Abstract
Background
Adenoid cystic carcinoma (ACCs) are malignant salivary gland tumors noteworthy for high rates of late failure with limited salvage therapy options. We have previously shown increased Akt signaling is common in ACC and the HIV protease inhibitor nelfinavir (NFV) inhibits in vitro tumor growth by suppressing Akt signaling. This phase II trial was conducted to determine progression free survival in response to NFV in patients with recurrent/end stage ACC who have failed standard therapies.
Methods
Eligible patients had recurrent or end stage ACC and measureable disease per Response Evaluation Criteria in Solid Tumors (RECIST) criteria. NFV was provided at 1250 mg twice daily.
Results
Among 15 trial participants, median progression free survival was 5.5 months (lower 95% bound 4.4 months). No patient achieved a RECIST partial or complete response to therapy.
Conclusion
NFV monotherapy does not result in a meaningful improvement in clinical outcomes among patients with recurrent ACC.
Keywords: adenoid cystic carcinoma, head and neck cancer, nelfinavir, Akt, salivary gland cancer
INTRODUCTION
Although relatively rare, accounting for less than 1% of head and neck cancers overall, adenoid cystic carcinoma (ACC) is one of the most common malignant salivary gland tumors and accounts for an estimated 14% of the salivary gland cancers occurring in the United States each year [1, 2]. ACC can arise from both major and minor salivary glands in a variety of head and neck locations, including the parotid and submandibular glands, oral cavity, oropharynx, nasal cavity/paranasal sinuses, and larynx [3]. Primary therapy typically consists of surgical resection and adjuvant radiation therapy. Relative to other head and neck cancers, ACCs are noteworthy for their indolent clinical course and a propensity for late failure as evidenced by recurrence free survival rates of 65% at 5 years, but only 30% at 15 years [3]. At the time of recurrence, salvage treatment options are often limited by the risk of severe treatment related morbidity with additional surgery or radiation. Furthermore, response rates with standard chemotherapies are poor [4], highlighting the need for novel/additional targeted therapies.
In previous work, we have used immunohistochemical techniques to examine the activity of oncogenic cell signaling pathways in a series of 9 paraffin embedded ACC tissue specimens. We found that 7/9 showed strong phosphorylated Akt expression, 5/9 strong phosphorylated MAP Kinase, and 0/9 strong EGFR expression [5]. Although no Akt pathway inhibitors are currently approved for treatment of head and neck cancers, we have previously shown that the HIV protease inhibitor nelfinavir (NFV) can suppress Akt pathway signaling in transformed cell lines [6], suggesting its potential use as a therapy in the challenging clinical circumstance of recurrent ACC. Subsequent in vitro testing in transformed cells revealed that NFV treatment at drug concentrations achievable in vivo in humans results in decreased Akt pathway activity, decreased tumor cell proliferation, and decreased clonogenic survival relative to controls [5]. Because NFV showed efficacy in inhibiting Akt activity in vitro, and because its safety profile is already well established [7](making a phase I trial unnecessary), we initiated a phase II study examining the efficacy of NFV therapy in patients with recurrent ACC who have failed standard therapies.
METHODS
Study Design
The primary objective for this phase II trial was to determine progression free survival in response to NFV therapy in patients with recurrent ACC who have failed all standard treatment options (http://www.clinicaltrials.gov NCT01065844). The protocol was approved by the IRB at the University of Iowa. Written, informed consent was obtained from all patients at the time of study entry. Funding for this study was provided through institutional sources.
Patients and Methods
Eligible patients were greater than 18 years of age, had a histological diagnosis of ACC, measureable disease per Response Evaluation Criteria in Solid Tumors (RECIST v1.1) criteria, ECOG performance status of 0-2 or Karnofsky performance status greater or equal to 50%, were staged as recurrent or end-stage, had failed all other therapy, and had clinical and/or imaging evidence of disease progression prior to trial enrollment. Patients who were pregnant, had known HIV, uncontrolled diabetes, a history of allergic reaction to NFV or related compounds, Hemophilia A or B, were undergoing concurrent anti-cancer therapy, or were taking medications contraindicated with NFV were excluded. Participants were also required to have normal organ and marrow function within 14 days of initiation of therapy (Leukocytes ≥3,000, ANC ≥1,500, platelets ≥100,000, total bilirubin and creatinine within institutional normal limits, AST/ALT≤2.5 × institutional upper limit of normal, and creatinine clearance ≥ 60 mL/min/1.73m2). Both patients with localized and metastatic disease were included in the study.
