Abstract
In the late 1960’s, only types A and B hepatitis were believed to exist, distinguished by circumstances of exposure and incubation periods. In the early 1970’s, studies of transfusion recipients were begun with the belief that hepatitis B would be responsible should transfusion-associated hepatitis develop. After discovery of the viruses of hepatitis A and B, neither agent was found responsible, hence non-A, non-B (NANB) hepatitis. Initial follow-up of these cases showed that ~ 50% developed chronic hepatitis based on persistence of serum enzymes for at least 6 months. Approximately 15 years later, after the hepatitis C virus had been identified as the cause for NANB hepatitis, chronic hepatitis was found to develop more frequently as indicated by persistent viral infection in over 80% of infected adults but in only about 50% of infected children or young women. Follow-up over 2 to 4 decades indicated that many infected persons developed progressive hepatic fibrosis, sometimes culminating in cirrhosis and/or liver cancer. Long-term natural history studies have proved to be challenging because disease onset is often silent and progression extremely slow. Differing strategies have been used to determine the natural history, the descriptions and results of which are presented in this review.
Keywords: cirrhosis, hepatitis C, natural history, prospective study, retrospective-prospective study, retrospective study
Viral hepatitis was originally believed to consist of two types only – infectious or type A hepatitis and serum or type B hepatitis (1). Cases were recognized through typical symptoms or jaundice, although there was already knowledge of the existence of anicteric hepatitis in the early 1960s (2). In the absence of specific diagnostic assays, the two types were distinguished by the circumstances of exposure and by the incubation periods – the faecal–oral route and a short incubation for hepatitis A, and percutaneous blood exposure and a long incubation period for hepatitis B. Thus, when epidemiological and immunoglobulin prophylaxis studies of transfusion-related hepatitis were initiated in the late 1960s and early 1970s (3–5), the belief was that if hepatitis should develop, it would definitely be hepatitis B.
The designs of these early transfusion studies were, in fact, unwittingly the source for the future discovery of hepatitis C. Firstly, study participants were monitored frequently with blood tests for the detection of biochemical abnormalities, especially the aminotransferases, generally bimonthly for 6 to 12 or more months. Secondly, the diagnosis of ‘hepatitis’ was based not on symptoms or jaundice but on predefined patterns of serum aminotransferase elevations, thus expanding the disease spectrum. Thirdly, blood samples obtained for monitoring purposes were all stored in freezers for future research purposes. The transfusion studies identified an ‘acute hepatitis’ that straddled the incubation periods of hepatitis A and B, most of which lacked the typical hepatitis-like symptoms or jaundice. Interestingly, in early 1974, the illness was briefly referred to as hepatitis C (6). Contemporaneous with these transfusion studies was the identification first of the hepatitis B virus (7) and then later the hepatitis A (8) virus, and the subsequent development of specific serological assays for the two viruses. Testing of stored samples was then begun anticipating that transfusion-associated hepatitis and hepatitis B would be found to be synonymous. Surprisingly, however, the samples were non-reactive first to the tests for hepatitis B and later, after the discovery of the agent, also non-reactive to the tests for hepatitis A, leading to the term, non-A, non-B (NANB) hepatitis (3). Initially, some considered this entity a non-specific hepatic reaction, but later, inoculation of chimpanzees with stored sera from the transfusion studies demonstrated the development of similar biochemical abnormalities in these animals, indicating that NANB hepatitis was indeed the result of a transmissible agent (9, 10). Proof that the hepatitis C virus (HCV) was responsible followed a decade and a half later, when the actual virus was identified and specific serological tests were developed (11, 12). Testing stored sera from both the blood donors and their recipients then confirmed that NANB hepatitis and HCV were one and the same (13).
Acute transfusion-associated hepatitis
The acute hepatitis cases identified in the transfusion studies were mostly unaccompanied by symptoms and were characterized by mild to moderately raised serum enzymes and generally normal serum bilirubin levels. Accordingly, the condition was initially regarded as a relatively inconsequential problem. However, continued serum enzyme monitoring revealed that, in approximately 50% of them, elevated serum enzyme values persisted beyond 6 months, fulfilling the definition of chronic hepatitis (3, 4). Nevertheless, concern remained somewhat muted because early biopsies revealed only mild chronic hepatitis. However, when repeat liver biopsies showed the presence in some instances of increasing necroinflammation, accompanied by advancing fibrosis and even cirrhosis, apprehension about the disease increased. Also of concern at that time were the occasional reports of hepatocellular carcinoma (HCC) identified in persons whose only history was an earlier bout of apparent transfusion-associated NANB hepatitis (14, 15). Thus, even before the recognition of HCV, treatment trials with interferons were initiated (16).
The discovery of HCV and the resultant serological and virological assays yielded additional information that intensified the concerns. While acute hepatitis was believed to advance to chronic hepatitis based on continuing enzyme elevations in about 50% of those with NANB hepatitis, HCV RNA was found to persist beyond 6 months in a far greater proportion, i.e. 75–85% of cases. Furthermore, reports of the detection of HCV in individuals with so-called cryptogenic cirrhosis and with HCC were emerging (17).
Other investigations were simultaneously discovering that NANB/C hepatitis was not confined to the transfusion setting. At first referred to as sporadic or community-acquired hepatitis C (18), the virus was identified under other circumstances of percutaneous exposure, such as among illicit drug users, healthcare workers exposed through a needlestick, recipients of such biologicals as clotting factor concentrates and later Rh immunoglobulin, newborns of infected mothers, and possibly through sexual contact (18–20). Like transfusion-associated hepatitis C, persons infected under these circumstances developed few or no symptoms, and yet many also advanced to chronic hepatitis C, albeit at differing rates (19–22).
Collectively, these data indicated that more information was needed about the long-term sequelae of chronic hepatitis C. But how was this to be accomplished when onset of the acute illness was mostly unrecognized because of the absence or paucity of clinical symptoms; available data indicated that progression from acute to chronic hepatic C occurred unobtrusively, and the rate of evolution was determined to be exceedingly slow, occurring over periods of two to four decades. Moreover, because antiviral therapy was being increasingly utilized, would it be possible to conduct a true ‘natural history’ study?
