, Bellamy C, Nalesnik MA, Kaneku H, Jennings LW, Isse K, Terasaki PI, Klintmalm GB, Demetris AJ. Acute liver allograft antibody-mediated rejection: an inter-institutional study of significant histopathological features. Liver Transpl. 2014;20:1244–1255. doi: 10.1002/lt.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Musat AI, Pigott CM, Ellis TM, Agni RM, Leverson GE, Powell AJ, Richards KR, D’Alessandro AM, Lucey MR. Pretransplant donor-specific anti-HLA antibodies as predictors of early allograft rejection in ABO-compatible liver transplantation. Liver Transpl. 2013;19:1132–1141. doi: 10.1002/lt.23707. [DOI] [PubMed] [Google Scholar]
- 59.Dar W, Agarwal A, Watkins C, Gebel HM, Bray RA, Kokko KE, Pearson TC, Knechtle SJ. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 60.O’Leary JG, Gebel HM, Ruiz R, Bray RA, Marr JD, Zhou XJ, Shiller SM, Susskind BM, Kirk AD, Klintmalm GB. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013;13:954–960. doi: 10.1111/ajt.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Bello A, Congy-Jolivet N, Muscari F, Lavayssiere L, Esposito L, Cardeau-Desangles I, Guitard J, Dorr G, Suc B, Duffas JP, et al. Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am J Transplant. 2014;14:867–875. doi: 10.1111/ajt.12651. [DOI] [PubMed] [Google Scholar]
- 63.Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012;18:984–992. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 64.O’Leary J, Kaneku H, Banuelos N, Jennings L, Klintmalm G, Terasaki P. Impact of IgG3 subclass and C1q-fixing donor specific antibodies on rejection and survival in liver transplantation. American Journal of Transplantation. 2015 doi: 10.1111/ajt.13153. in press. [DOI] [PubMed] [Google Scholar]
- 65.Castillo-Rama M, Sebagh M, Sasatomi E, Randhawa P, Isse K, Salgarkar AD, Ruppert K, Humar A, Demetris AJ. “Plasma Cell Hepatitis” in Liver Allografts: Identification and Characterization of an IgG4-Rich Cohort. Am J Transplant. 2013;13:2966–2977. doi: 10.1111/ajt.12413. [DOI] [PubMed] [Google Scholar]
- 66.Venick RS, McDiarmid SV, Farmer DG, Gornbein J, Martin MG, Vargas JH, Ament ME, Busuttil RW. Rejection and steroid dependence: unique risk factors in the development of pediatric posttransplant de novo autoimmune hepatitis. Am J Transplant. 2007;7:955–963. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 67.Sebagh M, Castillo-Rama M, Azoulay D, Coilly A, Delvart V, Allard MA, Dos Santos A, Johanet C, Roque-Afonso AM, Saliba F, et al. Histologic findings predictive of a diagnosis of de novo autoimmune hepatitis after liver transplantation in adults. Transplantation. 2013;96:670–678. doi: 10.1097/TP.0b013e31829eda7f. [DOI] [PubMed] [Google Scholar]
- 68.Mendes F, Couto CA, Levy C. Recurrent and de novo autoimmune liver diseases. Clinics in liver disease. 2011;15:859–878. doi: 10.1016/j.cld.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72. e54. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 70.Hubscher SG. What is the long-term outcome of the liver allograft? Journal of Hepatology. 2011;55:702–717. doi: 10.1016/j.jhep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Inui A, Sogo T, Komatsu H, Miyakawa H, Fujisawa T. Antibodies against cytokeratin 8/18 in a patient with de novo autoimmune hepatitis after living-donor liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11:504–507. doi: 10.1002/lt.20404. [DOI] [PubMed] [Google Scholar]
- 72.Huguet S, Vinh J, Johanet C, Samuel D, Gigou M, Zamfir O, Duclos-Vallee JC, Ballot E. Identification by proteomic tool of atypical anti-liver/kidney microsome autoantibodies targets in de novo autoimmune hepatitis after liver transplantation. Annals of the New York Academy of Sciences. 2007;1109:345–357. doi: 10.1196/annals.1398.041. [DOI] [PubMed] [Google Scholar]
- 73.Dragun D, Catar R, Philippe A. Non-HLA antibodies in solid organ transplantation: recent concepts and clinical relevance. Curr Opin Organ Transplant. 2013;18:430–435. doi: 10.1097/MOT.0b013e3283636e55. [DOI] [PubMed] [Google Scholar]
