SUMMARY
In PTEN-mutated tumors, we show that PI3Kα activity is suppressed and PI3K signaling is driven by PI3Kβ. A selective inhibitor of PI3Kβ inhibits the Akt/mTOR pathway in these tumors but not in those driven by receptor tyrosine kinases. However, inhibition of PI3Kβ only transiently inhibits Akt/mTOR signaling because it relieves feedback inhibition of IGF1R and other receptors and thus causes activation of PI3Kα and a rebound in downstream signaling. This rebound is suppressed and tumor growth inhibition enhanced with combined inhibition of PI3Kα and PI3Kβ. In PTEN deficient models of prostate cancer, this effective inhibition of PI3K causes marked activation of androgen receptor activity. Combined inhibition of both PI3K isoforms and androgen receptor results in major tumor regressions.
Keywords: PI3Kβ, PI3Kα, isoform-selective inhibitors, feedback inhibition, PTEN-mutated tumors, IGF1R/IRS1 signaling pathway, RTKs, prostate cancer, Triple negative breast cancer
INTRODUCTION
The PI3K/Akt/mTOR signaling pathway is aberrantly activated by mutation in many cancers (Cancer Genome Atlas, 2012). Dysregulation of PI3K signaling in these tumors results in increased pathway output and is accompanied by exaggerated physiologic feedback inhibition of receptor tyrosine kinase (RTK) signaling, which normally serves to limit the extent and duration of activation of the pathway. In model systems, PI3K signaling has been shown to be necessary for the maintenance of tumors in which it is activated (She et al., 2008). However, the therapeutic benefit of inhibitors of PI3K, Akt and mTOR has been modest (Fruman and Rommel, 2014). For instance, loss of function of PTEN due to mutations occurs in more than 40% of metastatic prostate cancers (Taylor et al., 2010), yet TORC1 inhibitors, such as rapamycin analogs, are inactive in this disease (Armstrong et al., 2013).
The modest efficacy of these inhibitors has been attributed to several factors, including the coexistence of other mutations and relief of feedback inhibition of physiologic signaling in tumors treated with pathway inhibitors (Chandarlapaty et al., 2011; Carracedo et al., 2008; Halilovic et al., 2010; Serra et al., 2011; Ihle et al., 2009). Many PI3K inhibitors and `dual-specificity inhibitors' that inhibit both mTOR and PI3K are not selective and off-target toxicity may limit pathway inhibition and efficacy (Brachmann et al., 2012; Toledo et al., 2011). Furthermore, PI3K subserves many key physiologic functions and its inhibition may be limited by hyperglycemia and other on-target toxicities (Martini et al., 2013).
Recently, isoform selective PI3K inhibitors have been developed and shown to have limited toxicity even at high doses (Juric et al., 2012). Several groups have found that in tumors with PI3Kα mutation or those in which PI3K signaling is driven by activated receptor tyrosine kinases, PI3Kα is the dominant isoform, whereas in tumors with PTEN mutations, PI3Kβ is dominant (Knight et al., 2006; Ni et al., 2012; Wee et al., 2008). Consistent with these data, PI3Kβ, but not PI3Kα was required for the development of prostate tumors in the anterior prostate (AP) of a Pten-deficient genetically engineered mouse (GEM) model (Jia et al., 2013; Jia et al., 2008).
These findings suggest that selective inhibitors of PI3Kβ will effectively inhibit PI3K signaling in tumors in which PTEN is inactivated and will therefore have significant antitumor activity. In this study we tested this hypothesis.
RESULTS
PI3Kβ inhibitors transiently inhibit PI3K signaling in PTEN-mutated models
AZD8186 (Figure S1A), an ATP-competitive inhibitor of PI3Kβ and PI3Kδ, was used in these studies. In the ActivX assay for inhibition of kinases, 250 nM AZD8186 appreciably inhibited only PI3Kβ (98% inhibition) and PI3Kδ (92% inhibition) and did not inhibit other kinases including mTOR (S.S., S.C., unpublished data). In an in vitro kinase assay using the catalytic domains of the different PI3K isoforms as substrates (See Supplemental Experimental Procedures), the IC50 of AZD8186 for PI3Kβ was 4 nM; PI3Kδ, 12 nM; PI3Kα, 35 nM, and PI3Kγ, 675 nM.
We determined the concentrations at which AZD8186 inhibits PI3K signaling and cell growth in BT-474, a HER2 dependent breast cancer cell line in which signaling is driven by PI3Kα (Torbett et al., 2008), and in LNCaP, a PTEN-mutated prostate carcinoma cell line. In BT-474, up to 250 nM AZD8186 had no effect on Akt/mTOR signaling, but 500 nM had some effect (Figure S1B). 250 nM AZD8186 also had little effect on BT-474 growth (Figure S1C), so we concluded that it is a selective inhibitor of PI3Kβ at this concentration.
Akt phosphorylation was significantly inhibited in the PTEN-mutated LNCaP exposed to 25 nM AZD8186 for two hours (Figure S1D). Thus, in these cells, AZD8186 inhibits PI3K signaling at concentrations more than 10-fold below those that inhibit PI3Kα or mTOR. LNCaP proliferation was correspondingly sensitive to AZD8186 (IC50 100 nM ± 92 nM) (Figure S1E). 250 nM AZD8186, a concentration that afforded selective inhibition of PI3Kβ, was used in subsequent studies.
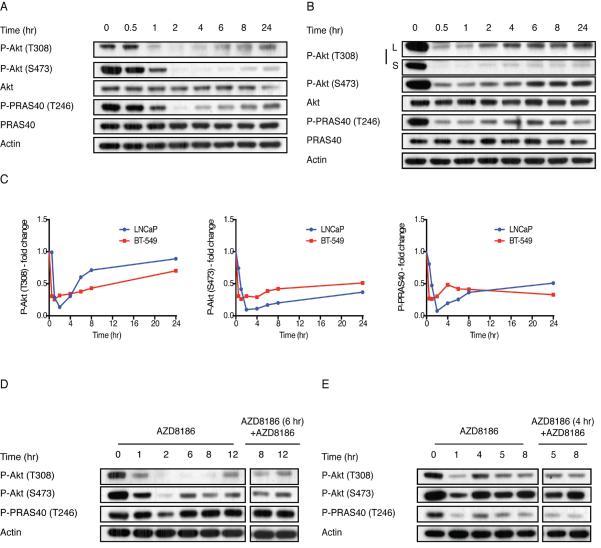
When LNCaP or the PTEN-mutated triple negative breast cancer (TNBC) cell line, BT-549 were exposed to 250 nM AZD8186, PI3K signaling was rapidly inhibited but subsequently rebounded (Figure 1A, B). Akt phosphorylation was maximally inhibited after thirty minutes of AZD8186 treatment of BT-549 and after two hours of treatment of LNCaP (Figure 1 A–C). Dephosphorylation of the Akt substrate PRAS40 occurs with the same kinetics (Figure 1 A–C). Rebound in the phosphorylation of Akt T308 and PRAS40 occurred in both cell lines, two hours after treatment of BT-549 and four hours afterwards in LNCaP (Figure 1A–C). Rebound of Akt S473 phosphorylation occurred more slowly, after six hours of drug treatment (Figure 1A–C). After 24 hours of treatment of LNCaP cells, phosphorylation of Akt T308 rises to 85% of baseline, P-Akt (S473) to 35% and P-PRAS40 to 50% of baseline (Figure 1A, C). In BT-549 cells the phosphorylation of Akt T308 rises to 70%, P-Akt S473 to 50% and P-PRAS40 to 30% of baseline (Figure 1B, C).
