Abstract
Conventional chemotherapy is the main treatment for cancer and benefits patients in the form of decreased relapse and metastasis and longer overall survival. However, as the target therapy drugs and delivery systems are not wholly precise, it also results in quite a few side effects, and is less efficient in many cancers due to the spared cancer stem cells, which are considered the reason for chemotherapy resistance, relapse, and metastasis. Conventional chemotherapy limitations and the cancer stem cell hypothesis inspired our search for a novel chemotherapy targeting cancer stem cells. In this review, we summarize cancer stem cell enrichment methods, the search for new efficient drugs, and the delivery of drugs targeting cancer stem cells. We also discuss cancer stem cell hierarchy complexity and the corresponding combination therapy for both cancer stem and non-stem cells. Learning from cancer stem cells may reveal novel strategies for chemotherapy in the future.
Keywords: Side effects, biomarkers, drug delivery system, multifunctional carrier, cancer hierarchy
Introduction: limitations of chemotherapy and corresponding strategies
Other than surgery, radiotherapy, endocrine therapy, and immunotherapy, chemotherapy is the main treatment for cancer. Patients with different cancers derive more survival benefits through chemotherapy not only at the early stage of disease, but also at the late stage. However, quite a few cancers develop drug resistance easily and cause relapse and metastasis. What are the reasons for conventional chemotherapy failure? First, conventional chemotherapy drugs such as paclitaxel mainly target proliferating cancer cells. Such drugs kill the majority of proliferating cancer cells, but cannot do so with dormant cancer cells, which can divide into proliferating cancer cells and cause relapses following chemotherapy [1,2]. Thus, targeting only proliferating cancer cells is less efficient. Conventional drugs such as cyclophosphamide kill both proliferating and dormant cancer cells [3,4]. However, multidrug-resistant mechanisms ensure that a number of cancer cells can resist and escape chemotherapy. These dormant or resistant cancer cells are the reason for conventional chemotherapy failure, and are considered cancer stem cells [5-7]. Recently, accumulating studies demonstrated that cancer stem cells, a cancer cell subpopulation with unlimited capacity for self-renewal, differentiation, and tumorigenesis, are the reason for relapse and metastasis [8,9]. Initially, conventional chemotherapy and radiotherapy kill most cancer cells and shrink tumors immediately, but the spared cancer stem cells eventually result in relapse and metastasis. A new therapy targeting a few cancer stem cells may not shrink the tumor in an obvious manner initially, but may eventually disappear due to the loss of self-renewal and proliferation [10] (Figure 1). Other than inhibiting cancer cells, normal tissues are harmed by conventional chemotherapy, which also causes many side effects, such as bone marrow suppression [11], nausea and vomiting [12], neurotoxicity [13,14], and temporary alopecia [15,16] due to the targeted drug delivery systems being less precise and the targeting drugs being less efficient. Therefore, enhancing conventional chemotherapy efficacy and reducing its side effects necessitates the search for potential efficient drugs targeting cancer stem cells and designing a novel drug delivery system to transfer such drugs only to cancer sites, and not normal tissues. Herein, we summarize the methods for enriching cancer stem cells, the search for new efficient drugs, and the delivery of targeted therapy drugs. We also discuss cancer stem cell hierarchy complexity and the corresponding target therapy strategies.
Figure 1.
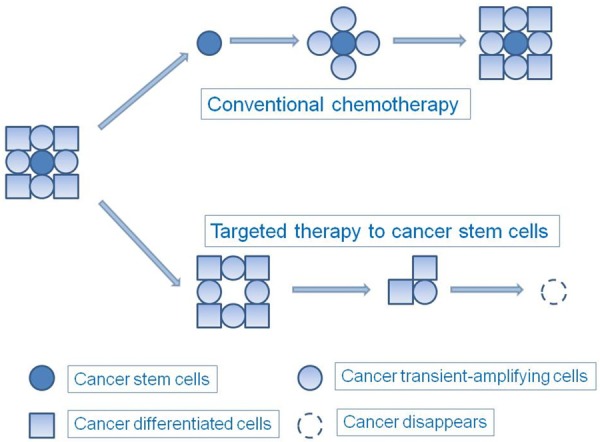
Effects of conventional chemotherapy and targeted therapy. Conventional chemotherapy initially kills most cancer cells and shrinks tumor size immediately, but the spared cancer stem cells eventually result in relapse and metastasis. Targeted therapy of cancer stem cells may not shrink the tumor size in an obvious manner at first, but the tumor may eventually disappear due to the loss of self-renewal and proliferation capacity.
Methods of enriching cancer stem cells
Prior to targeted therapy of cancer stem cells, culturing a stable cancer stem cell line for chemotherapy testing is necessary. Ikegaki et al. reported the production of several stable neuroblastoma stem cell lines via transient treatment using epigenetic modifiers. A stemness phenotype was maintained by the stable induced cancer stem cells for > 1.5 years in culture with sphere-forming medium [17], providing a new approach for obtaining a phenotypic stable cancer stem cell line. Searching for potential compounds that are preferentially efficacious against cancer stem cells instead of against normal stem cells and differentiated cells is useful. However, whether the suitability of the cancer stem cell line for screening targeted chemotherapy drugs and the suitability of the approach for obtaining other stable cancer stem cell line types remains doubtful. To date, culturing a stable cancer stem cell line is very difficult, as cancer stem cells tend to differentiate rapidly into cancer non-stem cells in vitro. However, some agents may be appropriate for cancer stem cell enrichment. The first is phenotypic isolation of cancer cells using specific markers that are mainly expressed in cancer stem cells. Bonnet et al. discovered in 1997 that CD34+CD38- leukemia cells had much quicker capacity for self-renewal, differentiation, and tumorigenesis than CD34-CD38+ leukemia cells. Thus, the authors considered CD34+CD38- subpopulations the initiating cells of leukemia [18]. Inspired by leukemia stem cell research, other researchers isolated CD44+CD24- breast cancer stem cells from patients with breast cancer in 2003 [19]. Subsequently, a number of specific markers were discovered that were related to cancer stem cells [20] (Table 1) and which were suitable for enriching cancer stem cells or as potential targets in cancer therapy. A recent study showed that intracellular autofluorescence was an exclusive marker in many epithelial cancer stem cell types. Autofluorescent cancer cells expressed high levels of pluripotency-associated genes, were enriched in sphere culture and during chemotherapy, and had strong capacity for invasion, metastasis, and tumorigenesis [21]. The mechanism of autofluorescence is similar to that of side population cells, a stem-like cell subpopulation [22-24] isolated by Hoechst 33342 efflux, a DNA-binding dye, which depends on the adenosine triphosphate (ATP)-binding cassette sub-family G member 2 [25]. The advantage of this biomarker is that, compared with biomarkers such as CD133, CD44, and aldehyde dehydrogenase (ALDH1), autofluorescent cancer stem cells can be isolated simply by fluorescence-activated cell sorting without requiring a monoclonal antibody or dye, which may affect cancer cells.