- 74.Ohe H, Uchida Y, Yoshizawa A, Hirao H, Taniguchi M, Maruya E, Yurugi K, Hishida R, Maekawa T, Uemoto S, et al. Association of Anti-Human Leukocyte Antigen and Anti-Angiotensin II Type 1 Receptor Antibodies With Liver Allograft Fibrosis After Immunosuppression Withdrawal. Transplantation. 2014;98:1105–1111. doi: 10.1097/TP.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 75.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farkash EA, Colvin RB. Diagnostic challenges in chronic antibody-mediated rejection. Nature reviews Nephrology. 2012;8:255–257. doi: 10.1038/nrneph.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: a schematic view based on pathogenesis. Transplantation. 2013;95:1073–1083. doi: 10.1097/TP.0b013e31827e6b45. [DOI] [PubMed] [Google Scholar]
- 78.Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol. 2012;24:115–121. doi: 10.1016/j.smim.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cousens LP, Najafian N, Mingozzi F, Elyaman W, Mazer B, Moise L, Messitt TJ, Su Y, Sayegh M, High K, et al. In vitro and in vivo studies of IgG-derived Treg epitopes (Tregitopes): a promising new tool for tolerance induction and treatment of autoimmunity. J Clin Immunol. 2013;33(Suppl 1):S43–49. doi: 10.1007/s10875-012-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Platt JL. Antibodies in transplantation. Discovery medicine. 2010;10:125–133. [PMC free article] [PubMed] [Google Scholar]
- 81.Chang AT, Platt JL. The role of antibodies in transplantation. Transplantation reviews. 2009;23:191–198. doi: 10.1016/j.trre.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leffell MS, Zachary AA. Anti-allograft antibodies: some are harmful, some can be overcome, and some may be beneficial. Discovery medicine. 2010;9:478–484. [PubMed] [Google Scholar]
- 83.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–2312. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 84.Adair A, Mitchell DR, Kipari T, Qi F, Bellamy CO, Robertson F, Hughes J, Marson LP. Peritubular capillary rarefaction and lymphangiogenesis in chronic allograft failure. Transplantation. 2007;83:1542–1550. doi: 10.1097/01.tp.0000266689.93615.cd. [DOI] [PubMed] [Google Scholar]
- 85.Escaned J, Flores A, Garcia-Pavia P, Segovia J, Jimenez J, Aragoncillo P, Salas C, Alfonso F, Hernandez R, Angiolillo DJ, et al. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: validation with endomyocardial sampling in cardiac allografts. Circulation. 2009;120:1561–1568. doi: 10.1161/CIRCULATIONAHA.108.834739. [DOI] [PubMed] [Google Scholar]
- 86.Greene AS, Tonellato PJ, Zhang Z, Lombard JH, Cowley AW., Jr Effect of microvascular rarefaction on tissue oxygen delivery in hypertension. Am J Physiol. 1992;262:H1486–1493. doi: 10.1152/ajpheart.1992.262.5.H1486. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Sun Q, Zhang M, Xie K, Chen J, Liu Z. Capillary dilation and rarefaction are correlated with intracapillary inflammation in antibody-mediated rejection. J Immunol Res. 2014;2014:582902. doi: 10.1155/2014/582902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimizu A, Yamada K, Sachs DH, Colvin RB. Persistent rejection of peritubular capillaries and tubules is associated with progressive interstitial fibrosis. Kidney International. 2002;61:1867–1879. doi: 10.1046/j.1523-1755.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 89.Dormond O, Dufour M, Seto T, Bruneau S, Briscoe DM. Targeting the intragraft microenvironment and the development of chronic allograft rejection. Hum Immunol. 2012;73:1261–1268. doi: 10.1016/j.humimm.2012.07.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bruneau S, Woda CB, Daly KP, Boneschansker L, Jain NG, Kochupurakkal N, Contreras AG, Seto T, Briscoe DM. Key Features of the Intragraft Microenvironment that Determine Long-Term Survival Following Transplantation. Front Immunol. 