Abstract
Lymphedema, a common complication of cancer treatment, is characterized by inflammation, fibrosis, and adipose deposition. We have previously shown that macrophage infiltration is increased in mouse models of lymphedema. Because macrophages are regulators of lymphangiogenesis and fibrosis, this study aimed to determine the role of these cells in lymphedema using depletion experiments. Matched biopsy specimens of normal and lymphedema tissues were obtained from patients with unilateral upper extremity breast cancer-related lymphedema, and macrophage accumulation was assessed using immunohistochemistry. In addition, we used a mouse tail model of lymphedema to quantify macrophage accumulation and analyze outcomes of conditional macrophage depletion. Histological analysis of clinical lymphedema biopsies revealed significantly increased macrophage infiltration. Similarly, in the mouse tail model, lymphatic injury increased the number of macrophages and favored M2 differentiation. Chronic macrophage depletion using lethally irradiated wild-type mice reconstituted with CD11b-diphtheria toxin receptor mouse bone marrow did not decrease swelling, adipose deposition, or overall inflammation. Macrophage depletion after lymphedema had become established significantly increased fibrosis and accumulation of CD4+ cells and promoted Th2 differentiation while decreasing lymphatic transport capacity and VEGF-C expression. Our findings suggest that macrophages home to lymphedematous tissues and differentiate into the M2 phenotype. In addition, our findings suggest that macrophages have an antifibrotic role in lymphedema and either directly or indirectly regulate CD4+ cell accumulation and Th2 differentiation. Finally, our findings suggest that lymphedema-associated macrophages are a major source of VEGF-C and that impaired macrophage responses after lymphatic injury result in decreased lymphatic function.
Keywords: lymphedema, fibrosis, inflammation, macrophages, diphtheria toxin, lymphatic function
lymphedema is a disease characterized by lymphatic fluid stasis, inflammation, and fibroadipose deposition that occurs commonly after lymphatic injury during the course of cancer treatment. Although breast cancer survivors make up the largest cohort of patients with secondary (i.e., iatrogenic) lymphedema, this complication also occurs commonly in patients treated for a variety of other solid tumors, including gynecological cancers, sarcomas, and melanomas (5, 22). In these individuals, progressive adipose deposition and tissue fibrosis results in increasing limb volume, heaviness, functional difficulties, increased susceptibility to infections, and rare but deadly secondary tumors.
Several clinical findings suggest that the pathology of lymphedema is a multistep sequence with lymphatic injury serving as the initiator of these events. This hypothesis is supported by the fact that lymphedema develops only in a subset of patients who undergo lymphadenectomy rather than uniformly in all patients who suffer lymphatic injury (21). In addition, some patients develop lymphedema even after seemingly trivial injury to the lymphatic system, suggesting that even minor disturbances in lymphatic function can initiate the pathological sequence (12). Finally, the development of lymphedema in the majority of patients occurs in a delayed fashion, usually months to years after the initial surgical injury, implying that intervening secondary events are necessary for the development of this pathological process (19).
Recent studies in our laboratory have suggested that tissue fibrosis is a key step in the development of lymphedema. This hypothesis is supported by the fact that fibrosis is a hallmark of lymphedema clinically (27), and progression of disease is characterized by deposition of fibroadipose tissues and luminal obliteration of collecting lymphatic vessels with proliferative smooth muscle cells (13). In addition, it is well recognized that fibrosis is a common cause of end-organ failure in a number of organ systems, including liver, lung, skin, kidney, and heart, suggesting that lymphedema may simply represent end-organ failure of the lymphatic system because of fibrosis (32). Fibrosis also provides a rationale for the delayed development of lymphedema because the progressive abnormal collagen deposition necessary to cause organ dysfunction takes time to accumulate. Finally, recent work in our laboratory has shown that interventions designed to decrease tissue fibrosis in lymphedema can potently increase lymphatic function and decrease the pathological consequences of lymphatic injury in preclinical mouse models (1–4, 38). Thus there is a strong rationale to understand the pathological mechanisms that regulate fibrosis in lymphedema, as understanding these concepts may provide novel preventative or treatment options for a disease that presently has no cure.
Our previous studies have characterized the inflammatory cell infiltrate associated with lymphedema both clinically and in mouse models. Using a variety of techniques, we have shown that >70% of the cells that accumulate in chronically lymphedematous tissues are CD4+ cells and that neutralization of these cell types using depleting antibodies or surgical lymphedema models in CD4 knockout mice markedly decreases fibrosis and lymphatic dysfunction after lymphatic injury (3). However, we have also previously reported that the number of macrophages in the lymphedematous tissues is increased substantially compared with controls (37). In addition, in a recent report using a mouse tail model of lymphedema, we found that animals lacking Toll-like receptors had decreased macrophage accumulation and increased fibrosis/adipose deposition (35). These findings are consistent with previous studies demonstrating that macrophages play active roles in the regulation of fibrosis in other organ systems (6, 33). In addition to other studies, consistent with known roles of macrophages in the regulation of lymphangiogenesis (7, 8, 23), we have shown that macrophages in lymphedematous tissues strongly express VEGF-C (33–36). However, although it is clear that lymphedema promotes macrophage infiltration, the precise role of these cells in the regulation of fibrosis and lymphangiogenesis in response to sustained lymphatic fluid stasis has not been assessed through direct loss-of-function studies.
The purpose of the present study was therefore to determine the role of macrophages in the regulation of pathological changes in response to sustained lymphatic fluid stasis after lymphatic injury. Using a conditional ablation model of macrophages expressing diphtheria toxin receptor (DTR), we show that decreasing macrophage accumulation after lymphatic injury results in increased fibrosis and impaired lymphatic function. In addition, we report that loss of macrophages in this setting is associated with increased CD4+ cell infiltration and expression of profibrotic cytokines. Finally, we report that conditional ablation of macrophages results in decreased expression of VEGF-C in the mouse tail lymphedema model.
MATERIALS AND METHODS
Clinical lymphedema specimens.
After informed consent and approval by the Institutional Review Boards of the MD Anderson and Memorial Sloan Kettering Cancer Centers, six women with stage 2 or 3 upper-extremity breast cancer-related lymphedema were recruited and underwent full-thickness skin biopsy of their normal and lymphedematous limbs. On the basis of the relative amount of lymphedema in the various regions of the arm, matched 5-mm biopsies (i.e., harvested from the same region of the arm on both sides) were obtained from a point ∼5–10 cm above or below the elbow on the dorsal surface of the limb. Specimens were fixed overnight in 10% formalin, paraffin-embedded, sectioned, and stained with the macrophage marker EGF-like module-containing mucin-like hormone receptor-like 1 (EMR-1) (Abcam, Cambridge, MA) using our previously published immunohistochemistry protocols (3).
Animals and mouse tail model of lymphedema.