A screening visit with full history and physical, including a CBC with differential, serum chemistries, ALT, AST, serum bilirubin, serum creatinine, and pregnancy test (in patients of childbearing potential), was obtained prior to initiation of NFV therapy. At each subsequent clinic visit, the subject completed the EORTC QLQ-30 Quality of Life survey, performance status and vital signs were obtained, and the patient was screened for concomitant medication use. Clinical visits were obtained on a monthly basis at study entry, or sooner as deemed necessary by the treating physician. The interval between visits was lengthened at the discretion of the treating physician. Imaging studies were obtained at a median interval of every 12.0 weeks after trial enrollment (range 7.0-14.4 weeks, standard deviation +/− 1.78 weeks), at the discretion of the treating physician.
NFV was provided at 1250 mg twice daily (a standard dose used in HIV therapy [8]) until: patients refused further treatment; or, developed progressive disease/symptoms, unacceptable adverse events attributed to NFV by the treating physician, or intercurrent illness preventing further treatment administration. Medical adverse events were graded using the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3) [9]. Toxicities requiring dose modification were defined as any grade 4 neutropenia, grade 3 neutropenia of more than 21 days, grade 4 thrombocytopenia, grade 3 ALT/AST elevation, any grade 3 metabolic lab abnormality lasting more than 7 days, or any grade 3 toxicity not explainable by other causes. In patients who developed a toxicity requiring dose modification, treatment was delayed for a minimum of 1 week. If the toxicity became grade 1 or 0 within four weeks off therapy, patients wishing to continue trial participation were allowed to resume NFV starting at a reduced dose (625 mg twice daily), which could later be increased (to a maximum dose of 1250 mg twice daily) at the discretion of the treating physician.
Statistics
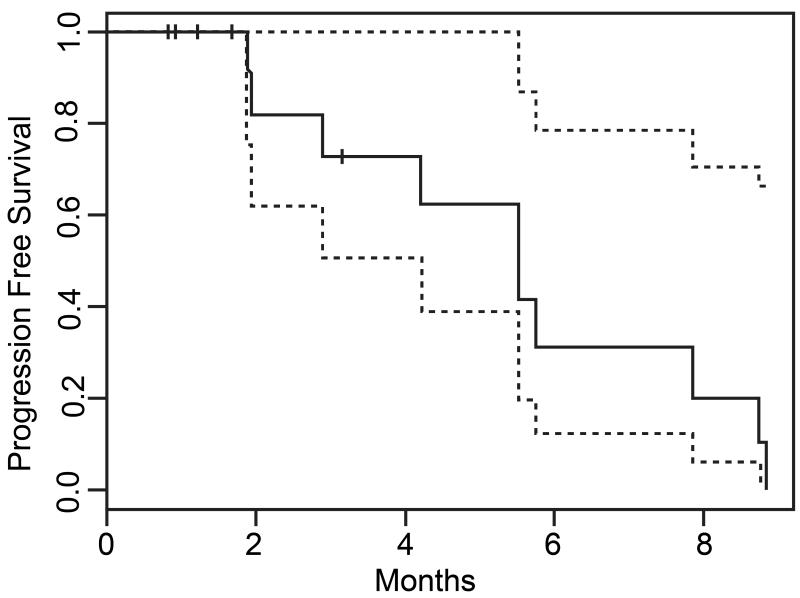
Treatment efficacy was determined by progression free survival, time to treatment failure, and measureable disease response per RECIST (version 1.1) criteria [10]. RECIST assessments were performed by one of two study authors, MMM or JMB. Best response assessments were based on a minimum time from study entry of 8 weeks. Progression free survival was defined as the time from study enrollment until disease progression by RECIST criteria or death from any cause. Patients in whom neither outcome had been observed at the time therapy was discontinued were treated as censored for the purposes of data analysis. Time to treatment failure was defined as the time from study enrollment until treatment was discontinued for any reason. Results of a previous phase II clinical trial showed a median progression free survival of 3.5 months among patients with recurrent or metastatic ACC treated with lapatinib monotherapy [11]. This trial was powered to test the statistical hypothesis that NFV therapy improves median progression free survival to at least 5.2 months in a similar patient cohort. To test a one-sided statistical hypothesis at the 0.10 level of significance with a power of 0.80, a minimum enrollment of 30 patients was estimated. Early trial termination was initiated after enrollment of 15 patients when interim analysis showed that no patient met RECIST criteria for a partial or complete response to NFV therapy and a significant number of patients discontinued treatments due to toxicity or poor tolerance.
