Summary
Immunological memory is a cardinal feature of adaptive immunity. We are now beginning to elucidate the mechanisms that govern the formation of memory T cells and their ability to acquire longevity, survive the effector-to-memory transition, and mature into multipotent, functional memory T cells that self-renew. Here, we discuss the recent findings in this area and highlight extrinsic and intrinsic factors that regulate the cellular fate of activated CD8+ T cells.
Keywords: T cells, memory, cell differentiation, viral, transcription factors, vaccination
Introduction
Cardinal features of memory T cells
The formation of immunological memory following acute infections or immunization is a hallmark of adaptive immunity, whereby an organism ‘remembers’ the original pathogen encountered and mounts more robust humoral and cellular responses to rapidly control reinfection and reduce the severity of disease (1–6). Immunological memory mainly consists of long-lived plasma cells that constitutively secrete high-affinity neutralizing antibodies, and memory B and T cells (5). Although the concepts and principles of immunological memory have governed the design and application of vaccines for centuries, the underlying mechanisms that regulate memory B- and T-cell formation remain poorly defined.
During viral infection, circulating virus-specific naive T cells engage with antigen-presenting dendritic cells (DCs) via the formation of immunological synapse (7–9). After this first 18- to 24-h initiation stage, activated T cells start to rapidly proliferate (10, 11). Pathogen-specific T cells can expand approximately 10,000 fold at a rate of 4–6 h per cell cycle and complete up to 20 cell divisions in a week’s time, during which they also differentiate and acquire effector functions and the ability to migrate to the sites of infection (3–5). These functional cytotoxic T lymphocytes (CTLs) then elaborate cytokines and granzymes to kill pathogen-infected cells and control the infection (3, 6). Following pathogen clearance, effector CD8+ T cells undergo equally precipitous contraction, wherein the majority of pathogen-specific effector CD8+ T cells die via apoptosis, but approximately 5–10% of them survive to further mature into long-lived protective memory CD8+ T cells (4–6). Memory CD8+ T cells can persist for very long periods of time in the absence of antigen (Ag) and provide life-long protection in mice and humans (12, 13). Typically, the number of memory CD8+ T cells remains quite stable overtime, largely through interactions with interleukin-7 (IL-7) and IL-15 that mediate survival and self-renewal (referred to as homeostatic proliferation or turnover) of memory CD8+ T cells (3, 6, 14).
As memory CD8+ T cells differentiate, a number of interesting stem cell-like properties are bestowed onto these cells, such as longevity, telomerase expression, and the ability to self-renew themselves through homeostatic turnover (4, 6). In addition, memory CD8+ T cells exist in a poised multipotent state because they persist as resting memory CD8+ T cells, but, upon reinfection and antigenic stimulation, they have the capacity to rapidly proliferate and differentiate into secondary effector CD8+ T cells (3, 6, 14). The combination of these functional attributes, with the sheer increase in the relative abundance of memory T cells compared with an unimmunized individual, constitute the basis of long-term T-cell-mediated immunity. In this review, we aim to summarize the latest progress made in the field with a focus on CD8+ T-cell memory ontogeny and maintenance.
Generating effector CD8+ T-cell heterogeneity and memory cell potential
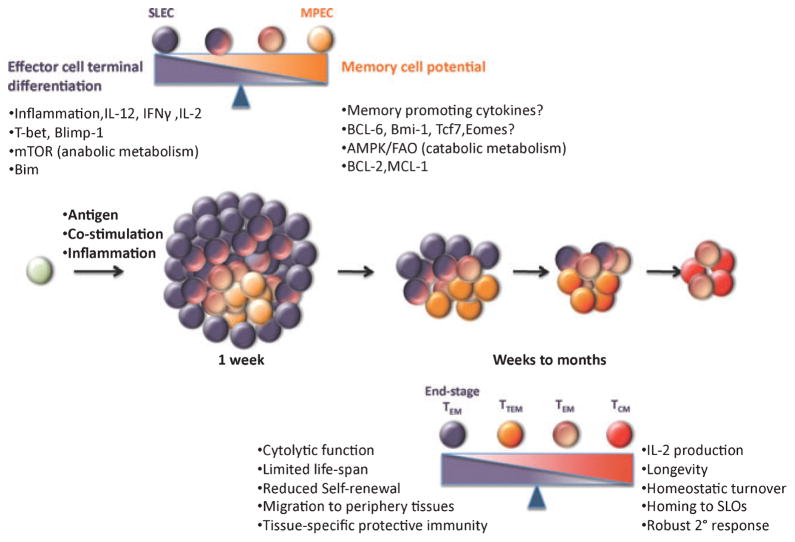
It is now evident that the populations of antigen-specific CD8+ T cells are quite heterogeneous, and not all effector CD8+ T cells, and memory CD8+ T cells for that matter, acquire these quintessential memory cell properties equivalently (6, 15). As effector CD8+ T cells expand and differentiate during a primary response most cells terminally differentiate into end-stage effectors that have a shortened lifespan and die following infection, whereas a smaller subset of cells differentiates into memory cell precursors that have increased memory cell potential (6, 15–17). An elegant study utilizing the transfer of single naive CD8+ T cells demonstrated that an individual precursor cell has the capacity to give rise to these differently fated effector cell populations and memory CD8+ T cells, indicating that naive CD8+ T cells are not pre-programmed to develop into effector or memory cells and that the specification of these fates occurred after T-cell activation (18). However, the diversity of the effector cell population is not limited to just these two cell subsets because additional types of effector CD8+ T cells exist that appear to display a mix of terminally differentiated and memory precursor cell properties and have intermediate lifespans and proliferative responses to secondary infections (19, 20, N.S. Joshi and S.M. Kaech, unpublished data). Thus, perhaps a more accurate description of effector CD8+ T-cell differentiation is that it occurs along a continuum with cells harboring greater memory cell potential and longevity on the one end and terminally differentiated effector cells on the other end, but in between these two extremes lies effector cells that exist in intermediate differentiation states (Fig. 1). Such a model helps to account for the numerous subsets of effector CD8+ T cells seen during immune responses as well as provides plasticity within an effector ‘lineage’, permitting cells to slide between differentiation states according to signal input. Additionally, it provides for the multipotency of memory cells to further differentiate along this continuum into terminal effector CD8+ T cells upon restimulation.
Fig. 1. Generation of effector CD8+ T cells and progressive differentiation into memory T cells.