Items requiring consideration for evaluating the natural history
As knowledge of hepatitis C began to grow, it became clear that in order to better understand the process of evolution of hepatitis C, consideration must be directed at the very least towards the following four questions:
What is the rate of spontaneous resolution of acute hepatitis C that brings the disease to an end, thus confining progression only to those in whom the virus persists?
Among those who do not spontaneously recover, what outcomes can be anticipated, with what frequency and over what duration of time?
What are the variables and risk factors that help predict that progression of chronic hepatitis is likely to occur?
What is the pathogenesis of disease persistence and progression?
Frequency of spontaneous virological resolution
Early studies found that spontaneous virological resolution occurred in about 15–25% of persons who developed transfusion-associated acute hepatitis C, and, conversely, that HCV infection persisted in 75–85% of infected persons (23, 24). Similar results were reported in later studies of diverse populations (HCV-infected blood donors, persons with ‘community-acquired’ infection, IV drug abusers and children with leukaemia), spontaneous resolution occurring in 14–29% of these cohorts (25–28). Later, a far higher rate of spontaneous resolution was noted among infected children (21, 29), young women (20, 22) and even some persons with community-acquired hepatitis C (30), the figures ranging between 42 and 45%. These data suggested that young age at the time of infection is an important determinant of the likelihood of spontaneous recovery.
Frequency and rates of serious outcomes
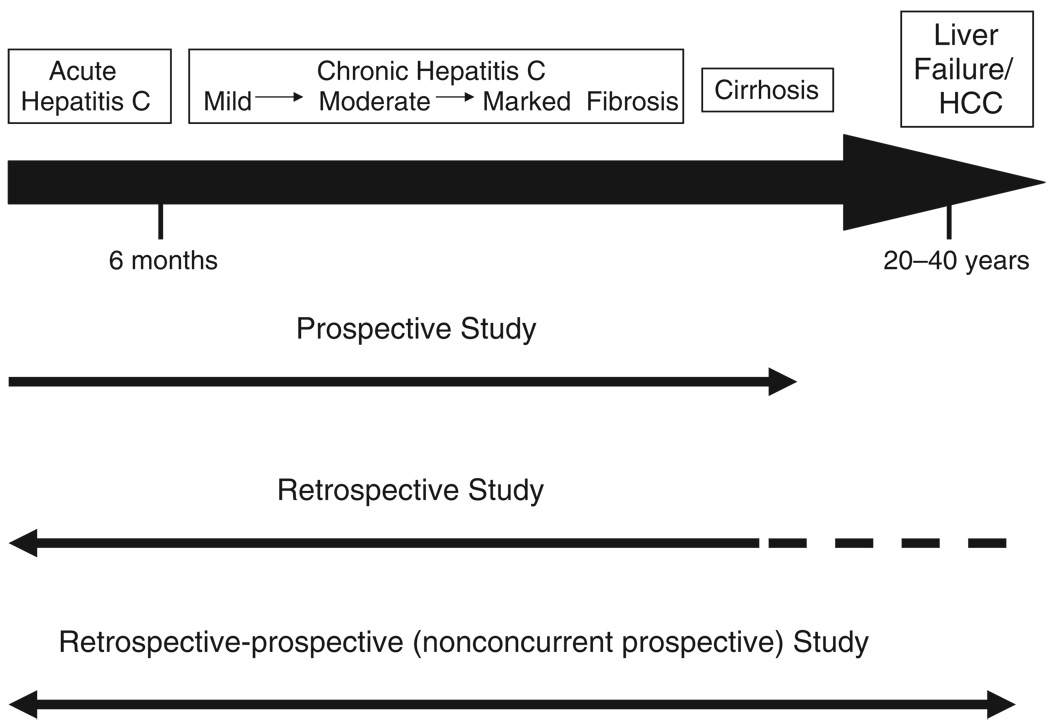
Infected persons whose acute HCV infection does not resolve are at risk for progressive liver disease, characterized by continuing hepatocellular inflammation and an increase in fibrosis from mild to advanced (bridging), followed by cirrhosis, first compensated then decompensated, and, finally, HCC. These developments occur over periods of two to four decades, however, so that investigators have used differing strategies to evaluate the frequency and rate of progression of the chronic liver disease (31) (Fig. 1).
Fig. 1.
Strategies used to establish the natural history of hepatitis C (31).
One strategy is to perform a prospective study beginning with persons who develop acute hepatitis C under observation. The advantages of this approach are that disease onset is precisely established (normal baseline serum enzymes and negative HCV RNA at transfusion, followed by appearance of HCV RNA and enzyme increases weeks to months later), and that all persons infected, whether or not they have symptoms, can be identified. This approach also permits recognition of spontaneous resolution. Furthermore, matched uninfected transfusion recipients can be selected as controls for a paired follow-up. The disadvantages are that the onset of acute hepatitis C is usually silent and therefore difficult to identify unless there is a recognized point-source outbreak, and that liver disease progression is exceedingly slow, so that decades of follow-up are required to establish a full outcome; moreover, antiviral treatment is likely to be instituted in the interim that will preclude a true ‘natural’ history study.
Another strategy is a retrospective study of a large cohort of persons with established chronic hepatitis C, tracing them back to their original exposure, presumed to be a first receipt of a transfusion, a needle-stick exposure or the first percutaneous use of illicit drugs. While offering the advantage of shortening the time needed in the study to determine the interval between ‘exposure’ and the current chronic hepatitis status, this approach suffers from the bias of focusing on living persons with HCV-related chronic liver disease without the ability to identify those who had spontaneously recovered, were too mild and asymptomatic to be recognized or conversely had already died from chronic liver disease. Moreover, the precise timing of disease onset cannot be accurately established. Retrospective studies thus tend to emphasize the more serious outcomes while de-emphasizing chronically infected individuals without overt liver disease progression, a so-called ascertainment bias.
The third strategy is the retrospective-prospective (non-concurrent prospective) study. This requires the serendipitous discovery of a distant well-characterized point-source outbreak of hepatitis C where data from all infected persons are recoverable, the ability to recall them to determine their current status and then to follow them prospectively. Provided data on all originally infected persons can be resurrected and the full cohort recalled, this strategy has the advantage of reducing the time needed to determine the outcome.
