Abstract
Chronic kidney disease is now recognized to be a worldwide problem associated with significant morbidity and mortality and there is a steep increase in the number of patients reaching end-stage renal disease. In many parts of the world, the disease affects younger people without diabetes or hypertension. The costs to family and society can be enormous. Early recognition of CKD may help prevent disease progression and the subsequent decline in health and longevity. Surveillance programs for early CKD detection are beginning to be implemented in a few countries. In this article, we will focus on the challenges and successes of these programs with the hope that their eventual and widespread use will reduce the complications, deaths, disabilities, and economic burdens associated with CKD worldwide.
Keywords: chronic kidney disease, epidemiology, surveillance
Recent findings from the Global Burden Disease 2010 study, to which the International Society of Nephrology has contributed, have highlighted chronic kidney disease (CKD) as an important cause for global mortality.1 The number of deaths from CKD has risen by 82.3% in the last two decades, the third largest increase among the top 25 causes of death, behind HIV/AIDS (396%) and diabetes.1 That kidney disease constitutes a public health priority is also underlined by the fact that, worldwide, the number of end-stage renal disease patients receiving renal replacement therapy (RRT) is estimated at >1.4 million, with an annual growth rate of 8%.2 With a population that is aging, steep increases in the incidence of type 2 diabetes mellitus and hypertension are driving this growth, putting an enormous burden on health-care resources.3 However, end-stage renal disease represents the tip of the iceberg; CKD occurs in ~10% of the population but it must not be assumed that kidney disease is entirely contained within the cardiovascular risk envelope. In the developing world, up to 40% of those identified with CKD in screening programs do not have diabetes or any cardiovascular disease.3,4 Such patients with kidney disease are often young, and the health and social costs of the progression of their kidney disease are high and prolonged. Thus, early recognition of CKD and concomitant comorbid conditions can potentially slow the progression to renal failure, increase longevity, improve quality of life, and reduce health-care costs.
Surveillance programs are just beginning to be implemented in a few countries. International Society of Nephrology encourages all members of the World Health Organization to recognize CKD as a major noncommunicable disease requiring the development of a specific health policy for its early detection and treatment.5 This would also entail the development of health information systems to capture data in order to better measure the incidence and prevalence of renal failure, track patient outcomes, and determine the true burden of disease. The following sections highlight some of these efforts with the hope of providing a platform that will discuss the challenges and successes of such efforts.
CKD SURVEILLANCE IN THE UNITED STATES OF AMERICA
The US Centers for Disease Control and Prevention’s National CKD Surveillance System
Background
In recent years, CKD has deservedly received attention as a public health problem. In 2006, based on a congressional mandate,6 the CDC launched its own CKD initiative2 that included the establishment of the first comprehensive CKD Surveillance System for the United States. Two teams from the University of California, San Francisco and the University of Michigan were funded to work collaboratively with the CDC in developing this system.
Approach and methodology
The principles and methodology for implementation of CKD surveillance in the United States have been published previously.7 In summary, the ‘passive’ surveillance approach is utilized, leveraging existing data from a variety of disparate data sources, broadly consisting of data from health-care systems (e.g., the Department of Veterans Affairs—VA, or other large administrative health data, e.g., Medicare), as well as data obtained from non-health-care sources (e.g., nationally representative surveys, such as the National Health and Nutrition Examination Survey—NHANES).
