Abstract
Purpose of review
The long-lived viral reservoir is a major obstacle to achieving a cure for HIV. Therapeutic strategies, such as early antiretroviral therapy (ART), may be a prerequisite to achieving long-term control of viral replication upon ART withdrawal.
Recent findings
HIV persistence is established early in acute HIV infection (AHI) with infection in long-lived memory CD4+ T cells. Studies conducted in nonhuman primates have suggested that this could occur as early as 3 days postinfection; however, the timing in humans is uncertain. ART during AHI significantly restricts the HIV reservoirs as compared with later treatment. Early ART, particularly prior to the detection of HIV immunoglobulin M, may also reduce the contribution of the long-lived central memory CD4+ T cells to the total HIV reservoir, a profile observed in individuals who naturally control HIV without ART.
Summary
It is clear that early ART has a greater impact in limiting the HIV reservoirs than later treatment. However, latently infected long-lived memory CD4+ T cells persist in most early treated individuals. Therefore, additional interventions will likely be required to eliminate all cells capable of producing replication-competent virus but treatment in AHI may be the critical first step in containing the HIV reservoirs.
Keywords: acute HIV infection, early antiretroviral therapy, HIV DNA, HIV reservoir, replication-competent virus
INTRODUCTION
The discovery of a small pool of latently infected cells that persist in HIV-infected individuals receiving suppressive antiretroviral therapy (ART) has considerably dampened the hope of eradicating HIV with ART alone [1–3]. This long-lived reservoir is often considered the major obstacle to achieving a cure for HIV and therapeutic strategies that could limit its size may be a prerequisite to achieving control of viral replication upon ART withdrawal, which is also called HIV remission [4,5]. Current evidence suggests that ART instituted early during acute HIV infection (AHI) is key to containing HIV reservoir establishment, particularly in long-lived CD4+ T cells, providing a basis for its critical role in achieving HIV remission [6■,7,8]. Indeed, a prime example is the case of the Mississippi child who started ART by 2 days of life for 18 months and subsequently was able to remain with low HIV reservoirs and virally suppressed for 27 months in the absence of ART [9,10]. Similarly, the VISCONTI cohort of adults in France was treated within the first 2 months of infection and was able to control viremia without ART for more than 5 years [11■■]. Through key published works, this review covers the timing and mechanisms of HIV reservoir establishment and the effects of early ART on the HIV reservoirs as well as future research directions.
The latent HIV reservoir is established early after infection
The mechanisms responsible for the establishment of HIV latency are not fully elucidated. Latently infected cells are rare in vivo and appear to arise when activated CD4+ T cells, the major targets cells for HIV infection, become infected and survive long enough to revert back to a resting memory state, which is nonpermissive for viral gene expression [12]. An alternative model proposes that HIV latency is directly established in resting CD4+ T cells, following interaction with dendritic cells and/or specific chemokines [13,14].
No matter how latently infected cells are generated, several lines of evidence clearly indicate that the latent reservoir is established early in infection, indicating that one or both of these mechanisms occur within the first few days or weeks after transmission. Indeed, initiation of ART as early as 10 days after the onset of symptoms of primary HIV infection (PHI) does not prevent the generation of latently infected carrying infectious HIV despite the successful control of plasma viremia shortly after institution of therapy [15■]. Studies in nonhuman primates (NHPs), which have the advantage of precisely defining the time and location of the reservoir establishment, have also demonstrated that lentiviruses seed their reservoir within a few days post-infection; large numbers of resting CD4+ T cells carry integrated simian immunodeficiency virus (SIV), and simian human immunodeficiency virus DNA [16] can be detected at the earliest stage of infection. In line with these results, Whitney et al. [17■■] recently demonstrated that treatment with ART 3 days post-SIVmac251 infection in rhesus macaques blocks the emergence of viral RNA and proviral DNA in peripheral blood but does not prevent the establishment of a viral reservoir in tissues. After discontinuation of ART following 24 weeks of fully suppressive therapy, virus rebounded in all animals [17■■]. These data indicate that the viral reservoir could be seeded substantially earlier than previously assumed [5]. Another study in SIVmac239-infected rhesus macaques showed that ART initiated prior to peak virus replication (day 12) significantly limits systemic virus dissemination and seeding of the reservoir in the peripheral blood and lymphoid tissues [18]. Notwithstanding the important information generated by these studies conducted in NHP, it is crucial to caution their application to HIV infection in humans as SIV and HIV may establish their reservoir in distinct cells and tissues, and with different efficiency and kinetics. For instance, resting CD4+ T and myeloid cells are essentially resistant to HIV infection due to the restriction factor, Sterile alpha motif domain-containing protein 1 and HD domain-containing protein 1 (SAMHD1) [19,20–22], a mechanism that is counteracted by the SIV protein vpx, which is absent in HIV-1. This suggests that SIV may be more prone to directly establishing latency in resting CD4+ T cells and myeloid cells when compared with HIV. Moreover, the amount of virus used to efficiently infect NHPs is usually much higher than the ones that cause natural HIV transmission in humans and ART may not be as effective in NHPs as in HIV-infected patients. Therefore, the establishment of latent viral reservoir in humans and NHPs may differ in numerous ways, and further studies are warranted to demonstrate that these experimental models can be used to investigate the mechanisms responsible for the establishment and persistence of the long-lived HIV reservoir in infected individuals.