2012;3:54. doi: 10.3389/fimmu.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furuya T, Murase N, Nakamura K, Woo J, Todo S, Demetris AJ, Starzl TE. Preformed lymphocytotoxic antibodies: the effects of class, titer and specificity on liver vs heart allografts. Hepatology. 1992;16:1415–1422. doi: 10.1002/hep.1840160618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawagishi N, Satomi S. ABO-incompatible living donor liver transplantation: new insights into clinical relevance. Transplantation. 2008;85:1523–1525. doi: 10.1097/TP.0b013e318173a70e. [DOI] [PubMed] [Google Scholar]
- 93.Aguilera I, Alvarez-Marquez A, Gentil MA, Fernandez-Alonso J, Fijo J, Saez C, Wichmann I, Nunez-Roldan A. Anti-glutathione S-transferase T1 antibody-mediated rejection in C4d-positive renal allograft recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:2393–2398. doi: 10.1093/ndt/gfm955. [DOI] [PubMed] [Google Scholar]
- 94.Hubscher SG. Antibody-mediated rejection in the liver allograft. Curr Opin Organ Transplant. 2012;17:280–286. doi: 10.1097/MOT.0b013e328353584c. [DOI] [PubMed] [Google Scholar]
- 95.Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, Hayashi P, Passannante A, Andreoni K. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17:357–368. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 96.Jain A, Demetris AJ, Kashyap R, Blakomer K, Ruppert K, Khan A, Rohal S, Starzl TE, Fung JJ. Does tacrolimus offer virtual freedom from chronic rejection after primary liver transplantation? Risk and prognostic factors in 1,048 liver transplantations with a mean follow-up of 6 years. Liver Transpl. 2001;7:623–630. doi: 10.1053/jlts.2001.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kakizoe S, Yanaga K, Starzl TE, Demetris AJ. Evaluation of protocol before transplantation and after reperfusion biopsies from human orthotopic liver allografts: considerations of preservation and early immunological injury. Hepatology. 1990;11:932–941. doi: 10.1002/hep.1840110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, Leverson GE, Bellingham JM, Fernandez LA, Foley DP, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bellamy CO. Complement C4d immunohistochemistry in the assessment of liver allograft biopsy samples: Applications and pitfalls. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17:747–750. doi: 10.1002/lt.22323. [DOI] [PubMed] [Google Scholar]
- 100.Matsunaga Y, Terada T. Peribiliary capillary plexus around interlobular bile ducts in various chronic liver diseases: An immunohistochemical and morphometric study. Pathol Int. 1999;49:869–873. doi: 10.1046/j.1440-1827.1999.00959.x. [DOI] [PubMed] [Google Scholar]
- 101.Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9:204–209. doi: 10.1002/hep.1840090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takemura M, Oguma S, Mori S, Ishii M, Starzl TE, Demetris AJ, Takahasi T. Peribiliary vascular diseases in rejected livers; computer-aided three-dimensional reconstruction and morphometry. Transplantation Proceedings. 1991;23:1409–1412. [PMC free article] [PubMed] [Google Scholar]
- 103.Matsumoto Y, McCaughan GW, Painter DM, Bishop GA. Evidence that portal tract microvascular destruction precedes bile duct loss in human liver allograft rejection. Transplantation. 1993;56:69–75. doi: 10.1097/00007890-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, Maekawa T, Uemoto S, Haga H. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18:1333–1342. doi: 10.1002/lt.23534. [DOI] [PubMed] [Google Scholar]
- 105.Yamada H, Kondou H, Kimura T, Ikeda K, Tachibana M, Hasegawa Y, Kiyohara Y, Ueno T, Miyoshi Y, Mushiake S, et al. Humoral immunity is involved in the development of pericentral fibrosis after pediatric live donor liver transplantation. Pediatr Transplant. 2012;16:858–865. doi: 10.1111/j.1399-3046.2012.01781.x. [DOI] [PubMed] [Google Scholar]
