Abstract
Cancer immunotherapy has proven to be challenging as it depends on overcoming multiple mechanisms that mediate immune tolerance to self-antigens. A growing understanding of immune tolerance has been the foundation for new approaches to cancer immunotherapy. Adoptive transfer of immune effectors such as antitumor monoclonal antibodies and Chimeric Antigen Receptor T cells bypasses many of the mechanisms involved in immune tolerance by allowing for expansion of tumor specific effectors ex vivo. Vaccination with whole tumor cells, protein, peptide, or dendritic cells has proven challenging, yet may be more useful when combined with other cancer immunotherapeutic strategies. Immunomodulatory approaches to cancer immunotherapy include treatment with agents that enhance and maintain T cell activation. Recent advances in the use of checkpoint blockade to block negative signals and so maintain the antitumor response are particularly exciting. With our growing knowledge of immune tolerance and ways to overcome it, combination treatments are being developed, tested and have particular promise. One example is in situ immunization that is designed to break tolerance within the tumor microenvironment. Progress in all these areas is continuing based on clear evidence that cancer immunotherapy designed to overcome immune tolerance can be useful for a growing number of cancer patients.
Keywords: Cancer immunotherapy, Immune Tolerance, Monoclonal antibodies, CARs, Vaccination, Checkpoint Blockade, Immunomodulation
Introduction
Rapid growth in our understanding of immunology over the past several decades has resulted in more effective vaccines against a variety of infectious diseases. This led to hope that similar successes would follow in cancer immunotherapy. Immune tolerance can be broadly classified into central tolerance based on immune editing that takes place in the thymus, and peripheral tolerance that involves suppression of autoreactive lymphocytes that have entered the periphery [1]. In this review, we will discuss immune tolerance and how it is addressed by current approaches to cancer immunotherapy.
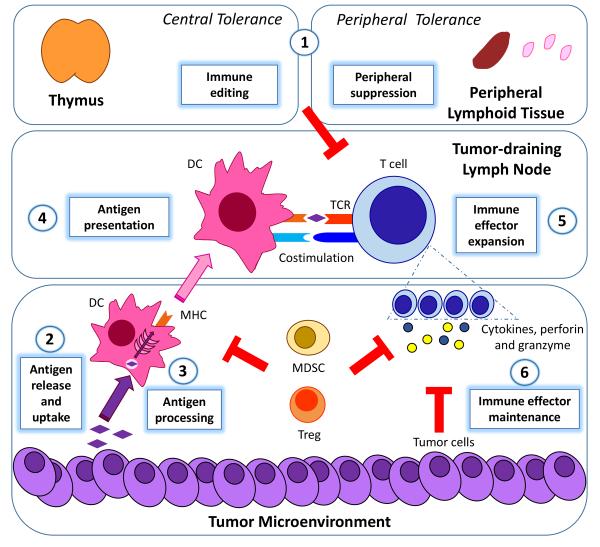
A discussion of immunotherapy strategies designed to break tolerance would benefit from a brief review of the immune response and how immune tolerance impacts on each point in the process. An effective antitumor immune response requires processing of tumor-associated antigens by dendritic cells (DCs), presentation of antigens to antigen-specific T cells, activation and proliferation of those T cells, and maintenance of the T cell response long enough for the T cells to effectively eliminate the cancer (Figure 1) [2]. Immune tolerance can result from suppression at any point in this process. A suboptimal immune response can result from limited antigen uptake and presentation [3]. T cells capable of responding to specific tumor antigens may be significantly reduced due to immune editing [4]. The ability of tumor-specific lymphocytes to be fully activated and to proliferate may be limited by a lack of effective co-stimulatory signals [5]. Even if a robust immune response is generated, it may not last long enough to induce tumor regression. Activated T cells need to efficiently traffic and accumulate at the tumor site [6]. They then need to resist exhaustion and immunosuppression in the tumor microenvironment. Multiple mechanisms employed by tumor cells, including alteration of the antigen presentation machinery or secretion of immunosuppressive factors that can induce apoptosis of lymphocytes or activate negative regulatory pathways, can induce tolerance and limit the effectiveness of the immune response [7]. Tumor cells that enhance immune tolerance either directly or indirectly have a selective survival advantage thereby resulting in their outgrowth [4].
Figure 1. Immune tolerance and the antitumor immune response.
