Abstract
Image-guided tumor ablation for early stage hepatocellular carcinoma (HCC) is an accepted non-surgical treatment that provides excellent local tumor control and favorable survival benefit. This review summarizes the recent advances in tumor ablation for HCC. Diagnostic imaging and molecular biology of HCC has recently undergone marked improvements. Second-generation ultrasonography (US) contrast agents, new computed tomography (CT) techniques, and liver-specific contrast agents for magnetic resonance imaging (MRI) have enabled the early detection of smaller and inconspicuous HCC lesions. Various imaging-guidance tools that incorporate imaging-fusion between real-time US and CT/MRI, that are now common for percutaneous tumor ablation, have increased operator confidence in the accurate targeting of technically difficult tumors. In addition to radiofrequency ablation (RFA), various therapeutic modalities including microwave ablation, irreversible electroporation, and high-intensity focused ultrasound ablation have attracted attention as alternative energy sources for effective locoregional treatment of HCC. In addition, combined treatment with RFA and chemoembolization or molecular agents may be able to overcome the limitation of advanced or large tumors. Finally, understanding of the biological mechanisms and advances in therapy associated with tumor ablation will be important for successful tumor control. All these advances in tumor ablation for HCC will result in significant improvement in the prognosis of HCC patients. In this review, we primarily focus on recent advances in molecular tumor biology, diagnosis, imaging-guidance tools, and therapeutic modalities, and refer to the current status and future perspectives for tumor ablation for HCC.
Key Words: Diagnosis, Hepatocellular carcinoma, Image-guided tumor ablation, Treatment outcome
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide. It is the sixth most common cancer and the third most common cause of cancer-related death [1]. In addition, HCC is the leading cause of death among patients with liver cirrhosis [2]. Its age-adjusted worldwide incidence has been estimated at 16 cases per 100,000 people [3].
For the treatment of HCC, minimally invasive locoregional therapies include radiofrequency ablation (RFA), ethanol injection, microwave ablation, cryoablation, irreversible electroporation (IRE), and high-intensity focused ultrasound (HIFU) ablation. Among these approaches, RFA is now accepted as a curative treatment for very early or early-stage HCC in several international HCC treatment guidelines [4,5,6,7]. In the instance of a patient with a small HCC measuring less than 2 cm in diameter, who is not a potential candidate for liver transplantation, RFA may serve as a first-line therapy alternative to resection, according to the recent Barcelona Clinic Liver Cancer treatment strategy [4]. Furthermore, the advantages of these tumor ablation techniques over invasive surgical resection includes a shorter hospital stay and reduced overall costs, increased preservation of surrounding hepatic tissues, decreased morbidity, and applicability for patients with advanced HCC who are not candidates for surgical resection [8]. Thus, an interventional oncologist should know the current status including recent advances and future challenges in the field of image-guided tumor ablation.
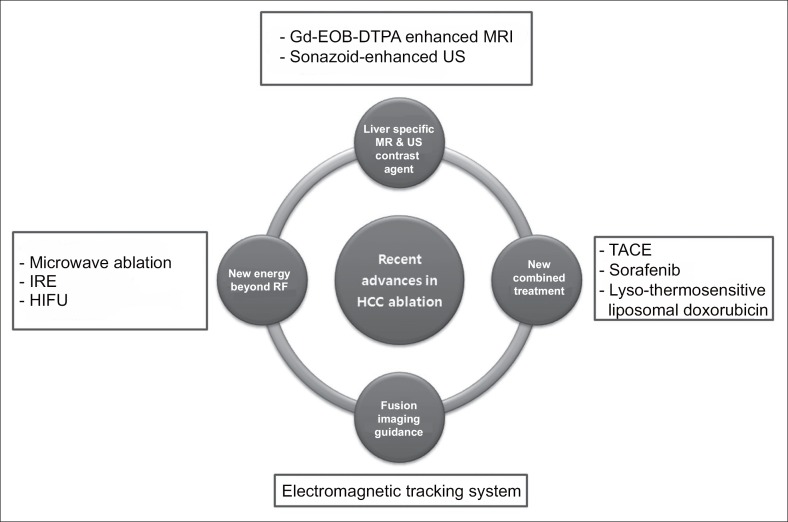
The aims of this review are to summarize the recent advances in ablative therapy for HCC, to discuss the biological mechanism of the ablation-related immune reaction, and to summarize the technical improvements in diagnosis, imaging-guidance tools, and treatment modalities (Fig. 1).
Fig. 1.
Recent advances in HCC ablation treatment.
