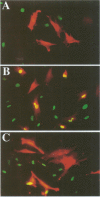
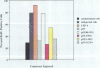
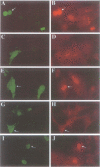
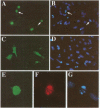
Abstract
The p53 tumor suppressor limits cellular proliferation by inducing either G1 arrest or apoptosis, depending on the cellular context. To determine if these pathways are mechanistically distinct, we have examined the effects of different p53 mutants in p53 null primary mouse embryo fibroblasts. We chose this system as it is highly physiological and ensures that the interpretation of the results will not be confounded by the presence of endogenous p53 or oncoproteins which target p53. Using single cell microinjection assays for both G1 arrest and apoptosis, with loss-of-function and chimeric gain-of-function mutants, we have demonstrated that transcriptional activation is critical for both processes. Replacement of the p53 activation domain with that of VP16, or replacement of the p53 oligomerization domain with that of GCN4, reconstituted both G1 arrest and apoptosis activities. However, despite the importance of transcriptional activation in both processes, the target gene requirements are different. The p21 cyclin-dependent kinase inhibitor, which has been shown to be a direct target of p53 and a component of the radiation-induced G1 arrest response, is dispensable for oncogene-induced apoptosis, suggesting that these two p53-dependent transcriptional pathways are distinct.
