SUMMARY
Mechanisms governing muscle satellite cell withdrawal from cell cycle to enter into quiescence remain poorly understood. We studied the role of angiopoietin 1 (Ang1) and its receptor Tie-2 in the regulation of myogenic precursor cell (mpc) fate. In human and mouse, Tie-2 was preferentially expressed by quiescent satellite cells in vivo and reserve cells (RCs) in vitro. Ang1/Tie-2 signaling, through ERK1/2 pathway, decreased mpc proliferation and differentiation, increased the number of cells in G0, increased expression of RC-associated markers (p130, Pax7, Myf-5, M-cadherin), and downregulated expression of differentiation-associated markers. Silencing Tie-2 had opposite effects. Cells located in the satellite cell neighborhood (smooth muscle cells, fibroblasts) up-regulated RC-associated markers by secreting Ang1 in vitro. In vivo, Tie-2 blockade and Ang1 overexpression increased the number of cycling and quiescent satellite cells, respectively. We propose that Ang1/ Tie-2 signaling regulates mpc self-renewal by controlling the return to quiescence of a subset of satellite cells.
INTRODUCTION
Adult skeletal muscle has a remarkable regenerative potential, which is mainly attributable to a population of precursors called satellite cells. In normal adult muscle, satellite cells are quiescent (Hawke and Garry, 2001). The ability of satellite cells to respond to both the routine turnover of myonuclei and muscle regeneration indicates that mechanisms must be in place to maintain a viable satellite cell pool throughout adult life span (Zammit et al., 2006). In vivo evidence of satellite cell self-renewal was obtained using grafts of intact single isolated myofibers into muscle (Collins et al., 2005) or fresh isolated quiescent satellite cells (Montarras et al., 2005). Ex vivo studies on isolated myofibers showed that after activation and proliferation, a small number of myogenic precursor cells (mpcs) do not undergo terminal differentiation, but retain the ability to restore the reserve pool of quiescent progenitor cells by a direct self-renewal (Zammit et al., 2004), probably involving asymmetric division (Kuang et al., 2007; Shinin et al., 2006). In cultures of myogenic cells, a subpopulation also constitutes the “reserve cells” (RCs); these noncycling undifferentiated cells may further give rise to both differentiated and new RCs, sharing many characteristics with muscle satellite cells (Beauchamp et al., 2000; Carnac et al., 2000; Friday and Pavlath, 2001; Kitzmann et al., 1998). Activated proliferating satellite cells/mpcs coexpress Pax7 and MyoD transcription factors. At time of differentiation, while the majority of mpcs exits the cell cycle to enter terminal myogenic differentiation and fuses into myotubes, the RC pool downregulates MyoD expression, maintains high levels of Pax7 expression, and is in the Go phase (Kitzmann et al., 1998; Zammit et al., 2004). Consequently, satellite cells, or at least a subset of them, are now considered as myogenic stem cells (Collins et al., 2005; Kuang et al., 2007; Perez-Ruiz et al., 2008).
The mechanisms controlling the withdrawal of myoblasts from the cell cycle to enter into terminal differentiation have been studied, while exit from the cell cycle to enter in the quiescence state remains poorly understood. In mice, several markers have been associated with quiescent satellite cells, including M-cad-herin (Beauchamp et al., 2000; Irintchev et al., 1994), syndecan 3 and 4 (Cornelison et al., 2001), CD34 (Beauchamp et al., 2000), calcitonin receptor (Fukada et al., 2007), and Myf5 (Beauchamp et al., 2000), although Myf5 negative satellite cells have been described to be even more capable of self-renewal than Myf5+ cells (Kuang et al., 2007). A large number of effectors have been shown to be involved in the regulation of proliferation and differentiation of myogenic cells, but few have been identified as direct regulators of quiescence and self-renewal of satellite/myogenic cells: in human cultures, p130 from the Rb family is involved in the RC pool constitution by blocking cell-cycle progression and differentiation (Carnac et al., 2000). In mice, Pax7 transcription factor is required for satellite cell maintenance and acquisition of a quiescent undifferentiated state (Olguin and Olwin, 2004; Oustanina et al., 2004). Calcium signaling, via calcineurin and NFAT, upregulates Myf5 expression in quiescent RCs at time of fate choice between self-renewal and myogenic differentiation (Friday and Pavlath, 2001). Wnt and Notch signalings are crucial regulators of mpc proliferation and differentiation that are finely regulated with time (Brack et al., 2008). Their role in myogenic cell self-renewal is not yet deciphered, although Notch activation alters RC recruitment into myotubes (Kitzmann et al., 2006) and β-catenin promotes self-renewal of satellite cells, likely through wnt pathway (Perez-Ruiz et al., 2008).
In adult normal skeletal muscle, satellite cells are located close to capillaries (Christov et al., 2007). In vitro, we have shown that endothelial cells (ECs) and mpcs have privileged interactions and may act in a paracrine way (Christov et al., 2007). One of the main molecular systems regulating vascular homeostasis is the angiopoietin (Ang)/Tie system (Shim et al., 2007). Ang1 binding to its tyrosine kinase Tie-2 endothelial receptor is required to maintain vascular integrity while Ang2 behaves as a context-dependent agonistic or antagonistic Tie-2 ligand (Shim et al., 2007). Ang1/ Tie-2 system is a pleiotropic system because it has been involved in various biological activities, including cell survival, proliferation, migration, chemotaxis, and quiescence (Fiedler and Augustin, 2006; Shim et al., 2007). It is involved in the quiescence of hematopoietic stem cells (HSCs): osteoblast-secreted Ang1 binds to Tie-2 borne by HSCs to promote their survival and maintenance in G0 (Arai et al., 2004). In migrating ECs, matrix-Ang1 bound to Tie-2 induces cell-matrix contacts involving ERK signaling, while in contacting ECs, Ang1/Tie-2 forms homotypic cell:cell interactions involving Akt signaling (Fukuhara et al., 2008; Saharinen et al., 2008). This indicates that signaling triggered by Ang1/Tie-2 may differ in the same cell according to the status of the cell and its environment.
Here, we explored the role of Ang1/Tie-2 signaling in the regulation of myogenic cell fate by using human cell culture system, in vivo labeling analyses, and murine strains allowing the tracing of specific cell lineages. We showed that Ang1 and Tie-2 are strongly expressed in quiescent undifferentiated RCs. We used several strategies to alter Ang1/Tie-2 signaling, and further analyzed myogenic cell fate (differentiation versus quiescence) to identify a new role for Ang1/Tie-2 interaction in the self-renewal of myogenic cells that proceeds in vitro and ex vivo on isolated myofibers. Ang1 binding to Tie-2 induces myogenic cells toward the RC status and is expressed by the most quiescent satellite cells in skeletal muscle in vivo. Finally, in vivo loss- and gain-of-function studies confirmed the role of Ang1/ Tie-2 in the regulation of satellite cell quiescence in skeletal muscle.
