Abstract
Background:
Methanol and DMSO are commonly used as carrier solvents for lipophilic chemicals in in-vitro experiments. However, very little information is available regarding the effects of these solvents on the expression of pro and anti-apoptotic genes and proteins.
Materials and Methods:
In this study, we examined the cytotoxic effects of methanol and dimethylsulfoxide at 0.5% (final concentrations recommended for in-vitro toxicity assays) on human breast cancer MCF-7 cells. We also investigated the effects of these solvents on the mRNA and immunocytochemical expression of apoptotic proteins BAX and BCL-2.
Results:
The results of neutral red cell viability assay showed that methanol and DMSO concentrations of 0.5% exhibited no cytotoxic effects on MCF-7 cells following a 24 hour exposure. Gene expression and Immunofluorescence results showed that methanol but not DMSO reduced the expression of the BAX pro-apoptotic protein, while both solvents did not alter the expression of the BCL-2 oncoprotein.
Conclusion:
Our results suggest that while methanol concentrations at 0.5% may be appropriate for in vitro toxicity studies in human breast cancer MCF-7 cells, it could alter the results of gene and protein expression experiments.
Keywords: Apoptosis, BAX, BCL-2, cytotoxicity, dimethylsulfoxide, MCF-7 cells, methanol
INTRODUCTION
Methanol and Dimethyl sulfoxide (DMSO) are part of the most commonly used solvents in in-vitro experiments because many experimental drugs are not readily water soluble.1,2,3,4,5 Methanol is a light, volatile, colorless, flammable liquid. At room temperature, it is a polar liquid, and is used as an antifreeze and solvent.6 DMSO is an organosulfur compound. It is colorless and is a polaraprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water.7 In many studies, methanol and DMSO are employed to deliver drugs to cells, and according to the NHK Neutral Red Uptake Cytotoxicity Assay Phase III (The National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods NTP-NICEATM 2003), the final DMSO or ethanol concentration for application to the cells must be 0.5% (v/v) in the vehicle controls and in all the test concentrations.8 It is thus crucial that the effects of the vehicles themselves at these concentrations on BAX and BCL2 gene and protein expression in these cells are evaluated. Hollebeck et al.,9 emphasized the importance of evaluating the effects that vehicles might have on key properties that are being investigated, as they found that even at low concentrations (0.1–0.5%), DMSO exhibited anti-inflammatory properties in the in-vitro intestinal Caco-2 cell model.
MCF-7 Cells have been used widely in cancer research, with many of these testing the effects of exogenous compounds on pro and antiapoptotic genes.10,11,12,13,14 Neoplastic cells share a common set of attributes or “acquired capabilities” that operate and are controlled at different spatial and temporal scales; including their abilities to generate their own mitogenic signals, resist exogenous growth-inhibition signals, evade apoptosis and senescence, proliferate without limit, acquire vasculature, and invade and metastasize distant sites.15 Apoptosis is a critical process that is dysregulated during tumourigenesis.16 The defective apoptosis pathways seen in human cancers often result from over-expression of antiapoptotic proteins, sometimes leading to cancer initiation when there is an upregulation of procancer variants of apoptotic genes.16 The induction of apoptosis occurs via two major pathways: The extrinsic (death receptor) pathway and the intrinsic (mitochondria) pathway.17 The intrinsic apoptotic pathway is under the control of mitochondrial pro-enzymes. Most cell death in vertebrates occurs via the mitochondrial pathway of apoptosis.18 This pathway is controlled by the B-cell CLL/lymphoma 2 (BCL-2) family of proteins and they regulate the integrity of the mitochondrial outer membrane. When apoptosis arises as a result of cooperation among these proteins, the two pro-death BCL-2 effector proteins: BCL-2-associated X protein (BAX) and BCL-2 antagonistic killer (BAK), disrupt the mitochondrial outer membrane in a process known as ‘mitochondrial outer membrane permeabilisation’ (MOMP).18 If MOMP takes place, proteins located in the mitochondrial inter-membrane compartment enter the cytosol and activate caspases and cysteine proteases that coordinate the disassembling of the cell.18 MOMP is opposed by the pro-survival BCL-2 proteins, such as BCL-2, BCL-W, BCL-xL, A1/Bfl1 and MCL-1, which impede the capacity of BAX and BAK to permeabilise the outer mitochondrial membrane.