A review of many, although by no means all, studies that used these differing strategies follows:
First group of ‘natural history’ studies
Prospective studies
Reported between 1991 and 1993, four early studies all involved follow-up of originally diagnosed transfusion-related NANB hepatitis (32–35) (Table 1). At the time of reporting, early version serological tests for hepatitis C had become available. Reports of progression to cirrhosis ranged between 7 and 15.6%, three studies commented on the development of HCC (0, 0.7 and 1.3%) and liver-related deaths ranged between 1.3 and 3.7%. The frequencies of serious outcomes were moderately low, a result presumably of the relatively short durations of follow-up, ranging from 7.6 to 16 years.
Table 1.
Long-term sequelae of chronic hepatitis C: studies before 2001
| Type of study | Author | Group | No. of patients | Interval from exposure (years) |
Cirrhosis (%) |
|---|---|---|---|---|---|
| Prospective | Di Bisceglie (32) | PTH | 65 | 9.7 | 12.3 |
| Tremolada (33) | PTH | 135 | 7.6 | 15.6 | |
| Mattsson (34) | PTH | 61 | 13.0 | 8.0 | |
| Koretz (35) | PTH | 80 | 16.0 | 7.0 | |
| Retrospective | Kiyosawa (36) | PTH | 231 | 10–29 | 35.1 |
| Tong (37) | PTH | 131 | 14–28 | 51.0 | |
| Niederau (38) | Various | 838 | 9–22 | 16.8 | |
| Yano (39) | PTH | 70 | NR | 50.0 | |
| Gordon (40) | PTH | 215 | 19.0 | 55.0 | |
| Gordon (40) | IDU | 195 | 20.0 | 21.0 | |
| Retrospective/ prospective |
Kenny-Walsh (20) | Y.wom | 376 | 17.0 | 2.0 |
| Vogt (21) | Children | 458 | 19.8 | 0.3 | |
| Wiese (22) | Y.wom | 1980 | 25.0 | 0.4 | |
| Thomas (27) | IDU | 1667 | 13.7 | 1.1 | |
| Locasciulli (28) | Post-leuk | 56 | 17.0 | 0 | |
| Casiraghi (29) | PTH* | 31 | 35.0 | 3.2 | |
| Rodger (30) | IDU | 98 | 25.0 | 4.0 | |
| Seeff (41) | Com.acq. | 17 | 45–50 | 5.9 | |
| Seeff (42) | PTH | 222 | 23.0 | 17.0 |
Post-transfusion hepatitis in infants.
Com.acq, community acquired; IDU, injection drug use; NR, not reported; post-leuk, post-leukemia; PTH, post-transfusion hepatitis; Y.wom, Rh immunoglobulin in young women.
Retrospective studies
Five studies undertaken a little later (between 1990 and 1998) reported higher rates of serious outcome, consisting of cirrhosis in 17–55%, HCC in 2–23% and liver-related deaths in 3.7 and 15.3% in the two studies in which it was reported (36–40) (Table 1). The lengths of follow-up ranged between 9 and 29 years. In one study, persons infected via transfusion had higher rates of morbidity and mortality than those infected through IV drug abuse (40). In another study, serial histology revealed that chronic hepatitis was identified a mean of 10 years, cirrhosis a mean of 21.2 years and HCC a mean of 29 years after transfusion, highlighting the slow pace of progression, when it occurs (39). These were clearly more disturbing results but appear to have been accounted for, in part, by referral bias.
Retrospective-prospective studies
At least 10 such studies were reported between 1992 and 2001 (20–22, 26–30, 41, 42), three concerning transfusion-related hepatitis (21, 29, 41), two of which involved transfused infants (21, 29), two involved young women infected by contaminated Rh immunoglobulin (20, 22), one a follow-up of persons cured of childhood leukaemia (28), while four were studies of patients with community-acquired, some drug abuse-related, hepatitis (26, 27, 30, 42) (Table 1). The follow-up intervals in eight of the studies ranged between 9 and 35 years (20–22, 26–28, 30, 41), and in the ninth, it was 45 years (42). Cirrhosis was identified in 0.3–5.9% of cases in eight of the studies and in 15% of the ninth study, the only one in which hepatitis among adults was transfusion related (41). This same study was the only one in which HCC developed (1.9%), and was associated with the highest liver-related death rate (2.8%). No liver-related deaths occurred in six of the remaining eight, the rates in the other two being 2.1 and 1%. In this group of studies, the most striking findings were that infected children and young women had the highest rates of spontaneous resolution and the lowest rates of development of cirrhosis and HCC, as well as of death from liver disease.
Added to the original report of the Irish women infected by Rh immunoglobulin (20) are at least four further studies of their natural history (43–46). The durations of follow-up in these studies ranged between 17 and 27 years. In the 27-year follow-up study, repeat liver biopsies showed no change in fibrosis in 49%, fibrosis regression in 24%, fibrosis progression in 27% and incomplete and complete cirrhosis in only 2.1% (46). A similarly low rate of serious outcome was reported in a follow-up study of the German women who had received contaminated Rh immunoglobulin (21). Twenty-five years after the infection, only 46% were viraemic, one-half losing the virus spontaneously and 6% through antiviral treatment. Overt cirrhosis was identified in 0.5%, bridging fibrosis in 1.5%, and only one of the 1833 studied women had developed HCC. In a study of 31 persons infected 35 years earlier through minitransfusions at birth, serious outcomes were low, similar to that found in young women and in a prior study in children (29). Among 11 biopsies performed in 16 viraemic persons, nine had mild fibrosis, one had moderate fibrosis and only one had marked fibrosis. Repeat liver biopsy of five of them 5 years later revealed no progression in four, and mild progression in the fifth, none developing cirrhosis, HCC or death from liver disease. Thus, even after follow-up periods of 27–35 years, infected infants and young women have continued to show exceedingly low rates of liver disease progression.
