Abstract
LAT (linker for activation of T cells) is a transmembrane adaptor protein that is highly tyrosine phosphorylated upon engagement of the T cell receptor (TCR). Phosphorylated LAT binds Grb2, Gads, and PLCγ1 to mediate T cell activation, proliferation, and cytokine production. T cells from mice harboring a mutation at the PLCγ1 binding site of LAT (Y136F) have impaired calcium flux and Erk activation. Interestingly, these T cells are highly activated, resulting in the development of a lymphoproliferative syndrome in these mice. CD4+ T cells in LATY136F mice are Th2 skewed, producing large amounts of IL4. Here we showed that the LATY136F T cells could also overproduce interleukin-6 (IL6) due to activated NFκB, AKT, and p38 pathways. By crossing LATY136F mice with IL6-deficient mice, we demonstrated that IL6 is required for uncontrolled T cell expansion during the early stage of disease development. Reduced CD4+ T cell expansion was not due to a further block in thymocyte development or an increase in the number of Treg cells, but was caused by reduction in cell survival. In aged IL6−/−LATY136F mice, CD4+ T cells began to hyperproliferate and induced splenomegaly; however isotype switching and autoantibody production were diminished. Our data indicated that the LAT-PLCγ1 interaction is important for controlling IL6 production by T cells and demonstrated a critical role of IL6 in the development of this lymphoproliferative syndrome.
Keywords: Cytokine, Autoimmunity, T cell Receptors, Cell Proliferation
Introduction
Cytokines play an important role in orchestrating immune responses, and are vital for T cell survival, proliferation, and differentiation. Aberrant cytokine production is thought to play a role in the development of inflammatory conditions, which can lead to autoimmunity. Interleukin-6 (IL6) is a cytokine that is found in high quantities in the joints and sera of patients with rheumatoid arthritis (RA). Within joint tissues, IL6 is produced by fibroblasts, activated macrophages, and lymphocytes. It activates vascular endothelial cells to upregulate adhesion molecules, which then promote leukocyte recruitment to the site of inflammation. IL6 is also important for B cell maturation to antibody-secreting plasma cells, leading to overproduction of autoantibodies, as seen in the patients with RA (1). Because of its many roles in instigating inflammation, IL6 has become a promising target for the treatment of RA and other autoimmune diseases.
IL6-mediated signaling has been well studied. Upon binding IL6, IL6Rα associates with gp130 and activates downstream signaling cascades. STAT1 and STAT3 are phosphorylated by JAK1/2, form homodimers, and translocate to the nucleus to activate their target genes (2). IL6-mediated STAT3 activation induces the gene transcription of BCL2, BCL-XL, cFOS, and TIMP, all of which regulate cell survival and proliferation. Additionally, IL6 association with IL6Rα can induce phosphorylation of the SHP-2 binding site on gp130, resulting in ERK activation (3, 4). Finally, IL6 signaling also activates PI3K, which is an important mediator of Akt activation and cell survival (5).
IL6 is mainly produced by macrophages during acute inflammation; however, it is also produced by T cells during chronic inflammation (3). IL6 plays an important role in T cell survival, differentiation, and cytokine production. It has been shown to inhibit apoptosis of naïve T cells (6, 7) and to regulate the Th17/Treg balance (8). IL6, together with TGF-β, promotes Th17 differentiation, while TGF-β alone promotes iTreg differentiation (8–10). Additionally, it has been reported that IL6 can lead to the production of other cytokines by T cells. T cell receptor (TCR) signaling in conjuncture with IL6 signaling can induce c-Maf expression and early IL4 production (11). In human T cells, specifically T follicular helper cells (Tfh), IL6 can induce IL21 production, which in turn causes plasma cell differentiation (12).
Importantly, IL6 has been implicated in the regulation of T cell homeostasis. Microbiota within the gut have been shown to activate dendritic cells to produce IL6 in a Myd88-dependent manner, which induces spontaneous T cell proliferation (13). Additionally, blocking antibodies against IL6R can alleviate CD8+ T cell proliferation during colitis (14). IL6 is also important for CD4+ T cell homeostatic proliferation. In mice harboring a mutation in gp130, which results in enhanced STAT3 activation, non-hematopoietic cells increase production of IL7, which in turn drives homeostatic proliferation of CD4+ T cells (15).
Linker for activation of T cells (LAT) is an adaptor molecule that is highly tyrosine phosphorylated upon TCR engagement with MHC-peptide complexes. Through its phosphorylated tyrosine residues, LAT recruits Grb2, Gads, and PLCγ1 (16). LAT functions to link TCR engagement to downstream signaling pathways that are important for T cell activation, proliferation, survival, and cytokine production. Among the tyrosine residues in murine LAT, Y136 is responsible for binding PLCγ1. The recruitment of PLCγ1 to the LAT signalosome is important for the hydrolysis of PIP2 to IP3 and DAG, leading to calcium mobilization and ERK activation (16, 17). In LATY136F mutant mice, thymocyte development is partially blocked at the DN3 (double negative) stage, indicating the important role for LAT-PLCγ1 signaling at the pre-TCR checkpoint. CD4+ T cells are able to exit the thymus and hyperproliferate in secondary lymphoid organs. These CD4+ T cells have an activated phenotype (CD44hiCD62Llo) and low levels of surface TCR. In addition, these T cells produce elevated amounts of IL4. Consequently, B cells are activated, undergo isotype switching, and produce high levels of serum IgE, IgG1, and anti-dsDNA antibodies (18, 19). In this study, we were interested in investigating the role of cytokines in spontaneous hyperproliferation of LATY136F T cells. Our results indicated that LATY136F T cells produced IL6, and that IL6 played an important role in the development of LAT-mediated autoimmunity.
Materials and Methods
Mice
LATY136F (LATm/m) and LAT−/− mice have been previously described (18, 20). Myd88−/−, IL6−/−, and IL6Rα−/− mice (generated by crossing IL6Rαf/f and CMV-Cre mice) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in specific pathogen-free conditions and were used in accordance with the National Institutes of Health guidelines. All experiments were approved by the Duke University IACUC.
Flow Cytometry and ELISA
Single cell suspensions were prepared from spleens, lymph nodes, or thymuses and were stained with fluorescent antibodies (BioLegend) in the presence of 2.4G2 (anti-FcγII/III receptor). Intracellular staining for transcription factors was performed after cells were fixed and permeabilized (BD Bioscience). Prior to intracellular staining for cytokines, cells were stimulated in vitro for 4 hours with PMA (20ng/mL) and ionomycin (0.5µg /mL) in the presence of Monesin. For inhibitor experiments, SB203580 (30µM), PD98059 (30µM), LY294002 (10µM), Akti-1/2 trifluoroacetate salt hydrate (2µM), or QNZ (2µM)(Sigma) was added to RPMI complete media supplemented with IL2 for 48 hours prior to PMA and ionomycin stimulation. For intracellular staining for pERK, WT splenocytes were incubated with different inhibitors for 48 hours, then stimulated with anti-CD3 (2C11) for 30 minutes prior to fixation and permeabilization. 7-aminoactinomycin D (7AAD) distinguished live cells (Invitrogen). Data were acquired on the FACSCanto II (BD Bioscience) and analyzed using FlowJo software. Anti-dsDNA and serum antibody ELISAs were performed as previously described (21).
