Abstract
BACKGROUND
Mutations in RPE65 cause Leber’s congenital amaurosis, a progressive retinal degenerative disease that severely impairs sight in children. Gene therapy can result in modest improvements in night vision, but knowledge of its efficacy in humans is limited.
METHODS
We performed a phase 1–2 open-label trial involving 12 participants to evaluate the safety and efficacy of gene therapy with a recombinant adeno-associated virus 2/2 (rAAV2/2) vector carrying the RPE65 complementary DNA, and measured visual function over the course of 3 years. Four participants were administered a lower dose of the vector, and 8 were administered a higher dose. In a parallel study in dogs, we investigated the relationship among vector dose, visual function, and electroretinography (ERG) findings.
RESULTS
Improvements in retinal sensitivity were evident, to varying extents, in six participants for up to 3 years, peaking at 6 to 12 months after treatment and then declining. No associated improvement in retinal function was detected by means of ERG. Three participants had intraocular inflammation, and two had clinically significant deterioration of visual acuity. The reduction in central retinal thickness varied among participants. In dogs, RPE65 gene therapy with the same vector at lower doses improved vision-guided behavior, but only higher doses resulted in improvements in retinal function that were detectable with the use of ERG.
CONCLUSIONS
Gene therapy with rAAV2/2 RPE65 vector improved retinal sensitivity, albeit modestly and temporarily. Comparison with the results obtained in the dog model indicates that there is a species difference in the amount of RPE65 required to drive the visual cycle and that the demand for RPE65 in affected persons was not met to the extent required for a durable, robust effect. (Funded by the National Institute for Health Research and others; ClinicalTrials.gov number, NCT00643747.)
Leber’s congenital amaurosis is a group of inherited, early-onset, severe retinal dystrophies that cause substantial sight impairment in childhood.1 One of the causes of this condition is mutations in the gene encoding RPE65 (retinal pigment epithelium–specific protein 65 kDa). The encoded retinoid isomerase converts all-trans retinyl esters to 11-cis retinal for the regeneration of visual pigment after exposure to light. RPE65 deficiency causes photoreceptor-cell dysfunction and impaired vision from birth. Severe dysfunction of rod photoreceptor cells, which are wholly reliant on retinal pigment epithelium–derived RPE65, causes severely impaired night vision. The function of cone photoreceptor cells, which mediate vision in daylight, is relatively preserved in childhood because cones have access to an alternative source of 11-cis retinal.2 However, progressive degeneration of both rod and cone photoreceptor cells, in association with local accumulation of toxic retinyl esters,3 results in severe sight impairment by early adulthood.
Augmentation of Rpe65 in animal models of Rpe65 deficiency can improve retinal and visual function, as assessed by means of electroretinography (ERG) and observation of vision-guided behavior, respectively.4-6 We and others have previously reported that gene-augmentation therapy for RPE65 deficiency can improve aspects of sight in human participants.7-10 However, the magnitude and durability of benefit reported to date in humans do not match those observed in animal models.11
Species-specific differences in the outcomes of gene therapy are largely unexplained, but they may reflect differences in pathophysiological mechanisms, vector tropism, or both. The rate of retinal degeneration in humans is variable but, relative to life span, is typically higher than in mouse and dog models.11 Studies in animals have shown that improvements in function are correlated with retinal thickness at the time of intervention.11 The finding that the retinas even in older Rpe65-deficient dogs can respond with an improvement in function, despite local accumulation of lipid metabolites and advanced photoreceptor-cell degeneration,12 indicates that the window of opportunity for benefit is not restricted to early disease. However, the durability of the benefit from RPE65 gene augmentation depends on protection against retinal degeneration, and in both mice and dogs, RPE65 gene augmentation promotes the survival of photoreceptors only when it is administered early in the course of the disorder.13,14 In one study involving human participants, intervention between the ages of 11 and 30 years failed to protect against degeneration despite sustained improvements in retinal function.11 However, the optimal window of opportunity for intervention in humans has yet to be determined.
Here we report the 3-year results of a phase 1–2 trial of gene therapy in humans with disease-causing mutations in RPE65. We also report the results of a parallel study in dogs to determine the relative demand for RPE65 protein and the effect of vector dose on retinal function.
METHODS
TRIAL DESIGN AND OVERSIGHT
We performed a phase 1–2 open-label trial involving 12 participants to evaluate the safety and efficacy of gene therapy with a recombinant adeno-associated virus 2/2 (rAAV2/2) vector, rAAV2/2.hRPE65p.hRPE65, carrying the RPE65 complementary DNA (cDNA), administered at two dose levels. The study was approved by the U.K. Gene Therapy Advisory Committee, the Medicines and Healthcare Products Regulatory Agency, and the Moorfields Research Governance Committee and was conducted in compliance with Good Clinical Practice guidelines in accordance with the European Clinical Trials Directive (2001 EU/20/EC) and the Declaration of Helsinki. Participants or their guardians gave written informed consent. The authors verified the data, made the decision to submit the manuscript for publication, and vouch for the completeness of the data, the accuracy of the analyses, and the fidelity of the study to the protocol, available with the full text of this article at NEJM.org.