Early antiretroviral therapy limits the size of the latent HIV reservoir
Although the reservoir is seeded very early in infection, the beneficial effect of starting ART during the first weeks or months after infection to reduce the size of the latent reservoir has been reported in numerous studies. Strain et al. [23■■] reported that after 12 months of ART, replication-competent virus could not be detected in all patients who initiated treatment before seroconversion, nor in the majority of individuals who initiated ART in the first 6 months after seroconversion [23■■]. These observations were confirmed in several studies indicating that HIV-infected individuals who start ART within the first few months of HIV infection display a reservoir of smaller magnitude compared with those who start later in infection [6■,24■–26■,27■■]. Of note, the association between early ART initiation and a reduced size of the reservoir has been reported in multiple groups of patients using different assays including total HIV DNA [23■■,25■,26■,27■■], integrated DNA [24■,27■■], as well as the quantitative viral outgrowth assay [6■,23■■,27■■]. Importantly, there is a gradual increase in the size of the reservoir during the first few weeks after infection. Individuals who start ART at the Fiebig [28] I stage of the disease [HIV RNA+ , p24 antigen-, HIV Immunoglobulin M (IgM-)] show a significantly lower frequency of CD4+ T cells harboring integrated HIV DNA when compared to Fiebig II (HIV RNA+, p24 antigen+ , HIV IgM–) and Fiebig III (HIV IgM+, HIV IgG–) individuals [25■]. Therefore, the benefits of early ART may be maximal during the first few weeks of infection.
The decay of the latent HIV reservoir may also be faster in individuals who start ART early after infection [27■■,29■], although the limited size of the initial pool of latently infected CD4+ T cells has been proposed to be the most important factor contributing to a smaller size of the reservoir after several years of therapy. Archin et al. [6■] demonstrated that although the frequency of cells harboring replication-competent HIV declines after the first year of ART in most acutely infected patients, no further decay is noted when this frequency reaches very low levels (less than 0.5 cells per million). Therefore, a subset of latently infected cells may persist indefinitely, even in patients who have started ART during acute infection. The identification of the phenotype of these cells, in which HIV may persist for decades, is currently an area of intense investigation. Indeed, the composition of the reservoir (i.e., its distribution in distinct memory CD4+ T cell subsets) may provide crucial information on the mechanisms of HIV persistence, and several recent studies indicate that early ART greatly influences this parameter.
Early antiretroviral therapy alters the distribution of the reservoir in CD4+ T-cell subsets
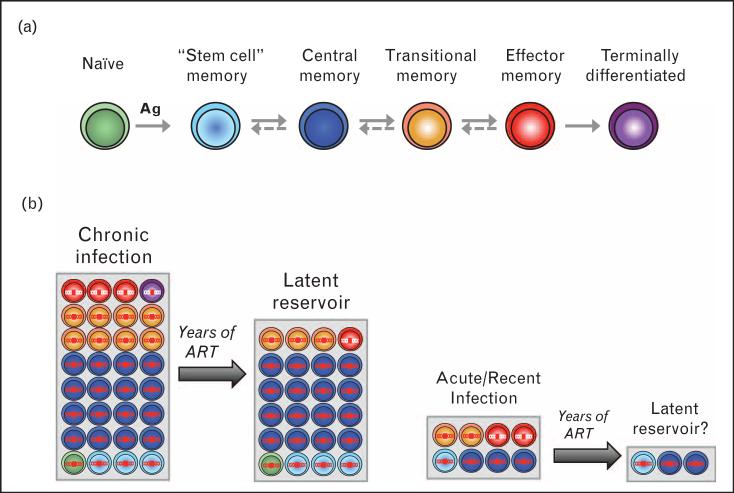
Resting memory CD4+ T cells are the predominantly infected cells in virally suppressed patients. The use of a combination of markers expressed by memory CD4+ T cells has led to the identification of three cellular reservoirs in which HIV persists after prolonged suppressive ART, namely central (TCM), transitional (TTM) and effector (TEM) memory CD4+ T cells [24■,30] (Fig. 1). Because of their very distinct functional and phenotypic properties [31], these three cellular reservoirs may support viral persistence through different mechanisms. For instance, the drastic differences in the activation status of TCM and TEM cells [24■,32] suggest that the former might represent an ideal reservoir for latent HIV, whereas the latter may be more prone to support residual levels of viral replication in the face of ART. The relative contribution of each subset to the pool of HIV-infected cells is highly variable between HIV-infected individuals [24■]. Several recent studies suggest that a relatively low infection frequency of TCM cells may be strongly associated with lack of disease progression in a variety of apparently unrelated clinical scenarios in untreated individuals. Long-term nonprogressors (LTNPs) display a low infection frequency of TCM cells, which is associated with an increased size of the TCM pool and a greater number of anti-gag CD8+ T cells. Of note, TCM from HLAB27/B57 LTNPs are markedly less infected than TCM from individuals who do not bear these protective alleles [33]. In addition, in the recently described VISCONTI cohort of posttreatment controllers (PTCs), who initiated ART early in infection and naturally control HIV replication in the absence of ART, TCM contribute very minimally to the total HIV reservoir [11■■]. Taken together, the relative protection of TCM cells in LTNPs and PTCs suggests that a TCM compartment devoid of HIV may be an important correlate of natural control of HIV replication.
