Abstract
Adjuvants for DNA vaccination are designed to promote transformation of transgenes into target cells and increase inflammation in the site of injection, with resultant immune cell recruitment. Numerous studies indicated cationic liposomes as effective adjuvants for DNA vaccination due to their ability to promote in vivo transfection and innate immune system activation. Commercial reagents as Adjuplex and in vivo-JetPEI are also intended to facilitate DNA vaccination. Here, we evaluate the adjuvant properties of cationic liposomes, Adjuplex and in vivo-JetPEI compared to injection of DNA without adjuvant. In mice vaccinated with piggyBac pDNA vaccines, we assessed in vivo antigen expression, innate immune responses in draining lymph nodes, and antigen-specific T cell responses in spleens and blood. Surprisingly, vaccination with DNA in PBS emerged as the most efficient in promoting in vivo transfection and consequent antigen expression, while the addition of adjuvant reduced the amount of antigen expressed. On the other hand, we discovered higher numbers of innate immune cells and activated dendritic cells in the lymph nodes of mice injected with adjuvants than those vaccinated in PBS. The analysis of eGFP-specific immune responses revealed that all the different immunizations induced functional antigen-specific T cells in spleens, although only T cells generated by non-adjuvant vaccination and Adjuplex were identified in the blood of vaccinated mice. These results provide insight into the effects of these 3 adjuvants and may facilitate appropriate use off adjuvants by researchers using DNA vaccines in laboratory animals.
Keywords: adjuvants, Adjuplex, cell mediated immunity, DNA vaccine, liposomes, innate immunity, JetPEI, piggyBac, transposase
Abbreviations
- s.c.
sub cutaneous
- eGFP
enhanced Green Fluorescent Protein
- i.p.
intra peritoneum
- IL-2
interleukin-2
- IFNγ
Interferon-γ
Introduction
Vaccination using DNA plasmids has emerged as an effective approach for generating robust antigen-specific immune responses in laboratory animals.1 Although DNA plasmids contain unmethylated CpG motifs that exert immune stimulatory effects, different adjuvants have been developed to increase the efficacy of these vaccines.2 In our previous study we demonstrated that strong antigen-specific T cell responses can be induced after subcutaneous injection of piggyBac integrating DNA plasmid vaccines in mice.3 In the present study we utilize the same experimental approach to assess the contribution of DOPE/DOTAP cationic liposomes, Adjuplex and in vivo-JetPEI on antigen expression and both innate and adaptive immune responses.4-6
Liposomes are synthetic spherical lipid layers that can encapsulate antigens and act as both a vaccine delivery vehicle and adjuvant. Liposomes have been used widely in experimental vaccines to enhance both humoral and cellular immunity, although no adjuvants based on liposomes have yet been registered for human use. A key advantage of liposomes is their versatility and plasticity. Liposome composition and preparation can be varied to include combinations of lipids with different charge, size and location of antigens. Adjuplex is a commercially available reagent based on a proprietary combination of lecithin and carbomer homopolymer that potentiates immune responses to vaccines without reactogenicity and has a demonstrated superiority to Alum, Freund's adjuvant, Ribi-R730, and numerous other experimental adjuvants. In laboratory animals, Adjuplex has been shown to improve the protective immune response to a variety of experimental human vaccines including HIV, influenza, rotavirus, and malaria. However, Adjuplex alone is non-immunogenic.7-10
In vivo-JetPEI is another commercially available reagent based on a linear polyethylenimine vehicle primarily designed for effective, reproducible delivery of in vivo nucleic acid. Studies have recently demonstrated the efficacy of in vivo-JetPEI as a vaccine adjuvant, although other studies previously reported that in vivo-JetPEI does not induce major pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12/IL-23 that are required for optimal antibody and T cell responses.11-13
We set out to compare these vaccination approaches and the results of this study were surprising in that vaccination with DNA plasmid alone was superior to combination with any of the 3 adjuvants. These findings will provide researchers with practical information about the mechanisms of action for these 3 adjuvants, which can be applied in designing effective DNA vaccines for experimentations in laboratory animals.