All experimental protocols were reviewed and approved by the IACUC committee at Memorial Sloan Kettering Cancer Center. Adult female C57BL/6J and CD11b-DTR [B6.FVB-Tg(ITGAM-DTR/EGFP)34Lan/J] mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were maintained in a temperature- and light-controlled environment with ad libitum access to food and water. Surgery was performed on animals ranging in age from 14–20 wk.
Tail lymphedema was established in mice according to our previously published methods (3). Briefly, a 2–3-mm portion of skin was circumferentially excised in a full-thickness manner at a point located 10 cm distal to the base of the tail. Deep lymphatic collecting vessels located along the lateral tail veins were identified and then microsurgically ligated, with care taken to avoid injury to the vascular structures. The wounds were then covered with a sterile dressing and allowed to heal by secondary intention. Control animals underwent a circumferential tail skin incision without deep lymphatic ligation or injury.
Macrophage depletion and bone marrow transplantation.
We used the CD11b-DTR mice as our model system to enable systemic, chronic macrophage depletion. These animals have been previously characterized and are transgenic animals that express the simian DTR gene under the regulation of the CD11b promoter, a gene that is highly expressed on macrophages (6, 31). Because the primate DTR is 100,000 times more avid at binding DT than the murine equivalent, administration of minute doses of the toxin results in targeted cell death and systemic depletion of CD11b-expressing cells. Although this system has been used previously in a number of settings for depletion of various cell types, the dosing and schedule of DT administration requires optimization in various transgenic lines. In addition, in some circumstances, repeated dosing of DT can cause systemic toxicity requiring extensive optimization if chronic cellular depletion is necessary. Therefore, in extensive preliminary experiments, we optimized the dosing schedule of DT (Sigma-Aldrich, St. Louis, MO) and used a treatment schedule of 2.5 ng/g intraperitoneally three times per week. Control animals received intraperitoneal injections of vehicle only (PBS) over the same time period.
To avoid systemic toxicity from repeat injections and to avoid potential confounding effects of DT ablation on nonmacrophage CD11b-expressing cells, we created bone marrow chimeras in wild-type (WT) animals reconstituted with bone marrow cells derived from CD11b-DTR mice using standard methods (17). Briefly, adult female WT mice were irradiated (950 rad) to ablate their bone marrow cells using a whole-animal irradiator, and 24 h later they were reconstituted with 5 × 106 donor bone marrow cells harvested from the femurs and humeri of female CD11b-DTR mice and delivered via lateral tail vein injection. Mice were allowed to recover for 4 wk before use in experiments.
Flow cytometry.
Flow cytometry was performed on tail tissues and lymph nodes as previously described (37). Briefly, single cell suspensions were created from whole lymph nodes by crushing the tissues between two glass slides in RPMI and filtering the suspension through a 70-μm filter. Single-cell suspensions were prepared from tail skin and subcutaneous tissue specimens by digesting tissues in a mixture of collagenase D, dispase, and DNaseI for 45 min (Roche, Nutley, NJ). The cell suspension was then passed through a 70-μm filter. All filtered single-cell suspensions were resuspended in a 2% FCS solution. Cells were then incubated in CD16/CD32 (eBiosciences, San Diego, CA) to block endogenous Fc receptors and analyzed using a Fortessa multicolor flow cytometer (BD Biosciences, San Jose, CA) with BD FACSDiva and FlowJo software (Tree Star, Ashland, OR). Cell populations were analyzed and defined using the cell surface markers B220, CD11b, CD45, CD206, F4/80, and Ly6G (all BioLegend, San Diego, CA) using three to five animals per group per experiment.
Tail volumes and lymphoscintigraphy.
Tail volume measurements were performed weekly using digital caliper measurements at 1-cm intervals beginning at the distal zone of lymphatic injury and calculated using the truncated cone formula (3). Lymphoscintigraphy using technetium-99m sulfur colloid (Tc99) was used to analyze lymphatic transport function and performed using our previously reported methods (24). Briefly, clinical-grade unfiltered Tc99-sulfur colloid was purchased from Nuclear Diagnostic Products (Rockaway, NJ) and used as supplied. The radiochemical purity of the Tc99-sulfur colloid was confirmed by the manufacturer using thin-layer chromatography (Whatman 3MM paper developed in normal saline) and was uniformly 92% or better. The preparation was also checked visually under a microscope to verify the absence of any aggregates. The administered activity for each mouse imaged was assayed in a radioisotope calibrator. Using a high-molecular-weight-conjugated tracer insured that activity was only seen in conjunction with the colloid (i.e., uptake limited to the lymphatics). Tc99 was injected in a small volume and under constant pressure in the distal portion of the tail, and decay-adjusted uptake was measured in the sacral lymph nodes using an X-SPECT camera (Gamma Medica, Northridge, CA). Analysis was completed using ASIPro software (CTI Molecular Imaging, Knoxville, TN), and regions of interests were quantified to measure peak and rate (i.e., slope of the best-fit line) uptake of Tc99.
Histology, cell counts, and fibrosis.
Tail tissues were harvested, briefly fixed in 4% paraformaldehyde (Affymetrix, Cleveland, OH), decalcified, and paraffin embedded using our previously published methods (3). Hematoxylin and eosin staining was completed using standard protocols. Adipose deposition area in the subcutaneous tissues was outlined and calculated using Pannoramic Viewer (3DHISTECH, Budapest, Hungary). Dermal thickness was measured in multiple quadrants in the lymphedematous tail and was quantified using Pannoramic Viewer.
Immunohistochemical and immunofluorescent staining was performed using our previous methods for CD45, DTR (both from R&D Systems, Minneapolis, MN), Gata-3 (BD Biosciences), CD4, collagen I, LYVE-1, and F4/80 (all from Abcam) (3). Negative control sections were incubated with isotype control antibody or secondary antibody alone, and specificity was confirmed using known positive and negative controls. Positively stained cells in the dermis and immediate subcutaneous areas were identified, and cell counts were performed in three random high-powered fields/animal by two reviewers who were blinded to the experimental groups. For analysis of lymphatic vessel counts, LYVE-1+ vessels were identified in the dermis of cross-sectional histological sections located 1.5 cm distal to the zone of lymphatic injury using scanned images, and a minimum of three areas were analyzed in each animal. For analysis of lymphatic vessel area, LYVE-1+ vessels were identified in the dermis of cross-sectional histological sections, and the luminal area was calculated in a random selection of five to eight vessels in each quadrant of the distal tail.
Sirius red scar index was calculated to analyze fibrosis and collagen deposition patterns as previously reported (1, 3). Briefly, tissues were stained with Sirius red (Direct Red 80; Sigma-Aldrich), washed extensively, and imaged using an Axio Scope microscope (Carl Zeiss, Jena, Germany). Birefringence patterns and hue, reflecting patterns of collagen deposition and organization, were analyzed in the dermis using Metamorph Offline Software (Molecular Devices, Sunnyvale, CA) in a minimum of three sections per animal per group. Scar index was calculated by comparing the ratio of orange/red to yellow/green staining and is expressed as an arbitrary number with higher numbers representing more fibrosis.