Figure 1. PI3Kα inhibition in PTEN mutant models leads to a transient inhibition of the Akt pathway.
(A, B) LNCaP (A) and BT-549 (B) cells were treated with 250 nM AZD8186 as indicated and the effect on the downstream signaling was analyzed. (C) The fold change in P-Akt (T308), P-Akt (S473) and P-PRAS40 levels following AZD8186 treatment was determined by densitometric analysis of the bands in (A) and (B), respectively. (D, E) LNCaP (D) and BT-549 (E) cells were treated with 250 nM AZD8186 for the indicated times. Lysates were immunoblotted with indicated antibodies (left panels). After 4 (BT-549 cells) or 6 (LNCaP cells) hours of AZD8186 treatment, AZD8186 was re-added to the cells (right panels) for the indicated times and lysates were immunoblotted with the indicated antibodies. See also Figure S1 and Table S1.
To determine the generality of the phenomenon, the effects of AZD8186 were assessed in a panel of eleven cell lines with PTEN inactivation. PI3K signaling was rapidly inhibited by the drug and then rebounded significantly hours later in all of the cell lines (Table S1). AZD8186 caused significant inhibition of cell growth in seven of the eleven cell lines, with IC50s for growth inhibition in the range of concentrations that selectively inhibit PI3Kβ. In cell lines with wild type PTEN, PI3Kβ inhibition did not inhibit PI3K signaling or cell proliferation (Table S1). An unrelated PI3Kβ inhibitor GSK-2636771 is selective against p110β at 1 μM and also caused rapid inhibition of PI3K signaling in LNCaP that rebounded after 4 to 8 hours (Figure S1F–H).
Rebound of Akt signaling after PI3Kβ inhibition is PI3Kα-dependent
Reactivation of Akt signaling after PI3Kβ inhibition is not likely to be due to decreasing intracellular drug concentrations, since re-addition of 250 nM AZD8186 to cells after the initial exposure to drug did not inhibit phosphorylation of Akt or PRAS40 (Figure 1D, E). We asked whether rebound is due to induction of other class 1 PI3K isoforms. Expression of p110γ or p110δ protein was not detected in LNCaP or BT-549 cells treated with the PI3Kβ-inhibitor (AZD8186, 250 nM), and expression of p110α, p110β and of p85 was unchanged (Figure S1I, S1J).
The selective PI3Kα inhibitor BYL719 (Fritsch et al., 2014) had no effect on the phosphorylation of Akt or Akt substrates in LNCaP or BT-549 cells (Figure 2A, S2A). However, in both cell lines, combining PI3Kβ (AZD8186) and PI3Kα (BYL719) inhibitors markedly reduced the rebound in phosphorylation of Akt and its substrates that occurred with AZD8186 alone (Figure 2A, S2A). Similar findings were obtained in the PTEN-mutated TNBC cell line HCC70 and the prostate cancer cell line PC3 (Figure S2B, S2C). Thus, in these cells, PI3Kα does not drive PI3K signaling, but is responsible for the rebound in PI3K signaling that occurs after PI3Kβ inhibition.
Figure 2. Reactivation of Akt signaling following PI3Kα inhibition is PI3Kβ-dependent.
(A) LNCaP cells were treated with AZD8186 (250 nM), BYL719 (2.5 μM) or the combination. Cells were collected at indicated times and the effect on Akt signaling and apoptotic markers was analyzed by immunoblotting. (B) LNCaP cells were treated with AZD8186 for various times. PI3Kβ was immunoprecipitated from cell lysate and PI3K activity assay was performed. The product, PI3P, was resolved by thin-layer chromatography and detected by autoradiography. A lane with a purified PI3Kβ was used as a positive control and a lane with IgG was used as a negative control for the assay (left panel). Whole cell lysates and immunoprecipitates were blotted with anti-PI3Kβ (left panel). The change in PI3Kβ activity was determined by densitometric analysis of the bands (right panel). (C) Cell growth of LNCaP cells treated as indicated was monitored with cell counter. The data represent mean ± SD of triplicate samples. (D) Apoptotic cell death of LNCaP cells treated as indicated for 48 hr was quantified by Annexin V staining. The data represent mean ± SD of duplicate samples. See also Figure S2.
These results suggest that PI3Kα activity is low in these cells and induced after PI3Kβ inhibition. To test this possibility, in vitro PI3K assays were performed on PI3Kα immunoprecipitated from lysates of LNCaP cells exposed to AZD8186 (Figure 2B). PI3Kα activity, as measured by the accumulation of P32-labeled, phosphatidylinositol 3-phosphate, was induced 3.9-fold four hours after PI3Kβ inhibition, reaching a peak (5-fold induction) at six to eight hours. Expression of the PI3Kα protein was unchanged (Figure 2B). We conclude that after inhibition of PI3Kβ in PTEN-mutated tumor cells, induction of PI3Kα kinase activity occurs and is in part responsible for the rebound in Akt/mTOR signaling.
The durable inhibition of PI3K signaling achieved by combining inhibitors of PI3Kβ and PI3Kα has biologic consequences. PI3Kβ inhibition causes significant slowing of LNCaP growth in culture, but proliferation persists and cell number begins to increase 3–4 days after drug addition (Figure 2C). PI3Kα inhibition has little effect, but the combination completely suppresses growth (Figure 2C), induces PARP and Caspase 3 cleavage (Figure 2A) and apoptosis (Figure 2D). Similar effects were noted in BT-549, HCC70 and PC3 cells treated with the combination (Figures S2D–G).
BAY806946 – a PI3K inhibitor that more potently inhibits PI3Kα than PI3Kβ (Glauer et al., 2013), had similar effects as BYL719 in LNCaP— no effect when given alone but significantly enhanced inhibition of signaling and suppression of growth when combined with the PI3Kβ inhibitor (Figure S2H, S2I). Thus, inhibition of PI3Kα suppresses the rebound in PI3K signaling in tumor cells exposed to PI3Kβ inhibitors and this is associated with enhanced inhibition of cell growth and induction of apoptosis.