In response to infections, naive CD8+ T cells become activated, proliferate rapidly, and differentiate into a heterogeneous pool of effector CD8+ T cells. Most cells terminally differentiate into end-stage effectors that have a shortened lifespan and die following infection, whereas a smaller subset of cells differentiates into memory cell precursors that can further mature into functional memory cells that self-renew. Exposure to high levels of proinflammatory cytokines, such as IL-12, IFN-γ, and IL-2, causes upregulation of T-bet and Bilmp-1 as well as increased mTOR activity in activated CD8+ T cells, which in turn promotes effector cells to terminally differentiate and die via Bim-dependent apoptosis. By contrast, a group of cells upregulate BCL-6, Bmi-1, Tcf7, and possibly Eomes when they either circumvent high levels of proinflammatory cytokines or possibly encounter pro-memory signals. They can also switch from mTOR-mediated anabolic state to AMPK/FAO-mediated catabolic state following growth factor withdrawal and survive effector-to-memory transition by upregulating anti-apoptosis factors, such as Bcl-2 and Mcl-1. Between these two cell subsets (SLEC in dark purple and MPEC in orange), additional types of effector CD8+ T cells exist that appear to display a mix of terminally differentiated and memory precursor cell properties and have intermediate lifespans and proliferative potential. After pathogen clearance, effector CD8+ T cells give rise to diverse subsets of memory T cells, including end-stage TEM, TTM, TEM, and TCM. End-stage TEM cells are more effector like, have poor recall ability, and gradually decay from the memory pool. TTM continually differentiate into TCM that persist for a long term via homeostatic turn over, acquire homing ability to SLOs, produce IL-2, and mount robust secondary response upon recall. TEM cells usually reside in periphery tissues and retain their cytolytic activity, thereby providing immediate effector function at the portal of pathogen entry, but exhibiting reduced proliferative capacity. IL, interleukin; IFN-γ, interferon-γ; mTOR, mammalian target of rapamycin; AMPK, 5′-adenosine monophosphate-activated protein kinase; FAO, fatty acid oxidation; SLEC, short-lived effector cell; MPEC, memory precursor effector cell; TEM, effector memory T cells; TTM, transitional TEM; TCM, ‘central’ memory CD8+ T cells; SLO, secondary lymphoid organ.
During many acute infections, a handful of markers have become helpful to dissect the effector population into subpopulations that have more or less memory cell potential (6, 15, 16, 21–23). Increased expression of IL-7Rα was the first marker found to distinguish memory precursor cells from terminally differentiated, shorter lived effector cells, and IL-7Rα is functionally required for their long-term survival (21, 23). Other proteins that co-segregate with increased IL-7Rα expression on antigen-specific CD8+ T cells include Bcl-2, CD27, CXCR3, and CD28 (6, 16, 22). Although the IL-7Rαhi effector CD8+ T cells preferentially survive following infection compared with IL-7Rαlo cells, there is some death in this population as the memory CD8+ T cells form (6, 15). However, the memory cells that descend from the IL-7Rαhi cells display the trademark memory cell properties most distinctly, such as increased proliferative responses to antigen, IL-2 production, and the ability to self-renew. Additionally, the IL-7Rαhi memory CD8+ T cells have the greatest capacity to develop into ‘central’ memory CD8+ T cells (TCM) that express lymph node-homing receptors and reside in lymphoid tissues (6) (Fig. 1).
By contrast, increased expression of CD57 and KLRG1 and decreased expression of IL-7Rα, CXCR3, CD27, and CD28 are associated with effector or memory CD8+ T cells that are cytotoxic and produce interferon (IFN)-γ but are relatively senescent (6, 15, 16, 24, 25). In addition, these types of CD8+ T cells tend to have the lower expression of IL-2 and telomerase, shortened telomeres, heightened levels of the cell cycle in inhibitor p27kip, and decreased in AKT signaling (26–28, T.W. Hand and S.M. Kaech, unpublished data). Moreover, in several mouse models of infection, antigen-specific KLRG1-expressing effector CD8+ T cells that do not express IL-7Rα have reduced longevity and recall response compared with cells that are KLRG1loIL-7Rhi (16, 21, 23, 29–33). In such settings, we have referred to KLRG1hiIL-7Rαlo CD8+ T cells as short-lived effector cells (SLECs) and KLRG1loIL-7Rαhi cells as to memory precursor effector cells (MPECs) (16). Lastly, some effector CD8+ T cells express markers of terminal differentiation (such as KLRG1), yet appear to survive the effector-to-memory transition phase. These cells tend to maintain higher expression of cytotoxic molecules longer after pathogen clearance compared with KLRG1loIL-7Rhi memory cells, are more prevalent in peripheral tissues like the liver and lung, and can be considered as effector memory (TEM) cells (6, 22, 34–36).
The diversity of effector and memory CD8+ T cells is considerably more expansive than that described above and cells that display ‘hybrid’ phenotypes are also found throughout immune responses. For instance, double-negative KLRG1loIL-7Rαlo effector CD8+ T cells are present during infection and may represent cells of an early effector state that have not reached terminal differentiation (19, 20). Moreover, double-positive KLRG1hiIL-7Rα hi cells are common in resting memory CD8+ T-cell populations. These cells have a longer half-life than KLRG1hiIL-7Rα lo cells, but it is reduced relative to KLRG1loIL-7Rhi memory cells. Moreover, compared to KLRG1loIL-7Rahi memory cells, KLRG1hiIL-7Rα hi memory cells tend to proliferate less to antigen and homeostatic cytokines and produce less IL-2. Some of these types of memory CD8+ T cells may represent memory CD8+ T cells that have experienced multiple infections as these cells accumulate TEM phenotypes, yet remain longer lived (37, 38). Although the categorization of effector and memory CD8+ T cells is helpful to dissect differences among subsets, further studies are required to illustrate the stabilities of each of these subsets based on the aforementioned surface makers. Furthermore, the inclusion of additional markers amplifies the heterogeneity and the spectrum of differentiation states that can be acquired by effector and memory CD8+ T cells.
Effector and memory cell fate-determining factors
The factors determine which effector CD8+ T cells die and which ones develop into memory cells has been a long-standing question in the field. Multiple signals have been proposed to be involved in this cell fate-determining process, including the strength and duration of TCR stimulation, inflammatory cytokines, transcriptional regulations, metabolic switches, and uneven segregation of lineage-determining factors. The strength and duration of these signals received by each individual T cell could be different, due to variations in temporal and spatial exposure, which could contribute to the heterogeneity of effector T cells. In this review, we cover recent advances in the field that regulates these processes.
Signal strength at the time of activation dictates effector T-cell differentiation and cell fate decisions
Naive T-cell activation, effector differentiation, and subsequent memory T-cell development are regulated by TCR signals, costimulation, and inflammations, which are often referred to as signals 1–3. These three signals are inseparable during infection settings in vivo. Therefore, the integration of strength and duration of all three signals influences memory T-cell differentiation. Although it is not exactly clear mechanistically, the progeny of activated naive T cells most likely ‘see’ different amounts of these signals and undergo divergent differentiation pathways during infection, which might account for the diverse nature of the effector T-cell pool. Nevertheless, which signals ‘decide’ whether an effector CD8+ T cell will die after pathogen clearance or differentiate into functional and long-lived memory T cells is not completely understood. Here, we discuss the recent findings in the individual and collaborative roles of these signals in memory T-cell development.
Role of antigenic stimulation in effector expansion and differentiation
CD8+ T cells specific to any peptide only consist of a small fraction of naive repertoire; therefore, robust proliferation is required to sufficiently combat rapidly replicating pathogens (39). It was thought that repeated antigenic stimulation may be required for the sustained effector T-cell expansion. However, recent studies demonstrated that a brief antigen exposure could set forth an ‘autopilot’ CD8+ T-cell response without the continued need for antigen stimulation (40–42). Activated CD8+ T cells then go through a number of divisions, acquire effector function, and give rise to memory cells (40–42).