Immune tolerance includes central tolerance (immune editing in the thymus) and peripheral tolerance (suppression of autoreactive lymphocytes in peripheral lymphoid tissue) (1). An effective antitumor immune response starts with tumor antigen release and uptake by DCs (2), antigen processing (3) and presentation by DCs in a stimulatory context to antigen-specific T cells (4), and the activation and proliferation of those T cells (5). The final step is the maintenance of the activated T cell response (6) to effectively eliminate the cancer in a tumor microenvironment rendered immunosuppressive by tumor cells and suppressive immune populations (MDSCs and Tregs). Immune tolerance can result from suppression at one or more of these steps. DC: dendritic cell; MHC: major histocompatibility complex; TCR: T cell receptor; MDSC: myeloid-derived suppressor cells; Treg: regulatory T cells.
Cancer immunotherapy strategies designed to break tolerance can be broadly classified based on the point in this process where the intervention takes place. Such strategies include 1) adoptive transfer of immune effectors, 2) vaccination, and 3) immunomodulatory therapy.
1) Adoptive transfer of immune effectors
The underlying advantage of adoptive transfer as an immunotherapeutic strategy is that immune effectors (such as antibodies and T cells) can be generated in large numbers ex vivo, bypassing the need for in vivo antigen presentation and immune effector proliferation.
1A) Tumor-specific monoclonal antibodies
Monoclonal antibodies (mAbs) represent an extremely valuable and successful class of cancer therapeutics. mAbs targeting the human epidermal growth factor receptor 2 HER-2 (trastuzumab), CD20 (rituximab), and the vascular endothelial growth factor VEGF (bevacizumab) are top-selling drugs, and a number of other mAbs have received FDA approval for the treatment of various solid tumors and hematological malignancies [8-10]. In addition to modifying tumor cell signaling cascades or tumor-stroma interactions, mAbs can also mediate antitumor activity via antibody-dependent cell mediated cytotoxicity, phagocytosis and complement-dependent cytotoxicity. There is growing evidence that, in some cases, mAb-induced tumor cell lysis can enhance uptake and cross-presentation of tumor antigens by DCs leading to the generation of adaptive immune responses [9,10]. This illustrates how manipulation of the immune system in one manner can impact on other aspects of the immune response.
Early advances in mAb therapy included assessing mAbs with different specificities, and modifying their structure to reduce their immunogenicity [8,10]. Ongoing studies are exploring ways to modify their structure to enhance interactions with immune effector cells including natural killer cells and DCs [11].
Immunoconjugates can combine the specificity of mAbs with the potency of cytotoxic moieties. Immunoconjugates include the anti-CD20 radioconjugates 90Y-ibritumomab tiuxetan and 131I-tositumomab for lymphoma, and a number of antibody drug conjugates that are showing promise in both hematologic malignancies and solid tumors [12]. While some may not consider immunoconjugates to be “immunotherapy” because the mechanism of tumor cell lysis is not limited to immune-mediated lysis, immunoconjugates do rely on immune recognition of the target antigen and provide an effective approach to overcoming immune tolerance.
Bispecific mAbs harness the cytolytic potential of T cells by binding to tumor antigens with one arm and an antigen on immune effector cells with the other, thereby retargeting the T cell, irrespective of its natural specificity, towards malignant cells expressing the target antigen. Examples of bispecific antibodies include anti-CD19/anti-CD20 (blinatumomab) and anti-EPCAM/anti-CD3 (catumaxomab) [8-11]. The rapid clearance of bispecific antibodies necessitates continuous infusion, which represents an ongoing challenge in their clinical utility [2]. However, bispecific mAbs do demonstrate clinical efficacy in some malignancies and development continues.
1B) Adoptive T cell transfer (ACT)
Adoptive T cell transfer involves removing lymphocytes from the tumor-bearing patient, expanding them ex vivo in the presence of various growth factors, and re-infusing them into the patient [13,14]. To enhance T cell activation, interleukin 2 (IL-2) co-infusion was employed. To enhance tumor specificity and efficacy of transferred cells, tumor-infiltrating lymphocytes were used based on the assumption they are rich in antitumor T cell populations. Employing these modifications, objective responses reaching 50% and durable remissions have been achieved in patients with metastatic melanoma treated with autologous tumor infiltrating lymphocytes [13,15].
