Abstract
Recent human study data has re-established the value of DNA vaccines, especially in priming high-level antigen-specific antibody responses, but also raised questions about the mechanisms responsible for such effects. Whereas previous reports have shown involvement of downstream signaling molecules in the innate immune system, the current study investigated the role of Aim2 as a sensor for DNA vaccines. The Aim2 inflammasome directs maturation of the pro-inflammatory cytokines IL-1β and IL-18 and an inflammatory form of cell death called pyroptosis. Both the humoral and cellular antigen-specific adaptive responses were significantly reduced in Aim2−/− mice in an IL-1β/IL-18 independent manner after DNA vaccination. Surprisingly, Aim2−/− mice also exhibited significantly lower levels of IFN-α/β at the site of injection. These results indicate a previously unreported link between DNA vaccine induced pyroptotic cell death and vaccine immunogenicity that is instrumental in shaping the antigen specific immune response to DNA vaccines.
Introduction
The discovery of DNA vaccine technology in the early 1990s was a major event in the history of vaccinology due to the many unique features of DNA immunization, including its ability to elicit balanced antibody and T cell immunity (1–6). However, in early clinical studies, the immunogenicity of DNA vaccines in humans was low when such vaccines were used alone. More recent human trials have demonstrated that DNA vaccines are actually extremely powerful in priming the host’s immune system to develop high level protective antibody responses against HIV-1 and pandemic influenza viruses (3, 7–11). Animal studies further demonstrated that DNA immunization is effective in eliciting higher levels of antigen-specific B cell responses (12). One mechanism to achieve such an outcome is that DNA vaccination is effective in eliciting higher germinal center (GC) B cell development via enhanced follicular helper T (Tfh) cells for the production of high quality antibody responses (13).
DNA vaccines produce immunogens in vivo, which are then presented to the immune system via the endogenous antigen processing pathway. At the same time, the DNA plasmid itself confers an intrinsic adjuvant effect that enhances the immune response generated towards the vaccine-encoded immunogens (13, 14), but the intracellular processes involved remain to be fully elucidated. Several pattern-recognition receptors (PRRs) have been identified which respond to DNA molecules (15, 16). One of the best-characterized DNA sensing PRRs is the endosomal TLR9, which is essential for the recognition of unmethylated CpG containing oligodeoxynucleotide (ODN) motifs commonly found in bacterial plasmids (17). As DNA vaccine plasmid backbones contain certain CpG:ODN motifs, it was initially thought that TLR9 would be critical for DNA vaccine immunogenicity. However, while DNA plasmid activates dendritic cells via TLR9, TLR9-deficient mice were able to mount immune responses comparable to wild-type mice (18, 19). Likewise, DNA immunization of MyD88 and TRIF-deficient mice yields robust immune responses, further suggesting that TLR signaling may be dispensable for DNA vaccine induced immunogenicity (20).
In recent years, it has become increasingly evident that the double-stranded nature of DNA itself functions as a potent activator of innate immune signals (21–25). Cytosolic DNA is a powerful initiator of type I IFN (IFN-αβ) in both immune and non-immune cells, functioning through a STING/TBK1/IRF3 dependent pathway that is independent of CpG motifs and TLRs. At the same time, it is not clear whether other components of the innate immune system beyond the STING/TBK1/IFN-αβ pathway are involved in the immunogenicity of DNA vaccines (20, 21). This is especially true in the case of inflammasome pathways. Inflammasomes regulate caspase-1 activity, ultimately resulting in cleavage of the pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into their active forms. Inflammasome activation also results in pyroptotic cell death; a suicidal form of cell death characterized by the release of damage associated molecular patterns (DAMPs) that further propagates innate immune signaling to surrounding bystander cells (26). One flavor of inflammasome contains absent in melanoma 2 (Aim2), which is a direct sensor of cytosolic DNA and a member of the PYHIN family (23). Aim2 contains a DNA binding HIN200 domain, as well as a pyrin domain. While Aim2’s role in orchestrating immune responses to both viral and bacterial pathogens is well characterized (27–29), the role of Aim2 in DNA vaccination is unknown.
Here we found that Aim2 and the adapter molecule Asc were required for the generation of optimal immunogen-specific antibody responses to a DNA vaccine expressing influenza HA immunogen in a mouse model. DNA vaccination leads to transcription of key components of the inflammasome. Importantly, the efficacy of DNA vaccination was independent of IL-1β and IL-18. Surprisingly, Aim2-deficient mice were unable to elicit a type I IFN response at the site of injection. Our data therefore establishes a novel role for Aim2 as a key player in the regulation of DNA vaccination.