During an infection, the extent of T-cell proliferation is governed by the amount of antigen available in vivo and the strength of TCR signal. By infecting mice with recombinant vaccinia virus strains that produced either high or low quantities of an ovalbumin (OVA) epitope, one group showed that the magnitude of the responding CTL population was proportional to epitope abundance (43). The strength of antigenic stimulation for CD8+ T-cell activation is determined by the binding affinity of TCR to MHC I–peptide complex as well as the duration of their interaction. A recent study elegantly demonstrated that T-cell receptor (TCR) transgenic CD8+ T cells were able to sufficiently respond to altered peptide antigens with a broad range of affinity during Listeria infection. Although strong TCR stimulation was favored for sustained T-cell expansion, it was not a prerequisite for complete activation. Even very low affinity antigens induced detectable effector and memory CD8+ T-cell generation. Nevertheless, other studies have shown that a prolonged time was required to commit naive T cells to proliferate under lower antigen doses than higher antigen doses in the presence of costimulation (44–46). Taken together, these studies suggested that naive T cells can incorporate the strength and duration of TCR signals to reach a threshold of full activation, resulting in antigen-independent effector development. Importantly, this initial TCR activation also instigates the developmental program that sufficiently drives long-lived memory cell formation.
Role of costimulation in memory development and maintenance
Costimulatory molecules (CD28, CD27, 4-1BB, and OX40) expressed by CD8+ T cells are important for T-cell activation, expansion, survival, and memory formation (4, 47). Antigenic stimulation in the absence of costimulatory signals was thought to induce tolerance or clonal depletion (48, 49). The roles of costimulatory molecules in generating successful effector CD8+ T-cell response are more critical when T cells are primed weakly or of short duration. Furthermore, costimulatory molecules have been shown to be involved in memory T-cell development. For example, a recent study has indicated that OX-40-deficient mice had reduced memory CD8+ T-cell formation after Listeria infection (50). Aside from OX-40, other costimulatory molecules, including CD28, CD27, and 4-1BB, have also been shown to contribute to memory CD8+ T-cell generation and/or longevity in variety of infection models (4, 51–53).
Costimulatory signals are also required for maximal maintenance of T-cell memory. IL-7 and IL-15, cytokines that are required for memory T-cell homeostatic turnover, have been reported to upregulate the costimulatory tumor necrosis factor receptor (TNFR) family members OX40 and 4-1BB, respectively, and this may provide additional survival signals to memory CD8+ T cells (53, 54). Several TNFR family members, including 4-1BB, recruit TNFR-associate factor-1 (Traf-1) for downstream signaling. Like 4-1BB and OX-40 deficiency, Traf-1 deficiency has a modest but significant effect upon memory CD8+ T-cell formation and survival (55, 56). Finally, 4-1BB and OX-40 may positively feedback on the expression of the IL-2Rα and IL-7Rα, thus further promoting T-cell survival (57). Thus, IL-7 and IL-15 provided in certain ‘niches’ may affect the interactions between cytokines, cytokine receptors, and costimulatory receptors to sustain T-cell survival and their long-term maintenance (54).
Role of inflammation in the effector and memory cell fate decisions
It has become increasingly clear that cytokine milieu initiated through pathogen-associated molecular patterns (PAMPs) upon pathogen encounter have immediate effects on activated CD8+ T cells and determine the clonal burst size, acquisition of effector functions, and modulate effector versus memory cell fate decisions (3, 48, 58). Early studies suggest that signal 3 provided by proinflammatory cytokines, mainly IL-12, IFN-γ, and IFN-αβ, is required to break the tolerance when naive T cells were activated with weak TCR stimulation both in vitro and in vivo (48, 59, 60). In addition, signal 3 also enhances T-cell expansion via promoting proliferative effector CD8+ T cells survival during various infections (60–63). Although the underlying mechanisms are incompletely clear, some studies suggested that IL-12 might enhance activated CD8+ T-cell survival by upregulating Bcl3 and/or inhibiting caspase-3 catalytic function (64, 65). Another important role of signal 3 is to promote activated CD8+ T cells to acquire effector function by means of producing cytotoxic cytokines, such as IFN-γ and TNF-α (48, 60, 66). Additionally, it is interesting to note that the responsiveness of CD8+ T cells to signal 3 for their optimal expansion and survival is largely dependent on the nature of pathogens. For instance, the expansion of pathogen-specific CD8+ T cells that are deficient of IFN-αβ receptor was greatly reduced during lymphocytic choriomeningitis virus (LCMV) infection, but the same CD8+ T cells expand normally during Listeria infection. On the contrary, CD8+ T-cell expansion during Listeria infection relies mainly on IL-12 signal, but IFN-αβ is not necessary (3, 6, 58, 67).
Accumulating evidence supports the concept that inflammation also plays a key role in effector and memory cell fate decisions (3, 6, 15, 16, 68–72). Original studies demonstrated that truncating Listeria infection using antibiotics blunted contraction of antigen-specific CD8+ T cells (70, 71). This observation was further confirmed in the setting of immunization with peptide-pulsed, mature DCs, in which delivery of antigen and costimulatory signals (signals 1 and 2) sufficed to generate memory CD8+ T cells, but the presence of adjuvants (such as CpG) during immunization accentuated not only the expansion of activated CD8+ T-cell population but also its subsequent contraction (33, 69, 72). Collectively, these data suggested that certain pro-inflammatory cytokines, primarily IL-12 and IFN-γ, intensified antigen-specific CD8+ T-cell expansion and differentiation but also induced terminal differentiation and a shortened lifespan (3, 6, 15, 16, 71–74) (Fig. 1). In addition to IL-12 and IFN-γ, other candidate cytokines that may have a similar role in effector and memory cell fate decisions are type I IFNs, other IL-12 family members, and IL-2 (75, 76).
Owing to the early production of IFN-γ and IL-12p70 during infection, one would presume that their effects on activated CD8+ T-cell proliferation, differentiation, and cell fate decision appear very early during Listeria infection and certain immunizations (77, 78, W. Cui and S.M. Kaech, unpublished data). Interestingly, pathogen-specific CD8+ T cells become refractory to IFN-γ but remain responsive to IL-12 soon after their activation (78, W. Cui and S.M. Kaech, unpublished observation). This observation suggests that IFN-γ and IL-12 may regulate effector and memory CD8+ T-cell differentiation in a coordinated manner. (77, 79). IFN-γ is produced mainly by NK cells during the initial stage of innate response to Listeria infection (72, 80) and plays an important role in the first line of defense by directly inhibiting viral or intracellular bacterial replication, upregulating major histocompatibility complex class II (MHC II) and macrophage function (81, 82). IFN-γ also enhances IL-12p70 production from macrophages and DCs by inducing IL-12p35 expression (72), and this creates a positive circuit in which IL-12 feeds back to further enhance IFN-γ production by natural killer (NK) cells (72, 77). IL-12 then directly acts on activated CD8+ T cells to augment their proliferation, differentiation, and formation of terminally differentiated CTLs (16, 32, 66, 72–74).