Little data is available in literature describing the effects of these solvents on apoptotic BAX/BCL-2 mRNA and protein expression. The objectives of the present study were to evaluate the effects of 0.5% DMSO and 0.5% Methanol first in a NHK Neutral Red Uptake Cytotoxicity/Viability Assay and then to determine their effects on BAX and BCL2 mRNA and protein expression in the breast MCF-7 Cells.
MATERIALS AND METHODS
Kits, reagents and antibodies
Methanol and DMSO (>99% pure) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The Neutral Red TOX-4 kit, Methanol, and ethylenediamine-tetraacetic acid (EDTA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit polyclonal anti-BAX antibody and mouse monoclonal anti-BCL2 were purchased from DAKO (DK-2600 Glostrup, Denmark). Secondary antibodies, Goat anti Rabbit Rhodamine conjugated and Goat anti Mouse FITC conjugated were purchased from Abcam (Cambridge, UK). The GeneJET RNA Purification kit, DNase I, RNase-free kit and O'GeneRuler Low Range DNA Ladder were purchased from Thermo Scientific (Pittsburgh PA. USA). High Capacity cDNA Reverse Transcription Kit and Power SYBR® Green PCR Master Mix were purchased from Life technologies (California, USA). Oligos for qPCR were purchased from Integrated DNA Technologies, Inc. (Coralville, Iowa, USA). Agarose D-1 Low EEO-GQT was purchased from Conda Laboratories (Madrid, Spain).
Cell culture and treatment
Human breast carcinoma MCF-7 cells (American Type Culture Collection Rockville, MD, USA) were cultured in DMEM medium (Gibco BRL, Gaithersburg, MD, USA) containing 10% Fetal Bovine serum (Gibco, USA). The cells were maintained in 25cm2 flasks (Corning[®]) at 37° C in a humidified atmosphere containing 5% CO2. The cells were incubated with methanol and DMSO at a 0.5% (v/v) final concentration for 24 hours. Control cells were exposed to the growth medium alone. This work was approved by the ethics committee of the University of Witwatersrand Medical School.
Neutral red uptake assay
The Neutral Red TOX-4 kit was obtained from Sigma (St. Louis, MO, USA). 96-well Falcon tissue culture plates were seeded with approximately 1000 cells/well, suspended in 100 μl DMEM. The plates were incubated for 24 hours to achieve ~60–70% attachment and confluence. The medium was removed and cells were rinsed with 200 μl of phosphate buffered saline (PBS) and subsequently treated with medium containing 0.5% (v/v) methanol and DMSO for 24 hours. The negative control consisted of untreated cells, left in culture medium. Another group of cells were also exposed to 100 ug/ml efavirenz and used as a positive control. 100 ug/ml EFV was selected as a positive control because it exhibited a significant cytotoxic effect on MCF-7 cells in a separate range finder experiment that we conducted. The plate consisted of four replicates for each treatment including negative and positive controls. After 24 hours, the medium was removed and manufacturer's protocol was followed for the assay.19 Cells were rinsed 3 times in 200 μl of PBS. Neutral red medium was added to each well and plates were incubated for 2 hours for the neutral red dye to be taken up by viable cells. Thereafter the NR solution was removed and cells were rinsed with PBS. The solubilising solution (1% acetic acid in 50% ethanol) was added to each well for 10 minutes in order to extract the dye. The absorbance of the extracted dye was measured in a microplate reader (Anthos 2010 Model 17–550 Austria) at a wavelength of 540 nm. Background absorbance was read at 690 nm and subtracted from the 540 nm measurement. All experiments were repeated in three technical repeats on three different days.