Western blotting
T cells were purified from spleens and lymph nodes using EasySep CD4+ purification kits (STEMCELL Technologies). Equal numbers of T cells from WT, LATm/m, and IL6−/−LATm/m mice were lysed, resolved on SDS-PAGE, and blotted with antibodies against the following proteins: Zap70, pLck, Lck, pERK, ERK2, pAkt (Ser473), Akt, pP38, P38, pNFκB, and NFκB (Cell Signaling).
T cell proliferation
For in vitro proliferation, splenocytes were loaded with 5µM CFSE and stimulated with plate-coated anti-CD3 (3µg/ml 2C11) or with PMA and ionomycin overnight. After 48 hours, CFSE dilution of CD4+ T cells was assessed by flow cytometry. For in vivo proliferation, 3×106 CD4+ T cells were sorted, loaded with CFSE, and transferred via i.v. injection to LAT−/− hosts for 6 days before CFSE dilution was assessed by FACS.
Real-time PCR
Total RNAs from purified CD4+ T cells or whole lymph nodes were isolated using TRIzol reagent (Invitrogen). Lymph nodes were homogenized with 1.5mm beads using the D1030 Beadbug homogenizer (Benchmark Scientific). cDNAs were synthesized with the SuperScript reverse transcriptase (Invitrogen). Cytokine RNAs were quantified using SYBR Green Super mix (Bio-Rad).
Immunofluorescence imaging
To determine the presence of autoantibodies, NIH3T3 cells were dropped onto 18 chamber slides, fixed with 1:1 acetone/methanol prior to incubation with serum (1:50), and stained with goat anti-mouse IgG-FITC and DAPI (Invitrogen). Cells were mounted using Fluoromount-G (SouthernBiotech), and examined using the Leica SP5 confocal microscope.
Results
LATY136F T cells overproduced IL6
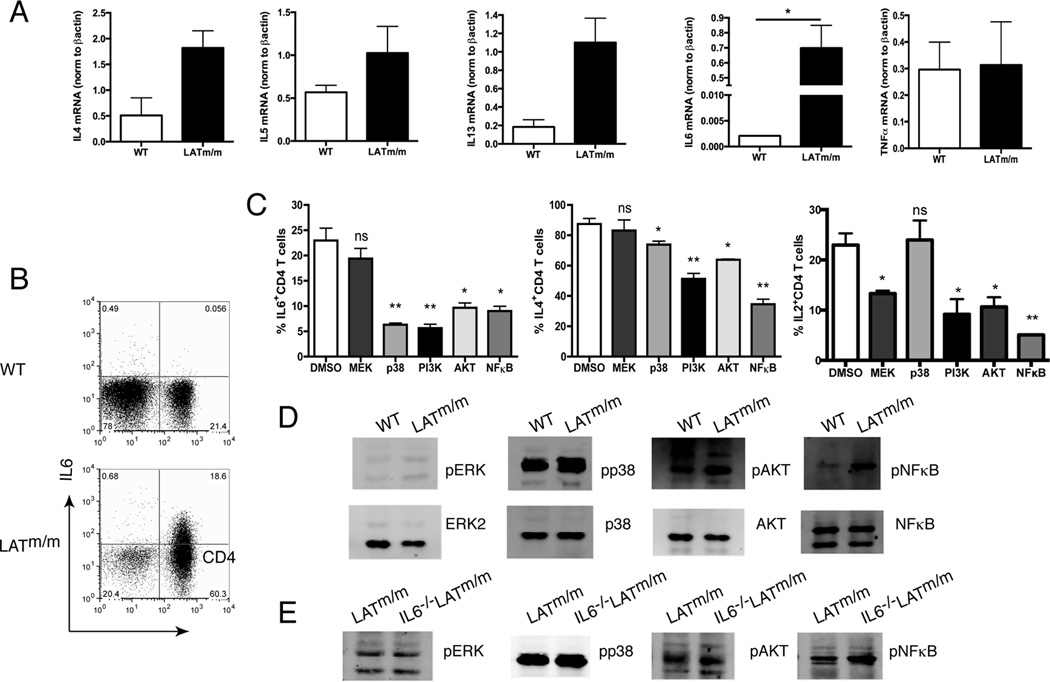
Published data indicate that CD4+ T cells from the LATY136F mice, designated as LATm/m mice here, are Th2 skewed (18, 19). We wanted to understand the effect of aberrant LAT-PLCγ1 signaling on the production of other cytokines. RNAs were isolated from WT and LATm/m CD4+ T cells to examine cytokine production at the level of transcription by real-time PCR analysis. As shown in Fig. 1A, CD4+ T cells from LATm/m mice had elevated levels of Th2 cytokines, such as IL4, IL5, and IL13, as expected. Interestingly, LATm/m T cells also had significantly elevated levels of IL6 RNA compared with WT CD4+ T cells (Fig. 1A). TNFα expression in WT and LATm/m T cells was similar (Fig. 1A). In addition, TNFα concentration in the sera of these mice, analyzed by multiplex assay, was also similar (data not shown), suggesting that the elevated IL6 was not a result of a systemic inflammatory response.
Figure 1. Increased production of IL6 by LATY136F T cells.
(A) Increased IL4, IL5, IL13, IL6, and TNFα production by real-time PCR analysis of cytokine transcripts in LATm/m CD4+ T cells compared to WT. Relative expression of cytokine RNA was normalized to β-actin. (B) Intracellular staining of IL6. WT and LATm/m splenocytes were stimulated with PMA and ionomycin for 4 hours prior to intracellular staining. (C) The effect of pharmacological inhibitors on cytokine production. LATm/m splenocytes were incubated with different inhibitors or DMSO in the presence of IL2 for 48 hours prior to stimulation with PMA and ionomycin and intracellular staining. Significance differences in cytokine production with various inhibitors (PD98059 for MEK1/2, SB203580 for p38, LY294002 for PI3K, trifluoroacetate for Akt1/2, and QNZ for NF-κB) is compared to DMSO treated cells. (D–E) Activation of signaling pathways in LATm/m T cells. Whole cell lysates from WT and LATm/m T cells (D) or LATm/m and IL-6−/−LATm/m T cells (E) without activation were analyzed by Western blotting with antibodies as indicated. Data are representative of 3–5 individual experiments. Mice used were 8–10 weeks old. Two-tailed t test; *, p<0.05, **, p<0.005.
Because of the potential role of IL6 in the regulation of T cell homeostasis (13–15), we chose to explore the role of IL-6 in T cell hyperproliferation and autoimmunity of LATm/m mice. To determine if the elevated IL6 was also seen at the protein level, we isolated splenocytes from 6 wk-old mice and stimulated them in vitro with PMA and ionomycin prior to intracellular staining for IL6. Our results showed that WT splenocytes had very little positive staining. In comparison, close to 25% of CD4+ T cells from LATm/m mice produced IL6 (Fig. 1B). Very few non-CD4+ T cells from LATm/m mice were IL6 positive. These data indicated that in addition to enhanced Th2 cytokine production, aberrant TCR signaling in LATm/m T cells also led to overproduction of IL6.