FIGURE 1.
The size and composition of the latent HIV reservoir is affected by early ART. (a) Subsets of CD4 T cells can be identified based on the levels of expression of several cellular markers including CD45RA, CCR7, CD27 and CD95. The pathway of T cell differentiation, that is, the sequence of development of the different T-cell subsets, remains elusive in humans, and it is unclear if the differentiation pathway is linear or branched, one-way or reversible [31]. (b) In chronically infected patients, the contribution of TCM cells to the overall pool of infected cells is important [30]. As these cells are long lived, they survive after years of ART and represent the major latent HIV reservoir in patients who started ART during chronic infection [24■]. The contribution of TSCM cells also increases with time [27■■]. Patients with acute or recent infection display a small size of the reservoir (as depicted by a reduced size of the grey box). In addition, their TCM cells may be relatively preserved from infection, with a greater contribution of short-lived cells. After years of ART, this may result in a reservoir of a very small magnitude. ART, antiretroviral therapy.
Can early ART initiation reproduces this skewed distribution of the reservoir by protecting TCM cells from infection? Preliminary data generated on samples from the RV254 study, an AHI study conducted by the Thai Red Cross AIDS Research Center and the US Military HIV Research Program, indicate that very early ART efficiently protects all memory CD4+ T-cell subsets from infection, including the long-lived TCM cells [7]. This is in agreement with other studies investigating the frequency of infection of distinct memory CD4+ T-cell subsets in study participants who initiated ART early in infection [8,26■,27■■]. Whether the TCM subset is more protected than the other subset by early ART is still unclear.
In addition to TCM, TTM and TEM cells, the recently described stem cell memory T cells, which share functional and self-renewal properties with TCM cells [34], may represent an additional relevant reservoir for HIV [35]. Importantly, early ART may significantly reduce the contribution of this extremely long-lived subset to the overall HIV reservoir [27■■].
Clinical trials of early antiretroviral therapy and the HIV reservoirs
There are, so far, only a limited number of studies evaluating the impact of ART initiated in AHI on the HIV reservoirs. A major limitation is the difficulty in identifying people in AHI; this is a narrow window that spans the first 4 weeks of infection when the HIV IgG antibody response is not yet detected by standard assays [28,36]. The diagnosis generally requires nucleic acid testing that is costly and not part of standard testing algorithms in most settings. Thus, studies of acutely treated individuals are usually small. The immediate initiation of ART is also challenging, and in many studies, ART is initiated weeks or months after AHI is identified.
Table 1 summarizes the findings of selected studies published between 2005 and 2014 [6■,25■, 27■■,37–39,40■■,41,42■]. Most enrolled less than 40 participants. They included individuals treated within a few weeks to 6 months after the onset of HIV with a handful including those treated during the earliest stages of AHI prior to HIV IgM detection [25■,42■]. These studies consistently demonstrated the superiority of early ART over late treatment in reducing the HIV reservoir size as defined by HIV DNA levels (total and integrated, 2-long terminal repeat (LTR) circles 2-LTR circles) and frequencies of resting CD4+ T cells that are capable of producing replication-competent virus. Occurrence of low-level viremia measured by single-copy HIV RNA assay is also less with early ART [6■]. The frequency of infected cells in the gastrointestinal tract was evaluated in two studies that observed a statistically significant decline in HIV DNA after early ART [25■,41]. Studies comparing standard vs. regimens intensified with integrase and entry inhibitors did not show a superior effect of the latter regimen, but these studies are greatly underpowered [42■,43]. Comparison between studies is limited by the highly variable timing of ART initiation, ART regimen and follow-up periods as well as the different measures of the HIV reservoir.
Table 1.