RESULTS
Patient Characteristics
Between October 26, 2009 and February 13, 2012, 15 patients underwent NFV therapy for recurrent ACC at the University of Iowa Hospitals and Clinics. Patient characteristics are listed in table 1. Median age of enrolled patients was 52 years (range 25 to 77). Sixty Percent (9/15) were female. Most patients (13/15) had primary disease locations in the head and neck, while one patient had a primary lung location and one patient presented with metastatic disease of unknown primary. All patients had failed previous cancer specific therapy, usually consisting of a combination of surgery (13 patients) and radiation therapy (14 patients), with or without chemotherapy (7 patients).
Table 1.
Baseline characteristics of patients undergoing nelfinavir therapy for recurrent adenoid cystic carcinoma.
| Patient Characteristics | Number (n=15) |
|---|---|
| Age | |
| Median (range) | 52 (25-77) |
| Gender | |
| Male | 6 |
| Female | 9 |
| Primary Disease Site | |
| Head and Neck | 13 |
| Other | 1(lung) |
| Unknown | 1 |
| Previous Therapy | |
| Surgery | 13 |
| Radiation | 14 |
| Chemotherapy | 7 |
| Off Study Reason | |
| Disease Progression | 10 |
| Adverse Event | 5 |
Progression Free Survival
Individual patient treatment characteristics are shown in Table 2. Of the 15 patients enrolled in the trial, 10 met the progression free survival analysis endpoint of radiographic disease progression by RECIST criteria. Patients who did not meet this endpoint (n=5) were censored from progression free survival analysis. No patients died during trial participation.
Table 2.
Treatment characteristics of patients with recurrent adenoid cystic carcinoma undergoing nelfinavir therapy. KPS = karnofsky performance status; S = surgery; C = chemotherapy(the number of systemic therapy regimens each patient received prior to trial enrollment is noted in parentheses), RT = radiation therapy. Duration of nelfinavir therapy (Treatment Duration) and progression free survival (PFS) are given in months. PD =progressive disease; SD = stable disease. LR = locally recurrent; M = metastatic. X=censored
| Patient | Sex | Age | KPS | Primary Site | Stage | Prior Therapy |
Treatment Duration |
PFS | Best Response |
Off Study Reason |
Adverse Event Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 25 | 90 | Lacrimal | LR | S, C(1), RT | 1.8 | 1.9 | PD | Progression | |
| 2 | M | 48 | 80 | Lacrimal | LR | S, C(2), RT | 4.2 | 4.2 | PD | Progression | |
| 3 | F | 49 | 60 | Nasopharynx | M | S, C(2), RT | 0.8 | X | - | Adverse Event |
Thrombocytopenia |
| 4 | F | 75 | 70 | Submandibular | M | S, RT | 1.2 | 1.9 | PD | Progression | |
| 5 | M | 77 | 60 | Maxillary Sinus | M | S, RT | 0.8 | X | - | Adverse Event |
Hyponatremia |
| 6 | F | 52 | 90 | Submandibular | LR | S, RT | 1.4 | X | - | Adverse Event |
Dizziness |
| 7 | M | 40 | 80 | Salivary | M | S, C(2), RT | 8.1 | 8.8 | SD | Progression | |
| 8 | F | 58 | 80 | Hard Palate | LR | RT | 3.2 | X | SD | Adverse Event |
Elevated Liver Enzymes |
| 9 | M | 65 | 70 | Parotid | M | S, RT | 5.5 | 5.8 | SD | Progression | |
| 10 | F | 57 | 70 | Lung | M | S, C(4), RT | 7.9 | 7.9 | SD | Progression | |
| 11 | F | 40 | 90 | Unknown | M | C(2) | 5.5 | 5.5 | SD | Progression | |
| 12 | F | 57 | 90 | Maxillary Sinus | M | S, RT | 1.2 | X | - | Adverse Event |
Elevated Liver Enzymes |
| 13 | M | 72 | 100 | Submandibular | M | S, RT | 8.7 | 8.7 | SD | Progression | |
| 14 | F | 40 | 90 | Inner/Middle Ear |
M | S, RT | 2.8 | 2.9 | PD | Progression | |
| 15 | M | 51 | 90 | Lacrimal | M | S, C(4), RT | 5.5 | 5.5 | SD | Progression |
The median duration of NFV therapy was 3.2 months, while the median progression free survival was 5.5 months (lower 95% confidence interval = 4.4 months, upper bound not estimable, Figure 1). The best RECIST response achieved by any patient during participation in the trial was stable disease, which was observed in 7 patients at some point during their trial participation. Among these 7 patients, 2 had documented stable disease at time points beyond 6 months after trial enrollment. No patient achieved a partial or complete response to NFV therapy.