TRIAL PARTICIPANTS
We included 12 participants (6 to 23 years of age) with early-onset, severe retinal dystrophy caused by mutations in RPE65 (Table 1). Their genotypes were confirmed at a National Health Service diagnostic laboratory. Residual enzyme function was estimated for the G40S and R91W mutations15 and the Y368H mutation16 by means of prediction of protein structure. For each participant, the eye with the poorer visual acuity was selected as the study eye; the contralateral eye served as an untreated control.
Table 1.
Participant Characteristics, Dosing, and Selected Outcomes.*
| Participant No. |
Age | RPE65 Mutation (% Residual Enzyme Function)† |
Vector Dose‡ |
Visual Acuity at Baseline§ |
Peak Retinal Sensitivity on Dark- Adapted Perimetry¶ |
Peak Retinal Sensitivity on Microperimetry |
Vision- Guided Ambulatory Navigation∥ |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Eye |
Control Eye |
Study Eye |
Control Eye |
Study Eye |
Control Eye |
|||||
| yr | vg | log MAR | dB-sr | |||||||
| 1 | 23 | Y368H (0.87) Y368H (0.87) |
1×1011 | 1.16 | 0.88 | 0 | 0 | 0 | 0 | −4 |
| 2 | 17 | IVS1+5g→a (NA) G40S (1.65) |
1×1011 | 1.52 | 1.62 | 0 | 0 | 0 | 0 | −1 |
| 3 | 18 | E6X (NA) D167Y (NA) |
1×1011 | 0.76 | 0.50 | 54 | 2 | 1.4 | 0.5 | 0 |
| 4 | 11 | K298fs (NA) Y368H (0.87) |
1×1011 | 0.91 | 0.75 | 0 | 0 | 1.0 | 0 | −1 |
| 5 | 23 | Y368H (0.87) Y368H (0.87) |
1×1012 | 0.36 | 0.31 | 26 | 8 | 0.8 | 0 | 8 |
| 6 | 17 | Y368H (0.87) Y368H (0.87) |
1×1012 | 0.68 | 0.53 | 46 | 7 | 1.5 | 0 | 2.5 |
| 7 | 10 | IVS1+5g→a (NA) IVS12–2a→g (NA) |
1×1012 | 0.44 | 0.46 | 0 | 0 | 0 | 0 | 0.5 |
| 8 | 10 | R91W (5.08) R91W (5.08) |
1×1012 | 0.69 | 0.64 | 20 | 0 | 0.3 | 0 | 5 |
| 9 | 6 | IVS1+5g→a (NA) IVS1+5g→a (NA) |
1×1012 | 0.82 | 0.89 | 0 | 0 | 0 | NT | NP |
| 10 | 6 | IVS1+5g→a (NA) Y368H (0.87) |
1×1012 | 0.80 | 0.70 | 10 | 0 | 0 | 0 | 7 |
| 11 | 13 | R124X (NA) F530fs (NA) |
1×1012 | 0.63 | 0.55 | 0 | 0 | 0 | 0 | −0.5 |
| 12 | 19 | G40S (1.65) G40S (1.65) |
1×1012 | 0.54 | 0.60 | 5 | 0 | 0.3 | 0 | 2.5 |
The term dB-sr denotes decibel–steradian units, MAR minimum angle of resolution, NA not applicable, NP the participant was not able to perform the test, NT the participant was not tested, and vg vector genomes.
Residual enzyme function was estimated for the G40S, R91W, and Y368H mutations by means of prediction of protein structure. Predictions are unavailable for the other mutations. Residual enzyme function is expressed as a percentage of normal enzyme function.
The injection volume was 0.9 ml in Participants 1 and 9 and 1 ml in all other participants; vector administration involved the fovea in all participants except Participants 9 and 11.
Values are the mean of three separate assessments at baseline.
Values are the number of locations with significant positive slope (P<0.05), as compared with the baseline measurement, as evaluated with the use of pointwise linear regression.
Values are the improvement in performance at an ambient illumination of 4 lux 6 months after vector administration, assessed as the difference in the number of errors made by the participant when the study eye and the control eye were used independently while navigating a course.
INTERVENTION
The vector-manufacture process and surgical delivery technique have been described elsewhere.7 To determine the effect of RPE65 gene supplementation on foveal cones, we aimed to include the fovea within the subretinal vector bleb (i.e., the area of neurosensory retina that is elevated by the subretinal injection of vector suspension) in each participant. We administered the lower dose (1×1011 vector genomes) to the first four participants and the higher dose (1×1012 vector genomes) to the subsequent eight participants. We measured vector dissemination and systemic immune responses as described in the Supplementary Appendix, available at NEJM.org.
OUTCOME MEASURES
We evaluated participants at baseline and at intervals for 3 years after vector administration. We measured visual acuity, contrast sensitivity, color vision, and spectral sensitivities. For the investigation of visual fields, we used microperimetry, Goldmann kinetic perimetry, and photopic and scotopic (dark-adapted) automated static perimetry; we also assessed vision-guided ambulatory navigation and performed color fundus photography, autofluorescence imaging, optical coherence tomography (OCT), and ERG. The primary safety outcome was the incidence of a grade 3 adverse event at 3 years, defined as either the loss of visual acuity by 15 or more letters on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart or severe unresponsive intraocular inflammation.
