Abstract
Idiopathic CD4 T lymphocytopenia (ICL) is a rare and severe condition with limited available data. We conducted a French multicenter study to analyze the clinical and immunologic characteristics of a cohort of patients with ICL according to the Centers for Disease Control criteria.
We recruited 40 patients (24 female) of mean age 44.2 ± 12.2 (19–70) years. Patients underwent T-lymphocyte phenotyping and lymphoproliferation assay at diagnosis, and experiments related to thymic function and interferon (IFN)-γ release by natural killer (NK) cell were performed. Mean follow-up was 6.9 ± 6.7 (0.14–24.3) years. Infectious, autoimmune, and neoplastic events were recorded, as were outcomes of interleukin 2 therapy.
In all, 25 patients had opportunistic infections (12 with human papillomavirus infection), 14 had autoimmune symptoms, 5 had malignancies, and 8 had mild or no symptoms. At the time of diagnosis, the mean cell counts were as follows: mean CD4 cell count: 127/mm3 (range, 4–294); mean CD8: 236/mm3 (range, 1–1293); mean CD19: 113/mm3 (range, 3–547); and mean NK cell count: 122/mm3 (range, 5–416). Most patients had deficiency in CD8, CD19, and/or NK cells. Cytotoxic function of NK cells was normal, and patients with infections had a significantly lower NK cell count than those without (p = 0.01). Patients with autoimmune manifestations had increased CD8 T-cell count. Proliferation of thymic precursors, as assessed by T-cell rearrangement excision circles, was increased. Six patients died (15%). CD4 T-cell count <150/mm3 and NK cell count <100/mm3 were predictors of death.
In conclusion, ICL is a heterogeneous disorder often associated with deficiencies in CD8, CD19, and/or NK cells. Long-term prognosis may be related to initial CD4 and NK cell deficiency.
Abbreviations: AIHA = autoimmune hemolytic anemia, CDC = Centers for Disease Control, CMV = cytomegalovirus, cpm = count per minute, CVID = common variable immunodeficiency, CXCR4 = C-X-C chemokine receptor type 4, HIV = human immunodeficiency virus, HLA = human leukocyte antigen, HPV = human papillomavirus, HTLV-1/2 = human T-cell lymphotropic 1/2, ICL = idiopathic CD4 T lymphocytopenia, IFN-γ = interferon-γ, IL = interleukin, JC virus = John Cunningham virus, LPA = lymphocyte proliferation assay, NK = natural killer, P = patient, PBMC = peripheral blood mononuclear cell, Pwd = pokeweed, SI = stimulation index, sj = signal joint, TREC = T-cell rearrangement excision circle
INTRODUCTION
Idiopathic CD4 T lymphocytopenia (ICL) is an immunodeficiency described in 1992 and characterized by the United States Centers for Disease Control (CDC) as absolute CD4 T-lymphocyte count <300/mm3 or <20% of total T cells on more than 1 cell count at least 6 weeks apart; no evidence of infection with human immunodeficiency virus (HIV)-1/2 or human T-cell lymphotropic 1/2 (HTLV-1/2); and lack of a defined immunodeficiency disease or therapy associated with depressed levels of CD4 T cells.24 Epidemiologic, clinical, and immunologic characteristics of the syndrome were described in 1993,9,10,13,24,25 and ICL is now considered a heterogeneous syndrome not caused by an infectious agent. Patients with ICL may have opportunistic infections such as disseminated Cryptococcus neoformans infection,32 Pneumocystis jirovecii pneumonia,20 and John Cunningham (JC) virus infection15 as a result of profound cell-mediated immune-response deficiency.
Few studies have focused on the pathophysiology of ICL. CD4 T-lymphocyte phenotyping revealed increased CD95 expression that could be responsible for excess apoptosis leading to lymphocytopenia.17,21 Moreover, the membrane expression of C-X-C chemokine receptor type 4 (CXCR4) was found impaired in T lymphocytes with ICL, and CXCR4 trafficking was improved with interleukin 2 (IL-2) treatment in some patients.22 Recently, mutations in nunc119,12 MAGT1,18 and Rag16 were found associated with CD4 T lymphocytopenia. In a prospective study of 39 patients, CD8 T lymphocytopenia (<180/mm3) and degree of CD4 T-cell activation measured by human leukocyte antigen DR (HLA-DR) expression were found associated with poor prognosis.33
We prospectively analyzed the clinical and immunologic characteristics and long-term prognosis of patients with ICL in a French multicenter cohort. We quantified lymphocyte subpopulations and mitogen/antigen-induced proliferation and explored proliferation of thymic precursors through quantification of T-cell rearrangement excision circles (TRECs) as well as natural killer (NK) cell cytotoxicity.
PATIENTS AND METHODS
Patient Selection
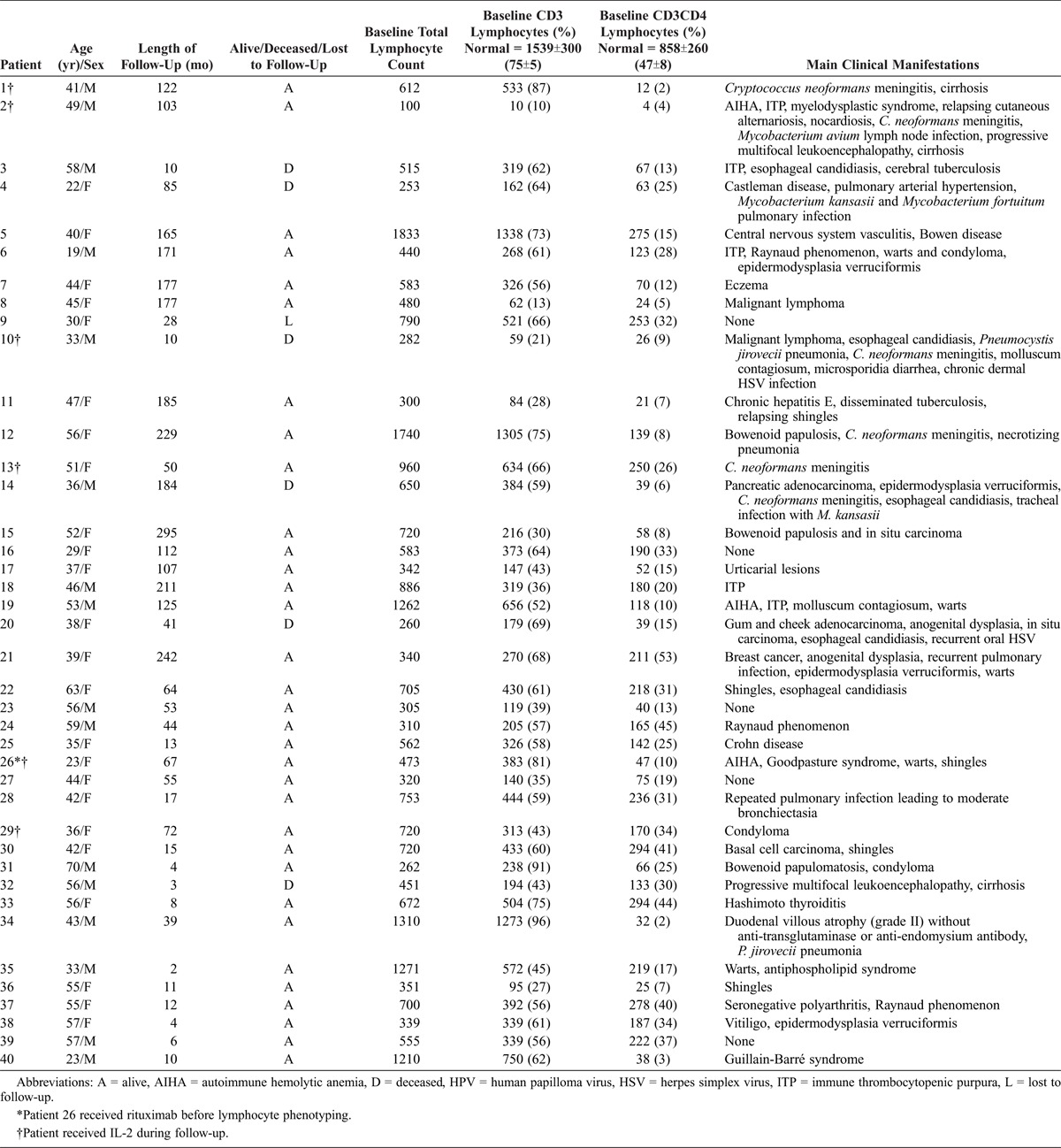
We prospectively included patients with CD4 T lymphocytopenia between January 1991 and June 2012. Diagnosis of ICL was based on absolute CD4 T-lymphocyte count <300/mm3 or <20% of total T cells on 2 cell counts at 6 weeks apart; no HIV-1/2 or HTLV-1/2 infection; and absence of defined immunodeficiency or therapy associated with decreased levels of CD4 T cells.24 Therefore, we systematically searched for a known primary or secondary immunodeficiency, including viral infection (for example, HIV, HTLV, transient viral infection), tuberculosis, malignancy (lymphoma or solid tumor), autoimmune and/or inflammatory disorders (for example, Sjögren syndrome, sarcoidosis, systemic lupus erythematosus, granulomatosis with polyangiitis) or other causes of acquired lymphocytopenia. Searches for a known immunodeficiency were performed according to knowledge at the time of diagnosis and during follow-up. Data from clinical files were retrospectively collected by 2 authors (AR and LM). Results for 6 patients were previously reported: Patient 1 (P1), P2, and P13;22 P12;14 P14;23 and P21.19