Materials and Methods
Animals
C57BL/6 mice were obtained from Taconic Laboratories. Aim2−/− mice were generated in house by K. Fitzgerald’s group at the University of Massachusetts Medical School (UMMS) as previously described (27). Aim2−/− mice were on a mixed B6/129 background and therefore B6×129 mice were utilized as controls. B6.129 (hereafter referred to as Aim2+/+) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were bred at UMMS. IL-1 receptor (Il-1r), IL-18 receptor (Il-18r), and Asc-deficient mice were produced in house. All mice were maintained in the Department of Animal Medicine at UMMS according to IACUC-approved protocols. Mice received 100 μg of codon optimized H1HA DNA vaccine expressing the full-length wild type HA protein from A/Texas/04/09 (pH1HA), divided between quadriceps, at 2 and 4 weeks. A third boosting immunization was delivered 1-week prior to sacrifice.
Gene Induction Analysis
C57BL/6 mice were shaved and immunized with 100 μg H1-TX04-09.tPA DNA vaccine plasmid intramuscularly injected into the hind quad muscle. Punch biopsies were harvested from the site of immunization at 6 hours, 12 hours, and 24 hours post immunization and snap frozen. RNA was isolated from tissue biopsies using TRIzol Reagent (Life Technologies). We analyzed gene expression using the Nanostring nCounter Analysis system (Nanostring Technologies). Each reaction contained 100 ng RNA in a 5 μl aliquot, plus reporter and capture probes. We also included 6 pairs of positive control and 8 pairs of negative control probes. Gene induction analysis and normalization was conducted using nSolver Analysis Software v1.1. Raw counts were normalized to naïve mice using 3 reference genes: Gapdh, Gusb, and Hprt1.
Cell Culture and Cytokine ELISA
Mouse BMDCs were generated from Aim2+/+ or Aim2−/− mice by culturing fresh bone marrow in R10 medium containing GM-CSF for 8 days at 37 ° C. Aim2+/+ and Aim2−/− immortalized BMDM were produced in house. Cells were first primed with 200 ng/ml LPS (Sigma Aldrich) for 4–5 hrs. prior to treatment with appropriate stimuli. All media was removed from the cells, and the appropriate stimulus was added. Poly (dA:dT) (Sigma Aldrich) DNA and H1-TX04-09.tPA DNA vaccine plasmid were transfected using Lipofectamine 2000 at a concentration of 1.5 μg/ml. ATP was added at a concentration of 1.25 μg/ml. Cultures were incubated 16–18 hours at 37 ° C, and supernatants were harvested. Cell culture supernatants were assayed for IL-1β (BD Biosciences, Franklin Lakes, NJ) by ELISA. Lactate dehydrogenase (LDH) release was used to measure pyroptotic cell death. LDH assays were performed using the Promega CytoTox96 Non-radioactive Cytotoxicity Kit according to manufacturer’s directions (Promega, Madison, WI)
Enzyme Linked Immunosorbent Assay (ELISA)
Microtiter plates were coated with transiently expressed H1HA antigen at ~1 μg/mL in PBS for 1 hour at room temperature and assayed as previously described (30). NaSCN displacement was performed at a serum dilution of 1:100. After washing of serum samples, NaSCN was added at various (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 M) concentrations in PBS for 15 min followed by 5 washes in EWB. The assay was then completed as above.
ELISPOT Assay
Splenocyte T and B cell ELISPOT reagents were obtained from Mabtech (Mariemont, OH). H1HA specific T cells were quantified per manufactures instructions. Positive controls were stimulated with 20 ng/ml PMA (Sigma-Aldrich, St. Louis, MO) and 500 ng/ml ionomycin (Sigma-Aldrich). The H1HA relevant peptide used was a CD8+ cell-restricted HA peptide (IYSTVASSL). H1HA-specific antibody secreting cells were detected by coating of MAIPSWU (Millipore, Billerica, MA, USA) plates with the transiently expressed H1HA antigen utilized for ELISA (~1.0 μg/well). Positive spots were visualized on a CTL Imager and counting was performed with Immunospot software (Cellular Technology Ltd., Shaker Heights, OH)
In Vivo Caspase-1 activation
Aim2+/+ and Aim2−/− mice were shaved and immunized with 100 μg H1-TX04-09.tPA DNA vaccine plasmid intramuscularly injected into the hind quad muscle. Punch biopsies were harvested from the site of immunization at 6 hours, 12 hours, and 24 hours post immunization and snap frozen. Cryopreserved tissue sections were generated and adhered to glass slides. Samples were then stained with a caspase-1 FAM/FLICA kit according to manufacturer’s instructions (ImmunoChemistry Technologies, Bloomington, MN). Stained slides were visualized on a confocal microscope. Sixteen independent fields were analyzed for fluorescence.
Quantitative Real Time-Polymerase Chain Reaction
Aim2+/+ and Aim2−/− mice were shaved and immunized with 100 μg H1-TX04-09.tPA DNA vaccine plasmid intramuscularly injected into the hind quad muscle. Punch biopsies were harvested from the site of immunization at 6 hours, 12 hours, and 24 hours post immunization and snap frozen. RNA was isolated from tissues biopsies using TRIzol Reagent (Life Technologies #15596-026), and cDNA was generated using the Bio Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). cDNA was then used for rt-PCR reactions on a Bio-Rad CFX-96 cycler. Primers sequences are available upon request.