Advances in Understanding of Biological Mechanisms of Tumor Ablation
RFA
The mechanism of cell death in RFA is based on the frictional heat generated using high-frequency alternating current. Around the ablative zone, heat-induced cell necrosis releases many immunogenic intracellular substrates including heat shock proteins (HSP), uric acid, ribonucleic acids, deoxyribonucleic acids and high mobility group protein B1 [9]. Among these substrates, RFA-induction of HSP70 plays a key role in stimulating the anti-tumor immune response. It involves various immune processes, such as a danger signal to the immune system by activating dendritic cells and an antigen chaperone to antigen-presenting cells [10,11]. In addition, an elevated serum level of HSP70 in patients after RFA correlates with improved survival [12].
Microwave Ablation
Microwave ablation uses electromagnetic (EM) waves to generate heat to thereby kill the tumor by direct hyperthermic injury, similar to the outcome of RFA. However, the induction of proinflammatory cytokines including interleukin-1 and interleukin-6 by microwave ablation is minimal compared with that induced by RFA and cryoablation in an animal model [13]. In humans, there is a significant inverse correlation between survival outcome and the extent of immune cell infiltration in patients treated with microwave ablation for HCC [14].
Cryoablation
In contrast to thermal energy-based ablative therapy, cryoablation induces cytotoxicity based on the Joule-Thomson theory. Low temperatures between −20°C and −40°C are produced within the tumor. There are four mechanisms that lead to cryoablative injury of tumors, which include 1) direct cell injury by cellular dehydration, 2) vascular injury and ischemia induced by endothelial damage to the microvasculature, 3) apoptosis incited by sublethal temperatures, and 4) immunomodulation [15]. Compared with RFA and lazer ablation, cryoablation is most affected by postablative immunogenicity. Some possible explanations suggest that thermal energy-based ablation induces protein denaturation, which in turn may leads to reduced quantities of anti-tumor antigens, and that heat-mediated coagulation of the tissues may prevent egress of intracellular products into the systemic circulation [16]. This unique enhanced anti-tumor immunogenicity produces cryoshock, which is only observed after cryoablation of hepatocytes. Although the rate of this major complication is very rare (up to 1%), it produces a serious systemic inflammatory response syndrome by proinflammatory mediators derived from Kupffer cells [17].
Advances in the Diagnosis of HCC
Technical advances in the diagnostic imaging of HCC during the recent decade include dynamic contrast-enhanced ultrasonography (DCEUS), new computed tomography (CT) techniques, and magnetic resonance imaging (MRI) with liver specific contrast agents. These imaging modalities can provide more accurate imaging information, even for tumor nodules smaller than 1 cm in diameter. The smaller tumors that can be detected are more challenging for the interventional oncologist in accurate targeting during ablation.
Dynamic Contrast-enhanced US
Levovist® (Schering, Berlin, Germany) is a first-generation contrast material used for US [18]. The contrast effect of this agent is based on microbubble-mediated destruction that results from external US exposure with a high mechanical index. DCEUS has been used mainly for the US characterization of hepatic tumors [19]. Various second-generation contrast agents are now available and include microbubbles containing sulfur hexafluoride (SonoVue®; BraccoSpA, Milan, Italy), perflutren protein (Optison®; GE Healthcare, Buckinghamshire, United Kingdom), or perfluorocarbon (Sonazoid®; GE Healthcare, New York City, NY, USA) [20]. All are constructed of a hard encapsulating shell of microbubbles, which provide better detail in the harmonic signals during DCEUS. Among these, Sonazoid® can be taken up by hepatic Kupffer cells, allowing postvascular phase imaging for the detection of HCC beginning 10 minutes after an intravenous injection. Double-contrast US using Sonazoid® (Kupffer and subsequent arterial phase by reinjection) improves the efficiency of HCC screening in high-risk patients [21] (fig. 2). The approach is one of the diagnostic tools for HCC included in the recent consensus-based guidelines established by the Japan Society of Hepatology [22].