RESULTS
Ang1 and Tie-2 Are Expressed by RCs and Quiescent Cells in Human Myogenic Cultures and by Human Satellite Cells In Vivo
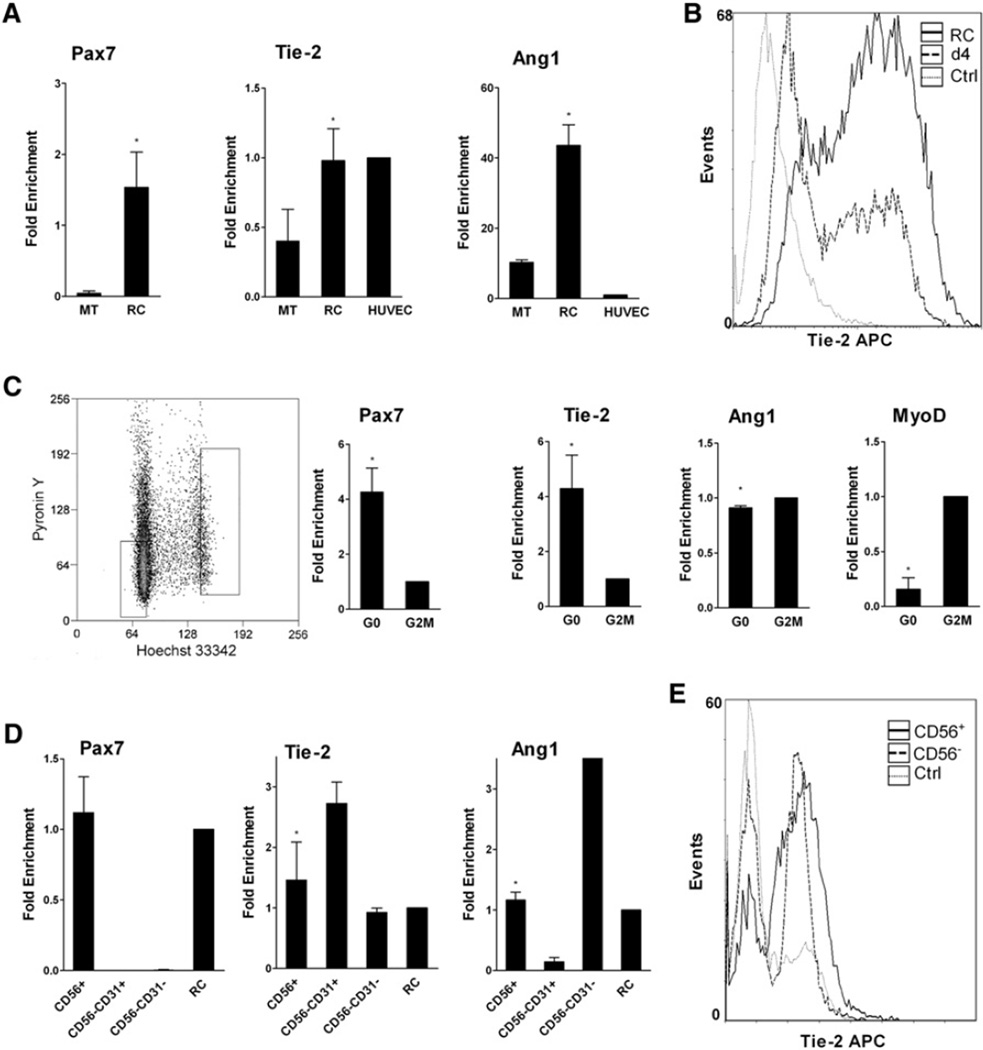
Expression of Angs and Tie-2 was analyzed in cultured human mpcs at various stages of differentiation (Chazaud et al., 2003) and, in differentiated cultures, in multinucleated myotubes (MTs) and RCs (that accounted for about 15%–20% of the cells and did not express MyoD) (Figure S1C). As expected, Pax7 expression decreased as cells differentiated and was higher in RCs compared to MTs (38-fold more, p <0.05) (Figure 1A and Figure S1A). Ang1 expression decreased with differentiation (p < 0.05) and was upregulated in RCs (4.2-fold more than in MTs, p < 0.05) (Figure 1A and Figure S1A). Tie2 expression increased with differentiation (p < 0.05) and was more expressed in RCs compared to MTs (2.5-fold more, p < 0.05) (Figure 1A and Figure S1A). Ang2 was expressed at a very low level at all stages (Figure S1A), while Tie-1 was not expressed by human mpcs (data not shown). Flow cytometry experiments confirmed the expression of Tie-2 at the surface of human mpcs: at day 4, 42.3 ± 10.3% of cells expressed Tie-2 while in RCs, 80.9 ±9.3% of cells expressed Tie-2 (p <0.01) (example is shown in Figure 1B).
Figure 1. Expression of Tie-2 and Ang1 by Human Myogenic Cells.
(A) RT-qPCR analysis of Pax7, Tie-2, and Ang1 expression in human RCs, MTs, and HUVECs. Results are means ± SEM.
(B) Flow cytometry analysis of Tie-2 expression in day 4 mpcs and RCs.
(C) G0 and G2/M cell-cycle fractions were sorted from day 4 mpcs and were analyzed for expression of various genes by RT-qPCR. Results are means ± SD.
(D) Freshly isolated human skeletal muscle-derived CD56+, CD56− CD31−, and CD56−CD31+ cell fractions were analyzed for Pax7, Tie-2, and Ang1 expression by RT-qPCR and compared to human RCs. Results are means ± SEM.
(E) Flow cytometry analysis of Tie-2 expression in freshly isolated human skeletal muscle-derived CD56+ and CD56− cell fractions.
Cultured human mpcs were sorted based on their cell-cycle status (Figure 1C). Compared to G2/M fraction, G0 cells expressed higher levels of Tie-2 (4-fold more, p < 0.0005) and Pax7 (p < 0.05) and lower levels of MyoD (p < 0.001) (Figure 1C).
Quiescent human satellite cells were isolated from normal adult muscle by magnetic cell sorting based on the surface marker CD56 and analyzed by RT-PCR and flow cytometry. CD56/NCAM is a reliable marker for quiescent satellite cells in normal human muscle (Illa et al., 1992). Human-muscle-derived CD56+ cells expressed Pax7 and also expressed Tie-2 and Ang1, as do the CD56− fractions that contain ECs and periECs (CD45−) and macrophages (CD45+) that are both Pax7− (Figure S1B). The CD56− fraction was further sorted into CD31+ and CD31− fractions. RT-qPCR results showed that CD56−CD31+, which corresponded to ECs, expressed 2-fold more Tie-2 than CD56+/satellite cells (Figure 1D). Finally, sorted CD56+ were analyzed by flow cytometry, and about 80% of sorted cells expressed Tie-2 at their surface (Figure 1E), confirming Tie-2 expression by satellite cells in vivo.
Ang1 Inhibits Human Mpc Growth and Apoptosis through Tie-2 Binding
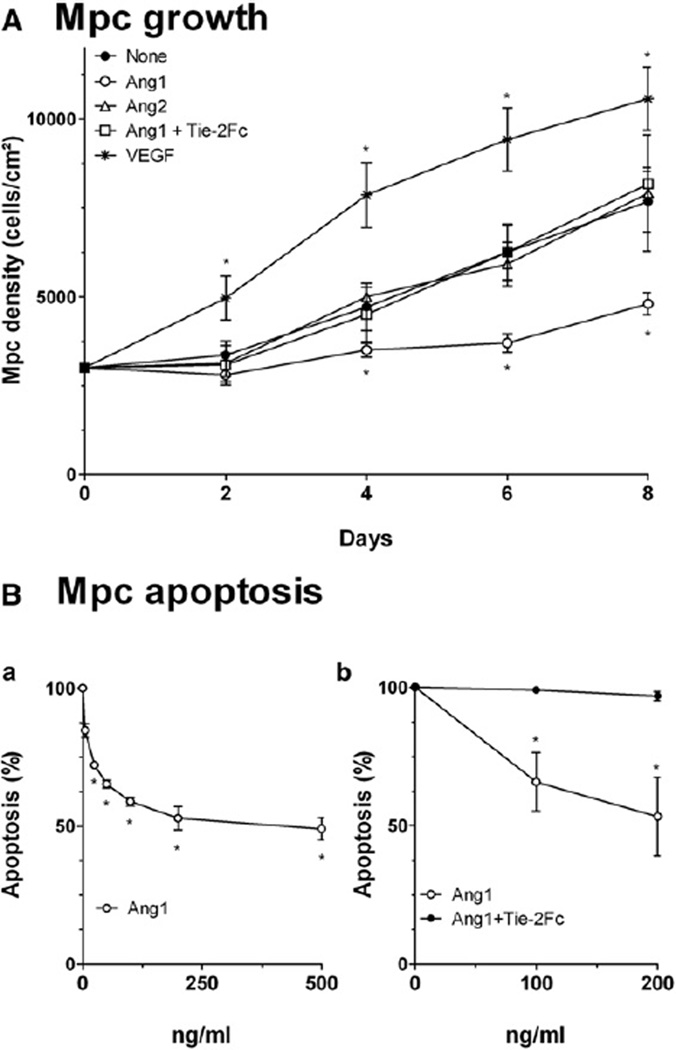
Ang1 inhibited mpc growth (p < 0.0001): at day 8, mpc density was decreased by 32% compared to control (Figure 2A). Tie-2 competitor Tie-2Fc totally inhibited the effect of Ang1, suggesting that Ang1’s effect was mediated by its receptor Tie-2 (Figure 2A). Ang2 had no effect on mpc growth (Figure 2A). Ang1 exerts an antiapoptotic effect on various cell types (Arai et al., 2004; Shim et al., 2007). Ang1 protected mpcs from staurosporine-induced apoptosis in a dose-dependent way (p < 0.0001), 51% of protection being observed at 500 ng/ml (Figure 2B). Here, again, Tie-2Fc totally abolished Ang1’s effect (Figure 2C).
Figure 2. Mpc Growth and Apoptosis.