Effects of early antiretroviral therapy on HIV reservoirs
| Reference | Sample size | Study population | Intervention | Timing of ART initiation | Treatment duration | Effect on reservoir size (measurea) | Main findings and/or comments |
|---|---|---|---|---|---|---|---|
| [25■] |
n = 15 3 Fiebig I 2 Fiebig II 8 Fiebig III 2 Fiebig IV |
Acutely infected Thais, mostly MSM with HIV clade CRF01_AE | MegaHAART regimen during AHI, defined as: Tenofovir Emtricitabine Efavirenz Raltegravir Maraviroc |
Fiebig stages I–IV | 24 weeks | Significant decline in total HIV DNAa copies in PBMCs and gut mucosal tissue; HIV DNA undetectable in 3/15 in blood and 3/7 in gut. After megaHAART, median total HIV DNA in PBMCs declined from 1513 / 106 CD4+ at baseline to 106 copies/106 at week 24 (P = 0.002). |
MegaHAART initiated in early Fiebig stages was associated with reduced reservoir size in blood and gut. |
| [6■] | n = 27 | Acutely infected individuals (CHAVI cohort) | Mathematical model to analyze frequency of latently infected cells post-ART | ART <45 days of estimated date of infection | ≥6 months | Replication-competent virus in resting CD4+ T cells serially measured by viral outgrowth assaya and SCAa. Positive correlation between the frequency of latently infected cells and the area under the pretreatment viral load curve; viremia frequently undetectable (<1 copy/ml) when resting cell infection frequency <0.3/million in resting CD4+ T cells. | Degree of latent infection can be limited by early ART during AHI. Few latently infected cells are generated after ART initiation. This study also showed that latency was founded immediately upon HIV infection. |
| [27■■] | n = 9 | Acutely infected individuals compared with reference cohort of chronically infected individuals (ART initiated >2 years postinfection] | ART initiation <6 months of infection (regimen not specified] | <6 months of infection | ≥10 years | Replication-competent virus measured by viral outgrowth assaya retrievable in 5/8 early ART patients; estimated frequency of CD4+ T cell with replication-competent virus higher in chronically infected patients than in early ART patients (P = 0.052). Statistically significant longitudinal decreases in total, integrated and 2-LTR HIV-1 DNAa in early ART patients (P = 0.0008). |
In patients treated during PHI, the decay of HIV-1 DNA in CD4+ T cells is faster and more pronounced than in chronically infected individuals. CD4+ T cells reservoirs in early treated patients are smaller but infection persists in long-lasting central memory and T memory stem cells. Time from HIV-1 acquisition to ART initiation correlated with total or integrated HIV-1 DNA after >10 years of ART. Levels of CD4+ T cell-associated 2-LTR HIV-1 DNA lower in early ART patients, particularly in effector memory CD4+ T cells. |
| [37] | n = 32 | Acutely and recently infected patients, mostly MSM with HIV-1 clade B | Standard first-line ART followed by interruption | Early ART defined as ≤60 days of infection (n = 24) vs. later start >60 days of infection (n = 8) | 1 8 months (median) | Average levels of cell-associated HIV-1 DNAa in early ART patients after treatment interruption 0.4 log10 (P = 0.03) lower compared with later starters. | Early ART within 60 days of HIV-1 infection resulted in lower levels of proviral HIV-1 DNA for ≥l-year post-ART interruption. |
| [38] |
n = 38 Six post-ART controllers Seven low viremic patients 25 noncontrollers |
Acutely infected patients who interrupted ART during PHI (ANRS C06 PRIMO study group) | Comparison of patient characteristics to predict HIV-1 control | ART initiated during first 3 months after HIV infection | Not specified | Post-ART controllers had lower median HIV-1 DNAa of 3.1 log10 copies/106 PBMC, compared with 3.4 log10 copies/106 PBMCs for post-ART noncontrollers (P = 0.09). | Lower HIV DNA levels at ART interruption were associated with post-ART HIV control, compared with patients who experienced viral rebound. |
| [39] | n is variable per number for whom available of patients data are | Patients treated during PHI in 39 health centers in 10 countries | Quadruple ART administered twice daily during PHI, defined as: (QUEST GE PROB3005 cohort] Zidovudine Lamivudine Abacavir Amprenavir |
Quadruple ART initiated during PHI | 48 weeks | Median cell-associated RNAa level decreased from 3.4 log copies/million PBMCs (n = 114) to 0.8 log copies/million PBMCs(n = 63) and median cell-associated DNAa level (n = 113) decreased from 2.8 log copies/million PBMCs (n = 113) to 1.6 log copies/million PBMCs (n = 63) after 48 weeks. | Data represent one of the largest cohorts of patients prospectively enrolled during PHI. ART initiation during PHI was associated with lower levels of cell-associated RNA and DNA than with ART initiation during chronic infection. |
| [40■■] | n = 34 | Patients in early HIV infection (Options Project, San Francisco, USA) |
Standard ART regimen | <6 months of infection, compared with later ART group (n = 32), untreated (n = 34) and HIV-negative controls (n = 1 9) | Not specified | Early ART predicted 4.8-fold lower HIV DNAa levels in PBMCs than with later ART initiation (P = 0.005). Early ART predicted lower cell-associated HIV RNAb levels (difference in signal-to-cutoff-ratio (S/Co) 3.2 (P = 0.035). |
Data suggest that early ART initiated within 6 months of infection is associated with lower HIV DNA and cell-associated reservoir sizes. This study shows that timing of ART initiation is a critical determinant of the HIV reservoir size. Early ART is also associated with lower levels of CD4+ and CD8+ T-cell activation. |
| [41] | n = 8 | Patients in AHI, defined as ≤3 bands on western blot, p24 antigen positive and/or proviral DNA positive | Raltegravir Emtricitabine Tenofovir |