STATISTICAL ANALYSIS
The outcomes for efficacy were descriptive in nature and were defined as any improvement in visual function greater than the test–retest variability for any assessment, determined by means of one-way analysis of variance with the use of multiple baseline measurements.17 A detailed description of the methods used in the dose–response study in the dog model, as well as the statistical analysis of the results in that model, can be found in the Supplementary Appendix.
RESULTS
VECTOR ADMINISTRATION
The bleb of subretinal vector extended to include the fovea in all eyes except two (in Participants 9 and 11). We observed no intraoperative adverse events. Subretinal vector was fully absorbed within 24 to 48 hours, as indicated by findings on OCT. Lacrimal fluid samples were weakly positive for vector DNA sequences at 1 day after surgery, but not at 30 days, in one participant (Participant 4) who was given the lower dose of vector and in two participants (Participants 7 and 10) who were given the higher dose. Vector DNA was not detected in the participants’ peripheral blood, saliva, or semen (semen was analyzed in one participant).
ADVERSE EVENTS
The ocular adverse events we observed included mild or transient intraocular inflammation in three participants who received the higher dose (Table S1 in the Supplementary Appendix). Macular thinning and a decline in visual acuity were also evident after subfoveal vector administration. The systemic adverse events we observed included those known to be associated with oral glucocorticoids.
RETINAL SENSITIVITY AND VISION-GUIDED AMBULATORY NAVIGATION
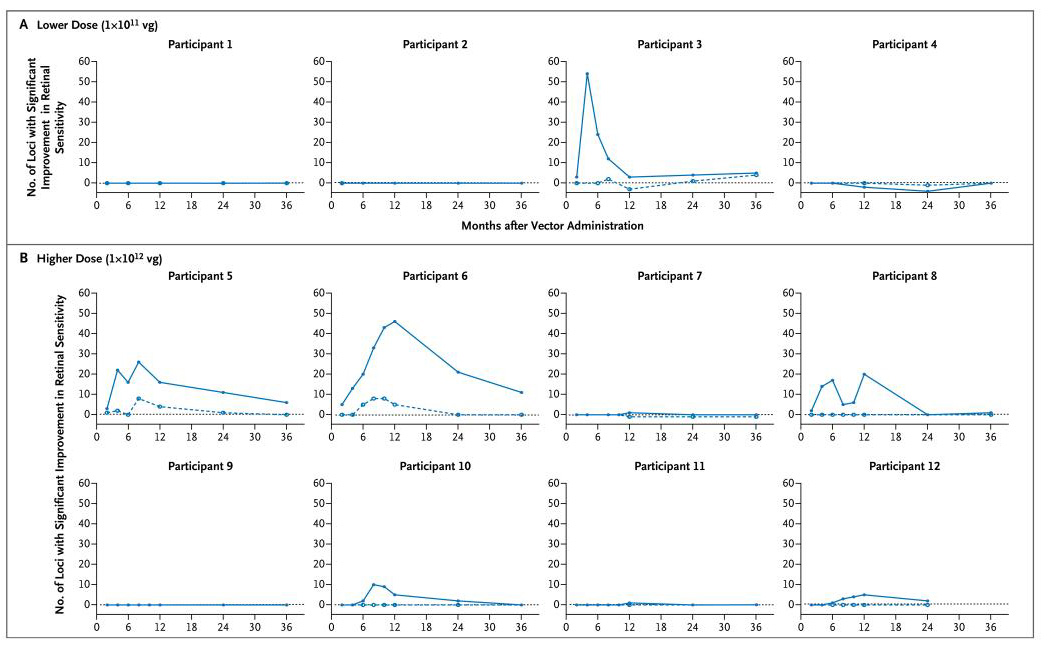
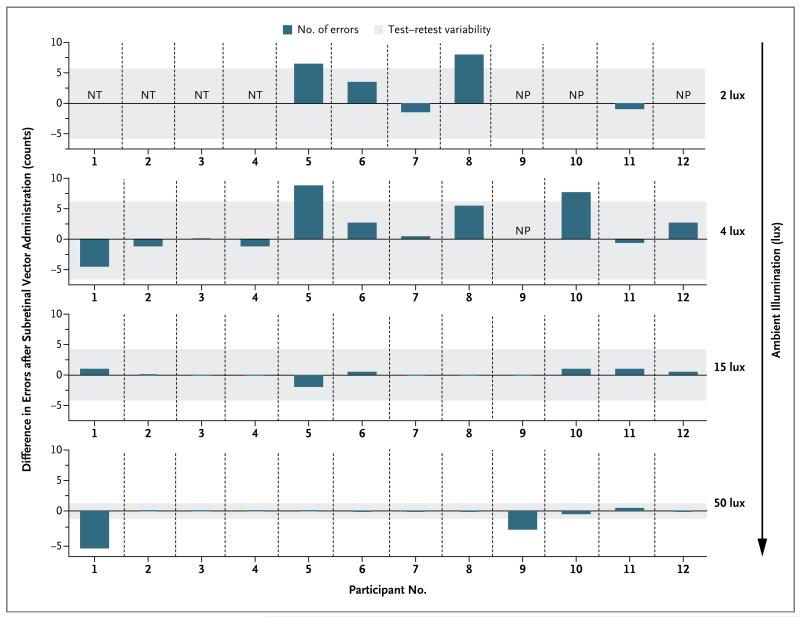
The outcomes for efficacy were primarily descriptive in nature. Improvements in retinal sensitivity were evident in the study eyes of six participants (Participants 3, 5, 6, 8, 10, and 12) on dark-adapted perimetry (Fig. 1) and in five of these participants (Participants 3, 5, 6, 8, and 12) on microperimetry (Fig. S1 and S2 in the Supplementary Appendix). For the first 4 months after vector administration, maximal improvement of rod function after vector administration was evident on dark-adapted perimetry only after extended dark adaptation (Fig. S3 in the Supplementary Appendix), with retinal sensitivity improving progressively for up to 4 hours during dark adaptation. Five of these participants (Participants 3, 5, 6, 8, and 10) reported subjective improvements in night vision, with three of them (Participants 5, 8, and 10) showing improvements in vision-guided ambulatory navigation (Fig. 2). One participant (Participant 4) reported subjectively improved night vision but had no consistent measurable improvement in retinal sensitivity or navigation.