Additional ‘natural history’ studies, 2001 and beyond
Two other retrospective studies of transfusion-related hepatitis C have since been reported with differing outcomes (Table 2). In a follow-up study from the UK, all-cause mortality was not significantly different after 16 years between infected and non-infected recipients (HR, 1.7) but liver-related mortality was (HR, 2.71) (47). Among the infected cases, liver-related mortality was 3.7%, thought to be contributed to by excess alcohol intake. In the other study of Austrian plasma donors infected a mean of 31 years earlier, 34% showed advanced fibrosis (F3/F4) or HCC on liver biopsy, 7.4% underwent liver transplantation (2/3 for hepatic decompensation and 1/3 for HCC) and 8.2% died, over one-half because of liver disease (48). Most patients were men, many with ‘unhealthy’ life styles, including alcoholism. The authors indicated that there was no significant mortality in the first 20 years but that both morbidity and mortality increased substantially thereafter.
Table 2.
Long-term sequelae of chronic hepatitis: studies post-2001
| Type of study | Author | Group | No. of patients | Interval from exposure (years) |
Cirrhosis (%) |
|---|---|---|---|---|---|
| Retrospective | Harris (47) | PTH | 924 | 16.0 | 2.4 |
| Ferenci (48) | Pl. donors | 485 | 31.0 | 34.0* | |
| Forns (49) | PTH | 116 | 24.0 | 39.0 | |
| Franchini (51) | B.dis | 102 | 15.0–34.0 | 6.9 | |
| Posthouwer (52) | B.dis | 120 | 21.0 | 5.0 | |
| Retrospective/Prospective | Mohan (50) | PTH | 60 | 13.0 | 1.6 |
Thirty-four per cent had advanced fibrosis or hepatocellular carcinoma.
B.dis, bleeding disorders; Pl. donors, plasma donors; PTH, post-transfusion hepatitis.
In another retrospective study of patients infected by various means approximately 20 years earlier, almost 40% had developed cirrhosis, 10.5% had developed hepatic decompensation, the frequency increasing over time, and 6% had died from liver disease (49) (Table 2). The authors concluded that the high rate of progressive liver disease could be accounted for by ascertainment bias and also by the fact that the original diagnoses had been made at ‘a significantly more advanced stage of the disease’.
In slight contrast to the earlier data among infected infants are the results from a more recent study involving children infected at birth or in early childhood, which indicate that HCV-related liver disease in children can progress fairly rapidly (Table 2). This retrospective-prospective study identified children infected at a mean age of 7.1 months through a transfusion look-back programme and through referrals to a children’s hospital (50). After a mean duration of infection of 13.4 years, liver biopsies in these mostly asymptomatic children revealed minimal to mild inflammation in 71%, absent or minimal fibrosis in 88% and bridging fibrosis in 12%. Repeat liver biopsies in four children 1–4 years later identified no change in three and cirrhosis in the fourth. It is noteworthy that children enrolled by referral had more severe liver disease than those enrolled through lookback, confirming the impact of selection bias.
Two studies of HCV-infected persons with inherited bleeding disorders reached differing conclusions (Table 2). In one, with a median follow-up of 25 years, 86% were still viraemic, 69% had non-progressive disease, 6.9% had developed cirrhosis, 4.5% had hepatic decompensation and 2.3% had HCC; 3.4% had died from liver disease (51). None were HIV co-infected. In the second study, reporting a 21-year follow-up, 35% had had spontaneous viral clearance and only 5% had developed cirrhosis, both of whom were also infected with HIV (52). Most of the mono-infected persons had normal biochemical indices and none had died.
Progression based on disease severity
Two groups that represent opposite ends of the disease spectrum progress at differing rates, namely those who are immune suppressed and those who are HCV infected but who have persistently normal aminotransferase levels.
Immunocompromised individuals
Hepatitis C virus-infected persons who are immunocompromised, either because of HIV co-infection (53–57) or through immunosuppressive treatments (58, 59), have a higher rate of progression to a more serious outcome than do those who are mono-infected. Relative to immunocompetent infected persons, immune suppressed persons have an approximately two-fold increase in the frequency and rate of development of cirrhosis.
Hepatitis C virus-infected persons with normal aminotransferase levels
Significant fibrosis is less common in persons with normal than with abnormal serum enzymes, but is reportedly present in 5–30% of them (60, 61). There has been some controversy as to whether persons with persistently normal enzymes do have potentially progressive liver disease. Studies of paired biopsies spaced 1.5–5 years apart have reported no fibrosis progression (62–66), while other studies of paired biopsies spaced 2.8–7 years apart have reported fibrosis progression in 17–28% (67–69).
Risk factors for disease progression
Factors that could conceivably play a role in progression of chronic hepatitis C include features of the virus, the host or items of environmental or extraneous origin (70–72).
Regarding the virus, attention has been focused on viral concentration and viral genotype, but there is little definitive evidence that these factors affect liver disease progression.
In contrast, there are numerous host factors that appear to influence fibrosis progression that include the demographical features of age, gender and race. As already noted, infection at a young age is associated with a slow rate of progression (21, 29), confirming other published data (73–75). Men with chronic HCV infection are more likely to progress to cirrhosis than are women as is evident from the data of the Irish and German women infected by the contaminated Rh immunoglobulin (20, 22), and as has been demonstrated in other reports (73, 75, 76). Paradoxically, given their high rate of evolution to HCC and their lower rate of response to treatment, African Americans appear to have a slow rate of progression to cirrhosis (77, 78). Genetic polymorphisms have been evaluated for their influence on disease progression, with a focus on major histocompatibilty class I and II alleles (79, 80) as well as on profibrogenic cytokines (81, 82). Metabolic factors with inter-relationships among steatosis (83, 84), diabetes (85, 86) and obesity (87, 88) have all been reported as affecting disease progression. As noted earlier, normal values of alanine aminotransferase and its associated lesser degree of histological inflammation appear to predict a lower level and rate of fibrosis progression (61, 69). Finally, the rate of progression is increased in the face of co-infection with HIV (53–57), hepatitis B (89, 90) and schistosomiasis (91, 92), as well as with the comorbidity of haemochromatosis (93).
Regarding extraneous issues, by far the most significant impact on disease progression is the cofactor of associated alcoholism (94–96), although the exact extent of alcohol intake likely to add to the injury is uncertain (97). Similarly, smoking has been reported to be associated with increased fibrosis progression to both cirrhosis and HCC (98, 99).