Results
Immunization with piggyBac DNA vaccines in PBS induces stronger antigen expression than DNA vaccination using cationic liposomes, Adjuplex, or JetPEI
In these studies pmhyGENIE-3 piggyBac vectors that catalyze the insertion of a transgene-containing transposon were used to vaccinate mice and generate antigen-specific immune responses. The efficacy of these DNA vaccines may be improved by including adjuvants that promote in vivo transfection and immune system activation. We designed experiments to compare 3 adjuvants with different chemical structure: DOPE/DOTAP cationic liposomes, Adjuplex and JetPEI (Fig. 1). Mice were injected with piggyBac integrating DNA plasmids formulated with PBS or DNA plasmids in combination with one of the 3 different adjuvants. Unvaccinated mice were used as controls. Innate immune responses were evaluated in lymph nodes of vaccinated mice 72h after the first immunization using pmhyGENIE-3-eGFP. Adaptive eGFP-specific T cell responses were then evaluated in spleen cells isolated 10 days after boost vaccination with pmhyGENIE-3-eGFP. The presence of eGFP-specific CD8+ T cells was also evaluated in the blood of these mice using eGFP-specific pentamers.
Figure 1.
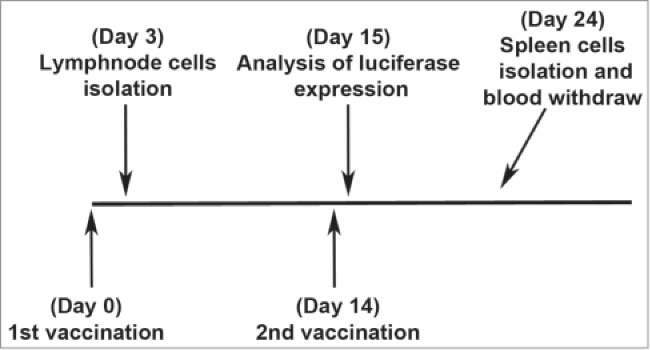
Experimental protocol to compare antigen expression, innate immunity, and antigen-specific T cell activation after DNA vaccination with or without adjuvants. Different groups of mice were injected with piggyBac integrating DNA plasmids formulated with each of the 3 different adjuvants. Unvaccinated and non-adjuvant DNA vaccinated mice were also used. Antigen expression was evaluated in mice after the second vaccination with pmhyGENIE-3-GL3. In other mice, innate immune responses were evaluated in inguinal lymph nodes after the 1st vaccination with pmhyGENIE-3-eGFP. In another group of mice, adaptive eGFP-specific immune responses were evaluated in spleen cells isolated 10 days after the second vaccination with pmhyGENIE-3-eGFP. Presence of eGFP-specific CD8+ T cells was also evaluated in blood of pmhyGENIE-3-eGFP vaccinated mice after the second vaccination.
The efficacy of the 3 adjuvants in promoting in vivo transfection and consequent antigen production was evaluated by measuring luciferase activity 24h after the second vaccination with pmhyGENIE-3-GL3 (Fig. 2). This luciferase encoding plasmid was used since in vivo protein expression is quantifiable using the IVIS imaging system that detects in vivo luciferase activity. Formulations of piggyBac plasmids diluted in PBS or combined with either liposomes, Adjuplex, or JetPEI were s.c. injected in the hind flank of mice. The day after the second vaccination a significantly higher activity of luciferase was detected in mice injected with DNA suspended in PBS. Plasmid inoculation using Adjuplex or JetPEI also induced significant luciferase expression, although at lower levels compared to DNA in PBS. Within the group of mice vaccinated using piggyBac plasmids in conjunction with liposomes, levels of transgene expression was not equal. IVIS imaging of luciferase activity showed that some mice exhibited a detectable luciferase signal while others did not (Fig. 2B). These data suggest that plasmid DNA diluted in PBS may be taken up by host cells following s.c. injection and produce considerable amounts of antigen. In contract, all 3 adjuvants interfered with this process as indicated by lower levels of luciferase activity produced after vaccination.
Figure 2.