Figure 1. Retinal Sensitivity Measured by Means of Dark-Adapted Perimetry.
Retinal sensitivity in the dark-adapted state was evaluated with the use of a pointwise linear regression for each of the 76 locations tested. The sum of loci with a significant positive slope (P<0.05) is plotted against time for the study eye (solid line) and contralateral control eye (broken line) for each of the 12 participants. Participants 3, 5, and 6 had a clear improvement in retinal sensitivity by approximately month 6, followed by a decline in sensitivity, which returned to near-baseline levels by 3 years after therapy. Participants 8, 10, and 12 also had some improvement. The term vg denotes vector genomes.
Figure 2. Vision-Guided Ambulatory Navigation.
The participants’ abilities to navigate a course at a range of illuminances 6 months after vector administration were assessed by measurement of the number of navigational errors made when the study eye and control contralateral eye were used independently. The dark blue bars indicate, for each participant tested, the difference between the study eye and control eye in the number of errors made. Bars that extend upward from 0 indicate fewer errors for the study eye than for the control eye for each participant. The gray-shaded areas indicate the test–retest variability, determined by one-way analysis of variance with the use of multiple baseline measurements. The four rows of data are results for each of the lighting conditions; only Participants 5 through 12 were tested at 2 lux. Participants 5, 8, and 10 had better performance with their study eyes at lower illuminances. Participants 1 and 9 had better performance with their untreated control eye at the highest illuminance. NP denotes that the participant was not able to perform the test, and NT denotes that the participant was not tested.
SPECTRAL SENSITIVITY
Measurement of spectral sensitivities at a fixed location in the superior retina after administration of the higher dose confirmed that two participants (5 and 6) had substantial improvements (10 to 100 times as high) in rod sensitivity that peaked at 12 months after treatment and subsequently declined (Fig. S4A in the Supplementary Appendix). For Participant 6, improvements were proportionately greater at 500 nm than at 600 nm, which indicated recovery of rod function. For Participant 5, similar improvements at 500 and 600 nm indicated an improvement in cone function. After rod bleaching (i.e., depigmentation of photopigment as a result of exposure to bright light), spectral sensitivities collapsed to become cone-like during the cone plateau and indicated improved cone photoreceptor-cell function in one participant (Participant 5) (Fig. S4B in the Supplementary Appendix). Other participants had only modest improvements in cone flicker sensitivity, and we found no evidence of improved cone function on dark-adapted perimetry with a red light stimulus.
VISUAL ACUITY
The participants had a wide range of visual acuities at baseline (Fig. S5 in the Supplementary Appendix). In one participant (Participant 11), an apparent improvement in visual acuity in the study eye was accompanied by a similar improvement in the contralateral untreated eye; with this exception, no consistent improvement in visual acuity was evident. A decline in visual acuity by more than 15 letters on the ETDRS chart was observed in two participants (Participants 3 and 7), and a more modest but sustained decline in acuity only in the study eye was apparent in one additional participant (Participant 12). In two of the participants with a decline in visual acuity (Participants 7 and 12), the decline was associated with a subjective deterioration of vision.
ERG
Rod photoreceptor-mediated responses measured with the use of ERG were undetectable at baseline, and we measured no significant sustained change in rod or cone responses in any participant; participants who had very-low-amplitude residual ERG findings retained the same level of function.