Immunocytochemistry of BAX and BCL2
For immunofluorescence analysis, the ABCAM[TM] double labelling procedure was followed. 1 × 103 cells were plated on cover slips 1 day before the experiment. Cells were treated with 0.5% (v/v) methanol and DMSO for 24 h. Following treatment, cells were washed three times with 0.5% BSA in PBS, followed by fixation in 10% phosphate buffered formalin for 10 min. The fixed cells were rinsed in PBS and permeablized with 0.05% Triton-X 100, washed and blocked with 10% Normal goat serum in PBS for 60 mins to eliminate non-specific binding of secondary antibody. Cells were then incubated with polyclonal rabbit antihuman BAX (1:1000 DAKO) and monoclonal mouse antihuman BCL-2 (1:100 DAKO) overnight at 4° C in a moist chamber. Following overnight incubation, coverslips were washed and incubated with secondary Antibody. The cells were washed and incubated for 2 hours with a FITC-conjugated goat anti-mouse antibody (1:500 Rockland) and Rhodamine-conjugated goat anti-rabbit antibody (1:1000 Rockland) in the dark. Slides were rinsed, nuclei counterstained in 4Ͳ,6-diamidino-2-phenylindole, dihydrochloride (DAPI) 300 nM for 5 mins, rinsed and mounted with fluoromount (Sigma). Negative control groups were set up to ensure that the secondary antibodies were specific for their primary antibodies. The primary antibodies were substituted with 0.1% BSA/PBS and the normal protocol was carried out. In a second negative control, the cells were treated in the same way as the experimental slides, but the secondary antibodies were substituted with PBS. The positive controls used in this study were HeLa cells, which had been previously shown to express BAX and HepG2 cells, shown to express BCL-2.
Cells were visualized using a Zeiss Laser Scanning Confocal Microscope 780 under a Zeiss 100X oil immersion objective. Slides were kept dark once fluorescent antibodies had been added to prevent bleaching. To avoid the fading of fluorochromes, images were analysed within the same time period. The image acquisition settings were set to remain constant for all exposures. Using the ZEN 2010 (Carl Zeiss, Germany) image analysis software, the intensity of the fluorescence of each micrograph was analysed. This was done by using the software to initially define the regions of interest (ROIs); namely, the nucleus and cytoplasm. The mean intensity of each ROI from the treatment groups was then analysed with the statistics software JMP® (Version 10.0 SAS Institute Inc., Cary, NC, USA). Data are reported as mean ± SEM. After verifying the normal distribution and the homogeneity of the variance using an F test (P < 0.05), a one way analysis of variance (where a significance level of P < 0.05 was set) was used to compare the results.
RNA extraction, cDNA synthesis, and real-time qPCR analysis
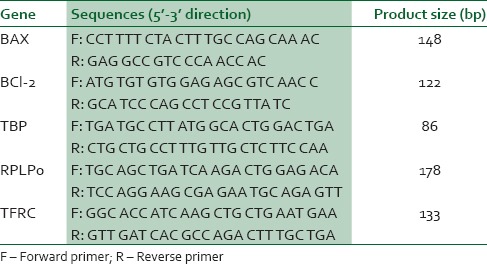
Cells were exposed to DMSO and methanol for 24 hours and total RNA extraction was performed using the GeneJET RNA Purification kit according to the manufacturer's instructions. RNA concentration and purity were determined by using the Nanodrop-1000 spectrophotometer. RNA integrity was checked by gel electrophoresis. According to the manufacturer's instructions, genomic DNA was removed from total RNA using the DNase I, RNase-free kit. The DNAase I treated RNA was again cleaned with the GeneJET RNA Purification kit, re quantified and stored at −80° C until used. According to the manufacturer's instructions, cDNA was synthesized using the MultiScribe™ Reverse Transcriptase from 700 ng RNA. The reverse transcriptase reaction was carried out in a GeneAmp® PCR System 9600 Thermal Cycler for 10 min at 25° C, 120 min at 37° C and then the enzyme was deactivated for 5 min at 85° C. cDNA aliquots were then utilized in qPCR reactions for BAX and BCL-2, with TBP, RPLP0 and TFRC used as the endogenous reference genes as described previously.20 Briefly, PCR reactions were amplified