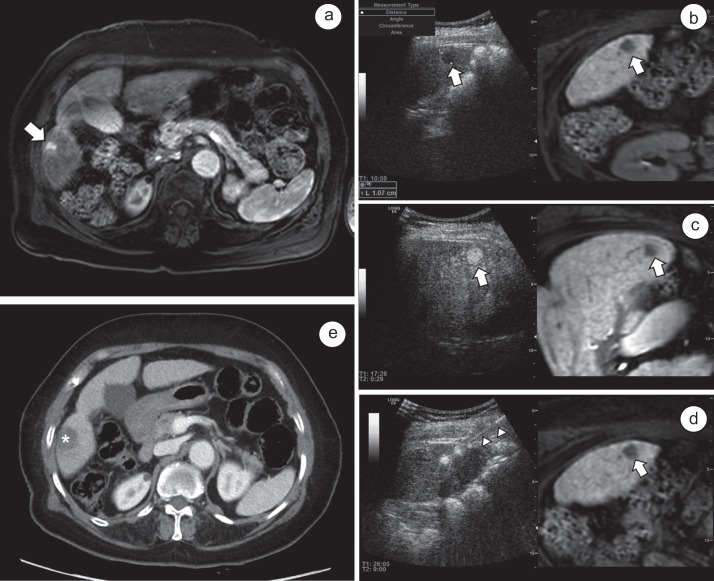
Fig. 2.
Double contrast US using Sonazoid® and imaging fusion US/MRI in RFA for HCC. a Arterial phase on MRI with Gd-EOB-DTPA enhancement shows a 1.1 cm sized-small HCC (white arrow) in segment 5 of the liver. b A contrast-enhance US with Sonazoid® shows the defect (white arrow) in the postvascular phase at 10 minutes after the injection of the contrast medium. c By defect-reperfusion US imaging, reinjection of an additional dose of Sonazoid® can confirm the tumor's hyper-vascularity (white arrow) before needle insertion. d RFA is performed using a 15-gauge electrode with a 2-cm active tip (white arrow heads) for the target tumor (white arrow). e ACT scan obtained immediately following RFA reveals complete tumor ablation with a sufficient tumor ablative margin (asterisk) without immediate major complications.
New CT Techniques
To acquire optimal arterial phase imaging with high spatial resolution is important for detection of small HCCs in patients with cirrhosis. The most important mechanism for the detection of a HCC on CT is by utilizing the property of altered intrinsic vascularity of the tissue, because many HCCs are more vascular than native liver parenchyma, the former which are supplied by tumor-feeding arteries. Therefore, to improve the sensitivity of CT for detecting HCCs, it is necessary to detect a small amount of iodinated contrast medium in the tumor tissue [23].
Recently, low-tube-voltage CT imaging (such as 80-kVp) has been introduced for increasing the photoelectric effect, thereby providing a high contrast to noise ratio for the identification of HCCs [24]. New noise reduction techniques including an adaptive statistical iterative reconstruction algorithm can be compensated for by increasing the overall noise level when utilizing low-tube-voltage CT imaging [25]. In addition, dual-energy CT can make virtual non-contrast images and iodine map images, which are synthesized from dual-energy data sets. It may improve the evaluation of the therapeutic response of transarterial chemoembolization (TACE) in patients with HCC and thereby lead to lower radiation doses by the replacement of a precontrast CT scan [26].
MRI With Liver-Specific Contrast Agents
Unlike previous gadolinium (Gd) chelate contrast agents for MRI, that are excreted mainly by the kidneys, gadobenate dimeglumine (Gd-BOPTA, MultiHance®; Bracco Diagnostics, Milan, Italy) and gadoxetate disodium (Gd-EOB-DTPA; available as Primovist® in Europe and Eovist® in the US through Bayer Healthcare Pharmaceuticals) are eliminated via the hepatobiliary system as hepatocyte-specific contrast agents [27]. They have dual action in dynamic MRI, allowing the evaluation of the hepatobilliary phase (i.e., the transitional phase) as well as the hemodynamics of the tumor on the arterial and portal venous phase, similar to conventional extracellular contrast agents. The enhancement of hepatic lesions during the arterial and portal phases depends on organic anionic transporting polypeptides (OATP) and multidrug resistance associated protein (MRP) expression. In most HCCs, the expression of OATP1B1/B3 is markedly decreased. When the expression of OATP1B1/B3 is maintained in some HCCs, the expression of MRP2 can be high [28]. These cellular mechanisms produce hypointense HCCs compared to the surrounding liver in the hepatobiliary phase [29]. However, 5%-10% of HCCs are iso- or hyperintense relative to the liver [30] and display less aggressiveness in terms of time to tumor recurrence after surgical resection compared to hypointense hepatobiliary phase HCCs [31].
Concerning the diagnostic performance of Gd-EOB-DTPA, a recent study reported a sensitivity of 91%-93% of contrast-enhanced MR with diffusion-weighted imaging for small HCCs (diameter <2 cm) [32]. With respect to the diagnosis of subcentimeter HCCs (diameter <1 cm), diagnostic Gd-EOB-DTPA MRI can be improved by adding hypointensity on the hepatobiliary phase images as ‘washout’ [33]. In a comparison with 64-slice multidetector CT, contrast-enhanced 3.0 Tesla MRI showed a better diagnostic performance for the detection of HCCs with diameters ≤2 cm in patients with chronic liver disease [34].