Reactivation of PI3Kα is IGF1R dependent
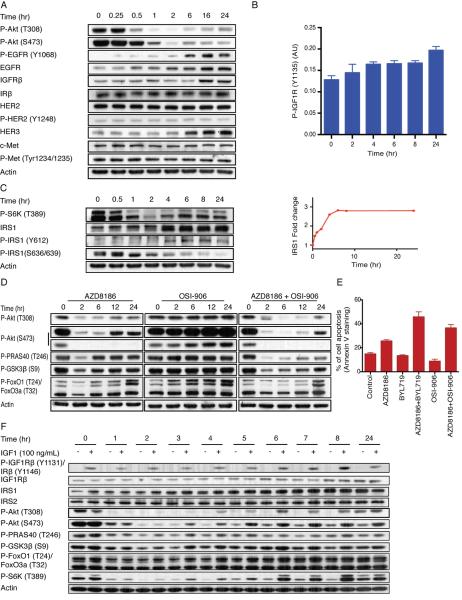
Activation of the PI3K pathway causes feedback inhibition of RTK signaling via Akt and mTOR dependent mechanisms (Chandarlapaty et al., 2011; O'Reilly et al., 2006; Rodrik-Outmezguine et al., 2011). We hypothesized that PI3Kβ inhibitors induce PI3Kα kinase activity by relieving this feedback. Antibody arrays were used to assess the effect of PI3Kβ inhibition on the phosphorylation of 49 RTKs. In LNCaP cells, phosphorylation of EGFR and HER2 were induced 24 hours after PI3Kβ inhibition (Figure S3A), but only induction of Y1068 EGFR phosphorylation was confirmed by immunoblotting (Figure 3A, S3B) and the EGFR inhibitor gefitinib (2 μM) prevented induction of EGFR Y1068 phosphorylation without affecting Akt rebound or inhibition of cell growth (Figure S3C–E).
Figure 3. PI3Kβ activation is IGF1R dependent.
(A) Expression and/or phosphorylation of several RTKs in LNCaP cells treated with 250 nM AZD8186 for the indicated times was examined by immunoblot analysis. (B) The phosphorylation of IGF1R (Y1131) in LNCaP cells treated with 250 nM AZD8186 for the indicated times was quantified by sandwich ELISA kit. The data represent mean ± SD of triplicate samples. (C) LNCaP cells were treated with 250 nM AZD8186 and collected at various time points. Immunoblotting analysis was performed as indicated. The change in the expression of IRS1 was determined by densitometric analysis of the bands. (D) LNCaP cells were treated with AZD8186 (250 nM), OSI-906 (2 μM), or the combination for the indicated times. Immunoblotting of lysates was used to examine the effect on downstream signaling. (E) LNCaP cells were treated with AZD8186, BYL719, OSI-906, or the combinations as indicated. Apoptotic cell death was quantified by Annexin V staining after 48 hours of treatment. Data are the mean percentage of Annexin V-positive cells ± SD. (F) LNCaP cells were treated with AZD8186 for various times. At the indicated time cells were stimulated with IGF-1 (100 ng/ml) for 10 min. Immunoblotting analysis was used to assay the effect on downstream signaling. See also Figure S3.
Previous work showed that inhibition of Akt/mTOR signaling reactivates the expression and/or activation of multiple RTKs that regulate the pathway, including IGF1R, insulin receptor (IR), and HER kinases (Chandarlapaty et al., 2011; Rodrik-Outmezguine et al., 2011). Treatment of LNCaP or BT-549 cells with the PI3Kβ inhibitor caused induction of the expression of HER3 and IGF1R (Figure 3A, S3F, S3G), but had no effect on the expression of IR (Figure 3A and S.S., N.R., unpublished data). Increased phosphorylation of HER3, IR, or IGF1R was not detected.
IR, IGF1R, and HER3 are key physiologic activators of the PI3K pathway. Induction of HER3 is unlikely to play a dominant role in reactivation of PI3Kα in these cells, since neither gefitinib (Figure S3C, S3D, S3H, S3I) nor lapatinib (S.S., unpublished data) affected Akt rebound or cell growth in LNCaP or BT-549 treated with AZD8186.
Activation of Akt/mTOR causes the feedback inhibition of IGF1R and IR signaling by inhibiting the expression and activation of both receptors and the expression of the IGF1R/insulin receptor substrate (IRS) proteins, IRS1 and IRS2 (Haruta et al., 2000; Simpson et al., 2001; Chandarlapaty et al., 2011; O'Reilly et al., 2006). ELISA assays showed that the expression level of IGF1R rises significantly from 1594 pg/mL to 3155 pg/ml after 8 hours of PI3Kβ inhibition and is induced 3-fold by 24 hours (Figure S3J). Induction of IGF1R expression was accompanied by a small increase in its phosphorylation as assessed by ELISA analysis–1.3-fold induction after 4 hours of AZD8186 treatment, reaching 1.5-fold induction after 24 hours (Figure 3B).
The increase in expression of IGF1R is accompanied by increased expression of the IRS1 protein in LNCaP (1.5-fold after one hour, 2.8-fold in 6 hours) and BT-549 cells (1.9-fold induction after 4 hours) (Figure 3C and S3K). In both models, mTOR activation is dependent on PI3Kβ, as demonstrated by the sensitivity of S6K phosphorylation to AZD8186 (Figure 3C, S3K). Activated S6K phosphorylates serine sites of IRS1 (S312 and/or S636/639) that causes the degradation of IRS1 (Haruta et al., 2000; Um et al., 2004). Increased IRS1 expression in cells treated with AZD8186 was associated with decreased phosphorylation of IRS1 S636/639 and increased phosphorylation at Y612, a site that plays a role in the ability of IRS1 to activate PI3K (Esposito et al., 2001) (Figure 3C, S3K). These data suggest that inhibition of PI3Kβ relieves feedback inhibition of IGF and insulin signaling by increasing IGF1R and IRS1 expression and thus causing reactivation of PI3Kα.
To test this hypothesis, we used OSI-906, a dual inhibitor of the IGF1R and IR kinases (IC50 0.035 μM for IGF1R, 0.075 μM for IR and IC50s greater than 10 μM against a panel of 45 other kinases) (Mulvihill et al., 2009). Combined treatment of LNCaP or BT-549 with OSI-906 (2 μM) and AZD8186 (250 nM) significantly diminished and delayed the rebound in Akt signaling observed with AZD8186 alone (Figure 3D, S3L). OSI-906 enhanced the growth inhibition caused by AZD8186 almost to the same degree as the PI3Kα inhibitor did (Figure S3M, S3N). OSI-906 alone had slight effects on Akt signaling in LNCaP cells (Figure 3D). In BT-549, OSI-906 alone induced PRAS40 and FOXO1/3 phosphorylation and had no effect on Akt or GSK3β phosphorylation (Figure S3L). Moreover, whereas the IGF1R/IR inhibitor alone did not induce apoptosis in LNCaP and BT-549 cells, combining AZD8186 and OSI-906 caused a marked increase in apoptosis compared to AZD8186 alone (Figure 3E, Figure S2G).
These data suggest that relief of feedback inhibition of IRS1 and IGF1R expression plays a major role in causing the observed reactivation of PI3Kα signaling. This conclusion is supported by a knockdown experiment: siRNA-mediated depletion of IRS1 in LNCaP cells reduced the rebound of Akt phosphorylation in LNCaP cells exposed to the PI3Kβ inhibitor whereas a scrambled siRNA control had no effect (Figure S3O).
