Abstract
Background
Epidural spinal cord stimulation is a promising technique for modulating the level of excitability and reactivation of dormant spinal neuronal circuits after spinal cord injury (SCI). We examined the ability of chronically implanted epidural stimulation electrodes within the cervical spinal cord to (1) directly elicit spinal motor evoked potentials (sMEPs) in forelimb muscles and (2) determine whether these sMEPs can serve as a biomarker of forelimb motor function after SCI.
New method
We implanted EMG electrodes in forelimb muscles and epidural stimulation electrodes at C6 and C8 in adult rats. After recovering from a dorsal funiculi crush (C4), rats were tested with different stimulation configurations and current intensities to elicit sMEPs and determined forelimb grip strength. Results: sMEPs were evoked in all muscles tested and their characteristics were dependent on electrode configurations and current intensities. C6(−) stimulation elicited more robust sMEPs than stimulation at C8(−). Stimulating C6 and C8 simultaneously produced better muscle recruitment and higher grip strengths than stimulation at one site.
Comparison with existing method(s)
Classical method to select the most optimal stimulation configuration is to empirically test each combination individually for every subject and relate to functional improvements. This approach is impractical, requiring extensively long experimental time to determine the more effective stimulation parameters. Our proposed method is fast and physiologically sound.
Conclusions
Results suggest that sMEPs from forelimb muscles can be useful biomarkers for identifying optimal parameters for epidural stimulation of the cervical spinal cord after SCI.
Keywords: Epidural stimulation, Cervical spinal cord injury, Dorsal funiculi crush, Motor evoked potentials
1. Introduction
Epidural electrical stimulation of the spinal cord, i.e., electrical enabling motor control (eEmc), is a promising therapy for the rehabilitation of sensorimotor function after spinal cord injury (SCI) (Edgerton and Roy, 2012; Alam and He, 2014; Dietz and Fouad, 2014). eEmc facilitates locomotion by stimulating the locomotor networks in the spinal cord more naturally than that occurs with functional electrical stimulation (FES), i.e., stimulating the muscles directly. eEmc delivered at the lumbosacral enlargement of the spinal cord in cats, rats, and mice enables weight-bearing standing and stepping (Gerasimenko et al., 2003; Saigal et al., 2004; Ichiyama et al., 2005; Gerasimenko et al., 2007; Courtine et al., 2008; Musienko et al., 2009). Importantly, eEmc at the lumbosacral cord in human subjects with a complete SCI has resulted in promising results such as in the initiation of voluntary leg movements along with gains in postural control, bladder, and sexual function (Minassian et al., 2004; Harkema et al., 2011; Angeli et al., 2014).
Restoration of arm and hand function is one of the highest priorities of individuals with a cervical SCI (Anderson, 2004). Currently, data on the effects of epidural stimulation to neuromodulate the cervical cord chronically are non-existent. Previous work in the rats has shown that instraspinal stimulation of the cervical segments of the spinal cord in rats elicits motor responses in multiple forelimb muscles (Sunshine et al., 2013) and selected stimulation parameters can facilitate functional reaching and grasping movements in monkeys (Zimmermann et al., 2011; Sharpe and Jackson, 2014). Chronic intraspinal stimulation of the spinal cord has been reported to improve forelimb function in SCI rats (Kasten et al., 2013; Mondello et al., 2014). Presently the biggest limitations for achieving effective therapeutic spinal cord stimulation procedures include the lack of critical information related to the optimum (1) electrode placement (position of active and reference electrode and location along the spinal cord), (2) stimulation parameters (frequency, intensity, duration, polarity, etc.), and mode of stimulation (monopolar, bipolar, or simultaneous stimulation). All of these parameters have the potential to affect the therapeutic outcome (Wongsarnpigoon and Grill, 2008; Capogrosso et al., 2013). Moreover, since the mechanisms through which eEmc exerts its therapeutic effect are only vaguely understood, a rapid access of an effective electrophysiological biomarker to identify the optimum parameters to maximize the therapeutic outcomes and minimize side effects becomes a high priority clinically (Wongsarnpigoon and Grill, 2011).
A method that is commonly adopted to select the most optimal eEmc technique to achieve the best functional outcome is to empirically test step-by-step all combinations of each stimulation parameter individually for every subject (Wongsarnpigoon and Grill, 2008). This approach, however, is impractical in that it involves several permutations of stimulation parameters that require extensively long experimental time and also lacks a physiological basis of how eEmc is being effective. Over the past decade, we have made considerable advances in determining the optimal eEmc parameters necessary for recovering some motor functions in paraplegics (Harkema et al., 2011; Sayenko et al., 2013). In the present work, we apply the principles that we have learned from our results using eEmc at the lumbosacral region of the spinal cord to the cervical region of the spinal cord.