Data were analyzed by initial symptoms of any infection known to be associated with lymphocytopenia and any symptom related to autoimmune diseases. During follow-up, unusual infections, neoplasms, and symptoms related to autoimmune diseases were recorded. Patients were classified into 3 groups based on clinical and/or laboratory manifestations at diagnosis or during follow-up: autoimmune/inflammation, infection, or malignancy. Patients could be classified in more than 1 group.
Methods
Laboratory and Immunologic Data
Laboratory tests were performed in each center. Immunologic assays and lymphocyte phenotyping were all performed in a single laboratory in Pitié-Salpêtrière hospital when ICL diagnosis was established. We collected laboratory results for leukocyte and lymphocyte counts; levels of hemoglobin, albumin, serum gammaglobulin, IgG, IgA, and IgM; antinuclear antibodies; C3, C4 complement components and CH50; hepatitis B surface antigen (HBs); antibodies for hepatitis B core (HBc), hepatitis C virus (HCV), HIV-1/2, HTLV-1/2, human herpes virus 8, and Epstein-Barr virus; and zinc.
Blood samples from healthy adult volunteer donors from the blood collection center at Pitié-Salpêtrière hospital were used as controls. Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-hypaque (Eurobio, Les Ulis, France). To label surface antigens, we used monoclonal antibodies for CD3-PC5 or CD3-FITC, CD4-RD1, CD8-ECD, CD16+56-PE, CD19-PC5, CD25-PE, CD28-FITC, CD45RA-PE, CD45RO-FITC, TCRγδ-PE, and HLA-DR-FITC from Beckman Coulter (Marseille, France) and for TCRαβ-FITC from Becton Dickinson.
For lymphocyte proliferation assay (LPA) of fresh PBMCs, cells were resuspended at 106/mL, and 100 μL cells were seeded in each well and stimulated with precoated anti-CD3 alone or with anti-CD28, IL-2, phytohemagglutinin or pokeweed (Pwd) in triplicate. After 3 days, thymidine was added for 18 hours, and proliferation was measured by thymidine incorporation. Results are expressed as mean ± SD count per minute (cpm). The stimulation index (SI) was calculated as the cpm ratio of (cells + stimuli)/(cells + medium). A positive test result was defined as the association of 1) cpm >35,000 (>5000 for Pwd) and 2) SI >35 (>5 for Pwd).
LPA stimulation with antigens against cytomegalovirus (CMV) (Behring, Marburg, Germany), tuberculin (Statens Serum Institut, Denmark), candidin (Sanofi Pasteur, Lyon, France), and streptococcal enzyme (Sigma-Aldrich, St. Louis, MO) was for 6 days and was otherwise similar to mitogen LPA. A positive test result was defined as cpm >3000 and SI >3.
Parallel quantification of the signal joint (sj)-TREC and the diversity βjunction β(DβJβ)-TRECs, together with CD3γ gene (used as a housekeeping gene) was performed for each sample using LightCycler technology (Roche Diagnostics, Meylan, France) with a technique adapted from Dion et al.8 Intrathymic precursor T-cell proliferation was evaluated by calculating the sj/βTREC ratio as described by Dion et al.7 sjTREC frequencies were adjusted to naive T-cell counts as defined by CD45RA and CCR7 expression.
PBMCs were incubated for 16 hours with 10 ng/mL IL-12 and 100 ng/mL IL-18 (R&D Systems) at 37°C or without (control). Golgi Stop and Golgi Plug solutions (BD Biosciences) were added for an additional 5 hours. Cells were stained for the cell-surface markers CD3 (ECD; #UCHT1) and CD56 (PC7; #N901; Coulter), fixed and permeabilized with BD cytofix/cytoperm solution (BD Biosciences), then stained for intracellular interferon-γ (IFN-γ) expression (Alexa-Fluor-700; #B27; BD Biosciences) and analyzed by flow cytometry. Percentage of intracellular IFN-γ producing cells was determined in CD3-CD56+ NK cells.
Statistical Analysis
Results are expressed as number and/or percentages for categorical variables and mean ± SD for continuous variables. Lymphocyte subpopulations and intracellular IFN-γ production were compared by Mann-Whitney test. Regression analysis was used for correlation analysis of lymphocyte subpopulations, with significance determined by Fisher exact test. The Kaplan-Meier method was used for survival analysis. Analysis of covariance (ANCOVA) was used for TREC analysis.
Ethical Considerations
This survey was conducted in compliance with the protocol of Good Clinical Practices and Declaration of Helsinki principles. Patients gave their consent to participate after being orally informed about the study protocol. In accordance with European regulation (Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations, and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use; Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data), observational studies of data obtained without any additional therapy or monitoring procedure in France do not need formal approval of an institutional review board or an independent ethics committee, and formal written consent from patients is not required for this kind of project.