Table 1.
Specific cancer stem cell markers
| Marker | |
|---|---|
| A2B5 | Glioblastoma [89] |
| ABCG2 | Melanoma [90] |
| ABCG5 | Melanoma [91] |
| ALDH1 | Breast [92,93], bladder [94], lung [95], colon [96], HNSCC [97], esophageal carcinoma [98] |
| ANTXR1 | Breast [99] |
| BMI1 | Colorectal [10] |
| CD19 | B-precursor ALL [100] |
| CD26 | Colorectal [101] |
| CD34 | AML[102], B-precursor ALL [100] |
| CD44 | Breast [103], colorectal [104], pancreatic [105], ovarian [106], gastric [107,108], HNSCC [109], AML [110], oral [111] |
| CD47 | AML [112] |
| CD90 | Liver [113] |
| CD105 | Renal [114] |
| CD110 | Colorectal [115] |
| CD117 | Ovarian [106] |
| CD123 | AML [116] |
| CD133 | Brain tumors [34,117,118], prostate [119], colon [36,120], lung [121], melanoma [90], pancreatic [26], ovarian [122], endometrial [123], liver [124] |
| CD166 | Colorectal [104], prostate [125], HNSCC [126] |
| CD271 | Melanoma [127] |
| CDCP1 | Colorectal [115] |
| CLL1 | AML [128] |
| DDX4 | Ovarian [129] |
| DNAJB8 | Renal cell carcinoma [130], colorectal [131] |
| EGFRvIII | Glioblastoma [132] |
| EpCAM | Liver [133], colorectal [104] |
| GD2 | Breast [134] |
| LGR5 | Colon [135] |
| MDR1 | Melanoma [136] |
| OCT4 | Osteosarcoma [137] |
| OV6 | Liver [138] |
| P27 | Breast [139] |
| SOX2 | Ovarian [140], cutaneous carcinoma [141] |
| SSEA1 | Glioblastoma [142] |
| SSEA4 | Oral [111] |
| TIM3 | AML [143] |
ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; ALDH: aldehyde dehydrogenase; ABCG: ATP-binding cassette superfamily G member; ANTXR1: anthrax toxin receptor 1; BMI1: B-lymphoma Moloney murine leukemia virus insertion region 1; CDCP1: CUB domain–containing protein 1; CLL1: C-type lectin-like molecule-1; DDX4: DEAD box polypeptide 4; DNAJB8: DnaJ homolog subfamily B member 8; EGFRvIII: epidermal growth factor receptor variant III; EpCAM: epithelial cell adhesion molecule; GD2: glycoprotein D2; LGR5: leucine-rich repeat G protein–coupled receptor 5; HNSCC: head and neck squamous cell carcinoma; MDR1: multi-drug resistance protein 1; OCT4: octamer-binding transcription factor 4; SOX2: sex-determining region Y-box 2; SSEA: stage-specific embryonic antigen; TIM3: T cell immunoglobulin- and mucin domain–containing molecule 3.
The second is enriching cancer stem cells through chemotherapy or radiotherapy. Conventional chemotherapy or radiotherapy are the main treatments for cancer. However, due to resistance, cancer stem cells can escape cytotoxicity and survive chemotherapy and radiotherapy. Therefore, cancer stems cells can be enriched using chemotherapy or radiotherapy. Hermann et al. reported that, following 5-day cultivation wih gemcitabine in vivo, pancreatic cancer stem cells were enriched up to 47.2% compared to 1.47% in a primary cancer cell line. In vivo tumor xenograft experiments showed that, compared to vehicle treatment, the pancreatic cancer stem cells were enriched by > 2 times following 3-week gemcitabine treatment [26]. Dylla et al. showed that colon cancer stem cells could survive cyclophosphamide or irinotecan treatment, and in xenogeneic tumors, were enriched. The chemoresistant cancer stem cells expressed more oncogenes, such as ALDH1A1, MYC, and MYB [27]. Bao et al. reported that CD133+ glioma stem cells were resistant to radiation by preferentially activating the DNA damage checkpoint response and increasing DNA repair capacity. After radiation, glioma stem cell frequency was increased in both in vitro culture and in vivo xenograft [28].
The third is inducing epithelial to mesenchymal transition (EMT). Mani et al. induced immortalized human mammary epithelial cells (HMLEs) into the mesenchymal state through ectopic expression of the Twist or Snail transcription factors, which both induce EMT in epithelial cells. The induced HMLEs formed mammospheres in suspension culture and soft agar colonies in vitro effectively, with high and low expression of the surface markers CD44 and CD24, respectively; the authors considered them mammary stem cells or mammary cancer stem cells [29]. The advantage of inducing EMT in cancer stem cells is that there are a large number of induced cancer stem cells and the state is much stabler, which is more suitable for cancer stem cell testing.
The fourth is serum-free cultivation using epidermal or fibroblast growth factor, and other factors. It was first used for enriching neural stem cells [30,31], and then was used with other normal stem cells such as mammary stem cells [32,33]. Due to the lack of specific cancer stem cell markers, it was used in the last decade to enrich cancer stem cells, such as that from brain [34], breast [35], colon [36], pancreatic [37], and prostate cancer [38]. The benefit of serum-free cultivation is that it preserves the state of stemness. This method preserves the stem-like characteristics of cancer stem cells enriched by other methods.