The purpose of the present study was to identify the optimal eEmc electrode sites and stimulation configuration for cervical spinal cord stimulation. To accomplish this we delivered eEmc at the cervical spinal cord of unanesthetized rats and measured the evoked sMEPs in multiple forelimb muscles when using different electrode combinations and stimulation current intensities. We also tested the grip strength of the rats at different stimulation configurations. We found that the optimal stimulation parameters not only produced robust sMEPs but also improved the grip strength of the SCI rats. The results indicate that sMEPs can be used as a useful biomarker for identifying the optimal parameters for eEmc of the cervical spinal cord after a SCI.
2. Materials and methods
All experimental procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Publication No. 86-23, revised 1985) and approved by the Animal Research Committee of the University of California, Los Angeles (UCLA).
2.1. Subjects
Five healthy female Long-Evan rats (270–350 g body weight) were studied. The rats were housed individually at a constant temperature of 25 °C and humidity of 40% and were maintained on a 12:12 h light:dark cycle. Water was supplied ad libitum and rat chow availability was monitored carefully based on weight gain. The rats were acclimated to the testing environment prior to all surgeries. Each rat was identified as right- or left-handed (preferred paw) during the standard reaching and grasping task (Whishaw and Tomie, 1989; McKenna and Whishaw, 1999).
2.2. Surgical procedures
All survival surgical procedures were conducted under aseptic conditions. The rats were given an analgesic (Buprenex, 0.01–0.05 mg/kg, s.c.) 45 min prior to surgery and were anesthetized with isoflurane gas (1.5–2.5%) administered via facemask to effect throughout the surgery. The surgery was performed with the rats placed on a heating pad (TP-500, Gaymar Industries Inc., Orchard Park, NY, USA) maintained at 27 °C to prevent hypothermia. The rats were given lactated ringers (5–6 cc; s.c.) immediately after the surgery and placed in an incubator maintained at 37 °C until fully recovered. Baytril, a general antibiotic, and buprenex were administered (5 mg/kg and 0.05 mg/kg, s.c., respectively) twice daily for 3 days post-surgery. Rodent food pellets, fresh fruit (orange and apple slices), and cereal (fruit loops) were placed on the bottom of the cage during the recovery period (1–2 weeks).
2.3. EMG electrode implantation
Select forelimb muscles (deltoid, biceps brachii, pronator teres, flexor digitorum, and extensor digitorum) relevant for performing reaching and grasping movements were implanted unilaterally (preferred paw side) with intramuscular recording electrodes as described previously (Roy et al., 1991). A skin incision was made along the sagittal suture of the skull and the connective tissue and the muscles covering the skull were reflected laterally. The skull was thoroughly dried and three stainless steel screws were firmly inserted into the exposed bone. A miniature connector (Omnetics, Minneapolis, MN, USA) was placed between the screws and rigidly affixed to the bone using dental cement. Skin and fascial incisions were made to expose the bellies of the forelimb muscles of interest. Two multistranded Teflon-coated stainless steel wires (AS632, Cooner Wire, Chatsworth, CA, USA) connected to gold plated amphenol pins in the head connector were passed subcutaneously to each muscle. The wires were passed into each muscle belly using a 23-gauge needle and a small notch (~0.5–1.0 mm) was made in the Teflon coating to make the electrode. The electrode wires then were anchored at both ends with 4.0 Ethilon suture. The EMG wires were coiled near each implant site for stress relief. Stimulation through the head connector was used to verify the proper placement of the electrodes in each muscle and the proper location was verified via dissection post-mortem. All exposed areas were kept moist with 0.9% saline washes. All incisions were closed in layers, i.e., investing fascia with 4.0 Vicryl and then the skin with 4.0 Ethilon.
2.4. Cervical spinal cord injury
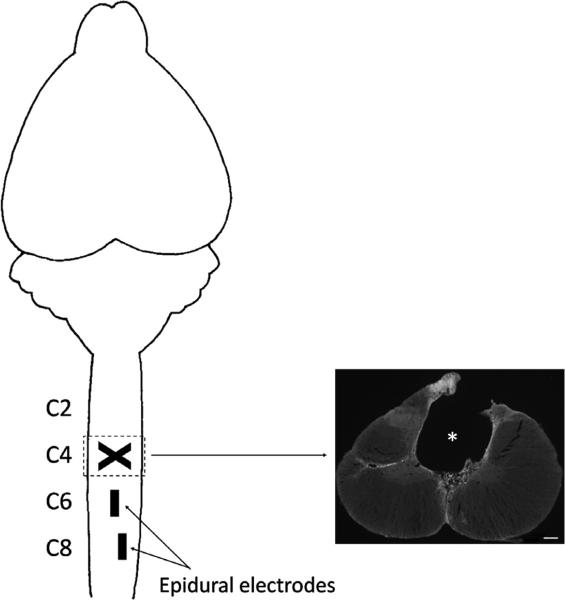
A longitudinal midline skin incision was made dorsal to the spinal column and the underlying neck muscles were reflected laterally to provide access to the vertebrae overlying the cervical spinal cord segments of interest. A partial laminectomy was performed at C3–C4 vertebrae to expose the spinal cord. The C4 dorsal funiculi were crushed (shown by a cross in Fig. 1) by placing the tips of fine forceps 2 mm apart (1 mm on each side of the mid-line), inserting the tips 2 mm in depth into the spinal cord, and then squeezing the tips together and holding them closed for 20 s. All exposed areas were kept moist with 0.9% saline washes.