Timing and coupling of signals 1, 2, and 3
The above findings demonstrate the close relationship between CD8+ T cells and antigen-presenting cells and NK cells (and probably other innate cells), and the cytokines they produce, such as IFN-γ and IL-12, during infection or immunization. Moreover, early studies have shown clear synergy between signals 1, 2 and 3 and that their coordinated exposure renders complete T-cell activation, proliferation, and development of cytotoxic activity (3, 6, 48). Interestingly, the coupling of these signals also modulates memory and effector cell fate decisions. Recent studies from our laboratory and others have shown that antigen stimulation and inflammatory cytokines, such as IL-12, need to be delivered simultaneously to induce maximal effector CD8+ T-cell differentiation and loss of memory cell properties (30, 33, 72). Likewise, shortening the duration of infection impedes the formation of KLRG1hiIL-7RloCD27loIL-2lo and effector cells and hastens the formation of memory cell properties (16, 33, 72, 83). The coupling of these signals during infection probably also explains why ‘latecomer’ cells (i.e. naive CD8+ T cells that are activated 2–3 days following the start of infection) preferentially generate memory-like CD8+ T cells; in this scenario, the initial peak of pro-inflammatory cytokines is in decline when the CD8+ T cells are activated (83, 84).
Although the precise details for how signals 1 and 3 integrate to influence effector and memory cell potential are unknown, one could postulate that TCR signals open up key cell fate-determining loci that are targets of IL-12/signal transducer and activator of transcription 4 (STAT4) signals. An example of such transcriptional regulation is IL-12Rβ2 itself. IL-12Rβ2 is silenced in naive T cells due to the inaccessible nature of its chromatin structure, but TCR activation is necessary and sufficient to open IL-12Rβ2 regulatory elements, through BAF complex-mediated modeling leading to early IL-12Rβ2 expression (85). Subsequent IL-12 signaling and STAT4 activation further augments IL-12Rβ2 expression, directly leading to enhanced IL-12 signaling and terminal differentiation (85).
Transcriptional and metabolic regulation of memory T-cell differentiation
Transcriptional regulation in effector and memory cell fate decisions
An activated CD8+ T cell is exposed to a myriad array of signals, such as cytokines, chemokines, growth factors, nutrients, and environmental cues. How these signals are transmitted into cells and translated into gene expression patterns that promote effector differentiation yet also preserve a long-lived and multipotent pool of cells that can self-renew is an important and complicated question. Here, we summarize the role of a few transcriptional regulators identified in this process during acute infections in mice (Fig. 1).
T-bet (encoded by Tbx21) was originally identified in CD4+ T cells as a T-box transcription factor responsible for T-helper 1 (Th1) lineage commitment, but, more recently, T-bet has been found to play an important role in effector and memory cell fate decisions (16, 73, 86–89). T-bet expression is induced initially by TCR signaling and augmented by IL-12 signals in activated CD8+ T cells (16, 73). When T-bet expression was examined in virus-specific CD8+ T cells, it was found to be elevated in the KLRG1hiIL-7Rlo shorter lived effector CD8+ T cells relative to the KLRG1loIL-7Rhi memory precursor effector CD8+ T cells (16, 90). This finding suggested that an expression gradient of T-bet acted like a rheostat to control the balance between terminal effector CD8+ T-cell differentiation and memory cell potential in effector CD8+ T cells. Higher amounts of T-bet instructed KLRG1hiIL-7Rlo terminal effector cell formation, but lower amounts appeared to permit normal memory cell formation. For example, T-bet-deficient memory CD8+ T cells expressed less IL-15Rβ chain (CD122), were defective in the expression of other MPEC-signature genes, and did not proliferate as well as wildtype cells to secondary infection (6, 15, 16, N.S. Joshi and S.M. Kaech, unpublished data). Eomesodermin (Eomes), another T-box factor expressed in activated CD8+ T cells, is also important for CD122 and perforin expression in CD8+ T cells (16, 76, 90). Eomes has an interesting relationship with T-bet because on the one hand, T-bet and Eomes appear to cooperate in CTL function and memory T-cell homeostasis. T-bet and Eomes coordinate the expression of CD122 in memory CD8+ T cells (16, 90), and CD8+ T cells that are doubly deficient in both genes are incapable of generating CTLs during LCMV infection. Instead, Tbx21−/− Eomes−/− CD8+ T cells abnormally differentiated into IL-17-producing CD8+ T cells that caused excessive neutrophil infiltration and a lethal inflammatory syndrome (86, 87). On the other hand, they also seem to work in opposition at some level because in contrast to T-bet, IL-12 paradoxically suppresses Eomes expression and Eomes expression preferentially increases relative to T-bet as memory CD8+ T cells form and mature (73). However, the mechanisms by which T-bet and Eomes cooperate or possibly antagonize each other at different loci remains to be elucidated.
Another pair of transcriptional regulators that function in memory CD8+ T-cell development is Blimp-1 and BCL-6. These are transcriptional repressors that mutually antagonize each other’s expression and are best known for regulating the development of germinal center B cells and long-lived plasma cells (91, 92). Blimp-1 is necessary for the formation of terminally differentiated, senescent, and long-lived plasma cells that reside in the bone marrow (93, 94). In T cells, genetic ablation of Blimp-1 causes a fatal lymphoproliferative autoimmune disorder, demonstrating a role for Blimp-1 in T-cell homeostasis (95, 96). Recently, the function of Blimp-1 in antigen-specific CD8+ T-cell development has been examined in greater detail (17, 97, 98). During acute LCMV infection, which is a systemic viral infection, Blimp-1 was expressed to a higher degree in terminally differentiated KLRG1hiIL-7Rlo effector CD8+ T cells relative to KLRG1loIL-7Rhi cells, and Blimp-1-deficient effector CD8+ T cells primarily developed into memory precursor cells and acquired central memory cell characteristics, such as CD62L expression, IL-2 production, and enhanced proliferative responses, more rapidly than their wildtype counterparts (17). However, Blimp-1 is important for maximizing the formation of certain effector functions in CD8+ T cells and the control of viral infections. For instance, Blimp-1 is essential in CD8+ T cells for the control of influenza infection, a localized respiratory infection, and defective viral control correlated with impaired effector cell migration to the lungs (97). In addition, Blimp-1 is critical to normal perforin, granzyme B and K expression, which is important for cytotoxic CD8+ T-cell activity (17, 97). During chronic LCMV infection, Blimp-1 was needed both for optimal effector functions as well as for expression of inhibitory receptors (such as PD-1, LAG3, CD160, and 2B4), which consequently decreases virus-specific CTL functions (i.e. induces CD8+ T-cell ‘exhaustion’) to prevent fatal immunopathology (98). Interestingly, rendering the virus-specific CD8+ T cells haplo-insufficient in Blimp-1 led to better viral control because this treatment provided a healthier balance between effector cell function and exhaustion. Limiting Blimp-1 by one-half was sufficient to sustain a level of CTL function but was insufficient for maximal expression of the inhibitory receptors.
In B cells, Blimp-1 and BCL-6 reciprocally antagonize each other to regulate the balance between germinal center B cells and plasma cells (92). Evidence suggests that this set of transcription factors acts similarly as a genetic switch in the regulation of effector and memory T-cell fate decisions. In the absence of Blimp-1, BCL-6 expression is increased, and this probably contributes the phenotypes of Blimp-1-deficient CD8+ T cells because BCL-6-overexpression in CD8+ T cells also recapitulates several of the phenotypes exhibited by Blimp-1-deficient cells such as increased formation memory CD8+ T cells (preferentially TCM cells) and increased proliferative responses to secondary immunization and homeostatic cytokines (99–101). Conversely, a BCL-6-deficiency in CD8+ T cells causes several of the opposite phenotypes (99–101). Additionally, BCL-6b, a homolog of BCL-6, is also important for generating memory CD8+ T cells that can respond robustly to secondary infection (102).