Advances in Imaging-Guiding Modalities and Assistive Technique for RFA
The accurate placement of an electrode or applicator at the planned site is essential to achieve complete tumor control with assistance of imaging guidance and assistive technique. For smaller tumors that are currently detected using the novel CT/MRI/US modalities, accurate imaging guidance is deemed more important.
Fusing Imaging System For Intervention
Recent technical advances of imaging fusion have enabled the overlay or side-by-side display of real-time US images combined with the established fusion of CT/MRI-acquired images during an interventional procedure [35]. Briefly, the magnetic field generator induces currents in the position sensor, which is mounted in the US transducer. When the US transducer moves, the change in the magnitude of the electrical current in the position sensor is converted into information concerning the transducer location in the US machine.
Applying this technique to tumor ablation enhances the identification of target lesions and the feasibility of the intervention [36,37]. In addition, fusion imaging is more useful for RFA in patients with small HCCs because the detectability of HCCs in planning US for RFA depends on the index tumor size [38]. Fusion imaging with conventional US and liver CT/MRI-acquired images for percutaneous RFA can reduce the chance of mistargeting and it can increase the lesion detectability of small (diameter <2 cm) HCCs [39]. Furthermore, fusion imaging combining conventional US and hepatobiliary phase Gd-EOB-DTPA enhanced MRI is more sensitive than conventional or DCEUS in detecting small or atypical HCCs [40]. However, despite the use of fusion imaging during RFA, mistargeting can occur in patients with a small subcapsular tumor due to liver deformation during breathing [41].
EM Tracking Under US Guidance
To date, three types of EM tracking guiding systems are available for imaging-guided tumor ablation for hepatic lesions under US guidance. A coaxial system using an EM-guiding trocar can target index tumors in the liver by the step-wise insertion of the trocar into the mass, removal of a stylet having a position sensor after proper placement, and insertion of a radiofrequency electrode through the trocar [42]. In the second system, installation of a detachable EM-position sensor at the base of a radiofrequency electrode can provide an expected pathway of the electrode during targeting [43]. Thirdly, a disposable EM-position sensor can be embedded in the distal tip of the radiofrequency electrode. A recent study using a custome-made agar phantom reported that an electrode housing an embedded EM-position sensor resulted in a shorter electrode placement time and a lower electrode pull-back rate for repositioning compared with the conventional electrode in both the “in-plane” and “out-of-plane” approaches [44].
Wider use of these EM tracking systems will allow for faster electrode placement into the target lesions than the conventional method, regardless of operator experience or the type of approach [44,45,46]. In addition, it will reduce the bleeding complications associated with intrahepatic vessel damage resulting from the repeated insertion of a needle and frequent changes in the needle passage during the procedure [47].
Contrast-enhanced US
Like fusion imaging, contrast-enhanced US increases RFA performance in terms of localization and targeting of a tumor. For example, Sonazoid® provides a postvascular phase, which lowers the HCC echogenic defect in the background of well-enhanced normal hepatic parenchyma [48]. This process enhances the contrast between a lesion and the liver for poorly visible tumors using conventional B-mode US. Over 80% of the tumors that remain inconspicuous even with the use of fusion imaging for RFA, can be visualized after Sonazoid® injection [49]. In addition, this approach enables the effective assessment of the early therapeutic response of HCC after RFA [50].
Artificial Material Infusion
A recent meta-analysis demonstrated that the percutaneous approach is the most frequently option used for RFA of hepatic tumors owing to its ease of accessibility and the use of various guiding modalities, such as fluoroscopy, US, CT, and MRI [51]. However, unintended collateral thermal injury during thermal energy-based ablation (when performing a percutaneous procedure) may be inevitable when the index tumor is located close to heat-vulnerable perihepatic structures like the abdominal wall or the diaphragm, gallbladder, or gastrointestinal tract [52]. In addition, hepatic dome lesions can complicate the optimal placement of an electrode. To avoid these problems, artificial material can be percutaneously infused into the peritoneal space to separate the liver from heat-vulnerable structures and to enhance the sonic window. Current popular artificial materials include 0.9% saline, 5% dextrose in water solution [53], thermo-reversible poloxamer [54], and hyaluronic acid gel [55]. However, abdominal adhesion associated with previous hepatic resection can be an obstacle for effective separation between the target tumor and perihepatic structures [56].