Pathogenesis of chronic hepatitis C
An extensive discussion of the mechanisms responsible for recovery from HCV infection is beyond the scope of this article. Control of HCV infection is clearly immunologically mediated as is evident by the appearance of virus-specific T cells with the occurrence of clinically apparent hepatitis C (100). Persistence of HCV infection results when innate responses are blunted, thus diminishing the direct antiviral effects and dampening the priming of adaptive responses (101). T-cell induction of interferon (IFN)-γ clears HCV infection, while a poor response to IFN-γ permits continued viral replication with its effects on cytokine release.
How does one process these reported variable outcomes
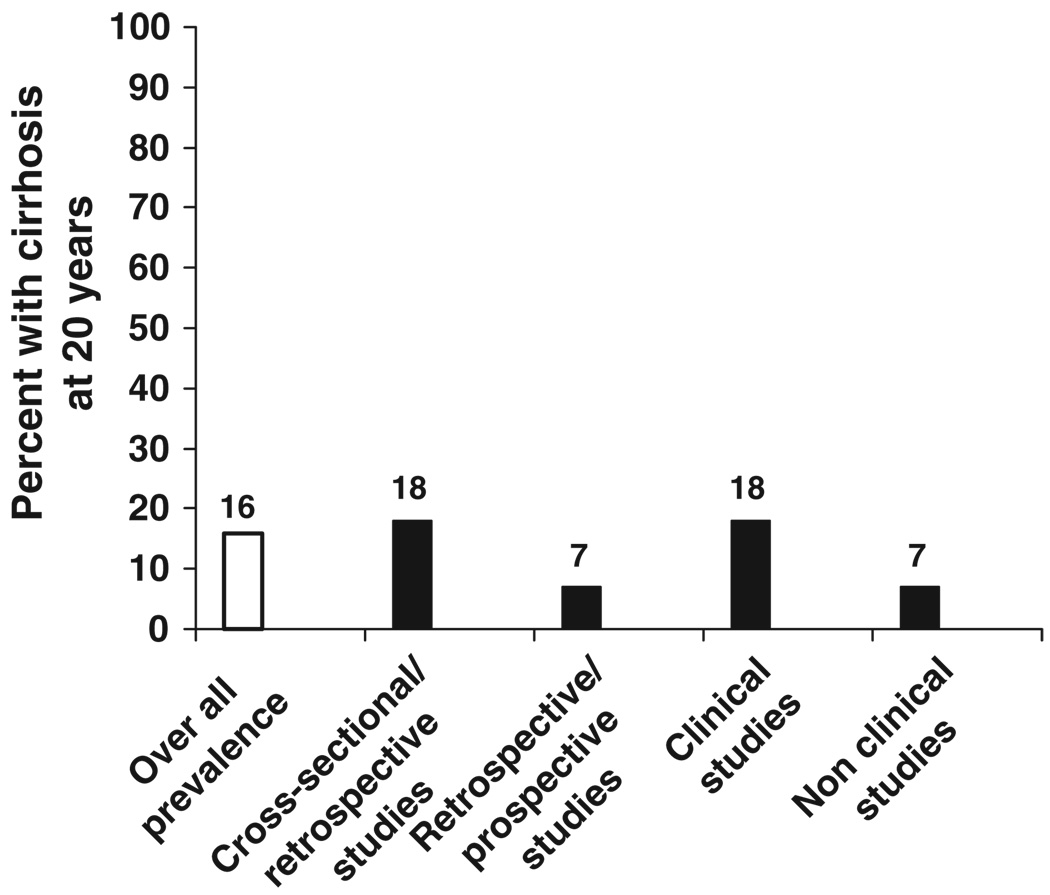
Because of the variable outcomes reported from these numerous studies, several efforts have been undertaken to process the data using meta-analyses and modelling. In 2001, Freemen and coworkers conducted a systematic review of then-published epidemiological studies (102). For inclusion in the analysis, each study was required to have at least 20 HCV-infected persons evaluated as well as information on age and duration of infection. Among 157 studies examined, 57 were selected for further evaluation. The studies could be consolidated into four categories: 33 comprised a liver clinic series (equivalent to a retrospective study), five were post-transfusion cohorts, 10 were blood donor series and nine were community-based cohorts. The likelihood of developing cirrhosis after 20 years of HCV infection was 22% (95% CI, 18–26%) for liver clinic studies, 24% (11–37%) for post-transfusion studies, 7% (4–10%) for community-based studies and 4% (1–10%) for blood donor studies. The high rate of progression in the liver clinic studies was thought to be a result of selection bias. A similar conclusion was reached by investigators in the UK, who, on conducting an analogous survey, concluded that ascertainment bias can convert a 20-year progression rate to cirrhosis of approximately 5% among community-based infected individuals to 20% among patients referred to the clinic, owing to the probability that the closer the patient is to the development of cirrhosis, the more likely the patient is to be referred to the liver clinic (103). In yet another study using empirical calibration of model parameter values, the authors concluded that the rates of progression to advanced liver disease may be lower than has been assumed previously (104). Most recently, researchers from Canada and Australia reported their data on estimating fibrosis progression using meta-analysis and metaregression (105) (Fig. 2). They winnowed down over 5000 citations to 111 studies, 100 classified as cross-sectional/retrospective and 11 as retrospective-prospective. The majority of studies had taken place in liver clinics. The overall estimated prevalence of cirrhosis at 20 years after infection was 16%, 18% for cross-sectional/retrospective and 7% for retrospective-prospective studies, 18% for studies in clinical studies and 7% for studies conducted in non-clinical settings.
Fig. 2.
Estimated prevalence of cirrhosis at 20 years after infection according to strategy of case identification. Meta-analysis and metaregression based on 111 reported natural history studies (105).