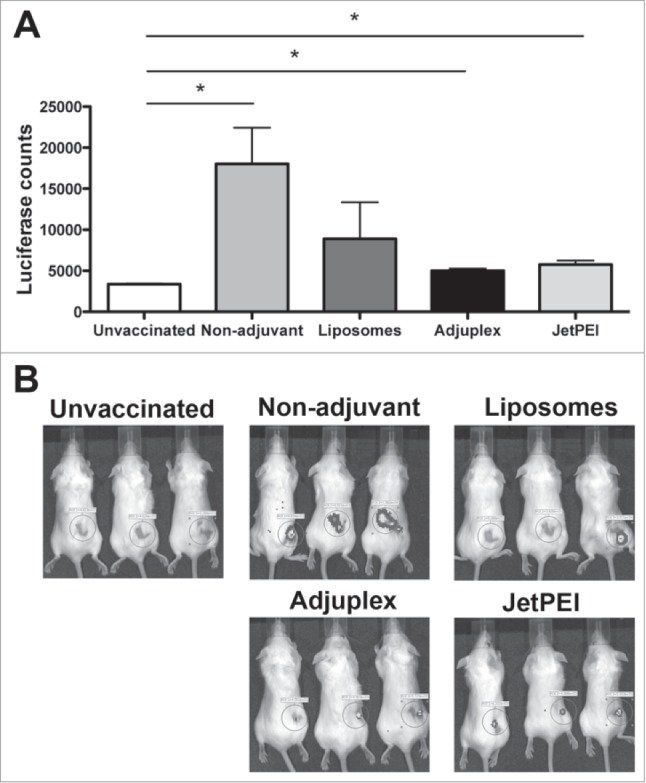
Vaccination with pmhyGENIE-3-GL3 diluted in PBS leads to improved expression of luciferase protein when compared with liposomes, Adjuplex, and JetPEI. (A) Luciferase activity from mice s.c. injected with plasmid DNA without adjuvants (Non-adjuvant) or combined with liposomes, Adjuplex or JetPEI. Control mice were not vaccinated. Data represent mean + SEM (N = 5/group). Statistical significance was evaluated using one-way ANOVA. Bonferroni multiple comparison test was performed to identify differences between control and vaccinated mice. *P < 0.05. (B) Representative IVIS images from mice unvaccinated or s.c. injected with pmhyGENIE-3-GL3 with or without adjuvants.
Adjuvants generate innate immune responses with activation of dendritic cells in lymph nodes of vaccinated mice
It has been demonstrated that adjuvants trigger innate immune responses and these responses are central to their adjuvant activity.14 Since innate responses typically precede adaptive responses, the effects of liposomes, Adjuplex and JetPEI were evaluated 3d after vaccination. Cells isolated from draining lymph nodes of vaccinated mice were enumerated and populations of immune cells identified using flow cytometry analysis. Results from mice vaccinated using the different adjuvants were compared with those from mice vaccinated without adjuvants and unvaccinated mice. A significantly larger number of cells were found after vaccination with liposomes or Adjuplex, compared with controls (Fig. 3A). Vaccination using JetPEI and non-adjuvant DNA did not increase lymph node cell numbers. Percentages of the different leukocyte populations were assessed using multicolor flow cytometer analysis, which allows the simultaneous identification of several different cell types in one sample.15 We identified surface markers for different types of leukocytes including T cells (CD3, CD4 and CD8), B cells (B220), granulocytes (Gr-1), monocytes/macrophages (CD11b) and dendritic cells (CD11c). Our data show that liposomes caused an increase in the relative abundance of cells involved in the innate immune response. In particular, a higher abundance of B cells, granulocytes and macrophages were found in mice injected with liposomes compared with unvaccinated mice. Mice vaccinated using Adjuplex exhibited a higher abundance of granulocytes and macrophages, while mice vaccinated using JetPEI and non-adjuvant vaccinated mice only exhibited increased abundance for macrophages (Fig. 3B, C, D).
Figure 3.
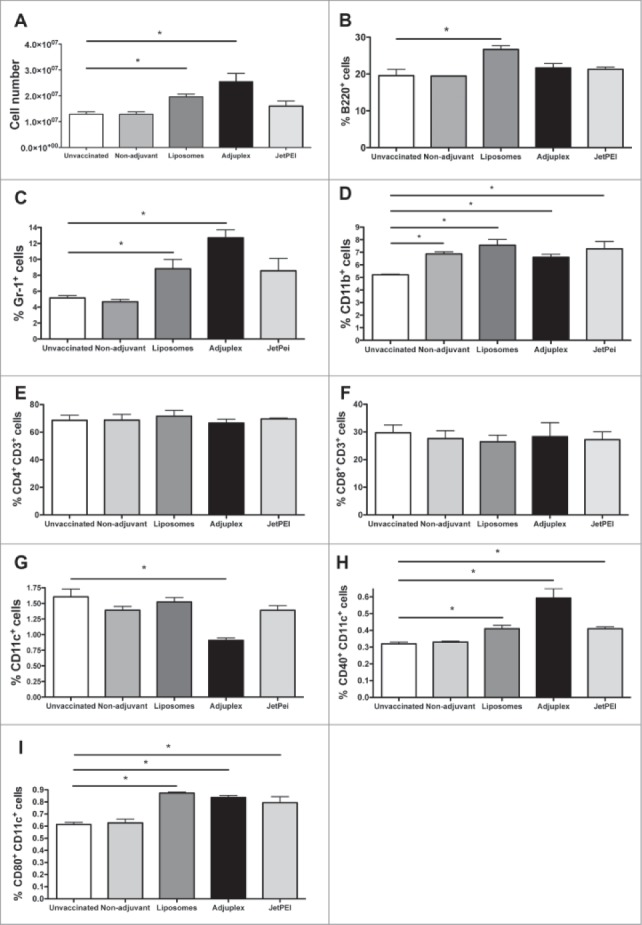
Analysis of lymph node cells after DNA vaccination in PBS, liposomes, Adjuplex, or JetPEI. Lymph nodes were isolated 72 h after vaccination with the different adjuvants and compared with lymph nodes from unvaccinated or non-adjuvant DNA vaccinated mice. Overall number of lymph node derived cells was assessed (A) and flow cytometric analysis performed to detect percentages of different immune cell populations. Results are shown for B220+ B cells (B), Gr-1+ granulocytes (C), CD11b+ macrophages (D), CD4+CD3+ T cells (E), CD8+CD3+ T cells (F) and CD11c+ dendritic cells (G). The activation of CD11c+ dendritic cells was also evaluated by analyzing the expression of CD40+ cells (H) and CD80+ cells (I). Data are expressed as mean + SEM (N = 5/group). Means were compared using one-way ANOVA followed by the Bonferroni multiple comparison test * P ≤ 0.05. Data of CD4+and CD8+cells are expressed as percentage of these cells over the population of total CD3+ cells. Data of Gr-1+, CD11b+and CD11c+cells are instead showed as percentage of the total number of lymph node cells.