These four methods can be used to enrich cancer stem cells (Figure 2). Their common drawback is that the enriched cancer cells are not pure cancer stem cells. Therefore, using two or more methods to enrich cancer stem cells is more suitable.
Figure 2.
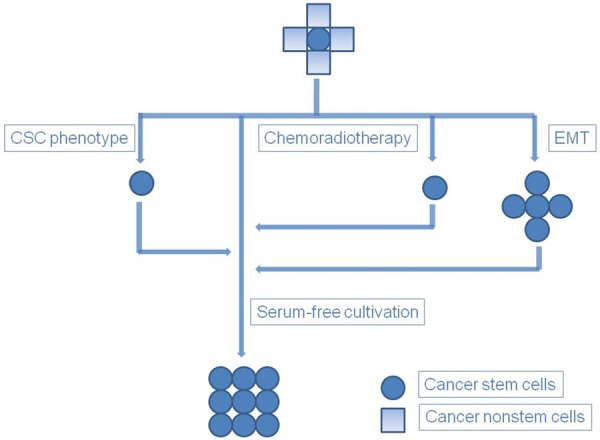
Cancer stem cell enrichment methods. Figure depicts four methods for enriching cancer stem cells (CSC): phenotypic isolation of cancer cells with specific cancer stem cell markers, conventional cytotoxic chemotherapy or radiotherapy, serum-free cultivation, and EMT. The stem-like characteristics of cancer stem cells enriched using other methods require preservation by serum-free cultivation.
Methods of searching for new efficient drugs
How do we search for new efficient drugs targeting cancer stem cells? A high-throughput screening platform may be one option (Figure 3). Gupta and colleagues screened 16000 compounds, eventually selecting salinomycin, which inhibits breast cancer stem cells 100-fold more effectively than paclitaxel, the main drug for breast cancer chemotherapy [39], which proved to be a breakthrough for screening drugs that target cancer stem cells. Many studies followed these findings [40-43]. However, some researchers were critical of the fact that salinomycin is very toxic in normal cells and causes lethal side effects, and may be not suitable for chemotherapy in vivo [44].
Figure 3.
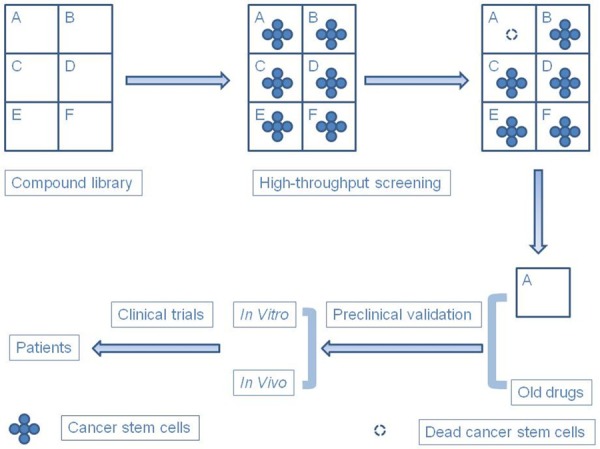
Methods for discovering new efficient drugs. There are two methods for discovering new efficient drugs: High-throughput screening, which is very useful for discovering new drugs among many compounds, and validation of old drugs targeting cancer stem cells.
Another option is validating old drugs that inhibit cancer stem cells efficiently (Figure 4), such as metformin, which is used for diabetes. Cancer risk is reduced in patients with diabetes who receive metformin [45-49]. Metformin inhibits cancer stem cell sphere-forming in vitro and xenografts in vivo, and sensitizes many cancers, such as breast [50-53], pancreatic [54,55], and colon cancer [56], and esophageal carcinoma [57] and glioma [58] to radiotherapy and chemotherapy. Phenformin, a related biguanide, inhibits lung cancer stem-like cell growth and invasive capacity in vitro [59], and affects the metabolic state of breast cancer stem cells [51]. In addition to metformin and phenformin, the anti-alcoholism drug disulfiram is markedly cytotoxic in cancer stem-like cells of breast cancer [60,61], hepatocellular carcinoma [62], and glioblastoma [63,64]. It inhibits self-renewal, induces apoptosis, and reverses drug resistance through mechanisms such as inducing reactive oxygen species, inhibiting the ALDH and nuclear factor-κB (NF-κB) pathways, downregulating glypican-3, inhibiting chymotrypsin-like proteasomal activity, and inactivating the ubiquitin-proteasome pathway. The antipsychotic drug thioridazine selectively targets leukemia stem cells via the dopamine receptors, but without being cytotoxic to normal blood stem cells [65]. Its anti-cancer potential was also reported in breast and gastric carcinoma [66,67]. Some dopamine analogues also inhibit glioblastoma stem cells efficaciously [68]. In addition to these drugs, more drugs targeting cancer stem cells need to be discovered and validated in clinical trials before clinical usage.
Figure 4.
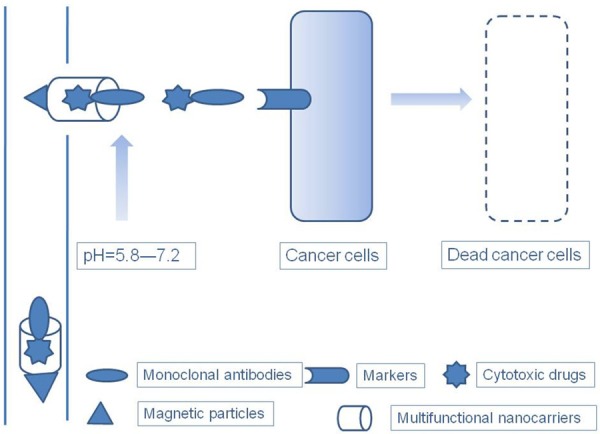
An ideal drug and drug delivery system. The ideal drug and drug delivery system should combine passive targeting aspects, e.g., enhanced permeability and retention (EPR) effect of the tumor; pH-, light-, and thermosensitive; and magnetic properties, with active targeting using monoclonal antibodies specific to cancer. Multifunctional nanocarriers are ideal carriers for chemotherapy drugs, where they adopt the tumor EPR effect, conjugate with one or more pH-, light-, and thermosensitive and magnetic particles, and load cytotoxic drugs and monoclonal antibodies targeting cancer.