We next wanted to elucidate the signaling pathways downstream of the mutant LAT that were required for increased IL6 production. We used different pharmacological inhibitors to block known pathways downstream of LAT and then determined their effect on IL6 production. LATm/m splenocytes were incubated with various inhibitors prior to stimulation with PMA and ionomycin to examine IL6 production. Interestingly, while on average 23% of DMSO-treated T cells produced IL6, treatment with both p38 and PI3K pathway inhibitors resulted in a 4-fold reduction in the percentage of IL6-producing CD4+ T cells (6.3% and 5.6% respectively). Downstream of PI3K, both AKT and NFκB have been demonstrated to induce IL6 (22, 23). In both tumor and RA model systems, IL17 can induce AKT phosphorylation resulting in IL6 production (24, 25). Additionally, NFκB binds the IL6 promoter and induces its transcription in many cell types (26). As shown in Fig. 1C, inhibition of AKT or NFκB resulted in more than a 2-fold reduction in the percentage of CD4+ T cells producing IL6 (9.7% and 9.0% respectively). In contrast, MEK1/2 inhibitor (PD98059) treatment caused no significant difference in IL6 production, but it did cause a two-fold reduction in IL2 production (Fig. 1C). The specificities of these inhibitors were confirmed by FACS analysis of WT splenocytes activated in the presence of these inhibitors. The p38 inhibitor had very little effect on TCR-mediated Erk phosphorylation, while the MEK1/2 inhibitor severely reduced its phosphorylation (data not shown).
We also examined the effect of various inhibitors on IL4 production. Among the different inhibitors, the PI3K and NFκB inhibitors impacted IL4 production more than the other three inhibitors (Fig. 1C). Treatment with the PI3K inhibitor resulted in a 1.7-fold reduction in the percentage of CD4+ T cells producing IL4 (51.3% compared to 91.2% with DMSO), while NFκB inhibition resulted in a 2.5-fold reduction in the percentage of CD4+ T cells producing IL4 (34.5%). These results indicated that both AKT and NFκB activation were required for increased IL6 and IL4 production in CD4+ LATm/m T cells.
To confirm the inhibitor data, we next wanted to see which of these pathways were activated in LATm/m CD4+ T cells. CD4+ T cells were isolated from aged matched 2–3 month old mice and equal numbers of cells were lysed without any stimulation. Zap70, which is upstream of LAT signaling and therefore should not be affected by the LAT mutation, was used as a loading control. As shown in Fig. 1D, the levels of p38, AKT, and NFκB proteins were all similar, yet the phosphorylation of these proteins was increased in LATm/m T cells, indicating that these signaling pathways were activated in the absence of LAT-PLCγ1 signaling (Fig. 1D). In contrast, there was no significant difference in Erk activation. Together, these results suggested that the LATY136F mutation caused the activation of p38, AKT, and NFκB pathways in CD4+ T cells, which resulted in elevated IL6 production.
Reduced expansion of LATY136F CD4+ T cells in the absence of IL6
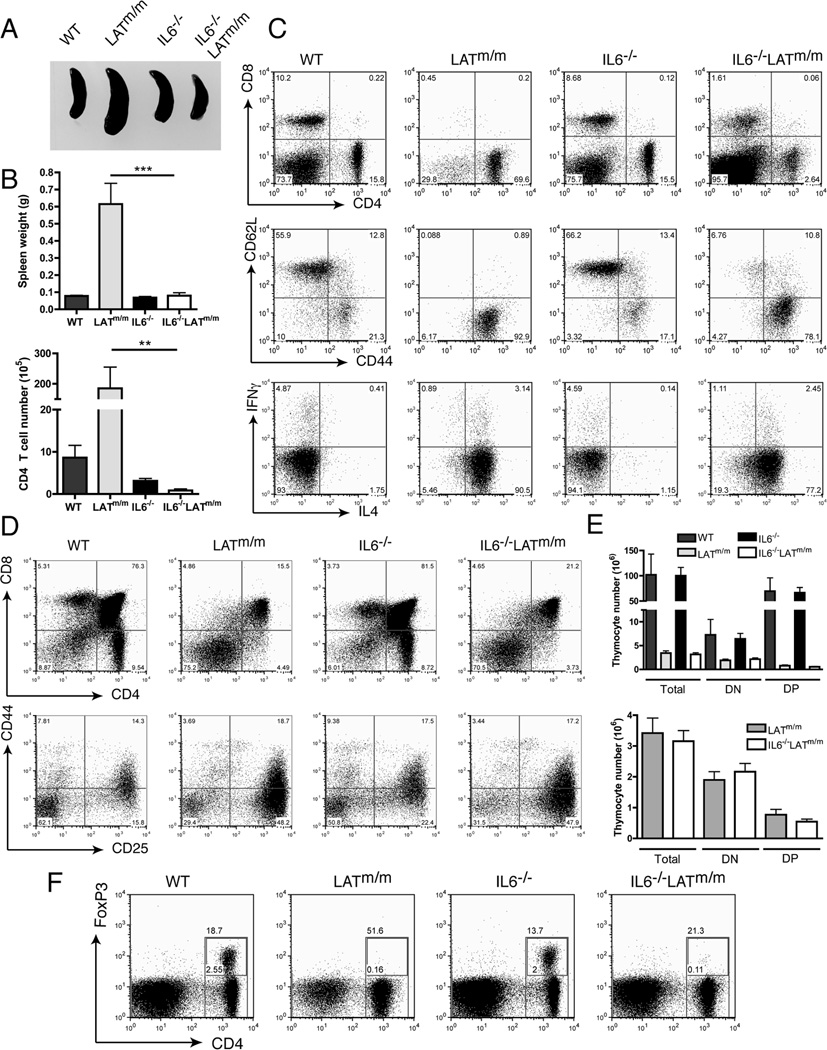
LATm/m mice display splenomegaly and lymphadenopathy due to hyperproliferating CD4+ T cells as early as 4 wks of age (18, 19). To assess the contribution of IL6 to the LATY136F-mediated autoimmune syndrome, IL6−/−LATm/m mice were generated, and disease development was compared to that in LATm/m mice at 6 wks of age. Age-matched WT and IL6−/− mice were used as controls. Remarkably, IL6−/−LATm/m mice had normal spleen size and weight, similar to control WT and IL6−/− mice (Fig. 2A, 2B). LATm/m mice had enlarged spleens and increased number of splenocytes as previously reported (data not shown). In agreement with the disparate size, spleens from LATm/m mice contained a large percentage of CD4+ T cells (~70%). Spleens from IL6−/−LATm/m mice, however, had dramatically reduced percentages of CD4+ T cells (2.6%), even compared to WT and IL6−/− control mice (~15%)(Fig. 2C). The number of CD4+ T cells in IL6−/−LATm/m mice was also reduced compared to that in LATm/m mice (Fig. 2B). Interestingly, while LATm/m mice lack peripheral CD8+ T cells, 4–6 wk-old IL6−/−LATm/m mice retained that population (Fig. 2C), although at much lower percentages than control mice (1.6% compared to 8.7% in IL6−/− mice or 10.2% in WT mice).
Figure 2. The effect of IL6 on LATY136F T cell homeostasis and development.
(A) Spleens from 6 wk-old WT, LATm/m, IL-6−/−, and IL-6−/−LATm/m mice. (B) Spleen weight and the numbers of CD4+ T cells from 4 to 6 wk-old mice. (C) Representative FACS plots of splenocytes. The expression of CD44 and CD62L was pre-gated on CD4+ cells. For intracellular staining of IFNγ and IL4, cells were stimulated with PMA and ionomycin for 4 hours. (D) Analysis of thymocyte development in 4 wk-old mice. (E) The numbers of different subsets of thymocytes. (F) IL6 deficiency had no effect on Treg cells in 6 wk-old IL-6−/−LATm/m mice. FoxP3 expression in CD4+ T cells was analyzed by intracellular staining. Data are representative of 3–5 individual experiments. Two-tailed t test; **, p<0.005. ***, p<0.001.