(A) Mpcs were grown with or without Ang1 ± Tie-2Fc, Ang2, or VEGF, and cell density was estimated every 2 days.
(B) Myogenic cell death was induced by staurosporine, mpcs were incubated with Ang1 (Ba) or with Ang1 ± Tie-2Fc (Bb), and apoptosis was evaluated. Results are means ± SEM.
Ang1 Favors the RC Status in Human Mpcs and the Self-Renewal of Mouse Satellite Cells
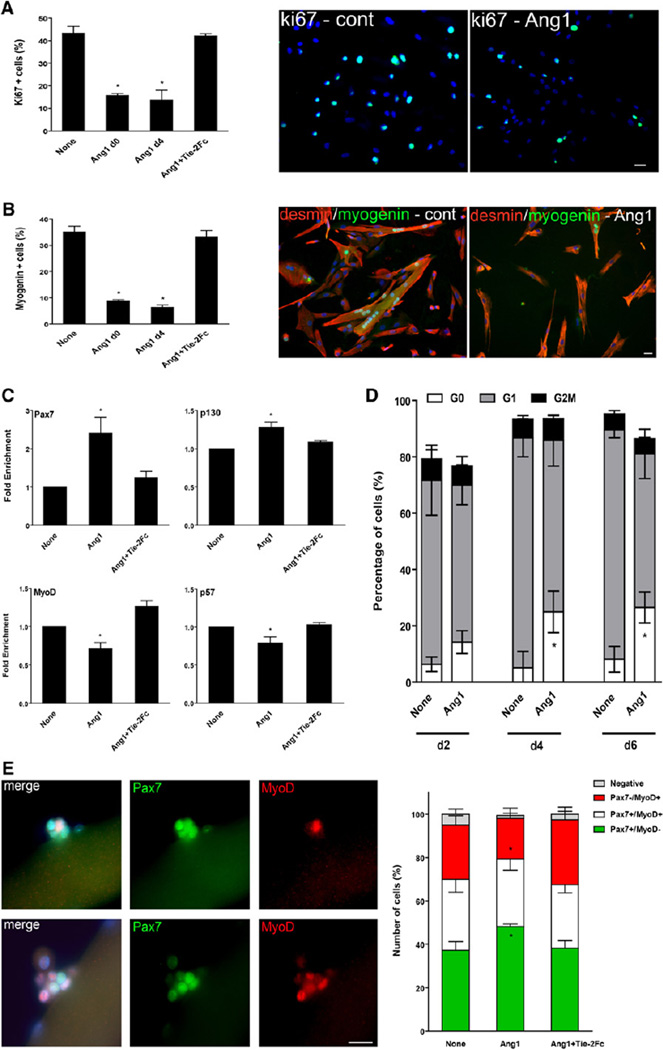
Addition of Ang1 decreased the number of Ki67+ cells by 66% (p < 0.05), indicating an inhibition of cell proliferation (Figure 3A). Accordingly, cyclin D1 expression was decreased by 44% (p < 0.005) in the presence of Ang1 (Figure S2A). The number of both myogenin+ cells and of myotubes was strongly reduced in the presence of Ang1 (by 75% [p < 0.05] and 85% [p < 0.001], respectively) (Figure 3B and Figure S2B). Addition of Tie-2Fc totally abolished the effects of Ang1 on proliferation and differentiation (Figures 3A and 3B and Figures S2A and S2B). In the absence of reliable anti-human Pax7 antibody, we performed RT-qPCR analyses of various markers associated with either mpc differentiation or self-renewal. In whole mpc cultures, Ang1 treatment increased both Pax7 and p130 expression (p < 0.005 and p < 0.05, respectively) and decreased both MyoD and p57 expression (p < 0.005 and p < 0.05, respectively) (Figure 3C).
Figure 3. Effects of Ang1 on Myogenic Cells.
(A and B) Mpcs were incubated with Ang1 ± Tie-2Fc from day 0 or from day 4 and then analyzed. (A) Mpc proliferation was evaluated after Ki67 immunolabeling. (B) The number of myogenin+ cells was evaluated after myogenin/desmin immunolabeling.
(C) Mpcs were incubated with Ang1 ± Tie-2Fc from day 0 and then analyzed for Pax7, p130, MyoD, and p57 expression by RT-qPCR.
(D) Mpcs were incubated with Ang1 ± Tie-2Fc from day 0, stained with Pyronin Y and Hoechst, and sorted for their cell-cycle status at days 2, 4, and 6. The number of cells in each cell-cycle phase is given as percentage of total events.
(E) Isolated mouse myofibers were incubated with Ang1 ± Tie-2Fc and stained for Pax7 (green) and MyoD (red). The number of Pax7+ and MyoD+ cells was counted in the clusters derived from satellite cells and reported as percentages. Pictures show examples of labeling observed in myofiber-associated cell clusters. Results are means ± SEM except in (D), where they are means ± SD. Bars, 10 µm.
We analyzed the mpc cell-cycle status in the presence of Ang1 and showed that the number of cells in G0 increased significantly from day 4, from 5% to 25%, and did not further increase afterward (p < 0.05) (Figure 3D). These results indicate that Ang1 favored the entry into quiescence and the appearance of RCs in mpc cultures.
We further studied the effect of Ang1 by the analysis of myogenic cell fate on isolated mouse single myofibers according to their expression of Pax7 and MyoD (Zammit et al., 2004). Although at early time of activation, Pax7+/MyoD− cells may represent early differentiating cells, 3 days after activation, Pax7+/MyoD− cells are considered as self-renewing cells (Zammit et al., 2004). Ang1 increased the number of Pax7+/MyoD− cells by 10% and decreased the number of Pax7−/MyoD+ cells (p < 0.05) (Figure 3E) in the clusters. Addition of Tie-2Fc reversed the effect of Ang1 (Figure 3E).
Ang1 effect on mpc growth and entry into G0 was noticeable from day 4 of culture, suggesting that mpcs are responsive to Ang1 signaling only at a given time. We added Ang1 at day 4 and found that Ang1 provoked the same response when added at day 0 or day 4 (Figures 3A and 3B). In the experiments below, Ang1 treatment was performed at day 4, and analyses were performed at day 5.
Tie2 Silencing Decreases RC Population within Human Mpcs
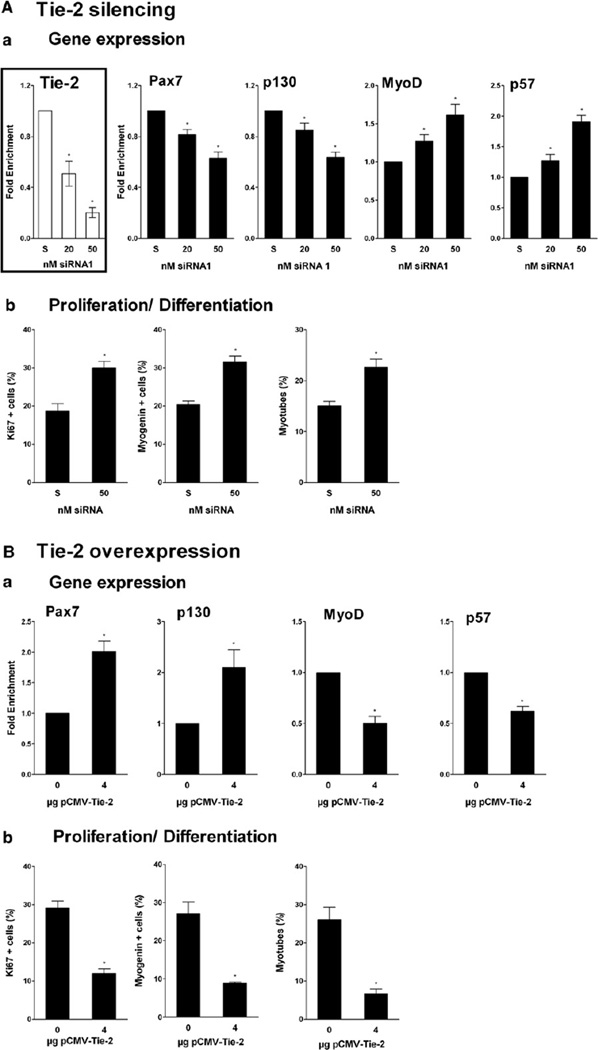
Tie-2 expression was inhibited by two different small interference RNAs (siRNA) in a dose-dependent way (p < 0.05) (Figure 4Aa and Figure S2C). In whole mpc cultures, Pax7 and p130 expression was inhibited in a dose dependent way (p < 0.001 and p < 0.05, respectively) (Figure 4Aa and Figure S2C). Inversely, MyoD and p57 expression was increased (p < 0.05) (Figure 4Aa and Figure S2C). Accordingly, the number of Ki67+ cells, of myogenin+ cells, and of myotubes was increased in the presence of Tie-2 siRNA (p < 0.005, p < 0.05, and p < 0.05, respectively) (Figure 4Ab). These data show that expression of Tie-2 was required for proper signaling of Ang1 to induce the appearance of RCs and that Ang1 binding to its Tie-2 receptor was required to commit responsive mpcs toward quiescence.