Protein and RNA analysis.
VEGF-C protein expression was quantified in total protein isolated from the tail tissues using ELISA (eBiosciences) as previously described (3). Briefly, skin and subcutaneous tissues were harvested and lysed using the Qiagen DNA/RNA/Protein mini kit using the manufacturer's methods (Qiagen, Valencia, CA). Protein concentration was quantified using the Bradford method, and VEGF-C expression was analyzed in triplicate samples of 20 μg of total protein per animal.
For analysis of tissue mRNA expression, a 5-mm section of distal tail skin was harvested, snap-frozen in liquid nitrogen, and homogenized in Trizol (Life Technologies, Carlsbad, CA). Total cellular RNA was extracted, and RNA quality and integrity were assessed using the Agilent bioanalyzer (Santa Clara, CA). Equal amounts of RNA were reverse transcribed into cDNA, and RT-PCR was performed with validated primers for Prox-1, IL-13, LYVE-1, and GAPDH using the TaqMan system as previously described (all reagents Life Technologies) (36). Results were obtained using the Viia7 software (Life Technologies) normalized to GAPDH and analyzed with the ΔΔ method to compare groups (25). Results are represented as fold change compared with controls.
Statistical analysis.
The Student's t-test was used to compare differences between two groups, whereas comparison of multiple groups was performed using ANOVA with post hoc tests (Tukey- Kramer) to compare differences between individual groups. Data were analyzed and presented graphically using GraphPad software (La Jolla, CA). Graphs are presented as box-and-whisker plots with the box representing the 25–75% data range, the line representing the sample median, and the whiskers representing the data minimum and maximum. In all experiments, a P value of <0.05 was considered significant. All experiments were performed in triplicate, and for each individual experiment 6–10 animals were used for each group unless otherwise noted.
RESULTS
Lymphedema results in increased macrophage infiltration.
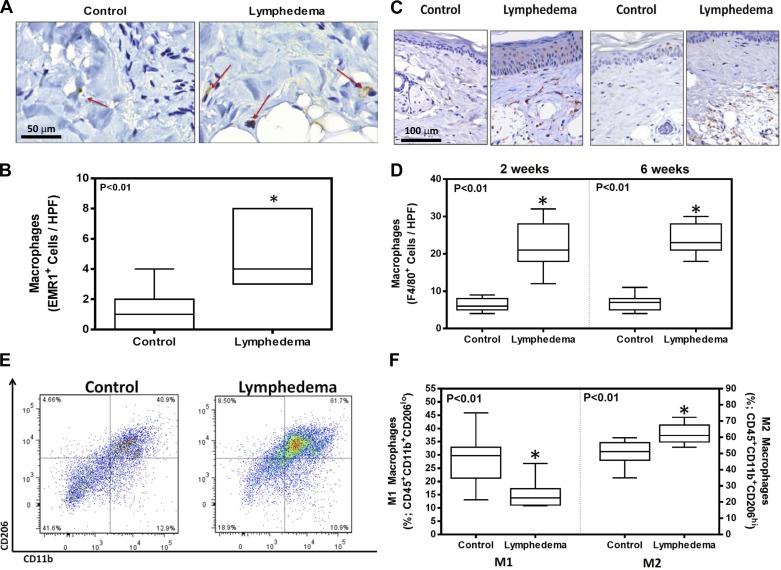
Analysis of matched skin biopsies from patients with upper-extremity breast cancer-related lymphedema demonstrated a greater than threefold increase in the number of macrophages (EMR-1+ cells) in the subcutaneous and dermal tissues of lymphedematous tissues compared with controls (Fig. 1, A and B; P < 0.01). Although macrophages were primarily present in the dermal areas, they could also be observed adjacent to adipocytes just below the dermis. Similarly, we found that lymphatic injury in the mouse tail model also results in significant accumulation (3.3–3.5-fold increase) of macrophages (F4/80+) in the dermis at an early time point after surgery and is sustained even 6 wk later (Fig. 1, C and D; P < 0.01). Using flow cytometry on single cell suspensions harvested from mouse tail tissues 6 wk after surgery, we found that the lymphedema results in a relative decrease in the percentage of M1 cells (CD11b+, CD206lo) and an increase in the percentage of M2 cells (CD11b+, CD206hi) compared with controls (Fig. 1, E and F).
Fig. 1.
Lymphedema results in increased macrophage infiltration. A: representative high-powered field (HPF) (×100) histological sections of matched control (normal limb) and lymphedematous tissues obtained from a patient with unilateral upper-extremity breast cancer-related lymphedema. Red arrows show EGF-like module-containing mucin-like hormone receptor-like 1 (EMR1) positively stained macrophages. B: cell counts of EMR+ macrophages in control and lymphedematous limbs of patients with breast cancer-related upper extremity lymphedema (n = 8; *P < 0.01). C: representative HPF (×80) histological images of tissues obtained from mice that underwent tail incision without lymphatic ligation (control) and lymphedematous tails 2 and 6 wk after surgery stained for F4/80+ macrophages. D: cell counts of F4/80+ cells in control and lymphedematous tail tissues 2 and 6 wk after tail surgery (n = 10; *P < 0.01). E: representative flow diagrams of tail tissues obtained from control and lymphedematous tail tissues 6 wk after surgery identifying cells that express CD206 and CD11b. F: quantification of M1 (CD11b+CD206lo) and M2 (CD11b+CD206hi) macrophages in control and lymphedematous tail tissues 6 wk after surgery (n = 8; *P < 0.01).
Chronic ablation of macrophages in CD11b-DTR mice is effective but has systemic toxicity.
As expected, acute treatment of the CD11b-DTR mouse with DT resulted in significant and dose-dependent depletion of macrophages 24 h after toxin administration. One dose of 25 ng/g of DT led to a marked decrease (nearly 80%) in splenic and lymph node macrophages (not shown), whereas smaller doses (2.5 ng/g) led to an ∼35% decrease in macrophage number. The highest doses of DT administered caused minor systemic toxicity when administered as a one-time dose; there was no discernable systemic effect from the lower doses used (<5 ng/g; not shown). Maximum macrophage depletion occurred by 24 h after DT administration, and cell numbers rebounded by 72 h (not shown). Therefore, we optimized a schedule of dosing to enable chronic and sustained depletion of macrophages. This was achieved with a dose of 2.5 ng/g administered intraperitoneally every 3 days and resulted in a nearly 75% depletion of macrophage numbers that was sustained for long periods (2–3 wk; not shown). Animals tolerated this dosing schedule well without evidence of systemic toxicity or weight loss for a period of 2–3 wk. However, when dosing was continued for longer periods as required by our study, we encountered increasing toxicity and decreased survival beginning at the 3-wk time point (not shown). Because long-term and sustained macrophage depletion was a primary goal of our studies, we devised other approaches to decrease systemic toxicity and achieve our goal.