We also analyzed cells involved in the adaptive immune response. Dendritic cells, CD4+ T cells and CD8+ T cells did not increase in relative abundance after vaccination with the different conditions, except in the case of Adjuplex that caused reduced levels of dendritic cells compared with controls (Fig. 3E, F, G). Analysis of dendritic cell activation markers, CD40 and CD80, indicated that vaccination using any of the 3 adjuvants promoted the maturation of dendritic cells as indicated by levels of these 2 co-receptors. In contrast, mice vaccinated without adjuvants showed the same levels of activated dendritic cells than unvaccinated mice (Fig. 3H, I).
Only DNA injection in PBS or formulated with adjuplex generates detectable antigen-specific CD8+ T cells in blood of vaccinated mice
Since eGFP represents a non-self protein that can serve as a vaccine antigen for mice, we utilized the plasmids encoding this protein to compare vaccine adjuvants. Pentamer reagents are available that detect mouse CD8+ T cells specific for the eGFP 200–208 epitope, which were used to evaluate eGFP specific CD8+ T cells generated in vaccinated mice. Results showed that, after both the primary and secondary vaccination with pmhyGENIE-3-eGFP in PBS or in combination with Adjuplex, significantly more eGFP-specific CD8+ T cells were detected in the blood of these mice compared to unvaccinated controls (Fig. 4). In contrast, DNA vaccination using liposomes or JetPEI did not produce significantly higher eGFP-specific CD8+ T cells in the blood.
Figure 4.
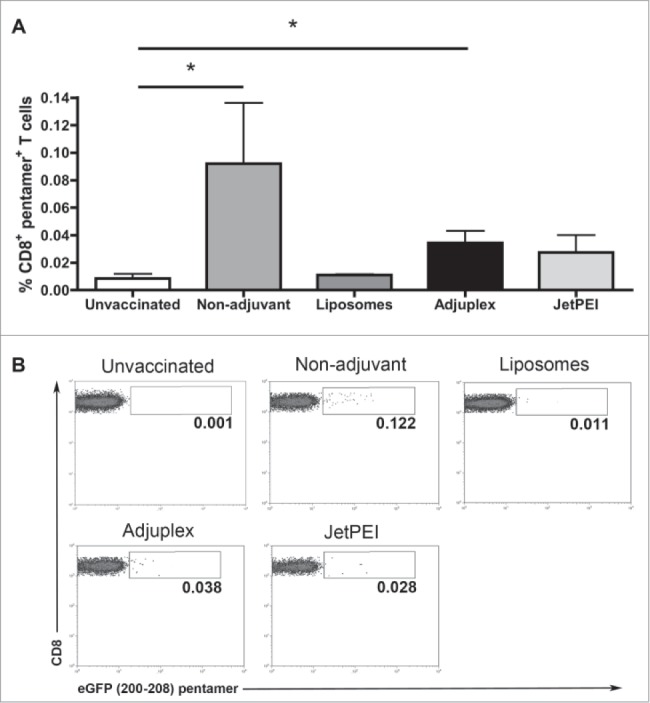
eGFP-specific CD8+ T cells were detected in blood of mice vaccinated with DNA in PBS (Non-adjuvant) or injected with Adjuplex. (A) Flow cytometric detection of CD8+ T cells specific for the eGFP epitope corresponding to amino acids 200–208 was performed using a fluorescent pentamer. Data are shown for blood cells collected 10 days after second vaccination and represent mean + SEM (N = 5/group). Statistical significance was evaluated using one-way ANOVA. Bonferroni multiple comparison test was performed to identify differences between groups. *P < 0.05. (B) Representative images from flow cytometry analysis of blood cells stained with eGFP 200–208 pentamers.