RESULTS
Clinical Manifestations
We prospectively included 59 patients in the study; 19 were secondarily excluded because they did not meet CDC criteria for ICL: 8 had a CD4 count ≥300/mm3, and the other exclusion criteria were sarcoidosis (n = 3), malignant lymphoma (n = 1), multiple myeloma (n = 1), Sjögren syndrome (n = 1), splenomegaly secondary to portal thrombosis (n = 1), anorexia nervosa (n = 1), exudative enteropathy (n = 1), common variable immunodeficiency (CVID) (n = 1), and tuberculosis (n = 1). We followed 40 patients (24 female; mean age 44.2 ± 12.2 [19–70] years at the time of diagnosis) for a mean of 6.9 ± 6.7 (0.14–24.3) years. Patient 21 had moderate hypogammaglobulinemia and decreased level of IgG but did not fulfill criteria for CVID. Patients’ clinical characteristics at the time of diagnosis are detailed in Table 1. Eleven patients received trimethoprim sulfamethoxazole prophylaxis.
TABLE 1.
Baseline Characteristics of 40 Patients With Idiopathic CD4 Lymphocytopenia (ICL)
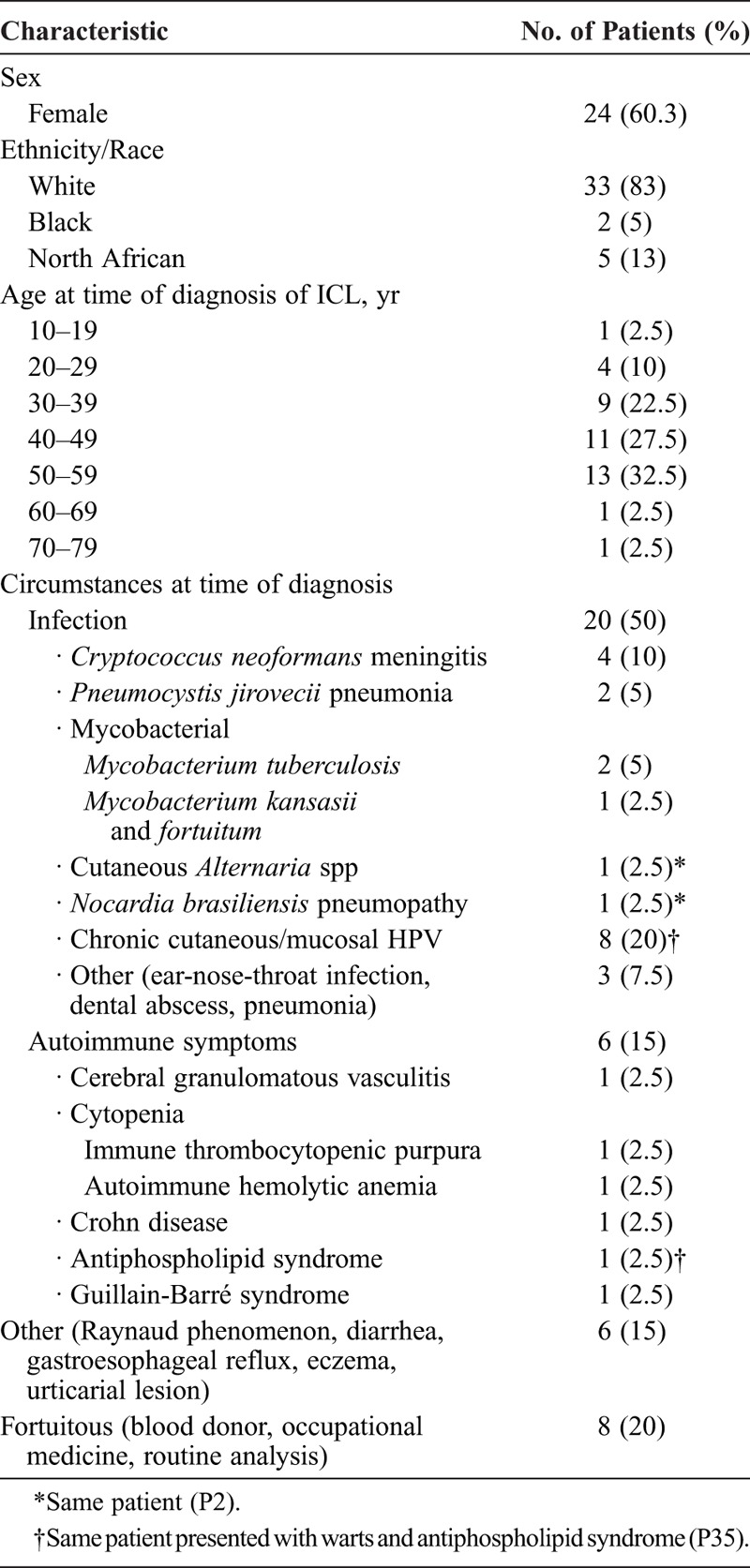
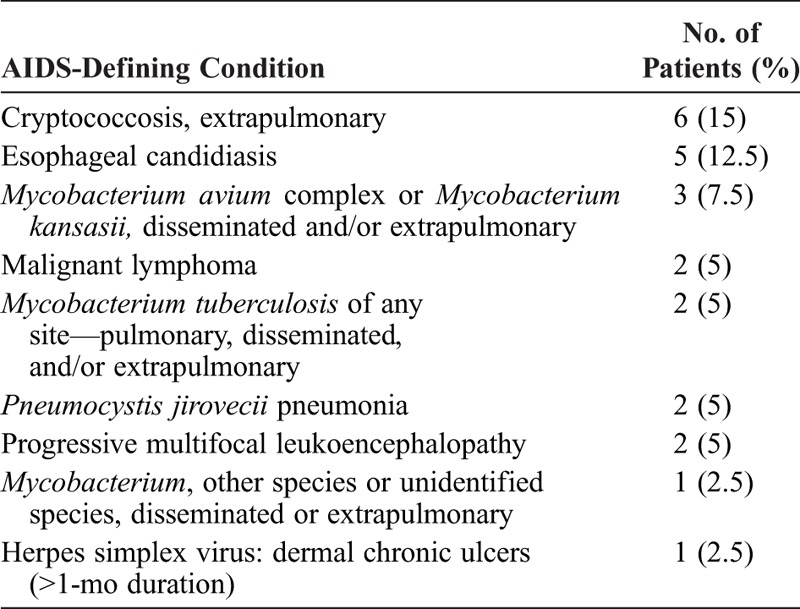
Infections developed in 25 patients at diagnosis or during follow-up. In total, 12 patients had extensive human papillomavirus (HPV)-related infection, including warts (n = 5), condyloma (n = 3), Bowenoid papulomatosis (n = 3), pseudo-epidermodysplasia verruciformis (n = 4), and anogenital dysplasia (n = 2), and 2 patients had molluscum contagiosum (n = 2). Cryptococcus neoformans infection was documented in 6 patients: all had evidence of meningitis and 1 (P1) underwent surgery for excavated pneumonia related to cryptococcal infection. At diagnosis or during follow-up, 14 patients had acquired immunodeficiency syndrome (AIDS)-defining conditions (Table 2). Other opportunistic infections were recurrent Alternaria species and Nocardia brasiliensis infections.
TABLE 2.