Advances in Therapeutic Modalities for Tumor Ablation
RFA
In clinical practice, it was often difficult in the early use of RFA to achieve a sufficient ablative margin for HCCs exceeding 3 cm in diameter [57]. However, a sufficient ablative margin is important in preventing local tumor progression after treatment because microsatellite nodules are more frequently identified in large HCCs compared with small HCCs [58]. Several electrodes with high-power generation have been developed to maximize the ablative performance of RFA; these include perfused [59], clustered, expandable, multi-tined, multipolar [60], monopolar with a multiple-electrode switching system [61], and a multiple switching system [62]. A recent prospective study using a monopolar, multiple-electrode switching system demonstrated excellent local tumor control after RFA for patients with small- and medium-sized HCCs, with a 3-year local tumor progression rate of only 11% [61].
Other Ablation Modalities
A third-generation microwave ablation system incorporating antenna cooling and high-power generation is now commercially available. It can rapidly create larger ablation zones compared with the RFA system. However, many antennae designs feature an elongated ablation zone of up to 6 cm in the longest axis that may increase the risk of burning of the abdominal wall and unintended areas. The recent development of a new antenna for more rounded and forward-weighted heating has enabled the treatment of smaller tumors [63]. In cryoablation, smaller cryoprobe diameters are associated with a lower cooling capacity, which has necessitated cryoprobes having large bore sizes. Now, an effective and thinner cryoprobe (17-gauge) is available in a nitrogen-based device. This system does not need large high-pressure gas tanks.
US-guided HIFU treatment for patients with hepatic metastasis offers effective local tumor control with minimal adverse effects [64]. MR-guided HIFU for liver lesions is still at an early stage due to the limited therapeutic window caused by the thoracic cage and respiratory motion. However, development of motion gating for MR-guided HIFU is being pursued [65]. In addition, oncolytic virus [66] and photodynamic [67] therapy have been introduced as ablative techniques for HCC. IRE is a new ablative technique that uses high-voltage, low-energy direct current to create nanopores in the cell membrane by the passage of electrons through adjacent cells. The Nanoknife® (Angiodynamic, Latham, NY, USA) is commercially available for this purpose. Although the clinical data of IRE in the treatment of HCC are very limited, a recent study reported an 18-month recurrence-free survival for patients with unresectable HCCs treated by IRE [68].
Advances in Combined Treatment with Tumor Ablation
Combination radiation treatment or TACE with RFA have shown promising clinical results in terms of tumor necrosis and disease-free survival compared with radiation treatment or RFA alone [69,70]. A recent meta-analysis reported that the combination of RFA and TACE has advantages in improving the overall survival rate for patients with intermediate and large-sized HCCs, compared to RFA monotherapy [71]. These synergistic effects may be due to the decreased blood flow into the target lesions with the additional elimination of adjacent micrometastasis. The recent development of molecular-targeted agents in the treatment for HCC has prompted synergies between locoregional tumor ablation and systemic chemotherapy. RFA combined with sorafenib, a vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor inhibitor, produces a therapeutic effect that is superior to RFA alone for small (diameter ≤3 cm) HCCs [72]. Sorafenib also decreases the level of hypoxia inducible factor-1α and VEGF A expression, which normally promote the progression of residual tumors after RFA [73]. There are also several new potential molecular target agents (e.g., lenvatinib, tivantinib, etc) for patients with HCC [74,75]. However, the exact role of these agents in combination treatment with tumor ablation warrants further evaluation, as in the recently completed STORM trial (NCT00692770), which failed to demonstrate an advantage of using sorafenib in preventing recurrence after local ablation or surgical resection. Targeted hyperthermia with RFA in combination with lyso-thermosensitive liposomal doxorubicin has been explored in a randomized controlled trial with the goal of improving effective drug delivery [76].
Conclusions
Randomized controlled trials in patients with resectable HCCs are problematic because it may be unethical to conduct head-to-head comparisons between tumor ablation and surgical resection. Nonetheless, the available clinical data indicates that recent advances in minimally invasive image-guided tumor ablation have been important in the management of early stage HCC. The full potential of tumor ablation for HCC as a locoregional treatment remains to be established. It is already clear that these therapeutic techniques with their technical advances, provide effective treatment outcomes with fewer complications in patients with various stages of HCC that are otherwise unattainable with other treatment options including TACE, surgical resection, target agent, and radiation therapy alone. Ultimately, customized combinations of other adjuvant treatments with the most appropriate ablation modality should prove important in optimizing clinical outcomes.
Conflicts of Interest
The authors report no potential conflicts of interest.
Financial support
This study was supported by a grant from Samsung Medical Center [GFO1130071].
