Abstract
AIM: Elevation of alanine aminotransferase (ALT) level is commonly seen among patients suffering from severe acute respiratory syndrome (SARS). We report the progression and clinical significance of liver derangement in a large cohort of SARS patient.
METHODS: Serial assay of serum ALT was followed in patients who fulfilled the WHO criteria of SARS. Those with elevated ALT were compared with those with normal liver functions for clinical outcome. Serology for hepatitis B virus (HBV) infection was checked. Adverse outcomes were defined as oxygen desaturation, need of intensive care unit (ICU) and mechanical ventilation and death.
RESULTS: Two hundred and ninety-four patients were included in this study. Seventy (24%) patients had elevated serum ALT on admission and 204 (69%) patients had elevated ALT during the subsequent course of illness. Using peak ALT > 5×ULN as a cut-off and after adjusting for potential confounding factors, the odds ratio of peak ALT > 5×ULN for oxygen desaturation was 3.24 (95%CI 1.23-8.59, P = 0.018), ICU care was 3.70 (95%CI 1.38-9.89, P = 0.009), mechanical ventilation was 6.64 (95%CI 2.22-19.81, P = 0.001) and death was 7.34 (95%CI 2.28-24.89, P = 0.001). Ninety-three percent of the survived patients had ALT levels normalized or were on the improving trend during follow-up. Chronic hepatitis B was not associated with worse clinical outcomes.
CONCLUSION: Reactive hepatitis is a common complication of SARS-coronavirus infection. Those patients with severe hepatitis had worse clinical outcome.
Keywords: SARS, Hepatitis, Hepatitis B virus, Coronavirus
INTRODUCTION
Severe acute respiratory syndrome (SARS) is caused by a novel coronavirus (SARS-coronavirus, SARS-CoV) infecting primarily the lung and the enteric tract[1-9]. Up to August 2003, there were 8422 reported cases worldwide and 916 cases died of this condition[10]. Although the outbreak of SARS is currently under control, the source of SARS-CoV has not been identified and the threat of SARS returning in winter persists.
Case series in Hong Kong and Toronto indicated that SARS is not merely a respiratory disease. Diarrhea and bleeding diathesis had also been reported in patients infected by SARS-CoV[11,12]. In our previous report, SARS-CoV was found in biopsy of small intestine and colon of patients with diarrhea[11]. Deranged liver functions were reported in 22-56% of patients at the time of hospital admission[13-17]. In a previous study, it has also been suggested that co-infection with hepatitis B virus (HBV) is associated with more severe respiratory disease[18]. The cause of impaired liver function, its clinical implication and association with HBV co-infection have not been fully explored.
In this study, we follow the natural course of hepatic involvement in SARS. The impact of liver derangement and chronic HBV infection on the clinical outcome of SARS patients is revisited.
MATERIALS AND METHODS
Patients
Patients in the present study were collected from a university medical center (Prince of Wales Hospital) and a community hospital (Princess Margaret Hospital) designated to look after SARS patients during the outbreak in Hong Kong. All patients fulfilled the case definitions of SARS by the World Health Organization, i.e., temperature above 38 °C, difficulty in breathing and cough, pneumonic changes on chest X-ray or high-resolution computed tomography, and contact history with SARS patients[19]. All patients were initially treated with empirical antibiotics including cefotaxime and clarithromycin (or levofloxacin) to cover common pathogens causing community-acquired pneumonia. Oseltamivir (Tamiflu) was also given to treat possible influenza infection when little was known about SARS during the early phase of the outbreak. If fever persisted for more than 48 h, all patients received corticosteroids and ribavirin treatment. The choice of corticosteroids was intravenous hydrocortisone 100 mg every 8 h or oral prednisolone 1 mg/kg body weight per day. Ribavirin was given at 400 mg every 8 h intravenously or 1200 mg thrice a day orally. Pulse intravenous methylprednisolone (500-1000 mg/d), up to a maximum dose of 3 g, was given when there were signs of radiological or clinical deterioration. Patients, with oxygen saturation that fell below 90% at room air, were offered supplementary oxygen through nasal cannula. Those who required more than 4 L/min oxygen would be transferred to intensive care unit (ICU) for close monitoring. Mechanical ventilation by CPAP was implemented, when patients could not achieve 90% oxygen saturation despite receiving 5 L/min oxygen or more. All patients were kept in hospital for monitoring for at least 3 wk before discharge. Liver enzymes were checked every 1-3 d during hospital stay, on discharge and on follow-up visits. Clinical outcomes were assessed at least 8 wk after the admission of patients.