Methods of delivering cancer-targeting drugs
Delivering anti-cancer drugs specific to cancer tissues and sustaining a stable high drug concentration improve anti-cancer efficacy and reduce the side effects in normal tissues. Some characteristics of cancer may be used to realize this. First, due to form and architecture abnormality of the newly formed blood vessels, the vascular endothelial cell interstitial space in cancer tissues is much looser than that in normal tissues. This allows anti-cancer drugs to infiltrate into the cancer tissues easily if the drug molecule is the same size as that of the gap between normal tissues and cancer tissues. In addition, the lack of effective lymphatic drainage ensures that the drug is much more easily retained in cancer tissues than in normal tissues. This is termed the enhanced permeability and retention effect, which is widely used in anti-cancer drugs modified with liposomes, nanomaterials, or high–molecular weight polymers [69]. Moreover, the pH values of normal tissues and cancer tissues differ. Due to the stronger glycolysis, cancer tissues generate more lactic acid, therefore the cancer microenvironment pH is about 5.8–7.2 (median, 7.0); under normal conditions, the pH is generally around 7.4 [70,71]. A pH-sensitive drug is designed to release slowly under normal conditions, but the stability of a complex drug decreases under the pH conditions of cancer tissue, such that it is released quickly mainly in cancer tissue and kills cancer cells specifically [72]. In addition to the specific cancer tissue physical and chemical properties, a complex drug can have light-sensitive [73], thermosensitive [74], and magnetic properties [75], and then illumination, heat, and magnetization external to the tumor location draw the drug specifically into the cancer tissues. However, special equipment is needed for each treatment session, rendering it less convenient.
The above methods for anti-cancer drug delivery are considered passive targeting. Conversely, active targeting delivers anti-cancer drugs by conjugating the complex drugs with monoclonal antibodies specific to the target cancer tissues. In comparison to normal cells, cancer cells have abnormal molecular expression, cell signaling pathways, and microenvironments, which are potential targets for guiding anti-cancer drugs with specific monoclonal antibodies [9,76,77]. For example, herceptin, or trastuzumab, a monoclonal antibody of human epidermal growth factor receptor 2 (HER2), which is overexpressed in some breast cancers, is widely used to treat HER2-positive breast cancer. Choi et al. reported that a herceptin-conjugated, doxorubicin-loaded multifunctional nanocarrier led to much higher cellular uptake and stronger cytotoxicity in HER2 overexpression breast cancer in vitro and shrank tumors significantly in vivo, compared to that not conjugated to herceptin [78]. Several studies have also shown that different herceptin-conjugated cytotoxic drugs loaded to multifunctional carriers improved therapy efficacy in HER2-positive breast cancer [79,80] and pancreatic cancer [81]. This active targeting method tends to inhibit cancer with high efficacy, and a greater number of specific cancer cell or cancer environment targets need to be discovered for potential active targeting drug design.
Recently, nanomedicine has come to the fore in cancer drug design and delivery [82,83]. Multifunctional nanocarriers combine passive and active targeting methods, which the ideal anti-cancer drugs should have, to enhance their efficacy and to reduce side effects (Figure 4). Chiang et al. designed a multifunctional nanocarrier with passive targeting pH-sensitive and magnetic particles, the active targeting herceptin, and the cytotoxic drugs doxorubicin (hydrophilic) and paclitaxel (hydrophobic). The complex compound, containing three agents, enhanced anti-cancer efficacy more efficiently than a nanocarrier with only one or two agents [79]. Its advantage is that it is more convenient, and patients with cancer would prefer one complex drug rather than the combination therapy in the present clinical treatment, which involves several drugs.
Learning from cancer stem cell hierarchy complexity
In addition to cancer stem cells, tumors contain the bulk of differentiated cancer cells. Currently, the relationship between cancer stem cells and differentiated cancer cells is not well known. According to the cancer stem cell hypothesis, cancer stem cells head the hierarchy, and can differentiate into transient amplifying and differentiated cancer cells [20,84]. Conversely, transient amplifying and differentiated cancer cells cannot dedifferentiate into cancer stem cells. The hierarchy is similar to that of normal stem cells; however, recent studies have disputed this. Takahashi and Yamanaka reported that pluripotent stem cells were generated directly from fibroblast cultures following the addition of four genes, namely Oct3/4, Sox2, c-Myc, and KLF4 [85]. On some occasions, differentiated cancer cells can also convert to cancer stem cells. Chaffer et al. reported that a subset of oncogene-transformed basal-like mammary epithelial cells spontaneously dedifferentiated into cancer stem-like cells with tumorigenesis capacity [86]. Schwitalla et al. reported a similar observation, where NF-κB– and β-catenin–transformed differentiated vellus cells dedifferentiated into stem-like cells and could form spheroids in vitro and cancer in vivo [87]. The cancer stem cell and differentiated cancer cell interconversion indicates a more complex cancer stem cell hierarchy. Therefore, therapy that only targets cancer stem cells may be less effective than initially presumed, and combination therapy targeting cancer stem cells and differentiated cancer cells may be more effective. Zhang et al. reported that paclitaxel, the main drug in breast cancer chemotherapy, combined with salinomycin, a high-throughput screening–validated drug that targets breast cancer stem cells [39], was more efficient for eradicating breast cancer and cancer stem cells compared to treatment with only one drug. The effect was improved when paclitaxel was modified with polyethylene glycol-block-polycaprolactone (PEG-b-PCL) polymeric micelles and octreotide, which targets somatostatin receptors overexpressed in breast cancer, and when salinomycin was modified with PEG-b-PCL polymeric micelles [88]. Ke et al. reported that using polymeric micelles for co-delivering thioridazine, which targets breast cancer stem cells effectively, and doxorubicin, a conventional cytotoxic drug used for treating breast cancer, was more effective for inhibiting both cancer stem and non-stem cells compared to delivering only one drug [66]. In conclusion, the complexity of cancer stem cell hierarchy teaches us that combination therapy should simultaneously target the bulk of cancer non-stem cells and some cancer stem cells (Figure 5).
Figure 5.