Figure 1.
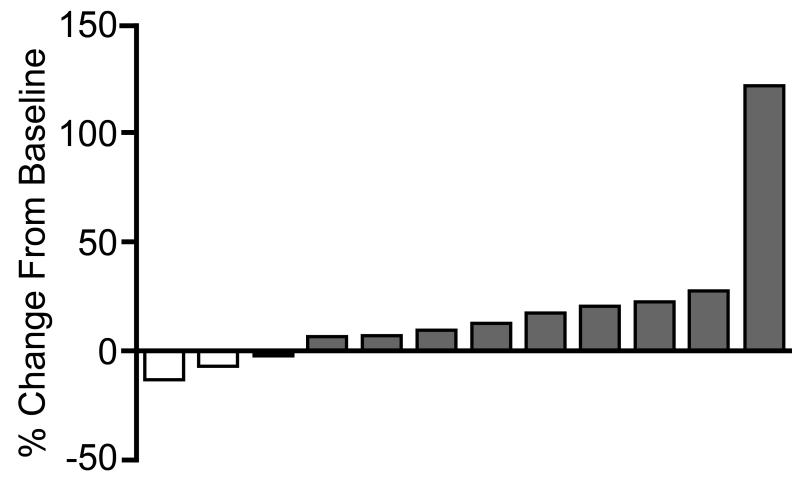
Among the 12 patients who participated in the trial long enough to undergo at least one follow up imaging assessment, 9 showed measureable tumor growth at the first follow up scan (4 patients who met RECIST criteria for progression and 5 patients who had tumor growth not meeting RECIST criteria). Though not meeting RECIST criteria for a partial response, 3 patients showed measureable disease regression at the first follow up scan after trial entry (Figure 2). Among these three patients, two ultimately developed progressive disease (at 8.7 and 5.5 months after study entry) and 1 withdrew due to toxicity without undergoing any further imaging assessments on trial.
Figure 2.
Toxicity
Five patients experienced grade 3 or higher medical adverse events or toxicities requiring dose modification that resulted in discontinuation of therapy. These included grade 4 hyponatremia, grade 3 thrombocytopenia, and grade 3 dizziness in 1 patient each, and grade 3 liver enzyme elevations in two patients. In three cases, the patient resumed NFV therapy after a treatment break but ultimately experienced symptom recurrences that led to trial withdrawal. One patient (severe hyponatremia) showed improvement in his laboratory measures after holding NFV and receiving medical therapy but was advised not to resume trial participation by his treating physician. The final patient (grade 3 thrombocytopenia) enrolled in hospice care shortly after trial withdrawal and did not undergo follow up lab evaluation. All other patients withdrew from trial participation after disease progression was noted on follow up imaging. Median time to treatment failure was 3.2 months.
Health related quality of life data was obtained for 13 of the 15 patients enrolled in the trial. Among these 13 patients, 7 showed worse QLQ-30 Global Health Status Scores at the first follow up evaluation after trial entry, 2 patients showed no change, and 4 patients showed improved scores. Surprisingly, among the 4 patients who met RECIST criteria for disease progression at the first follow up imaging evaluation after trial entry, 2 had improved QLQ-30 Global Health Status Scores.