Fig. 1.
Rat spinal cord injury model. A dorsal funiculi crush at the C4 spinal segment (shown by a cross) damages the dorsal corticospinal tract projections (shown in inset on right with a 0.2 mm white scale bar; * spinal cord lesion site). Epidural electrodes are implanted at the C6 and C8 spinal cord segments (shown by rectangles on the spinal cord).
2.5. Epidural electrode implantation
The epidural electrode implantation procedures have been described in detail previously (Ichiyama et al., 2005). A partial laminectomy was performed at the C6 and T1 vertebral levels to expose the C6 and C8 spinal cord levels. Wires from the head connector (see above) were passed subcutaneously to the laminectomy sites and then passed under the spinous processes of the remaining vertebrae between the partial laminectomies and above the dura mater. Stimulation electrodes were made by removing a small portion of the Teflon (~1 mm) to expose the stainless steel wire on the surface facing the spinal cord. The electrodes were secured in position by suturing the wire to the dura mater above and below the electrode using 8.0 Ethilon suture. Fig. 1 shows the placement of the electrodes. In addition, the Teflon was pulled gently over the cut end of the wires to prevent stimulation through this site. A loop was formed near the site of insertion of the wires to provide stress relief. A common ground wire (~1 cm of the Teflon removed at the distal end) was inserted subcutaneously near the shoulder on the dominant paw side. All exposed areas were kept moist with 0.9% saline washes. The incisions in the cervical region were closed using 4.0 Vicryl for the muscle and connective tissue layers and 4.0 Ethilon for the skin.
2.6. sMEP testing procedures
Six combinations of eEmc were examined at 2 Hz at different constant current intensities (Grass SIU5; Grass Instruments, Warwick, RI, USA): two bipolar (C6(−) C8(+) and C6(+) C8(−)) and four monopolar (C6(−) Ref(+), C6(+) Ref(−), C8(−) Ref(+) and C8(+) Ref(−)) configurations were tested. EMG from the forelimb muscles was recorded with the rat at rest. The EMG signal was filtered (band passed; 30 Hz–1 kHz) and amplified (1000×) using an analog amplifier (Differential AC amplifier Model 1700, AM-Systems Inc., Sequim, WA, USA). The signal was digitized at a 10 kHz sampling rate and stored on a computer using a data acquisition card (NI PCI-6052E, National Instruments Inc., Austin, TX, USA) using custom designed software written for LabVIEW (National Instruments Inc., Austin, TX, USA).
2.7. Grip strength testing procedures
The grip strength of each rat was determined with (40 Hz, 70% of threshold, all configurations) and without epidural stimulation using a custom-made grip strength meter. The rats were held while grasping a bar instrumented with a force transducer. The rat then was gently pulled away from the bar. The maximum grip strength was measured as the force recorded just prior to the rat releasing the bar. The force signals were digitized (10 kHz sampling rate; NIDAQ) and stored on a computer for further analysis.
2.8. Data analysis
The data were analyzed offline with a custom written program in MATLAB (MathWorks Inc., Natick, MA, USA). The program allowed detection of distinct raw EMG signals that corresponded to the rat's active and non-active periods. From the EMG signals during the non-active periods, sMEPs were analyzed from all tested forelimb muscles. A single trial of sMEPs within the raw EMG was defined as an evoked response from the start of the stimulation pulse up to a duration of 25 ms. The time window was divided further into an early (first 9 ms) and a late (last 16 ms) response. We also measured the peak amplitude of each response and compared across all electrode configurations. The sMEPs subsequently were rectified and averaged. We also measured the area under the rectified sMEP curves to determine the total muscle activation at a given stimulation current intensity and electrode configuration. For analysis of muscle recruitment curves, the current values were normalized by setting the minimum current as 0 (0%) and the maximum current as 1 (100%) for each electrode configuration. Grip strength (maximum force) was calculated from the peak output value of the transducer on the grip strength meter for each subject with each electrode configuration. The mean grip strength data were then normalized by scaling between 0 and 1 for each electrode configuration (here, 0 represents minimum and 1 represents maximum grip strength). All data are reported as mean (±standard error).