Conclusion
It is evident that hepatitis C is a progressive disease, and that a variable proportion of chronically infected persons will advance to end-stage liver disease, namely hepatocellular failure and/or HCC. Such persons are highly likely to die as a consequence of the liver disease unless they undergo liver transplantation. A presumably larger group of chronically infected individuals, primarily those who do not develop cirrhosis, are likely to die from other non-liver-related illnesses or traumatic events that represent the more common causes of death. Some will even progress through life without ever knowing that they are HCV infected, while others may suffer from varying degrees of fatigue and a decreased quality of life. In order to accurately establish the frequency of these variable outcomes, it would be necessary to mount a life-long study of a large cohort from the time of the initial infection and follow them until their demise, but for this to be a true natural history study, infected persons would have to forego treatment of the infection, clearly not an acceptable option. Moreover, it would be almost impossible to pursue a study of this duration, both for the infected cohort and for the investigator. The natural history has particular relevance for those infected at birth or in early childhood. Even though evolution to cirrhosis in the first two to three decades after infection among these individuals is relatively infrequent, it is unclear whether disease progression will begin to increase as this population ages. There is increasing evidence that disease progression does not follow a linear path and that it is likely to increase as the infected person ages (73, 105). Suffice it to say, because of the advent of effective treatment, the era of natural history studies of chronic hepatitis C has passed, except perhaps under rare circumstances. As treatment improves, the illness will come to be viewed as an infectious disease requiring treatment for all, with less concern about the long-term sequelae.
Footnotes
Conflicts of interest
The author has declared no conflicts of interest.
References
- 1.Krugman S, Giles JP, Hammond J. Infectious hepatitis: evidence for two distinctive clinical, epidemiological, and immunologic types of infection. JAMA. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 2.Hampers S, Prager D, Senior JR. Post-transfusion anicteric hepatitis. N Engl J Med. 1964;272:749–754. doi: 10.1056/NEJM196410082711501. [DOI] [PubMed] [Google Scholar]
- 3.Feinstone S, Kapikian AZ, Purcell RH, et al. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975;292:767–770. doi: 10.1056/NEJM197504102921502. [DOI] [PubMed] [Google Scholar]
- 4.Prince AM, Brotman B, Grady GF, et al. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet. 1974;2:241–246. doi: 10.1016/s0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LB, Zimmerman HJ, Wright EC, et al. A randomized, double-blind, controlled trial of the efficacy of immune serum globulin for the prevention of post-transfusion hepatitis. A Veterans Administration cooperative study. Gastroenterology. 1977;72:111–121. [PubMed] [Google Scholar]
- 6.Prince AM, Brotman B, Grady GF, et al. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis B virus. Lancet. 1974;2:241–246. doi: 10.1016/s0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg BS, Alter HJ, Visnich S. A “new” antigen in leukemia serum. JAMA. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 8.Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973;182:1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- 9.Alter J, Purcell RH, Holland PV, et al. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459–463. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- 10.Tabor E, Gerety RJ, Drucker JA, et al. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet. 1978;1:463–466. doi: 10.1016/s0140-6736(78)90132-0. [DOI] [PubMed] [Google Scholar]
- 11.Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 12.Kuo G, Choo QL, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 13.Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 14.Gilliam JH, III, Geisinger KR, Richter JE. Primary hepatocellular carcinoma after chronic NANB post-transfusion hepatitis. Ann Intern Med. 1984;101:794–795. doi: 10.7326/0003-4819-101-6-794. [DOI] [PubMed] [Google Scholar]
- 15.Kiyosawa K, Akahane Y, Nogata A, Furita S. Hepatocellular carcinoma after non-A, non-B post-transfusion hepatitis. Am J Gastroenterol. 1984;79:777–781. [PubMed] [Google Scholar]
- 16.Hoofnagle HJ, Mullen KB, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1989;315:1575–1578. doi: 10.1056/NEJM198612183152503. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Barrera JM, Calvet X, et al. Prevalence of antibody to hepatitis C in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1006–1008. doi: 10.1016/s0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- 18.Villarejos VM, Visona KI, Eduarte CA, Provost PJ, Hilleman MR. Evidence for viral hepatitis other than type A or type B among persons in Costa Rica. N Engl J Med. 1975;293:1350–1352. doi: 10.1056/NEJM197512252932606. [DOI] [PubMed] [Google Scholar]
- 19.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(Suppl. 3):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 20.Kenny-Walsh E for the Irish Hepatology Research Group. Clinical outcomes after hepatitis infection from contaminated anti-D immune globulin. N Eng J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 21.Vogt M, Lay T, Frosner G, et al. Prevalence and clinical outcomes of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–870. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 22.Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in hepatitis C (genotype 1) single-source outbreak in Germany: a 20-year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- 23.Aach RD, Stevens CE, Hollinger FB, et al. Hepatitis C virus infection in post-transfusion hepatitis. N Engl J Med. 1991;325:1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- 24.Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 25.Alter HJ, Conry-Cantilena C, Melpolder J, et al. Hepatitis C in asymptomatic blood donors. Hepatology. 1997;26(Suppl. 1):29S–33S. doi: 10.1002/hep.510260705. [DOI] [PubMed] [Google Scholar]
- 26.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1999;341:1899–1905. [Google Scholar]
- 27.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 28.Locasciulli A, Testa M, Pontisso P, et al. Prevalence and natural history of hepatitis C infection in patients cured of childhood leukemia. Blood. 1997;90:4628–4633. [PubMed] [Google Scholar]
- 29.Casiraghi MA, De Paschale M, Romano L, et al. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology. 2004;39:90–96. doi: 10.1002/hep.20030. [DOI] [PubMed] [Google Scholar]