Serological assays
The level of anti-coronavirus IgG antibody was measured by immunofluorescence assay. Paired sera from acute (taken within 7 d after the onset of fever) and convalescent (taken 14-21 d after the onset of fever) blood samples were tested at serial two-fold dilutions starting from 1:40. Positive serological evidence of coronavirus infection was defined as either having a seroconversion or ≥four-fold rise in antibody titer. Hepatitis B surface antigen (HBsAg) and hepatitis C antibodies (anti-HCV) were tested by commercially available enzyme-linked immunosorbent assay kits (Abbott GmBH Diagnostika, Wiesbaden-Delkenheim, Germany). Hepatitis B e antigen (HBeAg) and antibodies to hepatitis B e antigen (anti-HBe) were measured by ELISA (Sanofi Diagnostics, Pasteur, France).
Data analysis
Continuous variables were expressed as mean±SD for normal distribution data and median (range) if the distribution was skewed. Statistical analysis was performed by SPSS (version 11.0, Chicago). Categorical variables were compared by χ2 test and continuous variables by Student’s t test or Mann-Whitney U test as appropriate. Adverse clinical outcomes were defined as need of oxygen desaturation requiring oxygen supplementation, ICU admission, mechanical ventilation, liver decompensation and mortality. Liver decompensation was defined as development of hepatic encephalopathy associated with elevated serum bilirubin (>51 mmol/L) and prolonged prothrombin time (>16 s). As the reference ranges of alanine aminotransferase (ALT) levels were different between the two hospitals, ALT levels were expressed as folds of increase above the upper limit of normal (ULN) in individual laboratories. The relationships of peak ALT levels and various adverse clinical outcomes were compared by receiver operator characteristic curve. Baseline clinical characteristics with a P value <0.1 for adverse clinical outcomes on comparing patients with high peak ALT levels vs. those with lower ALT levels were adjusted by multivariate logistic regression analysis. All statistical tests were two-sided. P value <0.05 was statistically significant.
RESULTS
Clinical characteristics
Two hundred and ninety-four patients including 126 male (43%) and 168 female were included in this study. The median age of this cohort was 36 years, range 12-83 years. Two hundred and forty-three patients had paired blood samples checked for SARS-CoV serology and all had positive results. These patients were admitted on the third (range 0-11) day after the onset of fever. Thirty (10%) patients were found to have positive HBsAg and 214 patients had negative HBsAg. In 50 cases, HBsAg status was not checked during hospitalization. All patients in the Prince of Wales cohort had negative anti-HCV antibodies, and 7 of 12 HBV-infected patients had positive HBeAg. Anti-HCV and HBeAg status were not routinely monitored in the Princess Margaret Hospital cohort. Lamivudine (100 mg/d) was commenced in 20 of the 30 chronic hepatitis B patients on or before the commencement of corticosteroid treatment and was continued thereafter. Two chronic hepatitis B infected patients had co-existing liver cirrhosis. One of them had inoperable multi-focal hepatocellular carcinoma and the other was admitted for bleeding esophageal variceal. Forty-one (14%) patients had other co-morbid illnesses including hypertension (12), diabetes mellitus (5), end-stage renal failure (2), chronic rheumatic heart disease (2), ischemia heart disease (2), sick sinus syndrome (1), atrial fibrillation (1), asthma (1), chronic obstructive airway disease (1), bronchiectasis (1), old pulmonary tuberculosis (1), previous cerebrovascular accident (1), autism (1) and pregnancy (1).
Overall, 141 (48%) patients had oxygen desaturation, 50 (17%) required admission to ICU, 33 (11%) patients required mechanical ventilation, and 27 (9%) patients died. None of the patients developed liver decompensation after contracting SARS. All mortalities were due to respiratory failure related to SARS with or without sepsis and multi-organ failure. The outcome of the studied cohort is summarized in Figure 1.
Figure 1.