INFLAMMATORY AND IMMUNE RESPONSES
Either intraocular inflammation or immune responses occurred in five of the eight participants who received the higher dose (Participants 5 through 12) but in none of the four participants who received the lower dose (Participants 1 through 4). These responses appeared nondeleterious in all participants except Participant 7, in whom an episode of mild anterior uveitis was followed by focal pigmentary changes at the macula (Fig. S6 in the Supplementary Appendix) and a persistent reduction in visual acuity by 15 letters on the ETDRS chart (Fig. S5 in the Supplementary Appendix). At week 4, this participant had evidence of an increase in neutralizing AAV2 antibodies and a marginal increase in circulating T cells with reactivity to AAV2, as determined with the use of an enzyme-linked immunosorbent spot (ELISpot) assay, but no elevation in the titer of antibodies against RPE65 (Table S2 in the Supplementary Appendix). Two participants (Participants 8 and 9) had asymptomatic episodes of posterior intraocular inflammation in the study eye (Fig. S6 in the Supplementary Appendix); in one participant (Participant 8), this was associated with a temporary attenuation of the improvements in retinal sensitivity (Fig. 1, and Fig. S1 in the Supplementary Appendix). Intraocular inflammation in Participant 9 was associated with transiently increased circulating neutralizing antibodies to AAV2 (Table S2 in the Supplementary Appendix), but no adverse effect on visual function was evident. Fluorescein angiography and fundus autofluorescence imaging at 12 months after therapy showed no significant change from baseline in any participant (data not shown).
RETINAL THICKNESS
Of the 10 eyes in which subretinal vector administration involved the fovea (i.e., in all participants except 9 and 11), 6 had a sustained reduction in macular thickness (Participants 4 through 8 and 12) (Fig. S7A in the Supplementary Appendix). Macular thinning was typically apparent within 3 months and subsequently was relatively stable. Associated thinning of the photoreceptor-cell (outer nuclear) layer was evident, with variable disruption of the photoreceptor ellipsoid (inner segment) zone (Fig. S7B in the Supplementary Appendix).
DOSE–RESPONSE RELATIONSHIP IN RPE65-DEFICIENT DOGS
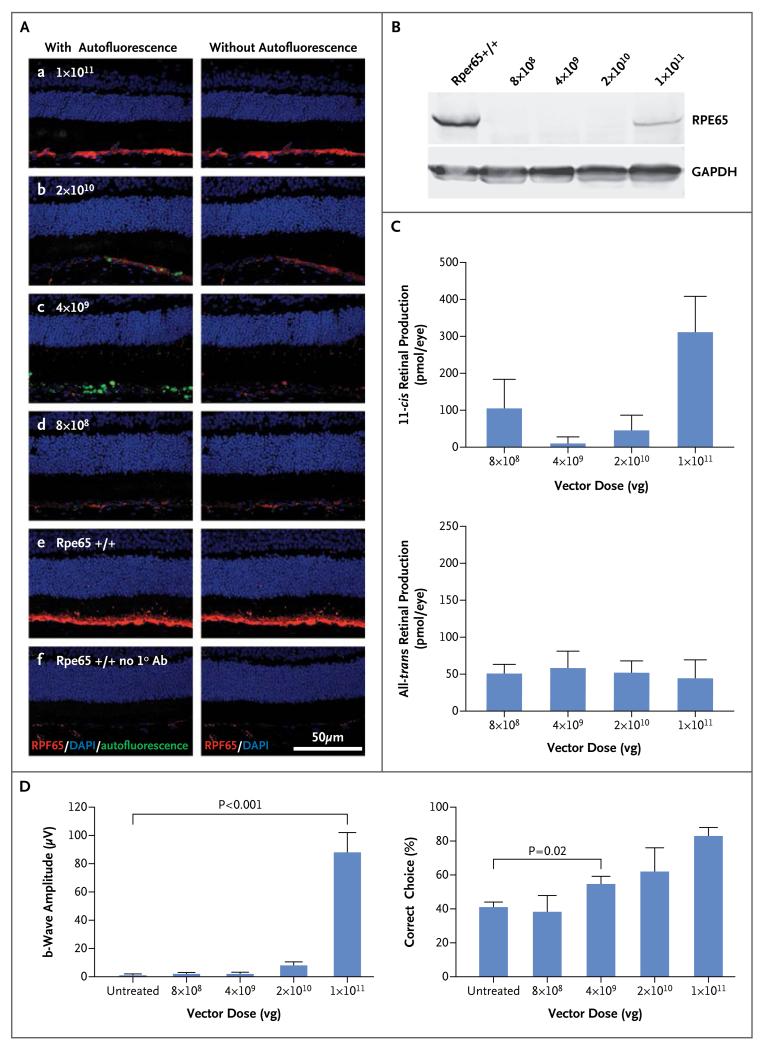
To investigate the differences in the responses to gene augmentation between dogs and humans with RPE65 deficiency, we estimated the normal demand for RPE65 in each species by measuring the endogenous expression of the protein and performed a dose–response study of Rpe65 gene augmentation in dogs. The expression of RPE65 messenger RNA in the human eye was 2.5 times as high as that in the dog eye (P = 0.02), which suggests that the human eye has a correspondingly higher requirement for RPE65 protein. The highest dose of rAAV2/2.hRPE65p.hRPE65 vector in dogs corresponded to the greatest amounts of RPE65 immunostaining (Fig. 3A), recombinant RPE65 protein (Fig. 3B), and 11-cis retinal (Fig. 3C), as well as the highest ERG response (Fig. 3D). The effect of gene augmentation on vision in dogs was strongly correlated with vector dose (R = 0.58, P<0.001) (Fig. 3D). Dogs administered the lower dose (4×109 vector genomes) had significantly better vision than untreated dogs (P = 0.02 by Student’s t-test) but no significant measurable improvement in ERG response.