2.9. Statistical analyses
Differences in the early and late evoked responses between all stimulation configurations were analyzed for all muscles (n = 60 bursts using analysis of variance measures (ANOVA)). Repeated measures ANOVA were used to compare grip strength among all stimulation configurations. Individual group differences were determined using the Tukey post hoc analysis. Differences between groups were considered statistically significant at P < 0.05. All statistical analyses were performed using MATLAB (MathWorks Inc., Natick, MA, USA) and GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
The rats received a dorsal funiculi crush at C4 that damaged the dorsal corticospinal tract and the dorsal columns bilaterally. Following the injury, the rats presented mild motor deficits of the forelimbs that were characterized by a moderate loss of inter-limb coordination during overground locomotion. These deficits gradually decreased over time and were not noticeable at the time of sMEP testing. All rats maintained some ability to use their forepaws to grip and hold food pellets although the success rate was impaired.
3.1. Different electrode configurations have different motor thresholds
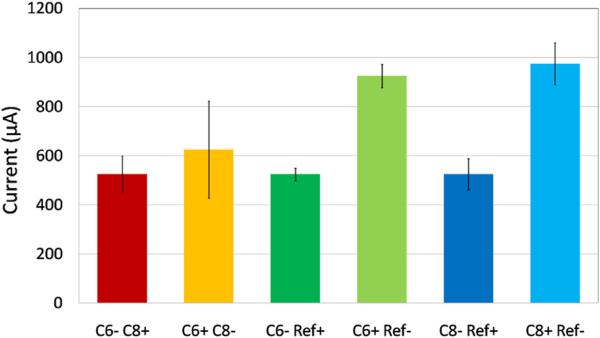
The threshold currents required to evoke sMEPs in the fore-limb muscles on the preferred paw side using cervical eEmc at distinct spinal cord segments in unanesthetized rats were determined. Different intensities of stimulation current were needed to produce sMEPs from the same muscles with different electrode configurations (Fig. 2). A minimum of 500–900 μA was required to produce sMEPs in all five muscles dependent on the different electrode configurations. The bipolar stimulation combinations generally showed lower stimulation thresholds compared to anodic monopolar stimulation configurations with rostral cathodic stimulation (C6− C8+) exhibiting a lower threshold compared to caudal cathodic stimulation (C6+ C8−). For monopolar stimulation configurations, all cathodic stimulations (C6− Ref+) and (C8− Ref+) had a lower threshold compared to anodic stimulation configurations.
Fig. 2.
Average minimum threshold current for each stimulation electrode configuration to elicit motor evoked responses (sMEPs) in all five forelimb muscles implanted with EMG electrodes (deltoid, biceps brachii, pronator teres, flexor digitorum, and extensor digitorum).
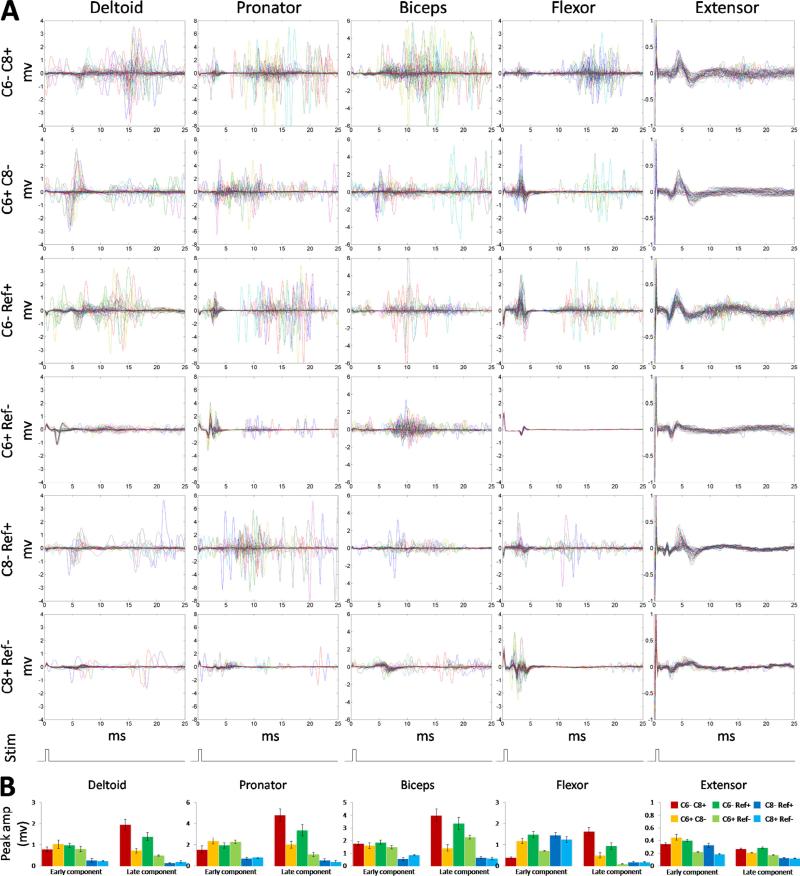
Fig. 3A shows the EMG responses at motor threshold of each muscle for all six configurations tested. All five muscles showed clear early responses (sMEPs within 9 ms), whereas late responses (sMEPs at 9–25 ms) were observed consistently in the deltoid, biceps brachii, pronator, and flexor muscles. The extensor muscle showed no late responses with any of the six stimulation configurations. sMEPs were more prominent during bipolar than most monopolar stimulation configurations. Cathodic C6 monopolar stimulation (C6− Ref+) showed sMEPs in all five muscles that were similar in amplitude and pattern to those observed with bipolar stimulation. The mean peak amplitudes of the early and late responses of the sMEPs for each muscle for all six stimulation configurations are shown in Fig. 3B. The late responses for C6 cathodic stimulation (both C6− C8+ and C6− Ref+) were significantly larger than most other tested stimulation configurations for all muscles.