- 106.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczek E, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. American Journal of Pathology. 1988;132:489–502. [PMC free article] [PubMed] [Google Scholar]
- 107.Bellamy CO, Herriot MM, Harrison DJ, Bathgate AJ. C4d immunopositivity is uncommon in ABO-compatible liver allografts, but correlates partially with lymphocytotoxic antibody status. Histopathology. 2007;50:739–749. doi: 10.1111/j.1365-2559.2007.02677.x. [DOI] [PubMed] [Google Scholar]
- 108.Bu X, Zheng Z, Yu Y, Zeng L, Jiang Y. Significance of C4d deposition in the diagnosis of rejection after liver transplantation. Transplant Proc. 2006;38:1418–1421. doi: 10.1016/j.transproceed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 109.Dankof A, Schmeding M, Morawietz L, Gunther R, Krukemeyer MG, Rudolph B, Koch M, Krenn V, Neumann U. Portal capillary C4d deposits and increased infiltration by macrophages indicate humorally mediated mechanisms in acute cellular liver allograft rejection. Virchows Arch. 2005;447:87–93. doi: 10.1007/s00428-005-1245-z. [DOI] [PubMed] [Google Scholar]
- 110.Haga H, Egawa H, Fujimoto Y, Ueda M, Miyagawa-Hayashino A, Sakurai T, Okuno T, Koyanagi I, Takada Y, Manabe T. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 2006;12:457–464. doi: 10.1002/lt.20652. [DOI] [PubMed] [Google Scholar]
- 111.Jain A, Mohanka R, Orloff M, Abt P, Romano J, Bryan L, Batzold P, Mantry P, Bozorgzadeh A. Characterization of CD4, CD8, CD56 positive lymphocytes and C4d deposits to distinguish acute cellular rejection from recurrent hepatitis C in post-liver transplant biopsies. Clin Transplant. 2006;20:624–633. doi: 10.1111/j.1399-0012.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 112.Krukemeyer MG, Moeller J, Morawietz L, Rudolph B, Neumann U, Theruvath T, Neuhaus P, Krenn V. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation. 2004;78:65–70. doi: 10.1097/01.tp.0000132324.14207.8b. [DOI] [PubMed] [Google Scholar]
- 113.Sakashita H, Haga H, Ashihara E, Wen MC, Tsuji H, Miyagawa-Hayashino A, Egawa H, Takada Y, Maekawa T, Uemoto S, et al. Significance of C4d staining in ABO-identical/compatible liver transplantation. Mod Pathol. 2007;20:676–684. doi: 10.1038/modpathol.3800784. [DOI] [PubMed] [Google Scholar]
- 114.Sawada T, Shimizu A, Kubota K, Fuchinoue S, Teraoka S. Lobular damage caused by cellular and humoral immunity in liver allograft rejection. Clin Transplant. 2005;19:110–114. doi: 10.1111/j.1399-0012.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 115.Troxell ML, Higgins JP, Kambham N. Evaluation of C4d staining in liver and small intestine allografts. Arch Pathol Lab Med. 2006;130:1489–1496. doi: 10.5858/2006-130-1489-EOCSIL. [DOI] [PubMed] [Google Scholar]
- 116.Bouron-Dal Soglio D, Rougemont AL, Herzog D, Soucy G, Alvarez F, Fournet JC. An immunohistochemical evaluation of C4d deposition in pediatric inflammatory liver diseases. Hum Pathol. 2008;39:1103–1110. doi: 10.1016/j.humpath.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 117.Aguilera I, Sousa JM, Gavilan F, Gomez L, Alvarez-Marquez A, Nunez-Roldan A. Complement component 4d immunostaining in liver allografts of patients with de novo immune hepatitis. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17:779–788. doi: 10.1002/lt.22302. [DOI] [PubMed] [Google Scholar]
- 118.Schmeding M, Dankof A, Krenn V, Krukemeyer MG, Koch M, Spinelli A, Langrehr JM, Neumann UP, Neuhaus P. C4d in acute rejection after liver transplantation--a valuable tool in differential diagnosis to hepatitis C recurrence. Am J Transplant. 2006;6:523–530. doi: 10.1111/j.1600-6143.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- 119.Silva MA, Mirza DF, Murphy N, Richards DA, Reynolds GM, Wigmore SJ, Neil DA. Intrahepatic complement activation, sinusoidal endothelial injury, and lactic acidosis are associated with initial poor function of the liver after transplantation. Transplantation. 2008;85:718–725. doi: 10.1097/TP.0b013e3181663366. [DOI] [PubMed] [Google Scholar]