Statistical analysis
All data is presented as the mean of individual mice +/− standard deviation (SEM). Statistical analysis was performed using a Student’s t test, a one-way ANOVA followed by a Tukey post-test, or a two-way ANOVA followed by a Bonferonni post-test.
Results
DNA vaccine plasmid induces expression of Aim2, caspase-1 and the inflammasome
While previous studies have mainly used non-coding DNA plasmid or DNA vaccines coding for marker proteins to study DNA-elicited innate immune responses, the current study tested a DNA vaccine (pH1HA) expressing the HA antigen of the type A influenza virus subtype H1N1 virus which was responsible for a pandemic influenza in 2009. HA is the major protective antigen in clinically licensed inactivated and live-attenuated influenza vaccines. DNA vaccines expressing HA have been shown to be immunogenic in eliciting HA-specific antibodies in both animal and human studies (2, 30–34). The expression of HA antigen by pH1HA used in the current study was confirmed by Western blot and its immunogenicity to elicit HA-specific antibody response was verified in a pilot mouse study (data not shown).
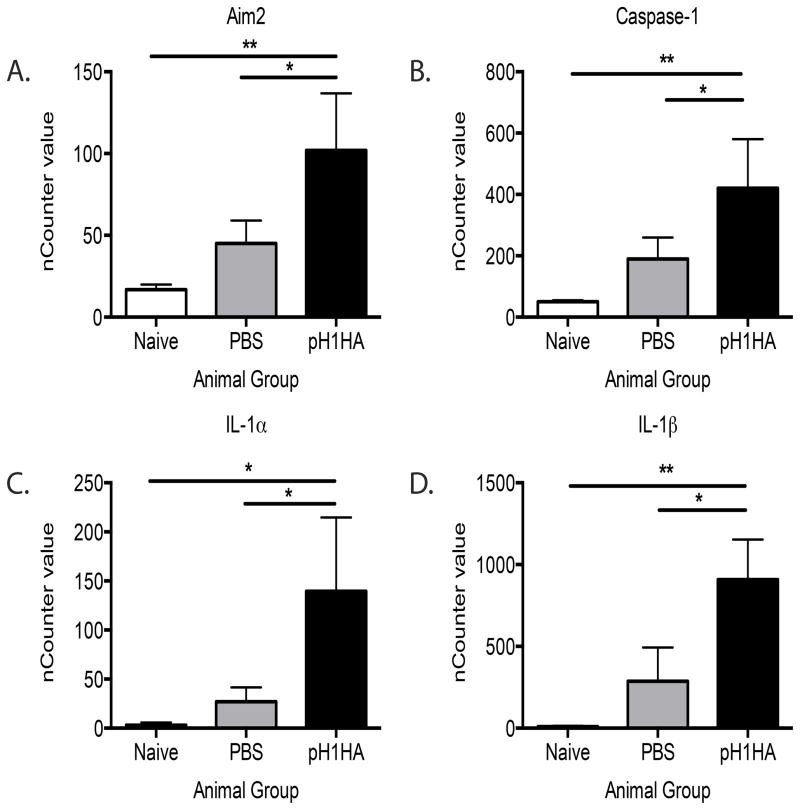
We first wanted to profile key immune response genes following DNA vaccine pH1HA using the Nanostring nCounter gene expression system, which includes a custom array encoding 50 innate immunity targets. Gene induction was quantified from wild-type C57BL/6 mice immunized with the pH1HA DNA vaccine. Messenger RNA was isolated and the expression of innate immune genes profiled using the Nanostring nCounter, and changes in gene induction quantified. Notably, Aim2 was induced ~6 fold within 12 hours of immunization when compared to naïve samples. Aim2 is a type I IFN inducible gene suggesting a potent ability of cells at the site of vaccination to recognize cytosolic plasmid vaccines (Figure 1A). In accordance with the induction of Aim2, caspase-1 was also highly upregulated. (Figure 1B). Most striking were the high levels of the inflammatory cytokines IL-1α and IL-1β (Figure 1C,D). These observations indicate that inflammasome components were present at the site of vaccination. To test if the inflammasome pathway was active at the site of vaccination we utilized a caspase-1 specific FAM/FLICA fluorescent stain to covalently label catalytically active caspase-1. Mature caspase-1 became apparent within 6 hours of immunization and reached a peak at 12 hours (Supplemental Figure 1). Collectively, these results led us to examine the role of the Aim2 inflammasome pathway in antigen specific immune responses elicited by pH1HA DNA vaccination.
Figure 1. Plasmid DNA vaccination induces the inflammasome.
Inflammasome activation at the site of immunization was quantified by Nanostring nCounter analysis 12 hours post DNA immunization. The site of injection was harvested and mRNA was isolated and expression levels were quantified for (A) Aim2, (B) caspase-1, (C) IL-1α, (D) IL-1β. Data are the averages ± SEM of 5 mice per group.