A unique aspect of this surveillance system is its comprehensiveness and the systematic approach adopted for the capture of major domains (topics) related to CKD and specific concepts within those topics (measures). Our teams analyze data from multiple data sources before uploading them in a standardized manner to the project website.8
RESULTS
With information from 20 different sources, the CKD Surveillance System has the most information about CKD available in the United States. The system provides information on the following key topics: burden of CKD (prevalence and incidence), burden of risk factors for CKD (prevalence of these conditions in the general population), awareness of CKD (both in the general population and among providers), health-care quality and processes of care for those with CKD, health consequences associated with CKD (including resource utilization), and health system capacity to deal with CKD. The surveillance system also provides information on special populations (e.g., CKD in children, CKD among organ transplant recipients, and so on), and tracks progress on CKD indicators related to the objectives of Healthy People 2020.9
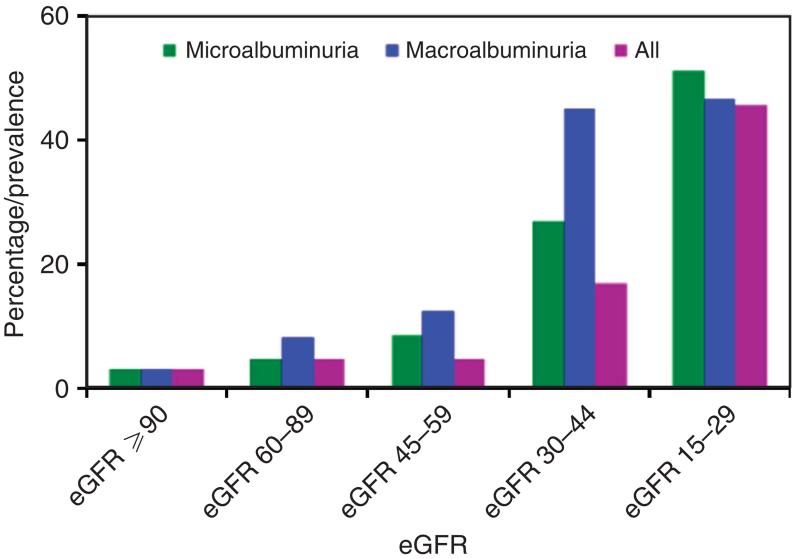
Under the six topics listed above, the system provides 136 measures, e.g., family history of CKD, with more detailed information. Specific indicators were developed for these measures, yielding ~200 charts with corresponding tables (an example is shown in Figure 1). Data are searchable and graphics customizable. The teams continue to review new topics and measures for inclusion, based on published evidence.
Figure 1. Percentage who were aware of their kidney disease by level of albuminuria and estimated glomerular filtration rate (eGFR), National Health and Nutrition Examination Survey, 1999–2010 Centers for Disease Control and Prevention.
Chronic Kidney Disease Surveillance System—United States. The US Department of Health and Human Services. http://www.cdc.gov/ckd/surveillance.
CHALLENGES
Integrating information from the wide array of disparate data sources available represents a key challenge. Meeting regulatory requirements for timely data procurement, data privacy, consensus on appropriate topics, measures, and denominators for population-based surveillance, handling of missing data, and maintaining an effective dissemination strategy are some of the other important challenges.
CONCLUSIONS
The US CKD Surveillance System was created to provide timely and detailed information on important aspects of CKD to ensure a systematic, comprehensive, and user-friendly product relevant to medical professionals, researchers, and policy makers alike. It has the potential to inform policy makers and have an impact on policy as well as provide impetus for implementation of robust population-based strategies to stem the burden of CKD, reduce complications and cost, and improve the lives of patients with CKD.
CKD IN EUROPE
The European Renal Association—European Dialysis and Transplant Association
The European Renal Association—European Dialysis and Transplant Association registry has an extensive amount of data on the incidence and prevalence of RRT from the majority of the European countries. However, data on the prevalence of CKD in Europe are still scarce. In 2008, Zhang and Rothenbacher10 reported about eight European studies investigating the prevalence of CKD in the general population in seven different countries. In 2012, McCullough11 found 13 studies that investigated CKD prevalence in diverse populations from seven European countries. Both reviews noted different definitions of CKD and this wide variation in definitions for prevalence of CKD complicates attempts to compare the burden of CKD between countries.
The aim of the European CKD Burden Consortium is (1) to compare the prevalence and progression of CKD in subjects not on RRT across Europe, and apply uniform definitions and (2) to explain potential international differences in CKD prevalence and progression by healthcare system characteristics.