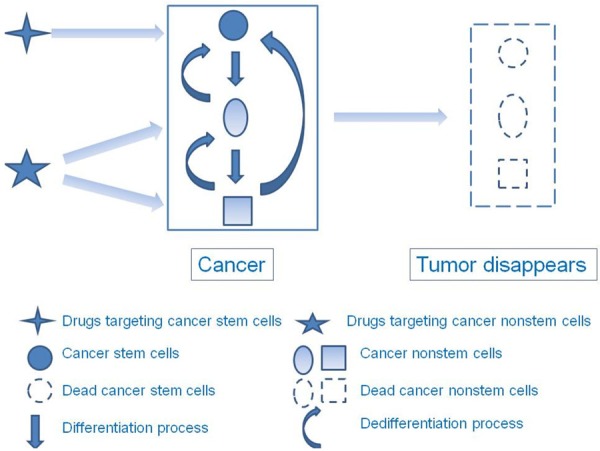
Complexity of cancer stem cell hierarchy and corresponding combination therapy. Cancer stem cells are usually at the top of the cancer hierarchy and can differentiate into transient amplifying and differentiated cancer cells. Cancer non-stem cells can also regain self-renewal and proliferation capacity through dedifferentiation under certain conditions. The complexity of cancer stem cell hierarchy indicates that the appropriate treatment strategy is combination therapy simultaneously targeting cancer stem and non-stem cells.
Conclusion
One solution to enhancing conventional chemotherapy efficacy and reducing its side effects is modifying the drug delivery system and discovering more efficient target drugs. An ideal drug delivery system must deliver drugs only to cancer sites and sustain high, stable drug concentrations. An ideal complex drug should target all cancer cells, and not normal cells; a multifunctional nanocarrier can meet both demands. Given cancer stem cell hierarchy complexity, a strategy involving combination therapy should be used to target both the bulk of differentiated cancer cells and the minority of cancer stem cells together. The bulk of stable cancer stem cells are indispensible for testing drug effects in the search for more efficient drugs that target cancer stem cells. The four cancer stem cell enrichment methods described earlier are phenotypic isolation of cancer cells with specific cancer stem cell markers, conventional cytotoxic chemotherapy or radiotherapy, suspension cultivation, and EMT. A high-throughput screening platform may be a better choice for screening efficient target drugs. Learning from cancer stem cells, combining new drug delivery systems, and new target drugs may reveal novel strategies for chemotherapy in the future.
Disclosure of conflict of interest
None.
References
- 1.Volk-Draper L, Hall K, Griggs C, Rajput S, Kohio P, DeNardo D, Ran S. Paclitaxel Therapy Promotes Breast Cancer Metastasis in a TLR4-Dependent Manner. Cancer Res. 2014;74:5421–5434. doi: 10.1158/0008-5472.CAN-14-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu H, He X, Zhang Z, Yin Q, Yu H, Li Y. A TPGS-incorporating nanoemulsion of paclitaxel circumvents drug resistance in breast cancer. Int J Pharm. 2014;471:206–213. doi: 10.1016/j.ijpharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Larocca A, Montefusco V, Bringhen S, Rossi D, Crippa C, Mina R, Galli M, Marcatti M, La Verde G, Giuliani N, Magarotto V, Guglielmelli T, Rota-Scalabrini D, Omedé P, Santagostino A, Baldi I, Carella AM, Boccadoro M, Corradini P, Palumbo A. Pomalidomide, cyclophosphamide, and prednisone for relapsed/refractory multiple myeloma: a multicenter phase 1/2 open-label study. Blood. 2013;122:2799–2806. doi: 10.1182/blood-2013-03-488676. [DOI] [PubMed] [Google Scholar]
- 4.Tazawa Y, Usukubo I, Takada K, Takekuma Y, Shibayama Y, Sugawara M. Schedule-dependent cytotoxicity of Etoposide and cyclophosphamide in P-glycoprotein-expressing human leukemic K-562 cells. Biol Pharm Bull. 2014;37:1323–1329. doi: 10.1248/bpb.b14-00207. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem. 2011;286:37813–37829. doi: 10.1074/jbc.M110.200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abubaker K, Latifi A, Luwor R, Nazaretian S, Zhu H, Quinn MA, Thompson EW, Findlay JK, Ahmed N. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden. Mol Cancer. 2013;12:24. doi: 10.1186/1476-4598-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W, Sydorenko N, Moon YC, Gibson L, Wang Y, Leung C, Iscove NN, Arrowsmith CH, Szentgyorgyi E, Gallinger S, Dick JE, O’Brien CA. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 11.Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014;27:440–446. doi: 10.1177/0897190014546108. [DOI] [PubMed] [Google Scholar]
- 12.Barton DL, Thanarajasingam G, Sloan JA, Diekmann B, Fuloria J, Kottschade LA, Lyss AP, Jaslowski AJ, Mazurczak MA, Blair SC, Terstriep S, Loprinzi CL. Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance) Cancer. 2014;120:3575–3582. doi: 10.1002/cncr.28892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezendam NP, Pijlman B, Bhugwandass C, Pruijt JF, Mols F, Caroline Vos M, Pijnenborg JM, van de Poll-Franse LV. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: Results from the population-based PROFILES registry. Gynecol Oncol. 2014;135:510–517. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Alberti P, Cavaletti G. Management of side effects in the personalized medicine era: chemotherapy-induced peripheral neuropathy. Methods Mol Biol. 2014;1175:301–322. doi: 10.1007/978-1-4939-0956-8_12. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichs K, Carstensen MH. Successful reduction of alopecia induced by anthracycline and taxane containing adjuvant chemotherapy in breast cancer - clinical evaluation of sensor-controlled scalp cooling. Springerplus. 2014;3:500. doi: 10.1186/2193-1801-3-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Płonka PM. Hair pigmentation disorders or 50 years of German-Polish alliance for study on a severe side effect of chemotherapy: Kostanecki’s legacy. Exp Dermatol. 2015;24:10–11. doi: 10.1111/exd.12560. [DOI] [PubMed] [Google Scholar]
- 17.Ikegaki N, Shimada H, Fox AM, Regan PL, Jacobs JR, Hicks SL, Rappaport EF, Tang XX. Transient treatment with epigenetic modifiers yields stable neuroblastoma stem cells resembling aggressive large-cell neuroblastomas. Proc Natl Acad Sci U S A. 2013;110:6097–6102. doi: 10.1073/pnas.1118262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hajj M, Wicha M, Benito-Hernandez A, Morrison S, Clarke M. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HG, Chen C, Yang H, Pan YF, Zhang XH. Cancer stem cell subsets and their relationships. J Transl Med. 2011;9:50. doi: 10.1186/1479-5876-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda-Lorenzo I, Dorado J, Lonardo E, Alcala S, Serrano AG, Clausell-Tormos J, Cioffi M, Megias D, Zagorac S, Balic A, Hidalgo M, Erkan M, Kleeff J, Scarpa A, Sainz B, Heeschen C. Intracellular autofluorescence: a biomarker for epithelial cancer stem cells. Nat Methods. 2014;11:1161–1169. doi: 10.1038/nmeth.3112. [DOI] [PubMed] [Google Scholar]