Involvement of Aim2 in cellular responses to DNA vaccine plasmid
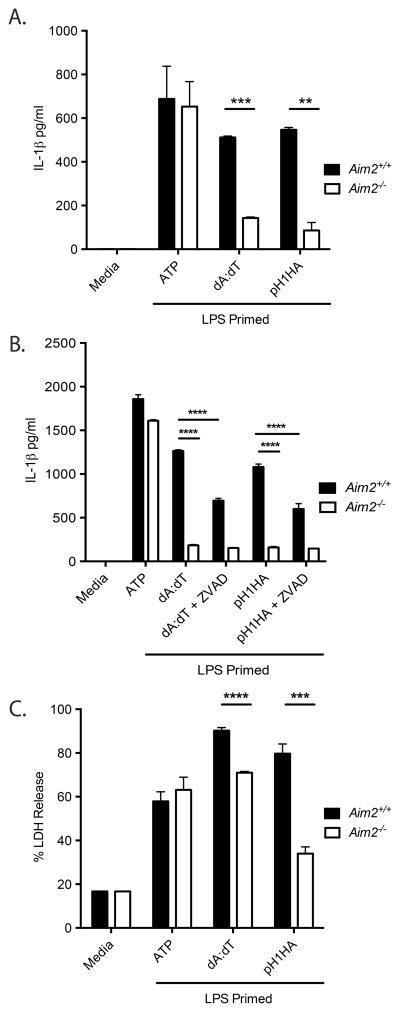
We first evaluated the ability of macrophages and dendritic cells to recognize pH1HA DNA vaccine by performing in vitro experiments. IL-1β production in response to pH1HA DNA vaccine was evaluated in BMDM collected from either Aim2+/+ or Aim2−/− mice (Figure 2A). As expected, both the synthetic B-form dsDNA poly(deoxyadenylic-deoxythymidylic) and pH1HA DNA vaccine induced a robust IL-1β response in Aim2+/+ BMDM as measured by ELISA. However, IL-1β production was abolished in Aim2−/− BMDM. Similar results were seen in BMDC (data not shown). Next, the pan-caspase inhibitor Z-VAD-FMK was included in BMDM cultures prior to adding pH1HA DNA vaccine (Figure 2B). This resulted in inhibited IL-1β production in Aim2+/+ macrophages, yielding IL-1β levels comparable to Aim2−/− wells, supporting the role of Aim2 in DNA vaccine mediated IL-1β maturation. Finally, pH1HA DNA vaccine induced an inflammatory form of cell death (pyroptosis) in Aim2+/+ macrophages, as measured by lactate dehydrogenase release (Figure 2C). This response was attenuated in Aim2−/− cells. Collectively, these data indicate that Aim2 acts as a sensor of DNA vaccine plasmid and regulates caspase-1 dependent IL-1β production and pyroptotic cell death in response to pH1HA DNA vaccines in vitro.
Figure 2. Aim2 is required for IL-1β production in response to DNA vaccines.
LPS (200 ng/ml) primed Aim2+/+ and Aim2−/− BMDMs were transfected with poly(dA-dT) or DNA vaccine plasmid for 18 hrs. (A) Secreted IL-1β in the culture supernatants was analyzed by ELISA. (B) Aim2+/+ and Aim2−/− BMDM were treated as above with the addition of the pan-caspase inhibitor Z-VAD-FMK and secreted IL-1β was quantified by ELISA. (C) Aim2+/+ and Aim2−/− BMDM were treated as above, and culture LDH amounts were reported as a percentage of lysed cellular controls. Data are presented as mean ± SEM from 3 independent experiments.
Effects of Aim2 deletion on pH1HA induced HA-specific immune responses
As it is evident that the Aim2 inflammasome recognizes and responds to pH1HA DNA vaccine in cultured cells, the role of Aim2 in pH1HA DNA vaccination was next examined in Aim2-deficient (Aim2−/−) and wild-type Aim2+/+ mice. The pH1HA DNA vaccine induced high-level HA-specific antibody responses in Aim2+/+ mice, but significantly lower antibody titers in Aim2−/− mice (Figure 3A). This reduction is isotype-dependent as Aim2−/− mice exhibited significantly lower levels of HA-specific IgG1, IgG2b, and IgG2c responses (data not shown). Likewise, Aim2−/− mice exhibited significantly reduced HA-specific circulating B cells as well as IFN-γ secreting CD8+ T cells in the spleen (Figure 3C,D). The role of Aim2 in regulating the maturation process of pH1HA-induced antibody responses was further confirmed by measuring the avidity of serum HA-specific antibodies in these mice (Figure 3B). Aim2+/+ mice required high concentrations of the chaotropic agent NaSCN to disrupt antigen/antibody complexes, while much lower concentrations of NaSCN were required for disassociation in Aim2−/− mice. To confirm the requirement for inflammasome signaling in DNA vaccine immunogenicity, we also quantified the adaptive response in Asc−/− mice. Asc-deletion similarly inhibited the generation of optimal HA-specific immune responses (Supplemental Figure 2A–C).