Figure 4. Effects of Tie-2 Silencing and Tie-2 Overexpression on Mpcs.
(A) Mpcs were incubated with Tie-2 siRNAs and scrambled control siRNA (S) and analyzed. (Aa) Tie-2, Pax7, p130, MyoD, and p57 expression were evaluated by RT-qPCR. (Ab) Mpc proliferation and differentiation were evaluated after Ki67 and myogenin/desmin immunolabeling, respectively.
(B) Mpcs were incubated with Tie-2-overexpressing plasmid and analyzed. (Ba) Pax7, p130, MyoD, and p57 expression were evaluated by RT-qPCR. (Bb) Mpc proliferation and differentiation were evaluated as in (Ab). Results are means ± SEM.
Tie-2 Overexpression Increases RC Population within Human Mpcs
In whole mpc cultures, Tie-2 overexpression induced an increase in Pax7 and p130 expression (p < 0.01 and p < 0.05, respectively), and inversely, a decrease in MyoD and p57 expression (p < 0.05) (Figure 4Ba). The number of Ki67+ cells, of myogenin+ cells, and of myotubes was decreased by Tie-2 overexpression (p < 0.05) (Figure 4Bb). These data show that overexpression of Tie-2 was associated with the increase of RCs within mpc culture, suggesting that endogenous Ang1 was sufficient to trigger a cell response when the receptor is overexpressed. Indeed, addition of Ang1 to overexpressing Tie-2 mpcs did not amplify the response of the cells (data not shown).
Ang1/Tie-2 Signaling Exerts Its Effect through the ERK1/2 Pathway
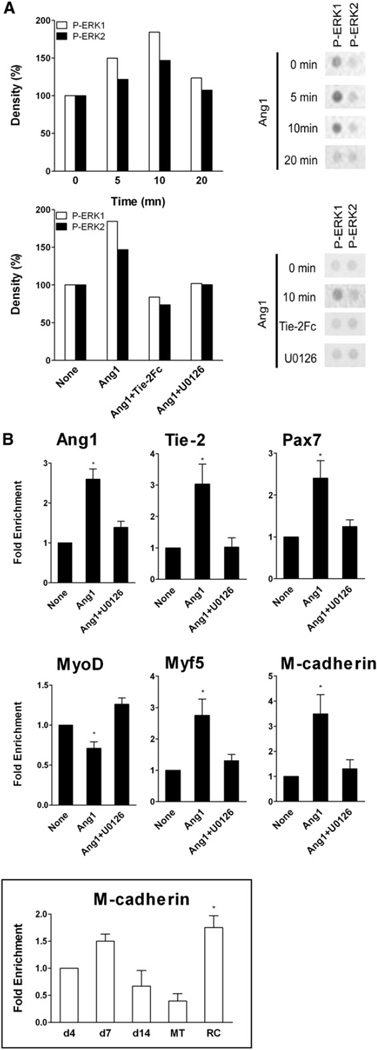
We investigated the signaling pathway triggered by Tie-2 binding using a Proteome Profiler Array (Figure S3A). Figure 5A shows that mpc treatment with Ang1 elicited a time-dependent increase in P-ERK1/2 protein level that reached a maximum at 10 min. We confirmed by western blot that Ang1/Tie-2 triggered only ERK1/2 signaling and not Akt signaling (Figure S3B and S3C). Addition of Tie-2Fc inhibited ERK1/2 phosphorylation (Figure 5A). U0126, a selective inhibitor of MEK1/2, an upstream effector of ERK1/2 phosphorylation, also abolished Ang1-induced ERK1/2 activation (Figure 5A). Inhibiting ERK1/2 signaling with U0126 almost totally inhibited the Ang1-induced expression of Ang1 and Tie-2 (p < 0.05) (Figure 5B), and also that of Pax7 (p < 0.005) whereas it restored that of MyoD (p < 0.05) (Figure 5B). Myf5 has also been shown to be upregulated in RCs (Friday and Pavlath, 2001) and Ang1 treatment markedly increased Myf5 expression (p < 0.05) that was inhibited by U0126 (p < 0.05) (Figure 5B). M-cadherin is expressed by quiescent satellite cells in vivo (Irintchev et al., 1994). Accordingly, we found that M-cadherin was expressed at a higher level by RCs compared to day 4 mpcs (1.75-fold) or MTs (4.5-fold) (p < 0.05) (Figure 5B, insert). Ang1 treatment strongly increased M-cadherin expression (p < 0.05); this upregulation was totally impaired by inhibition of the ERK1/2 signaling pathway (p < 0.05) (Figure 5B). These results show that Ang1/Tie-2 activated ERK1/2 signaling pathway that triggered expression of genes associated with the RC status.
Figure 5. Ang1/Tie-2 Intracellular Signaling.
(A) Mpcs were incubated with Ang1 during increasing times or with Ang1 ± Tie-2Fc or U0126 for 10 min. Blot density on phosphosignaling membrane array (example is shown on right panel) was expressed as percentages of signal density at time 0 or in untreated cells, respectively.
(B) Mpcs were incubated with Ang1 from day 4 ± U0126. Expression of various genes was evaluated by RT-qPCR. Insert: M-cadherin expression during in vitro myogenesis was evaluated by RT-qPCR. Results are means ± SEM.
Ang1 and Tie-2 Expression Is Positively Regulated by Ang1/Tie-2 Signaling
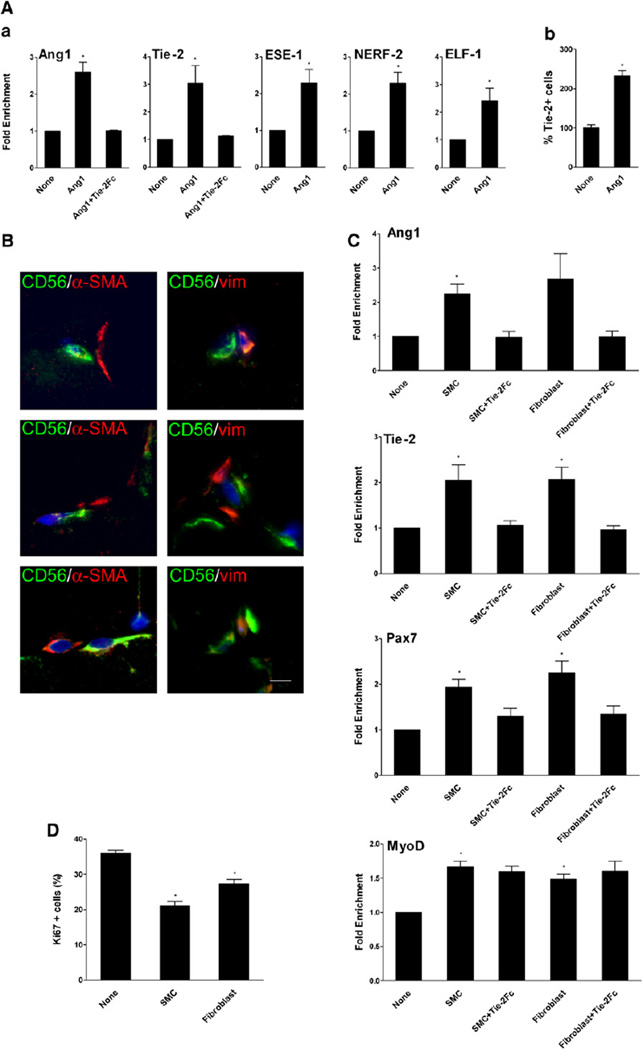
Transcription factors of the ETS family are involved in Ang/Tie gene regulation (Brown et al., 2004). Among these factors, ESE-1 has been shown to interact with Ang1 promoter and NERF-2 and ELF-1 have been shown to bind Tie-2 promoter and to stimulate Ang/Tie expression (Christensen et al., 2002). Ang1 stimulates Tie-2 expression via increased expression of NERF-2 (Christensen et al., 2002). In human mpcs, both Ang1 and Tie-2 expressions were upregulated upon Ang1 treatment (p < 0.005) (Figure 6A). Upregulation of Tie-2 expression was confirmed at protein level by flow cytometry analysis: 2.3-fold more myogenic cells expressed Tie-2 at their surface after Ang1 addition (p < 0.05) (Figure 6Ab). Ang1 treatment also increased ESE-1, NERF-2, and ELF-1 expression in mpc cultures (p < 0.01, p < 0.05, and p < 0.05, respectively) (Figure 6Aa), in accordance with a positive regulation loop. This effect was specific because other members of the ETS family were not upregulated in myogenic cells by Ang1 treatment (Figure S4A). It is important to note that addition of U0126 inhibited Ang1-induced expression of ESE-1, NERF-2, and ELF-1 (data not shown), indicating that ERK1/2 signaling pathway also regulated expression of these ETS transcription factors.