- 22.Richard V, Nair MG, Santhosh Kumar TR, Pillai MR. Side population cells as prototype of chemoresistant, tumor-initiating cells. Biomed Res Int. 2013;2013:517237. doi: 10.1155/2013/517237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin B, Zeng Y, Liu G, Wang X, Wang P, Song Y. MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int J Clin Exp Pathol. 2014;7:2934–2941. [PMC free article] [PubMed] [Google Scholar]
- 24.Boesch M, Zeimet AG, Reimer D, Schmidt S, Gastl G, Parson W, Spoeck F, Hatina J, Wolf D, Sopper S. The side population of ovarian cancer cells defines a heterogeneous compartment exhibiting stem cell characteristics. Oncotarget. 2014;5:7027–7039. doi: 10.18632/oncotarget.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Schuetz J, Bunting K, Colapietro A, Sampath J, Morris J, Lagutina I, Grosveld G, Osawa M, Nakauchi H, Sorrentino B. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 26.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Dylla S, Beviglia L, Park I, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke M, Hoey T, Lewicki J, Gurney A. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon R, Hao Y, Shi Q, Hjelmeland A, Dewhirst M, Bigner D, Rich J. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 29.Mani S, Guo W, Liao M, Eaton E, Ayyanan A, Zhou A, Brooks M, Reinhard F, Zhang C, Shipitsin M, Campbell L, Polyak K, Brisken C, Yang J, Weinberg R. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 31.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey D, Saxena M, Paranjape AN, Krishnan V, Giraddi R, Kumar MV, Mukherjee G, Rangarajan A. Phenotypic and functional characterization of human mammary stem/progenitor cells in long term culture. PLoS One. 2009;4:e5329. doi: 10.1371/journal.pone.0005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 35.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti M, Daidone M. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 36.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 37.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmody LC, Germain AR, VerPlank L, Nag PP, Muñoz B, Perez JR, Palmer MA. Phenotypic high-throughput screening elucidates target pathway in breast cancer stem cell-like cells. J Biomol Screen. 2012;17:1204–1210. doi: 10.1177/1087057112458317. [DOI] [PubMed] [Google Scholar]
- 41.Germain AR, Carmody LC, Nag PP, Morgan B, Verplank L, Fernandez C, Donckele E, Feng Y, Perez JR, Dandapani S, Palmer M, Lander ES, Gupta PB, Schreiber SL, Munoz B. Cinnamides as selective small-molecule inhibitors of a cellular model of breast cancer stem cells. Bioorg Med Chem Lett. 2013;23:1834–1838. doi: 10.1016/j.bmcl.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Germain AR, Carmody LC, Morgan B, Fernandez C, Forbeck E, Lewis TA, Nag PP, Ting A, VerPlank L, Feng Y, Perez JR, Dandapani S, Palmer M, Lander ES, Gupta PB, Schreiber SL, Munoz B. Identification of a selective small molecule inhibitor of breast cancer stem cells. Bioorg Med Chem Lett. 2012;22:3571–3574. doi: 10.1016/j.bmcl.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 43.Mezencev R, Wang L, McDonald JF. Identification of inhibitors of ovarian cancer stem-like cells by high-throughput screening. J Ovarian Res. 2012;5:30. doi: 10.1186/1757-2215-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowan K. High-throughput screening finds potential killer of cancer stem cells. J Natl Cancer Inst. 2009;101:1438–1439. doi: 10.1093/jnci/djp397. [DOI] [PubMed] [Google Scholar]
- 45.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 47.Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ, Lee YJ, Lee EK, Kook MC, Kim CG, Ryu KW, Kim YW. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: a nationwide cohort study. Aliment Pharmacol Ther. 2014;39:854–863. doi: 10.1111/apt.12660. [DOI] [PubMed] [Google Scholar]
- 48.Mei ZB, Zhang ZJ, Liu CY, Liu Y, Cui A, Liang ZL, Wang GH, Cui L. Survival benefits of metformin for colorectal cancer patients with diabetes: a systematic review and meta-analysis. PLoS One. 2014;9:e91818. doi: 10.1371/journal.pone.0091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, Song Q. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 50.Wahdan-Alaswad RS, Cochrane DR, Spoelstra NS, Howe EN, Edgerton SM, Anderson SM, Thor AD, Richer JK. Metformin-Induced Killing of Triple-Negative Breast Cancer Cells Is Mediated by Reduction in Fatty Acid Synthase via miRNA-193b. Horm Cancer. 2014;5:374–389. doi: 10.1007/s12672-014-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9:e87979. doi: 10.1371/journal.pone.0087979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu P, Davis M, Blackwelder AJ, Bachman N, Liu B, Edgerton S, Williams LL, Thor AD, Yang X. Metformin selectively targets tumor-initiating cells in ErbB2-overexpressing breast cancer models. Cancer Prev Res (Phila) 2014;7:199–210. doi: 10.1158/1940-6207.CAPR-13-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182:50–59. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8:e76518. doi: 10.1371/journal.pone.0076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, Majumdar AP. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9:e84369. doi: 10.1371/journal.pone.0084369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, Johnson RL, Song S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol. 2014;45:567–574. doi: 10.3892/ijo.2014.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato A, Sunayama J, Okada M, Watanabe E, Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T, Kitanaka C. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med. 2012;1:811–824. doi: 10.5966/sctm.2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnamurthy S, Ng VW, Gao S, Tan MH, Yang YY. Phenformin-loaded polymeric micelles for targeting both cancer cells and cancer stem cells in vitro and in vivo. Biomaterials. 2014;35:9177–9186. doi: 10.1016/j.biomaterials.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br J Cancer. 