Figure 3. Dose-Dependent Responses to Gene-Augmentation Therapy in Dogs.
Panel A shows the results of an immunohistochemical analysis demonstrating levels of RPE65 (in red) after administration of the indicated doses of vector (subpanels a–d). RPE65 signals are similar to wild-type levels (subpanel e) only at the highest dose. No signal is detected in negative control samples without primary antibody (subpanel f). Autofluorescent signal (in green) in the subretinal space, shown in subpanels on the left, has been removed in the subpanels on the right to facilitate visualization of RPE65. DAPI denotes 4′,6-diamidino-2-phenylindole, and GAPDH glyceraldehyde 3-phosphate dehydrogenase. Panel B shows the results of a Western-blot analysis indicating levels of recombinant RPE65 that are appreciable but lower than wild-type levels at the highest dose of injected vector. Panel C shows that the highest level of 11-cis retinal production corresponds to the highest dose of vector. The term vg denotes vector genomes. In Panel D, electroretinographic findings (left graph) show substantial restoration of the b-wave only at the highest dose of vector, whereas vision-guided behavior (right graph) shows dose dependency, with improved function evident even at lower doses of vector.
DISCUSSION
In 2008, we reported the preliminary results of this trial, which showed increased retinal sensitivity in 1 of 3 participants after intraocular administration of rAAV2/2.hRPE65p.hRPE65.7 Here, we report the outcomes in all 12 participants after 3 years. The magnitude of improved sensitivity within the treated area and the absence of an improvement of the same magnitude in the untreated contralateral eye provide evidence that such improvements are the consequence of RPE65 supplementation. Improved retinal sensitivity was apparent in a greater proportion of participants administered the higher dose (5 of 8 participants) than of those administered the lower dose (1 of 4 participants), indicating a possible dose–response effect; however, the number of participants in each group was small, and therefore the difference in proportions is suggestive rather than definitive. Even at the higher dose, the level of improvement in retinal sensitivity differed widely among participants, and no improvement was of a magnitude that could be detected by means of ERG. These observations are consistent with those of other investigators.18,19 In contrast, the responses after the same intervention in animal models of Rpe65 deficiency were predictably robust.
In common with the findings of Jacobson et al.,19 but in contrast to those of Maguire et al.,20 we identified no clear correlation between response and the age of the participant. In fact, the greatest improvements were evident in older participants (17, 18, and 23 years of age), and contrary to expectation, improvements in retinal sensitivity were of lower magnitude in younger participants who had the greatest preservation of retinal structure. The weaker effect in younger participants is unexplained but, in the context of a relatively well-preserved population of photoreceptor cells, the provision of a limited supply of 11-cis retinal may fail to meet a threshold required by individual cells for improved function. The majority of participants had at least one missense mutation known to mediate residual isomerase activity (Table 1), but we identified no clear correlation between participants’ genotypes and their response to the intervention.
Maximal retinal sensitivity was reached only after a substantially extended period of dark adaptation. This finding is consistent with a previous report of incomplete restoration of dark adaptation,21 indicating that the kinetics of the reconstituted retinoid cycle can remain abnormally slow after RPE65 gene supplementation. The level of RPE65 protein expressed may be insufficient to support normal provision of 11-cis retinal. Cideciyan et al.,21 citing evidence that RPE65 haploinsufficiency may not delay dark adaptation,22 suggested an alternative explanation, in which diffusion or transport of 11-cis retinal to photoreceptor cells is limited by an undefined “resistive barrier.” They suggested that such a barrier may be enhanced in RPE65 deficiency by the accumulation of all-trans-retinyl esters, lipid droplets, or disorganized rod outer segments3 or by retinal detachment that is induced temporarily by vector delivery.23 However, our finding that the improvements in retinal sensitivity were of greater magnitude in older participants than in younger participants suggests that the limitation results not from any progressively resistant barrier to diffusion but rather from limited enzymatic activity that fails to meet the demand of the surviving populations of photoreceptor cells.
Although RPE65 insufficiency typically causes severe rod photoreceptor-cell dysfunction, cone-mediated vision is relatively well preserved in the early stages. Whereas rod photoreceptor-cell function is critically dependent on the provision of 11-cis retinal by the retinal pigment epithelium, cone photoreceptor cells have an alternative source of the enzyme.2,24,25 Despite vector having been delivered to the fovea in the majority of participants, there was no improvement in foveal function, a finding consistent with results of a previous study.19 We identified improved extrafoveal cone function in only one participant. Other investigators have reported an improvement in the function of extrafoveal cone photoreceptor cells, as indicated by variably improved visual acuity in extrafoveal-fixating eyes19 and at very low levels of visual acuity.20 The question of why RPE65 gene supplementation improves the function of extrafoveal cones but not that of foveal cones remains unresolved.