Figure 6. Paracrine Effect of Neighboring Cells.
(A) Mpcs were incubated with or without Ang1 ± Tie-2Fc, and (Aa) expression of various genes, including Ang1, Tie-2, and transcription factors involved in the regulation of Ang1 and Tie-2 expression, was evaluated by RT-qPCR. (Ab) Expression of Tie-2 was analyzed by flow cytometry.
(B) Normal human muscle sections were immunolabeled with anti-α-SMA (red) or anti-vimentin (red) and anti-CD56 (green) antibodies. Bar, 5 µm.
(C and D) Mpcs were incubated with conditioned medium from SMCs and fibroblasts ± Tie-2Fc. Expression of several genes was evaluated by RT-qPCR (C), and mpc proliferation was evaluated after Ki67 immunolabeling (D). Results are means ± SEM.
Smooth Muscle Cells and Fibroblasts Secrete Ang1 that Enhances Ang1, Tie-2, and Pax7 Expression
We have previously shown that the majority of satellite cells are close to capillaries (Christov et al., 2007). Thus, in their neighborhood, satellite cells must encounter, in addition to the myofiber, ECs, periECs such as smooth muscle cells (SMCs) and pericytes, and interstitial cells of fibroblastic type. We previously showed that conditioned medium from human SMCs and fibroblasts had a negative effect on mpc growth (Christov et al., 2007). We first studied whether these cells are in close proximity in human muscle. Anti-α-SMA (used to label SMCs) and anti-vimentin (used to label fibroblastic cells) antibodies labeled interstitial cells, likely periECs, that may neighbor satellite cells (labeled by CD56 antibodies) (Figure 6B). Conditioned media of various cell types that are located in the vicinity of satellite cells were tested on mpc cultures. Conditioned medium from mpcs, from ECs, and from differentiated MTs had no significant effect on Pax7, Ang1, and Tie-2 expression (Figure S4B). In contrast, conditioned media from both SMCs and fibroblasts strongly up-regulated the expression of Ang1,ESE-1 (data not shown), Tie-2, and Pax7 (p < 0.05) (Figure 6C). Accordingly, mpc proliferation was decreased upon addition of SMC- or fibroblast- conditioned medium (p < 0.01) (Figure 6D). Addition of Tie-2Fc in the conditioned media abolished this activity (Figure 6C), indicating that the effect was mediated, primarily if not exclusively, by Ang1. Indeed, SMCs and fibroblasts expressed much higher levels of Ang1 than ECs and mpcs (data not shown).
Conditioned medium of all cell types stimulated MyoD expression (p < 0.05) (Figure 6C and Figure S4B). However, addition of Tie-2Fc did not compromise MyoD upregulation (Figure 6C), indicating that this stimulating effect was not triggered by Ang1/Tie-2 signaling but was induced by other factors released by the cells.
Tie-2 Is Strongly Expressed by Quiescent Satellite Cells In Vivo
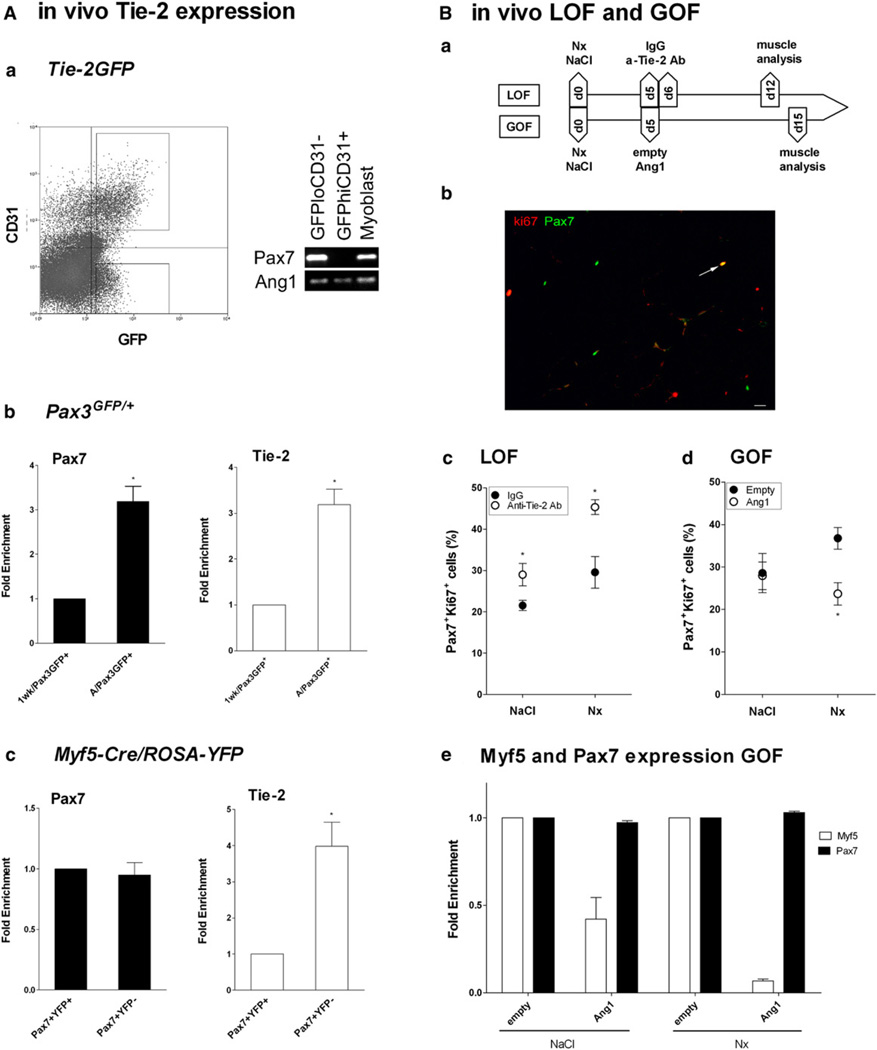
We used various mouse transgenic strains that label different cell lineages. In the Tg:Tie-2-GFP mouse, GFP signal was too faint to be observed by immunochemistry on tissue section, except in ECs (data not shown). However, after cell sorting, we identified two GFP+ populations in skeletal muscle: GFPhiCD31+ ECs and GFPloCD3− cells; the latter expressed Pax7, Ang1, and thus, Tie-2 (Figure 7Aa). These data confirmed that in mouse as in human (Figures 1D and 1E), satellite cells in normal muscle express both Tie-2 and Ang1. Sorted cells were cytospun and individually immunolabeled for Pax7 staining: 89.6 ± 3.5% of GFPlo (Tie-2) cells were Pax7+, indicating that they were truly satellite cells. We isolated GFP+ satellite cells from skeletal muscle of adult (2 months) and postnatal (1 week) Pax3GFP/+ mice (Montarras et al., 2005). The lower level of Pax7 expression in satellite cells of 1-week-old mice reflected that they were undergoing activation (Figure 7Ab). We observed that adult quiescent Pax3/GFP+ satellite cells expressed 3-fold more Tie-2 than satellite cells isolated from young, growing postnatal muscle (Figure 7Ab). We also analyzed satellite cells sorted from Myf5-Cre*ROSA-YFP adult muscle in which Myf5/YFP− satellite cells self-renew 4-fold more than Myf5/YFP+ satellite cells (Kuang et al., 2007). We observed that sorted YFP− satellite cells expressed Tie-2 4-fold more than YFP+ satellite cells (Figure 7Ac).
Figure 7. In Vivo Association between Ang1/Tie-2 System and Satellite Cell Quiescence.