Our findings suggest that, in PTEN-mutated tumors, PI3Kβ activation causes feedback inhibition of IGF1R signaling. If this is the case, the signaling response to IGF should be blunted unless the feedback is relieved. To determine whether this is so, we assessed IGF1 induction of PI3K signaling in LNCaP at various times after PI3Kβ inhibition with AZD8186 (Figure 3F). In control LNCaP cells, IGF1 stimulation for 10 minutes did not stimulate the pathway (time 0). After treatment of the cells with AZD8186 for one or two hours, PI3K signaling was inhibited, but IGF1 stimulation did not cause phosphorylation of Akt, its substrates, or S6K. After three hours of treatment, IGF1 clearly induced phosphorylation of Akt S473, GSK3β, PRAS40 and S6K (Figure 3F). IGF1R expression and induction of IGF1R phosphorylation were unchanged at that time, nor had a rebound in PI3K signaling occurred in cells untreated with IGF1 (Figure 3F). The data is consistent with enhanced transduction of the IGF1R signal three hours after PI3Kβ inhibition (Figure 3F). Induction continued to increase up to seven hours after PI3Kβ inhibition and was maintained for at least 24 hours. Increased IRS1 and IGF1R expression and rebound in PI3K signaling in the absence of IGF1 stimulation became evident three, six and five hours after PI3Kβ inhibition respectively, whereas increased IGF1R phosphorylation in response to IGF1R was noted six hours afterwards (Figure 3F). Thus, transduction of the activated IGF1R signal is suppressed in these tumors and enhanced after PI3Kβ inhibition. This enhancement likely plays a role in the induction of PI3Kα signaling that occurs after PI3Kβ inhibition. Induction of IRS1 plays a major role in this process and induction of IGF1R expression probably does as well, but the kinetics in Figure 3F show that this is a complex phenomenon and other factors are undoubtedly involved.
Combined inhibition of PI3Kα and PI3Kβ enhances PI3K pathway inhibition in vivo
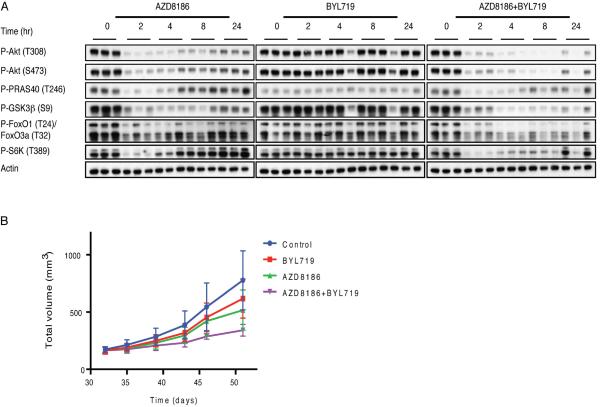
The optimal single agent murine dose of AZD8186 was determined to be 75 mg/kg BID, causing maximal inhibition of LNCaP xenograft growth without weight loss at this or even higher doses (100 mg/kg BID) (Figure S4A, S4B). This dose of AZD8186 had only minor effects on PI3Kα signaling in BT-474 xenografts and no significant effects on tumor growth (Figure S4C, S4D, Table S2). Mice bearing PTEN mutant LNCaP xenografts were treated with a single dose of 75 mg/kg of AZD8186, a single dose of 25 mg/kg of BYL719 or the combination. Two hours after AZD8186 administration, reduced phosphorylation of Akt, PRAS40, GSK3β, FOXO1/3 and S6K was observed and persisted for four hours but, by eight hours, Akt signaling had rebounded from its nadir (Figure 4A). By 24 hours, phosphorylation of S6K and PRAS40 rose to initial levels, whereas phosphorylation of Akt, GSK3β and FOXO1/3 had risen significantly but remained below untreated levels (Figure 4A). 25 mg/kg BYL719 (Fritsch et al., 2014) had little effect on PI3K signaling in LNCaP but did prevent the rebound observed with AZD8186 8 hours after drug administration and substantially reduced the rebound at 24 hours (Figure 4A). Similar results were obtained with these regimens in PTEN mutant PC3 prostate cancer xenografts (Figure S4E).
Figure 4. Inhibition of the PI3K pathway in mice bearing LNCaP tumors.
(A) Mice (n=3 mice/time point) bearing LNCaP tumors were randomized to receive vehicle, 75 mg/kg AZD8186, 25 mg/kg BYL719 or the combination of both, and treated for the specified times. The tumors were analyzed by immunoblotting to assay downstream signaling. (B) Mice bearing LNCaP tumors were randomized to receive vehicle, AZD8186 75 mg/kg twice per day, BYL719 25 mg/kg once per day, or the combination for 25 days. Tumor size was measured twice per week. The results are presented as the mean tumor volume ± SEM (n = 5 mice/group). See also Figure S4 and Table S2.
We next examined whether the IGF1R pathway drives PI3Kα reactivation in vivo in LNCaP xenografts. 75 mg/kg OSI-906 or 25 mg/kg BYL719 both significantly reduced the rebound observed with AZD8186 (Figure S4F). These data suggest that in these tumors, feedback activation of PI3Kα following PI3Kβ inhibition in vivo is driven at least in part through IGF1R or IR signaling.
Neither AZD8186 nor BYL719 had significant effects on LNCaP, HCC70 or PC3 xenograft growth (Figure 4B, S4G, S4H Tables S2). Combined treatment, results in significant slowing, but not arrest, of the growth of LNCaP tumors ((65% tumor growth inhibition (TGI)) (Figure 4B, Table S2), tumor regression in HCC70 (143% TGI), (Figure S4G, Table S2) and significant slowing of PC3 (84% TGI) (Figure S4H, Table S2). None of these treatments had a significant effect on the weight of the mice (Figure S4I, S4J).
Thus, prolonged inhibition of PI3K signaling by combining PI3Kα and PI3Kβ inhibitors is associated with enhanced inhibition of tumor growth. A similar experiment was performed with combined inhibition of PI3Kβ and the IGF1R/IR inhibitor OSI-906, but administration of either the latter alone or the combination was not tolerated due to more than 10% weight loss after three days of treatment (S.S., N.R., unpublished data). These results suggest that prevention of rebound with the PI3Kα inhibitor may be more feasible than with a dual IGF1R/IR kinase inhibitor.
Combined PI3Kα, PI3Kβ and androgen receptor inhibition potently inhibits PTEN mutant prostate tumors in vivo
Durable inhibition of PI3K signaling was achieved with combined inhibition of PI3Kβ and PI3Kα but was not sufficient to arrest the growth of the PTEN-mutated androgen receptor (AR)-dependent LNCaP prostate cancer model. AR and PI3K driven signaling are the two most commonly activated pathways in prostate cancer. We have recently shown that inhibition of either pathway relieves feedback inhibition of the other (`reciprocal feedback') and that combined inhibition of both is required to adequately inhibit tumor growth (Carver et al., 2011).