Vaccination with piggyBac DNA in combination with liposomes or Adjuplex generates functional CD8+ T cells that produce IFNγ
We next investigated whether the higher numbers of CD8+ T cells from mice vaccinated without adjuvants or with Adjuplex corresponded to increased function of these cells. Different groups of mice were given primary and secondary s.c. injections in the hind flank with 40 μg/mouse of integrating plasmid formulated with the different adjuvants as described in the Materials and Methods section. Conventional re-stimulation assays were used to test functionality of CD8+ T cells. Specifically, spleens were removed from the eGFP plasmid DNA vaccinated mice on day 28 and splenocytes pulsed with eGFP peptide (200–208 epitope). Intracellular staining and flow cytometry analyses were used to measure the level of IFNγ within the CD8+ T cells. For all of the DNA formulations, with or without adjuvants, results showed significantly higher numbers of IFNγ producing splenocytes after stimulation with eGFP peptide (Fig. 5). Interestingly, the strongest IFNγ responses in CD8+ T cells were in mice vaccinated without adjuvants.
Figure 5.
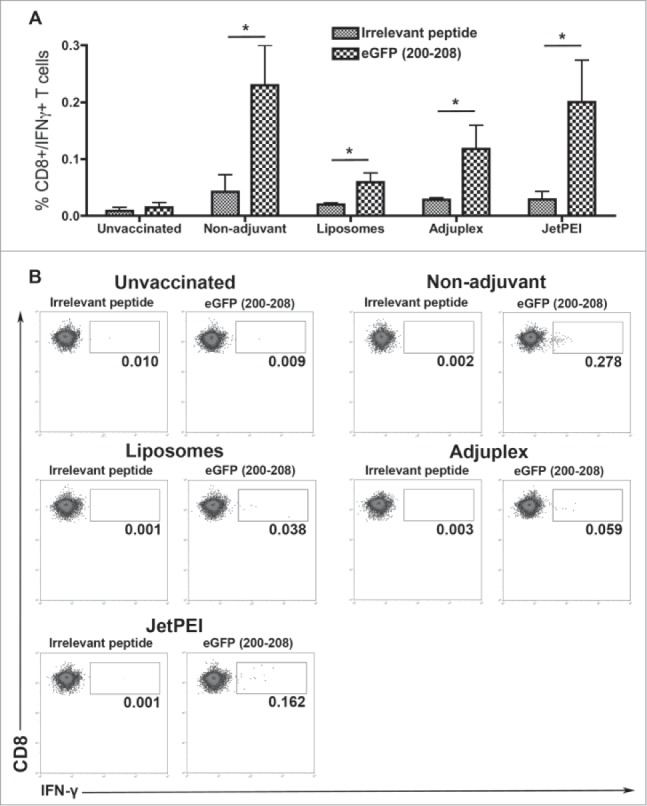
Vaccination with piggybac DNA plasmids formulated with PBS or different adjuvants generates antigen-specific CD8+ T cell responses in spleens. Data from flow cytometric detection of intracellular staining for IFNγ in CD8+ T cells after pulsing with an irrelevant peptide or with eGFP 200–208 peptide. Data represent mean + SEM (N = 5/group). Means of each group were compared using Bonferroni multiple comparison test after ANOVA analysis. *P < 0.05.