for 40 cycles prior, then the AmpliTaq Gold® DNA polymerase was activated for 10 min at 95° C. Each cycle consisted of a denaturing step for 15 sec at 95° C, and annealing/extension step for 1 min at 60° C. PCR amplification was performed in a final volume of 20 μL using the Power SYBR® Green PCR Master Mix with the ABI 7500 real-time PCR machine. Primer sequences and PCR product sizes are indicated in Table 1. To confirm the absence of nonspecific amplification, PCR products were separated on 3% agarose gels, stained with ethidium bromide and images acquired with the BioRad Gel Doc® XR (Model 170–8170 Segrate, Milan. Italy). Melt curves were generated for each PCR product. The relative mRNA expression levels of target genes in each sample were calculated using the qbasePLUS software (Biogazelle, Zulte, Belgium). The expression stability of the reference genes was evaluated using qbasePLUS version 2.3.21 PCR base line Cq values were imported into the qbasePLUS software. The relative quantity of each target/sample combination was scaled to the average Cq of corresponding target (scale set to untreated control in the qbasePLUS software). The relative expression of specific genes in the experiment were normalised as a ratio to the amount of the two most stably expressed reference genes according to the -[ΔΔC] T method of Livak and Schmittgen.22
Table 1.
Oligonucleotide sequences used for qPCR
Statistical analysis
Statistical analysis was performed using JMP® (Version 10.0 SAS Institute Inc., Cary, NC). Data are reported as mean ± SEM. After verifying the normal distribution and the homogeneity of the variance using an F test (P < 0.05), a one way analysis of variance (where a significance level of P < 0.05 was set) was used to compare the results. All means were then compared using the Tukey-Kramer HSD.
RESULTS
Neutral red assay
Methanol and DMSO, individually at 0.5% (v/v) concentration did not have any significant (P < 0.0001) cytotoxic effect on MCF-7 cells following a 24 hour incubation as demonstrated by the Neutral Red assay [Figure 1]. Methanol and DMSO at 0.5% (v/v) concentration did not exhibit any significantly different cytotoxic or viability effect from each other (P < 0.0001). 100 ug/ml efavirenz, which was used as a positive control, however exhibited a significant cytotoxic effect on MCF-7 cells [Figure 1].
Figure 1.
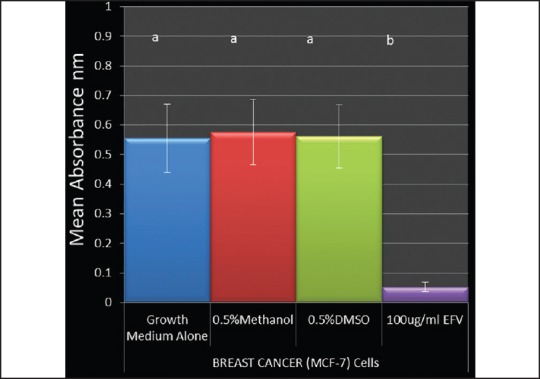
Effects of 0.5% Methanol and 0.5%DMSO on the percentage viability of breast cancer (MCF-7) cells. Cells were incubated with 0.5% Methanol and 0.5% DMSO for 24. Graphs were plotted with mean + SEM percentage viability from a mean of three independent experiments. Groups not connected by the same letter are significantly different (P < 0.05)
Immunofluorescence of BAX and BCL2 proteins in 0.5% DMSO and 0.5% methanol exposed MCF-7 cells
Immunofluorescence of the cells was assessed. The untreated group [Figure 2a] shows the expression and co localisation of BAX and BCL2 proteins. Figure 2b illustrates the effect of Methanol on expression of BAX and BCL-2 in MCF-7 cell cultures. While BCL-2 expression is not altered, BAX protein expression evidently is decreased in the 0.5% methanol exposed MCF-7 cell cultures [Figure 2b] compared to the untreated cells seen in Figure 2a. Figure 2c shows the effect of DMSO on expression of BAX and BCL-2 in MCF-7 cells. BAX and BCL-2 were not altered in the 0.5% DMSO exposed MCF-7 cells compared to the untreated [Figure 2a]. These observations were confirmed by statistically analysing the mean fluorescence intensities of BAX and BCL-2 in the nuclei and cytoplasm between the untreated and the treated groups, (Graphical representation shown in Figure 3). There are no differences in fluorescence intensities of BAX and BCL-2 between MCF-7 cells exposed to growth medium alone and 0.5% DMSO. 0.5% methanol however significantly reduced the expression of BAX but not BCL-2 in MCF-7 cells [Figure 3].