2013;109:1876–1885. doi: 10.1038/bjc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiba T, Suzuki E, Yuki K, Zen Y, Oshima M, Miyagi S, Saraya A, Koide S, Motoyama T, Ogasawara S, Ooka Y, Tawada A, Nakatsura T, Hayashi T, Yamashita T, Kaneko S, Miyazaki M, Iwama A, Yokosuka O. Disulfiram eradicates tumor-initiating hepatocellular carcinoma cells in ROS-p38 MAPK pathway-dependent and -independent manners. PLoS One. 2014;9:e84807. doi: 10.1371/journal.pone.0084807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hothi P, Martins TJ, Chen L, Deleyrolle L, Yoon JG, Reynolds B, Foltz G. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget. 2012;3:1124–1136. doi: 10.18632/oncotarget.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer. 2012;107:1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, Levadoux-Martin M, Lee JB, Giacomelli AO, Hassell JA, Fischer-Russell D, Trus MR, Foley R, Leber B, Xenocostas A, Brown ED, Collins TJ, Bhatia M. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 66.Ke XY, Lin Ng VW, Gao SJ, Tong YW, Hedrick JL, Yang YY. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35:1096–1108. doi: 10.1016/j.biomaterials.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 67.Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M, Tan Z, Ding Q, Zhang L, Lu J, Wu X, Liu Y. Thioridazine, an antipsychotic drug, elicits potent antitumor effects in gastric cancer. Oncol Rep. 2014;31:2107–2114. doi: 10.3892/or.2014.3068. [DOI] [PubMed] [Google Scholar]
- 68.Visnyei K, Onodera H, Damoiseaux R, Saigusa K, Petrosyan S, De Vries D, Ferrari D, Saxe J, Panosyan EH, Masterman-Smith M, Mottahedeh J, Bradley KA, Huang J, Sabatti C, Nakano I, Kornblum HI. A molecular screening approach to identify and characterize inhibitors of glioblastoma stem cells. Mol Cancer Ther. 2011;10:1818–1828. doi: 10.1158/1535-7163.MCT-11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 71.Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 72.Gao GH, Li Y, Lee DS. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J Control Release. 2013;169:180–184. doi: 10.1016/j.jconrel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Allison RR. Photodynamic therapy: oncologic horizons. Future Oncol. 2014;10:123–124. doi: 10.2217/fon.13.176. [DOI] [PubMed] [Google Scholar]
- 74.Dicheva BM, Koning GA. Targeted thermosensitive liposomes: an attractive novel approach for increased drug delivery to solid tumors. Expert Opin Drug Deliv. 2014;11:83–100. doi: 10.1517/17425247.2014.866650. [DOI] [PubMed] [Google Scholar]
- 75.Wegscheid ML, Morshed RA, Cheng Y, Lesniak MS. The art of attraction: applications of multifunctional magnetic nanomaterials for malignant glioma. Expert Opin Drug Deliv. 2014;11:957–975. doi: 10.1517/17425247.2014.912629. [DOI] [PubMed] [Google Scholar]
- 76.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sotiropoulou PA, Christodoulou MS, Silvani A, Herold-Mende C, Passarella D. Chemical approaches to targeting drug resistance in cancer stem cells. Drug Discov Today. 2014;19:1547–1562. doi: 10.1016/j.drudis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Choi WI, Lee JH, Kim JY, Heo SU, Jeong YY, Kim YH, Tae G. Targeted antitumor efficacy and imaging via multifunctional nano-carrier conjugated with anti-HER2 trastuzumab. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Chiang CS, Hu SH, Liao BJ, Chang YC, Chen SY. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine. 2014;10:99–107. doi: 10.1016/j.nano.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Lee AL, Wang Y, Cheng HY, Pervaiz S, Yang YY. The co-delivery of paclitaxel and Herceptin using cationic micellar nanoparticles. Biomaterials. 2009;30:919–927. doi: 10.1016/j.biomaterials.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 81.Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 2011;7:859–870. doi: 10.1016/j.nano.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi P, Gustafson JA, MacKay JA. Genetically engineered nanocarriers for drug delivery. Int J Nanomedicine. 2014;9:1617–1626. doi: 10.2147/IJN.S53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 86.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic non-stem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials. 2012;33:679–691. doi: 10.1016/j.biomaterials.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 89.Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, Ouafik L, Figarella-Branger D. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol. 2010;20:211–221. doi: 10.1111/j.1750-3639.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta C. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 91.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra J, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky S, Birnbaum D, Viens P, Wicha M. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass S, Jiang F. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang F, Qiu Q, Khanna A, Todd N, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass S, Katz R. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Chen Y, Hsu H, Tseng L, Huang P, Lu K, Chen D, Tai L, Yung M, Chang S, Ku H, Chiou S, Lo W. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 98.Minato T, Yamamoto Y, Seike J, Yoshida T, Yamai H, Takechi H, Yuasa Y, Furukita Y, Goto M, Bando Y, Tangoku A. Aldehyde dehydrogenase 1 expression is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2013;20:209–217. doi: 10.1245/s10434-012-2535-8. [DOI] [PubMed] [Google Scholar]
- 99.Chen D, Bhat-Nakshatri P, Goswami C, Badve S, Nakshatri H. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer Res. 2013;73:5821–5833. doi: 10.1158/0008-5472.CAN-13-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kong Y, Yoshida S, Saito Y, Doi T, Nagatoshi Y, Fukata M, Saito N, Yang SM, Iwamoto C, Okamura J, Liu KY, Huang XJ, Lu DP, Shultz LD, Harada M, Ishikawa F. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22:1207–1213. doi: 10.1038/leu.2008.83. [DOI] [PubMed] [Google Scholar]
- 101.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, Tan VP, Yau TC, Poon RT, Wong BC. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 103.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 106.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lau WM, Teng E, Chong HS, Lopez KA, Tay AY, Salto-Tellez M, Shabbir A, So JB, Chan SL. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630–2641. doi: 10.1158/0008-5472.CAN-13-2309. [DOI] [PubMed] [Google Scholar]