Discussion
DNA vaccines have some beneficial features in comparison with conventional vaccines in that they are easy to produce, relatively inexpensive, homogeneous, heat stable, and believed to be safer than subunit or viral vector-based vaccines. Over the past decade or more, numerous DNA vaccines have been constructed and tested for efficacy and some promising results have been reported. However, it is apparent from studies that vaccine immunogenicity requires improvement.2 Careful selection of adjuvants or DNA delivery systems can enhance elicited immune responses. Traditionally, approaches available to augment vaccine-elicited responses have been limited to aluminum salts or Freund's type adjuvants. Advances in molecular biology and recombinant techniques now allow the inclusion of various cytokines, as well as immunomodulatory molecules, in DNA vaccine formulations. Newer generation adjuvants also have been developed and show promising results in enhancing DNA-elicited immunity.17,18
An ideal adjuvant for DNA vaccination should first promote in vivo transfection of the genetic material such as plasmid DNA or linear RNA into cells of target tissue. In addition, it is also necessary that activation of innate and adaptive immune cells occur to produce strong and sustained antibody or cell-mediated responses. We compared 3 different adjuvants that can be used in combination with DNA vaccination for experimental purposes. Mice were vaccinated with either liposomes, Adjuplex or JetPEI and evaluated for antigen production and immune system activation. As controls we used unvaccinated mice and also mice vaccinated with DNA diluted in PBS. Surprisingly, vaccination using DNA suspended in PBS emerged as the most efficient in promoting in vivo transfection and consequent antigen expression, while inclusion of any adjuvant reduced significantly the amount of antigen produced. When we analyzed the effects of the different adjuvants in lymph nodes of vaccinated mice, we discovered higher numbers of innate immune cells and activated dendritic cells in mice injected with the adjuvants compared to unvaccinated controls or DNA in PBS. The analysis of the adaptive eGFP-specific immune responses generated by DNA vaccination in formulation with the adjuvants or PBS revealed that all the different immunizations induced functional antigen-specific T cells in spleens. Only non-adjuvant vaccination and Adjuplex generated significantly more antigen-specific T cells in the blood of vaccinated mice. We also investigated type-1 interferons in lymph nodes after vaccination due to the role this cytokine plays in DNA vaccine immunogenicity. The results were highly variable and not statistically significant, so these data were not included in this study.
These data indicate that adjuvants reduce the ability of DNA vaccines to enter into the cells after subcutaneous injection and thus hinder antigen expression. The adjuvants were effective in boosting innate immune stimulation, as indicated by the higher levels of dendritic cell maturation. Despite this fact, the 3 adjuvants did not lead to stronger specific-adaptive immune responses in spleens compared to plasmid with no adjuvant. Circulating antigen-specific T cells are a hallmark of effective vaccination, and our evaluation of eGFP-specific T cells in the blood of mice DNA vaccinated showed higher numbers without the inclusion of adjuvant or with Adjuplex. In the case of smaller amounts of antigen, Adjuplex can still strongly promote the development of antigen-specific T cells, probably due to its particular mechanism of action. In fact, Adjuplex displayed a different pattern of activation in T cells resident in the lymph node of vaccinated mice when compared with the other 2 adjuvants. Similar to liposomes and JetPEI, Adjuplex promoted the development of activated dendritic cells expressing CD40 and CD80, but it was the only adjuvant to reduce the frequency of dendritic cells that express CD11c. A phenomenon that can be possibly explained by the down-regulation of the CD11c molecules on the surface of activated dendritic cells, as previously observed after activation of toll like receptors caused by adjuvants.19 It is important to emphasize that we prepared the mixture between DNA and the different adjuvants following the manufacturers' directions. It is possible that higher transfection efficiencies and consequent stronger immune responses may be achieved by performing more extensive titration experiments to discover the optimal DNA/adjuvant ratio for each adjuvant.
The results of this study can provide researchers with detailed information on these 3 adjuvants and assist them in identifying the right conditions for their experiments using DNA vaccines in laboratory animals.
Materials and Methods
Mice
Female BALB/cJ mice at 4–8 weeks of age (Jackson Immunolabs, #000651) were maintained in a specific pathogen-free animal facility for at least 1 week before experiments. Procedures were performed in accordance with institutional guidelines approved by University of Hawaii Institutional Animal Care and Use Committee (IACUC).
Vaccination procedures
In experiments to evaluate antigen expression, mice were injected s.c. with 40 μg of piggyBac integrating plasmid DNA expressing luciferase, pmhyGENIE-3-GL3. In other experiments, analyses of immune responses were conducted in mice injected with a piggyBac integrating plasmid DNA expressing eGFP, pmhyGENIE-3-eGFP.3,20 Each mouse was immunized with plasmid DNA diluted in 100μl of PBS or formulated in 100μl of DOTAP/DOPE liposomes (Avanti Polar Lipids, #850725 and #890890) or 100μl of adjuvants Adjuplex (Sigma-Aldrich, #A0362) or in vivo-JetPEI (Polyplus transfection, # 201).
Adjuvants
Liposomes containing DOTAP and DOPE (Avanti Polar Lipids, Alabaster, AL, USA) in a 1:1 molar ratio were prepared by drying the lipid mixture in chloroform under a stream of nitrogen as a thin layer in a 10-mL round-bottomed tube. The lipid film was hydrated in PBS to give a final concentration of 10 mg lipids/mL and then sonicated with Avanti sonicator. The lipid suspension (100 μl/mouse) was mixed with DNA (40 μg/mouse) and incubated for 10 min before injection into mice. For each mouse vaccinated using Adjuplex, 40 μg of DNA in 80 μl of PBS were mixed to 20 μl of adjuvant, 10 min before injection. JetPEI vaccination was formulated by mixing together 2 10% glucose solutions, one containing 40 μg of DNA and the other including 3 μl of in vivo-JetPEI. After mixing the 2 solutions, mice were injected within 10 min.