Fig. 3.
(A) The threshold stimulation currents for inducing sMEPs in all five forelimb muscles for the six electrode configurations are shown. Each panel shows multiple plots (superimposed) of sMEPs induced by a single stimulation current. The last row indicates the stimulation pulse at time 0 s. (B) Average (±SEM) peak amplitude of the early (0–9 ms) and late (9–25 ms) responses of sMEPs for all six stimulation electrode configurations for each of the five muscles. * Significantly different from C6– C8+. + Significantly different from C6– Ref+.
3.2. Motor-evoked responses at different stimulation currents
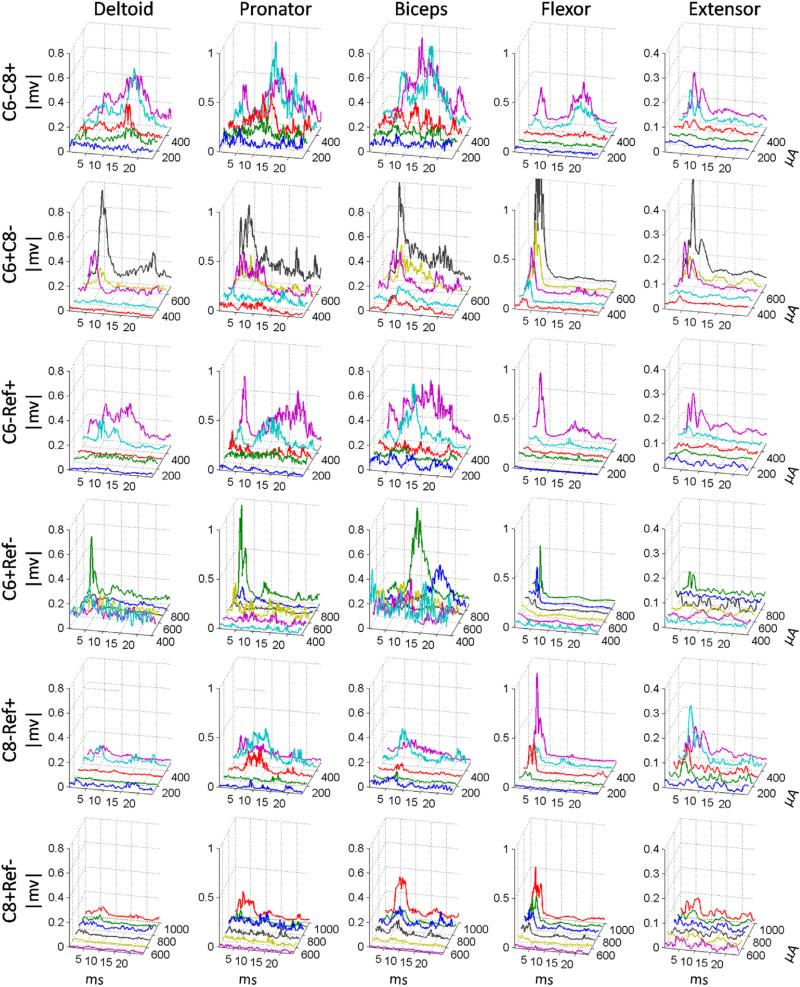
The rectified sMEPs from all tested muscles at different current intensities for all electrode configurations are shown in Fig. 4. In general, these values were consistently larger at the higher current intensities with the most robust effects observed with the bipolar (C6− C8+ and C6+ C8−) and the C6 cathodic (C6− Ref+) configurations. These same configurations also showed good overall rectified sMEPs in all five muscles at low current intensities. In contrast, the C6 and C8 anodic configurations did not show rectified sMEPs until very high stimulation currents (~800 μA).