First, a literature review was performed to identify all European studies that could provide data on CKD prevalence. Studies on CKD prevalence were performed in diverse populations, such as adult general populations and elderly general populations.
The representatives of all eligible studies received a questionnaire on the availability of data and health-care system characteristics. We received completed questionnaires from 44 studies with data on CKD prevalence from 21 different countries. On the basis of the new Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline12 and the availability of data in participating studies, standardized definitions for CKD prevalence were established (see Table 1). Although the use of the chronicity criterion is recommended by the Kidney Disease: Improving Global Outcomes, we did not include it in the definition as it was only available in four studies.
Table 1. Definition for CKD prevalence.
| Definition | Criteria |
|---|---|
| 1 | Albuminuria ≥30mg/g And/or eGFRa <60 ml/min/1.73 m2 |
| 2 | eGFRa <60 ml/min/1.73 m2 |
| 1 and 2 | No chronicity criterionb |
Abbreviation: eGFR, estimated glomerular filtration rate.
eGFR calculated using the CKD-EPI formula.
Chronicity criterion (persistence of albuminuria or decreased eGFR for ≥3 months) could not be used as only four studies had this available.
These standardized definitions and the CKD-EPI equation are being used to extract the prevalence of CKD by means of a syntax for various subgroups such as age group, gender, diabetic status, and body mass index status. In some countries, CKD prevalence data collection is limited to a specific age group (e.g., ages 65 years and older), and therefore we will provide only an age-specific prevalence number for these countries.
All CKD prevalence data will be age- and sex-standardized to the EU27 population. Data from about 19 general population-based studies originating from 13 countries across Europe will be included. The national differences in comorbidities and health-care system characteristics will be used to explain the potential differences in CKD prevalence. Later in 2013, the data collection on the progression of CKD will start.
Finally, identifying the prevalence and progression of CKD together with the international differences in health-care system characteristics should lead to a complete overview of the burden of CKD in Europe.
Funding
The research leading to these results has received funding from QUEST and the European Community’s Seventh Framework Programme under grant agreement number HEALTH-F2-2009-241544 (SysKID).
CKD SURVEILLANCE IN AUSTRALIA
CKD Queensland Registry
CKD is a major public health problem in Australia.13 The ANZDATA registry constitutes an excellent, long-term repository of information on patients on RRT in Australia,14 but the only CKD monitoring systems are through eKiDNAA coalition of several renal specialist users of Audit 4 software15 and now, on a regional basis, through the registry of CKD Queensland (CKD.QLD).16
The CKD.QLD registry, established in 2009, is a research and practice improvement platform in which all public renal specialty practices in the state of Queensland participate under the jurisdiction of Queensland Health. Queensland is the second largest state in Australia with a geographical area of 1,852,642 km2 and a population of 4.6 million, whose composition is representative of the aggregate Australian population of 23 million.17 This collaborative of public renal services embraces 80 senior practitioners (doctors and nurses) across 12 major hospital-based regional hubs, many with multiple clinics. There are various CKD service delivery models, which include hospital-associated practices, community clinics, and nurse practitioner–led clinics, with and without allied multidisciplinary teams.
The CKD.QLD registry will ultimately include all prevalent CKD patients from these clinics and recruit new patients as they are referred. Information that has been captured for clinical purposes in these renal practices in various formats, which include Excel, Audit 4, and web-based programs, is consolidated by the CKD.QLD registry into a repository and manipulated into standard definitions.
Recruitment of patients, with informed consent, began in June 2011, with 5,000 of an estimated 10,900 patients enrolled so far. By May 2014, 2,500 people will have been followed for 2 or more years, and 4,500 will have been followed for at least 1 year. Cross-sectional profiles of patients have been described in five of the service hubs, with longitudinal outcomes for at least a year described in all. In one metropolitan site, 47% of CKD patients with a follow-up for at least 12 months had been hospitalized on one or more occasions for reasons other than, or independent of, RRT.