Chimeric CD11b-DTR have sustained macrophage depletion without systemic toxicity.
Macrophages are hematopoietic cells; therefore, we reasoned that creation of bone marrow chimeras transferring the bone marrow from CD11b-DTR mice into WT mice would decrease the toxicity and off-target effects of chronic DT treatment while still enabling sustained macrophage depletion (Fig. 2A). In the chimeric animals, bone marrow immunofluorescence revealed that over 75% of the macrophages coexpressed DTR (Fig. 2B). Furthermore, >80% of macrophages present in lymphedematous tail tissue were bone marrow derived and coexpressed F4/80 and DTR (not shown). Consistent with this, we found that chimeric mice were highly sensitive to DT treatment such that even low doses of DT administered three times per week (2.5 ng/g) resulted in a greater than threefold decrease in the percentage of macrophages in peripheral lymph nodes as assessed by flow cytometry (Fig. 2, C and D; P < 0.01). This treatment also substantially decreased the number of macrophages in lymphedematous tail tissues as assessed by immunohistochemical localization of F4/80+ cells (>2-fold decrease; Fig. 2, E and F; P < 0.01). More importantly, chimeric mice displayed no evidence of systemic toxicity or weight loss (not shown). Because CD11b is also expressed in variable levels in other cell types derived from the granulocyte lineage, we also found modest (30%) but significant reductions in the number of bone marrow neutrophils after DT administration (not shown; P < 0.05).
Fig. 2.
Chimeric CD11b/diphtheria toxin receptor (DTR) have sustained macrophage depletion without systemic toxicity. A: diagrammatic representation of bone marrow chimeras created from wild-type (WT) mice reconstituted with bone marrow from CD11b-DTR mice. B: immunofluorescent staining of chimeric bone marrow showing costaining of CD11b and DTR. C: representative flow diagram of macrophages (B220+/CD11b+) harvested from the peripheral lymph nodes of animals treated with vehicle control or DT (3 wk after 3 times weekly treatment). D: quantification of macrophages in peripheral lymph nodes of mice treated with or without DT for 3 wk (*P < 0.01). E: representative immunohistochemical staining of lymphedematous tail tissues harvested 3 wk after 3 times weekly treatment with or without DT. Arrows show positively stained macrophages. F: quantification of F4/80+ cells per HPF (×80 magnification) in lymphedematous tissues of mice treated with or without DT for 3 wk (n = 8; *P < 0.01).
Depletion of macrophages does not alter tail volumes or adipose deposition.
To determine the temporal effects of macrophage depletion after lymphatic injury, chimeric WT/CD11b-DTR animals underwent tail skin and deep lymphatic excision and were treated with DT to deplete macrophages beginning either immediately after surgery (immediate DT) or starting 3 wk postoperatively (3 wk DT). Control animals were treated with vehicle only (PBS; i.e., not depleted). DT treatments were continued every 3 days for 3 wk, and animals were killed 6 wk after surgery.
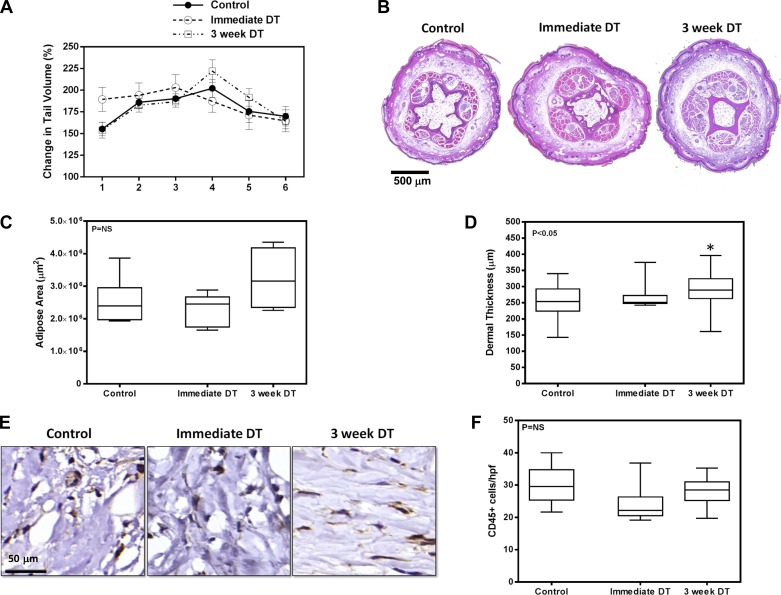
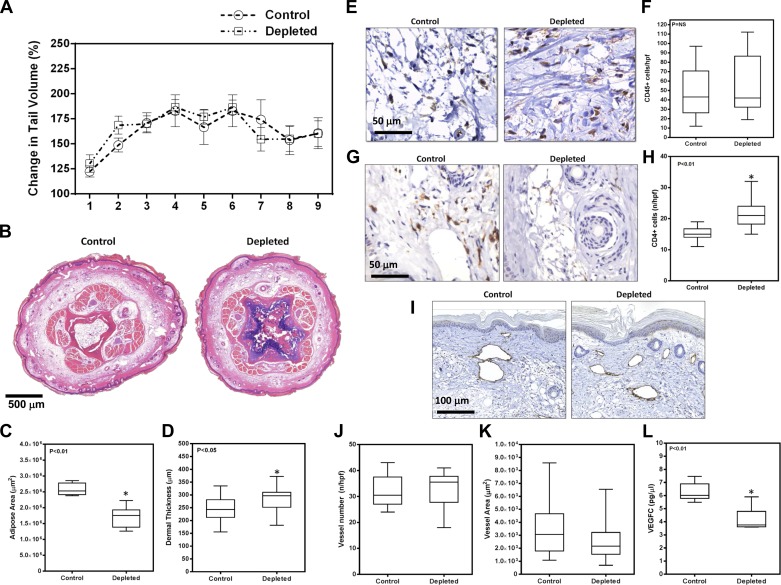
We were somewhat surprised to find no significant differences in tail volume measurements at any time point postoperatively (Fig. 3A). Similarly, although there was a modest increase in adipose area and dermal thickness in the 3-wk DT group, these differences only achieved statistical significance for dermal thickness when compared with controls, and the relative differences were modest (Fig. 3, B–D). In addition, analysis of inflammation 6 wk after surgery using the pan-leukocyte marker CD45 in histological sections showed no differences in overall tissue inflammation as a consequence of immediate or 3-wk DT macrophage depletion (Fig. 3, E and F). Taken together, these findings suggest that macrophage infiltration either immediately after lymphatic injury or even after lymphatic stasis has become established does not significantly contribute to adipose deposition or overall inflammatory reactions associated with interstitial fluid stasis.
Fig. 3.