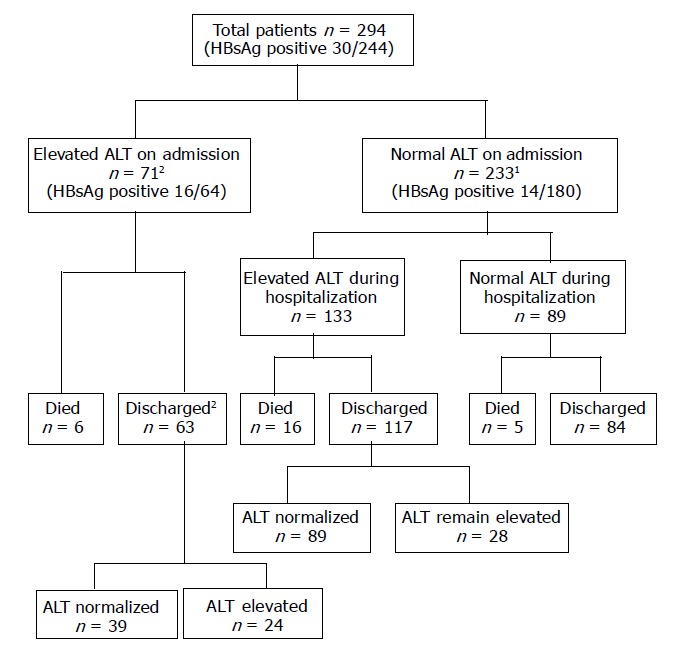
Clinical outcomes of patients included in the study. ALT, alanine aminotransferase. 1One patient did not have serial ALT results. 2Two patients did not have follow-up ALT results after discharge.
Liver enzyme derangement on admission
Seventy (24%) patients, including 15 chronic hepatitis B-infected patients, had elevated ALT levels on admission. The median ALT levels on admission was 0.55 (0.16-26.09) times upper limit of laboratory normal. The proportion of patients with different ALT levels, serum bilirubin and prothrombin time on admission are shown in Figure 2. Two chronic hepatitis B infected patients were admitted with icteric flare-up of chronic hepatitis B on lamivudine treatment and contracted SARS during their hospital stay. They had elevated serum bilirubin to more than 150 mmol/L and one of them had prolonged prothrombin time to 17 s. All other patients who had elevated ALT levels had normal serum bilirubin levels and had no evidence of hepatic decompensation.
Figure 2.
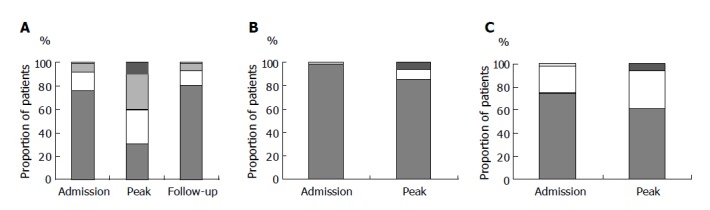
A: Proportion of patients with different ALT levels at initial visit (n = 294), at peak ALT (n = 293; 1 missing data) and on last follow-up (n = 264; 27 patients died, 3 missing data). B: Proportion of patients with different serum bilirubin levels at initial visit (n = 294) and at peak bilirubin (n = 293, 1 missing data). C: Proportion of patients with different prothrombin time at initial visit (n = 293, one missing data) and at peak prothrombin time (n = 283, 11 missing data).
Progression of liver derangement on follow-up
During the course of illness, 204 (69%) patients, including 23 HBsAg-positive patients, had elevated ALT levels. Majority of patients had ALT levels elevated at day 5-7 from fever onset and ALT peaked at the end of second week (Figure 3). The median peak ALT levels was 1.53 (0.28-316.25) times ULN. The proportion of patients with different peak ALT levels, serum bilirubin and prothrombin time are shown in Figure 2. Twenty-eight (9.5%) patients had ALT raised to over 5×ULN; among them 7 patients had elevated serum bilirubin (median 83 mmol/L, range 48-231 mmol/L) and 10 patients had elevated prothrombin time (median 20 s, range 14-56 s). Among the 138 patients in the Prince of Wales Hospital cohort, the median peak alkaline phosphatase (ALP) level was 0.78 (range 0.31-19.38) times ULN and 40 (29%) patients had experienced elevated ALP during the course of illness. None of these patients developed hepatic encephalopathy. Seven of the 28 (25%) patients died of SARS and multi-organ failure. On the other hand, only 5 of 89 (6%) patients who had persistently normal ALT died.