There are some differences between the CKD.QLD registry patients and those starting RRT in Australia. Notably, the CKD population includes older people with no gender difference, unlike the male-dominated RRT population, and has a greater proportion of patients with renal vascular disease but a lower proportion with diabetic nephropathy.
The CKD.QLD registry also provides a platform for interactive research, with data from the registry as the critical hub. Current and planned projects include epidemiologic studies, practice improvement projects, biomarker research, clinical trials, and health economic evaluations. We are also establishing a CKD Bio-bank, the first in Australia. Four PhD students are engaged in projects on epidemiology, renal supportive and palliative care, and genomics.
Significant challenges to the registry include the time required to gain informed consent and the different degree of detail of captured data among sites. Data capture will be simplified with unrolling of the Queensland Health’s electronic health record system. The biggest challenge, however, is that of ongoing funding to support registry activities. Currently, the CKD.QLD registry is funded by Queensland Health (in-kind), Amgen, Roche, Australia’s National Health and Medical Research Council, and the Colonial Foundation of Australia. We expect the translational outcomes generated through the registry to attract long-term funding from Queensland Health, the chief potential benefactor.
The CKD.QLD registry, endorsed by Kidney Health Australia and supported by Amgen, is also promoting development of a national collaborative CKD surveillance network.
Funding
The CKD.QLD registry is supported by Amgen, Roche, NHMRC Australia (Australian Fellowship—Wendy Hoy), the Colonial Foundation of Australia (an untied grant to Wendy Hoy), and Queensland Health in-kind.
CKD SURVEILLANCE IN ENGLAND
CKD domain of the UK Primary Care Quality and Outcomes Framework (QoF)
In 2005, a national strategy for early detection and prevention of CKD was published as part of a comprehensive policy to improve outcomes and experience of care across the whole kidney care pathway.18 All UK laboratories adopted automatic estimated glomerular filtration rate (eGFR) reporting using the four-variable MDRD equation, supported by a national isotope dilution mass spectrometry traceable creatinine measurement quality control program. Creatinine and eGFR results were transferred electronically to primary care electronic patient record systems. In 2006, a CKD domain was introduced into the UK Primary Care QOF, a pay-for-performance system, that required all primary care practices to establish a register for adults with CKD stages 3–5, measure and achieve blood pressure targets, and prescribe renin–angiotensin system blockers.19
The National Institute for Health and Clinical Excellence produced a national clinical guideline for the early identification and management of CKD in adults in primary and secondary care in 2008.20 Despite concerns about the accuracy of MDRD eGFR as a measure of kidney function and challenges to the concept of CKD, especially in older people, the approach had broad support from health-care professional and patient organizations. Postgraduate and continuing professional development education helped address the knowledge gap, and the primary care training curriculum was revised to include kidney disease. Initially, there was an increase in referrals to secondary care but this has now stabilized.
Impact
In 2006, 1.9 million people previously unrecognized as having CKD were registered in primary care, many from pre-existing chronic disease programs including diabetes and hypertension.19 This represented 3.2% of the adult population but variance between practices in CKD registrations on the QOF system was high reflecting largely a lack of confidence in discussing kidney disease with patients and uncertainty regarding the importance of CKD. People with CKD are now also identified by the NHS Health Check program that started in 2008 and offers vascular risk assessment to all those between 40 and 75 years of age, as well as by routine care. Currently two-thirds of those expected to have CKD stages 3–5, based on the nationally representative Health Survey for England prevalence projections, are registered with the QOF system. Practice variation ascertainment has now fallen, although it is still present.19,21
In one primary care study of over 10,000 confirmed CKD stage 3–5 patients, the proportion achieving blood pressure target (145/80) increased from 42% before the induction of QoF CKD registers to 50% in the 2 years post QoF and this was maintained in the most recent period.22 In 2010/11, CKD patients achieving blood pressure (BP) <140/85 in the last 15 months varied by Primary Care Trust from 69 to 81%, and from 86 to 95% for patients with hypertension and proteinuria, respectively, who were prescribed a RAS inhibitor.19,23 Quantification of albuminuria was added to QoF in 2009, raising awareness of proteinuria as an independent vascular risk factor. Guidance on renin–angiotensin system blockade is now restricted to those with proteinuria.