Figure 2.
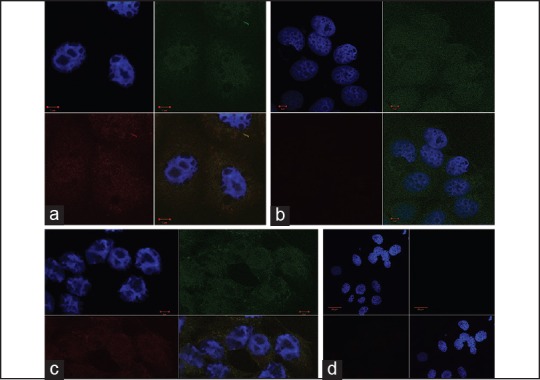
Fluorescent micrographs of MCF-7 cells unexposed to either solvent (a) exposed to 0.5% Methanol (b) and 0.5% DMSO (c). D is negative control. BCL-2 was stained with monoclonal anti-BCL-2 and Goat Anti-Mouse IgG FITC conjugated (Upper Right Quadrant- showing the nuclear and cytoplasmic expression of the oncogene BCL-2). BAX was stained with polyclonal rabbit anti-BAX and Goat anti-Rabbit IgG Rhodamine conjugated (Lower Left quadrant- showing the nuclear and cytoplasmic expression of BAX). Nucleus was counterstained with DAPI (Upper left quadrant). The lower right quadrant shows the combined image. 2D shows fluorescent micrographs of MCF-7 cells unexposed to either solvent. Cells were incubated with the secondary antibodies but not the primary antibodies to reveal non-specific staining. The nucleus was counterstained with DAPI (Upper left quadrant). The lower right quadrant represents the combined image
Figure 3.
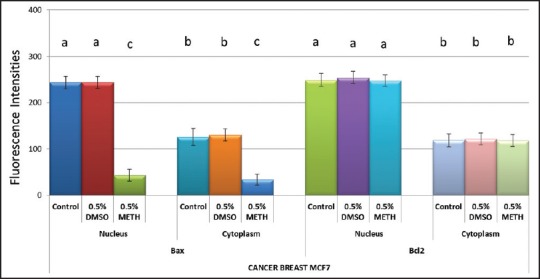
Effects of 0.5% Methanol and 0.5% DMSO on BAX and BC-2 protein expression in breast cancer (MCF-7) cells. Cells were incubated with 0.5% Methanol and 0.5% DMSO for 24 hours. Data (mean ± SEM), are represented as fluorescence intensities of protein expression relative to values from the untreated cells and representative of 3 independent experiments for immunofluorescence staining. Groups not connected by the same letter are significantly (P < 0.05) different
BAX and BCL-2 mRNA expression in 0.5%DMSO and 0.5%methanol exposed MCF-7 cells
In the MCF-7 cells exposed to 0.5% DMSO, the differences in fold changes were not significantly (P < 0.05) different from the untreated controls for BAX and BCL-2 [Figure 4]. The cells exposed to 0.5% methanol demonstrated significant differences (P < 0.05) in fold changes when compared to the untreated controls for BAX but not for BCL-2 [Figure 4]. In the 0.5% methanol exposed cells, BAX was significantly down-regulated. This result agrees with the immunofluorescence results.
Figure 4.