Figure 3.
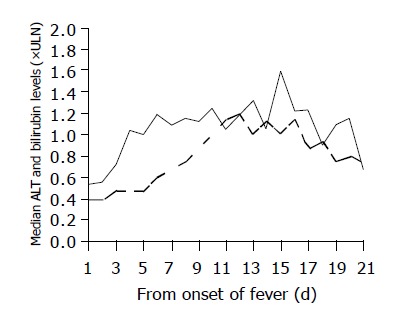
Median ALT and serum bilirubin levels of patients admitted to Prince of Wales Hospital (n = 138) from the day of fever onset to day 21 at hospital discharge. Both ALT and bilirubin levels were expressed as folds of ULN. Only patients who had at least one elevated ALT level during admission were included (n=104). ULN, upper limit of laboratory normal; solid line, serum ALT; broken line, serum bilirubin.
The area under the receiver operator characteristic curve of peak ALT for oxygen desaturation, need of ICU care, mechanical ventilation and mortality were 0.70 (95%CI 0.64-0.75; P<0.001), 0.72 (95%CI 0.65-0.79; P<0.001), 0.71 (95%CI 0.62-0.80, P<0.001) and 0.65 (95%CI 0.54-0.76, P = 0.011), respectively. Using the coordinate of peak ALT > 5×ULN, the sensitivity and specificity for any adverse outcomes were 15-27% and 93-95% respectively. Patients who had peak ALT over 5’ ULN had significant male predominance, more co-existing co-morbid conditions, more chronic hepatitis B patients and marginally higher serum creatinine levels as compared to those who had lower peak ALT levels (Table 1). Using peak ALT > 5×ULN as a cut-off and after adjusting these potential confounding factors, the odds ratio of peak ALT > 5×ULN for oxygen desaturation was 3.24 (95%CI 1.23-8.59, P = 0.018), ICU care was 3.70 (95%CI 1.38-9.89, P = 0.009), mechanical ventilation was 6.64 (95%CI 2.22-19.81, P = 0.001) and death was 7.34 (95%CI 2.28-24.89, P = 0.001).
Table 1.
Univariate analysis of baseline clinical characteristics among patients with peak ALT levels > 5×ULN vs with patients with peak ALT levels ≤ 5×ULN.
| Peak ALT >5×ULN | Peak ALT ≤5×ULN | P | |
| Number of patients | 28 | 265 | |
| Age (yr) | 40±14 | 39±15 | 0.68 |
| Male | 17 (61%) | 109 (41%) | <0.001 |
| Co-morbid | 4 (14%) | 37 (14%) | 0.002 |
| Hemoglobin (g/dL) | 13.4±2.1 | 13.4± 1.5 | 0.94 |
| White cell count (×109/L) | 5.3±2.3 | 5.0±2.0 | 0.49 |
| Neutrophil (×109/L) | 4.2±1.9 | 3.8±1.9 | 0.30 |
| Lymphocyte (×109/L) | 0.8±0.5 | 0.8±0.5 | 0.90 |
| Platelet (×109/L) | 146±53 | 151±53 | 0.64 |
| Creatinine (mmol/L) | 83 (481-318) | 79 (43-214) | 0.09 |
| HBsAg | 6/25 (24%) | 24/218 (11%) | <0.001 |
| Oxygen supplementation | 21 (75%) | 120 (45%) | <0.001 |
| ICU care | 11 (39%) | 39 (15%) | <0.001 |
| Mechanical ventilation | 9 (32%) | 24 (9%) | 0.012 |
| Death | 7 (25%) | 20 (8%) | 0.017 |
Excluding 27 patients who died and 3 patients who had no follow-up ALT results, 84 of 264 (32%) patients had persistently normal ALT levels during the entire course of illness. Among the 180 patients who had elevated ALT levels, 128 (71%) had ALT subsequently normalized and 37 (21%) patients had ALT on downward trend at the last follow-up visit. The remaining 8% of patients still had elevated ALT levels on the last follow-up.