Fewer patients were referred late for consideration of RRT and unplanned initiation of dialysis has fallen in the United Kingdom from 28% in 2005 to 21% in 2010.24 An increasing number of people start RRT with a pre-emptive transplant and over the past 4 years the incidence of RRT has levelled off at 109 PMP.24 This is despite the increasing age of the population and more obesity and diabetes, and it may represent a range of factors including better management of CKD progression and increasing provision of conservative kidney care as an alternative choice to RRT in those with frailty and multi-morbidity.
CKD identification and management were introduced into the national Cardiovascular Disease Outcomes Strategy published in 2013. Introduction of a professionally led national primary care CKD audit and quality improvement program and acute kidney injury initiatives to improve safety and medicine management in CKD are all being planned or implemented.
CKD SURVEILLANCE IN JAPAN
CKD-Japan cohort
CKD is prevalent (12.9%) in Japan. Until now, most epidemiological studies in Japan have been performed in mild CKD. CKD-Japan cohort was launched to investigate the comorbidities, treatment, and clinical outcomes, and to detect risk factors for CKD progression, death, cardiovascular disease, QOL, hospitalization, and medical cost in Japanese patients with CKD stage 3–5 (eGFR 10–59).25 Study design is summarized in Table 1. Although 91.9% were hypertensive, BP was relatively well controlled and ACEI or ARB was used in most patients. Compared with the Western countries, although a history of ischemic heart disease was much lower (<15%), stroke history was more common (11.5%). Diabetic patients had higher incidence of comorbidities.26 The prevalence of anemia (hemoglobin<11.5 g/dl) is high, although the number of patients treated with erythropoiesis stimulating agents is low.27 From a data of 1,075 patients who underwent ambulatory blood pressure monitoring, factors affecting BP differences between office and home and the patterns of nocturnal BP change were analyzed. Masked hypertension was high (31%) among CKD patients, suggesting that home BP is critical for appropriate BP control. Low GFR, diabetes, and winter season were associated with higher BP and abnormal BP patterns.28 Inverse relationship between left ventricular hypertrophy and eGFR was demonstrated.29 All data are now being fixed and further analyzed to elucidate the predictive factors for CKD progression, ESKD development, and cardiovascular disease, as well as factors associated with QOL and medical costs. The CKD-Japan cohort has been implemented smoothly according to its time line because of a strong connection with Japanese Society of Nephrology and funding support from industry. Collaboration with CRIC also raised the motivation of our study members. Towards the future of the CKD-Japan cohort, we must address the issues including fund-raising, follow-up of patients for longer periods, and participation in the international network for CKD cohort.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White SL, Chadban SJ, Jan S, et al. How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ. 2008;86:229–237. doi: 10.2471/BLT.07.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Feehally J. Health burden of kidney disease recognized by UN. Nat Rev Nephrol. 2012;8:12–13. doi: 10.1038/nrneph.2011.191. [DOI] [PubMed] [Google Scholar]
- 6.United States Congress Senate Committee on Appropriations Subcommittee on Departments of Labor, Health and Human Services, Education, and Related Agencies . Departments of Labor, Health and Human Services, Education, and related agencies appropriations for fiscal year 2006: hearings before a subcommittee of the Committee on Appropriations, United States Senate, One Hundred Ninth Congress, first session, on H.R. 3010, an act making appropriations for the Departments of Labor, Health and Human Services, and Education, and related agencies, for the fiscal year ending September 30, 2006, and for other purposes. Government Printing Office; Washington, DC: [Google Scholar]
- 7.Saran R, Hedgeman E, Plantinga L, et al. CKD Surveillance Team Establishing a national chronic kidney disease surveillance system for the United States. Clin J Am Soc Nephrol. 2010;5:152–161. doi: 10.2215/CJN.05480809. PMID: 19965534. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Chronic Kidney Disease Surveillance System—United States. U.S. Department of Health and Human Services; [accessed 30 January 2014]. Available at http://www.cdc.gov/ckd/surveillance. [Google Scholar]
- 9.U.S. Department of Health and Human Services [accessed 30 January 2014];Healthy people 2020: chronic kidney disease. Available at http://www.healthypeople.gov/2020.