Figure 3. Optimal DNA vaccine immunogenicity requires Aim2.
Wild-type Aim2+/+ and Aim2−/− mice were immunized intramuscularly with a pH1HA encoding DNA vaccine at weeks 2 and 4. (A) HA-specific IgG titers were analyzed fourteen days post second immunization. Anti-HA binding avidity was quantified via ELISA and reported as molar concentration of sodium thiocyanate required to displace anti-HA serum antibodies to 2x pre-bleed levels (B). For ELISPOT, spleens were harvested at termination 7 days following a 3rd boosting immunization. HA-specific antibody secreting B cells (C) or IFN-γ secreting T cells (D) in mice immunized with either pH1HA or empty vector. Data are the averages ± SEM of 5 mice per group.
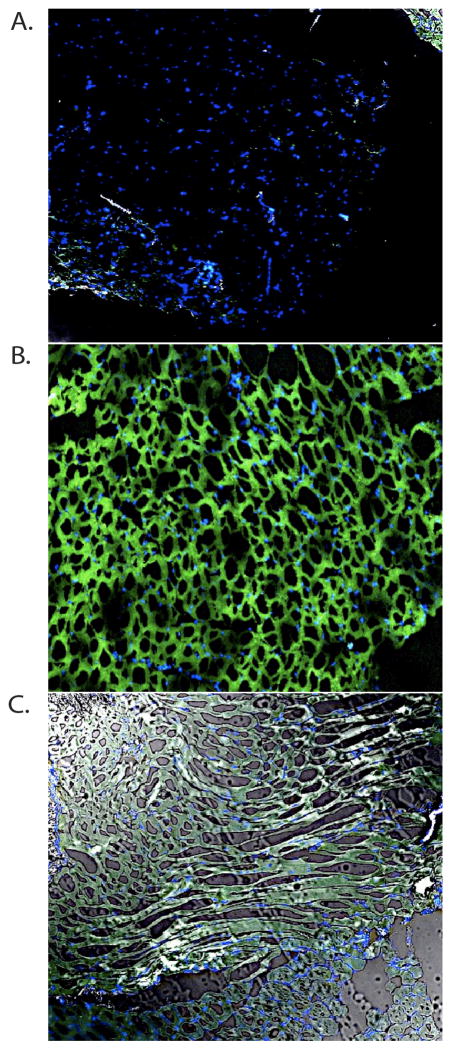
Aim2 deficient mice fail to cleave caspase-1 into its active form
Since DNA vaccination resulted in high levels of caspase-1 activation in Aim2+/+ mice (Supplemental Figure 1), we analyzed Aim2−/− mice for their ability to generate catalytically active caspase-1 using the FAM/FLICA assay (Figure 4). Aim2−/− mice demonstrated a clear reduction in caspase-1 activation at the 12-hour peak time point when compared to Aim2+/+ controls.
Figure 4. Aim2-deficient mice exhibit diminished caspase-1 activation at the site of immunization.
(B) Wild-type Aim2+/+ and (C) Aim2−/− mice were immunized intramuscularly with pH1HA vaccine and caspase-1 activation was quantified by FAM/FLICA staining 12 hours post immunization. (A) PBS injected controls were utilized for comparison. The site of injection was harvested and cryopreserved for tissue sectioning. FAM/FLICA staining was visualized by confocal microscopy and is representative of 3 mice per group.
Effects of IL-1 and IL-18 deletion on vaccine induced HA-specific immune responses
As inflammasome signaling ultimately results in the downstream cleavage of pro-IL-1β and pro-IL18 into their respective active forms, the role of IL-1β and IL-18 signaling in DNA vaccine was next investigated (Supplemental Figure 3). Surprisingly, both of these cytokines were dispensable for the DNA vaccine response as mice lacking the IL-1r or the IL-18r mounted normal vaccine responses. Total serum HA-specific IgG titers were similar to wild-type C57BL/6 mice in both Il-1r−/− and Il-18r−/− mice. Likewise, no significant difference was seen in total HA-specific B or CD8+ T cell numbers as measured by ELISPOT. This data is in line with previously published reports demonstrating little impact on DNA vaccination following MyD88 deletion (20).