Full text
PDF
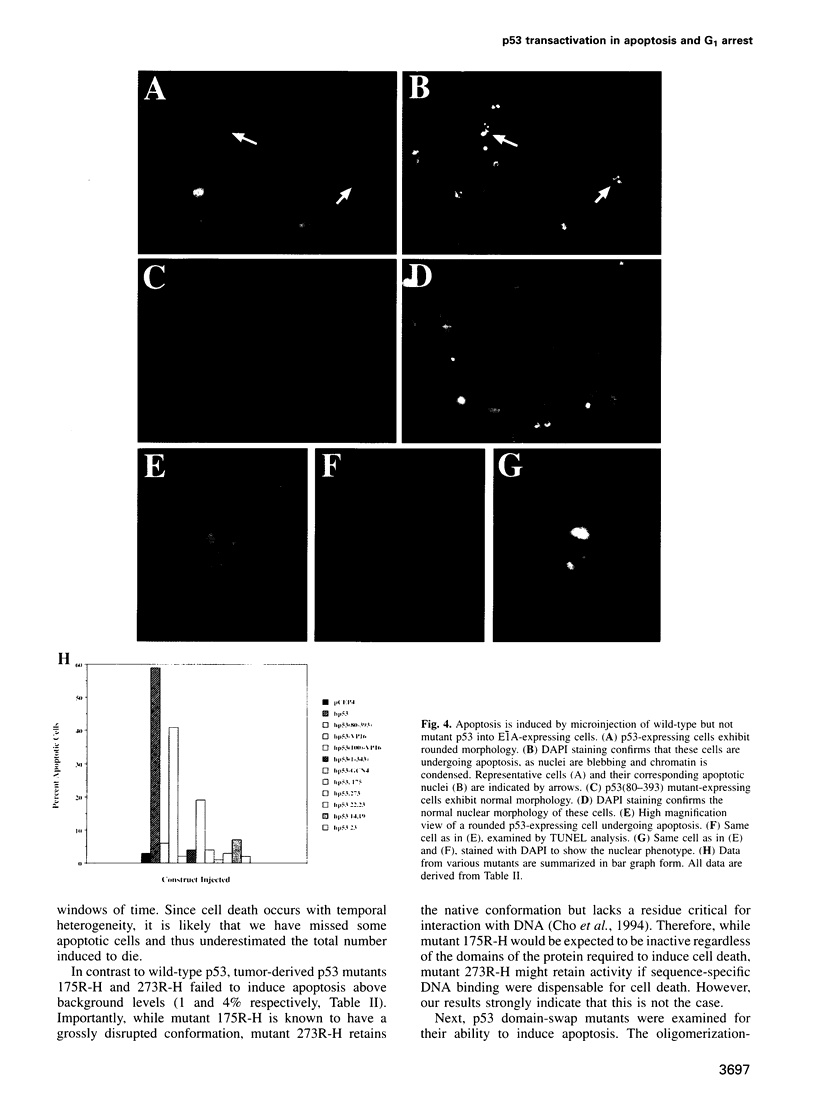

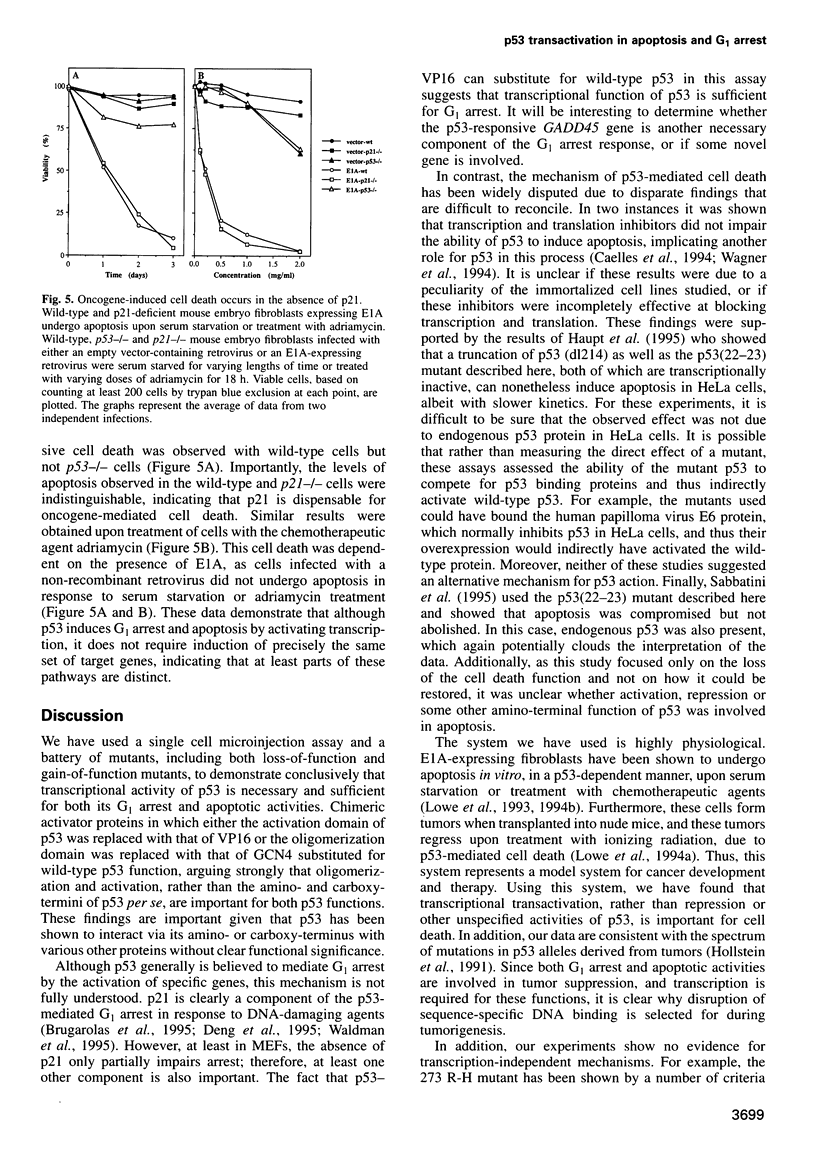


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugarolas J., Chandrasekaran C., Gordon J. I., Beach D., Jacks T., Hannon G. J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995 Oct 12;377(6549):552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Caelles C., Helmberg A., Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994 Jul 21;370(6486):220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Canman C. E., Gilmer T. M., Coutts S. B., Kastan M. B. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 1995 Mar 1;9(5):600–611. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994 Jul 15;265(5170):346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Fisher D. E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994 Aug 26;78(4):539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Friend S. p53: a glimpse at the puppet behind the shadow play. Science. 1994 Jul 15;265(5170):334–335. doi: 10.1126/science.8023155. [DOI] [PubMed] [Google Scholar]
- Guillouf C., Graña X., Selvakumaran M., De Luca A., Giordano A., Hoffman B., Liebermann D. A. Dissection of the genetic programs of p53-mediated G1 growth arrest and apoptosis: blocking p53-induced apoptosis unmasks G1 arrest. Blood. 1995 May 15;85(10):2691–2698. [PubMed] [Google Scholar]