AIDS-Defining Condition at Time of Diagnosis or During Follow-Up in 40 Patients With ICL
At diagnosis or during follow-up (Table 3), 14 patients had autoimmune and/or inflammatory manifestations, including immune thrombocytopenic purpura (ITP) (n = 5), autoimmune hemolytic anemia (AIHA) (n = 3), central nervous system vasculitis (n = 1), Goodpasture syndrome (n = 1), grade II duodenal villous atrophy (n = 1), Crohn disease (n = 1), antiphospholipid syndrome (n = 1), seronegative polyarthritis (n = 1), vitiligo (n = 1), Guillain-Barre syndrome (n = 1) and Hashimoto thyroiditis (n = 1). These manifestations were not related to therapy except for the AIHA and Goodpasture syndrome of P26, which appeared during and 3 years after IL-2 therapy, respectively.
TABLE 3.
Clinical Manifestations During Long-Term Follow-Up of 40 Patients With ICL
We observed 2 cases of malignant lymphoma (null phenotype lymphoma and non-Hodgkin lymphoma) and 3 cases of solid tumor. One patient had multicentric Castleman disease and pulmonary arterial hypertension. Three patients had cirrhosis of unknown etiology.
Overall, 8 patients had mild or no symptoms. Eight patients had infections and autoimmunity, 4 patients had infections and malignancy. No patient had autoimmunity and malignancy, and no patient had infectious, autoimmune, and malignant manifestations (Figure 1A).
FIGURE 1.
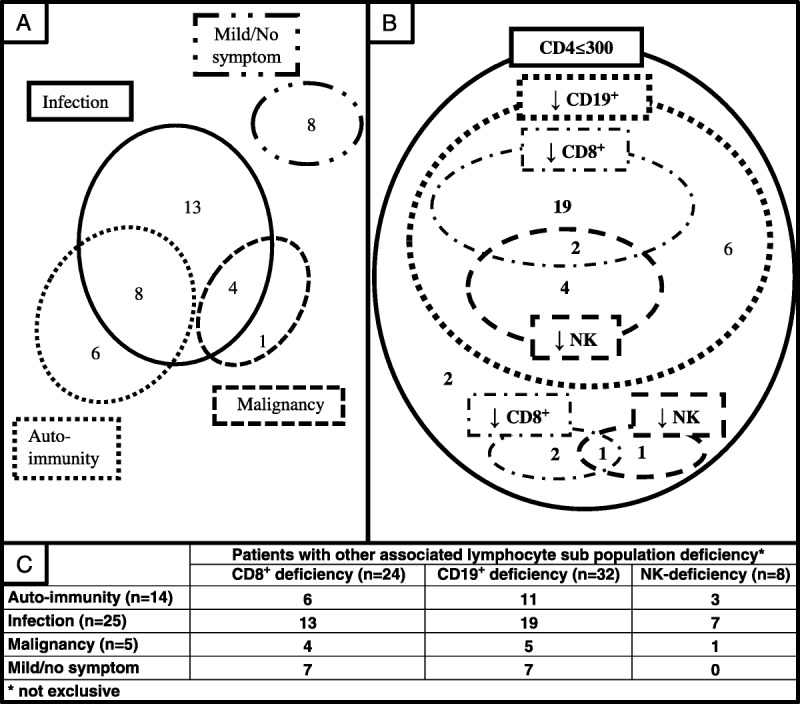
Biological heterogeneity of patients with idiopathic CD4 lymphocytopenia (ICL) at diagnosis and clinical manifestations during follow-up. A. Classification of patients with ICL by main clinical manifestations (n = 40); (B) lymphocyte subpopulation deficiencies (n = 37); and (C) clinical manifestation according to subpopulation-associated cell deficiency. CD19, CD8, and NK cell counts were available for only 37 patients at diagnosis.
Immunologic Characteristics
At the time of diagnosis, mean CD4 count was 127/mm3 (4-294). Mean CD8, CD19, and NK cell counts were 236/mm3 (1-1293), 113/mm3 (3-547), and 122/mm3 (5-416), respectively (Table 4). In total, 24/40 patients had CD8 T-cell deficiency, 32/38 had CD19 B-cell deficiency, and 8/39 had CD3-CD16+CD56+ NK cell deficiency (Figure 1B). Absolute CD4, CD19, CD8, and NK cell counts were positively correlated with severity of lymphocytopenia (Figure 2A). Proportions of each lymphocyte population were modified by severity of lymphocytopenia: that of CD8 T cells decreased with the severity of lymphocytopenia (p = 0.01). Residual CD4 T cells showed increased expression of markers of activation (HLA-DR+) and memory (CD45RO+) (Figure 2B) and reduced expression of a naive marker (CD45RA+) (data not shown).
TABLE 4.
Detailed Immunologic Data for 40 Patients With ICL, at Time of Diagnosis
FIGURE 2.
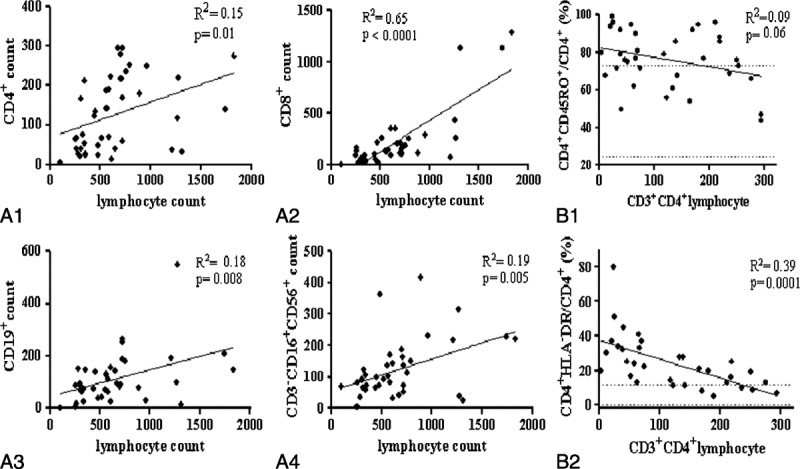
Descriptive analysis of lymphocyte populations and subpopulations. Positive correlations of CD4 (A1), CD8 (A2), CD19 (A3), and CD3-CD16+CD56+ NK (A4) counts and lymphocyte count. Negative correlations of CD45RO+ memory (B1) and HLA-DR+ –activated (B2) residual CD4 lymphocytes and CD4 lymphocytopenia severity. Dashed lines represent normal values ± 2 SDs. Abbreviations: R2 = goodness-of-fit of linear regression, p = probability that slope is null.
TABLE 4.
No caption available.
T-cell proliferation in the presence of mitogens was normal after stimulation with anti-CD3 antibody in 18/38 patients. On co-stimulation with anti-CD28 antibody or exogenous IL-2, proliferation was normal in 23/38 and 20/36 patients, respectively (Figure 3). B-cell proliferation was normal in 19/31 patients. Proliferation induced by CMV, streptococcal enzyme, tuberculin, and candidin antigens was defective in 25/34, 28/34, 26/35, and 17/27 patients, respectively (see Figure 3). We note that patients with infections showed significantly lower response to tuberculin-induced proliferation (p = 0.0008).
FIGURE 3.