Depletion of macrophages does not alter tail volumes or adipose deposition. A: tail volumes of mice treated with vehicle control, DT beginning immediately after surgery (immediate DT), or DT beginning 3 wk after surgery (3-wk DT). B: representative cross-sectional histological sections of control, immediate DT, and 3-wk DT-treated mice (×2.5 magnification) harvested 6 wk after surgery. Sections were harvested 1.5 cm distal to the zone of lymphatic injury. C: quantification of adipose area in control, immediate DT, and 3-wk DT groups from cross-sectional histological sections harvested 6 wk after surgery. D: dermal thickness of various groups analyzed in cross-sectional histological sections (n = 8; *P < 0.05). E: representative HPF photomicrograph (×80) of tail tissues stained for CD45. F: quantification of CD45+ cells in tail sections of mice treated with vehicle control, immediate DT, or DT 3 wk after surgery.
Depletion of macrophages increases tissue fibrosis and impairs lymphatic function.
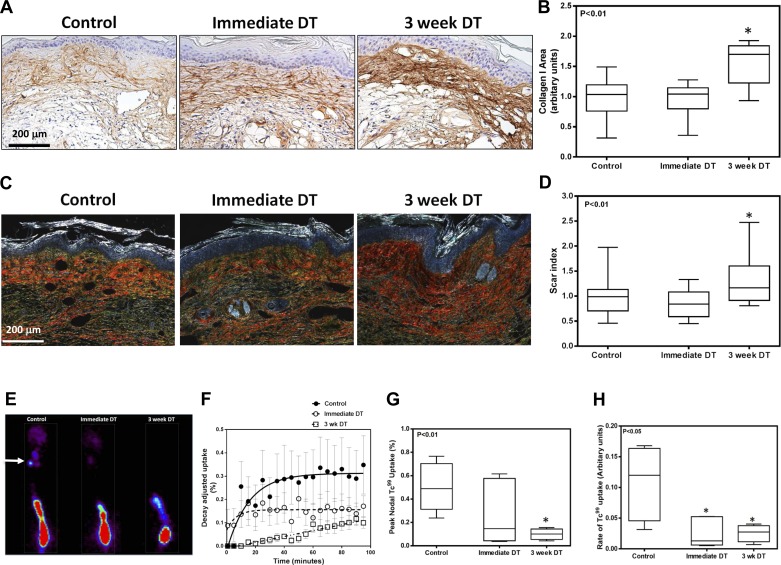
Consistent with our observation of increased dermal thickness in the 3-wk DT animals, histological analysis of tissue sections from DT-treated and control animals harvested 6 wk after surgery demonstrated that depletion of macrophages beginning at 3 wk postoperatively resulted in a significant increase in collagen type I deposition compared with control animals (Fig. 4, A and B). Thickened collagen bundles could easily be observed in the subdermal and dermal areas of the skin surrounding capillary lymphatics in this area. This observation was confirmed with Sirius red staining demonstrating increased scar index indicative of increased organized collagen fiber deposition in the 3-wk DT group (Fig. 4, C and D). We also noted decreased lymphatic function in the 3-wk DT group as reflected by markedly decreased Tc99 transport (Fig. 4, E–H). This analysis demonstrated a decrease both in peak nodal uptake by the sacral lymph nodes (P < 0.01 for 3-wk DT group) as well as a significant decrease in the rate of Tc99 uptake in all macrophage-depleted animals, suggesting that interstitial transport capacity is greatly diminished after macrophage depletion.
Fig. 4.
Depletion of macrophages increases tissue fibrosis and impairs lymphatic function. A: representative HPF photomicrographs (×40) of tail sections stained for type I collagen. B: quantification of type I collagen staining in tail sections of mice treated with vehicle control, immediate DT, or DT beginning 3 wk after surgery (n = 8; *P < 0.01). C: representative photomicrographs of tail sections stained with Sirius red and imaged using polarized light microscopy (×40 magnification). D: scar index of various groups quantified from Sirius red-stained tissues (n = 8; *P < 0.01). E: representative Tc99 heat maps of tails from control, immediate DT, and 3 wk postsurgery DT-treated animals. Hot spot at the bottom of the photograph is the injection site. Small areas of uptake at the top of the picture are the sacral lymph nodes (white arrow). F–H: graphs depicting decay adjusted uptake (F), peak nodal uptake (G) (n = 8; *P < 0.01), and rate of uptake (H) (n = 8; *P < 0.05) in the sacral lymph nodes of animals in various groups.
Macrophage depletion increases CD4+ cell infiltration and Th2 differentiation.
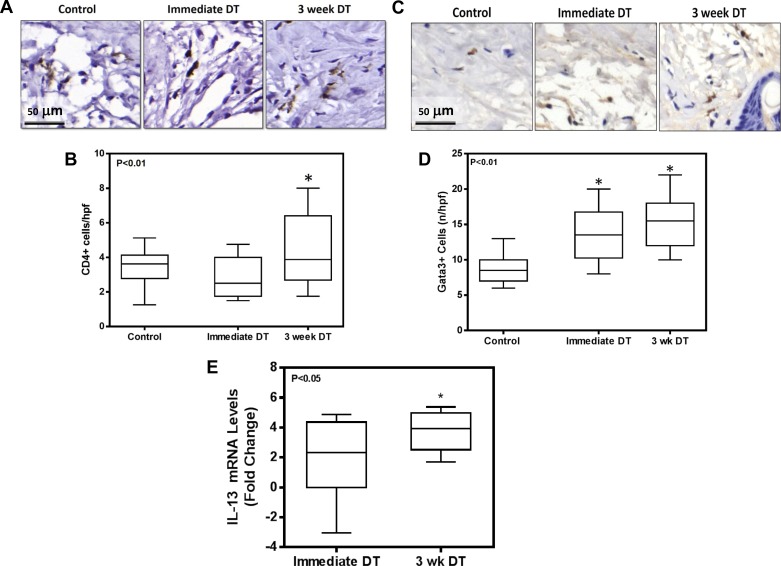
We have previously shown that CD4+ cells play a significant role in the regulation of fibrosis in lymphedema (3, 37). Therefore, we next sought to determine how macrophages regulate CD4+ cell responses. Consistent with our observation of increased fibrosis in the 3-wk DT group, we found that depletion of macrophages 3 wk after lymphatic injury resulted in modest but significant increases in the number of CD4+ cells infiltrating the distal lymphedematous tail tissues (Fig. 5, A and B). In addition, we noted increased numbers of cells that stained positive for Gata-3, a transcription factor necessary for Th2 differentiation of naïve CD4+ cells, suggesting that Th2 differentiation was increased in animals depleted of macrophages either immediately after or beginning 3 wk after surgery (Fig. 5, C and D). This observation was supported by a greater than threefold increase in the gene expression of IL-13, a Th2 cytokine, in macrophage-depleted animals (Fig. 5E; P < 0.05). Taken together, these findings suggest that macrophage accumulation after lymphatic injury can regulate or modulate CD4+ cell infiltration and Th2 differentiation.