- Haffner R., Oren M. Biochemical properties and biological effects of p53. Curr Opin Genet Dev. 1995 Feb;5(1):84–90. doi: 10.1016/s0959-437x(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Rowan S., Shaulian E., Vousden K. H., Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995 Sep 1;9(17):2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Jacks T., Remington L., Williams B. O., Schmitt E. M., Halachmi S., Bronson R. T., Weinberg R. A. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994 Jan 1;4(1):1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Tung K. S., Tourtellotte W. G., Brown G. A., Korsmeyer S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995 Oct 6;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Lin J., Chen J., Elenbaas B., Levine A. J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994 May 15;8(10):1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W. Cancer therapy and p53. Curr Opin Oncol. 1995 Nov;7(6):547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Jacks T., Housman D. E., Ruley H. E. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2026–2030. doi: 10.1073/pnas.91.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993 Apr;7(4):535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- Malkin D. p53 and the Li-Fraumeni syndrome. Cancer Genet Cytogenet. 1993 Apr;66(2):83–92. doi: 10.1016/0165-4608(93)90233-c. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Oliner J. D. Discerning the function of p53 by examining its molecular interactions. Bioessays. 1993 Nov;15(11):703–707. doi: 10.1002/bies.950151102. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
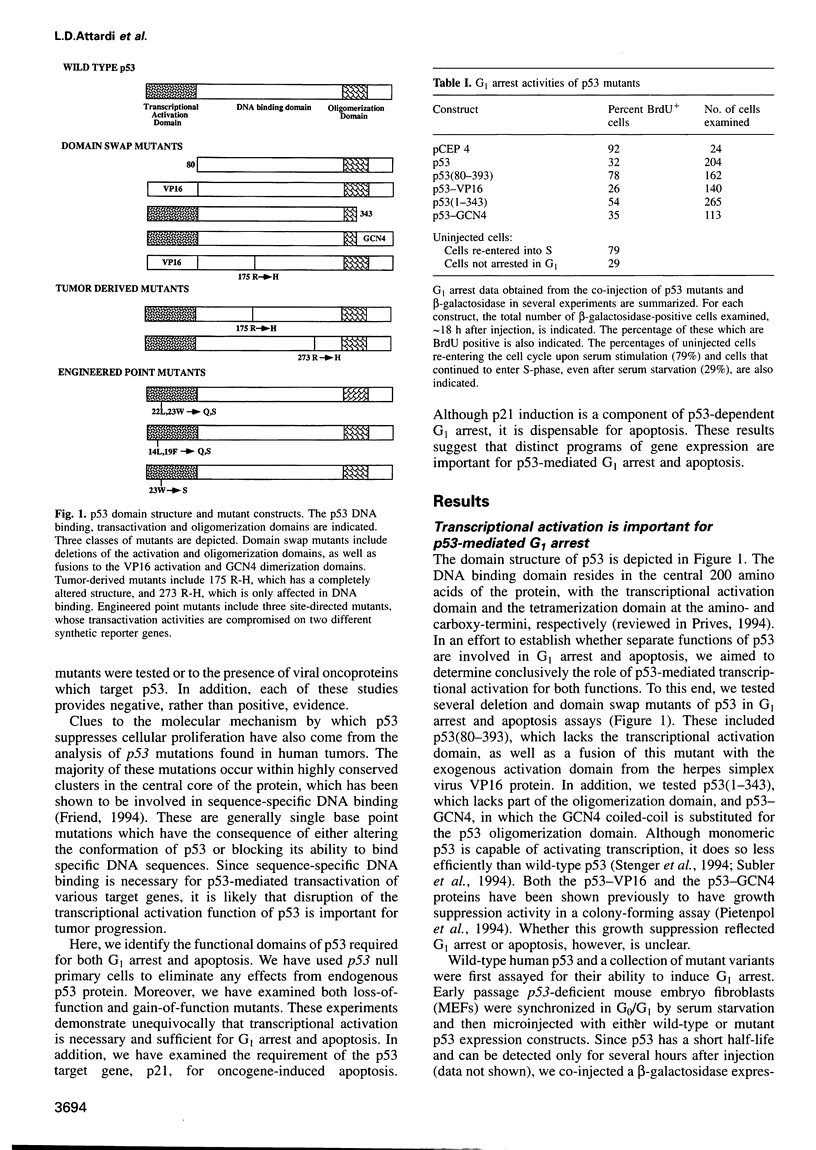
- Pietenpol J. A., Tokino T., Thiagalingam S., el-Deiry W. S., Kinzler K. W., Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C. How loops, beta sheets, and alpha helices help us to understand p53. Cell. 1994 Aug 26;78(4):543–546. doi: 10.1016/0092-8674(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Purdie C. A., Harrison D. J., Peter A., Dobbie L., White S., Howie S. E., Salter D. M., Bird C. C., Wyllie A. H., Hooper M. L. Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene. 1994 Feb;9(2):603–609. [PubMed] [Google Scholar]
- Purdie C. A., Harrison D. J., Peter A., Dobbie L., White S., Howie S. E., Salter D. M., Bird C. C., Wyllie A. H., Hooper M. L. Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene. 1994 Feb;9(2):603–609. [PubMed] [Google Scholar]
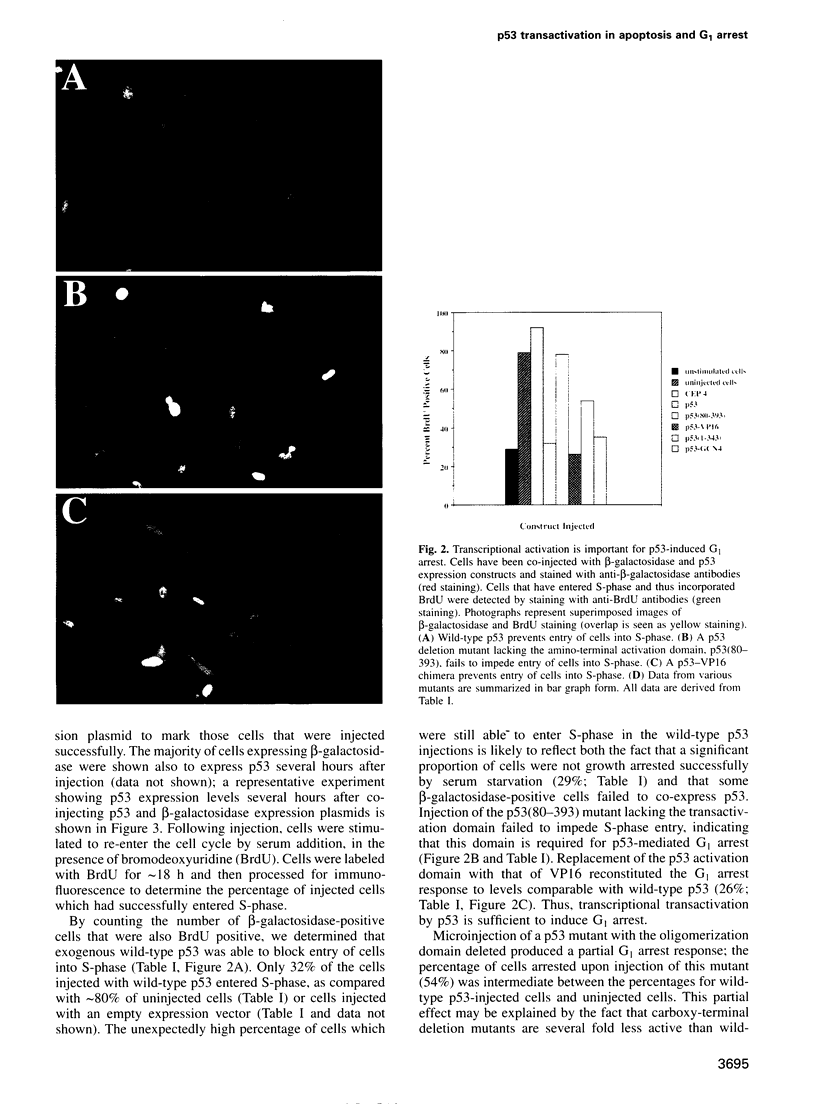