- 109.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 111.Noto Z, Yoshida T, Okabe M, Koike C, Fathy M, Tsuno H, Tomihara K, Arai N, Noguchi M, Nikaido T. CD44 and SSEA-4 positive cells in an oral cancer cell line HSC-4 possess cancer stem-like cell characteristics. Oral Oncol. 2013;49:787–795. doi: 10.1016/j.oraloncology.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 114.Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 115.Gao W, Chen L, Ma Z, Du Z, Zhao Z, Hu Z, Li Q. Isolation and phenotypic characterization of colorectal cancer stem cells with organ-specific metastatic potential. Gastroenterology. 2013;145:636–646. e635. doi: 10.1053/j.gastro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 116.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, Gearing DP, Vairo G, Lopez AF, Dick JE, Lock RB. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 117.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 118.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 119.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 120.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 121.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 122.Baba T, Convery P, Matsumura N, Whitaker R, Kondoh E, Perry T, Huang Z, Bentley R, Mori S, Fujii S, Marks J, Berchuck A, Murphy S. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 123.Rutella S, Bonanno G, Procoli A, Mariotti A, Corallo M, Prisco MG, Eramo A, Napoletano C, Gallo D, Perillo A, Nuti M, Pierelli L, Testa U, Scambia G, Ferrandina G. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15:4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 124.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 125.Jiao J, Hindoyan A, Wang S, Tran LM, Goldstein AS, Lawson D, Chen D, Li Y, Guo C, Zhang B, Fazli L, Gleave M, Witte ON, Garraway IP, Wu H. Identification of CD166 as a Surface Marker for Enriching Prostate Stem/Progenitor and Cancer Initiating Cells. PLoS One. 2012;7:e42564. doi: 10.1371/journal.pone.0042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan M, Yang X, Wang L, Clark D, Zuo H, Ye D, Chen W, Zhang P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol Cell Proteomics. 2013;12:3271–3284. doi: 10.1074/mcp.M112.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 129.Kim KH, Kang YJ, Jo JO, Ock MS, Moon SH, Suh DS, Yoon MS, Park ES, Jeong N, Eo WK, Kim HY, Cha HJ. DDX4 (DEAD box polypeptide 4) colocalizes with cancer stem cell marker CD133 in ovarian cancers. Biochem Biophys Res Commun. 2014;447:315–322. doi: 10.1016/j.bbrc.2014.03.144. [DOI] [PubMed] [Google Scholar]
- 130.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, Sokolovskaya A, Matsuzaki J, Yamada R, Fujii R, Kampinga HH, Kondo T, Hasegawa T, Hara I, Sato N. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72:2844–2854. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 131.Morita R, Nishizawa S, Torigoe T, Takahashi A, Tamura Y, Tsukahara T, Kanaseki T, Sokolovskaya A, Kochin V, Kondo T, Hashino S, Asaka M, Hara I, Hirohashi Y, Sato N. Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells. Cancer Sci. 2014;105:389–395. doi: 10.1111/cas.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Emlet DR, Gupta P, Holgado-Madruga M, Del Vecchio CA, Mitra SS, Han SY, Li G, Jensen KC, Vogel H, Xu LW, Skirboll SS, Wong AJ. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014;74:1238–1249. doi: 10.1158/0008-5472.CAN-13-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Battula VL, Shi Y, Evans KW, Wang RY, Spaeth EL, Jacamo RO, Guerra R, Sahin AA, Marini FC, Hortobagyi G, Mani SA, Andreeff M. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Invest. 2012;122:2066–2078. doi: 10.1172/JCI59735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barker N, van Es J, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters P, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 136.Keshet G, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves A, Schachter J, Amariglio N, Rechavi G. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368:930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 137.Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, Steindler DA, Gibbs CP. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–5655. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, Yan HX, Fu J, Chen Y, Zhang HL, Zheng LY, He YQ, Li YQ, Wu FQ, Zou SS, Li Z, Wu MC, Feng GS, Wang HY. OV6+ tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57:613–620. doi: 10.1016/j.jhep.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 139.Choudhury S, Almendro V, Merino VF, Wu Z, Maruyama R, Su Y, Martins FC, Fackler MJ, Bessarabova M, Kowalczyk A, Conway T, Beresford-Smith B, Macintyre G, Cheng YK, Lopez-Bujanda Z, Kaspi A, Hu R, Robens J, Nikolskaya T, Haakensen VD, Schnitt SJ, Argani P, Ethington G, Panos L, Grant M, Clark J, Herlihy W, Lin SJ, Chew G, Thompson EW, Greene-Colozzi A, Richardson AL, Rosson GD, Pike M, Garber JE, Nikolsky Y, Blum JL, Au A, Hwang ES, Tamimi RM, Michor F, Haviv I, Liu XS, Sukumar S, Polyak K. Molecular profiling of human mammary gland links breast cancer risk to a p27(+) cell population with progenitor characteristics. Cell Stem Cell. 2013;13:117–130. doi: 10.1016/j.stem.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bareiss PM, Paczulla A, Wang H, Schairer R, Wiehr S, Kohlhofer U, Rothfuss OC, Fischer A, Perner S, Staebler A, Wallwiener D, Fend F, Fehm T, Pichler B, Kanz L, Quintanilla-Martinez L, Schulze-Osthoff K, Essmann F, Lengerke C. SOX2 expression associates with stem cell state in human ovarian carcinoma. Cancer Res. 2013;73:5544–5555. doi: 10.1158/0008-5472.CAN-12-4177. [DOI] [PubMed] [Google Scholar]
- 141.Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, Schober M. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014;5:4511. doi: 10.1038/ncomms5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jan M, Chao MP, Cha AC, Alizadeh AA, Gentles AJ, Weissman IL, Majeti R. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A. 2011;108:5009–5014. doi: 10.1073/pnas.1100551108. [DOI] [PMC free article] [PubMed] [Google Scholar]