Measurement of antigen expression
Bioluminescent signals from luciferase-vaccinated mice were monitored using the IVIS Lumina (Perkin Elmer, Waltham, MA, USA) after i.p. injections of 0.1 mL of 15 mg/mL D-luciferin. Regions of interest were identified around the injection sites and quantified as total photon counts using Living Image™ software. The values were expressed as an average of all vaccinated mice for every treatment group.
Measurement of immune responses
Spleen cells of immunized mice (1 × 106) were stimulated for 7 h with 10 μg/mL eGFP peptide (200-208 epitope) in the presence of 5 IU/mL IL-2 (BioLegend, #589102). For detection of IFN-γ in the cytoplasm, Golgi-plug (BD Biosciences, # 555029) was added 6 hours before staining. Fixation/permeabilization kits were used in combination with anti-CD3-PECy5, anti-CD8-APC, and anti-IFN-γ-PE (eBiosciences, #15-0031, #17-0081, #12-7311). Data were collected using the FACScalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Blood (200 μL) was collected from tails of BALB/c immunized mice. PE-labeled H2-Db pentamer (PENT) presenting the eGFP 200-208 epitope was purchased from Proimmune (#198) and cellular staining protocol performed as recommended by the manufacturer. Briefly, 2 × 106 cells were stained with 10 μL of PENT at room temperature for 10 min, washed and further incubated for 20 min with anti-CD3-FITC, anti-CD8-APC and anti-CD16/32 PE-Cy5.5 (eBiosciences, #11-0032, #17-0081, #35-0161). PENT+/CD8+ cells were analyzed on a FACSCalibur Flow Cytometer and analyzed using FlowJo software. Inguinal lymph nodes were removed 72 h after vaccination and population of immune cells stained as described previously15 using FACScalibur flow cytometer. The following antibodies were used: anti-CD3-FITC, anti-CD4-PE/Cy7, anti-CD8-APC, anti-Gr1-PerCP/Cy5.5, anti-CD11c-PE, anti-B220-PE/TR and anti-CD11b-APC/Cy7 (BioLegend, #100306, #116016, #100712, #108427, eBiosciences #12-0114-81, BD Pharmigen #551489, #557657). CD3+ cells were previously gated before identification of CD4+ and CD8+ T cell populations. CD11c+ cells were previously gated before measuring their activation using antibodies anti- CD40-PE/Cy5 and anti-CD80-APC (eBiosciences #15-0401-81, # 17-0801-81).
Statistical analyses
All statistical tests were performed using GraphPad Prism (GraphPad 5.0). Means were compared using one-way ANOVA followed by the Bonferroni multiple comparison test. Statistical significance values are indicated in the figure legends.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Ann Hashimoto for assistance with the animal husbandry.
Funding
This research was supported by NIH grants R01AI089999 (P.H), 5P20RR024206 (S.M); G122P20GM103457-06A1 (J.U.); Hawaii Community Foundation grant 14ADVC-64492; Buzzi Unicem Foundation for the study of Mesothelioma grant FBU-P31 (P.B.) and core facilities supported by P20GM103516, P20RR016453, G12RR003061, G12MD007601.