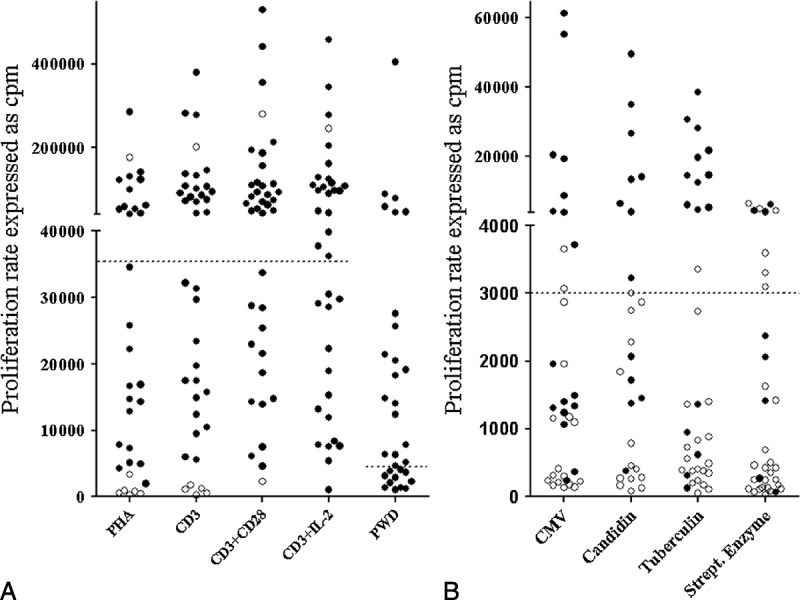
A. T-lymphocyte proliferation induced by mitogens (PHA, CD3, CD3+CD28, CD3+IL-2, PWD). B. T-lymphocyte proliferation induced by antigens (CMV, candidin, tuberculin, streptococcal enzyme). A positive test result is defined as both thymidine incorporation (cpm) above threshold (dashed line) and a positive stimulation index (SI). Positive and negative SIs are represented by black (positive) and open (negative) dots. Abbreviations: CMV = cytomegalovirus, cpm = count per minute, PHA = phytohemagglutinin, PWD = pokeweed.
Follow-Up and Prognosis
Patients with infection manifestations during follow-up had a significantly lower NK cell count at diagnosis; patients with autoimmune symptoms had significantly higher CD3+, CD8+, and CD8+CD45RO+ T-cell counts; and patients with malignancy had low CD4+CD45RA+ and CD8+CD45RO+ T-cell counts (Table 5). Six patients (15%) died during follow-up. The causes of death were cerebral infection with Mycobacterium tuberculosis identified on autopsy, misdiagnosed as toxoplasmosis (P3); anaplastic lymphoma (null phenotype) (P10); pancreatic adenocarcinoma (P14); epidermoid carcinoma of the cheek (P20); respiratory failure related to pulmonary arterial hypertension (P4); and multiorgan failure due to septic shock related to Escherichia coli infection (P32). We identified initial CD4 T-cell count <150/mm3 and low NK cell count (<100/mm3) or a low NK cell count (<100/mm3) as prognostic markers of increased mortality (Figure 4).
TABLE 5.
Main Clinical Manifestations During Follow-Up (Infection, Autoimmunity, Malignancy) According to Initial Lymphocytic Features for 40 Patients With ICL
FIGURE 4.
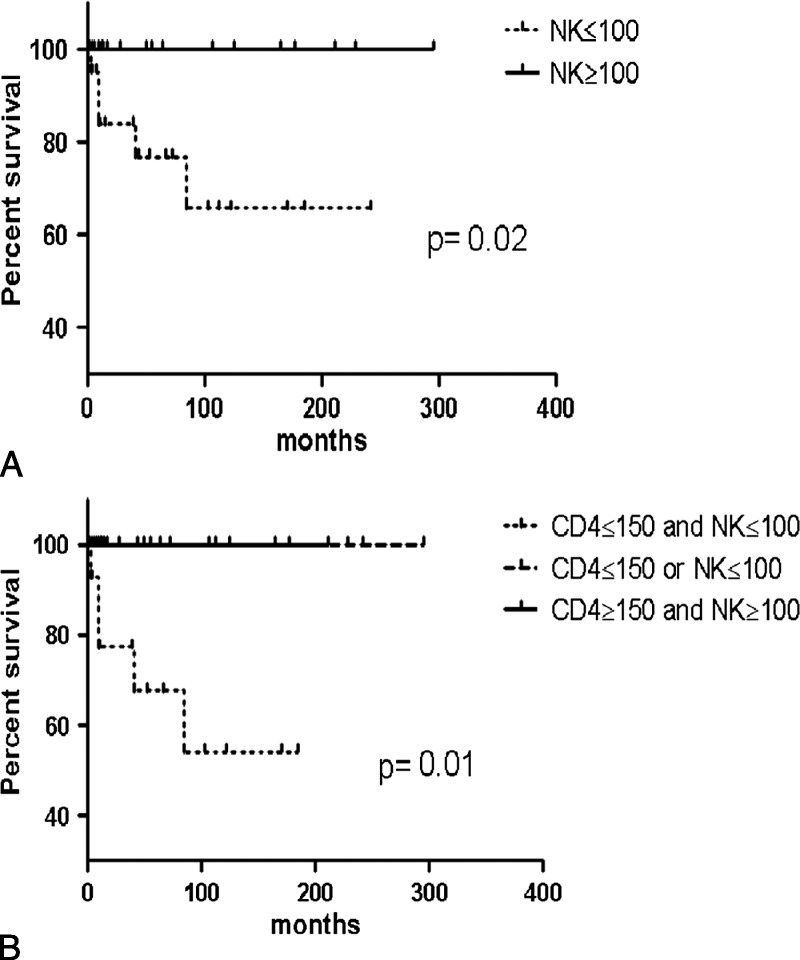
Survival in 40 patients with ICL by lymphocyte subpopulation counts. Kaplan-Meier survival curves by (A) initial NK cell count and (B) combined CD4 T-lymphocyte and NK cell count.
In the current study, 6 patients (P1, P2, P10, P13, P26, P29) received recombinant IL-2 treatment. CD4 T-lymphocyte count was increased, but remained below normal values in 4 of these patients. Treatment was stopped in 2 patients because of injection intolerance (P29) and AIHA 6 months after treatment initiation (P26). Treatment was stopped for lack of efficacy in P2, who died from a null-phenotype lymphoma. For patients with response to recombinant IL-2 treatment, the treatment was stopped 3 (P13) and 5 (P1) years later, without any new opportunistic infection. Response to treatment was sustained after IL2 treatment interruption, but remained under normal values in 1 patient (P13).
TRECs Analysis
Eighteen ICL patients and 34 healthy controls were investigated for TRECs in peripheral blood. It is noteworthy that sjTREC frequency (sjTREC/PBMC) was identical in ICL patients and healthy controls (data not shown). However, when adjusted to naive T-cell counts, the sjTREC frequency (sjTRECs/105 naive T-cells) was higher in ICL patients than in healthy controls (p < 0.0001; ANCOVA) (Figure 5A). Finally, patients with ICL had higher sj/βTREC ratio than controls (p = 0.01; ANCOVA) (Figure 5B). Taken together, these results suggest that in the investigated ICL patients, thymic function is supra normal as compared to age-matched healthy individuals.
FIGURE 5.