AHI, compared with patients treated during chronic infection (n = 8) | 52 weeks | In acutely treated patients: Total HIV-1 DNAa levels in PBMCs reduced in both groups, with more pronounced decrease in PHI patients at week 52 - from 4594 copies per 106 cells at baseline to 494 at week 52 (P = 0.0003). Total HIV-1 DNA in colon tissue biopsiesa reduced from 2125 copies per 500 ng DNA at baseline to 113 at week 52 (P = 0.001). |
Levels of HIV-1 DNA in PBMCs and gut tissue were lower in PHI vs. chronically infected patients at week 52. |
| [42■] |
n = 36 (randomized) n = 34 met criteria for primary endpoint analysis |
Acutely infected patients defined as plasma HIV-1 RNA levels >5000 copies/ml with negative or indeterminate EIA | Once daily fixed combination 3-drug Pl-based cART regimen vs. 5-drug regimen intensified with raltegravir and maraviroc (1:2 ratio] | ART initiated at randomization in AH | Approximately 2 years | At week 48, undetectable single-copy assaya in 3/1 1 in 3-drug arm vs. 9/21 in 5-drug arm (P = 0.46). No statistically significant differences in cell-associated HIV-1 DNAa in PBMCs or replication-competent virus in resting CD4+ T cells at week 96 between 3-drug vs. 5-drug arm. |
No statistically significant effect on the HIV reservoir of intensified 5-drug cART vs. 3-drug cART initiated during early infection. |
| [11■■] | n = 14 | PTCs who controlled viremia for several years post-ART interruption (VISCONTI cohort) | Standard ART | Combination ART initiated within 10 weeks of PHI | Approximately 3 years | PBMC-associated HIV-1 DNAa levels in PTCs during infection control (median 1.71 log copies/106 PBMC) similar to those in spontaneous HIV controllers and lower than patients who started ART during chronic HIV infection. In the absence of ART, HIV-1 DNAa levels in PBMC declined in 5/8 and was stable in 2/8. Replication-competent virus detected in at least one T-cell subset (TCM, TTM, TEM) in 7/7 tested patients. Low frequency of infection in TCM compared with TTM. |
Control of viremia post-ART interruption was associated with low HIV blood reservoir size. This finding suggests that limiting the HIV reservoir is crucial for control of viral replication off ART. Long-lived HIV-infected CD4+ T-cell subsets contributed minimally to the total resting HIV reservoir in PTCs. |
| [23■■] | n = 27, MSM | Subgroups of MSM recruited from PHI studies in San Diego and Los Angeles, CA between 1996 and 1999 | Standard ART | ART initiation before or <6 months after seroconversion with viral <50 copies/ml load, compared with 17 patients who initiated ART during chronic infection and with viremia <50 copies/ml for 3 - 6 years | Duration of study | Total CAI in resting CD4+ T cellsa was undetectable after 1 year of ART (<0.07 IUPM) in 9/9 patients who initiated ART before seroconversion and in 6/8 patients who initiated ART <6 months after seroconversion, compared with none of the 17 controls who started ART during chronic infection | This study contributed to the quantitative understanding of the dynamics of long-lived cellular reservoirs of HIV-1 in patients during PHI compared with chronic infection |
Fiebig stages: Fiebig I (RNA+, p24 antigen–, HIV IgM–), Fiebig II (p24 antigen–, HIV IgM–), Fiebig III (HIV IgM+, HIV IgG–, western blot), Fiebig IV (HIV IgG–, western blot indeterminate).
AHI, acute HIV infection; ART, antiretroviral therapy; CAI, cell-associated infectivity; IUPM, Infectious units/million cells; MSM, men who have sex with men; PBMC, peripheral blood mononuclear cells; PHI, primary HIV infection; PI, protease inhibitor; PTC, posttreatment controllers; SCA, single-copy assay; TCM, central memory T cells; TEM, effector memory T cells; TTM, transitional memory T cells.
for references of special interest.
for references of outstanding interest.
FUTURE RESEARCH
Most of the studies that have demonstrated the ability of early ART at reducing the size of the latent reservoir have been conducted on cells isolated from peripheral blood. Little information is known on the establishment of the reservoir in tissues (particularly the major anatomical HIV reservoirs such as the gut and lymph nodes) at the earliest stage of infection. This limitation is obviously attributable to the difficulty in accessing these samples, but also to the lack of a sensitive, reproducible and flexible assay that can measure a few latently infected cells harboring replication-competent HIV. Alleviating this technical limitation would also allow a better understanding of the relative importance of each memory T-cell subset to HIV persistence, as well as the capacity of early ART at interfering with the establishment of latency in these discrete subsets of cells.
The persistence of infection in TCM after 10 years of ART in the study by Buzon et al. [27■■] suggests that treatment within the first 6 months of infection is not early enough, but it remains unknown what the window of opportunity to intervene is before reaching the ‘point of no return.’ Theoretically, the earlier ART is initiated (i.e., Fiebig stages I and II) [28], the higher should be the chance for HIV remission. However, the PTCs in the VISCONTI cohort were mainly those treated in Fiebig IV and V stages [11■■]. Understanding the precise timing of ART initiation that has the greatest impact on HIV reservoirs will require careful characterization of patients at time of ART initiation for their history of HIV exposure and viro-logic and serologic markers of HIV. A concerted effort to evaluate and identify AHI staging system that could predict outcomes relevant to HIV cure (e.g., HIV reservoir size) would be a major advancement. Additionally, there is a dearth of data on the HIV-specific immune responses following early ART. It is possible that such immune responses are blunted with rapid treatment and viral suppression, as evidenced by the higher proportion of early treated individuals who remain HIV seronegative compared with the later treatment initiators [44,45]. Knowledge on the association between existing immune responses and HIV reservoirs after AHI treatment will inform future immune-based strategies to eliminate latently infected cells.