- 120.Martelius T, Halme L, Arola J, Hockerstedt K, Lautenschlager I. Vascular deposition of complement C4d is increased in liver allografts with chronic rejection. Transpl Immunol. 2009;21:244–246. doi: 10.1016/j.trim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Hoshino J, Kaneku H, Everly MJ, Greenland S, Terasaki PI. Using donor-specific antibodies to monitor the need for immunosuppression. Transplantation. 2012;93:1173–1178. doi: 10.1097/TP.0b013e31824f3d7c. [DOI] [PubMed] [Google Scholar]
- 122.Feng S, Demetris AJ, Ekong U, Girnita A, Kanaparthi S, Soppe C, Tchao N, Isse K. Serum and Tissue DSA Subclass, Stellate and Endothelial Phenotype Monitoring in ITN029ST Tolerance Pediatric LIver Transplant Recipients over 5+ Years of Follow-up. Joint International Conference of ILTS, ELITA & LICAGE; London. 2014. [Google Scholar]
- 123.Banff Working G, Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 124.Banff Working G. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl. 2012;18:1154–1170. doi: 10.1002/lt.23481. [DOI] [PubMed] [Google Scholar]
- 125.Demetris AJ, Lunz JG, 3rd, Randhawa P, Wu T, Nalesnik M, Thomson AW. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transpl Int. 2009;22:120–141. doi: 10.1111/j.1432-2277.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 126.Benitez C, Londono MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martinez-Llordella M, Lopez M, Angelico R, Bohne F, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 127.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA : the journal of the American Medical Association. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 128.Wong T, Nouri-Aria KT, Devlin J, Portmann B, Williams R. Tolerance and latent cellular rejection in long-term liver transplant recipients. Hepatology. 1998;28:443–449. doi: 10.1002/hep.510280223. [DOI] [PubMed] [Google Scholar]
- 129.Bohne F, Martinez-Llordella M, Lozano JJ, Miquel R, Benitez C, Londono MC, Manzia TM, Angelico R, Swinkels DW, Tjalsma H, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. The Journal of clinical investigation. 2012;122:368–382. doi: 10.1172/JCI59411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, Miyagawa A, Sakashita H, Tsuruyama T, Ohe H, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606–614. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
- 131.Egawa H, Miyagawa-Hayashino A, Haga H, Teramukai S, Yoshizawa A, Ogawa K, Ogura Y, Okamoto S, Kaido T, Uemoto S. Non-inflammatory centrilobular sinusoidal fibrosis in pediatric liver transplant recipients under tacrolimus withdrawal. Hepatol Res. 2012;42:895–903. doi: 10.1111/j.1872-034X.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 132.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 133.Sanada Y, Matsumoto K, Urahashi T, Ihara Y, Wakiya T, Okada N, Yamada N, Hirata Y, Mizuta K. Protocol liver biopsy is the only examination that can detect mid-term graft fibrosis after pediatric liver transplantation. World J Gastroenterol. 2014;20:6638–6650. doi: 10.3748/wjg.v20.i21.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paterno F, Shiller M, Tillery G, O’Leary JG, Susskind B, Trotter J, Klintmalm GB. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012;12:2526–2531. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee CF, Eldeen FZ, Chan KM, Wu TH, Soong RS, Wu TJ, Chou HS, Lee WC. Bortezomib is effective to treat acute humoral rejection after liver transplantation. Transplant Proc. 2012;44:529–531. doi: 10.1016/j.transproceed.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 136.Isse K, Lesniak A, Grama K, Roysam B, Minervini MI, Demetris AJ. Digital transplantation pathology: combining whole slide imaging, multiplex staining and automated image analysis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:27–37. doi: 10.1111/j.1600-6143.2011.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