- 10.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough K. Measuring the population burden of chronic kidney disease: a systematic literature review of estimated prevalence of impaired kidney function. Nephrol Dial Transplant. 2012;27:1812–1821. doi: 10.1093/ndt/gfr547. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 13.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 14. [accessed on 28 February 2013]; http://www.anzdata.org.au/anzdata/AnzdataReport/34thReport/2011c01_stockflow_v1.2.pdf.
- 15.Boudville N, Kemp A, Moody H, et al. Factors associated with the progression of chronic kidney disease in nephrology practices in Australia. Nephron Clin Prac. 2012;121:36–41. doi: 10.1159/000342375. [DOI] [PubMed] [Google Scholar]
- 16.Venuthurupalli SK, Hoy EW, Healy GH, et al. CKD.QLD: chronic kidney disease surveillance and research in Queensland, Australia. Nephrol Dial Transplant. 2012;27:iii139–iii145. doi: 10.1093/ndt/gfs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [accessed on 08 May 2013]; http://www.abs.gov.au/ausstats/abs@.nsf/Products/3235.0~2010~Main+Features~Queensland?OpenDocument.
- 18.Renal Services Framework . National Service Framework for Renal Services Part Two: Chronic Kidney Disease, Acute Renal Failure and End of Life Care. Department of Health; London: 2005. [Google Scholar]
- 19.Quality Outcomes Framework. at http://qof.hscic.gov.uk.
- 20.National Institute for Clinical Excellence . Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care (CG73) NICE; London: 2008. [PubMed] [Google Scholar]
- 21.Roderick P, Roth M, Mindell J. Chronic Kidney Disease, Chapter 8 in Health Survey for England 2010. Natcen; London: 2011. [Google Scholar]
- 22.Karinaratne K, Stevens P, Irving J, et al. The impact of pay for performance on the control of blood pressure in people with chronic kidney disease stage 3-5. Neph Dial Transplant. 2013;28:2107–2116. doi: 10.1093/ndt/gft093. [DOI] [PubMed] [Google Scholar]
- 23.NHS Kidney Care . Atlas of Variation in Healthcare for People with Kidney Disease. NHS Kidney Care; London: 2012. [Google Scholar]
- 24.UK Renal Registry Reports at www.renalreg.com/Reports/reports.html.
- 25.Imai E, Matsuo S, Makino H, et al. CKD-JAC Study Group Chronic Kidney Disease Japan Cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31:1101–1107. doi: 10.1291/hypres.31.1101. [DOI] [PubMed] [Google Scholar]
- 26.Imai E, Matsuo S, Makino H, et al. Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol. 2010;14:558–570. doi: 10.1007/s10157-010-0328-6. [DOI] [PubMed] [Google Scholar]
- 27.Akizawa T, Makino H, Matsuo S, et al. Chronic Kidney Disease Japan Cohort Study Group Management of anemia in chronic kidney disease patients: baseline findings from Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol. 2011;15:248–257. doi: 10.1007/s10157-010-0396-7. [DOI] [PubMed] [Google Scholar]
- 28.Iimuro S, Imai E, Watanabe T, et al. Chronic Kidney Disease Japan Cohort Study Group Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol. 2013;8:721–730. doi: 10.2215/CJN.06470612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitta K, Iimuro S, Imai E, et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:730–742. doi: 10.1007/s10157-012-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]