CONCLUSION
It is clear that early ART results in lower reservoir size which could delay the time to viral rebound when ART is interrupted, as has been demonstrated in the Mississippi child. However, reaching sustained HIV remission or cure will require not only reduction size of the HIV reservoir, but also, to modify the distribution of the replication-competent HIV reservoir in CD4+ T cells and tissues. ART initiated during AHI, however, may be the first critical step in the path aimed at HIV remission or cure by limiting the HIV reservoir establishment prior to employing additional interventions to eliminate all cells capable of producing HIV.
KEY POINTS.
HIV persistence is established early in AHI in long-lived memory CD4+ T cells that are nonpermissive for viral gene expression, therefore, not eliminated by immune surveillance or ART.
ART instituted during AHI can reduce the HIV reservoir size to a greater extent than when treatment is given in chronic HIV. However, infection generally persists in memory CD4+ T cells in most early treated individuals.
Recent data suggest that treatment in the earliest AHI stage (Fiebig I) may protect central memory CD4+ T cells from infection and skew the distribution of latently infected cells to the shorter lived memory CD4+ T cells as is the case in individuals who control HIV without ART (elite controllers and PTCs).
ART in AHI may be the first critical step in the path to achieve HIV remission by containing HIV reservoir seeding prior to instituting additional interventions to eliminate all latently infected cells.
Acknowledgements
None.
Financial support and sponsorship
The given work was supported in part by grants to N.C. from the amfAR (ARCHE grants 108687-54 and 108928-56), the National Institutes of Health (1R21 AI113096-01 and 1U19AI096109 – DARE: Delaney AIDS research enterprise to find a cure) and a grant to J.A. from the National Institute of Allergy and Infectious Diseases (R01HD080435-01). This work was also partly supported by the CARE – Collaboratory of AIDS Researchers for Eradication (U19AI096113); David M. Margolis PI.
Footnotes
Conflicts of interest
J.A. has received honorarium for speaking engagement and advisory board participation from Gilead and ViiV. Healthcare. Other authors declared no potential conflicts of interest relevant to this work.
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Finzi D. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. [23 July 2014];Science [internet] 1997 278:1295–1300. doi: 10.1126/science.278.5341.1295. http://www.sciencemag.org/cgi/doi/10.1126/science.278.534 1.1295. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. [28 July 2014];Science [internet] 1997 278:1291–1295. doi: 10.1126/science.278.5341.1291. http://www.sciencemag.org/cgi/doi/10.1126/science.27 8.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A [internet] 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=24285&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks SG, Autran B, Berkhout B, et al. Nat Rev Immunol [internet] Vol. 12. Nature Publishing Group; 2012. [25 January 2014]. Towards an HIV cure: a global scientific strategy. pp. 607–614. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3595991&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng K, Siliciano RF. HIV: early treatment may not be early enough. [23 July 2014];Nature [internet] 2014 :1–2. doi: 10.1038/nature13647. http://www.ncbi.nlm.nih.gov/pubmed/25043038. [DOI] [PubMed]
- 6■.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. [15 July 2014];Proc Natl Acad Sci U S A [internet] 2012 109:9523–9528. doi: 10.1073/pnas.1120248109. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3386138&tool=pmcentrez&rendertype=Abstract. [This study is unique because it characterizes replication-competent virus in resting CD4+ T cells after early ART and describes the kinetics by which resting cell infection is established.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ananworanich J, Vandergeeten C, Chomchey N, et al. Early ART intervention restricts the seeding of the HIV reservoir in long lived central memory CD4 T cells. Conference on Retroviruses and Opportunistic Infections. 2013 [Google Scholar]
- 8.Cheret A, Bacchus C, Melard A, et al. Early HAART in primary HIV infection protects TCD4 central memory cells and can induce HIV remission. Conference on Retroviruses and Opportunistic Infections [internet] 2014 http://croi2014.org/sites/default/files/uploads/CROI2014_Final_Abstracts.pdf.
- 9.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. [20 February 2014];N Engl J Med [internet] 2013 369:1828–1835. doi: 10.1056/NEJMoa1302976. http://www.ncbi.nlm.nih.gov/pubmed/24152233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIAID Mississippi baby’ now has detectable HIV, researchers find [internet] 2014 http://www.niaid.nih.gov/news/newsreleases/2014/pages/mississip pibabyhiv.aspx.