Fig. 5.
Macrophage depletion increases CD4+ cell infiltration and Th2 differentiation. A: representative HPF (×80) photomicrographs of tail sections stained for CD4+ cells. B: quantification of CD4+ cells per HPF in tissue sections harvested from animals in various groups (n = 8; *P < 0.01). C: representative HPF (×80) photomicrographs of tail sections stained for Gata-3+ cells. D: quantification of Gata-3+ cells per HPF in various experimental groups (n = 8; *P < 0.01). E: IL-13 gene expression relative to control animals in experimental animals treated with DT either immediately after surgery or beginning 3 wk postoperatively. Data are presented as fold change vs. control corrected for GAPDH expression (n = 8; *P < 0.05).
Macrophage depletion decreases VEGF-C expression.
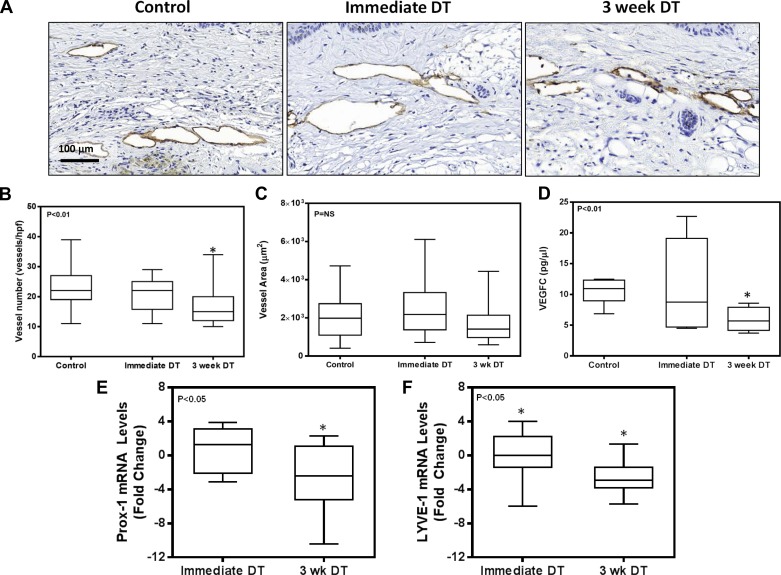
Analysis of LYVE-1+ vessel counts in the tail tissues of experimental and control mice demonstrated that depletion of macrophages 3 wk after surgery modestly (1.5-fold) but significantly decreased the total number of capillary lymphatic vessels (Fig. 6, A and B; P < 0.05). However, the lymphatic vessel luminal area was unchanged (Fig. 6C). Consistent with the decreased number of LYVE-1+ vessels in the 3-wk DT group, we noted a decrease in the expression of VEGF-C protein in tissue samples harvested from animals in this group (Fig. 6D). These changes were reflected in our qPCR findings analyzing Prox-1 and LYVE-1 expression among the control, immediate DT, and 3-wk DT groups. Using the control group as the standard for the ΔΔ method, we found a nearly fourfold decrease in the expression of these lymphatic markers in the 3-wk DT group compared with control animals (Fig. 6, E and F). In contrast, we found no significant differences in VEGF-C or Prox-1 expression in animals treated with DT immediately after surgery.
Fig. 6.
Macrophage depletion decreases VEGF-C expression. A: representative photomicrographs (×40) of tissue sections stained with LYVE-1 in various experimental groups. B: quantification of the number of LYVE-1+ vessels in tail tissue sections (n = 8; *P < 0.01). C: quantification of LYVE-1+ vessel area in tail tissue sections. D: VEGF-C protein expression in total cellular protein harvested from tail tissue sections of various experimental groups (n = 8; *P < 0.01). E and F: expression of Prox-1 (E) and LYVE-1 (F) in tail tissues in various experimental groups. Values are expressed as mRNA level fold change relative to control animals that were not treated with DT (n = 8; *P < 0.05).
Depletion of macrophages after lymphedema is established decreases adipose deposition.
In our initial set of experiments, we sought to determine the role of macrophages on the development of pathological changes associated with lymphedema beginning shortly after lymphatic injury. We next sought to determine how changes in macrophage infiltration modulate the pathological effects of sustained lymphatic stasis after lymphedema had become established. To accomplish this, we performed tail skin and lymphatic excision surgery on WT/CD11b-DTR bone marrow chimera mice and allowed them to recover for a period of 6 wk. We have previously shown that, at 6 wk, mice have significant lymphedema changes in the tail, including adipose deposition, fibrosis, and persistent CD4+ cell accumulation (3). Beginning at the 6-wk time point, animals were depleted of macrophages using thrice weekly DT injections for a period of 3 wk, and pathological changes were assessed.
Similar to our findings with immediate or early macrophage depletion, we found no significant differences in tail volumes after DT administration in animals treated after lymphedema had become established (Fig. 7A). However, in contrast to our earlier findings, we did note a modest (1.5-fold) but significant decrease in adipose deposition in these animals after macrophage depletion, whereas dermal thickness was increased (Fig. 7, B and C). Although we found no overall differences in tissue inflammation as assessed by CD45+ cell counts (Fig. 7, D and E), we again noted that there was a significant increase in the number of CD4+ cells in tissues obtained from macrophage-depleted animals (Fig. 7, F and G). There were no significant differences between groups when comparing LYVE-1+ vessel counts or LYVE-1 vessel area; however, similar to our earlier observation, we noted a decrease in the concentration of VEGF-C protein by ELISA (Fig. 7, H–K).
Fig. 7.
Depletion of macrophages after lymphedema is established decreases adipose deposition. A: tail volumes of mice treated with or without DT beginning 6 wk after surgery when lymphedema had become established. B: representative cross-sectional histology of tail sections stained with hematoxylin and eosin (×2.5 magnification). C: quantification of adipose area in control and depleted animals (n = 8; *P < 0.01). D: quantification of dermal thickness in control and depleted animals (n = 8; *P < 0.05). E: representative HPF photomicrographs (×80) of tail tissues harvested from control and DT-depleted animals stained for CD45. F: quantification of CD45+ cells in tail tissues of control and macrophage-depleted animals. G: representative HPF photomicrographs (×80) of tail tissues harvested from control and DT-depleted animals stained for CD4. H: quantification of CD4+ cells per HPF in tail tissues of control and macrophage-depleted animals (n = 8; *P < 0.01). I: representative photomicrograph of tail tissues from control and macrophage-depleted animals stained with LYVE-1. J: quantification of LYVE-1+ vessels/HPF in control and experimental animals. K: quantification of LYVE-1+ vessel areas in control and experimental animals. L: VEGF-C protein expression in total cellular protein harvested from tail tissue sections of experimental and control animals (n = 8; *P < 0.01).