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.European Association For The Study Of The Liver European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis. 2014;32:764–777. doi: 10.1159/000368020. [DOI] [PubMed] [Google Scholar]
- 7.Lin SM. Local ablation for hepatocellular carcinoma in taiwan. Liver Cancer. 2013;2:73–83. doi: 10.1159/000343843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol. 2007;17:2062–2070. doi: 10.1007/s00330-007-0587-0. [DOI] [PubMed] [Google Scholar]
- 9.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, Eiz-Vesper B. Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood. 2009;113:3008–3016. doi: 10.1182/blood-2008-06-162727. [DOI] [PubMed] [Google Scholar]
- 11.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haen SP, Gouttefangeas C, Schmidt D, Boss A, Clasen S, von Herbay A, Kosan B, Aebert H, Pereira PL, Rammensee HG. Elevated serum levels of heat shock protein 70 can be detected after radiofrequency ablation. Cell Stress Chaperones. 2011;16:495–504. doi: 10.1007/s12192-011-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad F, Gravante G, Bhardwaj N, Strickland A, Basit R, West K, Sorge R, Dennison AR, Lloyd DM. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg. 2010;200:500–506. doi: 10.1016/j.amjsurg.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Dong BW, Zhang J, Liang P, Yu XL, Su L, Yu DJ, Ji XL, Yu G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia. 2003;19:119–133. doi: 10.1080/0265673021000017154. [DOI] [PubMed] [Google Scholar]
- 15.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 16.Jansen MC, van Hillegersberg R, Schoots IG, Levi M, Beek JF, Crezee H, van Gulik TM. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery. 2010;147:686–695. doi: 10.1016/j.surg.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Chapman WC, Debelak JP, Blackwell TS, Gainer KA, Christman JW, Pinson CW, Brigham KL, Parker RE. Hepatic cryoablation-induced acute lung injury: Pulmonary hemodynamic and permeability effects in a sheep model. Arch Surg. 2000;135:667–672. doi: 10.1001/archsurg.135.6.667. discussion 672-663. [DOI] [PubMed] [Google Scholar]
- 18.Schlief R, Schurman R, Niendorf HP. Basic properties and results of clinical trials of ultrasound contrast agents based on galactose. Ann Acad Med Singapore. 1993;22:762–767. [PubMed] [Google Scholar]
- 19.Wen YL, Kudo M, Zheng RQ, Ding H, Zhou P, Minami Y, Chung H, Kitano M, Kawasaki T, Maekawa K. Characterization of hepatic tumors: value of contrast-enhanced coded phase-inversion harmonic angio. AJR Am J Roentgenol. 2004;182:1019–1026. doi: 10.2214/ajr.182.4.1821019. [DOI] [PubMed] [Google Scholar]
- 20.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200–206. doi: 10.1016/s0720-048x(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Hatanaka K, Kumada T, Toyoda H, Tada T. Double-contrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol. 2011;106:368–370. doi: 10.1038/ajg.2010.432. [DOI] [PubMed] [Google Scholar]
- 22.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M, HCC Expert Panel of Japan Society of Hepatology Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Yoon JH, Joo I, Woo HS. Recent advances in ct and mr imaging for evaluation of hepatocellular carcinoma. Liver Cancer. 2012;1:22–40. doi: 10.1159/000339018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Kim SH, Park HS, Kim GH, Lee JY, Lee JM, Han JK, Choi BI. Added value of 80 kVp images to averaged 120 kVp images in the detection of hepatocellular carcinomas in liver transplantation candidates using dual-source dual-energy MDCT: results of JAFROC analysis. Eur J Radiol. 2011;80:e76–e85. doi: 10.1016/j.ejrad.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology. 2010;254:145–153. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Lee JM, Kim KW, Klotz E, Kim SH, Lee JY, Han JK, Choi BI. Dual-energy computed tomography to assess tumor response to hepatic radiofrequency ablation: potential diagnostic value of virtual noncontrast images and iodine maps. Invest Radiol. 2011;46:77–84. doi: 10.1097/RLI.0b013e3181f23fcd. [DOI] [PubMed] [Google Scholar]
- 27.Thian YL, Riddell AM, Koh DM. Liver-specific agents for contrast-enhanced MRI: role in oncological imaging. Cancer Imaging. 2013;13:567–579. doi: 10.1102/1470-7330.2013.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K, Tomoda K. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 30.Lee SA, Lee CH, Jung WY, Lee J, Choi JW, Kim KA, Park CM. Paradoxical high signal intensity of hepatocellular carcinoma in the hepatobiliary phase of Gd-EOB-DTPA enhanced MRI: initial experience. Magn Reson Imaging. 2011;29:83–90. doi: 10.1016/j.mri.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, Choi BI. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology. 2013;267:776–786. doi: 10.1148/radiol.13120775. [DOI] [PubMed] [Google Scholar]