In addition to being hyperproliferative, LATm/m CD4+ T cells are also characterized by their activated phenotype, low levels of surface TCR, and exaggerated Th2 cytokine production (18, 19). While CD4+ T cells in IL6−/−LATm/m mice did not expand greatly, we wanted to evaluate if they displayed the other characteristics of LATY136F T cells. Surface staining of 4–6 wk-old mice revealed that the vast majority of CD4+ T cells in IL6−/−LATm/m mice had an effector/memory-like phenotype (CD44hiCD62Llo)(Fig. 2C); however, the number of naïve T cells (CD44loCD62Lhi) was increased compared with that in LATm/m mice. Furthermore, staining of TCRβ revealed that both CD4+ and CD8+ T cells in IL6−/−LATm/m mice had very low levels of TCRβ at the cell surface similar to LATm/m T cells (data not shown). To assess cytokine production, splenocytes were stimulated with PMA and ionomycin for 4 hours prior to intracellular staining. Like LATm/m T cells, the vast majority of IL6−/−LATm/m T cells had the capacity to produce large amounts of IL4; therefore, they were also heavily Th2 skewed (Fig. 2C). In addition, T cells from IL6−/−LATm/m mice had similar phosphorylation of p38, AKT, and NFκB as LATm/m T cells, suggesting that the activated PI3K and p38 pathways seen in LATm/m T cells were not caused by enhanced signaling through IL6 (Fig. 1E). Together, these results indicated that even though IL6 deficiency did not change the activated phenotype of LATm/m T cells, IL6 was important in driving uncontrolled CD4+ T cell expansion in LATm/m mice.
IL6 deficiency had no effect on the development of LATY136F T cells
Since the LATY136F mutation causes a partial block at the pre-TCR checkpoint (DN3) during thymocyte development, we next wanted to determine if the reduced number of T cells seen in the periphery of IL6−/−LATm/m mice was due to a further block in thymocyte development. Thymocytes from 4 wk-old IL6−/−LATm/m, LATm/m, and control mice were analyzed. Staining for CD4 and CD8 revealed a similar block in the DN compartment. Both LATm/m and IL6−/−LATm/m mice had 75.2% and 70.5% DN thymocytes respectively (Fig. 2D). Further analysis of the DN compartment showed that thymocyte development was partially blocked at DN3 and the percentages of the DN1-DN4 subsets were similar between LATm/m and IL6−/−LATm/m mice (Fig. 2D). Furthermore, the numbers of total, DN, and DP thymocytes were comparable in LATm/m and IL6−/−LATm/m mice (Fig. 2E). Thus, the reduction in the number of CD4+ T cells seen in the periphery of IL6−/−LATm/m mice was not due to a further block in thymocyte development.
No effect of IL6 deficiency on regulatory T cells in LATY136F mice
We next wanted to elucidate the mechanism by which IL6 regulates T cell expansion in secondary lymphoid organs of LATm/m mice. It is known that IL6 inhibits Treg differentiation and promotes Th17 differentiation from naïve T cells (9, 10, 27). Since LATm/m mice lack functional Tregs (28, 29), it is possible that IL6 deficiency in LATm/m mice may restore the development or function of regulatory T cells. To detect the presence of Tregs, T cells from 6 wk-old mice were stained intracellularly for FoxP3, the master regulator of Tregs. Similar to LATm/m mice, IL6−/−LATm/m mice were devoid of FoxP3+CD4+ T cells (Fig. 2F). This indicated that the reduced expansion of CD4+ T cells in IL6−/−LATm/m mice was not due to the appearance of Tregs that suppressed T cell expansion. It also suggested that the absence of Tregs in LATm/m mice was not due to an overabundance of IL6 in lymphoid tissues, but rather an intrinsic role of LAT-PLCγ1 signaling in the development or maintenance of Tregs.
Myd88 signaling is not required for LATY136F T cell proliferation
Studies have demonstrated that toll-like receptor (TLR)-mediated IL6 production can induce T cell homeostatic proliferation (13). Additionally, naïve T cells express TLRs, such as TLR 1, 2, 3, 6, and 7, while activated CD4+ T cells express TLR4 and TLR5 (30, 31). TLR-mediated signaling may potentially intersect with aberrant TCR signaling in LATm/m T cells to induce IL6 production. We next tested whether TLR signaling was important for IL6 production in LATm/m mice by crossing them to Myd88−/− mice to ablate TLR signaling.
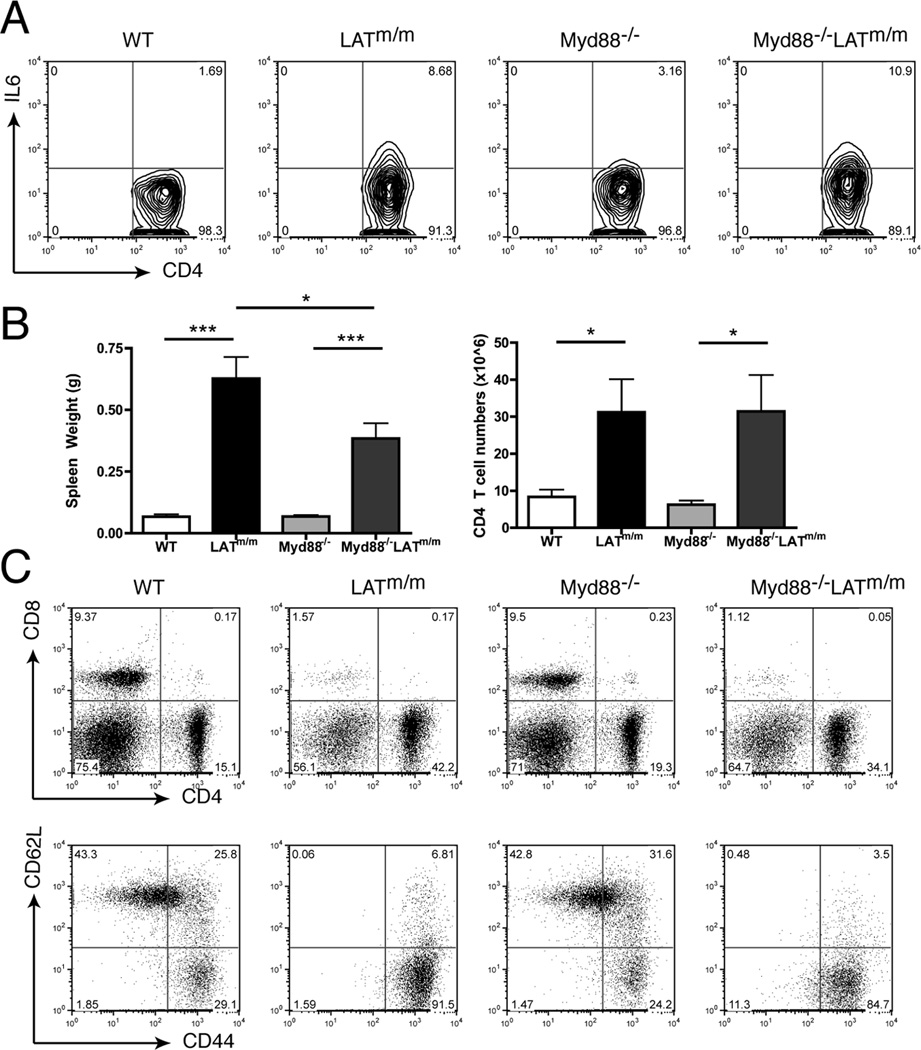
Similar to IL6−/−LATm/m mice, we analyzed Myd88−/−LATm/m mice at 4–6 wks of age for IL6 production and the development of autoimmunity. We first analyzed the ability of Myd88−/−LATm/m T cells to produce IL6. As shown in Fig. 3A, 10.9% of Myd88−/−LATm/m T cells produced IL6, which was comparable to LATm/m T cells (8.7%). To assess autoimmunity, we first analyzed spleen weight and the number of CD4+ T cells, which were similar in Myd88−/− and WT mice (Fig. 3B). Myd88−/−LATm/m mice had significantly larger spleens and increased number of CD4+ T cells compared to Myd88−/− and WT control mice; however, the splenomegaly was not as drastic as that in LATm/m mice (Fig. 3B). The spleens of Myd88−/−LATm/m mice contained ~34% CD4+ T cells with virtually no CD8+ T cells (Fig. 3C). While the percentage of CD4+ T cells varied, the average number of CD4+ T cells in the spleen was not significantly different between Myd88−/−LATm/m mice and LATm/m mice. Additionally, Myd88 deficiency did not rescue the activation status of LATm/m T cells, similar to IL6 deficiency (Fig. 3C). T cell hyperproliferation in Myd88−/−LATm/m mice was similar to that seen in LATm/m mice, and it did not recapitulate the phenotype seen in IL6−/−LATm/m mice. These data indicated that TLR signaling through Myd88 did not play a major role in IL6 production and T cell expansion in LATm/m mice.