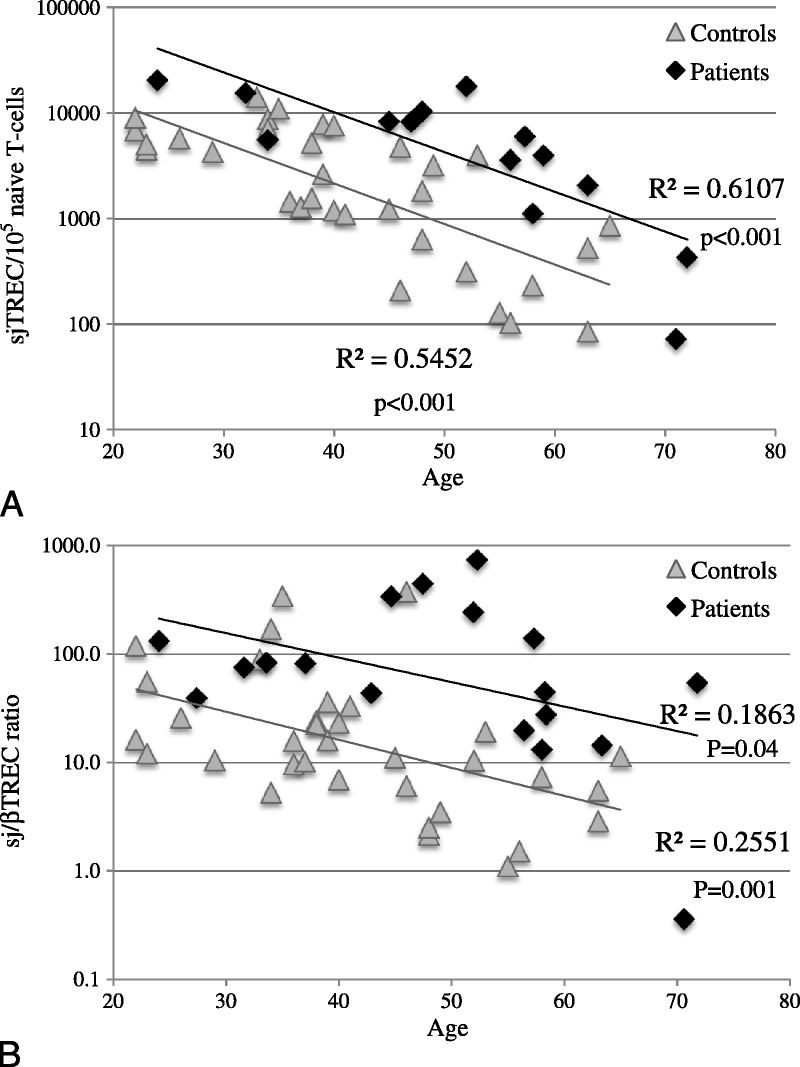
T-cell rearrangement excision circles (TRECs) in patients with ICL and healthy control individuals. Quantification of (A) sjTRECs/105 naive T cells and (B) sj/βTREC ratio in patients with ICL and healthy controls. Abbreviations: R2 = Spearman correlation, p = associated probability.
IFN-γ Release Assay by NK Cells
Nine ICL patients and 9 healthy controls underwent NK cell function analysis by IFN-γ release after treatment with IL-12 and IL-18. The frequency of IFN-γ–producing NK cells did not significantly differ between ICL patients and healthy controls, with a median of 23.4% (11.6%–41.0%) and 31.5% (13.2%–48.8%), respectively (p < 0.113) (Figure 6).
FIGURE 6.
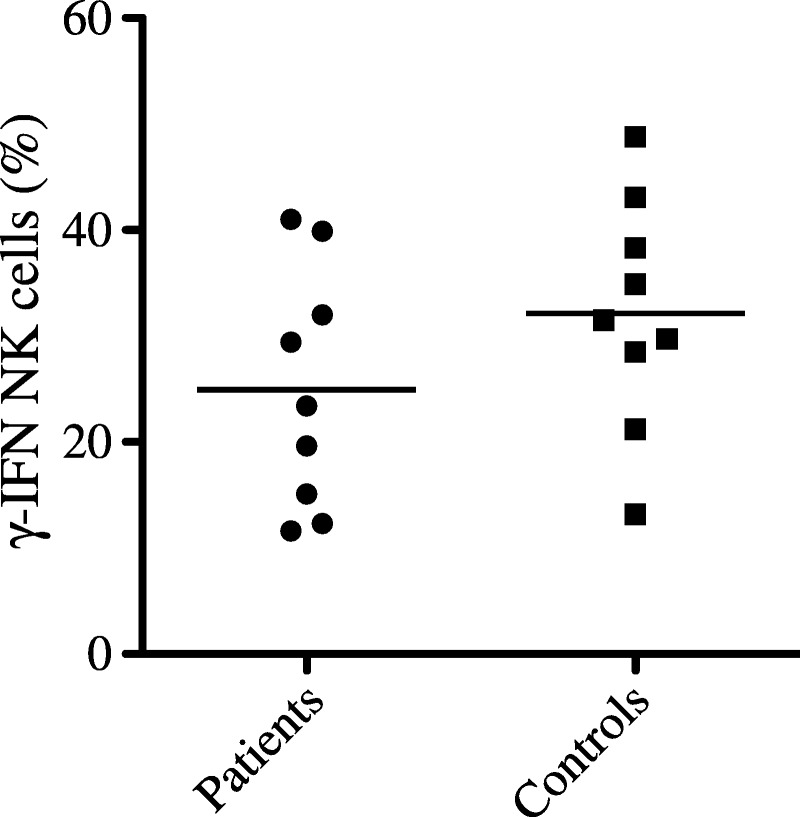
IFN-γ production by NK cells from patients with ICL and healthy control individuals. Peripheral blood mononuclear cells were collected from ICL patients and healthy donors to determine the proportion of IFN-γ–producing NK cells after overnight treatment with IL-12 and IL-18. Samples were gated on CD3-CD56+ NK cells. Horizontal bars indicate the mean.
DISCUSSION
We report the clinical and immunologic characteristics and long-term follow-up of 40 patients with ICL in a French multicenter cohort. Patients received a diagnosis of ICL after other etiologies of CD4 lymphocytopenia were excluded.
In our series, patients had opportunistic infections that have already been described in the setting of ICL, including Cryptococcus neoformans meningitis,34 Pneumocystis jirovecii pneumonia,20 and mycobacterial26 and JC virus15 infection. However, to our knowledge, Nocardia infection was previously reported in only 2 patients with ICL,11,28 and cutaneous Alternaria species infection has never been described. Although ICL patients have been reported with HPV infection,2 the follow-up of these patients revealed that mucocutaneous lesions due to HPV were common in ICL and can be responsible for neoplasms and/or require surgical treatment. Thus, the true prevalence of HPV infection in ICL patients may be underestimated, and these patients should be carefully examined to detect anogenital lesions.
Autoimmune symptoms are common in patients with ICL, as in other primary or secondary immunodeficiencies.4 We found autoimmune cytopenia in 6 of 40 patients (15%), which represents the most common autoimmune symptom. However, to our knowledge, central nervous system vasculitides and Goodpasture syndrome have not been previously reported in ICL. The disruption of immune tolerance remains of unknown origin. Patients show a relative increase in activated CD25+CD4+ or HLA-DR+CD4+ T cells. This hyperactivated state could result from CD4 T lymphocytopenia (inverse correlation of HLA-DR+CD4 T lymphocytes and CD4 T cells) and could be responsible for autoimmune manifestations.