An improvement in retinal sensitivity was evident within 1 to 2 months after vector administration, with progressive improvement for 6 to 12 months. The sensitivity subsequently declined, although the maintenance of improvement above preintervention levels was still evident after 3 years in two participants (5 and 6). These findings are consistent with those of other studies, in which progressive retinal thinning11 and a progressive decline in function from the peak improvement11,26 suggest ongoing retinal degeneration despite improved function in surviving cells.
In Rpe65-deficient mice and dogs, intervention with RPE65 gene supplementation before substantial degeneration confers improvements in retinal function of a magnitude measurable by means of ERG and can protect against progressive degeneration.13,14 In trials involving humans to date, although RPE65 gene supplementation can improve retinal function and there is some evidence of a dose response, neither the magnitude of improvement nor its durability has matched that observed in animal models. This disparity in response between species may reflect differences in both the extent of established retinal degeneration at the point of intervention and the ability to meet cellular requirements for RPE65. In animal models, the effect that Rpe65 gene augmentation has on protection against degeneration is highly dependent on the timing of intervention,11,13,14 and because the onset of retinal degeneration, relative to life span, is earlier in humans than in mice and dogs, even earlier intervention is likely to be required for the most durable benefit in humans. In Rpe65-deficient dogs, we found that the effect of RPE65 gene supplementation is highly dose-dependent, with a particularly steep dose–response curve between doses of 1×1011 and 2×1010 vector genomes. Although lower doses resulted in improvements in visual behavior, the effect was insufficient to generate measurable responses on ERG, and only higher doses have been associated with protection against progressive degeneration.14 We also found that RPE65 expression in humans is greater than that in dogs, which suggests that the demand for RPE65 in the human is higher. Taken together, these findings suggest that more efficient delivery of RPE65 at an appropriately early point in disease progression will have a greater effect on retinal function and will better protect against progressive degeneration.
Instances of intraocular inflammation and immune responses to AAV2 point to dose-limiting toxic effects at the higher dose and suggest that the provision of RPE65 would be more safely enhanced by improvement of the efficiency of the vector construct rather than by administration of a higher dose of vector genomes. We also measured a reduction in central macular thickness after subfoveal delivery of the vector. This finding is consistent with that in a previous study19 and is most likely a direct consequence of temporary detachment of the neurosensory retina. Although an adverse effect of retinal detachment on macular function is predictable,27 we considered that the risk of harm to foveal function from temporary detachment might be modest, as compared with the potential for benefit from the restoration of retinoid cycling. However, given the lack of evidence of benefit to foveal cone photoreceptor cells to date, delivery techniques should be considered that minimize the height and duration of any foveal detachment, possibly through the use of multiple injections. Careful attention should also be given to the extent of retinal area targeted; evidence that non–cell-autonomous mechanisms can contribute to retinal cell death28,29 suggests that widespread administration to viable retina may help promote increased protection against degeneration.
Our results provide further evidence for improved rod-photoreceptor function in response to AAV2-mediated RPE65 gene supplementation. In dogs, substantial improvements in retinal function were measurable by means of ERG and could protect against degeneration, but they were highly dose-dependent. In humans, the improvements in retinal sensitivity were modest even in participants with relatively mild retinal degeneration and failed to protect against ongoing degeneration. We conclude that gene therapy with an rAAV2/2 vector carrying the RPE65 cDNA led to temporary, variable, and incomplete restoration of retinal function in humans, which partly reflects a persistent unmet demand for RPE65.
Supplementary Material
Acknowledgments
Supported by the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL (University College London) Institute of Ophthalmology, the U.K. Department of Health, RP Fighting Blindness, the Special Trustees of Moorfields Eye Hospital, the Sir Jules Thorn Charitable Trust, the Wellcome Trust, the European Union (European Vision Institute–Genoret and Clinigene programs), the Medical Research Council, Foundation Fighting Blindness, Fight for Sight, the Biotechnology and Biological Sciences Research Council, Research to Prevent Blindness, the Ulverscroft Foundation, Fighting Blindness (Ireland), the Myers-Dunlap Endowment for Canine Health, and the Hal and Jean Glassen Memorial Foundation.
We thank Andrew Dick, Alan Bird, Andrew Webster, and Zdenek Gregor; Catey Bunce for statistical advice; Vijay Anand, Kanom Bibi, Eliza Burton, Adrienne Chen, Yanai Duran, Sneha Haria, Felicia Ikeji, Sarah Kalwarowsky, Kelly Mackenzie, Janice Querubin, Anthony Robson, Hannah Roche, Andy Rider, Vincent Rocco, Sety Shekouhi, Chris Timms and the staff of Moorfields/UCL (NIHR) Biomedical Research Centre; and the participants and their families.