Co-infection with hepatitis B virus
Two of the 30 HBsAg-positive patients died despite lamivudine treatment. One patient was admitted for icteric flare-up of chronic hepatitis B and the other had liver cirrhosis admitted for esophageal variceal bleeding. Both patients acquired SARS during their hospital stay. Seven patients had persistently normal ALT levels throughout the admission and follow-up visits (six were on lamivudine). Twenty-one HBsAg-positive patients had elevated ALT during the SARS illness. Among them, 10 patients (6 on lamivudine) had transient elevation of ALT which subsequently returned to normal levels, 8 (5 on lamivudine) had declining levels of ALT and 3 patients (1 on lamivudine) had persistently elevated ALT levels. In this series, co-infection with viral hepatitis B was not associated with higher peak ALT level, increased risk of oxygen desaturation, ICU admission, mechanical ventilation or mortality (Table 2).
Table 2.
Clinical outcome of patients with and without chronic hepatitis B.
| HBsAg positive | HBsAg negative | P | |
| Number of patients | 30 | 214 | |
| Peak ALT (×ULN) | 2.2 (0.6-26.1) | 1.5 (0.3-316.3) | 0.16 |
| Oxygen supplementation | 17 (57%) | 104 (49%) | 0.68 |
| ICU care | 5 (17%) | 30 (14%) | 0.70 |
| Mechanical ventilation | 3 (10%) | 19 (9%) | 0.84 |
| Death | 2 (7%) | 14 (7%) | 0.98 |
DISCUSSION
Although SARS is primarily a pulmonary disease, liver derangement was commonly observed[13-17]. In this study, approximately a quarter of patients had elevated ALT on admission, and a further 45% of patients who had normal ALT on admission had ALT elevation during the course of illness. In a majority of patients ALT levels started to elevate towards the end of first week and peak at the end of second week. High peak ALT level appears to be an independent predictor of more severe illness and worse clinical outcome. Most patients however had transient elevation of ALT, which normalize spontaneously with the recovery of SARS.
The underlying cause for ALT elevation was uncertain but several mechanisms were worth considering. Direct pathogenic effect of SARS-CoV on the liver is unlikely due to the failure of identification of SARS-CoV or specific hepatitis features in the liver at autopsy in previous reports[20-23]. Elevated ALT might be related to the prescribed medications including antibiotics and high dose ribavirin. As a quarter of patients had elevated ALT levels on admission well before the prescription of drugs, drug treatment should not be the cause of liver enzyme derangement in these patients. Furthermore, many patients had ALT > 5×ULN. This is exceedingly rare with antibiotic and ribavirin given at these dosages[24]. Although chronic hepatitis C virus infection has only been excluded in about half of the cases in this series (the Prince of Wales Hospital cohort), the prevalence of chronic hepatitis C is very low among the general population in Hong Kong.
We believe that the elevation of liver enzyme is a reactive response towards SARS-CoV infection[25]. The hepatic acute phase response involving cytokine release from inflammatory cells is a defense reaction of the body against the causative agent to protect the vital functions of the liver[26]. The liver enzyme elevation in SARS is not typical of cholestasis secondary to sepsis as majority of patients did not have accompanied elevation of ALP. This is usually a transient reaction and therefore majority of patients have ALT levels returned to normal after recovery.
Although SARS-CoV is not a direct cause of liver injury, results from this study indicated that gross liver enzyme elevation, as indicated by high peak ALT levels, is an independent factor associated with poor clinical outcome. Peak ALT over 5×ULN increased the risk of mortality by seven-folds. Although age and co-morbid illnesses had been found to have significant negative impact on prognosis in SARS[6,13,18,27], in this study, the age of patients did not differ significantly between those with high or low peak ALT levels. Baseline ALT levels have not been found to be associated with any adverse clinical outcome in the past probably because of the delayed elevation of ALT levels in most patients. In fact most immunological damage of SARS in the lungs occurred in the second week of the illness[12,28]. The level of ALT elevation may reflect the severity of acute phase response, which in turn may reflect the severity of tissue damage in SARS[17]. We believe that the elevated ALT can serve as a surrogate marker to predict the clinical outcome of SARS.
We did not find any difference in various adverse clinical outcomes among chronic hepatitis B patients as compared to the HBsAg-negative patients. In our cohort, 16 (53%) chronic hepatitis B patients had elevated ALT levels upon admission and two of them suffered from icteric flare-up of hepatitis. Perhaps those patients who suffered from severe flare-up of hepatitis or decompensated liver cirrhosis might have a higher risk of mortality[29]. Lamivudine was not prescribed to one-third of the patients but none of them died or developed hepatic decompensation.