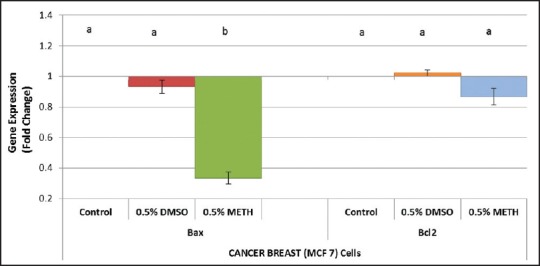
Effects of 0.5% Methanol and 0.5% DMSO on BAX and BCL-2 mRNA expression in breast cancer (MCF-7) cells. Cells were incubated with 0.5% Methanol and 0.5% DMSO for 24 hours. Data (mean ± SEM), are represented as fold changes of gene expression relative to values from the untreated cells (defined as 1) and representative of 3 independent experiments for RNA extraction. Groups not connected by the same letter are significantly (P < 0.05) different
DISCUSSION
Methanol and DMSO are widely used in in-vitro studies as carrier solvents for lipophilic chemicals.3,23 The concentration dependent cytotoxicity of these solvents in some cell lines has been previously reported, and the results suggest that not all cells respond in the same way to DMSO.4 Da Violante et al.,24 reported that DMSO concentration of 10% had no cytotoxic effects on Caco2/TC7 cells whereas Qi et al.,4 showed that following a 24 h treatment, DMSO concentrations of 0.25% or less caused little or no damage to postnatal hair cells in cochlear organotypic cultures, but DMSO concentrations of 0.5% or higher are toxic to both hair cells in postnatal rat cochlear cultures. The reasons for these cellular differences in DMSO cytotoxicity remain unclear. Formate, the toxic metabolite of methanol inhibits the mitochondrial electron transport chain, but only in certain tissues, including the retina, optic nerve, and basal ganglia.25,26 Similar concentrations of formate in the heart and kidney produced no toxicity.
Much work has been published on the effects of DMSO on various parameters within cell biology, but not as much work has been done on the effects of methanol on cell biology. The effects of these solvents on anti and pro-apoptotic protein expression at recommended concentrations commonly used for toxicity tests are not well characterized. The present study shows that methanol and DMSO concentrations of 0.5% exhibited no cytotoxic effects on MCF-7 cells following a 24 hour exposure using the neutral red assay compared to negative controls. This result concurs with reports by Hollebeck et al.,9 that DMSO concentration up to 1% had no lethal effect on Caco-2 cells for up to 24 hours. However, immunofluorescence and mRNA expression results from this study showed that methanol but not DMSO reduced the expression of the pro-apoptotic BAX, while both solvents did not alter the expression of the BCL-2 oncoprotein. Hypothetically, BCL-2 over-expression renders a survival advantage for cancer cells.27 This investigation reveals a reduction in the expression of the BAX mRNA and protein in methanol exposed cells which will cause an imbalance in the BCL-2 versus BAX ratio in vitro. These results suggest that while methanol concentrations at 0.5% may be appropriate for toxicity studies in human breast cancer MCF-7 cells, it may also confound the results of protein and mRNA expression experiments. This should therefore be taken into consideration when assessing the protein and mRNA expression effects of bioactive compounds requiring methanol as vehicle, in order to avoid miss-interpretation of the results.
ACKNOWLEDGEMENTS
We thank Dr. Clem Penny from the department of Internal Medicine, University of the Witwatersrand, Johannesburg, South Africa for providing the MCF-7 Cells. This work was supported by the South African Medical Research Council.
Footnotes
Source of Support: The authors declare no conflicts of interests with regards to this article.
Conflict of Interest: None declared.