Inhibitor of DNA binding 2 (Id2) is an inhibitor of the E protein family of transcription factors, and this family of proteins regulates many aspects of lymphocyte development and maintenance (103). Recently, a role for Id2 in memory CD8+ T-cell generation in the murine model of Listeria infection has been described (104). Id2 mRNA is upregulated in antigen-specific CD8+ T cells at the peak of expansion and persists into the memory phase. Id2-deficient CD8+ T cells initially expanded well but then started to die off as a result of increased expression of several pro-apoptotic molecules. Consequently, this led to significantly smaller numbers of effector and memory CD8+ T cells after infection, but, interestingly, the frequency of CD62LhiCD8+ T cells was increased considerably in the absence of Id2 (104).
Given memory CD8+ T cells resemble hematopoietic stem cell (HSC) in some ways, such as their ability to maintain a multipotent state and self-renew themselves, some transcription factors involved in stem cell longevity and maintenance have been proposed to be involved in memory T-cell development. Indeed, comparison of genome-wide transcription profiles between HSCs and memory T and B cells identified overlap in a small set of genes, suggesting, to some degree, the possibility of a shared genetic program between these different long-lived cell types (105).
Bmi-1, a member of the polycomb repressive complex, is rapidly expressed upon TCR ligation in primary CD8+ T cells and enhances their proliferative responses. Knockdown of Bmi-1 by RNAi impaired CD8+ T-cell proliferation and effector cytotoxicity, and conversely, Bmi-1 overexpression increased their proliferative responses to homeostatic cytokines. Interestingly, upon restimulation, Bmi-1 is induced in KLRG1loIL-7Rhi memory precursor cells but not in KLRG1hiIL-7Rlo effector cells, suggesting that Bmi-1 is important for the long-term maintenance and protection of memory CD8+ T cells (106). Another study has shown Wnt3a or inhibitors of GSK3β-induced Wnt signaling ceased activated CD8+ T-cell differentiation and promoted the formation of a subset of CD44loCD62hiSca-1hi self-renewing multipotent CD8+ T cells that have been referred to as memory stem cells (107). This Wnt-like signaling is associated with increases in Tcf7 and Lef1 expression, and both of these transcription factors are found in T cells with the increased potential to form memory cells in vivo (107). In the future, as we elucidate the transcriptional networks that regulate effector and memory CD8+ T-cell development, it will be interesting to delve deeper into the potential overlap in genetic programs between stem cells and memory T cells as well as to identify how these transcriptional pathways intersect and cross-regulate each other to ultimately determine the differentiation state and cell fate of effector T cells.
Metabolic switch during effector-to-memory transition
After explosive proliferation, CD8+ effector T cells halt their division and gradually enter a resting quiescent stage, whereby they periodically turnover or homeostatically proliferate (4, 6). Between these two distinct phases, pathogen-specific CD8+ T cells undergo a dramatic metabolic switch. In resting cells (including naive T cells), cellular homeostasis is maintained largely by chemical energy through oxidative phosphorylation in mitochondria (108). Upon activation, effector CD8+ T cells increase glucose uptake and switch to anabolic metabolism, which is characterized by heightened mammalian target of rapamycin (mTOR) activity and glycolysis (108). Presumably, this anabolic state is necessary for activated CD8+ T cells to undergo robust proliferation and increase protein and membrane synthesis during viral infection because perturbation of mTOR function, such as with a high dose rapamycin treatment, greatly blunts effector cell expansion (109). However, after pathogen clearance, the effector CD8+ T cells appear to ‘reset’ back to a catabolic state to survive mitogen and growth factor withdrawal (110).
Recent studies have shed some light on how these two metabolic states are regulated in CD8+ T cells during and after infection. One study showed that inhibiting mTOR activity by rapamycin treatment enhances the magnitude, quality, and the rate of memory formation during LCMV infection (109). Likewise, another report suggested that the activation of an inhibitor of mTOR, 5′-adenosine monophosphate-activated protein kinase (AMPK), which can be induced by cellular stress and adenosine triphosphate (ATP) deprivation, promotes the transition from an anabolic effector cell to a catabolic memory cell via induction of fatty acid oxidation (FAO). This model was supported by experiments examining TNF receptor-associated factor 6 (TRAF6)-deficient CD8+ T cells, which were defective in FAO and generated very few memory CD8+ T cells after Listeria infection. Metformin, an anti-diabetic drug and activator of AMPK, can partially bypass the need of TRAF-6 and restore memory cell generation in TRAF6-deficient CD8+ T cells (110). Collectively, these results suggest that two opposing metabolic pathways – PI3K/Akt/mTOR-mediated cellular growth and AMPK/FAO-mediated cellular homeostasis – have to be tightly controlled to ensure the proper effector differentiation and memory development (Fig. 1).
Although the precise molecular mechanisms of how these metabolic pathways control cell fate decisions remain unclear, one could postulate that mTOR kinase may modulate specification of effector or memory cell fates via gene expression. Consistent with this notion, earlier studies have found that mTOR is required for Th1, Th2, and Th17 CD4+ effector T-cell development, whereas it represses Treg development. Specifically, mTOR increases the activity of STATs and the expression of their downstream lineage-determining transcription factors (such as T-bet, GATA3, and RORγt). mTOR also suppresses Foxp3 expression in Tregs (111). A recent study further extended this paradigm to CD8+ T cells by showing that IL-12/STAT4-mediated mTOR activity sustains T-bet and simultaneously suppresses Eomes expression. Therefore, mTOR integrates environmental cues and acts as a rheostat to control T-cell fate decisions via modulation of T-bet and Eomes expression (112). Given that increased T-bet expression drives formation of short-lived, terminally differentiated KLRG1hiIL-7Rlo effector CD8+ T cells during viral infection, this finding helps to explain why inhibition of mTOR via rapamycin or metformin promotes memory cell formation in the above studies. Thus, these drugs offer promising therapeutic approaches for future vaccine development against infection and tumors.
CD4+ T-cell help and the role of IL-2
While it is well established that CD8+ memory T-cell development requires CD4+ T-cell help, the type(s) of CD4+ T cells that provide help and the precise signals involved remain elusive. Earlier studies using non-infectious immunization models suggested that CD4+ T cells can help antigen-presenting DCs via CD40L–CD40 ligation, which conditions the DCs to effectively stimulate naive CD8+ T-cell activation (113–115). Subsequent studies showed that CD40L-mediated DC ‘licensing’ of CD8+ T-cell effector responses by CD4+ T cells can be bypassed during several types of infections. However, these studies also showed that CD4+ T-cell help is indispensable for memory CD8+ T-cell development, function, and long-term maintenance following several acute infections (116, 117). Although the defects in memory CD8+ T cells vary between the different models of infection, typically fewer memory CD8+ T cells are maintained, and those that form tend to persist in a more effector-like state, with elevated expression of KLRG1, reduced IL-7R, CD27, IFN-γ, and IL-2 expression. They also have reduced proliferative responses to homeostatic cytokines and secondary infection and are devoid of TCM cells (116–118). In the case of LCMV infection, the virus is initially controlled in the absence of CD4+ T cells, but later, the virus recrudesces, indicating that CD4+ T cells (and probably the antibody responses) are critical to complete viral clearance in certain infections. This probably contributes to the defects in memory CD8+ T cells in these situations (119).