Fig. 4.
Average rectified sMEPs for each of the five muscles induced by spinal cord stimulation at different current intensities using all six stimulation electrode configurations. Each 3-D panel indicates the rectified sMEPs with increasing stimulation current intensities for one stimulation electrode configuration. X-axis is latency, Y-axis is current intensity, and Z-axis is rectified voltage amplitudes.
3.3. Differential recruitment of proximal and distal forelimb muscles
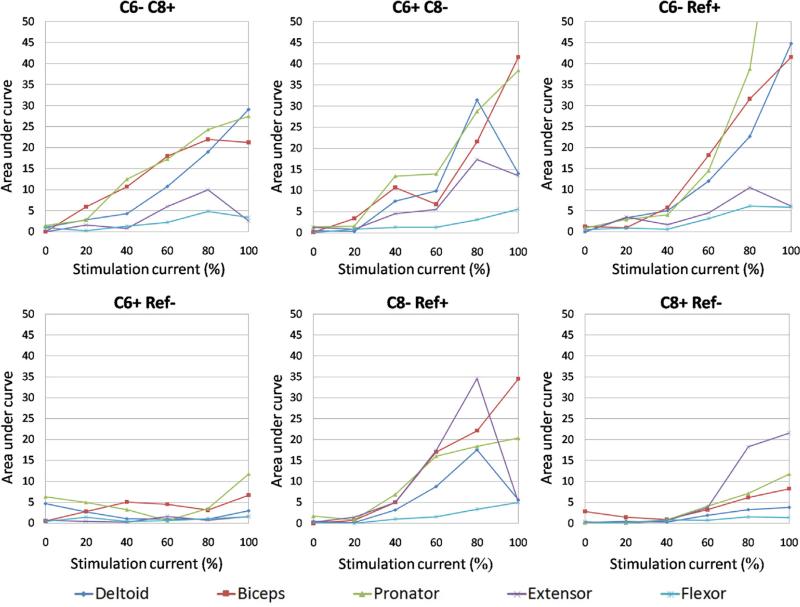
Robust muscle recruitment, as determined by a consistent increase in the amplitude of the sMEPs with increasing current intensity, was observed in the deltoid, biceps, and pronator muscles (Fig. 5). The two bipolar stimulation configurations had similar recruitment patterns for most muscles, although the recruitment of the extensor muscle was more robust with the C6+ C8− than the C6− C8+ configuration. As expected, C6− Ref+ monopolar stimulation showed a saturating muscle recruitment pattern for the deltoid, biceps, and pronator muscles that occurred at and above 80% of the maximum stimulation current tested. The C8− Ref+ configuration produced considerably higher amplitudes of recruitment in the digit extensor muscle compared to other stimulation configurations. The anodic monopolar stimulation configurations (C6+ Ref− and C8+ Ref−) showed minimal increases in muscle recruitment with increasing current intensities. The digit flexor muscle showed similar recruitment patterns with two bipolar (C6− C8+ and C6+ C8−) and two cathodic monopolar (C6− Ref+ and C8− Ref+) configurations. The variable recruitment patterns among electrode configurations are most likely related to the location of specific motoneuronal pools in the spinal cord as well as the projections of sensory input to those motor pools.
Fig. 5.
Muscle recruitment patterns for all six stimulation electrode configurations. Each panel indicates recruitment pattern for each muscle as a function of increasing stimulation current intensities. X-axis indicates an increase in the stimulation current from threshold to saturation expressed as a percentage of saturation and the Y-axis indicates the integrated area under the rectified sMEP curves.
3.4. Grip strength with continuous stimulation
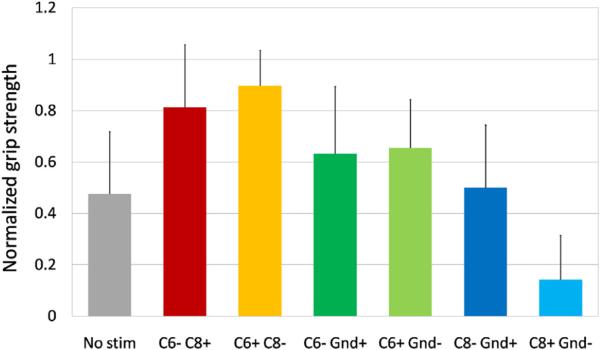
We tested the grip strength for all rats without and with eEmc (40 Hz) using all electrode configurations (Fig. 6). Although the differences were not statistically significant, the rats could generate somewhat higher grip forces than without stimulation using all configurations except C8 anodic monopolar (C8+ Ref−) configuration. The maximum grip force was generated during C6+ C8− bipolar stimulation. The other bipolar stimulation configuration (C6− C8+) also resulted in higher grip forces compared to all monopolar configurations and without stimulation.