The immune response is lineage dependent
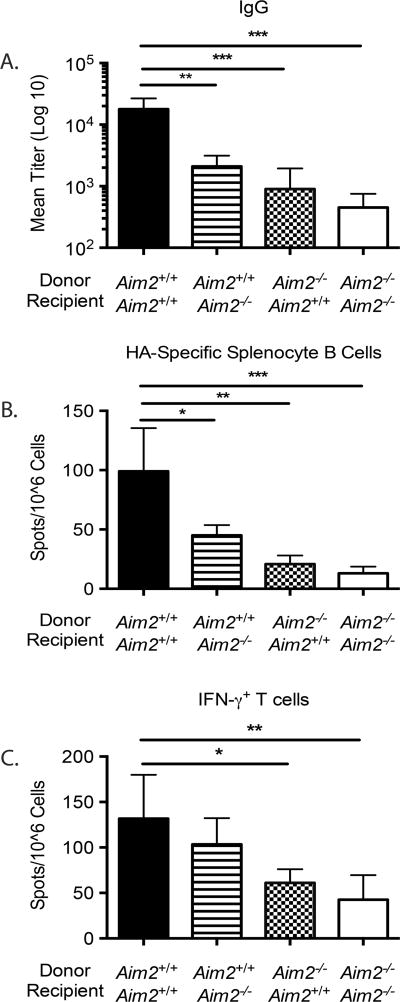
Previously published data has demonstrated the requirement for both hematopoietic and non-hematopoietic cell lineages in DNA vaccination (20). To further elucidate the role of Aim2, bone marrow chimeric mice were generated by transferring bone marrow from Aim2+/+ mice into Aim2−/− mice, or vice versa (Figure 5). Aim2+/+ and Aim2−/− mice reconstituted with Aim2−/− bone marrow exhibited strong defects in both the T cell response and HA-specific IgG production. Interestingly, transfer of Aim2+/+ bone marrow into Aim2−/− rescued the T cell response, but only partially rescued the humoral response. While HA-specific IgG levels were impaired compared to Aim2+/+ mice reconstituted with Aim2+/+ bone marrow, they were significantly higher than Aim2−/− bone marrow reconstituted mice. This would support that Aim2 is required in both the hematopoietic and non-hematopoietic lineages for optimal humoral responses, but deficiency in non-hematopoietic lineages does not affect CD8+ T cell responses.
Figure 5. Contribution of hematopoietic and non-hematopoietic cells to DNA vaccine induced immunogenicity.
Bone marrow chimeric mice were immunized with a pH1HA vaccine as described in Figure 2. Fourteen days post second immunization, sera from chimeric mice were analyzed for HA-specific IgG titers (A). Spleens were harvested at termination 7 days following the 3rd immunization. Frequency of HA-specific B cells (B) or IFN-γ+ T cells (C) were reported as spots per million splenocytes in mice immunized with pH1HA vaccine. Data are the averages ± SEM of 5 mice per group.
Aim2−/− mice lack IFN-αβ signaling
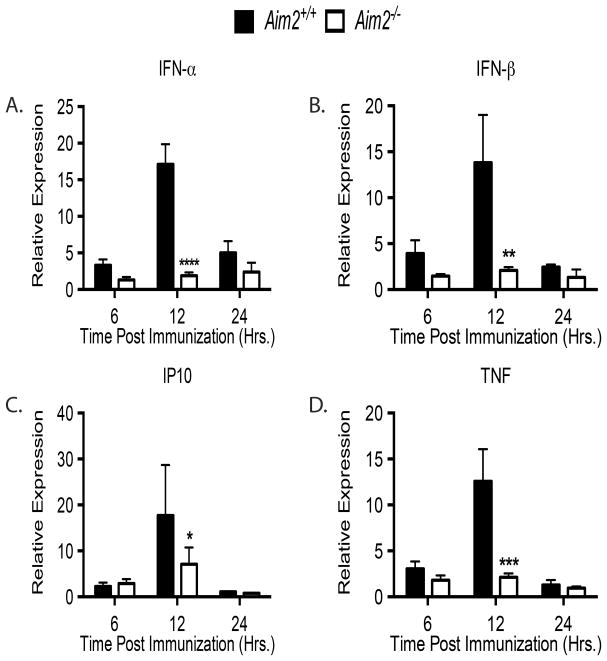
The failure of Aim2−/− mice to generate optimal adaptive immune responses implies a defect in immune priming at the site of injection. While the major function of the Aim2 inflammasome is to regulate caspase-1 activation, resulting in IL-1β, IL-18 and cell death pathways, our data indicate that IL-1β and IL-18 are not responsible for the Aim2 dependent effects we observed. We therefore endeavored to quantify IFN-α/β as it has been reported to play a key role in the immune response to B-DNA (25, 35–37). In addition, it has been established that IFN-α/β signaling is required for DNA vaccination (20, 21). Aim2 does not control DNA induced IFN-α/β production directly. Rather the STING pathway mediates these effects. Since the IFN-α/β response is so critical for DNA vaccination, we performed a detailed kinetic analysis measuring IFN-α/β expression in Aim2+/+ and Aim2−/− mice. To ensure we only detect DNA vaccine-induced IFN-α/β, we limited our measurements to the site of immunization. Aim2+/+ and Aim2−/− mice were immunized with the pH1HA DNA vaccine, and punch biopsies were collected from the site of injection. Quantitative rt-PCR analysis of mRNA clearly shows that Aim2−/− mice have reduced IFN-α and IFN-β expression compared to Aim2+/+ controls, with expression peaking at 12 hours post immunization in wild-type mice (Figure 6). Intriguingly, IFN-αβ expression in Aim2−/− mice peaked at 6 hours post immunization and remained static throughout the time course. Consistent with the decrease in IFN-α/β, there was a corresponding decrease in the IFN stimulated gene, IP10. We also noticed a significant decrease in TNF. Asc−/− mice had similar levels of IFN-α/β, further confirming the requirement for inflammasome signaling (Supplemental Figure 2D). These data suggest a previously unreported role for Aim2 in regulating local IFN-α/β levels following DNA vaccination. As Aim2 controls cell death at the site of infection, it is likely that Aim2 dependent cell death liberates endogenous DAMP danger signals, which might in turn elicit IFN-α/β via the Aim2-independent STING/TBK1 pathways. This broad defect in IFN-α/β signaling likely explains the defects we observed in Aim2-deficient mice treated with DNA vaccines.