In conclusion, we found that elevation of transaminase is a very common feature in SARS. The ALT elevation is usually transient and likely to be reactive in nature. Co-infection with HBV in the absence of liver cirrhosis or reactivated hepatitis does not affect the natural course of the disease.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 5.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Peñaranda S, Bankamp B, Maher K, Chen MH, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 6.Yeh SH, Wang HY, Tsai CY, Kao CL, Yang JY, Liu HW, Su IJ, Tsai SF, Chen DS, Chen PJ. Characterization of severe acute respiratory syndrome coronavirus genomes in Taiwan: molecular epidemiology and genome evolution. Proc Natl Acad Sci USA. 2004;101:2542–2547. doi: 10.1073/pnas.0307904100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chim SS, Tsui SK, Chan KC, Au TC, Hung EC, Tong YK, Chiu RW, Ng EK, Chan PK, Chu CM, et al. Genomic characterisation of the severe acute respiratory syndrome coronavirus of Amoy Gardens outbreak in Hong Kong. Lancet. 2003;362:1807–1808. doi: 10.1016/S0140-6736(03)14901-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan HL, Tsui SK, Sung JJ. Coronavirus in severe acute respiratory syndrome (SARS) Trends Mol Med. 2003;9:323–325. doi: 10.1016/S1471-4914(03)00135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Summary table of SARS cases by country, 1 November 2002 - 7 August 2003. Available from: http: //www.who.int/csr/sars/country/2003_08_15/en/
- 11.Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Ng MH, Yu LM, Hui DS, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 14.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 15.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 16.Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, Lee PO, Ng TK, Ng WF, Lee KC, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wong WM, Ho JC, Ooi GC, Mok T, Chan J, Hung IF, Ng W, Lam YM, Tam WO, Wong BC, et al. Temporal patterns of hepatic dysfunction and disease severity in patients with SARS. JAMA. 2003;290:2663–2665. doi: 10.1001/jama.290.20.2663. [DOI] [PubMed] [Google Scholar]
- 18.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Case definition for surveillance of severe acute respiratory syndrome (SARS) Available from: http: //www.who.int/csr/sars/caedefinition/en (accessed August 29, 2003)
- 20.MacPhee PJ, Dindzans VJ, Fung LS, Levy GA. Acute and chronic changes in the microcirculation of the liver in inbred strains of mice following infection with mouse hepatitis virus type 3. Hepatology. 1985;5:649–660. doi: 10.1002/hep.1840050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy GA, MacPhee PJ, Fung LS, Fisher MM, Rappaport AM. The effect of mouse hepatitis virus infection on the microcirculation of the liver. Hepatology. 1983;3:964–973. doi: 10.1002/hep.1840030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan HL, Leung WK, To KF, Chan PK, Lee N, Wu A, Tam JS, Sung JJ. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am J Med. 2004;116:566–567. doi: 10.1016/j.amjmed.2003.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JZ. Severe acute respiratory syndrome and its lesions in digestive system. World J Gastroenterol. 2003;9:1135–1138. doi: 10.3748/wjg.v9.i6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Investigator Brouchure. Ribavirin (SCH 18908). A nucleoside analogue, broad-spectrum antiviral agent for the oral treatment of chronic hepatitis C in combination with INTRON A (interferon alfa-2b recombinant [SCH 30500] Schering Plough 1997 [Google Scholar]
- 25.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buschenfelde KHM, Gerken G. Immune mechanisms in the production of liver diseases. In Zakin D and Boyer TD ed: Hepatology. A Textbook of Liver Diseases, ed 4. Philadelphia, Saunders; 2003. pp. 1127–1163. [Google Scholar]
- 27.Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu KL, Lu SN, Changchien CS, Chiu KW, Kuo CH, Chuah SK, Liu JW, Lin MC, Eng HL, Chen SS, et al. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71:125–128. [PubMed] [Google Scholar]
- 29.Hui AY, Chan HL, Liew CT, Chan PK, To KF, Chan CP, Sung JJ. Fatal outcome of SARS in a patient with reactivation of chronic hepatitis B. Am J Med. 2003;115:334–336. doi: 10.1016/S0002-9343(03)00363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