- 32.Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 33.Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, Choi BI. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748–760. doi: 10.1148/radiol.14131996. [DOI] [PubMed] [Google Scholar]
- 34.Hwang J, Kim SH, Lee MW, Lee JY. Small (≤ 2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-multirow detector CT. Br J Radiol. 2012;85:e314–e322. doi: 10.1259/bjr/27727228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227–239. doi: 10.14366/usg.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, Ueshima K, Shiozaki H. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol. 2008;190:W335–341. doi: 10.2214/AJR.07.3092. [DOI] [PubMed] [Google Scholar]
- 37.Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013;201:1141–1147. doi: 10.2214/AJR.13.10532. [DOI] [PubMed] [Google Scholar]
- 38.Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, Jeon HJ. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194:W396–400. doi: 10.2214/AJR.09.3171. [DOI] [PubMed] [Google Scholar]
- 39.Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013;24:958–965. doi: 10.1016/j.jvir.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Kunishi Y, Numata K, Morimoto M, Okada M, Kaneko T, Maeda S, Tanaka K. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:106–114. doi: 10.2214/AJR.10.6039. [DOI] [PubMed] [Google Scholar]
- 41.Lim S, Lee MW, Rhim H, Cha DI, Kang TW, Min JH, Song KD, Choi SY, Lim HK. Mistargeting after fusion imaging-guided percutaneous radiofrequency ablation of hepatocellular carcinomas. J Vasc Interv Radiol. 2014;25:307–314. doi: 10.1016/j.jvir.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Hakime A, Deschamps F, De Carvalho EG, Barah A, Auperin A, De Baere T. Electromagnetic-tracked biopsy under ultrasound guidance: preliminary results. Cardiovasc Intervent Radiol. 2012;35:898–905. doi: 10.1007/s00270-011-0278-8. [DOI] [PubMed] [Google Scholar]
- 43.Tomonari A, Tsuji K, Yamazaki H, Aoki H, Kang JH, Kodama Y, Sakurai Y, Maguchi H. Feasibility of the virtual needle tracking system for percutaneous radiofrequency ablation of hepatocellular carcinoma. Hepatol Res. 2013;43:1352–1355. doi: 10.1111/hepr.12096. [DOI] [PubMed] [Google Scholar]
- 44.Kang TW, Lee MW, Choi SH, Rhim H, Lim S, Song KD, Min JH, Choi SY, Lim HK, Yang J. A novel electrode with electromagnetic tip tracking in ultrasonography-guided radiofrequency ablation: A phantom, ex vivo, and in vivo experimental study. Invest Radiol. 2015;50:81–87. doi: 10.1097/RLI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 45.Hakime A, Barah A, Deschamps F, Farouil G, Joskin J, Tselikas L, Auperin A, de Baere T. Prospective comparison of freehand and electromagnetic needle tracking for US-guided percutaneous liver biopsy. J Vasc Interv Radiol. 2013;24:1682–1689. doi: 10.1016/j.jvir.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 46.Ewertsen C, Nielsen KR, Nielsen MB. Freehand biopsy guided by electromagnetic needle tracking: a phantom study. Ultraschall Med. 2011;32:614–618. doi: 10.1055/s-0031-1281852. [DOI] [PubMed] [Google Scholar]
- 47.Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27:1166–1173. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 48.Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952–4959. doi: 10.3748/wjg.v17.i45.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Min JH, Lim HK, Lim S, Kang TW, Song KD, Choi SY, Rhim H, Lee MW. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014;20:61–70. doi: 10.3350/cmh.2014.20.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishigaki Y, Hayashi H, Tomita E, Suzuki Y, Watanabe N, Watanabe S, Watanabe C, Takagi Y, Kato T, Naiki T. Usefulness of contrast-enhanced ultrasonography using sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2015;45:432–440. doi: 10.1111/hepr.12370. [DOI] [PubMed] [Google Scholar]
- 51.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, Teratani T, Shiina S, Ohtomo K. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25(Suppl 1):S57–S68. doi: 10.1148/rg.25si055505. [DOI] [PubMed] [Google Scholar]
- 53.Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196:907–913. doi: 10.2214/AJR.10.4584. [DOI] [PubMed] [Google Scholar]