Figure 6. Aim2-deficiency limits IFN-αβ production at the site of injection.
Aim2 +/+ and Aim2−/− mice were immunized intramuscularly with pH1HA vaccine, and the site of injection was harvested 12 hours later. Total RNA was isolated from tissue biopsies and subjected to rt-PCR for (A) IFN-α, (B) IFN-β, (C) IP10, and (D) TNF. Reported expression levels are relative to naïve Aim2 +/+ expression. Data are the averages ± SEM of 5 mice per group.
Discussion
The innate immune pathways governing DNA vaccination remain to be fully characterized. Recent reports have established the STING/TBK1/IFN-αβ axis as required for DNA vaccine immunogenicity (20, 21); however, the PRR(s) required for IFN-αβ production remain to be identified in this context. Likewise, the involvement of other innate immune signaling pathways is unclear. In particular, the requirement for the inflammasome signaling machinery in DNA vaccine elicited antigen-specific immune responses has not been examined. Here, we identified the Aim2 inflammasome as a DNA vaccine sensor with the ability to regulate the antigen-specific adaptive immune response. Whereas previous reports have focused on downstream signaling molecules, this is the first report to identify a DNA sensor that is required for DNA vaccine immunogenicity.
The failure of Aim2−/− mice to generate optimal adaptive immune responses implies a defect in immune priming at the site of injection. While the function of Aim2 has been well characterized, how and where Aim2 interacts with the vaccine plasmid remains unclear. Interestingly, immunization of bone marrow chimeras revealed varying degrees of necessity for Aim2 signaling in both the hematopoietic and non-hematopoietic lineages. Aim2 is required in both cell lineages for optimal humoral responses, as chimeric mice lacked high levels of anti-HA antibodies. This may be attributed to impaired IL-6 production in Aim2−/− mice possibly limiting B-cell survival and CD4+ T cell expansion as has been reported in several studies (Supplemental Figure 4) (38). In addition, recent reports have described the effect of DNA priming on T follicular helper cell generation (13). Even more surprising, Aim2-deletion in non-hematopoietic cells did not impact CMI, as mice reconstituted with Aim2+/+ bone marrow presented similar levels of IFN-γ to Aim2+/+. This provides further evidence for a defect in immune cell priming.
Of note, deletion of IL-1β and IL-18 signaling in DNA vaccination did not impact DNA vaccine immunogenicity, confirming previous reports describing minimal effect of MyD88 deletion on DNA vaccine immunogenicity (39). This stands in stark contrast to the importance of IL-1β and IL-18 inflammatory signaling in the early stages of pathogenic infection. Why IL-1β and IL-18 signaling are dispensable remains unclear, but one possible reason is the highly immunostimulatory nature of the DNA vaccine plasmid itself, as DNA is a potent inducer of both IFN-α/β and several NFκB regulated immune genes. In addition, the level of several chemokines, such as MIP-1α/β and MCP-1, remained unchanged (data not shown), allowing for recruitment of monocyte and lymphocyte populations.
Most intriguingly, the reduction of IFN-α/β levels at the site of immunization in Aim2−/− suggests a previously unknown relationship between Aim2 and local IFN-α/β production. Aim2 is not known to mediate IFN-α/β production directly, hinting at an indirect link between these two divergent pathways. We propose that decreased pyroptotic cell death in Aim2−/− mice results in diminished DAMP release, limiting cellular signaling and bystander cell activation. The release of cellular DNA by pyroptotic cells may augment IFN-α/β production by surrounding cells, possibly through the STING/TBK1 signaling axis, further propagating the immune response.
In conclusion, our results indicate that Aim2 plays a significant role in DNA vaccination. Aim2−/− mice failed to generate optimal immune responses upon DNA vaccination, exhibiting decreased serum IgG levels and T cell cytokine production, demonstrating the necessity for Aim2 signaling in DNA vaccine immunogenicity. In addition, we reported a previously unknown function for Aim2 in augmenting IFN-α/β production at the site of immunization. Our results provide a deeper understanding of the cellular mechanisms through which DNA vaccines stimulate both the innate and adaptive immune pathways to enhance the immune responses targeting the encoded antigen.