- Sabbatini P., Lin J., Levine A. J., White E. Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 1995 Sep 1;9(17):2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., Bae I., Chen C. Y., Gilmer T. M., Kastan M. B., O'Connor P. M., Fornace A. J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Stenger J. E., Tegtmeyer P., Mayr G. A., Reed M., Wang Y., Wang P., Hough P. V., Mastrangelo I. A. p53 oligomerization and DNA looping are linked with transcriptional activation. EMBO J. 1994 Dec 15;13(24):6011–6020. doi: 10.1002/j.1460-2075.1994.tb06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subler M. A., Martin D. W., Deb S. Overlapping domains on the p53 protein regulate its transcriptional activation and repression functions. Oncogene. 1994 May;9(5):1351–1359. [PubMed] [Google Scholar]
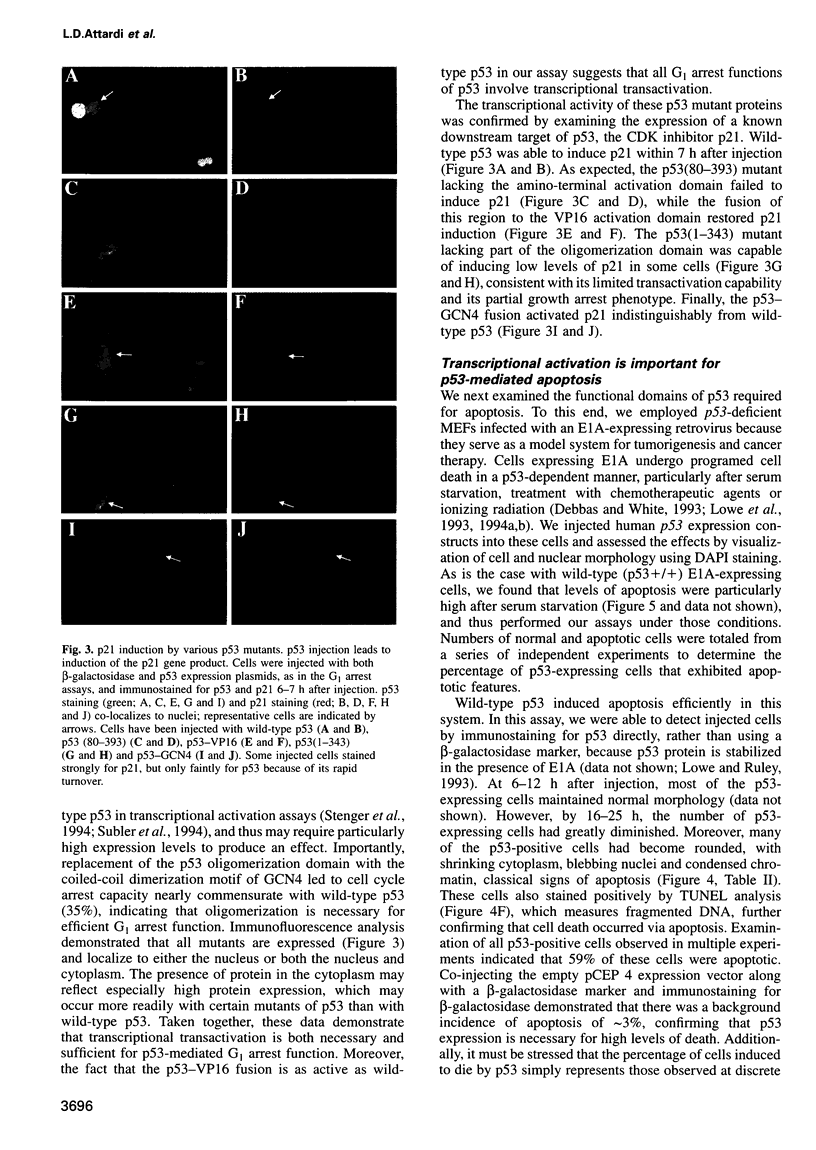
- Wagner A. J., Kokontis J. M., Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994 Dec 1;8(23):2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- Waldman T., Kinzler K. W., Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995 Nov 15;55(22):5187–5190. [PubMed] [Google Scholar]
- Wang X. W., Yeh H., Schaeffer L., Roy R., Moncollin V., Egly J. M., Wang Z., Freidberg E. C., Evans M. K., Taffe B. G. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995 Jun;10(2):188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]