(A) Satellite cells were sorted from skeletal muscle of various mouse strains and analyzed for Pax7 and Tie-2 expression by RT-PCR and RT-qPCR. (Aa) CD31 −GFPlo and CD31+GFPhi cell populations were sorted from Tg:Tie-2-GFP mice. (Ab) GFP+ cells were sorted from 1-week-old (1 wk/Pax3GFP+) and adult normal (A/Pax3GFP+) Pax3GFP/+ mice. (Ac) YFP+ and YFP− cells were sorted from adult normal Myf5-Cre/ROSA-YFP mice. Results are means ± SEM.
(B) Loss-of-function (LOF) and gain-of-function (GOF) studies were performed as shown on the scheme (Ba): muscle was injured with notexin (Nx) or not (Nacl). In LOF, at days 5 and 6, blocking anti-Tie-2 antibodies (a-Tie-2Ab or IgG) were i.m. injected, and muscle was analyzed at day 12. In GOF, at day 5, Ang1 plasmid (Ang1) (or empty plasmid) (empty) was electroporated, and was muscle analyzed at day 15. (Bb) Example of muscle-section labeling for Pax7 (green) and ki67 (red) (arrow shows double-positive satellite cell). Bar, 10 µm. (Bc) LOF and (Bd) GOF analysis of satellite cell staining. (Be) Whole-muscle expression of Pax7and Myf5 genes by RT-qPCR in GOF experiments. Results are means ± SEM.
In Vivo, the Number of Quiescent Satellite Cells Is Reduced by Blockade of Tie-2 and Increased by Overexpression of Ang1
We performed in vivo experiments, i.e., loss of function in which Tie-2 was inhibited by i.m.-injected blocking antibodies and gain of function in which Ang1 was overexpressed by the myofibers after electroporation (scheme in Figure 7Ba). We controlled in mouse myoblast cultures that the blocking Tie-2 antibody inhibited phosphorylation of Tie-2 receptor upon Ang1 addition (Figure S5A). We checked that Ang1 expression was increased in muscle after plasmid electroporation (Figure S5B) and that the ERK pathway was activated in whole muscle in this condition (Figure S5C). As these functional experiments were run in both normal and postinjury regenerating muscle, we controlled that antibody and plasmid treatments did not alter nor delay the time course of regeneration in toxic-injured muscles, as shown by the similar number of normal, centrally nucleated, and basic regenerating myofibers at time of analysis (Figure S5D). Also, as Ang1/Tie-2 signaling is involved in regulation of vessel homeostasis, we checked that the number of vessels (CD31+ structures) per myofiber was not altered by both antibody and plasmid treatment (Figure S5E).
The number of Pax7+ki67+ cells, which are cycling satellite cells, was counted as a percentage of the total number of Pax7+ cells (Figure 7Bb). When Tie-2 was inhibited in both normal (LOF-NaCl) and regenerating (LOF-Nx) muscle, the number of cycling satellite cells was increased by 38 and 55%, respectively (both p < 0.05) (Figure 7Bc). When Ang1 was overexpressed in normal (GOF-NaCl) muscle, the number of cycling satellite cells remained unchanged. However, after a cycle of regeneration (GOF-Nx), the number of cycling satellite cells was reduced by 36% in the Ang1-treated muscle (p < 0.05) (Figure 7Bd). Finally, in GOF experiments, whole muscle Pax7 and Myf5 expression was evaluated by RT-qPCR at day 15, i.e., at a time point when only satellite cells expressed these two transcription factors. Overexpression of Ang1 in the muscle triggered a decrease in Myf5 expression, while that of Pax7 was unchanged, suggesting that the number of the most uncommitted satellite cells, which do not express Myf5 (Kuang et al., 2007), increased upon Ang1 treatment. These data suggest that inhibiting/preventing Ang1/Tie-2 binding activates satellite cells, while overexpressing Ang1 triggers their entry into quiescence.
DISCUSSION
In the present study, we have shown that Ang1 and its receptor Tie-2 are expressed by quiescent satellite cells in vivo and quiescent RCs in vitro. We have obtained evidence that Ang1 binding to Tie-2 prevents apoptosis; decreases growth, proliferation, and differentiation of mpcs in culture; and triggers their entry into G0. Conversely, silencing of Tie-2 expression has strictly opposite effects and further confirms that Ang1 signals via its receptor Tie-2. Ang1/Tie-2 exert their effect through ERK1/2 signaling to control upregulation of markers associated with the RC phenotype (p130, Pax7, Myf-5, M-cadherin) and downregulation of markers associated with myogenic differentiation (MyoD, p57, myogenin). Ex vivo, Ang1 treatment increases the number of Pax7+/MyoD− cells in differentiating clusters derived from satellite cells on single myofibers. In vivo, Tie-2 is highly expressed by the most quiescent satellite cells in murine muscle. Functionally, blocking Tie-2 induces an increase in cycling satellite cells, while conversely, inducing overexpression of Ang1 by myofibers triggers the entry into quiescence of satellite cells in regenerating muscle. Finally, our data suggest that neighboring interstitial cells could be a source of Ang1 that acts on satellite cells to promote their quiescence. Altogether, these observations show that Ang1 binding to Tie-2 is involved in the regulation of the return to a quiescent state of a subset of satellite cells.
Among the Ang/Tie family, only Ang1 and its receptor Tie-2 were shown to be expressed by human and murine myogenic cultures and satellite cells. These results contrast with those obtained by Dallabrida et al. (2005), who stated that neither murine C2C12 nor human myogenic cells express Tie-2. However, we found that both murine and human satellite cells express Tie-2, based on RT-PCR, RT-qPCR, and flow cytometry analyses; in the Tg:Tie-2-GFP mouse, isolated GFP+(lo)CD31− cells express Pax7 transcription factor, which further confirms that satellite cells do express Tie-2. Our data show that Tie-2 expression varies according to the status of the myogenic cell. Tie-2 is more expressed by RCs in vitro, compared to MTs, and is also more expressed by cells in G0. In vivo, Tie-2 is more expressed by quiescent Pax3+ than by activated Pax3+ satellite cells. Furthermore, Tie-2 is more expressed by Myf5− satellite cells, which have been shown to self-renew/return to quiescence much more than Myf5+ satellite cells (Kuang et al., 2007). Altogether, these data show that the more the satellite cell is quiescent, the more it expresses Tie-2.
We show here that Ang1 protects mpcs from induced apoptosis in a dose-dependent manner. Ang1 anti-apoptotic activity has been demonstrated in ECs (Fiedler and Augustin, 2006; Shim et al., 2007) and in other cell types (Arai et al., 2004). As activation of Ang1/Tie-2 inhibited mpc growth while triggering survival signals, we hypothesized that it may promote the emergence of nonproliferating nondifferentiating cells. We used three ways to alter Ang1/Tie-2 system, i.e., activation by ligand addition and overexpression and silencing of the receptor. Activating the system is associated with a decrease in both the number of proliferating cells and differentiating cells. Accordingly, MyoD, which is associated with the commitment into differentiation and p57, involved in cell-cycle exit associated with myogenic differentiation (Reynaud et al., 2000), is downregulated. Activation of Ang1/Tie-2 signaling triggers an increase of the RC-associated markers that are as follows: (1) p130 protein, which is involved in the maintenance of RCs by blocking cell cycle progression (Carnac et al., 2000); (2) Pax7, the main transcription factor described today in the satellite/myogenic cell self-renewal (Zammit et al., 2004); and (3) Myf5, which is a myogenic transcription factor that has been shown to be, in cell cultures, strongly expressed in RCs (Friday and Pavlath, 2001). We also observed that M-cadherin expression is increased by Ang1 treatment. We found that M-cadherin is highly expressed by RCs compared to MTs (Charrasse et al., 2006); in vivo, M-cadherin is localized on quiescent satellite cells, on the side that lays the myofiber (Irintchev et al., 1994). Silencing Tie-2 expression has strictly opposite effects on mpc behavior and expression of these four markers. We furthermore confirmed in the single fiber model that Ang1 treatment increases the number of Pax7+/MyoD− cells, while the number of Pax7−/MyoD+ cells, which are committed to differentiation, is reduced in the clusters derived from activated satellite cells.