Inhibition of PI3Kβ activates AR in LNCaP cells as shown by the induction of the expression of three AR target genes, TMPRSS2, NKX3.1 and PSCA (Figure S5A). As shown previously (Carver et al., 2011), the potent AR inhibitor MDV3100 causes activation of Akt signaling (Figure S5B). To examine if activation of Akt after AR-inhibition is PI3K dependent we treated LNCaP cells with the AR inhibitor and added the PI3Kβ inhibitor or the PI3Kα inhibitor six hours later. AZD8186 addition caused marked inhibition of Akt signaling. BYL719 also reduced the induction of Akt phosphorylation by MDV3100, but to a much lesser extent (Figure S5C). Thus, it appears that induction of PI3K signaling in response to AR inhibition is mediated by both isoforms.
Addition of BYL719 to the combination of AZD8186 and MDV3100 prevented the rebound in the phosphorylation of Akt as well as the rebound induced by AZD8186 alone (Figure S5D). The combination of MDV3100 and BYL719 had only a mild effect on Akt signaling consistent with the dominant role of PI3Kβ in activating Akt (Figure S5D).
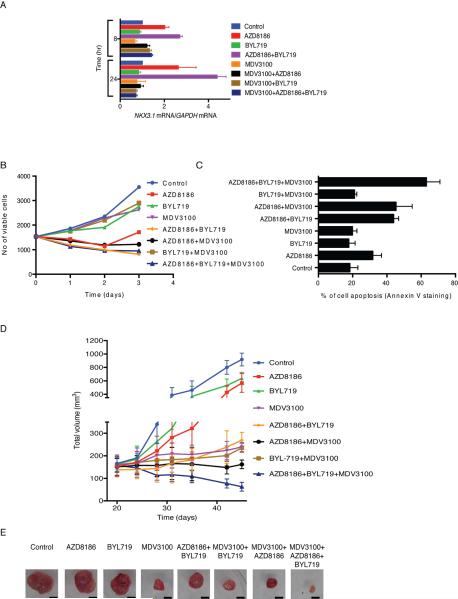
Inhibition of PI3Kβ with AZD8186 induced the mRNA expression of the AR-target gene NKX3.1, 2.7-fold after 24 hours (Figure 5A). In contrast, the PI3Kα inhibitor BYL719 had no effect, consistent with its lack of effect on PI3K signaling. However, BYL719 enhanced the induction of NKX3.1 observed with AZD8186 alone (4.4-fold above control; 1.7-fold above AZD8186). The AR inhibitor MDV3100 reduced the expression of NKX3.1 (Figure 5A) and strongly blunted the induction of AR activity by the PI3K inhibitors eight hours after combined treatment and completely suppressed the effect 24 hours after treatment (Figure 5A).
Figure 5. Combined inhibition of PI3Kα, PI3Kβ and AR potently inhibits LNCaP tumors in vitro and in vivo.
(A) LNCaP cell were treated as indicated and expression of NKX3.1 normalized to GAPDH was determined using qRTPCR. Error bars represent the SD of triplicate samples. (B, C) LNCaP cell were treated as indicated and cell growth was monitored with cell counter (B, error bars represent the SD of triplicate samples) or apoptotic cell death was quantified by Annexin V staining after 2 days of treatment (C, data are the mean percentage of Annexin V- positive cells ± SD of two independent experiments). Levene's test for equality of variance was followed by an unpaired, two-tailed Student t-test. (D) Castrated mice bearing LNCaP tumors were randomized to vehicle, AZD8186 (75 mg/kg) twice per day, BYL719 (25 mg/kg) once per day, MDV3100 (30 mg/kg) once per day or the different combinations for 3 weeks. Tumor size was measured twice per week. The results are presented as the mean tumor volume ± SEM (n = 5 mice/group). (E) Representative images of tumors following 3 weeks of treatment as in (D) Scale bar determined using the diameter of the 10 cm plate as a scale, represents 1 cm. See also Figure S5 and Table S3.
These results suggest that the antitumor effects of the PI3K inhibitors may be attenuated by their induction of AR signaling. In tissue culture, combined inhibition of PI3Kβ (AZD8186) and AR (MDV3100) inhibited LNCaP proliferation more than either drug alone (Figure 5B). Combining AZD8186 with MDV3100 or BYL719 completely suppressed growth and the triple combination was no better (Figure 5B). However, there were significant differences in induction of cell death, as measured by Annexin V staining (Figure 5C). Neither MDV3100 nor BYL719, alone or in combination, induced apoptosis, whereas AZD8186 (250 nM) caused a 1.7-fold induction (Figure 5C). Combining BYL719 with AZD8186 enhanced the effects of the latter-2.4-fold above control, 1.4-fold above AZD8186 alone (Figure 5C), suggesting that the rebound in PI3Kα activity has a protective effect on the tumor cell. Combining AR inhibition with PI3Kβ inhibition caused a similar increase. Combining both PI3K inhibitors with AR inhibition caused the greatest induction of apoptosis—a 3.4-fold increase of Annexin V stained cells relative to the control. The results imply that feedback reactivation of either pathway in response to inhibition of the other serves to limit apoptosis (Figure 5C).
We investigated the effects of suppressing both signaling pathways in vivo. Mice bearing LNCaP tumors were castrated and treated for three weeks with vehicle, individual drugs or different combinations. BYL719 and AZD8186 had only minor effects on tumor growth when administered alone (36% and 40% TGI, respectively) (Figure 5D). Combining both PI3K isoform inhibitors had a much greater effect (78% TGI), but the tumors continued to grow slowly. Similar effects were noted when the AR inhibitor MDV3100 was administered alone or in combination with BYL719 (90% and 93% TGI). The combination of PI3Kβ (AZD8186) and AR (MDV3100) inhibitors was superior and completely inhibited tumor growth (99% TGI), (Figure 5D, Table S3). However only the triple combination of AZD8186, BYL719 and MDV3100 caused significant tumor regression, which continued throughout the course of treatment (113% TGI), (Figure 5D, Table S3). Images of representative tumors from each treatment group reflect the effects depicted in the growth curve (Figure 5E).
Combined inhibition of PI3K and AR was also studied in a GEMM with prostate cancer caused by conditional deletion of Pten (PB-Cre;Ptenlox/lox) (Carver et al., 2011). In control mice, tumors grew slowly, with a 12.5% increase in the size of established tumors over 30 days. Because of the slow growth of these models, we considered a 30% decline in MRI tumor volume as a significant antitumor effect.
We assessed the effects of administration of the PI3Kβ inhibitor AZD8186 for 30 days and compared the results with three combination therapies - AZD8186 and the PI3Kα inhibitor BYL719, AZD8186 and the AR inhibitor MDV3100, and the triple combination of AZD8186, BYL719 and MDV3100. After treatment with 75 mg/kg AZD8186 for 30 days, tumor masses comprised of intraductal carcinoma of the prostate had increased in size by 2.86%, suggesting a modest inhibition of growth compared to controls (Figure 6A, 6B). Akt S473 phosphorylation was still detectable by immunohistochemistry, at slightly reduced levels. By contrast, in mice treated with the combination of PI3Kα and PI3Kβ inhibitors, there was a marked reduction in Akt phosphorylation (S473) (Figure 6B) however, intraductal prostatic carcinoma persisted and only slight inhibition of tumor growth at 30 days compared to AZD8186 alone (mean volume reduction of 6.1%) (Figure 6A, 6B).