REFERENCES
- 1.Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol Biochem Behav. 1995;50:521–6. doi: 10.1016/0091-3057(94)00331-9. [DOI] [PubMed] [Google Scholar]
- 2.Wallace JL, McKnight W, Del Soldato P, Baydoun AR, Cirino G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J Clin Invest. 1995;96:2711–8. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehm BR, Wolf SC, Bertsch D, Klaussner M, Wesselborg S, Schüler S, et al. Effects of nebivolol on proliferation and apoptosis of human coronary artery smooth muscle and endothelial cells. Cardiovasc Res. 2001;49:430–9. doi: 10.1016/s0008-6363(00)00253-4. [DOI] [PubMed] [Google Scholar]
- 4.Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen TH, Wang YH, Wu YH. Developmental exposures to ethanol or dimethylsulfoxide at low concentrations alter locomotor activity in larval zebrafish: Implications for behavioral toxicity bioassays. Aquat Toxicol. 2011;102:162–6. doi: 10.1016/j.aquatox.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 6.CDC – The Emergency Response Safety and Health Database: Systemic Agent: METHANOL – NIOSH n.d. [Last accessed on 2014 Apr 20]. Available from: http://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750029.html .
- 7.Novak KM, editor. Drug Facts and Comparisons. 56th ed. St. Louis, Missouri: Wolters Kluwer Health; 2002. p. 619. [Google Scholar]
- 8.NTP-NICEATM. Test Method Protocol for the NHK Neutral Red Uptake Cytotoxicity Assay Phase III – Validation Study: November 4, 2003. Prepared by the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NTP-NICEATM) [Last accessed on 2014 May 15]. Available from: http://iccvam.niehs.nih.gov/methods/acutetox/invidocs/phIIIprot/nhkphIII.pdf .
- 9.Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, et al. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011;206:268–75. doi: 10.1016/j.toxlet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Levenson AS, Jordan VC. MCF-7: The first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–8. [PubMed] [Google Scholar]
- 11.Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR, Park JS, et al. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J Agric Food Chem. 2004;52:1715–9. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- 12.Min KN, Joung KE, Kim DK, Sheen YY. Anti-cancer effect of 3-(4-dimethylamino phenyl)-N-hydroxy-2-propenamide in MCF-7 Human Breast Cancer. Environ Health Toxicol. 2012;27:e2012010. doi: 10.5620/eht.2012.27.e2012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LP, Zhao F, Wang Y, Zhao LL, Li QP, Liu HW. Antitumor effect of resorcinol derivatives from the roots of Ardisia brevicaulis by inducing apoptosis. J Asian Nat Prod Res. 2011;13:734–43. doi: 10.1080/10286020.2011.587412. [DOI] [PubMed] [Google Scholar]
- 14.Moon D, McCormack D, McDonald D, McFadden D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J Surg Res. 2013;180:208–15. doi: 10.1016/j.jss.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Grizzi F, Chiriva-Internati M. Cancer: Looking for simplicity and finding complexity. Cancer Cell Int. 2006;6:4. doi: 10.1186/1475-2867-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eum KH, Lee M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol Cell Biochem. 2011;348:61–8. doi: 10.1007/s11010-010-0638-8. [DOI] [PubMed] [Google Scholar]
- 18.Llambi F, Green DR. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet Dev. 2011;21:12–20. doi: 10.1016/j.gde.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–24. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 20.Adefolaju GA, Theron KE, Hosie MJ. Effects of HIV protease, nucleoside/non-nucleoside reverse transcriptase inhibitors on Bax, Bcl-2 and apoptosis in two cervical cell lines. Biomed Pharmacother. 2014;68:241–51. doi: 10.1016/j.biopha.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 24.Da Violante G, Zerrouk N, Richard I, Provot G, Chaumeil JC, Arnaud P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol Pharm Bull. 2002;25:1600–3. doi: 10.1248/bpb.25.1600. [DOI] [PubMed] [Google Scholar]
- 25.Sharpe JA, Hostovsky M, Bilbao JM, Rewcastle NB. Methanol optic neuropathy: A histopathological study. Neurology. 1982;32:1093–100. doi: 10.1212/wnl.32.10.1093. [DOI] [PubMed] [Google Scholar]
- 26.Treichel JL, Henry MM, Skumatz CM, Eells JT, Burke JM. Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology. 2003;24:825–34. doi: 10.1016/S0161-813X(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 27.Sjöström J, Bergh J. How apoptosis is regulated, and what goes wrong in cancer. BMJ. 2001;322:1538–9. doi: 10.1136/bmj.322.7301.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]