Figure 3. Myd88 in not required for IL6 production in LATm/m T cells.
(A) IL6 production. Splenocytes from WT, LATm/m, Myd88−/−, and Myd88−/−LATm/m mice were stimulated for 4 hours with PMA and ionomycin before intracellular staining. (B) Spleen weight and the numbers of CD4+ T cells. (C) FACS analysis of splenocytes. Cells were pre-gated on CD4+ cells for CD44 vs. CD62L expression. Data are representative of 3 individual experiments. All data were obtained from the analysis of 4–6 wk-old mice. Two-tailed t test; *, p<0.05, ***, p<0.001.
Defect in T cell survival in the absence of IL6
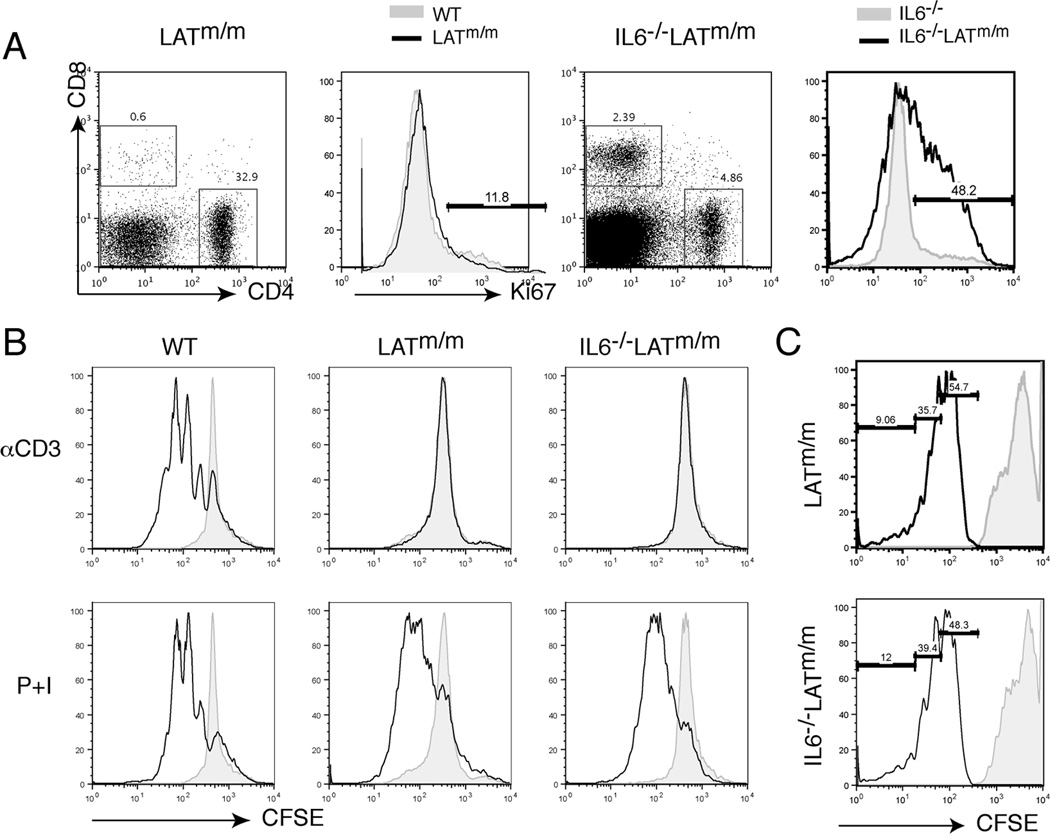
In addition to its role in T cell differentiation, IL6 is also important for both T cell survival and proliferation (6, 7, 13, 15). To examine if IL6 deficiency blocked proliferation in IL6−/−LATm/m T cells, we examined T cell proliferation by Ki67 staining, which marks cells that are in active phase of cell cycle, not resting cells. While the percentage of CD4+ T cells in IL6−/−LATm/m mice was relatively small (~5%), almost half of those T cells were in active proliferating phase (Fig. 4A). Even though LATm/m mice had enlarged spleens by 6 wks of age due to uncontrolled expansion of CD4+ T cells, only 11% of them were actively proliferating, similar to WT and IL6−/− T cells. This large Ki67+ population in IL6−/−LATm/m mice may be a factor of the lymphopenic environment. This result suggested that there was not a defect in T cell proliferation in the absence of IL6.
Figure 4. IL6 deficiency on LATY136F T cell survival.
(A) T cell proliferation. Ki67 intracellular staining was pre-gated on CD4+ T cells. (B) Splenocytes from 4–6 week old mice were stained with 5 µM CFSE and stimulated with plate-bound anti-CD3 (2C11) or PMA and ionomycin for 24 hours. Cells were cultured for additional 48 hours, harvested, and analyzed by FACS. The solid black line represents stimulated cells, and the shaded histogram represents unstimulated cells. (C). 3×106 CD4+ T cells were sorted, stained with CFSE , and transferred into LAT−/− host mice. Mice were sacrificed 6 days later and CFSE dilution in CD4+ T cells was analyzed by FACS.
To further confirm that T cell proliferation was not affected by IL6 deficiency in IL6−/−LATm/m, we examined their proliferation by CFSE dilution both in vitro and in vivo. To assess T cell proliferation in vitro, splenocytes were labeled with CFSE and then stimulated through the TCR using plate bound anti-CD3 or by PMA and ionomycin for 24 hours. CFSE dilution was analyzed 48 hours later. As seen in Fig. 4B, neither LATm/m T cells nor IL6−/−LATm/m T cells proliferated in response to TCR engagement likely due to low surface TCR levels, both cells diluted CFSE similarly in response to PMA and ionomycin stimulation. To access in vivo proliferation, 3×106 CD4+ T cells were sorted from LATm/m or IL6−/−LATm/m mice, stained with CFSE, and transferred into LAT−/− hosts, which are deficient in T cells. CFSE dilution was analyzed 6 days later. Similar slow homeostatic proliferation was detected for both LATm/m and IL6−/−LATm/m T cells (Fig. 4C).