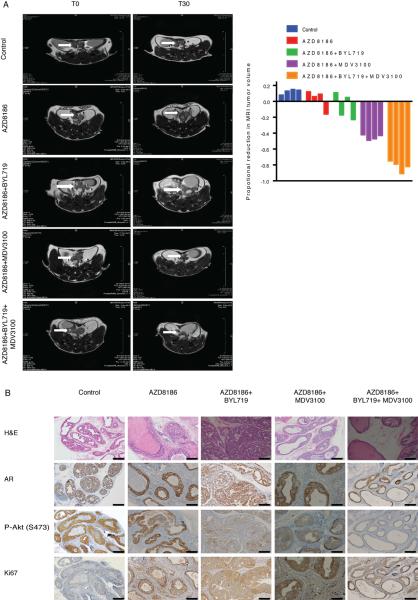
Figure 6. Triple therapy of PI3Kα, PI3Kβ and androgen receptor inhibitors potently inhibits Ptenlox/lox prostate tumors in vivo.
(A) Intact mice were treated with vehicle and castrated PB-Cre;Ptenlox/lox mice were treated with AZD8186 (75 mg/kg) twice per day alone, AZD8186 in combination with either BYL719 (25 mg/kg) once per day or MDV3100 (30 mg/kg) once per day, or the triple combination. Representative Magnetic Resonance Imaging (MRI) images of mice at initiation (T0) and completion of study (30 days, T30) are shown at the left and the waterfall plot depicting proportional change in tumor tumor volume of treated mice (n=4 per group) is shown at the right. (B) Representative images of histological and immunohistochemical staining for P-Akt (S473), AR and ki67 of tumors from mice treated as in (A). Scale bar represents 50 μm.
In our experience, MDV3100 causes a 19.2% reduction in the growth of this model in 30 days (Carver et al., 2011). Combined inhibition of PI3Kβ and AR led to a dramatic increase in antitumor efficacy compared to either alone (mean tumor volume reduction of 45%), but despite PI3Kβ inhibition some persistence of P-Akt was observed. Triple therapy (AZD8186, BYL719 and MDV3100) was even more efficacious with a near complete pathologic response (mean volume reduction of 84%), which suggests that suppression of feedback reactivation of PI3Kα enhances antitumor activity (Figure 6). A reduction in nuclear AR and Akt S473 phosphorylation occurs and only microscopic evidence of persistent focal prostatic intraepithelial neoplasia were observed compared with AZD8186 alone (Figure 6B). Thus, in both models combined therapy with AR, PI3Kα and PI3Kβ inhibitors is required for maximal tumor regression. None of the treatments caused weight loss in the mice bearing LNCaP tumors (Figure S5E) and nor were they associated with lethargy or feeding abnormalities in the GEMM.
DISCUSSION
Tumors with mutant PIK3CA or PTEN have not been especially sensitive to PI3K pathway inhibitors. Early studies focused on the use of selective mTOR and Akt inhibitors, but PI3K signals through other downstream targets in addition to Akt/mTOR. Moreover, whereas PI3K, Akt and mTOR inhibitors all relieve feedback inhibition of receptor tyrosine kinases, only PI3K inhibitors suppress rather than reactivate PI3K. Inhibition of PI3K may therefore be more effective therapeutically than inhibitors of downstream elements of the pathway and recent preclinical work showing that PI3K inhibitors have more potent antitumor activity than Akt inhibitors supports this idea (Will et al., 2014; Ebi et al., 2013). Early clinical experience with inhibitors of pan-class 1 PI3K was also disappointing however (Martini et al., 2013). This may have been due to a variety of factors, including the off-target toxicity of non-selective drugs, the toxic effects of PI3K inhibition, and the adaptation of the signaling network to inhibition of the pathway.
Selective isoform-specific inhibitors now offer the possibility of effective and potent inhibition of PI3K in tumors. Quite selective PI3Kα, β and δ inhibitors have been developed and have limited toxicity in preclinical models and early clinical trials (Juric et al., 2012; Furman et al., 2014; Gopal et al., 2014). Such drugs could be given at high enough doses to effectively suppress PI3K signaling in tumors dependent on particular PI3K isoforms. For instance, PI3Kδ is selectively expressed in lymphoid cells and PI3Kδ inhibitors have been shown to have impressive clinical activity in a variety of B-cell neoplasms (Furman et al., 2014; Gopal et al., 2014). Similarly, PI3Kα inhibitors have significant activity in hormone dependent breast cancers with mutant PIK3CA (Juric et al., 2012).
We show here that PI3K signaling and tumor cell growth were sensitive to selective inhibition of PI3Kβ in PTEN-mutated tumor cells but not in those driven by HER2 or mutant PIK3CA. PI3Kβ inhibition potently inhibited Akt/mTOR signaling in PTEN-mutated tumors, whereas PI3Kα inhibition had no effect. However, inhibition of Akt/mTOR signaling by PI3Kβ inhibition was transient because inhibition of Akt/mTOR relieves feedback inhibition of RTKs, thus reactivating PI3Kα.
Akt/mTOR signaling is driven by the dominant PI3K isoform in the tumor, whether it is PI3Kα or PI3Kβ. We and others have shown that activation of Akt and mTOR causes feedback inhibition of RTK signaling by a complex and incompletely characterized set of mechanisms. Akt phosphorylates and inhibits FOXO family transcription factors, thereby reducing the expression of HER3, IR and IGF1R - key physiologic activators of PI3K signaling (Chandarlapaty et al., 2011; Muranen et al., 2012; Garrett et al., 2013). Akt also activates mTOR, which then inhibits RTK signaling in a complex manner that includes phosphorylation and degradation of IRS1 and IRS2, and phosphorylation and stabilization of the PI3K inhibitory protein Grb10 (Yu et al., 2011; Hsu et al., 2011; Simpson et al., 2001; Haruta et al., 2000; O'Reilly et al., 2006). Thus, activation of Akt is expected to inhibit both receptor signaling and expression and inhibitors of mTOR and Akt have been shown to relieve this feedback and to reactivate PI3K signaling.
In the models we studied, PI3Kβ inhibition variably induced multiple receptors, IRS1, and IRS2 and the induction of PI3K rebound was always reduced by an inhibitor of IGF1R/IR. Inhibitors of HER2 or HER1 affected rebound only in a few models and usually to a lesser degree (S.S., N.R., unpublished data). The data suggests that the dominant cause of rebound in the tumors we studied in detail is relief of Akt/mTOR-dependent feedback inhibition of IGF1R and IRS1 expression. It is likely that in other cellular contexts, other receptors are also involved as the ligand environment and steady state expression of receptors will dictate the major mechanism of PI3Kα suppression in a given tumor. This model has several implications. RTKs selectively activate PI3Kα so it is clear why tumors in which these receptors are dysregulated are dependent upon this isoform. Loss of PTEN increases the half-life of PIP3 and results in a marked activation of Akt signaling. It is not clear why PTEN-mutated tumors are selectively dependent upon GPCR activation of PI3Kβ with little or no input from RTK activated PI3Kα (Knight et al., 2006; Dbouk and Backer, 2010; Fritsch et al., 2013). It is possible that PTEN loss occurs preferentially in tumors derived from particular lineages in which signaling is dependent on GPCRs. Our findings suggest a model in which PTEN loss leads to a hyperactivation of Akt/mTOR that is expected to potently feedback inhibit receptor tyrosine kinase-mediated activation of PI3Kα. Residual PI3Kβ activity, GPCR-dependent or not, is sufficient to maintain Akt activation and suppression of RTK dependent PI3Kα activation. This model predicts that ligand activation of Akt will be suppressed in such tumors. We did find that IGF did not activate Akt in PTEN mutant LNCaP cells. However, pharmacologic inhibition of PI3Kβ is predicted to reduce feedback inhibition of receptor signaling and cause a PI3Kα-dependent rebound in Akt/mTOR signaling. In support of the model, we find that the ability of IGF to activate Akt phosphorylation is restored as a function of time after PI3Kβ inhibition.