More recently, there has been considerable excitement about adoptive transfer of T cells engineered to express chimeric antigen receptors (CARs). CARs are chimeric single-chain constructs composed of antibody-derived complementarity-determining region (CDR) fused to a T cell receptor (TCR) signaling domain. Genetic transfer of CAR genes to autologous T cells results in T cells that get activated and proliferate in vivo upon contact with their antigen. Clinically, this can lead to both lysis of a large tumor burden and development of immunologic memory towards that specific target antigen [10,13]. Preclinical and clinical evaluation has led to stepwise improvements in the constructs used to produce CARs. Clinical studies, particularly those involving CD19-based CARs for the treatment of B cell malignancy, are showing great promise [16]. In one recent study, 14 out of 20 (82%) patients with CD19+ chronic lymphocytic leukemia achieved a complete response [17].
There are challenges related to the use of CARs. CAR therapy as it currently exists is labor intensive and expensive. It is associated with significant toxicities due to the cytokine storm that results from massive numbers of activated T cells. Loss of the target antigen expression can lead to development of resistance [16]. An additional challenge with CARs is that normal cells that express even low concentrations of the target antigen can be targeted. This autoreactivity could be long-lasting and difficult to manage given the emergence of CAR-based immunologic memory. This concern has limited the types of antigens that can serve as targets for CARs [2]. Despite these challenges, we are in a period of rapid improvement in CAR constructs, development of standard protocols that allow for more automated generation of CARs, careful identification of patient populations most likely to benefit from CAR therapy with acceptable toxicities, and an improved and standardized approach to managing toxicities.
2) Vaccination
Therapeutic cancer vaccines supply the patient’s immune system with tumor antigens, peptides, or whole tumor cells. Some degree of immune tolerance already exists in such patients who already have antigen-expressing tumor on board. Various strategies have been used to help vaccines break this tolerance through selection and modification of the antigen, use of antigen presenting cells such as DCs, and use of immune adjuvants.
Vaccination to prevent infections that can lead to cancer, such as human papilloma virus (HPV), have been highly successful in large part because there is no need to break tolerance to such foreign antigens. Cervarix®, a prophylactic vaccine against human papillomavirus virus (HPV)-associated cervical carcinoma, was granted FDA approval in 2009 [2]. Therapeutic cancer vaccines have proven to be much more challenging irrespective of whether the vaccine includes antigen in the form of whole tumor cells, tumor antigens or peptides. Immune editing, suppression of antigen processing and presentation, and difficulty maintaining a robust immune response can all limit the efficacy of cancer vaccines. Nevertheless, we are making stepwise progress in understanding how to overcome such challenges. One consistent finding is that such vaccines are more likely to be effective in patients who have minimal disease burden where the number of malignant cells is smaller, and immune tolerance is easier to overcome [18].
2A) Whole cell vaccines
Cell-based vaccines allow the host to generate immune responses against a variety of tumor-associated antigens and so lead to a more diverse immune response, which is helpful if immune tolerance is stronger for one antigen than another [19]. An example of such vaccines is GVAX, which is composed of two irradiated prostate cancer cell lines that secrete granulocyte-macrophage colony stimulating factor (GM-CSF). Clinical evaluation of GVAX failed to show a survival benefit in randomized clinical trials although some of the correlative science suggested development of an immune response [19]. GVAX involved immunization with allogeneic cell lines, which might not be the best way to generate an immune response directed towards antigens expressed by the patient’s tumor. Use of modified autologous cells is more challenging logistically. Nevertheless, ongoing studies are exploring this possibility. There is also active investigation into further genetic modification of the tumor cell, fusion of the tumor cell with antigen presenting cells, and use of various immune adjuvants to enhance its ability to break tolerance [19,20].
2B) Vaccination with protein antigen or peptides
Immunization using intact peptides derived from tumor associated antigens has the potential to overcome immune tolerance by bypassing the need for antigen processing. Such peptides can bind directly to MHC molecules (most commonly class I) on DCs, thereby providing a robust activating signal to T cells [21]. While peptides have been found to be safe and relatively easy to synthesize, initial clinical trials of immunization with free peptides demonstrated limited efficacy [2]. Addition of immune stimulants to further enhance activation of peptide-specific T cells may help some in this regard. Positive results were seen in a study exploring the combination of short peptides derived from glycoprotein 100 (gp100), a melanocyte differentiation antigen, with high-dose IL-2 for patients with advanced melanoma. In this Phase III clinical trial, the response rate was higher and progression-free survival longer with vaccine and IL-2 than with IL-2 alone [22].Another successful approach is a Phase III clinical trial which evaluated subcutaneous GM-CSF with a keyhole limpet hemocyanin (KLH) anti-idiotype fusion antibody (Id-KLH + GM-CSF) in patients with follicular lymphoma. The patient-specific Id protein vaccine significantly prolonged disease-free survival compared with the control vaccine (KLH + GM-CSF) [23].