During follow-up, 6 patients died (15%). The prognosis has substantially improved since the first report of 38.3% mortality by Smith et al in 1993.24 In the current series, death was related to immunodeficiency in all patients. We identified initial CD4 T-cell count ≤150/mm3 and NK cell count ≤100/mm3 or a low NK cell count (<100/mm3) as prognostic markers of death. However, the function of NK cells, when investigated, was preserved in patients with ICL. HLA-DR expression in CD4 T cells was associated with poor prognosis in a study by Zonios et al,33 probably because activation markers are associated with lymphocytopenia. Among our 40 patients, 4/25 with a CD8 count ≤180/mm3 and 2/15 with a CD8 count >180/mm3 died. Thus, we do not confirm that a CD8 count ≤180/mm3 can be considered a factor of poor prognosis.33 However, in the study by Zonios et al, CD8 T-cell count was a predictor of opportunistic-related death and not all-cause death. Although not statistically significant, 4 of 5 patients who died had a negative LPA in the presence of antigens. Thus, LPA negativity may be a prognostic marker in ICL, although this result needs to be confirmed in larger studies. Of our 40 patients, 5 (12.5%) remained free of symptoms during a mean follow-up of 6.9 ± 6.7 years. Although fortuitous detection of asymptomatic patients with ICL has been reported,1,3,6 probably only a minority of patients remain asymptomatic. In addition, at the end of follow-up, all but 1 of the patients with available CD4 T-cell counts showed persistent CD4 T-cell counts, as was previously reported.33,34
On univariate analysis, we identified a link between CD45RA+CD4+, CD45RA+CD8+, CD8, and NK cell counts and clinical manifestations. However, the level of significance was low, and the results need to be confirmed in further studies.
In the current study, 6 patients were treated with IL-2 with various outcomes. In a previous series of 6 patients with ICL and impaired expression of CXCR4 in CD4 T lymphocytes, 4 received recombinant IL-2 (including P1, P2, and P13 in the current study) and for 3 of these, the impaired CXCR4 expression was normalized. In our study, the only patient showing no response to IL-2 treatment in vivo did not show improved LPA results in the presence of IL-2 (P2), whereas other patients with response to IL-2 treatment in vivo showed improved LPA results in the presence of IL-2. Previously, 7 other patients were successfully treated with recombinant IL-2 (± IFN-γ).5,22,26,27,29–31 Thus, patients with ICL and severe manifestations may benefit from this treatment, even though an increase in lymphocyte count is inconsistent.22 Further studies are needed to evaluate the efficacy of this treatment in patients with ICL.
In the present study, at the time of diagnosis, mean CD4 T-cell count was 127/mm3 (4-294), and 35/37 patients with available lymphocyte subpopulation counts had associated CD8, CD19, and/or NK cell deficiency. The association of deficiency in CD4 T and B lymphocytes or NK cells has been reported.33 Our data confirm the data from the literature showing that in most cases, CD4 T-lymphocyte deficiency is not isolated in patients with ICL, and that probably the designation of this syndrome is not optimal. Thus, we propose to stratify patients according to associated cell deficiency even though larger studies are needed to better identify homogenous subgroups of patients and characterize the underlying molecular mechanism. Recent data reveal that mutations in nunc119,12 MAGT1,18 and Rag16 and defects in CXCL12–CXCR4 signaling and trafficking could be responsible for ICL.22 Results of the current study support the idea that the mechanism(s) underlying ICL might also cause deficiencies in T cells, B cells, and/or NK cells. The results of TREC analysis suggest an increased proliferation of both intrathymic precursor T cells and peripheral naive T cells in ICL patients. These compensatory proliferations should lead to increased naive T-cell counts through both enhanced thymic output and naive T-cell cycling. This is, however, insufficient to maintain normal peripheral T-cell counts, suggesting an accelerated maturation of recent thymic emigrant with increased peripheral turn-over.
In conclusion, 35% of our patients with ICL had autoimmune manifestations. Opportunistic infections were common, with one-third exhibiting HPV-related clinical manifestations. In these patients, CD4 T lymphocytopenia was often associated with CD8, CD19, and/or NK cell deficiency. The outcome has improved since ICL was first described. We found that a CD4 T-cell count <150/mm3 and low NK cell count (<100/mm3) or a low NK cell count (<100/mm3) may represent a poor prognosis. Larger studies are needed to better identify the patients who might benefit from IL-2 therapy.
Acknowledgments
The authors thank the members of the French Idiopathic CD4 Lymphocytopenia Study Group for including patients, and thank the patients who participated in the study.
Footnotes
Financial support and conflicts of interest: This study was funded by a grant from the Direction de la Recherche Clinique et du Développement of the Assistance Publique-Hôpitaux de Paris. The authors listed below have received financial support (personal or institutional) from the listed pharmaceutical or medical device companies, unrelated to the present work. GC: MSD France; ND: Janssen, Boerhinger, Expanscience, Roche; LG: Actelion, Roche, LFB Biotechnologies, CSL Behring; LM: LFB Biotechnologies, CSL Behring, Pfizer, Actelion, Lilly, Roche.
*The French Idiopathic CD4 T Lymphocytopenia Study Group: Lassoued K, Schmit JL, Amiens; Gaillat J, Annecy; Krivitzky A, Saadoun D, Avicenne hospital, Bobigny; El Guedj M, Cayenne; Berthou C, Brest; Fior R, Clamart; Boibieux A, La Croix Rousse hospital, Lyon; Bernard L, Montpellier; Dupin N, Mouthon L, Pelisse N, Régent A, Cochin hospital, Paris; Lortholary O, Necker hospital, Paris; Boissonas A, Paul Brousse hospital, Paris; Amoura Z, Autran B, Carcelin G, Darras-Jolly C, Debré P, Hubert P, Leblond V, Malloum K, Vidailhet M, Pitié-Salpêtrière hospital, Paris; Brouet JC, Clauvel JP, Fermand JP, Fieschi C, Galicier L; Morel P, Oksenhendler E, Saint-Louis hospital, Paris; Blum L, Pontoise; Vallantin X, Perpignan; Bestel B, Saint-Quentin; Maigre M, Saumur, Cartron G, Tours.