- 30.Rodger AJ, Roberts S, Lanigan A, et al. Assessment of long-term outcomes of community-acquired hepatitis C infection in a cohort with sera stored from 1971–1975. Hepatology. 2000;32:582–587. doi: 10.1053/jhep.2000.9714. [DOI] [PubMed] [Google Scholar]
- 31.Seeff LB. Strategies for assessing the long-term consequences of hepatitis C virus infection. Minerva Gastroenterol Dietol. 2000;46:207–216. [PubMed] [Google Scholar]
- 32.Di Bisceglie AM, Goodman ZD, Ishak KG, et al. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 33.Tremolada F, Cassin C, Alberti A, et al. Long-term follow-up of NANB (type C) post-transfusion hepatitis. J Hepatol. 1992;16:273–281. doi: 10.1016/s0168-8278(05)80657-9. [DOI] [PubMed] [Google Scholar]
- 34.Mattsson L, Sonnerborg A, Weiland O. Outcome of acute asymptomatic NANB hepatitis: a 13-year follow-up study of hepatitis C virus markers. Liver. 1993;13:274–278. doi: 10.1111/j.1600-0676.1993.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 35.Koretz RL, Abbey H, Coleman E, et al. NANB post-transfusion hepatitis: looking back on the second decade. Ann Intern Med. 1993;119:110–115. doi: 10.7326/0003-4819-119-2-199307150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Kiyosawa K, Sodeyama T, Tanaka E, et al. Interrelationship of blood transfusion, NANB hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C. Hepatology. 1996;12:671–675. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 37.Tong MJ, El-Farra NS, Reikes AR, et al. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 38.Niederau C, Lange S, Heinges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 39.Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 40.Gordon SC, Eloway RS, Long JC, et al. The pathology of hepatitis C as a function of mode of transmission: blood transfusion versus intravenous drug abuse. Hepatology. 1993;18:1338–1343. [PubMed] [Google Scholar]
- 41.Seeff LB, Hollinger FB, Alter HJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 42.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 43.Albloushi SS, Murray FE, Callagy G, et al. Changes in liver histopathology in women infected with hepatitis C through contaminated anti-D immunoglobulin injections in Ireland. Eur J Gastroenterol Hepatol. 1998;10:69–73. doi: 10.1097/00042737-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Fanning L, Kenney E, Sheehan M, et al. Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatology. 1999;29:904–907. doi: 10.1002/hep.510290310. [DOI] [PubMed] [Google Scholar]
- 45.Barrett S, Goh J, Coughlan B, et al. The natural course of hepatitis C virus infection after 22 years in a unique homogeneous cohort: spontaneous viral clearance and chronic HCV infection. Gut. 2001;49:423–430. doi: 10.1136/gut.49.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine RA, Sanderson SO, Ploutz-Snyder R, et al. Assessment of fibrosis progression in untreated Irish women with chronic hepatitis C contracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol. 2006;4:1271–1277. doi: 10.1016/j.cgh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Harris HE, Ramsay ME, Andrews NJ. Survival of a national cohort of hepatitis C virus infected patients, 16 years after exposure. Epidemiol Infect. 2006;134:472–477. doi: 10.1017/S0950268805005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferenci P, Ferenci S, Datz C, et al. Morbidity and mortality in paid Austrian plasma donors infected with hepatitis C at plasma donation in the 1970s. J Hepatol. 2007;47:31–36. doi: 10.1016/j.jhep.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Forns X, Ampurdanes S, Sanchez-Tapias J, et al. Long-term follow-up of chronic hepatitis C in patients diagnosed at a tertiary-care center. J Hepatol. 2001;35:265–271. doi: 10.1016/s0168-8278(01)00088-5. [DOI] [PubMed] [Google Scholar]
- 50.Mohan P, Colvin C, Glymph C, et al. Clinical spectrum and histopathological features of chronic hepatitis C infection in children. J Pediatr. 2007;150:168–174. doi: 10.1016/j.jpeds.2006.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franchini M, Rossetti G, Tagliaferri A, et al. The natural history of chronic hepatitis C in a cohort of HIV-negative Italian patients with hereditary bleeding disorders. Blood. 2001;98:1836–1841. doi: 10.1182/blood.v98.6.1836. [DOI] [PubMed] [Google Scholar]
- 52.Posthouwer D, Fischer K, van Erpecum KJ, et al. The natural history of childhood-acquired hepatitis C infection in patients with inherited bleeding disorders. Transfusion. 2006;46:1360–1366. doi: 10.1111/j.1537-2995.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 53.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 54.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 55.Thomas DL, Shih JW, Alter HJ, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 56.Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 57.Sulkowski MS. The HIV-coinfected patients. Managing viral hepatitis. J Acquir Immune Defic Syndr. 2007;45:S36–S37. doi: 10.1097/QAI.0b013e318068d0f4. [DOI] [PubMed] [Google Scholar]
- 58.Castellino S, Lensing S, Riely C, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St. Jude Children’s Research Hospital hepatitis C seropositive cohort. Blood. 2004;103:2460–2466. doi: 10.1182/blood-2003-07-2565. [DOI] [PubMed] [Google Scholar]
- 59.de Latour RP, Levy V, Asselah T, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 60.Puoti C, Guido M, Mangia A, et al. Clinical management of HCV carriers with normal aminotransferase levels. Dig Liver Dis. 2003;35:362–369. doi: 10.1016/s1590-8658(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 61.Puoti C, Castellacci R, Montagnese F. Hepatitis C virus carriers with persistently normal aminotransferases: healthy people or true patients? Dig Liver Dis. 2000;32:634–643. doi: 10.1016/s1590-8658(00)80850-6. [DOI] [PubMed] [Google Scholar]
- 62.Martinot-Peignoux M, Boyer N, Cazals-Hatem D, et al. Prospective study on anti-hepatitis C virus-positive patients with persistently normal serum alanine transaminase with and without detectable serum hepatitis C virus RNA. Hepatology. 2001;34:1000–1005. doi: 10.1053/jhep.2001.28458. [DOI] [PubMed] [Google Scholar]
- 63.Persico M, Persico E, Suozzo R, et al. Natural history of hepatitis C virus carriers with persistently normal amino-transferase levels. Gastroenterology. 2000;118:760–764. doi: 10.1016/s0016-5085(00)70145-4. [DOI] [PubMed] [Google Scholar]
- 64.Sangiovanni A, Morales R, Spinzi G, et al. Interferon alfa treatment of HCV RNA carriers with persistently normal transaminase levels: a pilot nonrandomized controlled study. Hepatology. 1998;7:853–856. doi: 10.1002/hep.510270330. [DOI] [PubMed] [Google Scholar]