Many challenges remain if peptide-based vaccines are to be effective. These include selecting the right peptide, heterogeneous expression of the peptide by tumor cells, enhancing activation of DCs at the site of immunization to optimize activation of T cells, avoiding development of peripheral tolerance once an immune response has been generated, and blocking the immunosuppressive mechanisms employed by tumors. It is likely peptide vaccines will need to be combined with other approaches to overcoming immune tolerance that go beyond the use of traditional immune adjuvants if they are to be useful clinically.
2C) Vaccination with antigen-pulsed DCs
DC-based vaccines were developed based on the realization that DCs are required to process and present tumor antigens but may be functionally defective, or present in inadequate numbers, in cancer patients [24]. To overcome this problem, DCs are generated from various patient-derived precursors by culturing them in vitro in the presence of cytokine and growth factor cocktails that induce DC expansion and maturation. DCs can then be loaded with tumor antigens, peptides or genetically modified to produce a tumor-associated antigen, and administered back to the patient [24]. Despite the complexities of ex vivo manipulation, some evidence for clinical efficacy has been observed with DC-based vaccines, and a vaccine for metastatic hormone-refractory prostate cancer (sipuleucel-T) was granted FDA approval in 2010. This vaccine uses a fusion protein of prostatic acid phosphatase (PAP) and GMCSF for loading DCs. Sipuleucel-T prolonged median survival by 4.1 months in a Phase III trial [25].
DC biology is extremely complex, and DC subsets vary considerably in their ability to process and present antigen and activate T cells [24]. Some subsets of DCs can actually enhance immune tolerance. Various approaches to improving DC-based vaccines by enhancing antigen uptake, inducing maturation and increasing antigen presentation in an immunogenic instead of a tolerogenic context have been used. These include modifying the manner of DC exposure to antigen and provision of other activation signals including toll-like receptor (TLR) agonists (TLR4, TLR7 and TLR9), several interleukins, and immunostimulatory mAbs [26-29]. While promising, their clinical utility as components of therapeutic DC-based vaccines for the treatment of cancer remains unclear.
3) Immunomodulatory therapy
A growing understanding of mechanisms of immune tolerance has led to identification of cell populations in the tumor microenvironment that contribute to local immunosuppression. Myeloid derived suppressor cells (MDSCs) can be abundant in the tumor microenvironment and have profound suppressive effects on T cells. MDSCs can also activate T regulatory cells (Tregs), which inhibit the function of effector T cells via multiple mechanisms. The presence of MDSCs and Tregs at the tumor site or in peripheral blood has been shown to correlate with poor prognosis in several types of cancer. Other substances produced by tumors, including IL-10 and VEGF, can block the differentiation of myeloid DCs and lead to accumulation of immature DCs with reduced expression of costimulatory molecules (CD80/CD86), leading to T cell anergy (Figure 1) [2,7,30,31].
Efforts geared towards selectively targeting the immunosuppressive cells in the tumor microenvironment are ongoing [2], but to date have proven challenging. Alternatively, a broad variety of agents that could impact on immune tolerance induced by the tumor microenvironment have been used to tip the balance from immune tolerance to immune reactivity.
3A) Enhancing T cell activation
Interleukins, including IL-2, IL-7, IL-12 and IL-15 have been investigated either as single agents or in combinatorial vaccine approaches. They each have complex effects, but in general stimulate T cell activation. Immunogenic T cell activation also depends on receiving a co-stimulatory signal from DCs that would lead to T cell activation and proliferation. mAbs capable of delivering costimulatory signals are being clinically evaluated. Examples include CD40, OX40 (CD134), 4-1BB (CD137), and GITR (CD357) [28,29].
3B) Checkpoint blockade
Immune checkpoints tightly regulate the magnitude of the T cell response and are critical for avoiding autoimmunity. However, they also limit the robustness and duration of desirable antitumor immune responses. Molecules that play a key role in checkpoint regulation include the T cell surface molecules cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death-1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3), and lymphocyte activation gene-3 (LAG-3). In the tumor microenvironment, the expression of these markers by intratumoral lymphocytes results in hyporesponsiveness sometimes described as immune exhaustion. A number of these molecules are also highly expressed on Tregs and are employed to suppress effector T cells [32]. As such, these molecules are highly attractive as targets for reversing immune tolerance.