Supplementary Material
Footnotes
Funding: This study was supported in part by NIH grants 5 U19 AI082676, 5 P01 AI082274 and 5R33AI087191, and Bill and Melinda Gates Foundation grant OPP1033112.
References
- 1.Wang R, Epstein J, Baraceros FM, Gorak EJ, Charoenvit Y, Carucci DJ, Hedstrom RC, Rahardjo N, Gay T, Hobart P, Stout R, Jones TR, Richie TL, Parker SE, Doolan DL, Norman J, Hoffman SL. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10817–10822. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Hackett A, Jia N, Zhang C, Zhang L, Parker C, Zhou A, Li J, Cao WC, Huang Z, Li Y, Lu S. Polyvalent DNA vaccines expressing HA antigens of H5N1 influenza viruses with an optimized leader sequence elicit cross-protective antibody responses. PloS one. 2011;6:e28757. doi: 10.1371/journal.pone.0028757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Zhang C, Zhang L, Li J, Huang Z, Lu S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine. 2008;26:2100–2110. doi: 10.1016/j.vaccine.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, Nabel GJ. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy JS, Co M, Green S, Longtine K, Longtine J, O’Neill MA, Adams JP, Rothman AL, Yu Q, Johnson-Leva R, Pal R, Wang S, Lu S, Markham P. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6–001) in healthy adult volunteers. Vaccine. 2008;26:4420–4424. doi: 10.1016/j.vaccine.2008.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:3947–3957. doi: 10.1016/j.vaccine.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS, Ledgerwood JE, Golding H. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. The Journal of infectious diseases. 2013;208:413–417. doi: 10.1093/infdis/jit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, Bailer R, Pearce MB, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS, Vrc, Teams VRCS. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clinical and vaccine immunology: CVI. 2012;19:1792–1797. doi: 10.1128/CVI.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, Bailer R, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS, Team VRCS. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. The Lancet infectious diseases. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Wang S, Lu S. Pilot Study on the Use of DNA Priming Immunization to Enhance Y. pestis LcrV-Specific B Cell Responses Elicited by a Recombinant LcrV Protein Vaccine. Vaccines. 2014;2:36–48. doi: 10.3390/vaccines2010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristin Hollister YC, Wang Shixia, Wu Hao, Mondal Arpita, Clegg Ninah, Lu Shan, Dent Alexander. The role of follicular helper T cells and the germinal center in HIV-1 gp120 DNA prime and gp120 protein boost vaccination. Human Vaccines & Immunotherapeutics. 2014;10 doi: 10.4161/hv.28659. epub: ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 18.Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, Heit A, Wagner H. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. Journal of immunology. 2003;171:5908–5912. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 19.Babiuk S, Mookherjee N, Pontarollo R, Griebel P, van Drunen Littel-van den Hurk S, Hecker R, Babiuk L. TLR9−/− and TLR9+/+ mice display similar immune responses to a DNA vaccine. Immunology. 2004;113:114–120. doi: 10.1111/j.1365-2567.2004.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nature immunology. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nature immunology. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 27.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature immunology. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantari P, Deoliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A, Latz E, Gazzinelli RT, Golenbock DT, Fitzgerald KA. Dual Engagement of the NLRP3 and AIM2 Inflammasomes by Plasmodium-Derived Hemozoin and DNA during Malaria. Cell reports. 2014;6:196–210. doi: 10.1016/j.celrep.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. Journal of neurochemistry. 2014 doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Taaffe J, Parker C, Solorzano A, Cao H, Garcia-Sastre A, Lu S. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. Journal of virology. 2006;80:11628–11637. doi: 10.1128/JVI.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Parker C, Taaffe J, Solorzano A, Garcia-Sastre A, Lu S. Heterologous HA DNA vaccine prime--inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suguitan AL, Jr, Cheng X, Wang W, Wang S, Jin H, Lu S. Influenza H5 hemagglutinin DNA primes the antibody response elicited by the live attenuated influenza A/Vietnam/1203/2004 vaccine in ferrets. PloS one. 2011;6:e21942. doi: 10.1371/journal.pone.0021942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Jia N, Li J, Han Y, Cao W, Wang S, Huang Z, Lu S. Optimal designs of an HA-based DNA vaccine against H7 subtype influenza viruses. Hum Vaccin Immunother. 2014;10 doi: 10.4161/hv.28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almansour I, Chen H, Wang S, Lu S. Cross reactivity of serum antibody responses elicited by DNA vaccines expressing HA antigens from H1N1 subtype influenza vaccines in the past 30 years. Hum Vaccin Immunother. 2013;9:2049–2059. doi: 10.4161/hv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature medicine. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 36.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. The Journal of experimental medicine. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS one. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends in immunology. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.