- 54.Johnson A, Sprangers A, Cassidy P, Heyrman S, Hinshaw JL, Lubner M, Puccinelli J, Brace C. Design and validation of a thermoreversible material for percutaneous tissue hydrodissection. J Biomed Mater Res B Appl Biomater. 2013;101:1400–1409. doi: 10.1002/jbm.b.32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasegawa T, Takaki H, Miyagi H, Nakatsuka A, Uraki J, Yamanaka T, Fujimori M, Sakuma H, Yamakado K. Hyaluronic acid gel injection to prevent thermal injury of adjacent gastrointestinal tract during percutaneous liver radiofrequency ablation. Cardiovasc Intervent Radiol. 2013;36:1144–1146. doi: 10.1007/s00270-013-0546-x. [DOI] [PubMed] [Google Scholar]
- 56.Kang TW, Lee MW, Hye MJ, Song KD, Lim S, Rhim H, Lim HK, Cha DI. Percutaneous radiofrequency ablation of hepatic tumours: factors affecting technical failure of artificial ascites formation using an angiosheath. Clin Radiol. 2014;69:1249–1258. doi: 10.1016/j.crad.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 58.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 59.Seror O, N'Kontchou G, Tin-Tin-Htar M, Barrucand C, Ganne N, Coderc E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation with internally cooled versus perfused electrodes for the treatment of small hepatocellular carcinoma in patients with cirrhosis. J Vasc Interv Radiol. 2008;19:718–724. doi: 10.1016/j.jvir.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, Choi BI. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 61.Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, Yoon JH, Kim YJ, Han JK, Choi BI. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589–600. doi: 10.1148/radiol.13121736. [DOI] [PubMed] [Google Scholar]
- 62.Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, Choi BI. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014;15:235–244. doi: 10.3348/kjr.2014.15.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation—what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang JH, Crum LA. Current status of clinical high-intensity focused ultrasound. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:130–133. doi: 10.1109/IEMBS.2009.5335244. [DOI] [PubMed] [Google Scholar]
- 65.Auboiroux V, Petrusca L, Viallon M, Muller A, Terraz S, Breguet R, Montet X, Becker CD, Salomir R. Respiratory-gated mrghifu in upper abdomen using an mr-compatible in-bore digital camera. Biomed Res Int. 2014;2014:421726. doi: 10.1155/2014/421726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu YC, Chen YJ, Yu YR, Lai YH, Cheng JC, Li YF, Shen CH, Tai CK. Replicating retroviral vectors for oncolytic virotherapy of experimental hepatocellular carcinoma. Oncol Rep. 2012;28:21–26. doi: 10.3892/or.2012.1789. [DOI] [PubMed] [Google Scholar]
- 67.Cheon YK, Cho YD, Moon JH, Jang JY, Kim YS, Kim YS, Lee MS, Lee JS, Shim CS. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102:2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 68.Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12:233–241. doi: 10.7785/tcrt.2012.500317. [DOI] [PubMed] [Google Scholar]
- 69.Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, Yamamoto H. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269:927–937. doi: 10.1148/radiol.13130387. [DOI] [PubMed] [Google Scholar]
- 70.Solazzo S, Mertyna P, Peddi H, Ahmed M, Horkan C, Goldberg SN. RF ablation with adjuvant therapy: comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia. 2008;24:560–567. doi: 10.1080/02656730802070768. [DOI] [PubMed] [Google Scholar]
- 71.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukuda H, Numata K, Moriya S, Shimoyama Y, Ishii T, Nozaki A, Kondo M, Morimoto M, Maeda S, Sakamaki K, Morita S, Tanaka K. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radio-frequency ablation—propensity score matching analysis. Radiology. 2014;272:598–604. doi: 10.1148/radiol.14131640. [DOI] [PubMed] [Google Scholar]
- 73.Xu M, Xie XH, Xie XY, Xu ZF, Liu GJ, Zheng YL, Huang GL, Wang W, Zheng SG, Lü MD. Sorafenib suppresses the rapid progress of hepatocellular carcinoma after insufficient radiofrequency ablation therapy: an experiment in vivo. Acta Radiol. 2013;54:199–204. doi: 10.1258/ar.2012.120249. [DOI] [PubMed] [Google Scholar]
- 74.Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):50–55. doi: 10.1159/000333259. [DOI] [PubMed] [Google Scholar]
- 75.Galuppo R, Ramaiah D, Ponte OM, Gedaly R. Molecular therapies in hepatocellular carcinoma: what can we target? Dig Dis Sci. 2014;59:1688–1697. doi: 10.1007/s10620-014-3058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poon RT, Borys N. Lyso-thermosensitive liposomal doxorubicin: an adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 2011;7:937–945. doi: 10.2217/fon.11.73. [DOI] [PubMed] [Google Scholar]