DISCUSSION
Though approved for use in HIV as an anti-retroviral therapy, NFV has also shown promise as an anti-neoplastic agent in a number of pre-clinical studies. The anti-neoplastic effect of NFV is thought to be mediated by inhibition of the Akt signaling pathway. We have previously shown that Akt up-regulation is common in ACC, and NFV mediated Akt inhibition results in decreased tumor cell survival in vitro[5]. Therefore, we chose to initiate a phase II clinical trial investigating the potential efficacy of NFV in the treatment of ACC patients who have failed traditional surgical, radiation, and/or chemotherapies.
Our results are noteworthy for a large proportion of patients who experienced medical adverse events leading to termination of trial participation. Most of the adverse events observed in our patient population, including liver enzyme elevations, dizziness, and thrombocytopenia, have also been observed among HIV patients undergoing NFV therapy[12, 13]. In addition, although hyponatremia is not commonly reported among HIV patients undergoing NFV therapy, diarrhea is a common side effect, which may have accounted for/contributed to the severe hyponatremia that resulted in trial termination for one participant in our trial.
In addition to our own work, there have been a number of pre-clinical studies demonstrating anti-tumor effects of NFV in dozens of cancer cell lines representing a broad range of cancer subtypes, including hepatocellular, breast, head and neck, and prostate carcinomas; multiple myeloma; glioblastoma multiforme; liposarcoma, and others [6, 14-19]. In spite of these promising pre-clinical studies, the efficacy of NFV as a cancer monotherapy has to our knowledge never been demonstrated in a clinical trial. Likewise, a large retrospective multicenter study of HIV patients (an at risk population for multiple cancers) undergoing NFV therapy did not show significantly different cancer incidence rates compared to non NFV treated patients [20]. These results should not be interpreted to mean that NFV is ineffective in cancer therapy. Indeed, most of the aforementioned pre-clinical studies show that NFV has greater impact as a radio or chemosensitizer, rather than a single modality intervention; and, several phase I clinical trials have shown promising results using NFV in combination therapy regimens among patients with non-small cell lung and pancreatic cancers [21, 22], spurring additional phase II trials that are currently underway.
Not surprisingly, the medical literature regarding NFV in cancer therapy parallels that generally reported with other Akt pathway inhibitors. A number of PI3 Kinase/Akt pathway inhibitors have been tested, or are currently under investigation in phase I/II clinical trials, and several have shown some efficacy in treating a number of cancer subtypes [23]. Patients with PI3 Kinase mutations seem to derive the most benefit from Akt axis inhibition as evidenced by results of a clinical study at the MD Anderson Cancer Center in which response rates of 35% were observed, compared to rates of only 6% among patients without PI3 Kinase mutations [24]. As with NFV, several reports suggest that combination therapies are more effective than Akt inhibitors as monotherapy, particularly given that Akt signaling is thought to play a key role in mediating resistance to chemo, radiation and hormonal anti-cancer therapies [23, 25].
Here, we report the results of NFV as a cancer therapy in the specific setting of recurrent adenoid cystic carcinoma. Despite good preclinical evidence, we were unable to demonstrate a clinically meaningful improvement in patient outcomes in response to NFV in the setting of ACC that has failed previous therapy. Our observed median progression free survival (5.5 months) does represent a slight improvement relative to a previously published progression free survival rate of 3.5 months in response to lapatinib monotherapy [11]; however, this finding is not unexpected considering the favorable selection criteria used in our study design. In addition, the frequency of RECIST assessment was every 8 weeks in the previous study, whereas in our study it was assessed at a median interval of 12 weeks. This difference in methods may have artificially prolonged the progression free survival observed in our work.
Several patients in our study did achieve a limited duration of radiographic stable disease by RECIST criteria (less than a 20% increase in tumor size), 2 patients with documented stable disease for more than 6 months. However, any such positive effect is likely of minimal practical clinical significance for the following reasons: 1) no patient achieved a RECIST partial or complete response to therapy at any point during trial participation, 2) 9/12 assessable patients showed radiographic evidence of tumor growth at the first follow up scan after trial enrollment, 3) a large proportion of patients in our trial (33%) experienced medical adverse events leading to early trial termination and a median time to treatment failure of just 3.2 months, and 4) our findings do not represent an improvement over previously published reports of ACC response to palliative treatment with a more traditional chemotherapy regimen of cisplatin and 5-fluorouracil [26]. As such, our results are consistent with previous clinical and pre-clinical studies demonstrating that Akt pathway inhibitors in general, and NFV specifically, are minimally effective as cancer monotherapies. This does not rule out their potential utility in combination therapy regimens, either with radiation, conventional chemotherapy, or other targeted therapies. We suggest that such an approach would be the most appropriate strategy for future clinical trials investigating NFV and other Akt pathway inhibitors in the treatment of ACC.