Analysis of intracellular signaling showed that Ang1/Tie-2 signals through the ERK1/2 pathway. It has been shown in ECs that such pathways may be activated by Ang1 binding to Tie-2 (Harfouche et al., 2005). Our results are in accordance with previous work showing that Myf5 expression is controlled by the ERK1/2 pathway (Perez-Ruiz et al., 2007). Moreover, it has been shown that ERK1/2 pathway may induce reversible quiescence and antiapoptotic signaling in a myoblast cell line (Reed et al., 2007) and in fibroblasts (Black et al., 2000). Transcription factors of the ETS family are involved in the regulation of expression of Ang1 and Tie-2 (Christensen et al., 2002). We confirmed here that Ang1 and Tie-2 expression is specifically regulated by ESE-1, NERF-2, and ELF-1, respectively, through ERK1/2 pathway (data not shown). Moreover, activation of Tie-2 with Ang1 further stimulates both Ang1 and Tie-2 expression, indicating a positive regulatory loop.
Coculture experiments show SMC and fibroblast supernatants stimulated Ang1, Tie-2, and Pax7 expression and accordingly decreased mpc proliferation. This paracrine signal likely occurrs via Ang1 because it is blocked by Tie-2Fc. Supernatants from all cell types stimulate MyoD expression in a Tie-2-independent way, as well as the number of MTs in the mpc cultures (data not shown). This is likely due to the effect of other factors secreted by these cell types on the main mpc population (Hawke and Garry, 2001) that is not responsive to Ang1 signaling.
Ang1 induces the same response in human mpcs when added from day 0 and from day 4 of culture. Moreover, Ang1 significantly increases the number of cells in G0 phase of the cell cycle only from day 4 of culture. This time point is marked by a decrease in proliferation concomitant with the onset of differentiation (Chazaud et al., 2003; Shefer et al., 2006). This is a critical time when cells can either irreversibly undergo terminal differentiation or return to quiescence to self-renew. Treatment of G2/M synchronized mpc cultures with Ang1 did not force cells to become quiescent when treatment was applied within the first 2 days (data not shown). This may simply result from the fact that Tie-2 is not expressed enough by G2/M cells to respond to Ang1. But these results also suggest that mpcs must reach a state of competence to be able to respond to Ang1 and/or that the number of competent cells increases in the culture to be detectable in our assay. In Tie-2-overexpressing mpcs, Ang1 provokes the same cell response as in normally expressing Tie-2 cells, indicating that the subset of cells able to return to quiescence is not unlimited. This is confirmed by the plateau of the number of cells that enter in G0 upon Ang1 treatment with time. Regulation of mpc responsiveness is unknown, but it has been recently shown that some regulatory molecular machineries, such as Wnt and Notch, must be tightly time-regulated for proper proliferation and differentiation of myogenic cells (Brack et al., 2008).
In vivo functional experiments cannot rule out side effects of Ang1/Tie-2, especially on vessel stability (Shim et al., 2007), that may interfere indirectly on satellite cell activation. However, we do not notice histological alteration, we showed no difference in the time course of regeneration, and we found similar number of vessels per myofiber between sham and treated muscles. Loss-of-function experiments show that preventing Ang1/Tie-2 binding in regenerating muscle increases the number of cycling satellite cells. Even in nonregenerating muscle, destabilizing Ang1/Tie-2 ligation induces satellite cell activation, although to a much less extent. Gain-of-function experiments show that in a regenerating muscle, overexpression of Ang1 in the muscle triggers an increase of the number of satellite cells that have entered into quiescence. Of note, in nonregenerating muscle, overexpressing Ang1 does not alter homeostasis of already quiescent satellite cells. However, in both cases, analyzing Pax7 and Myf5 expression at this time point, i.e., when only satellite cells express these two transcription factors, shows that Myf5 expression is decreased upon Ang1 overexpression while expression of Pax7 is unchanged, indicating that the number of the most uncommitted satellite cells that do not express Myf5 and that are capable of more self-renewal (Kuang et al., 2007) is increased upon Ang1 treatment.
It is tempting to hypothesize that Ang1/Tie-2-dependent signaling affects only cells that have reached a state of competence after a phase of proliferation. In this scheme, only a subset of cells would become responsive and return to quiescence. Whether this occurs through an autocrine or paracrine mechanism remains to be investigated more thoroughly. We have observed that mpcs in culture express and produce Ang1, and part of them are able to become quiescent RCs. These data from cell cultures and those from single-fiber experiments suggest such a self-regulation of myogenic cell homeostasis indicative of an autocrine loop that could operate to regenerate RCs. However, from our results, one cannot exclude a contribution of Ang1 originating from neighboring cell types, such as perivascular and interstitial cells, that would participate to the maintenance of the quiescent status of these responsive Tie-2+ Pax7+ satellite cells.
We propose that Ang1/Tie 2 signaling is involved in the control of the return of a subset of muscle precursor cells to quiescence that ensures self-renewal of adult muscle stem cells.
EXPERIMENTAL PROCEDURES
Mouse Strain
C57/B6 and Tg(TIE2GFP)287Sato/J mouse strain were purchased from Jackson Laboratories. Pax3GFP/+ mice were described in Montarras et al. (2005). Mice were bred and used according to French legislation for animal care and handling. Myf5-Cre*ROSA26-YFP mice were described in Kuang et al. (2007). Experiments were conducted at 4–8 weeks of age.
Satellite Cell Sorting from Mouse Muscle
Mouse tibialis anterior (TA) muscle was digested in DMEM containing collagenase B 0.2% (Roche Diagnostics Corp.) at 37°C for 45 min. Cells were sorted with a MoFlo cytometer (Dako) after CD31 staining (PE-conjugated anti-CD31 antibody, BD Biosciences). Muscle from Myf5-Cre*ROSA26-YFP mice was dissected and digested in collagenase-dispase. Muscle mononucleated cells were stained as described by Kuang et al. (2007) with an additional positive selection with CD34. Pax3/GFP+ satellite cells were sorted as GFP+ cells from muscles (diaphragm, pectoralis , and abdominal) of Pax3GFP/+ mice as described in Montarras et al. (2005).
In Vivo Functional Experiments
Injury was induced by notexin injection (10 µl, 12.5 µg/µl) (Notechis scutatus, Latoxan) into the right TA of anesthetized mice, and the left TA was injected by 0.9% saline solution. In gain-of-function experiments, TAs were electrotransfered at day 5 by pcDNA3.1-Ang1/V5-His plasmid encoding full-length murine ang-1 (or an empty plasmid (pcDNA 3.1/V5-His) as a control) in 50 ml of 0.9% saline solution. Non-treated notexin TA muscles were treated with bovine hyaluronidase (50 µl, 0.4 units/µl) 2 hr before muscle electrotransfer. Six square-wave 130 V/cm pulses, lasting 60 ms each with a 100-ms interval, were then applied with a BTX electrocell manipulator. Muscles were collected at day 15. In loss-of-function experiments, TA muscles were i.m. injected at days 5 and 6 with goat anti-mouse Tie-2 blocking antibody (20 mg/kg, 10 µl, R&D Systems) or Goat immunoglobulin G (IgG, R&D Systems). Muscles were collected at day 12.
Satellite Cell Sorting from Human Muscle
Normal human muscle sample was digested in HAM F12-HEPES 10 mM, EDTA 1 mM containing 0.15% pronase E (Sigma) at 37°C for 1 hr. CD56+, CD56−CD45+, CD56−CD45−, CD56−CD31−, and CD56−CD31+ cells were sorted using magnetic beads (Miltenyi Biotec).
Cell Culture
Human mpcs were cultured as previously described (Chazaud et al., 2003) in HAM F12 medium containing 15% fetal calf serum (FCS) (growth medium) (Invitrogen) or in HAM F12 5% FCS to induce differentiation. Mpcs were collected at day 4 (undifferentiated cells), day 7 (differentiating cells), and day 14 (fully differentiated cells). RCs and MTs were isolated from day 14 cultures as described (Kitzmann et al., 1998). In brief, human RCs were isolated using a short trypsinization (trypsin 0.1%/EDTA 0.1 mM, 30 s) that specifically removed MTs and left quiescent undifferentiated RCs adherent to the dish. The MRC-5 human fibroblastic cell line (ATCC), human umbilical vascular endothelial cells and human dermal microvascular endothelial cells (Promo-cell), and human SMCs (kindly provided by Dr. S. Eddahibi [INSERM U841, Créteil, France]) were cultured as previously described (Christov et al., 2007). Conditioned media were prepared from cells cultured in 24-well plates at 15,000 cell/cm2 density and incubated for 24 hr in DMEM serum-free medium.