Fig. 6.
Mean (±SEM) normalized (to maximum force) grip strength with no stimulation and with 40-Hz stimulation at sub-threshold current levels for all six stimulation electrode configurations.
4. Conclusion and discussion
The present study was performed to determine the feasibility of using electrophysiological-based biomarkers for identifying optimal sites for electrode placement along the spinal cord for predicting the more successful forelimb performance in response to eEmc stimulation after a cervical SCI in unanesthetized rats. The present study highlights the effects of varying epidural stimulation electrode configurations and the stimulation currents on sMEPs and grip strength after a bilateral dorsal funiculi crush. We were able to identify the more effective stimulation configurations to generate patterns of sMEPs that can serve as biomarkers for predicting the more effective motor performance. The motor threshold to evoke muscle responses was a direct function of different electrode configurations and/or stimulation current intensities. Lower stimulation thresholds and larger motor responses (both early and late sMEPs) were observed with bipolar compared to monopolar stimulation configurations. Cathodic bipolar stimulation to the rostral spinal cord (C6) had lower stimulation threshold values compared to cathodic bipolar stimulation to the caudal spinal cord (C8). These results demonstrate that the electrode site, polarity and stimulation intensity can be used to fine-tune the performance of the upper limb after a cervical SCI. This specificity was reflected in the grip strength measures. The rats generally produced higher grip forces during bipolar stimulation than monopolar or no stimulation.
Based on the somatotopic organization of motoneuronal pools in the cervical spinal cord of the rat (McKenna et al., 2000; Tosolini and Morris, 2012), it is not surprising that stimulation at C6 and C8 spinal segments in the present study evoked responses from all muscles tested. At the same time our data show that differences in the reflex organization of evoked potentials in forelimb muscles are dependent on the sites of stimulation. In particular, cathodic bipolar stimulation at C6 evoked potentials dominated by the late components, whereas stimulation at C8 was dominated by the early components of the evoked responses. These observations indicate that the electrical fields produced by C6 vs. C8 stimulation involved different neuronal circuits. Since eEmc primarily activates dorsal afferents (Gerasimenko et al., 2006; Capogrosso et al., 2013), early responses were observed more consistently with all stimulation configurations suggesting possible monosynaptic responses. With a more intense electric field generated by bipolar compared to monopolar eEmc, more sMEPs (specifically, more late responses) were generated by bipolar than monopolar stimulation. This specificity was reflected in the grip strength measures: the rats generally produced higher grip forces during bipolar stimulation than monopolar or no stimulation.
The electric field generated by eEmc also may have activated the more medial spinal column than the lateral column. This was reflected on sMEPs of deltoid, biceps brachii, pronator teres muscles with low stimulation threshold. In contrast high stimulation currents were required to produce sMEPs on flexor digitorum and extensor digitorum muscles. These two muscles also had low or no late responses.
Similar to previous work with neuromodulation of the hindlimbs with eEmc (Ichiyama et al., 2005; Gerasimenko et al., 2007; Courtine et al., 2009), it appears that eEmc neuromodulation therapies also can modulate a range of functionally specific cervical neural networks. Varying combinations of excitatory and inhibitory effects produced by using different electrode placements, stimulation strengths, frequencies, pulse shapes etc. can be used to fine-tune this neuromodulation and presumably motor performance. It is clear that the frequencies used diagnostically (2 Hz or less) will be considerably lower and less variable than the wide range of stimulation parameters necessary to be effective therapeutically, e.g., frequency and intensity (Lavrov et al., 2008; Wongsarnpigoon and Grill, 2011; Gad et al., 2013b; Angeli et al., 2014). Specifically, if changes in electrode combinations and/or stimulation currents can influence the motor threshold, then similar changes are likely to influence the therapeutic outcomes achieved with stimulation intensities below the motor threshold (Wongsarnpigoon and Grill, 2011). This study provides an initial effort in the development of procedures for identifying electrophysiological biomarkers that could apply more widely than to forelimb grip strength.
Our assumption is that the more optimal stimulation configurations that activate a given combination of motor pools, and thus muscles, during the diagnostic assessment can be used to predict the more optimal eEmc configurations for achieving immediate and/or chronic therapeutic effects after a SCI. Based on our previous studies of sMEPs in the hindlimb muscles after a complete SCI (Gerasimenko et al., 2006; Gad et al., 2013a) and the present results shown in Fig. 3, there are several electrophysiological responses to 2 Hz stimulation that could be important features of a biomarker that would be effective in predicting motor performance. For example, a wide range of response profiles with respect to amplitudes, delays, and levels of synchrony and randomness of potentials could serve as biomarkers. It does appear that, as in most hindlimb muscles, there is a prominence of early and late responses in the forelimb muscles, but with the early response tending to be more highly synchronized. In spite of this degree of synchronization this early response cannot be assumed to be attributable to a single type of circuitry, e.g., a monosynaptic pathway. The later and more randomly appearing responses are probably reflecting activation of continuously varying spinal networks as must occur routinely in in vivo situations. Thus, we suggest that the intrinsic properties of the spinal networks generate highly unsynchronized EMG bursting patterns as occurs during routine motor tasks. To more specifically identify the interneurons will be challenging, particularly in assessing these networks post-injury when the responses are likely to reflect a wide range of mechanisms that underlie functional neuronal plasticity that is so pervasive after a SCI.