The model also suggests that receptor mediated activation of PI3Kα is more sensitive to Akt/mTOR feedback inhibition than PI3Kβ activation is. If so, PI3Kβ inhibitors would cause reactivation of PI3Kα and a significant rebound in Akt phosphorylation that would not reach baseline levels. We observe that this is the case. In an accompanying manuscript, Costa et al., show complementary results with a selective inhibitor of PI3Kα, which inhibits Akt/mTOR signaling in tumors with RTK activation or PI3K mutation, but not in those with PTEN inactivation. Inhibition of signaling by the PI3Kα inhibitor was also associated with rebound, which, in this case was sensitive to PI3Kβ inhibition.
Reactivation of PI3Kα prevents durable inhibition of signaling by PI3Kβ inhibitors and may limit their clinical efficacy. AZD8186 effectively inhibits the growth of PTEN-mutated tumor cells in vitro but induces only minimal apoptosis and has modest antitumor activity in xenograft models. In contrast, combining PI3Kα and PI3Kβ inhibitors caused durable inhibition of PI3K signaling, increased apoptosis, and enhanced antitumor activity.
In normal cells, both PI3Kα and PI3Kβ contribute to the effects of the pathway on metabolism and growth (Foukas et al., 2006; Jia et al., 2008). This may be the basis for the lower toxicity of isoform dependent inhibitors. This paper and that of Costa et al., suggest that relief of feedback inhibition of the other isoforms may contribute as well. Combined therapy with PI3Kα and β inhibitors may cause greater `on-target' toxicity. We have recently shown that intermittent administration of a PI3Kα preferential inhibitor has potent antitumor activity in vivo (Will et al., 2014). Intermittent schedules may allow effective PI3K pathway inhibition without inordinate toxicity.
It has become increasingly clear that activation of PI3K signaling leads to feedback inhibition of androgen and estrogen receptor signaling in prostate and breast cancer. In PTEN mutant prostate cancer, we have shown that AR activation causes feedback inhibition of PI3K signaling as well (Carver et al., 2011). This reciprocal feedback inhibition of the two pathways limits the therapeutic effects of inhibitors of either. PI3K pathway inhibition has been almost completely ineffective in prostate cancer. In fact, mTOR inhibitors have been shown to activate AR and increase PSA in patients with advanced prostate cancer (H.I.S, unpublished data). We show here that PI3Kβ inhibition induces the expression of AR-dependent gene expression and that combined inhibition of PI3Kβ and PI3Kα enhances this effect, supporting the conclusion that suppression of PI3Kα rebound significantly increases inhibition of pathway output. Given this marked activation of AR-dependent gene expression, it is not surprising that combined PI3K isoform inhibition has only modest antitumor effects in vivo in AR-dependent prostate cancer models. Similarly, AR inhibition causes tumor stasis, but not regression in these models. In contrast, the three-drug combination results in marked regression in both the GEM and xenograft model.
The antitumor effects of selective mTOR and Akt inhibitors may be limited because of their relief of feedback inhibition of PI3K. Isoform selective PI3K inhibitors have already shown significant antitumor activity in tumors driven by PI3Kα or PI3Kδ (Juric et al., 2012; Furman et al., 2014; Gopal et al., 2014). PI3Kδ is selectively expressed in lymphoid cells and PI3Kδ inhibitors have been shown to have marked benefit in a variety of B-cell neoplasms (Furman et al., 2014; Gopal et al., 2014). Similarly, PI3Kα inhibitors such as BYL719 have significant single agent activity in PI3Kα mutant breast cancers (Juric et al., 2012). In this paper and in that of Costa et al., combined inhibition of PI3Kβ and PI3Kα was shown to durably suppress PI3K signaling in tumors in which the pathway is activated by PTEN inactivation or mutational or receptor activation of PI3Kα. We believe these combinations represent promising therapeutic strategies for a variety of PI3K dependent tumors. These depend on the use of potent, selective inhibitors and the suppression of pathways that are reactivated in response to relief of feedback.
Experimental Procedures
Cell culture
Prostate cancer cell lines (LNCaP and VCaP) and the human breast cancer cell lines (HCC70, ZR-75-1, BT-549 and BT-474) were obtained from the American Type Culture Collection. LNCaP, HCC70, ZR-75-1, BT-549, PC3, LNCaP-AR, MEL-190, MEL-39, MEL-267, U-87MG and IGROV1 were maintained in RPMI-1640 medium. BT-474, MDA-MB-468 and SKBR3 cells were maintained in a 1:1 mixture of DME: F12 medium. VCaP cells were maintained in DMEM medium. All media were supplemented with 4 mM glutamine, 100 units/mL each of penicillin and streptomycin, and 10% heat-inactivated fetal bovine serum.
Targeted pathway inhibitors
The inhibitors that were used are described in Supplemental experimental procedures.
In vitro kinase assays
We utilized two different PI3K in vitro kinase assays in this work.
PI3Kα kinase activity assay
To measure intracellular PI3Kα kinase activity, LNCaP cells were treated with 250 nM AZD8186 and lysates were prepared. Kinase assays were performed on PI3Kα immunoprecipitated in 250 μM ATP containing 10 μCi [γ32P] ATP with phosphatidylinositol as substrate. The product, phosphatidylinositol 3-phosphate, was resolved by thin layer chromatography and detected by autoradiography. This assay was described previously (Rodrik-Outmezguine et al., 2011; She et al., 2005) and detailed in Supplemental experimental procedures.
Determination of isoform selectivity of AZD8186
The inhibition of PI3Kβ, PI3Kα, PI3Kγ and PI3Kδ was evaluated in a Kinase Glo based enzyme activity assay using the catalytic domain of each of the class I PI3K isoforms, as described in Supplemental experimental procedures.
Immunoblot analysis
Cell lysates were separated by SDS-PAGE as described in Supplemental experimental procedures including a list of antibodies that were used.
siRNA Transfection
Accell siRNA was used according to the manufacturer's protocol as described in Supplemental experimental procedures.
RTK Arrays
Cells were washed with cold PBS and lysed in NP-40 lysis buffer. 250 μg of cell lysates was used to perform the Phospho-RTK arrays (R&D Systems) following the manufacturer's instructions.