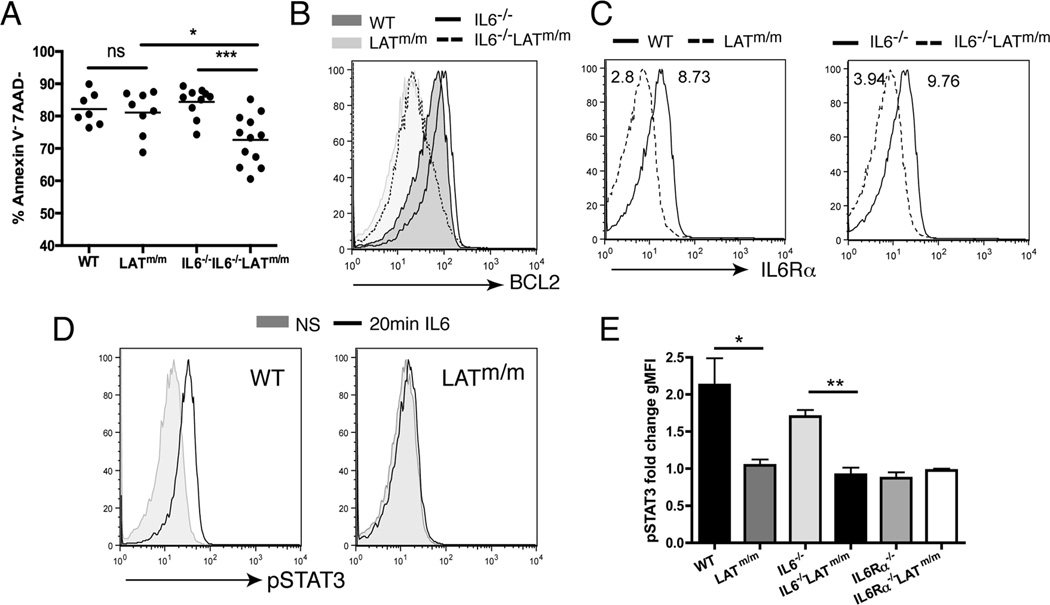
To examine the role of IL6 in LATm/m T cell survival, we examined cell death by staining with Annexin V and 7AAD. CD4+ T cells were pre-gated to remove doublets, and then the percentage of live cells (Annexin V−7AAD−) was determined. WT and IL6−/− spleens contained on average 82.2% and 84.4% live CD4+ T cells, respectively (Fig. 5A). LATm/m mice had an average of 81.1% live CD4+ T cells in their spleens, which was similar to WT and IL6−/− mice. In contrast, 72.6% of CD4+ T cells in IL6−/−LATm/m spleens were live, which was significantly reduced compared to WT, IL6−/−, and LATm/m T cells (Fig. 5A). Previous studies have demonstrated that IL6 signaling induces the expression of the anti-apoptotic molecules, BCL2 and BCL-XL, to rescue T cells from cell death (3, 6, 32). Intracellular staining of T cells from 6 week-old mice showed that IL6−/−LATm/m CD4+ T cells expressed low levels of BCL2 similar to LATm/m CD4+ T cells (Fig. 5B). Since BCL2 expression was not further reduced in IL6−/−LATm/m CD4+ T cells compared to LATm/m CD4+ T cells, we were able to conclude from these data that the effect of IL6 on the survival of CD4+ T cells in LATm/m mice is less likely through the regulation of BCL2 expression.
Figure 5. IL6 deficiency on LATY136F T cell survival.
(A) The percentage of live (Annexin V−7AAD−) CD4+ T cells. (B) Analysis of BCL2 expression in CD4+ T cells by intracellular staining. (C) IL6Rα surface expression on CD4+ T cells. WT and IL6−/− (solid line); LATm/m and IL6−/−LATm/m (dashed line). The numbers labeled in the FACS plots represent geometric mean florescent intensity. (D–E) pSTAT3 activation. Splenocytes were either treated with IL6 (1µg/ml) for 20 minutes or left untreated before pSTAT3 staining. Mice were used at 6 wks of age. Data are representative of 3 independent experiments. Two-tailed t test; *, p<0.05, **, p<0.005, ***, p<0.001.
While it has been shown that IL6 can rescue antigen-stimulated T cells from cell death (33), it was also reported that the effect of IL6 on T cell survival is restricted to naïve T cells, and there is little effect on activated T cells due to differential signaling and expression of IL6Rα (7). LATm/m T cells spontaneously develop an activated phenotype. Therefore, these cells may not be able to signal through the IL6Rα like naïve T cells. Indeed, when we examined IL6Rα expression, LATm/m CD4+ T cells expressed lower levels of surface IL6Rα compared to WT T cells (Fig. 5C). While some cytokine receptors become internalized upon engagement with their ligands, this is likely not the case here, as IL6−/−LATm/m CD4+ T cells also expressed low surface IL6Rα, despite the absence of IL6.
To test whether low IL6Rα expression could still allow for STAT3 activation, splenocytes were left unstimulated or stimulated in vitro with IL6 for 20 minutes, prior to intracellular staining for pSTAT3. As shown in Fig. 5D, STAT3 phosphorylation was induced in WT T cells upon IL6 stimulation while STAT3 phosphorylation was unchanged in LATm/m T cells. Quantitation of fold changes of STAT3 phosphorylation from unstimulated to stimulated showed that WT T cells had approximately a 2.1-fold increase in pSTAT3 (Fig. 5E). STAT3 phosphorylation was not increased in LATm/m T cells. In addition, there was no detectable pSTAT3 in LATm/m T cells at 5, 10, or 30 minutes after IL6 stimulation either (data not shown). As controls, IL6Rα−/− and IL6Rα−/−LATm/m splenocytes were also stimulated with IL6, and those T cells showed a lack of STAT3 phosphorylation (0.87 and 0.98 fold change in MFI respectively), similar to LATm/m T cells (Fig. 5E). Together, these data suggested that it is unlikely that IL6 acts directly on T cells in LATm/m mice to promote cell survival.