Late macrophage depletion increases fibrosis and impairs lymphatic function.
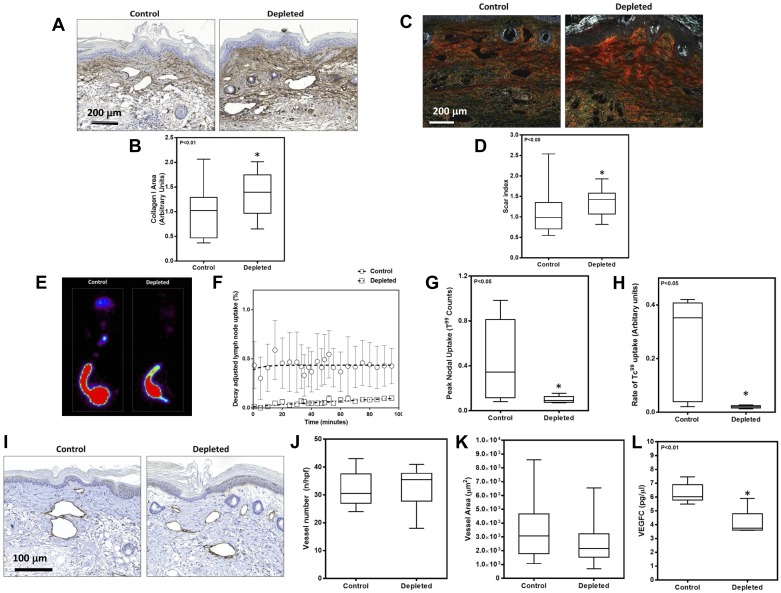
Consistent with our observations in animals depleted of macrophages shortly after lymphatic injury, we found that late depletion of these cells also results in increased fibrosis as assessed by collagen I immunostaining and Sirius red birefringence (Fig. 8, A–D). Similar to our earlier observations, we noted dense collagen fiber deposition in the dermal areas with prominent staining surrounding capillary lymphatic structures. This increase in fibrosis was reflected in a significant impairment in lymphatic transport as assessed by Tc99 lymphoscintigraphy. Macrophage-depleted animals displayed a greater than 4-fold decrease in peak nodal uptake and a greater than 12-fold decrease in the rate of Tc99 uptake compared with controls (Fig. 8, E–H). In addition, although we found no differences in LYVE-1+ vessel number or diameter, we did note significant decreases in VEGF-C protein expression after macrophage depletion (Fig. 8, I–L). Taken together, these findings suggest that macrophage inflammatory reactions after lymphedema has become established contribute either directly or indirectly to adipose deposition, exert an antifibrotic effect, and regulate VEGF-C expression.
Fig. 8.
Late macrophage depletion increases fibrosis and impairs lymphatic function. A: representative photomicrographs (×40) of tail tissues from control and macrophage-depleted animals stained for type I collagen. B: quantification of type I collagen staining area in experimental and control animals (n = 8; *P < 0.01). C: representative photomicrographs (×40) of tail sections stained with Sirius red and imaged using polarized light microscopy. D: scar index of control and experimental animals quantified from Sirius red-stained tissues (n = 8; *P < 0.05). E: representative Tc99 heat maps from control and macrophage-depleted animals. F–H: graphs depicting decay adjusted uptake (F), peak nodal uptake (G), and rate of uptake (H) in the sacral lymph nodes of animals in control and macrophage depleted animals (n = 8; *P < 0.05). I: representative photomicrographs (×40) of tissue sections stained with LYVE-1 in various experimental groups. J: quantification of the number of LYVE-1+ vessels in tail tissue sections. K: quantification of LYVE-1+ vessel area in tail tissue sections. L: VEGF-C protein expression in total cellular protein harvested from tail tissue sections of control and macrophage-depleted animals (n = 8; *P < 0.01).
DISCUSSION
In the present study, consistent with our previous publications and the work of others, we have shown that lymphedema, both clinically and in the mouse tail model, results in a significant accumulation of macrophages. Although the mechanisms that regulate macrophage homing, proliferation, and differentiation in lymphedema remain unknown, several lines of evidence suggest that this process is regulated by T cell inflammatory reactions. For example, our group has previously shown that depletion or loss of T cells in general, or CD4+ cells in particular, results in significant attenuation of macrophage accumulation in the mouse tail model of lymphedema (3). We have also shown that lymphedema-induced CD4+ cell inflammatory response is necessary for increased expression of inflammatory cytokines (e.g., interferon-γ, IL-4, and IL-6) that regulate macrophage migration and proliferation (3, 37). In addition, we have previously shown that lymphedema increases Th2 inflammatory responses, thus providing a rationale for the M2-biased macrophage inflammatory responses we observed in lymphedematous mouse tail tissues in the present study (3). The hypothesis that T cell inflammatory responses are necessary for macrophage migration and proliferation in lymphedema is also supported by studies on adipose tissue inflammation in obese individuals and mouse models demonstrating that T cell inflammatory reactions precede, and are necessary for, macrophage infiltration (16). Because a major component of the pathology of lymphedema involves abnormal adipose deposition, it is possible that similar inflammatory reactions to adipose inflammation drive macrophage migration to lymphedematous tissues. It is likely, however, that macrophage infiltration in lymphedema is regulated by multiple mechanisms because it is known that inflamed adipose tissues, as is characteristic of lymphedema and obesity, can directly promote macrophage migration and proliferation through the release of free fatty acids by necrotic adipocytes and elaboration of macrophage chemotactic factors such as monocyte chemoattractant protein 1 (10, 28). Taken together, these studies suggest that T cells and adipose tissue deposition play critical roles in the regulation of macrophage migration and proliferation in lymphedema. Future studies should address these pathways in more detail.
An interesting observation of our study was that lymphedema in the mouse tail model favored M2 differentiation of tissue macrophages. This finding is important because M2, or alternatively activated macrophages, are known to play key roles in the regulation of lymphangiogenesis by producing VEGF-C and because these cell types contribute to tissue remodeling by regulating production of matrix metalloproteinases and collagen by other cell types (15, 23, 38). Our observation is supported by previous studies demonstrating that tissue macrophages that accumulate in response to lymphedema express high levels of VEGF-C, consistent with M2 differentiation status (29, 35, 36). In addition, the known roles of M2 macrophages provide a rationale for our finding that depletion of macrophages after lymphatic injury significantly decreases the expression of VEGF-C and expression of lymphatic markers while increasing tissue fibrosis compared with controls. The combined effect of these changes may therefore be responsible for diminished lymphatic function that we observed after macrophage depletion. Taken together, these findings suggest that macrophage infiltration in lymphedema is reactive to ongoing inflammatory responses, and macrophages in this setting act to augment lymphangiogenesis and diminish fibrosis. This role is in contrast to CD4+ cells, which our laboratory has previously shown to contribute, either directly or indirectly, to the pathology of lymphedema.