Appendix
The authors’ full names and academic degrees are as follows: James W.B. Bainbridge, Ph.D., F.R.C.Ophth., Manjit S. Mehat, F.R.C.Ophth., Venki Sundaram, M.D., F.R.C.Ophth., Scott J. Robbie, Ph.D., F.R.C.Ophth., Susie E. Barker, Ph.D., Caterina Ripamonti, Ph.D., Anastasios Georgiadis, Ph.D., Freya M. Mowat, B.V.Sc., Ph.D., Stuart G. Beattie, Ph.D., Peter J. Gardner, Ph.D., Kecia L. Feathers, B.S., Vy A. Luong, B.Sc., Suzanne Yzer, M.D., Ph.D., Kamaljit Balaggan, Ph.D., F.R.C.Ophth., Ananth Viswanathan, Ph.D., F.R.C.Ophth., Thomy J.L. de Ravel, M.D., Ph.D., Ingele Casteels, M.D., Ph.D., Graham E. Holder, Ph.D., Nick Tyler, Ph.D., Fred W. Fitzke, Ph.D., Richard G. Weleber, M.D., Marko Nardini, Ph.D., Anthony T. Moore, F.R.C.Ophth., Debra A. Thompson, Ph.D., Simon M. Petersen-Jones, D.Vet.Med., Ph.D., Michel Michaelides, M.D., F.R.C.Ophth., L. Ingeborgh van den Born, M.D., Ph.D., Andrew Stockman, Ph.D., Alexander J. Smith, Ph.D., Gary Rubin, Ph.D., and Robin R. Ali, Ph.D.
The authors’ affiliations are as follows: the UCL (University College London) Institute of Ophthalmology (J.W.B.B., M.S.M., V.S., S.J.R., S.E.B., C.R., A.G., F.M.M., S.G.B., P.J.G., V.A.L., K.B., A.V., G.E.H., F.W.F., M.N., A.T.M., M.M., A.S., A.J.S., G.R., R.R.A.) and the Department of Civil, Environmental, and Geomatic Engineering (N.T.), UCL, and Moorfields Eye Hospital (J.W.B.B., M.S.M., V.S., S.J.R., A.G., K.B., G.H., A.M., M.M.), London, and the Department of Psychology, Durham University, Durham (M.N.) — all in the United Kingdom; the College of Veterinary Medicine, Michigan State University, East Lansing (F.M.M., S.M.P.-J.), and the Kellogg Eye Center, University of Michigan Medical School, Ann Arbor (K.L.F., D.A.T., R.R.A.); the Center for Human Genetics, KU Leuven (T.J.L.R.), and the Department of Ophthalmology, UZ Leuven, Campus Sint-Rafaël (I.C.) — both in Leuven, Belgium; Rotterdam Eye Hospital, Rotterdam, the Netherlands (S.Y., L.I.B.); and the Oregon Retinal Degeneration Center, Ophthalmic Genetics Service, Casey Eye Institute, Oregon Health and Science University, Portland (R.G.W.).
Footnotes
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research (NIHR) or the U.K. Department of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Moiseyev G, Chen Y, Nikolaeva O, Ma JX. An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species. FEBS J. 2011;278:2913–26. doi: 10.1111/j.1742-4658.2011.08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 4.Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–8. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–82. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annear MJ, Bartoe JT, Barker SE, et al. Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther. 2011;18:53–61. doi: 10.1038/gt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 8.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banin E, Bandah-Rozenfeld D, Obolensky A, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21:1749–57. doi: 10.1089/hum.2010.047. [DOI] [PubMed] [Google Scholar]
- 11.Cideciyan AV, Jacobson SG, Beltran WA, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annear MJ, Mowat FM, Bartoe JT, et al. Successful gene therapy in older Rpe65-deficient dogs following subretinal injection of an adeno-associated vector expressing RPE65. Hum Gene Ther. 2013;24:883–93. doi: 10.1089/hum.2013.146. [DOI] [PubMed] [Google Scholar]
- 13.Bemelmans AP, Kostic C, Crippa SV, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3(10):e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mowat FM, Breuwer AR, Bartoe JT, et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther. 2013;20:545–55. doi: 10.1038/gt.2012.63. [DOI] [PubMed] [Google Scholar]
- 15.Philp AR, Jin M, Li S, et al. Predicting the pathogenicity of RPE65 mutations. Hum Mutat. 2009;30:1183–8. doi: 10.1002/humu.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Izumi T, Hu J, et al. Rescue of enzymatic function for disease-associated RPE65 proteins containing various mis-sense mutations in non-active sites. J Biol Chem. 2014;289:18943–56. doi: 10.1074/jbc.M114.552117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology. 2013;120:1283–91. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poehner WJ, Fossarello M, Rapoport AL, et al. A homozygous deletion in RPE65 in a small Sardinian family with autosomal recessive retinal dystrophy. Mol Vis. 2000;6:192–8. [PubMed] [Google Scholar]
- 23.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Müller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–9. [PubMed] [Google Scholar]
- 24.Kaylor JJ, Yuan Q, Cook J, et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat Chem Biol. 2013;9:30–6. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang PH, Wheless L, Crouch RK. Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein. J Neurosci. 2011;31:10403–11. doi: 10.1523/JNEUROSCI.0182-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson SG, Cideciyan AV, Roman AJ, et al. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372:1920–6. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schocket LS, Witkin AJ, Fujimoto JG, et al. Ultrahigh-resolution optical coherence tomography in patients with decreased visual acuity after retinal detachment repair. Ophthalmology. 2006;113:666–72. doi: 10.1016/j.ophtha.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18:4076–82. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.