REFERENCES
- 1.Aledort LM, Operskalski EA, Dietrich SL, Koerper MA, Gjerset GF, Lusher JM, Lian EC, Mosley JW. Low CD4+ counts in a study of transfusion safety. The Transfusion Safety Study Group. N Engl J Med. 1993;328:441–442. [DOI] [PubMed] [Google Scholar]
- 2.Alisjahbana B, Dinata R, Sutedja E, Suryahudaya I, Soedjana H, Hidajat NN, Soetikno RD, Oktaliansah E, Deng A, Rady P, Tyring S, Gaspari AA. Disfiguring generalized verrucosis in an Indonesian man with idiopathic CD4 lymphopenia. Arch Dermatol. 2010;146:69–73. [DOI] [PubMed] [Google Scholar]
- 3.Busch MP, Valinsky JE, Paglieroni T, Prince HE, Crutcher GJ, Gjerset GF, Operskalski EA, Charlebois E, Bianco C, Holland PV, et al. Screening of blood donors for idiopathic CD4+ T-lymphocytopenia. Transfusion. 1994;34:192–197. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham-Rundles C, Murray HW, Smith JP. Treatment of idiopathic CD4 T lymphocytopenia with IL-2. Clin Exp Immunol. 1999;116:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeHovitz JA, Feldman J, Landesman S. Idiopathic CD4+ T-lymphocytopenia. N Engl J Med. 1993;329:1045–1046. [DOI] [PubMed] [Google Scholar]
- 7.Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, Dalloul A, Boulassel MR, Debre P, Routy JP, Grossman Z, Sekaly RP, Cheynier R. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. [DOI] [PubMed] [Google Scholar]
- 8.Dion ML, Sekaly RP, Cheynier R. Estimating thymic function through quantification of T-cell receptor excision circles. Methods Mol Biol. 2007;380:197–213. [DOI] [PubMed] [Google Scholar]
- 9.Duncan RA, von Reyn CF, Alliegro GM, Toossi Z, Sugar AM, Levitz SM. Idiopathic CD4+ T-lymphocytopenia—four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393–398. [DOI] [PubMed] [Google Scholar]
- 10.Fauci AS. CD4+ T-lymphocytopenia without HIV infection—no lights, no camera, just facts. N Engl J Med. 1993;328:429–431. [DOI] [PubMed] [Google Scholar]
- 11.Goktay F, Mansur AT, Ersahin M, Adaleti R, Gunes P. Idiopathic CD4+ T lymphocytopenia with epidermodysplasia verruciformis-like skin eruption, Nocardia farcinica brain abscesses and pulmonary tuberculosis: a case report with fatal outcome. J Dermatol. 2011;38:930–933. [PubMed] [Google Scholar]
- 12.Gorska MM, Alam R. A mutation in the human Uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood. 2012;119:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho DD, Cao Y, Zhu T, Farthing C, Wang N, Gu G, Schooley RT, Daar ES. Idiopathic CD4+ T-lymphocytopenia—immunodeficiency without evidence of HIV infection. N Engl J Med. 1993;328:380–385. [DOI] [PubMed] [Google Scholar]
- 14.Hubert P, Bergeron F, Ferreira V, Seligmann M, Oksenhendler E, Debre P, Autran B. Defective p56Lck activity in T cells from an adult patient with idiopathic CD4+ lymphocytopenia. Int Immunol. 2000;12:449–457. [DOI] [PubMed] [Google Scholar]
- 15.Iwase T, Ojika K, Katada E, Mitake S, Nakazawa H, Matsukawa N, Otsuka Y, Tsugu Y, Kanai H, Nakajima K. An unusual course of progressive multifocal leukoencephalopathy in a patient with idiopathic CD4+ T lymphocytopenia. J Neurol Neurosurg Psychiatry. 1998;64:788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijpers TW, Ijspeert H, van Leeuwen EM, Jansen MH, Hazenberg MD, Weijer KC, van Lier RA, van der Burg M. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood. 2011;117:5892–5896. [DOI] [PubMed] [Google Scholar]
- 17.Laurence J, Mitra D, Steiner M, Lynch DH, Siegal FP, Staiano-Coico L. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J Clin Invest. 1996;97:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallet V, Nicand E, Sultanik P, Chakvetadze C, Tesse S, Thervet E, Mouthon L, Sogni P, Pol S. Brief communication: case reports of ribavirin treatment for chronic hepatitis E. Ann Intern Med. 2010;153:85–89. [DOI] [PubMed] [Google Scholar]
- 20.Matsuyama W, Tsurukawa T, Iwami F, Wakimoto J, Mizoguchi A, Kawabata M, Osame M. Two cases of idiopathic CD4+ T-lymphocytopenia in elderly patients. Intern Med. 1998;37:891–895. [DOI] [PubMed] [Google Scholar]
- 21.Roger PM, Bernard-Pomier G, Counillon E, Breittmayer JP, Bernard A, Dellamonica P. Overexpression of Fas/CD95 and Fas-induced apoptosis in a patient with idiopathic CD4+ T lymphocytopenia. Clin Infect Dis. 1999;28:1012–1016. [DOI] [PubMed] [Google Scholar]
- 22.Scott-Algara D, Balabanian K, Chakrabarti LA, Mouthon L, Dromer F, Didier C, Arenzana-Seisdedos F, Lortholary O. Idiopathic CD4+ T-cell lymphocytopenia is associated with impaired membrane expression of the chemokine receptor CXCR4. Blood. 2010;115:3708–3717. [DOI] [PubMed] [Google Scholar]
- 23.Seligmann M, Autran B, Rabian C, Ferchal F, Olive D, Echard M, Oksenhendler E. Profound and possibly primary “idiopathic CD4+ T lymphocytopenia” in a patient with fungal infections. Clin Immunol Immunopathol. 1994;71:203–207. [DOI] [PubMed] [Google Scholar]
- 24.Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. An investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373–379. [DOI] [PubMed] [Google Scholar]
- 25.Spira TJ, Jones BM, Nicholson JK, Lal RB, Rowe T, Mawle AC, Lauter CB, Shulman JA, Monson RA. Idiopathic CD4+ T-lymphocytopenia—an analysis of five patients with unexplained opportunistic infections. N Engl J Med. 1993;328:386–392. [DOI] [PubMed] [Google Scholar]
- 26.Sternfeld T, Nigg A, Belohradsky BH, Bogner JR. Treatment of relapsing Mycobacterium avium infection with interferon-gamma and interleukin-2 in an HIV-negative patient with low CD4 syndrome. Int J Infect Dis. 2010;14(Suppl 3):e198–201. [DOI] [PubMed] [Google Scholar]
- 27.Trojan T, Collins R, Khan DA. Safety and efficacy of treatment using interleukin-2 in a patient with idiopathic CD4(+) lymphopenia and Mycobacterium avium-intracellulare. Clin Exp Immunol. 2009;156:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venzor J, Hua Q, Bressler RB, Miranda CH, Huston DP. Behcet’s-like syndrome associated with idiopathic CD4+ T-lymphocytopenia, opportunistic infections, and a large population of TCR alpha beta+ CD4- CD8- T cells. Am J Med Sci. 1997;313:236–238. [DOI] [PubMed] [Google Scholar]
- 29.Warnatz K, Draeger R, Schlesier M, Peter HH. Successful IL-2 therapy for relapsing herpes zoster infection in a patient with idiopathic CD4+ T lymphocytopenia. Immunobiology. 2000;202:204–211. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm M, Weissinger F, Kunzmann V, Muller JG, Fahey JL. Idiopathic CD4+ T cell lymphocytopenia evolving to monoclonal immunoglobulins and progressive renal damage responsive to IL-2 therapy. Clin Immunol. 2001;99:298–304. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz-Demirdag Y, Wilson B, Lowery-Nordberg M, Bocchini JA, Jr, Bahna SL. Interleukin-2 treatment for persistent cryptococcal meningitis in a child with idiopathic CD4(+) T lymphocytopenia. Allergy Asthma Proc. 2008;29:421–424. [DOI] [PubMed] [Google Scholar]
- 32.Zaharatos GJ, Behr MA, Libman MD. Profound T-lymphocytopenia and cryptococcemia in a human immunodeficiency virus-seronegative patient with disseminated tuberculosis. Clin Infect Dis. 2001;33:E125–128. [DOI] [PubMed] [Google Scholar]
- 33.Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, Adelsberger JW, Metcalf JA, Polis MA, Kovacs SJ, Kovacs JA, Davey RT, Lane HC, Masur H, Sereti I. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zonios DI, Falloon J, Huang CY, Chaitt D, Bennett JE. Cryptococcosis and idiopathic CD4 lymphocytopenia. Medicine (Baltimore). 2007;86:78–92. [DOI] [PubMed] [Google Scholar]