References
- 1.Lu S. Immunogenicity of DNA vaccines in humans: it takes two to tango. Hum Vaccin 2008; 4:449-52; PMID:18443427; http://dx.doi.org/ 10.4161/hv.4.6.6179 [DOI] [PubMed] [Google Scholar]
- 2.Sasaki S, Takeshita F, Xin KQ, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods 2003; 31:243-54; PMID:14511957; http://dx.doi.org/ 10.1016/S1046-2023(03)00140-3 [DOI] [PubMed] [Google Scholar]
- 3.Bertino P, Urschitz J, Hoffmann FW, You BR, Rose AH, Park WH, Moisyadi S, Hoffmann PR. Vaccination with a piggyBac plasmid with transgene integration potential leads to sustained antigen expression and CD8(+) T cell responses. Vaccine 2014; 32:1670-7; PMID:24513010; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant–‘the long and winding road’. Drug Discov Today 2009; 14:541-51; PMID:19508916; http://dx.doi.org/ 10.1016/j.drudis.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 5.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med 2009; 206:2967-76; PMID:19995959; http://dx.doi.org/ 10.1084/jem.20091181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492-503; PMID:21029960; http://dx.doi.org/ 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, et al.. Proof of principle for epitope-focused vaccine design. Nature 2014; 507:201-6; PMID:24499818; http://dx.doi.org/ 10.1038/nature12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta PK, Mukherjee P, Dhawan S, Pandey AK, Mazumdar S, Gaur D, Jain SK, Chauhan VS. Production and preclinical evaluation of Plasmodium falciparum MSP-119 and MSP-311 chimeric protein, PfMSP-Fu24. Clin Vaccine Immunol 2014; 21:886-97; PMID:24789797; http://dx.doi.org/ 10.1128/CVI.00179-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob G, Hicks MJ, Wee S, Rosenberg JB, De BP, Kaminsky SM, Moreno A, Janda KD, Crystal RG. Anti-cocaine vaccine based on coupling a cocaine analog to a disrupted adenovirus. CNS Neurol Disord Drug Targets 2011; 10:899-904; PMID:22229312; http://dx.doi.org/ 10.2174/187152711799219334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, McKee K, Tran K, O'Dell S, Schmidt SD, Phogat A, Forsell MN, Karlsson Hedestam GB, Mascola JR, Wyatt RT. Biochemically defined HIV-1 envelope glycoprotein variant immunogens display differential binding and neutralizing specificities to the CD4-binding site. J Biol Chem 2012; 287:5673-86; PMID:22167180; http://dx.doi.org/ 10.1074/jbc.M111.317776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Haes W, Rejman J, Pollard C, Merlin C, Vekemans M, Florence E, De Smedt SC, Grooten J, Vanham G, De Koker S, et al.. Lipoplexes carrying mRNA encoding Gag protein modulate dendritic cells to stimulate HIV-specific immune responses. Nanomedicine 2013; 8:77-87; PMID:22891862; http://dx.doi.org/ 10.2217/nnm.12.97 [DOI] [PubMed] [Google Scholar]
- 12.Jordan BJ, Vogel S, Stark MR, Beckstead RB. Expression of green fluorescent protein in the chicken using in vivo transfection of the piggyBac transposon. J Biotechnol 2014; 173:86-9; PMID:24452099; http://dx.doi.org/ 10.1016/j.jbiotec.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 13.Bonnet ME, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res 2008; 25:2972-82; PMID:18709489; http://dx.doi.org/ 10.1007/s11095-008-9693-1 [DOI] [PubMed] [Google Scholar]
- 14.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O'Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A 2008; 105:10501-6; PMID:18650390; http://dx.doi.org/ 10.1073/pnas.0804699105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann PR, Gurary A, Hoffmann FW, Jourdan-Le Saux C, Teeters K, Hashimoto AC, Tam EK, Berry MJ. A new approach for analyzing cellular infiltration during allergic airway inflammation. J Immunol Methods 2007; 328:21-33; PMID:17825315; http://dx.doi.org/ 10.1016/j.jim.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertino P, Panigada M, Soprana E, Bianchi V, Bertilaccio S, Sanvito F, Rose AH, Yang H, Gaudino G, Hoffmann PR, et al.. Fowlpox-based survivin vaccination for malignant mesothelioma therapy. Int J Cancer 2013; 133:612-23; PMID:23335100; http://dx.doi.org/ 10.1002/ijc.28048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheerlinck JY. Genetic adjuvants for DNA vaccines. Vaccine 2001; 19:2647-56; PMID:11257404; http://dx.doi.org/ 10.1016/S0264-410X(00)00495-3 [DOI] [PubMed] [Google Scholar]
- 18.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol 1999; 17:1075-81; PMID:10545912; http://dx.doi.org/ 10.1038/15058 [DOI] [PubMed] [Google Scholar]
- 19.Singh-Jasuja H, Thiolat A, Ribon M, Boissier MC, Bessis N, Rammensee HG, Decker P. The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology 2013; 218:28-39; PMID:22445076; http://dx.doi.org/ 10.1016/j.imbio.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 20.Urschitz J, Kawasumi M, Owens J, Morozumi K, Yamashiro H, Stoytchev I, Marh J, Dee JA, Kawamoto K, Coates CJ, et al.. Helper-independent piggyBac plasmids for gene delivery approaches: strategies for avoiding potential genotoxic effects. Proc Natl Acad Sci U S A 2010; 107:8117-22; PMID:20404201; http://dx.doi.org/ 10.1073/pnas.1003674107 [DOI] [PMC free article] [PubMed] [Google Scholar]