Human IGF Signaling Array
Human cytokine arrays (Quantibody; RayBiotech Inc.) were used to determine protein concentrations in cells. 250 μg of cell lysate was taken and analyses were performed in accordance with the manufacturer's instructions. Imaging was performed using the Quantibody Array Testing Software (RayBiotech Inc.).
IGF1Rβ (Y1131) Sandwich ELISA kit
PathScan Sandwich ELISA Kit was used to determine phosphorylation of IGF1R (Y1131) in LNCaP cells treated with 250 nM AZD8186. 500 μg of cell lysate was taken and analyses were performed in accordance with the manufacturer's instructions (Cell signaling).
Analysis of cell cycle and subG1
Cells were plated in 6 well plates in triplicate and were treated with drug or vehicle (DMSO) the following day for five days. Both adherent and floating cells were harvested, and the cell nuclei were prepared as described previously (She et al., 2008).
Analysis of cell growth
Cell growth was monitored by counting cells with a cell counter. Only viable (adherent) cells were counted. Curves represent different treatments. Error bars represent the SD of triplicate samples.
Analysis of cell apoptosis
Cell death was quantified by Annexin V (BioVision) staining, followed by flow cytometric analyses as described in Supplemental experimental procedures.
RT-PCR Analysis
Equal amounts of input RNA were used in a real-time PCR as described in Supplemental experimental procedures.
Mouse models
Pten null GEM, BT-474 and LNCaP and BT-474 xenografts models were used as described in Supplemental experimental procedures.
Animal studies were carried out under protocol 09-05-009 approved by the MSKCC Institutional Animal Care and Use Committee. Institutional guidelines for the proper, humane use of animals in research were followed.
MRI animal Imaging
We used MRI to assess treatment response in our prostate GEM models as described in Carver et al., 2011 and in Supplemental experimental procedures.
Supplementary Material
SIGNIFICANCE.
PI3K inhibition by PI3Kβ inhibitors is transient because of relief of feedback, so that combined inhibition of PI3Kα and PI3Kβ is required for maximal pathway inhibition and antitumor activity. Because of their greater selectivity and the ability to titrate inhibition of each isoform with these drugs, their combined administration may cause less toxicity than less specific pan-PI3K inhibitors and be easier to combine with other therapies. Prostate cancer treatment with PI3K signaling inhibitors has been ineffective because it causes activation of androgen receptor. Triple therapy with PI3Kα and PI3Kβ selective inhibitors combined with a potent androgen receptor inhibitor suppresses the reciprocal feedback activation of both pathways and results in near complete suppression of AR dependent prostate tumors in vivo.
ACKNOWLEDGMENTS
This work has been supported by funds from the NCI P50-CA92629 SPORE in Prostate Cancer, Prostate Cancer Foundation, Breast Program Project P01 CA094060, and Cancer Center Support Grant P30 CA008748, Stand Up To Cancer PI3K Dream Team Grant, and the Damon Runyon Foundation. The authors are grateful to Yogesh Tengarai Ganesan for his help with Annexin V staining, Alice Can Ran Qin for helping with the mice experiments and Xiaodong Huang, Rudy Tieu, Juan Qiu from the animal core facility.
J.W is co-inventror of MDV3100. C.B.T., H.U. and S.T.B. are AstraZeneca employees and shareholders. C.L.S. serves on the board of Novartis Pharmaceutical and co-inventor of MDV3100. J.B. serves as a consulter to Novartis Pharmaceutical. N.R. serves on the Scientific Advisory Board of AstraZeneca and is an advisor for Novartis Pharmaceutical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armstrong AJ, Shen T, Halabi S, Kemeny G, Bitting RL, Kartcheske P, Embree E, Morris K, Winters C, Jaffe T, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clinical genitourinary cancer. 2013;11:397–406. doi: 10.1016/j.clgc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJ, Kazic-Legueux M, Laborde L, Hattenberger M, Stauffer F, Vaxelaire J, et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Molecular cancer therapeutics. 2012;11:1747–1757. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas. N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer discovery. 2012;2:311–319. doi: 10.1158/2159-8290.CD-12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. Akt inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk HA, Backer JM. A beta version of life: p110beta takes center stage. Oncotarget. 2010;1:729–733. doi: 10.18632/oncotarget.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, Della Pelle P, Song Y, Yano S, Mino-Kenudson M, et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:21124–21129. doi: 10.1073/pnas.1314124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Cama A, Quon MJ. Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology. 2001;142:2833–2840. doi: 10.1210/endo.142.7.8283. [DOI] [PubMed] [Google Scholar]
- Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, Kauffmann A, Guthy D, Erdmann D, De Pover A, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:610–619. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauer J, Pletz N, Schon M, Schneider P, Liu N, Ziegelbauer K, Emmert S, Wulf GG, Schon MP. A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo. Blood Cancer J. 2013;3:e141. doi: 10.1038/bcj.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. The New England journal of medicine. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, Rosen N. PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer research. 2010;70:6804–6814. doi: 10.1158/0008-5472.CAN-10-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Molecular endocrinology. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle NT, Lemos R, Jr., Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer research. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Gao X, Lee SH, Maira SM, Wu X, Stack EC, Signoretti S, Loda M, Zhao JJ, Roberts TM. Opposing effects of androgen deprivation and targeted therapy on prostate cancer prevention. Cancer discovery. 2013;3:44–51. doi: 10.1158/2159-8290.CD-12-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric D, Rodon J, Gonzalez-Angulo AM, Burris HA, Bendell J, Berlin JD, Middleton MR, Bootle D, Boehm M, Schmitt A, Rouyrre N, Quadt C, Baselga J. Abstract CT-01: BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in human study. Cancer Research. 2012;72(8) Supplement 1. [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in Cancer: Any Good News? Front Oncol. 2013;3:108. doi: 10.3389/fonc.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, Buck E, Foreman K, Landfair D, O'Connor M, Pirritt C, Sun Y, Yao Y, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153–1171. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, Gao S, Mills GB, Brugge JS. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, Riddle S, Benes C, Eck M, Roberts T, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer discovery. 2012;2:425–433. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of Akt signaling. Cancer discovery. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the Akt kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Molecular cancer therapeutics. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- Simpson L, Li J, Liaw D, Hennessy I, Oliner J, Christians F, Parsons R. PTEN expression causes feedback upregulation of insulin receptor substrate 2. Molecular and cellular biology. 2001;21:3947–3958. doi: 10.1128/MCB.21.12.3947-3958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, Oyarzabal J, Pastor J, Bischoff JR, Fernandez-Capetillo O. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nature structural & molecular biology. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbett NE, Luna-Moran A, Knight ZA, Houk A, Moasser M, Weiss W, Shokat KM, Stokoe D. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J. 2008;415:97–110. doi: 10.1042/BJ20080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C. PTEN-deficient cancers depend on PIK3CB. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, Huang X, Monian P, Jiang X, de Stanchina E, et al. Rapid Induction of Apoptosis by PI3K Inhibitors Is Dependent upon Their Transient Inhibition of RAS-ERK Signaling. Cancer discovery. 2014;4:334–347. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.