- 11■■.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Posttreatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. [22 February 2014];PLoS Pathog [inter net] 2013 9:e1003211. doi: 10.1371/journal.ppat.1003211. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3597518&tool=pmcentrez&rendertype=Abstract. [This article reports the results of the VISCONTI cohort, a group of 14 posttreatment controllers who controlled viremia for several years post-ART interruption. This study characterized various CD4+ T-cell subsets and showed that viremic control may be associated with the low contribution of long-lived cells to the HIV reservoir.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siliciano RF, Greene WC. HIV latency. [30 July 2014];Cold Spring Harb Perspect Med [internet] 2011 1:a007096. doi: 10.1101/cshperspect.a007096. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3234450&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh S, Solomon A, Wightman F, et al. Brief report CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4 z T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:1–3. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 14.Evans V, Kumar N, Filali A, et al. Myeloid dendritic cells induce HIV-1 latency in nonproliferating CD4+ T cells. [16 August 2014];PLoS Pathog [internet] 2013 9:e1003799. doi: 10.1371/journal.ppat.1003799. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3855553&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15■.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A [internet] 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=21169&tool=pmcentrez&rendertype=Abstract. [This work is important in that it showed that ART initiation as early as 10 days after the onset of symptoms in PHI did not prevent the establishment of latently infected resting CD4+ T cells harboring proviral HIV DNA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura Y, Sadjadpour R, Mattapallil JJ, et al. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc Natl Acad Sci U S A [internet] 2009;106:8015–8020. doi: 10.1073/pnas.0903022106. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2683103&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17■■.Whitney JB, Hill AL, Sanisetty S, et al. Nature [internet] Nature Publishing Group; 2014. [21 July 2014]. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. http://www.nature.com/doifinder/10.1038/nature13594. [This work showed that the reservoir was seeded very early after SIV infection in rhesus monkeys (<3 days), during the eclipse phase and before detectable viremia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okoye AA, Rohankhedkar M, Reyes M, et al. Early treatment in acute SIV infection limits the size and distribution of the viral reservoir. Conference on Retroviruses and Opportunistic Infections [internet] 2014 http://croi2014.org/sites/default/files/uploads/CROI2014_Final_Abstracts.pdf.
- 19.Laguette N, Sobhian B, Casartelli N, et al. SAMHD1 is the dendriticand myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. [14 July 2014];Nature [internet] 2011 474:654–657. doi: 10.1038/nature10117. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3595993&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hrecka K, Hao C, Gierszewska M, et al. Nature [internet] Vol. 474. Nature Publishing Group; 2011. [12 August 2014]. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. pp. 658–661. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3179858&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldauf H-M, Pan X, Erikson E, et al. Nat Med [internet] Vol. 18. Nature Publishing Group; 2012. [12 August 2014]. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. pp. 1682–1687. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3828732&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descours B, Cribier A, Chable-Bessia C, et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology [internet] 2012;9:87. doi: 10.1186/1742-4690-9-87. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3494655&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23■■.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [This study is important in that it provided one of the first quantitative assessments of the dynamics of long-lived HIV-1 cellular reservoirs in patients treated during PHI. Data showed that replication-competent virus could not be detected in all subjects who initiated treatment before seroconversion, nor in the majority of patients who started ART less than 6 months post-seroconversion.] [DOI] [PubMed] [Google Scholar]
- 24■.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. [14 July 2014];Nat Med [internet] 2009 15:893–900. doi: 10.1038/nm.1972. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2859814&tool=pmcentrez&rendertype=Abstract. [This work characterized central memory and transitional memory CD4+ T cells as major cellular reservoirs for HIV infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. [22 February 2014];PLoS One [internet] 2012 7:e33948. doi: 10.1371/journal.pone.0033948. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3316511&tool=pmcentrez&rendertype=Abstract. [This work characterizes the earliest immunological and virological events in peripheral blood and gut following HIV infection (90% in Fiebig I–III) as well as a novel megaHAART (mega-highly active antiretroviral therapy) therapeutic approach.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26■.Josefsson L, Von Stockenstrom S, Faria NR, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. [30 July 2014];Proc Natl Acad Sci U S A [internet] 2013 110:E4987–E4996. doi: 10.1073/pnas.1308313110. http://www.pubmedcentral.nih.gov/articler-ender.fcgi?artid=3870728&tool=pmcentrez&rendertype=Abstract. [This study genetically characterized HIV-1 DNA from naïve and memory CD4+ T cells from eight patients and showed that early ART resulted in lower HIV-1 reservoirs in the blood and the gut of these patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27■■.Buzon MJ, Martin-Gayo E, Pereyra F, et al. Long-term antiretroviral treatment initiated in primary HIV-1 infection affects the size, composition and decay kinetics of the reservoir of HIV-1 infected CD4 T cells. [30 June 2014];J Virol [internet] 2014 doi: 10.1128/JVI.01046-14. http://www.ncbi.nlm.nih.gov/pubmed/24965451. [This study is the first to examine the long-term effect of early ART initiation (<6 months of infection). This study found that early treatment could accelerate the decay of productively infected CD4+ T cells and measured replication-competent virus in patients treated during PHI after 10 years of treatment. Patients treated during PHI have a reduced reservoir size, but a long-lasting reservoir (CD4+ T central memory and memory stem cells) is effectively established despite early treatment.] [DOI] [PMC free article] [PubMed]