- 65.Ghany MG, Kleiner DE, Alter H, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 66.Boccato S, Pistis R, Noventa F, et al. Fibrosis progression in initially mild chronic hepatitis C. J Viral Hepatitis. 2006;13:297–302. doi: 10.1111/j.1365-2893.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 67.Hui CK, Belaye T, Montegrande K, et al. A comparison in the progression of liver fibrosis in chronic hepatitis C between persistently normal and elevated transaminases. J Hepatol. 2003;38:511–517. doi: 10.1016/s0168-8278(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 68.Ryder SD, Irving WL, Jones DA, et al. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cividini A, Rebucci C, Silini E, et al. Is the natural history of hepatitis C virus carriers with normal aminotransferase really benign? Gastroenterology. 2001;121:1526–1527. doi: 10.1053/gast.2001.30115. [DOI] [PubMed] [Google Scholar]
- 70.Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis: modifiable and nonmodifiable factors. Gastroenterology. 2008;134:1699–1714. doi: 10.1053/j.gastro.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 71.Massard J, Ratziu V, Thabut D, et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatology. 2006;44:S19–S24. doi: 10.1016/j.jhep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Alberti A, Vario A, Ferrari A, et al. Review article: chronic hepatitis C – natural history and cofactors. Aliment Pharmacol Ther. 2005;22(Suppl. 2):74–78. doi: 10.1111/j.1365-2036.2005.02602.x. [DOI] [PubMed] [Google Scholar]
- 73.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 74.Minola E, Prati D, Suter F, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 75.Deuffic-Burban S, Poynard T, Valleron AJ. Quantification of fibrosis progression in patients with chronic hepatitis C using a Markov model. J Viral Hepatitis. 2002;9:114–122. doi: 10.1046/j.1365-2893.2002.00340.x. [DOI] [PubMed] [Google Scholar]
- 76.Di Martino V, Lebray M, Myers RP, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 77.Wiley TE, Brown J, Chan J. Hepatitis C virus infection in African Americans: its natural history and histological progression. Am J Gastroenterol. 2002;97:700–706. doi: 10.1111/j.1572-0241.2002.05555.x. [DOI] [PubMed] [Google Scholar]
- 78.Sterling RK, Stravitz RT, Luketic VA, et al. A comparison of the spectrum of chronic hepatitis C between Caucasians and African Americans. Clin Gastroenterol Hepatol. 2004;2:469–473. doi: 10.1016/s1542-3565(04)00164-8. [DOI] [PubMed] [Google Scholar]
- 79.Kuzushita N, Hayashi N, Moribe T, et al. Influence of HLA haplotypes on the clinical courses of individuals infected with hepatitis C virus. Hepatology. 1998;27:240–244. doi: 10.1002/hep.510270136. [DOI] [PubMed] [Google Scholar]
- 80.Tillmann HL, Chen DF, Trautwein C, et al. Low frequency of HLA-DRB1*11 in hepatitis C virus induced end stage liver disease. Gut. 2001;48:714–718. doi: 10.1136/gut.48.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kunzler S, Baumann M, Schirmacher F, et al. Prediction of progressive liver fibrosis in hepatitis C virus infection by serum and tissue levels of transforming growth factor-β. J Virol. 2001;8:430–437. doi: 10.1046/j.1365-2893.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- 82.Promrat K, McDermott DH, Gonzalez CM, et al. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352–360. doi: 10.1053/gast.2003.50061. [DOI] [PubMed] [Google Scholar]
- 83.Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 84.Adinolphi L, Gambardella M, Andreana A, et al. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 85.Ratziu V, Munteanu M, Charlotte F, et al. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39:1049–1055. doi: 10.1016/s0168-8278(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 86.Monto A, Alonzo J, Watson J, et al. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:720–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 87.Hourigan LF, Macdonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- 88.Ortiz V, Berenguer M, Rayon JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 89.Pontisso P, Gerotto M, Benvegnu L, et al. Co-infection by hepatitis B virus and hepatitis C virus. Antiviral Ther. 1998;3:137–142. [PubMed] [Google Scholar]
- 90.Gaeta GB, Stomaiula G, Precone DF, et al. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian srudy. J Hepatol. 2003;39:1036–1041. doi: 10.1016/s0168-8278(03)00470-7. [DOI] [PubMed] [Google Scholar]
- 91.Kamal S, Madwar M, Bianchi L, et al. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C coinfected with S. mansoni. Liver. 2000;20:281–289. doi: 10.1034/j.1600-0676.2000.020004281.x. [DOI] [PubMed] [Google Scholar]
- 92.Derbala MF, Al Kaabi SR, El Dweik NZ, et al. Treatment of hepatitis C virus genotype 4 with peginterferon alfa-2a: impact of bilharziasis and fibrosis stage. World J Gastroenterol. 2006;12:5692–5698. doi: 10.3748/wjg.v12.i35.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonkovsky H, Banner BF, Rothman AL. Iron and chronic viral hepatitis. Hepatology. 1997;25:759–768. doi: 10.1002/hep.510250345. [DOI] [PubMed] [Google Scholar]
- 94.Ostapowicz G, Watson KG, Locarnini SA, et al. The role of alcohol in the progression of liver disease caused by hepatitis C virus. Hepatology. 1998;27:1730–1735. doi: 10.1002/hep.510270637. [DOI] [PubMed] [Google Scholar]
- 95.Harris DR, Gonin R, Alter HJ, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134:120–124. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 96.Hutchison SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a metaanalysis. Hepatology. 2005;3:1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 97.Monto A, Patel K, Bostrom A, et al. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826–834. doi: 10.1002/hep.20127. [DOI] [PubMed] [Google Scholar]
- 98.Pessione F, Ramond M-J, Njapoum C, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001;34:121–125. doi: 10.1053/jhep.2001.25385. [DOI] [PubMed] [Google Scholar]
- 99.Mori M, Hari M, Wada I, et al. Prospective study of hepatitis B and C viral infection, cigarette smoking, alcohol consumption and other factors associated with hepatocellular carcinoma risk in Japan. Am J Epidemiol. 2000;151:131–139. doi: 10.1093/oxfordjournals.aje.a010180. [DOI] [PubMed] [Google Scholar]
- 100.Thimme R, Bukh J, Spangenberg HC, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence and disease. Proc Natl Acad Sci USA. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas DL. Seeff LB Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 103.Fu B, Tom BDM, Delahooke T, Alexander GJM, Bird SM. Event-biased referral can distort estimation of hepatitis C virus progression rate to cirrhosis, and of prognostic influences. J Clin Epidemiol. 2007;60:1140–1148. doi: 10.1016/j.jclinepi.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 104.Salomon JA, Weinstein MC, Hammitt JK, Soldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 105.Thein H-H, Yo Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–438. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]