IL6 in LATY136F mediated autoimmunity
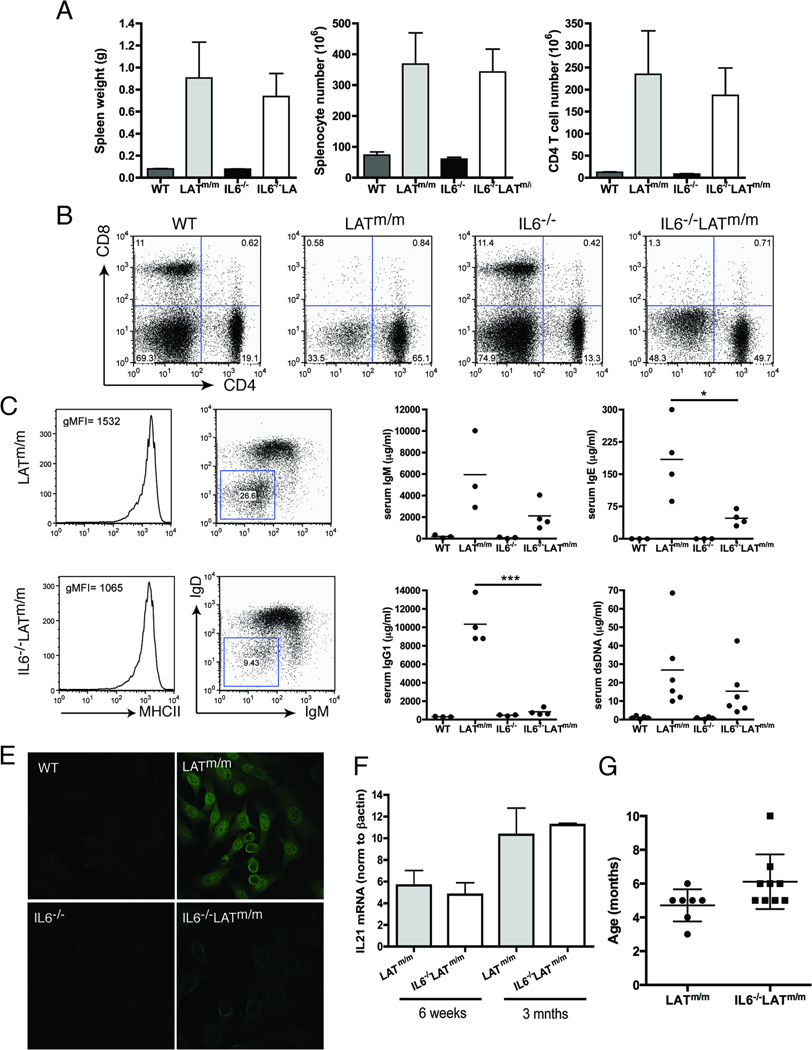
Finally, we wanted to determine if this regulation of T cell expansion by IL6 persisted in aged mice. To examine this, 3 month-old IL6−/−LATm/m mice were analyzed for T cell expansion and development of autoimmunity. Again, IL6−/− mice had normal spleen sizes and similar T cell populations as WT mice. In contrast to young mice, 3 month-old IL6−/−LATm/m mice now displayed severe splenomegaly, with large percentages of CD4+ T cells (49.7% compared to young mice with ~3%)(Fig. 6A, 6B). Aged IL6−/−LATm/m mice had a T cell profile similar to LATm/m mice, with close to half of the splenocytes being CD4+ T cells and very few CD8+ T cells (Fig. 6B). From these data, we were able to conclude that over time, there is a compensatory mechanism that promotes expansion and survival of CD4+ T cells in the absence of IL6.
Figure 6. The development of an autoimmune syndrome in aged IL6−/−LATm/m mice.
(A) Spleen weight, splenocyte number, and CD4+ T cell number. WT, LATm/m, IL6−/−, and IL6−/−LATm/m mice were 3 months old. (B) FACS analysis of splenic CD4 and CD8 expression. (C) MHC class II, IgD, and IgM expression on CD19+B220+ B cells from LATm/m and IL6−/−LATm/m mice. (D) Serum levels of IgM, IgG1, IgE, and anti-dsDNA antibodies. (E) Autoantibody production. NIH3T3 cells were incubated with sera from different mice followed by anti-mouse IgG-FITC. (F) IL21 mRNA levels from whole lymph nodes. RNAs were isolated from inguinal lymph nodes in Trizol reagent using a Beadbug homogenizer, and 5µg RNA was used for reverse-transcription. cDNAs were normalized to β-actin. (G) Long-term survival of LATm/m and IL6−/−LATm/m mice. The numbers indicated the average age of mice that either died or had to be sacrificed. Data are representative of 3–5 independent experiments. Two-tailed t test; *, p<0.05, ***, p<0.001.
The other aspect of LATY136F-mediated disease is the effect of CD4+ T cells on B cells. B cells become activated and express high levels of MHC class II, CD80, and CD86. They undergo isotype switching, resulting in high levels of serum IgG1 and IgE, and autoantibodies (18, 19). The number of B cells in IL6−/−LATm/m mice was not significantly different compared to LATm/m mice (data not shown). B cells from 3 month-old IL6−/−LATm/m mice also had similar upregulation of MHC class II; however, while 26.6% of LATm/m B cells had undergone class switching, only 9.4% of IL6−/−LATm/m B cells were IgD−IgM− (Fig. 6C). Furthermore, analysis of serum antibodies from 3 month-old mice demonstrated significantly less serum IgG1 and IgE in IL6−/−LATm/m mice compared to LATm/m mice, although still more than WT and IL6−/− mice (Fig. 6D). Interestingly, quantitation of anti-dsDNA antibodies by ELISA revealed that IL6−/−LATm/m had lower levels of autoantibodies than LATm/m mice. This result was also confirmed by immunofluorescence staining of NIH3T3 cells with the sera from these mice. Staining with the LATm/m sera revealed marked nuclear and cytoplasmic staining (Fig. 6E). While detectable, staining of NIH3T3 cells with IL6−/−LATm/m sera was much weaker.
It has previously been demonstrated that the role of IL6 on antibody production is indirectly mediated through induction of IL21 by T cells (34). Therefore, we wanted to examine IL21 levels in LATm/m mice with and without IL6. Since the phenotype seen in the spleen was mirrored in the lymph nodes, we homogenized whole LNs for preparation of total RNAs for real-time PCR analysis of IL21. There was no difference in IL21 mRNA levels in LATm/m and IL6−/−LATm/m lymph nodes (Fig. 6F). We also examined whether reduced early T cell survival and less autoantibody production in IL6−/−LATm/m mice had any effect on the survival of these mice. On average, LATm/m mice lived for 4.7 months (Fig. 6G). IL6−/−LATm/m mice appeared to have better long-term survival and lived average of 6.1 months, although this difference was not statistically significant. Together, our data demonstrated that in LATm/m mice, IL6, which is produced mainly by T cells, plays an important role in early T cell survival and B cell class switching and autoantibody production.
Discussion
Published studies show that the LAT-PLCγ1 interaction is essential in regulating T cell homeostasis and controlling production of Th2 cytokines. Our results here demonstrated that this interaction is also important for modulating IL6 production. IL6 is normally produced by innate cells such as monocytes and macrophages at the site of acute inflammation, and also by T cells during chronic inflammation (3). Its expression is induced upon activation of IL1, TNFα, or TLR signaling pathways, all of which depend on the transcription factor NFκB. Our results showed that basal phosphorylation of both NFκB and AKT was enhanced in T cells with the LATY136F mutation. Moreover, inhibition of these proteins reduced the ability of these T cells to produce IL6. Interestingly, p38 activation was also enhanced in LATm/m T cells, and inhibition of p38 also prevented LATm/m T cells from producing IL6. It has been demonstrated that in cardiac myocytes, MKK6 activates NFκB-mediated IL6 production in a p38 dependent manner (35). Thus, it is possible that in LATm/m T cells, p38 is enhancing NFκB-mediated IL6 production.