Cell Cycle
Cells were collected and incubated in HBSS containing 2% FCS containing 10 µM Hoechst 33342 (Sigma) at 37°C for 45 min. Pyronin Y (Sigma) that reflects RNA content was added (2.5 µg/ml) for 45 min at 37°C. Cells were sorted with an Influx 500 cell sorter (Cytopeia). Gates were placed at the edge of the cell populations to limit the contamination by cells in S phase (Figure 1). RNase (Invitrogen)-treated cells were used as control.
Cell Treatments and Preparation
Mpcs were seeded at 3 × 103 cells/cm2 in HAMF12 medium 10% FCS. At day 4, recombinant human Ang1 (500 ng/ml) (R&D Systems) or 24 hr conditioned media was added ± Tie-2Fc (4 µg/ml). At day 5, cells were collected and cell pellets were kept frozen for analysis of gene expression. At day 6, cells were treated for analysis of cell proliferation and differentiation. In some experiments, Ang1 was added at day 0 then every 2 days. In other experiments, U0126 (25 µM Sigma) was added 60 min before Ang1 addition.
Mpc Growth
Mpcs were grown in HAM F12 medium containing 10% FCS. Ang1 (500 ng/ml), Ang2 (500 ng/ml), VEGF (25 ng/ml), and Tie-2Fc (saturating concentration 4 µg/ ml) (R&D Systems) were added every 2 days. At each time point, mpc density was determined after trypsinization.
Mpc Apoptosis
Mpcs were plated in 96-well plates at 2 × 105 cells/cm2. Cells were incubated with staurosporine (0.5 µM) to induce apoptosis in serum-free advanced DMEM-F12 medium (Invitrogen) containing or not containing effectors and further incubated for 6 hr. Apoptosis was measured using Cell Death Detection ELISAPlus kit (R&D Systems).
Immunolabeling
Human mpcs cultured on coverslips were immunolabeled with primary antibodies, anti-Ki67 (Abcam), anti-myogenin (BD Biosciences), anti-desmin (Abcam), and Anti-MyoD1 antibody (Dako), and mpc proliferation and differentiation were evaluated as previously described (Arnold et al., 2007). Normal human skeletal frozen muscle sections were immunolabeled with primary antibodies: mouse anti-CD56 (Beckman Coulter 6602705), rabbit anti-a-smooth muscle actin (Abcam 5694), and goat anti-vimentin (Sigma). Mouse muscle cryosections were double-labeled with Pax7 antibodies (Developmental Studies Hybridoma Bank) and ki67 antibodies (Abcam) or labeled with CD31 antibodies (Abcam) revealed by FITC- and cy3-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.).
Flow Cytometry Analysis
Both human cultured mpcs and CD56+ cells extracted from muscle biopsies were labeled with anti-human Tie-2-APC antibodies (R&D Systems) and analyzed on a FC500 Beckman Coulter cytometer.
Tie-2 Silencing
Mpcs were seeded at 5 × 103 cells/cm2 in growth medium and were transfected at day 0 with two different validated siRNA-Tie-2 or scrambled control siRNA-Fluorescein using HiPerfect transfection reagent according to the supplier’s protocol (QIAGEN). Cells were collected at day 6.
Tie-2 Overexpression
Mpcs were seeded at 5 × 103 cells/cm2 in growth medium and were transfected at day 0 with 0–6 µg of pCMV-Tie-2 plasmid encoding full-length human Tie-2 (TEK OmicsLink expression Clone, Genecopoeia) using FuGene as a transfection reagent (Roche Diagnostics Corp.). Cells were collected at day 6. Tie-2 expression was increased in a dose-dependent way (p < 0.05). At 4 µg, Tie-2 expression was increased more than 200-fold.
Ang1/Tie-2 Intracellular Signaling Analysis in Human
100 µg of total cell protein extracts were treated according to the supplier’s instructions in Proteome Profiler Human Phospho-MAPK Array Kit (R&D Systems). Validation of the array was performed using IGF-1-treated mpcs (50 ng/ml, Abcys) that showed positive signals for phospho-ERK1/2 and phos-pho-Akt, as previously shown (Foulstone et al., 2004).
Floating Myofibers
Single fibers were isolated from Extensor Digitorum Longus as described in Zammit et al. (2004). At either day 0 or day 2, Ang1 (500 ng/ml) ± Tie-2Fc (4 µg/ml) was added. After 72 hr of incubation, myofibers were proceeded for Pax7/MyoD immunostaining as previously described (Zammit et al., 2004).
PCR and qPCR
Total RNAs were extracted using the RNeasy Mini Kit (QIAGEN) and were reverse transcripted using Superscript II Reverse Transcriptase (Invitrogen). cDNA extracts were amplified as described in Supplemental Experimental Procedures.
Statistical Analyses
Except Proteome ProfilerTM array, all experiments were performed using at least 3 different primary cultures or animals in independent experiments. The Student’s t test and ANOVA were used for statistical analysis. p < 0.05 was considered significant and is indicated in figure legends by an asterisk.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Bassez, R. Mounier, E. Petit, S. Tajbakhsh, and C. Torre for their precious help. Cell sorting was performed on cytometry facility of Institut Jacques Monod, CNRS, Paris, France, and IM3, IMRB, INSERM, Cre´teil, France. This work was supported by AFM and ANR.
Footnotes
SUPPLEMENTAL DATA
Supplemental Data include Supplemental Experimental Procedures, one table, and five figures and can be found with this article online at www.cell.com/cell-stem-cell/supplemental/S1934-5909(0900285-9).
REFERENCES
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Am Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1071–1081. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr. Biol. 2000;10:1119–1122. doi: 10.1016/s0960-9822(00)00699-0. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brown C, Gaspar J, Pettit A, Lee R, Gu X, Wang H, Manning C, Vol-and C, Goldring SR, Goldring MB, et al. ESE-1 is a novel transcriptional mediator of angiopoietin-1 expression in the setting of inflammation. J. Biol. Chem. 2004;279:12794–12803. doi: 10.1074/jbc.M308593200. [DOI] [PubMed] [Google Scholar]
- Carnac G, Fajas L, L’Honore A, Sardet C, Lamb NJ, Fernandez A. The retinoblastoma-like protein p130 is involved in the determination of reserve cells in differentiating myoblasts. Curr. Biol. 2000;10:543–546. doi: 10.1016/s0960-9822(00)00471-1. [DOI] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen RA, Fujikawa K, Madore R, Oettgen P, Varticovski L. NERF2, a member of the Ets family of transcription factors, is increased in response to hypoxia and angiopoietin-1: a potential mechanism for Tie2 regulation during hypoxia. J. Cell. Biochem. 2002;85:505–515. doi: 10.1002/jcb.10148. [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ. Res. 2005;96:e8–e24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Huser C, Crown AL, Holly JM, Stewart CE. Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFalpha. Exp. Cell Res. 2004;294:223–235. doi: 10.1016/j.yexcr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Friday BB, Pavlath GK. A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J. Cell Sci. 2001;114:303–310. doi: 10.1242/jcs.114.2.303. [DOI] [PubMed] [Google Scholar]
- Fukada SI, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular Signature of Quiescent Satellite Cells In Adult Skeletal Muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Harfouche R, Abdel-Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann. Neurol. 1992;31:46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Bonnieu A, Duret C, Vernus B, Barro M, Laoudj-Chenivesse D, Verdi JM, Carnac G. Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J. Cell. Physiol. 2006;208:538–548. doi: 10.1002/jcp.20688. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev. Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ruiz A, Gnocchi VF, Zammit PS. Control of Myf5 activation in adult skeletal myonuclei requires ERK signalling. Cell. Signal. 2007;19:1671–1680. doi: 10.1016/j.cellsig.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz A, Ono Y, Gnocchi VF, Zammit PS. {beta}-catenin promotes self-renewal of skeletal-muscle satellite cells. J. Cell Sci. 2008;121:1373–1382. doi: 10.1242/jcs.024885. [DOI] [PubMed] [Google Scholar]
- Reed SA, Ouellette SE, Liu X, Allen RE, Johnson SE. E2F5 and LEK1 translocation to the nucleus is an early event demarcating myoblast quiescence. J. Cell. Biochem. 2007;101:1394–1408. doi: 10.1002/jcb.21256. [DOI] [PubMed] [Google Scholar]
- Reynaud EG, Leibovitch MP, Tintignac LA, Pelpel K, Guillier M, Leibovitch SA. Stabilization of MyoD by direct binding to p57(Kip2) J. Biol. Chem. 2000;275:18767–18776. doi: 10.1074/jbc.M907412199. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol. Cancer Res. 2007;5:655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 2006;8:677–682. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beau-champ JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.