Although a variety of techniques have been described for macrophage depletion, we chose the CD11b-DTR transgenic model because this protocol enabled us to effectively and chronically deplete tissue macrophages for long periods of time in a cost-effective manner. This approach required a significant amount of work for optimization; however, when optimized, we were able to successfully deplete macrophages systemically and in lymphedematous tissues. In contrast, although the use of clodronate liposomes is well described and effective for systemic macrophage ablation (11, 30), previous studies have shown that this approach is less effective for depletion of tissue macrophages compared with circulating cells because liposomes do not cross endothelial cell membranes (9). Similarly, other studies have depleted macrophages systemically by using small molecule inhibitors or neutralizing antibodies directed against colony-stimulating factor 1 or its receptor, as this signaling pathway is essential for macrophage proliferation and differentiation (20). However, although this approach is effective and decreases macrophage cell populations significantly in short-term studies, the use of these treatments for more chronic conditions such as lymphedema is hampered by the relative high cost of these reagents when administered over long periods of time.
We were somewhat surprised to find that macrophage depletion in the mouse tail model did not significantly decrease overall inflammatory responses but in fact was associated with increased CD4+ cell infiltration. Although this finding is somewhat counterintuitive, a possible explanation may be that macrophages that accumulate in response to lymphedema play an anti-inflammatory or immunosuppressive role. This concept is supported by our observation of M2-biased macrophage responses, as these cells are known to have immunosuppressive effects and limit inflammatory responses in other physiological settings (14). Thus, although macrophage depletion in our study decreased the number of tissue macrophages, the generalized inflammatory responses (and more specifically T-cell infiltration) to lymphedema may have been augmented because of loss of M2 macrophages, resulting in a net lack of difference in overall number of inflammatory cells. Alternatively, it is possible that depletion of macrophages had little effect on overall inflammatory response to lymphedema because macrophages make up only a small proportion of cells in the inflammatory milieu of chronic lymphedema. This hypothesis is supported by our previous studies demonstrating that the vast majority (>70%) of leukocytes present in lymphedematous tissues are CD4+ cells (3).
An interesting finding in the present study was that depletion of macrophages was associated with a significant increase in collagen deposition, fibrosis, and impaired lymphatic function. Previous studies have shown that macrophages can promote both pro- and antifibrotic effects and that these responses are organ specific and differ temporally even within the same organ system (6). For example, macrophages have been shown to promote liver and renal fibrosis by producing profibrotic cytokines such as transforming growth factor-β1, activating fibroblasts, promoting migration of myofibroblasts, increasing collagen synthesis, and decreasing extracellular matrix turnover (26, 33). In other circumstances, macrophages can aid in reversal of fibrosis by phagocytosing apoptotic or injured cells, directly removing collagen from the extracellular matrix, promoting breakdown of extracellular matrix products by increasing production of matrix metalloproteinases, and decreasing the expression of profibrotic cytokines. Although the precise mechanisms by which macrophages regulate fibrosis in lymphedema remain unknown and require additional study, our present study suggests that this response involves interactions with CD4+ cells (more specifically with Th2-differentiated cells) because macrophage depletion significantly increased these responses. This concept is supported by previous studies in pathogenic responses and fibrosis in response to schistosomiasis infections (18).
In conclusion, using a mouse model as well as clinical specimens of lymphedema, we have shown that macrophage infiltration is significantly augmented in response to lymphatic injury and lymphedema. In addition, we have demonstrated that the majority of macrophages present in lymphedema are M2 differentiated and that depletion of macrophages either before or after lymphedema is established results in decreased VEGF-C expression and increased fibrosis, collagen deposition, and CD4+ cell infiltration. Our findings, therefore, suggest that macrophage infiltration in response to lymphedema is reactive, aiming to decrease overall inflammation and inhibit fibrosis. However, further studies are needed to test this hypothesis and define the mechanisms that regulate macrophage migration and function after lymphatic injury.
GRANTS
This work was supported by NIH R01 HL111130-01 (B. Mehrara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.G., D.A.C., D.C., J.Z., and B.J.M. conception and design of research; S.G., D.A.C., J.S.T., N.J.A., W.J.J., I.L.S., J.C.G., and J.Z. performed experiments; S.G., J.S.T., N.J.A., W.J.J., I.L.S., and J.C.G. analyzed data; S.G., J.S.T., W.J.J., I.L.S., J.C.G., and B.J.M. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G. and B.J.M. edited and revised manuscript; S.G., D.A.C., J.S.T., N.J.A., W.J.J., I.L.S., J.C.G., D.C., J.Z., and B.J.M. approved final version of manuscript.
REFERENCES
- 1.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202–3214, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avraham T, Yan A, Zampell JC, Daluvoy SV, Haimovitz-Friedman A, Cordeiro AP, Mehrara BJ. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am J Physiol Cell Physiol 299: C589–C605, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27: 1114–1126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 116: 5138–5149, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res 96: 1193–1199, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Goldman j Rutkowski J, Shields J, Pasquier M, Cui Y, Schmökel H, Willey S, Hicklin D, Pytowski B, Swartz M. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 21: 1003–1012, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 26: 5213–5219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihara M, Hara H, Hayashi Y, Narushima M, Yamamoto T, Todokoro T, Iida T, Sawamoto N, Araki J, Kikuchi K, Murai N, Okitsu T, Kisu I, Koshima I. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 7: e41126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32: 463–488, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92: 1368–1377, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19: 1264–1272, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockson SG. Secondary lymphedema: is it a primary disease? Lymphat Res Biol 6: 63–64, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann NY Acad Sci 1131: 147–154, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 72: 161–171, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307: H165–H172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Shen B, Liu X, Fan Y, Qiu J. Macrophages regulate renal fibrosis through modulating TGFbeta superfamily signaling. Inflammation 15: 15, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Suami H, Pan WR, Taylor GI. Changes in the lymph structure of the upper limb after axillary dissection: radiographic and anatomical study in a human cadaver. Plast Reconstr Surg 120: 982–991, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 25: 2062–2068, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 294: H1326–H1334, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol 184: 1167–1184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30: 245–257, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan A, Avraham T, Zampell J, Aschen S, Mehrara B. Mechanisms of lymphatic regeneration after tissue transfer. PLoS One 6: e17201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zampell J, Elhadad S, Avraham T, Weitman E, Aschen S, Yan A, Mehrara BJ. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol 302: C709–C719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampell JC, Yan A, Avraham T, Daluvoy S, Weitman ES, Mehrara BJ. HIF-1α coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J 26: 1027–1039, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 7: e49940, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Wang J, Gao J, Guo Y, Chen X, Wang B, Gao J, Rao Z, Chen Z. Alternatively activated RAW264.7 macrophages enhance tumor lymphangiogenesis in mouse lung adenocarcinoma. J Cell Biochem 107: 134–143, 2009. [DOI] [PubMed] [Google Scholar]