A few mechanisms have been implicated in the impaired memory CD8+ T-cell responses seen in the absence of CD4+ T cells. One is the heightened expression of the death receptor TNF-related apoptosis-inducing ligand (TRAIL) found after re-stimulation of ‘unhelped’ memory CD8+ T cells that leads to increased death and consequently decreased expansion of the secondary effector CD8+ T cells (120, 121). However, increased TRAIL expression alone does not account for all the defects in unhelped memory CD8+ T cells because mice doubly deficient in TRAIL and CD4+ T cells displayed only modest effects on memory T-cell erosion and secondary recall responses after Listeria and LCMV infection (122, 123).
Another critical signal for ‘helping’ memory CD8+ T cells is IL-2 (4, 124, 125). Despite seemingly normal primary CD8+ T-cell expansion, effector differentiation, and memory cell formation, antigen-specific TCR transgenic CD8+ T cells lacking the high-affinity IL-2 receptor α chain (CD25) were defective in mounting robust secondary proliferative responses (125). More interestingly, the functionality of CD25-deficient memory CD8+ T cells could be restored when exogenous IL-2 was administered at the time of immunization, indicating that early IL-2 signals during the primary phase of CD8+ T-cell activation and expansion were needed later for optimal memory CD8+ T-cell function (125).
Recent studies extended these findings by showing that the ‘amount’ of IL-2 signaling present early during CD8+ T-cell responses mattered; that is, intense or prolonged IL-2 signaling promoted effector cell potential (i.e. increased terminal effector CD8+ T-cell differentiation) and decreased memory cell potential (20, 75, 76). During LCMV, vesicular stomatitis virus, and vaccinia virus infection, CD25 expression tracks tightly with IL-2 production, and interestingly, high amounts of surface CD25 distinguishes effector CD8+ T cells that are competent to terminally differentiate into shorter lived KLRG1hi effector cells (20, 75, 76). In the absence of CD25, the CD8+ T cells had slightly blunted contraction, and fewer KLRG1hiIL-7Rlo effector cells formed (20). Additionally, CD25-deficient CD8+ T cells have reduced cytolytic activity due to the absence of IL-2-induced Eomes/STAT5-dependent transcription of perforin (Prf1) (76). These findings suggest that higher amounts of IL-2 signaling promotes the formation of terminally differentiated, short-lived CTLs, whereas lower amounts of IL-2 signaling are needed to instill a high proliferative capacity in the resulting memory CD8+ T cells. Akin to the model of IL-12 signaling in effector CD8+ T-cell development (16), these findings suggest that CD8+ T-cell differentiation is largely dictated by the strength of inflammatory signals present during infection.
In addition to these early forms of CD4+ T-cell help that act during the primary stage of a CD8+ T-cell response, other studies demonstrate that long-term memory CD8+ T-cell maintenance may also be reliant on ‘tonic’ signals from CD4+ T cells. In these experiments, ‘helped’ memory CD8+ T cells (i.e. those generated in wildtype mice) were transferred into CD4-deficient hosts, and their numbers gradually decayed (126). The nature of the signal(s) lacking in the absence of CD4+ T cells remains to be characterized, but it appears to be distinct from IL-2, as the maintenance of CD25−/− memory CD8+ T cells was relatively unaffected after LCMV infection (75). Thus, there appear to be many forms of CD4+ T-cell help that can impact effector and memory CD8+ T-cell differentiation and function. To aide in this investigation, it will be important to better understand when and where these two cell types ‘talk’ to each other during infection and which CD4+ T-cell subsets carry out the different forms of help. Ultimately, how CD4 T cell help affects the biochemical, metabolic, and genetic make-up of the CD8+ T cells will be necessary to dissect.
Memory T-cell conversion and maintenance
To reattain homeostasis and prevent immunopathology and autoimmunity, many effector CD8+ T cells die following pathogen clearance (47). However, a small portion of effector cells survive this effector-to-memory transition and convert into functional memory cells that persist for a long term through cytokine-dependent self-renewal. Here, we discuss some factors that regulate memory T-cell conversion and their homeostasis.
Apoptosis versus survival during contraction phase
Apoptosis or programmed cell death can occur through two major pathways: the death receptor pathway (an extrinsic pathway) and the mitochondrial pathway (an intrinsic pathway) (127, 128). Typically, the death receptor pathway initiates via interaction of TNFR family members with their extracellular ligands such as TNF-α, Fas ligand, and TRAIL (127–129). The mitochondrial pathway is initiated by disruption of the mitochondrial membrane from cellular stress, which leads to the release of cytochrome c into cytoplasm and the formation of apoptosome. Both pathways end with the downstream ‘effector’ caspase-3 and -7 activation that results in apoptosis (127, 130).
Recent findings suggest that both pathways are involved in effector T-cell death during the contraction phase. Following acute infection, viral specific CD8+ T-cell contraction is profoundly attenuated in TNFRI and TNFRII double-deficient mice due to their overlapping function (131). Fas, however, does not appear to play a critically important role in acute effector CD8+ T-cell contraction on its own (132), although it seems to account for the reduced numbers of antigen-specific T cells during chronic infections or autoimmune settings in the presence of persistent antigen (133–135). The Bim-induced intrinsic pathway has been found to play a central role in regulating CD8+ T-cell contraction, whereby Bim-deficient effector CD8+ T cells have prolonged survival after pathogen removal (134–138) (Fig. 1). However, in the context of Bim deficiency, the function of Fas in activated CD8+ T-cell death can be seen following acute infection because a double deficiency in Bim and Fas increases memory T-cell formation more than a Bim deficiency alone (134, 135). Despite an incomplete understanding of the molecular mechanism, Bim activity is probably controlled by the relative level of anti-apoptotic molecules Bcl-2, Bcl-XL, A1, and MCL-1 (139–141). Both Bcl-2 and MCL-1 have been shown to be required for sufficient memory T-cell survival (142, 143) (Fig. 1). Although Bim-deficient T cells do not contract initially at the same rate, they do gradually decay and reach the similar numbers as wildtype memory T cells (132, 134, 135, 137). This observation suggests that there are alternative signals in limitation that ultimately govern the number of memory T cells that can persist.
Common γ chain cytokines IL-7, IL-15, and IL-21 in memory maintenance
IL-7 and IL-15 are critical cytokines that regulate the transition from effector-to-memory CD8+ T cells, as both of these cytokines are critical to the survival and homeostasis of memory T cells (6, 14, 47). In general, it is thought that IL-7 primarily supports survival, whereas IL-15 induces basal level of homeostatic turnover, but both factors cooperate to support both cell processes (14, 19, 21, 47, 144–146). For instance, antigen-specific CD8+ T cells require IL-7R to form normal numbers of memory CD8+ T cells, and in the absence of both IL-15 and IL-7, essentially no memory CD8+ T cells form after infection. Likewise, if IL-7 or IL-15 levels are elevated via transgenic overexpression or administration of exogenous cytokines, greater numbers of memory CD8+ T cells persist under these conditions (14, 21, 27, 47, 147–150).