Our knowledge of these negative regulators, and our ability to use checkpoint blockade to turn off suppressive signals, is one of the most rapidly moving and exciting areas in both cancer immunology and clinical oncology. Checkpoint blockade mAbs that recognize receptor or ligand, and interfere with their interaction, can enhance the antitumor immune response and have been found to be effective clinically in some recent studies [33]. The anti-CTLA-4 mAb ipilimumab (Bristol Myers-Squibb) received FDA approval in 2011 for the treatment of metastatic melanoma based on a phase III trial demonstrating the first overall survival benefit in this population of patients [34]. Autoimmune side effects to anti-CTLA-4 therapy include enteropathies and endocrinopathies [11,32]. Preliminary preclinical and clinical studies exploring PD-1 blockade that target either PD-1 or its ligand PD-L1, are also promising. Two anti-PD-1 mAbs (MDX-1106 and CT-011) are in clinical trials for solid tumors and advanced hematologic malignancies as is a mAb targeting PD-L1 (MDX-1105) [11,32]. Evaluation of blockade of TIM-3 and LAG-3 is also moving towards the clinic, with preclinical studies suggesting blocking these pathways may be particularly effective if used in combination with PD1 blockade [35].
An ongoing challenge is learning how to use these agents to optimize tumor regression while avoiding unacceptable toxicity due to enhanced autoreactivity of T cells to benign cells. Despite this challenge, there is no doubt that checkpoint blockade is a successful immunotherapeutic strategy, and will be increasingly valuable in the clinic. Medical oncologists are accustomed to dealing with the toxicities of cytotoxic drugs, but at present are less familiar with identifying and managing the autoimmune toxicities of checkpoint blockade. This will need to change over the next decade as we learn more about how to use checkpoint blockade, and it becomes a component of cancer immunotherapy for an increasing number of patients.
3C) In situ immunization
Many of the processes that contribute to immune tolerance take place, at least in part, in the tumor microenvironment. As we learn more about such processes, there is increased interest in altering the microenvironment directly in a manner that overcomes immune tolerance and allows for development of a systemic antitumor response. Such modification of the tumor microenvironment with the goal of enhancing the antitumor response is not a new idea. Over a century ago, Dr. William Coley injected microorganisms that we now know include a number of TLR agonists into tumors and observed systemic antitumor responses [2]. Similarly, radiation therapy can generate immune responses capable of eradicating distant disease at sites outside the field of radiation (abscopal effect) [36].
Combination therapy is likely to be needed to modify various aspects in the tumor microenvironment for in situ immunization to be effective. Ideally, such an approach would include therapy that induces immunogenic tumor cell death, enhances antigen uptake and presentation, facilitates T cell activation and maintains the T cell response. While this is complex, preliminary studies suggest it can be effective [37].
Conclusion
We are currently in an incredibly exciting time in cancer immunotherapy, as we learn more about immune tolerance and how to overcome it. Decades of disappointing clinical trials of cancer vaccines that were based on poor understanding of the immune system [2], led some oncologists and cancer researchers at the turn of the century to view cancer immunotherapy as a “failed hypothesis”. No one would contend that today. The value of mAbs that target cancer is undeniable. Checkpoint blockade mAbs that do not target the cancer directly but instead modify the immune response have clear clinical efficacy. CAR T cell therapy has shown to be incredibly effective in select cancer types, and will surely have a role in the cancer immunotherapy of the future. There are still challenges with using vaccination to treat established cancers, but a growing number of studies suggest we are making progress there as well.
These various approaches to breaking immune tolerance towards cancer have their impact at different points in the complex interaction between the malignancy and the immune system. Therapeutic approaches to cancer therapy are designed to either bypass immune editing, or address mechanisms that contribute to peripheral tolerance (Table 1). Over the next 10 years, we will learn more about the value of each of these approaches individually, and more importantly, how to combine them into comprehensive strategies that enhance the immune response to cancer.
Table 1.