A published study demonstrated that β-catenin is constitutively degraded in peripheral T cells. Interestingly, a non-degradable form of β-catenin can act as a negative regulator of the LAT-PLCγ1 signaling cascade (36). Because WNT and β-catenin signaling can induce an inflammatory response and IL6 production, we analyzed β-catenin expression in those CD4+ T cells by Western blotting. β-catenin protein levels were slightly elevated in LATm/m T cells compared to WT peripheral T cells (data not shown). Thus, it is possible that the LATY136F mutation may cause sustained β-catenin expression, while drives IL-6 expression in these T cells.
Signaling through TLRs is a major mechanism for IL6 induction. Our data showed that LATY136F CD4+ T cells were able to produce IL6 in a Myd88-independent manner. Additionally, Myd88−/−LATm/m mice had CD4+ T cells that were hyperproliferative and activated. These result suggested that TLR-mediated Myd88 activation is neither required for T cell IL6 production nor for IL6-induced expansion of T cells in LATm/m mice. Our intracellular staining of IL6 suggested that macrophages and B cells were not the main producers of IL6 in LATm/m mice. Although our data indicted that CD4+ LATm/m T cells increased IL6 production, we cannot rule out the possibility that non-hematopoietic cells may contribute to IL6 production in these mice. The liver is able to produce IL6 as part of an acute phase response and muscle cells can also produce IL6. Because our multiplex assay for different cytokines did not detect high levels of IL6 in the serum of LATY136F mice (unpublished data), it is possible that IL6 is acting locally in secondary lymphoid organs, and not part of a systemic inflammatory response. Additionally, endothelial cells and fibroblasts can produce IL6. These cells may also contribute to local IL6 production in the spleens and lymph nodes of LAT mutant mice.
Ki67 intracellular staining indicated that IL6−/−LATm/m T cells were not defective in proliferation compared with LATm/m T cells. Additionally, when we transferred IL6−/−LATm/m or LATm/m T cells into LAT−/− mice (T cell deficient) to examine homeostatic proliferation by CFSE labeling, we did not observe a difference in CFSE dilution. Previous studies have shown that IL6 signaling plays an important role in IL7 production in vivo. In mice with a mutation in gp130 (a subunit of the IL6R) that causes enhanced STAT3 signaling, IL7 production is enhanced, thus driving abnormal CD4+ T cell proliferation (15). It has been shown that IL7, rather than the TCR-MHC interaction, is crucial for LATm/m T cell proliferation as they fail to expand in IL7-deficient hosts (37). Interestingly, we observed similarly normal IL7-mediated CFSE dilution in our transfer experiments, suggesting that IL7-dependent proliferation is not regulated by IL6 in the LATm/m mice. Additionally, we did not detect any difference in IL7 levels between IL6−/−LATm/m and LATm/m lymph nodes by Western blotting and RT-PCR analysis (data not shown). We cannot rule out the possibility that IL6 is acting locally to promote IL7 or other survival factors. Since the absence of IL6 did not affect the production of other cytokines, such as IL2 and IL4, other factors such as MCL1 or BCL-XL might be important in promoting T cell survival (2).
Our data showed that in the absence of IL6, there were fewer live T cells in young LATm/m mice. Our results suggested that IL6 does not act in an autocrine manner to directly promote T cell survival. We have observed differences in the expansion of LATm/m T cells when they were transferred into RAG−/− and LAT−/− mice, suggesting that B cells might promote their survival or proliferation (unpublished observations). It is possible that IL6 produced by T cells may cause B cells to produce a survival factor for T cells. Furthermore, IL6 has a known role in promoting T cell migration, both as a direct chemoattractant and through the regulation of chemokines such as CXCL10, CCL4, CLL5, and CCL11 (38, 39). In addition to its role in T cell survival, IL6 may also be important in this system to promote trafficking from the thymus to the secondary lymphoid organs, which may also contribute to the low numbers of T cells in the spleens of young IL6−/−LATm/m mice.
Despite the delayed T cell expansion in young IL6−/−LATm/m mice, by 3 months of age, IL6−/−LATm/m mice had enlarged spleens similar to LATm/m mice, due to the huge expansion of CD4+ T cells. Interestingly, while B cell numbers were comparable in IL6−/−LATm/m and LATm/m mice, antibody class-switching and production were reduced in the absence of IL6. Previous studies have demonstrated that IL6 has an indirect role in B cell antibody production through increasing IL21 production by T cells (34). When we examined IL21 levels in lymph nodes, there was no difference between IL6−/−LATm/m and LATm/m mice. How IL6 affects antibody class-switching and production in LATm/m mice remains to be further investigated.
IL6 is a cytokine that is found in high concentrations in many autoimmune syndromes, such as SLE and rheumatoid arthritis. It is a therapeutic target for dampening inflammation in these patients (40). IL6 is also highly expressed in germinal centers in patients with Castleman’s disease, a type of lymphoproliferative disorder (41). Our work established a critical role for LAT-PLCγ1 signaling in the regulation of IL6 production by T cells, and subsequent B cell class-switching and autoantibody production. Modulating LAT signaling may prove to be a novel target for controlling T cell-mediated cytokine production in human diseases.
Acknowledgements
The authors thank the Duke University Cancer Center Flow Cytometry facility.
This work was supported by National Institutes of Health Grants AI048674 and AI093717.
Footnotes
Disclosures
The authors have no financial conflicts of interest
References
- 1.Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008;4:767–775. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 3.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegiel B, Bjartell A, Culig Z, Persson JL. Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int J Cancer. 2008;122:1521–1529. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 7.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- 12.Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol Cell Biol. 2012;90:802–811. doi: 10.1038/icb.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, Ishigame H, Iwakura Y, Kitamura H, Nishimura T. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawa S, Kamimura D, Jin GH, Morikawa H, Kamon H, Nishihara M, Ishihara K, Murakami M, Hirano T. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med. 2006;203:1459–1470. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 18.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, Samelson LE, Love PE. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 19.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan SA, Zhu M, Bao S, Lewis CA, Ou-Yang CW, Zhang W. The role of LAT-PLCgamma1 interaction in gammadelta T cell development and homeostasis. J Immunol. 2014;192:2865–2874. doi: 10.4049/jimmunol.1302493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng WP, Su CM, Tang CH. FAK activation is required for TNF-alpha-induced IL-6 production in myoblasts. J Cell Physiol. 2010;223:389–396. doi: 10.1002/jcp.22047. [DOI] [PubMed] [Google Scholar]
- 24.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6:R120–R128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol. 2010;184:2476–2486. doi: 10.4049/jimmunol.0902876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobek V, Birkner N, Falk I, Wurch A, Kirschning CJ, Wagner H, Wallich R, Lamers MC, Simon MM. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6:R433–R446. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol. 2009;41:385–396. doi: 10.1165/rcmb.2008-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 34.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- 36.Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-gamma1 phosphorylation. J Immunol. 2011;186:784–790. doi: 10.4049/jimmunol.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S, Genton C, Lucas B, DiSanto JP, Acha-Orbea H, Malissen B, Malissen M. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol. 2008;180:1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
- 38.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissenbach M, Clahsen T, Weber C, Spitzer D, Wirth D, Vestweber D, Heinrich PC, Schaper F. Interleukin-6 is a direct mediator of T cell migration. Eur J Immunol. 2004;34:2895–2906. doi: 10.1002/eji.200425237. [DOI] [PubMed] [Google Scholar]
- 40.Heath I. A wolf in sheep's clothing: a critical look at the ethics of drug taking. BMJ. 2003;327:856–858. doi: 10.1136/bmj.327.7419.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori T, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74:1360–1367. [PubMed] [Google Scholar]