As described above, the expression of IL-7R is dynamically regulated in the antigen-specific CD8+ T cells during an infection. Naive CD8+ T cells express IL-7R, but IL-7R expression precipitously falls on activated CD8+ T cells as they expand and differentiate during infection (21, 23, 29, 151). At the peak of expansion, most effector CD8+ T cells are IL-7Rlo; however, a smaller subset of IL-7Rhi cells exist that express greater amounts of Bcl-2 and serine protease inhibitor 2A (Spi2a) and preferentially survive and generate long-lived memory CD8+ T cells that self-renew (21, 23, 27, 29, 146, 152, 153).
Given the IL-7Rα expression pattern on effector CD8+ T cells, it was initially predicted that IL-7R repression was the underlying cause for effector CD8+ T-cell death and contraction following infection. However, this model proved to be incorrect because enforced IL-7R expression, via an IL-7R transgene, on all virus-specific CD8+ T cells did not save the terminally differentiated KLRG1hi effector CD8+ T cells that are naturally IL-7Rlo (27, 154). These results indicated that IL-7R repression is not causal to effector cell death and that expression and IL-7 signaling is not an instructive signal for memory T-cell formation (27, 47, 154).
The essential role of IL-15 in memory T-cell maintenance and homeostasis was first identified because it could induce memory T-cell proliferation in vitro (155, 156) and fewer polyclonal memory CD8+ T cells were found in IL-15−/− and IL-15rα−/− mice (157, 158). Subsequently, the effects of IL-15 on effector and memory CD8+ T-cell development during acute viral and bacterial infections were examined and these studies showed that IL-15 was needed for memory CD8+ T-cell homeostasis and drove the basal turnover of the memory CD8+ T cells. In the absence of IL-15, the antigen-specific memory CD8+ T-cell population slowly eroded over time. Additionally, in some infections, but not all, IL-15 is needed for maximal effector cell expansion and this may be due in part to necessity of IL-15 for the survival of KLRG1hiIL-7Rlo effector CD8+ T cells (16, 19, 159, 160).
IL-15 is induced by type I IFNs, and macrophages and DCs are the primary producers and presenters of IL-15 to CD8+ T cells (14, 159, 161). Interestingly, a recent paper elegantly illustrated that the cell type most important for presenting IL-15 to memory CD8+ T cells varied according to the type of memory T cell (TCM versus TEM) and the tissue the memory CD8+ T cells resided in. For instance, DC-presented IL-15 selectively supported TCM, whereas macrophage-presented IL-15 supported both TCM and TEM cells and was the most important cell type to maintain memory CD8+ T cells in the bone marrow (162).
IL-21, the most recently identified common γ chain cytokine, is closely related to IL-2 and is mainly produced by CD4+ T cells and NKT cells (163, 164). IL-21 is an important inducer of Blimp-1 and BCL-6, and it regulates B-cell differentiation into plasma cells, T-follicular helper function, and Th17 development (164–170). IL-21 can also synergize with IL-2 and IL-15 to promote cytotoxic CD8+ T-cell differentiation (163, 164). For instance, combinations of IL-15 and IL-21 augment effector CD8+ T-cell proliferation, survival, and cytotoxic activity. Microarray analyses further revealed that this was in part through the upregulation of granzyme B and c-Jun (171). In line with this finding, IL-21r−/− mice have reduced effector CD8+ T-cell expansion and cytotoxicity after immunization (171, 172). For these reasons, it was surprising that a recent study also found that IL-21 suppresses the antigen-induced expression of CD44, IFN-γ, granzyme B, and Eomes. These IL-21-treated effector cells rendered better anti-tumor immunity when adoptively transferred into tumor-bearing mice (172). IL-21 can also synergize with IL-7 and IL-15 to induce memory CD8+ T-cell homeostatic proliferation, but IL-21 has little effect on this process alone (171). The role of IL-21 in CD8+ T-cell expansion, differentiation, and memory formation during acute LCMV infection is largely dispensable, but during chronic LCMV infection, it is critical to sustain the function of virus-specific CTLs (173–175).
Memory T-cell subsets
Memory T-cell populations are heterogeneous and consist of multiple subsets that vary in their homing characteristics, effector function, and proliferative functions. Currently, two subsets of memory T cells, TCM and TEM, have been best characterized. TCM cells express a high level of CD62L and CCR7 and efficiently home to lymph nodes, whereas effector memory cells lack the expression of these molecules and reside mainly in non-lymphoid peripheral tissues. However, these differences in localization are not absolute, and both subsets can be found to some degree in the different tissues (176–178). In addition, when circulating memory cells enter skin, liver, lung, and intestinal mucosa sites, they can upregulate ‘tissue-specific’ markers, acquire effector function, migratory, and protective abilities upon encountering distinct environmental cues (179–183). Furthermore, tissue-resident TEM cells are thought to provide immediate effector function at the portal of pathogen entry because they express perforin and granzyme B and can kill direct ex vivo, but, typically, they exhibit reduced proliferative capacity (176, 177). TCM cells undergo homeostatic turnover, mount robust proliferative responses, and rapidly elaborate effector molecules upon secondary stimulation (36, 184–186). Together, it is presumed that the generation of these two functionally distinct memory subsets increases the host’s defensive capacities. Although this central paradigm of memory subset categorization is useful, it should by no means be considered absolute. It becomes increasingly evident that heterogeneity exists within these subsets themselves, and surface markers such as CD62L and CCR7 are not coordinately regulated in all cell types.
The identification of these two memory subsets led to studies examining how they develop and are maintained during immune responses. Several models have been proposed in the past, but whether these two memory subsets are completely distinct lineages or inter-convertible remains a matter for debate. Some studies suggested that these two subsets represent fixed populations that are ‘separated at the birth’ and that they do not convert over time (187–189). Early study in comparison of human blood CD8+ TCM and TEM cells support this notion, although TCR repertoire analysis revealed some overlap between these lineages, as much as one-third in mouse splenic memory CD8+ T cells (190, 191). By contrast, other studies proposed that TEM can convert to TCM in the absence of antigen (35, 36, 186, 191, 192). Lastly, recent observations have suggested that TCM might be able to convert to TEM upon their arrival to non-lymphoid tissues (193, 194). Adoptively transferred resting TCM cells modulate their cytolytic functions by induction of granzyme B and also lose their expression of CD27 when they enter into the liver and lung (193, 194).
Based on collective work, we recently proposed that there exist at least three distinct populations of memory CD8+ T cells early after an acute viral infection (6). First, there is a subset of TEM cells that descend from IL-7Rhi MPECs. Some cells within this subset may remain as TEM, but others possess the ability to mature into TCM cells and represent the convertible or transitional TEM (TTM) (35, 36, 186, 189). Second, there is a population of TCM cells that form either early during infection or derive from aforementioned TTM cells. These memory cells obtain the ability to efficiently self-renew and homeostatically turn over (36). Third, there remains a population of end-stage effector cells that enter the memory phase. They descend from terminally differentiated effector cells that can be marked as IL-7RloKLRG1hiCD62Llo. These resting terminal effector cells display finite lifespan and do not efficiently convert to TCM cells, self-renew, or persist for a long term (16) (Fig. 1). The separation of end-stage TEM from transitional TEM cells might help to explain some apparently disparate results in the field. For instance, the overall reduction in signal strength and interclonal competition during T-cell activation by increasing naive T-cell precursor frequency favors TTM cell development (35). Furthermore, differences in the tropism and proportion of TCM, TTM, and end-stage TEM at the time of secondary challenge may account for the differences in protective immunity against a variety of pathogens (34, 36, 195–197). Recent studies have identified additional useful markers to further dissect different recall abilities among subsets of memory cells (22), and such approaches will broaden our view of memory cell heterogeneity, lineage relationship, and function in response to various infections.