Footnotes
Conflicts of Interest/Disclosures: None
REFERENCES
- 1.Chummun S, et al. Adenoid cystic carcinoma of the head and neck. Br J Plast Surg. 2001;54(6):476–80. doi: 10.1054/bjps.2001.3636. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG YJ, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute; Bethesda, MD: 2007. SEER Program, NIH Pub. No. 07-6215. 2007. [Google Scholar]
- 3.Khan AJ, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96(3):149–58. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 4.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42(8):759–69. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, et al. Signaling pathways in adenoid cystic cancers: implications for treatment. Cancer Biol Ther. 2009;8(20):1947–51. doi: 10.4161/cbt.8.20.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta AK, et al. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74(3):928–33. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gathe JC, Jr., et al. Long-term (120-Week) antiviral efficacy and tolerability of fosamprenavir/ritonavir once daily in therapy-naive patients with HIV-1 infection: an uncontrolled, open-label, single-arm follow-on study. Clin Ther. 2006;28(5):745–54. doi: 10.1016/j.clinthera.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Micromedex Healthcare Series. Drugdex System. Truven Health Analytics; Greenwood Village, CO: 2013. [Google Scholar]
- 9.National Cancer Institute, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. 2006. [Google Scholar]
- 10.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Agulnik M, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25(25):3978–84. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz M, et al. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)-1 protease, to treat HIV infection. J Infect Dis. 1998;177(6):1533–40. doi: 10.1086/515312. [DOI] [PubMed] [Google Scholar]
- 13.Gathe JC, Jr., et al. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir /ritonavir versus twice-daily nelfinavir in naive HIV-1-infected patients. AIDS. 2004;18(11):1529–37. doi: 10.1097/01.aids.0000131332.30548.92. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, et al. Antitumour effects of a protease inhibitor, nelfinavir, in hepatocellular carcinoma cancer cells. J Chemother. 24(3):161–6. doi: 10.1179/1973947812Y.0000000011. [DOI] [PubMed] [Google Scholar]
- 15.Guan M, Fousek K, Chow WA. Nelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancer. FEBS J. 279(13):2399–411. doi: 10.1111/j.1742-4658.2012.08619.x. [DOI] [PubMed] [Google Scholar]
- 16.Guan M, et al. Nelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6. Clin Cancer Res. 17(7):1796–806. doi: 10.1158/1078-0432.CCR-10-3216. [DOI] [PubMed] [Google Scholar]
- 17.Bono C, et al. The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo. Haematologica. 97(7):1101–9. doi: 10.3324/haematol.2011.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian X, et al. Modulation of CCAAT/enhancer binding protein homologous protein (CHOP)-dependent DR5 expression by nelfinavir sensitizes glioblastoma multiforme cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Biol Chem. 286(33):29408–16. doi: 10.1074/jbc.M110.197665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruning A, et al. Tamoxifen enhances the cytotoxic effects of nelfinavir in breast cancer cells. Breast Cancer Res. 12(4):R45. doi: 10.1186/bcr2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crum-Cianflone NF, et al. The impact of nelfinavir exposure on cancer development among a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2009;51(3):305–9. doi: 10.1097/QAI.0b013e3181aa13c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner TB, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26(16):2699–706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 22.R R, et al. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J Thorac Oncol. 2012;7(4):709–15. doi: 10.1097/JTO.0b013e3182435aa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogita S, Lorusso P. Targeting phosphatidylinositol 3 kinase (PI3K)-Akt beyond rapalogs. Target Oncol. 6(2):103–17. doi: 10.1007/s11523-011-0176-7. [DOI] [PubMed] [Google Scholar]
- 24.Janku F, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 10(3):558–65. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martelli AM, et al. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 3(4):371–94. doi: 10.18632/oncotarget.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill ME, et al. Cisplatin and 5-fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol. 1997;33(4):275–8. doi: 10.1016/s0964-1955(97)00026-2. [DOI] [PubMed] [Google Scholar]