HIGHLIGHTS.
Dorsal funiculi were injured at C4 spinal cord in adult female rats.
Epidural stimulation electrodes were implanted chronically at C6 and C8.
Potentials evoked in forelimb muscles by cervical spinal stimulation were examined.
Evoked potentials were dependent on electrode configuration and current intensity.
Evoked potentials are useful biomarkers to identify optimal stimulation parameters.
Acknowledgements
This research project was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) NIH 1U01EB015521, the Christopher & Dana Reeve Foundation, and the RFBR No. 13-04-12030 ofi-m, and by Russian Scientific Fund project No. 14-45-00024. The authors thank Maynor Herrera for his assistance in surgeries; Benita Jin and Jonathan Keyes for the animal care; Sharon Zdunowski for her technical support.
Footnotes
Conflict of interest
V. Reggie Edgerton, Roland R. Roy and Yury Gerasimenko – researchers on the study team hold shareholder interest in NeuroRecovery Technologies. Drs. Edgerton, Roy, and Gerasimenko also hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- Alam M, He J. Lower-limb neuroprostheses: restoring walking after spinal cord injury. In: Naik GR, Guo Y, editors. Emerging theory and practice in neuroprosthetics. IGI Global; Hershey, PA, USA: 2014. pp. 153–80. [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33:19326–40. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–42. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137:654–67. doi: 10.1093/brain/awt262. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. A new age for rehabilitation. Eur J Phys Rehabil Med. 2012;48:99–109. [PubMed] [Google Scholar]
- Gad P, Lavrov I, Shah P, Zhong H, Roy RR, Edgerton VR, et al. Neuromodulation of motor-evoked potentials during stepping in spinal rats. J Neurophysiol. 2013a;110:1311–22. doi: 10.1152/jn.00169.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, et al. Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J Neuroeng Rehabil. 2013b;10:108. doi: 10.1186/1743-0003-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol. 2003;33:247–54. doi: 10.1023/a:1022199214515. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods. 2006;157:253–63. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, et al. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–36. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–44. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Kasten MR, Sunshine MD, Secrist ES, Horner PJ, Moritz CT. Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. J Neural Eng. 2013;10:044001. doi: 10.1088/1741-2560/10/4/044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Dy CJ, Fong AJ, Gerasimenko Y, Courtine G, Zhong H, et al. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J Neurosci. 2008;28:6022–9. doi: 10.1523/JNEUROSCI.0080-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J Neurosci. 1999;19:1885–94. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–96. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–16. doi: 10.1038/sj.sc.3101615. [DOI] [PubMed] [Google Scholar]
- Mondello SE, Kasten MR, Horner PJ, Moritz CT. Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci. 2014;8:21. doi: 10.3389/fnins.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Pavlova NV, Selionov VA, Gerasimenko Iu P. Locomotion induced by epidural stimulation in decerebrate cat after spinal cord injury. Biofizika. 2009;54:293–300. [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–9. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:430–40. doi: 10.1109/TNSRE.2004.837754. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol. 2013;111:1088–99. doi: 10.1152/jn.00489.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AN, Jackson A. Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. J Neural Eng. 2014;11:016005. doi: 10.1088/1741-2560/11/1/016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng. 2013;10:036001. doi: 10.1088/1741-2560/10/3/036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Olfaction directs skilled forelimb reaching in the rat. Behav Brain Res. 1989;32:11–21. doi: 10.1016/s0166-4328(89)80067-1. [DOI] [PubMed] [Google Scholar]
- Wongsarnpigoon A, Grill WM. Computational modeling of epidural cortical stimulation. J Neural Eng. 2008;5:443. doi: 10.1088/1741-2560/5/4/009. [DOI] [PubMed] [Google Scholar]
- Wongsarnpigoon A, Grill WM. Effects of stimulation parameters and electrode location on thresholds for epidural stimulation of cat motor cortex. J Neural Eng. 2011;8:066016. doi: 10.1088/1741-2560/8/6/066016. [DOI] [PubMed] [Google Scholar]
- Zimmermann JB, Seki K, Jackson A. Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng. 2011;8:054001. doi: 10.1088/1741-2560/8/5/054001. [DOI] [PMC free article] [PubMed] [Google Scholar]