Summary of how various immunotherapeutic strategies impact on immune tolerance. Therapeutic approaches to cancer therapy are designed to either bypass immune editing (central tolerance) or address mechanisms that contribute to peripheral tolerance (outlined in Figure 1).
|
Immunotherapeutic Strategy |
Immune Editing |
Antigen Release and Uptake by DCs |
Antigen Processing |
Antigen Presentation |
Immune Effector Expansion |
Immune Effector Maintenance |
|---|---|---|---|---|---|---|
| Tumor-specific mAbs | BP | BP | BP | BP | + | |
|
Adoptive T Cell
Transfer |
BP | BP | BP | BP | + | |
| Whole Cell Vaccines | + | |||||
|
Vaccination with
Protein Antigen |
+ | |||||
|
Vaccination with
Peptide |
BP | + | ||||
|
Vaccination with
Antigen-Pulsed DCs |
BP | BP | + | |||
|
Enhancing T Cell
Activation |
+ | + | ||||
| Checkpoint Blockade | + | |||||
|
In Situ Immunization
and Combination Therapies |
+ | + | + | + | + |
BP Bypassed by this approach to cancer immunotherapy
+ Focus of this approach to cancer immunotherapy
Many key questions remain. Historically, insufficient understanding of the immune system has been a barrier to advancing immunotherapy for cancer. With increasing understanding combined with promising clinical results, we now have the basis and the rationale for intelligently designing and combining various approaches to cancer immunotherapy with the goal of breaking tolerance to cancer.
Acknowledgments
Financial Support: G.J.W. is supported by P30 CA86862 and P50 CA97274 from the National Cancer Institute.
Footnotes
Conflict of interest: None.
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollander N. Immunotherapy for B-cell lymphoma: Current status and prospective advances. Front Immunol. 2012;3:3. doi: 10.3389/fimmu.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Vacchelli E, Fridman WH, et al. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1(1):28–37. doi: 10.4161/onci.1.1.17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29(36):4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May C, Sapra P, Gerber H. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem Pharmacol. 2012;84(9):1105–1112. doi: 10.1016/j.bcp.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Mathur R, Weiner GJ. Picking the optimal target for antibody-drug conjugates. Am Soc Clin Oncol Educ Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e103. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Vacchelli E, Eggermont A, et al. Trial watch: Adoptive cell transfer immunotherapy. Oncoimmunology. 2012;1(3):306–315. doi: 10.4161/onci.19549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overwijk WW. Breaking tolerance in cancer immunotherapy: Time to ACT. Curr Opin Immunol. 2005;17(2):187–194. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin-2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 16.Kershaw MH, Westwood JA, Slaney CY, Darcy PK. Clinical application of genetically modified T cells in cancer therapy. Clin Trans Immunol. 2014;3:e16. doi: 10.1038/cti.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupp SA, Frey NV, Aplenc R, Barrett DM, Chew A, Kalos M, et al. T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) produce significant in vivo proliferation, complete responses and long-term persistence without Gvhd in children and adults with relapsed, refractory ALL. Poster Pres Am Soc Hematol. 2013;122(21):67. [Google Scholar]
- 18.Bocchia M, Bronte V, Colombo MP, et al. Antitumor vaccination: Where we stand. Haematologica. 2000;85(11):1172–1206. [PubMed] [Google Scholar]
- 19.Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int Rev Immunol. 2011;30(2-3):150–182. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 20.Browning MJ. Antigen presenting cell/tumor cell fusion vaccines for cancer immunotherapy. Hum Vaccin Immunother. 2013;9(7):1545–1548. doi: 10.4161/hv.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slingluff CL., Jr. The present and future of peptide vaccines for cancer: Single or multiple, long or short, alone or in combination? Cancer J. 2011;17(5):343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. Gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. N Engl J Med. 2011 Jun 2;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011 Jul 10;29(20):2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010 Jul 29;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 26.Galluzzi L, Senovilla L, Vacchelli E, et al. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vacchelli E, Galluzzi L, Eggermont A, et al. Trial watch: FDA-approved toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1(6):894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacchelli E, Galluzzi L, Eggermont A, et al. Trial watch: Immunostimulatory cytokines. Oncoimmunology. 2012;1(4):493–506. doi: 10.4161/onci.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolchok JD, Yang AS, Weber JS. Immune regulatory antibodies: Are they the next advance? Cancer J. 2010;16(4):311–317. doi: 10.1097/PPO.0b013e3181eb3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: Mechanisms and clinical development. J Cell Biochem. 2011;112(8):1969–197. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 32.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: An overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 33.Harshman LC, Drake CG, Wargo JA, Sharma P, Bhardwaj N. Cancer Immunotherapy Highlights from the 2014 ASCO Meeting. Cancer Immunol Res. 2014 Aug;2(8):714–719. doi: 10.1158/2326-6066.CIR-14-0119. [DOI] [PubMed] [Google Scholar]
- 34.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: Radiation abscopal effects, PAMPs, and DAMPs. Clin Cancer Res. 2012;18(17):4522–4525. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 37.Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: A phase I/II study. J Clin Oncol. 2010;28(28):4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]