- 28.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. [22 February 2014];AIDS (London, England) [internet] 2003 17:1871–1879. doi: 10.1097/00002030-200309050-00005. http://www.ncbi.nlm.nih.gov/pubmed/12960819. [DOI] [PubMed] [Google Scholar]
- 29■.Chun T-W, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. [28 July 2014];J Infect Dis [internet] 2007 195:1762–1764. doi: 10.1086/518250. http://www.ncbi.nlm.nih.gov/pubmed/17492591. [This study showed evidence for the decay rate of the latently infected resting CD4+ T cells in individuals on ART.] [DOI] [PubMed] [Google Scholar]
- 30.Brenchley JM, Hill BJ, Ambrozak DR, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appay V, Van Lier RAW, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry. [12 July 2014];Part A: J Int Soc Anal Cytol [internet] 2008 73:975–983. doi: 10.1002/cyto.a.20643. http://www.ncbi.nlm.nih.gov/pubmed/18785267. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Fo R, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Descours B, Avettand-Fenoel V, Blanc C, et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. [17 August 2014];Clin Infect Dis [internet] 2012 54:1495–1503. doi: 10.1093/cid/cis188. http://www.ncbi.nlm.nih.gov/pubmed/22441653. [DOI] [PubMed] [Google Scholar]
- 34.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. [9 July 2014];Nat Med [internet] 2011 17:1290–1297. doi: 10.1038/nm.2446. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3192229&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. [9 July 2014];Nat Med [internet] 2014 20:139–142. doi: 10.1038/nm.3445. http://www.ncbi.nlm.nih.gov/pubmed/412925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ananworanich J, Fletcher JLK, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. [22 February 2014];Retrovirology [internet] 2013 10:56. doi: 10.1186/1742-4690-10-56. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3669623&tool=pmcentrez&rendertype=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianella S, Von Wyl V, Fischer M, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. [16 August 2014];Antivir Ther [internet] 2011 16:535–545. doi: 10.3851/IMP1776. http://www.ncbi.nlm.nih.gov/pubmed/21685541. [DOI] [PubMed] [Google Scholar]
- 38.Goujard C, Girault I, Rouzioux C, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. [16 August 2014];Antivir Ther [internet] 2012 17:1001–1009. doi: 10.3851/IMP2273. http://www.ncbi.nlm.nih.gov/pubmed/22865544. [DOI] [PubMed] [Google Scholar]
- 39.Hoen B, Cooper D, Lampe FC, et al. Predictors of virological outcome and safety in primary HIV type 1-infected patients initiating quadruple anti-retroviral therapy: QUEST GW PROB3005. [16 August 2014];Clin Infect Dis [internet] 2007 45:381–390. doi: 10.1086/519428. http://www.ncbi.nlm.nih.gov/pubmed/17599319. [DOI] [PubMed] [Google Scholar]
- 40■■.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. [22 February 2014];J Infect Dis [internet] 2013 208:1202–1211. doi: 10.1093/infdis/jit311. http://www.ncbi.nlm.nih.gov/pubmed/23852127. [This work showed that ART initiation within 6 months of infection is associated with lower HIV reservoir size as measured by HIV DNA levels and cell-associated RNA levels, compared with later ART initiation. Finding suggests that the timing of ART initiation is a critical factor in the size of the HIV reservoir. Early ART initiation can further minimize chronic CD4+ and CD8+ T-cell activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koelsch KK, Boesecke C, McBride K, et al. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. [28 July 2014];AIDS (London, England) [internet] 2011 25:2069–2078. doi: 10.1097/QAD.0b013e32834b9658. http://www.ncbi.nlm.nih.gov/pubmed/21860347. [DOI] [PubMed] [Google Scholar]
- 42■.Markowitz M, Evering TH, Garmon D, et al. A randomized open-label study of 3- versus 5-drug combination antiretroviral therapy in newly HIV-1-infected individuals. [16 August 2014];J Acquir Immune Defic Syndr (1999) [internet] 2014 66:140–147. doi: 10.1097/QAI.0000000000000111. http://www.ncbi.nlm.nih.gov/pubmed/24457632. [This study is unique in that it compared the effect of 3-drug vs. 5-drug regimen in acutely infected patients and comprehensively assessed immunological and virological responses to intensified therapy. The 5-drug regimen failed to reveal statistically significant differences to the 3-drug regimen on measures of the HIV reservoirs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ananworanich J, Vandergeeten C, Schuetz A, et al. HIV reservoir size and immunity in blood and sigmoid colon of acute HIV-infected Thai subjects following 5- and 3-drug HAART. Conference on Retroviruses and Opportunistic Infections. 2012 [Google Scholar]
- 44.Hare CB, Pappalardo BL, Busch MP, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis [internet] 2006;42:700–708. doi: 10.1086/500215. http://www.ncbi.nlm.nih.gov/pubmed/16447118. [DOI] [PubMed] [Google Scholar]
- 45.Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. [17 August 2014];AIDS (London, England) [internet] 2014 28:1015–1020. doi: 10.1097/QAD.0000000000000178. http://www.ncbi.nlm.nih.gov/pubmed/24384692. [DOI] [PubMed] [Google Scholar]