Concluding remarks
A better understanding of the signals and genetic pathways that govern memory CD8+ T-cell differentiation and long-term maintenance is essential to aid both prophylactic and therapeutic vaccine designs. Detailed dissection of inflammatory cytokines that modulate effector and memory differentiation will help to define specific cytokine adjuvants that preferentially promote robust memory formation and prevent effector T cells from senescent terminal differentiation. Further deciphering of how innate signals and tissue environmental cues regulate the differentiation states and longevity of memory T cells is critical to maximizing protective immunity against various pathogens. Furthermore, the availability of innovative techniques such as intravital imaging, genome-wide transcriptional profiling, and signal cell-level analyses should provide more opportunities for our further understanding of memory T-cell differentiation.
References
- 1.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, et al. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 3.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 6.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 8.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Germain RN, et al. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 10.Henrickson SE, et al. In vivo imaging of T cell priming. Sci Signal. 2008;1:pt2. doi: 10.1126/stke.112pt2. [DOI] [PubMed] [Google Scholar]
- 11.Henrickson SE, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau LL, et al. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 13.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 14.Surh CD, et al. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 15.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 16.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stemberger C, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obar JJ, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 22.Hikono H, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns KS, et al. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 24.Akondy RS, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibegbu CC, et al. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 26.Plunkett FJ, et al. The loss of telomerase activity in highly differentiated CD8+ CD28−CD27− T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 27.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci USA. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 29.Huster KM, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keppler SJ, et al. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 32.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 33.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann MF, et al. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 35.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 36.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 37.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masopust D, et al. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 41.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 43.Wherry EJ, et al. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 44.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 45.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 46.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 47.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 48.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 49.Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Curr Opin Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mousavi SF, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 52.Hendriks J, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 53.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 54.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Sabbagh L, et al. A critical role for TNF receptor-associated factor 1 and Bim down-regulation in CD8 memory T cell survival. Proc Natl Acad Sci USA. 2006;103:18703–18708. doi: 10.1073/pnas.0602919103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 57.Lee SJ, et al. CD134 costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 58.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 60.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtsinger JM, et al. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 62.Kolumam GA, et al. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aichele P, et al. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 64.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol. 2005;174:600–604. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 65.Palmer EM, et al. IL-12 decreases activation-induced cell death in human naive Th cells costimulated by intercellular adhesion molecule-1. I. IL-12 alters caspase processing and inhibits enzyme function. J Immunol. 2001;167:749–758. doi: 10.4049/jimmunol.167.2.749. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal P, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson LJ, et al. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 68.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 69.Badovinac VP, et al. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 70.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 71.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 72.Cui W, et al. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takemoto N, et al. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 74.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 75.Kalia V, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8(+) T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 78.Haring JS, et al. In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-gamma receptor. J Immunol. 2005;175:3117–3122. doi: 10.4049/jimmunol.175.5.3117. [DOI] [PubMed] [Google Scholar]
- 79.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 80.Sercan O, et al. Innate immune cells contribute to the IFN-gamma-dependent regulation of antigen-specific CD8+ T cell homeostasis. J Immunol. 2006;176:735–739. doi: 10.4049/jimmunol.176.2.735. [DOI] [PubMed] [Google Scholar]
- 81.Pestka S, et al. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 82.Platanias LC. Introduction: interferon signals: what is classical and what is nonclassical? J Interferon Cytokine Res. 2005;25:732. doi: 10.1089/jir.2005.25.732. [DOI] [PubMed] [Google Scholar]
- 83.Sarkar S, et al. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 84.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Letimier FA, et al. Chromatin remodeling by the SWI/SNF-like BAF complex and STAT4 activation synergistically induce IL-12Rbeta2 expression during human Th1 cell differentiation. EMBO J. 2007;26:1292–1302. doi: 10.1038/sj.emboj.7601586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 87.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 89.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 90.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tunyaplin C, et al. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 92.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 94.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 95.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 96.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 97.Kallies A, et al. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 98.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ichii H, et al. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int Immunol. 2007;19:427–433. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- 100.Ichii H, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 101.Ichii H, et al. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 102.Manders PM, et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cannarile MA, et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 105.Luckey CJ, et al. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rao RR, et al. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 114.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 115.Schoenberger SP, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 116.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 117.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Northrop JK, Wells AD, Shen H. Cutting edge: chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. J Immunol. 2008;181:865–868. doi: 10.4049/jimmunol.181.2.865. [DOI] [PubMed] [Google Scholar]
- 119.Thomsen AR, et al. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J Immunol. 1998;161:4583–4590. [PubMed] [Google Scholar]
- 120.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 121.Hamilton SE, et al. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 122.Badovinac VP, et al. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 123.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bachmann MF, et al. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 125.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 128.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 129.Lakhani SA, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lakhani S, Flavell RA. Caspases and T lymphocytes: a flip of the coin? Immunol Rev. 2003;193:22–30. doi: 10.1034/j.1600-065x.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 131.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pellegrini M, et al. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou S, et al. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hughes PD, et al. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weant AE, et al. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 136.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 137.Wojciechowski S, et al. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 139.Willis SN, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 140.Zhu Y, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu X, et al. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 142.Wojciechowski S, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 144.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 145.Goldrath AW, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Osborne LC, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buentke E, et al. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;106:1949–1956. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]
- 148.Kieper WC, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nanjappa SG, et al. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest. 2008;118:1027–1039. doi: 10.1172/JCI32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chandele A, et al. Formation of IL-7Ralpha-high and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–3088. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 153.Liu N, et al. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5:919–926. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- 154.Haring JS, et al. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 155.Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood. 1996;88:230–235. [PubMed] [Google Scholar]
- 156.Kanai T, et al. IL-15 stimulates the expansion of AIDS virus-specific CTL. J Immunol. 1996;157:3681–3687. [PubMed] [Google Scholar]
- 157.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 158.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 160.Yajima T, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 161.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 162.Mortier E, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 163.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 164.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 165.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 166.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 167.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ozaki K, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 169.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 170.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 176.Masopust D, et al. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 177.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 178.Reinhardt RL, et al. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 179.Masopust D, et al. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 180.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 181.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 185.Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–2468. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- 186.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sallusto F, Lanzavecchia A. Exploring pathways for memory T cell generation. J Clin Invest. 2001;108:805–806. doi: 10.1172/JCI14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 189.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baron V, et al. The repertoires of circulating human CD8(+) central and effector memory T cell subsets are largely distinct. Immunity. 2003;18:193–204. doi: 10.1016/s1074-7613(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 191.Bouneaud C, et al. Lineage relationships, homeostasis, and recall capacities of central-and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zaph C, et al. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 195.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 197.Huster KM, et al. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]