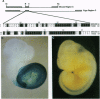
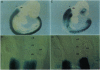
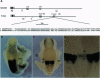
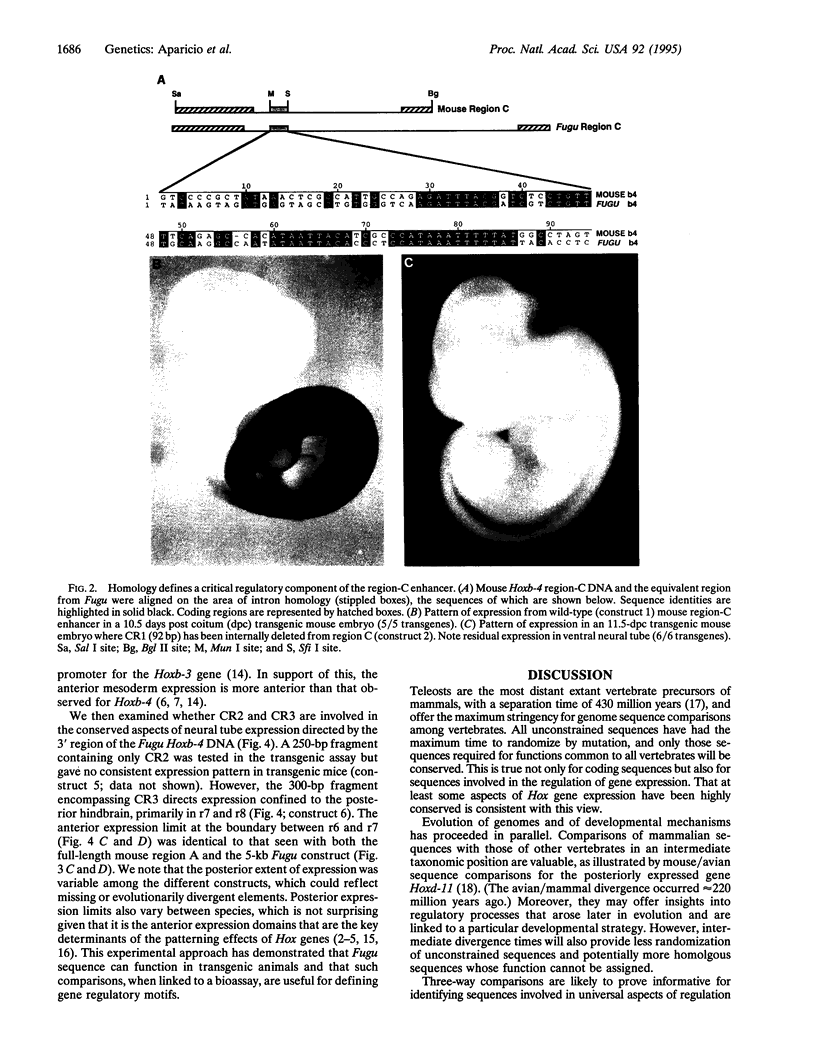
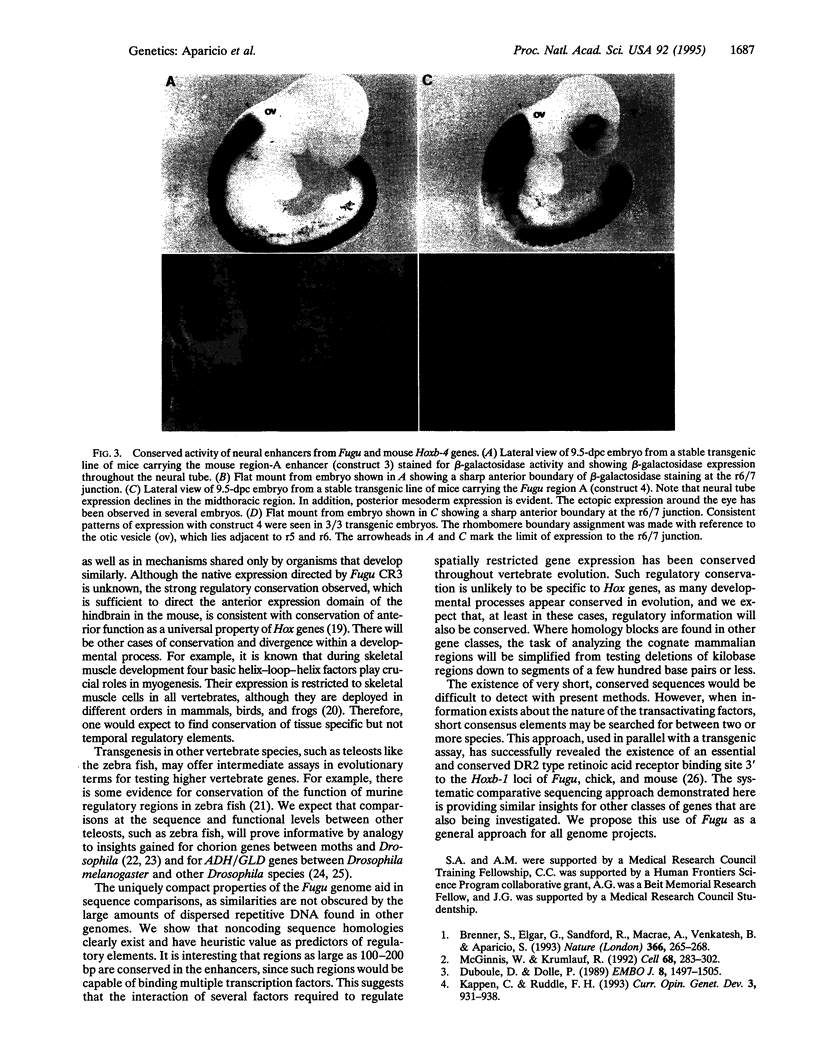
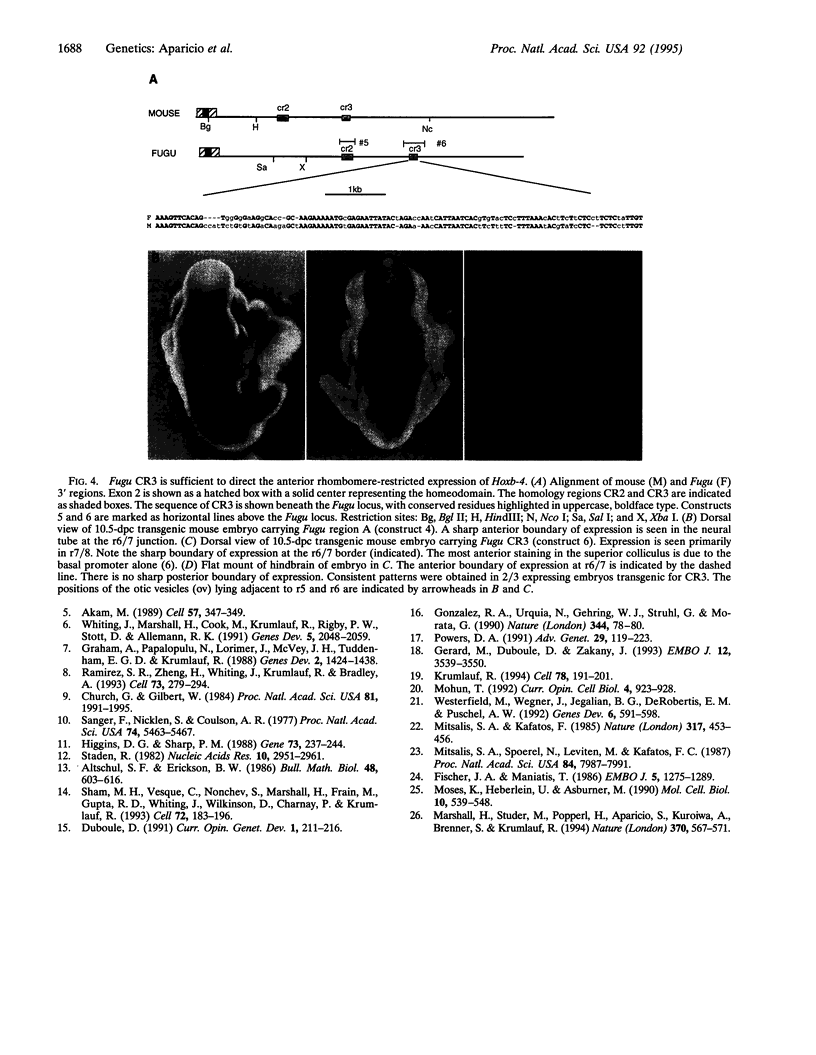
Abstract
Comparative vertebrate genome sequencing offers a powerful method for detecting conserved regulatory sequences. We propose that the compact genome of the teleost Fugu rubripes is well suited for this purpose. The evolutionary distance of teleosts from other vertebrates offers the maximum stringency for such evolutionary comparisons. To illustrate the comparative genome approach for F. rubripes, we use sequence comparisons between mouse and Fugu Hoxb-4 noncoding regions to identify conserved sequence blocks. We have used two approaches to test the function of these conserved blocks. In the first, homologous sequences were deleted from a mouse enhancer, resulting in a tissue-specific loss of activity when assayed in transgenic mice. In the second approach, Fugu DNA sequences showing homology to mouse sequences were tested for enhancer activity in transgenic mice. This strategy identified a neural element that mediates a subset of Hoxb-4 expression that is conserved between mammals and teleosts. The comparison of noncoding vertebrate sequences with those of Fugu, coupled to a transgenic bioassay, represents a general approach suitable for many genome projects.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989 May 5;57(3):347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Erickson B. W. Optimal sequence alignment using affine gap costs. Bull Math Biol. 1986;48(5-6):603–616. doi: 10.1007/BF02462326. [DOI] [PubMed] [Google Scholar]
- Brenner S., Elgar G., Sandford R., Macrae A., Venkatesh B., Aparicio S. Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature. 1993 Nov 18;366(6452):265–268. doi: 10.1038/366265a0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. Patterning in the vertebrate limb. Curr Opin Genet Dev. 1991 Aug;1(2):211–216. doi: 10.1016/s0959-437x(05)80072-3. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Regulatory elements involved in Drosophila Adh gene expression are conserved in divergent species and separate elements mediate expression in different tissues. EMBO J. 1986 Jun;5(6):1275–1289. doi: 10.1002/j.1460-2075.1986.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Reyes A., Urquia N., Gehring W. J., Struhl G., Morata G. Are cross-regulatory interactions between homoeotic genes functionally significant? Nature. 1990 Mar 1;344(6261):78–80. doi: 10.1038/344078a0. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Lorimer J., McVey J. H., Tuddenham E. G., Krumlauf R. Characterization of a murine homeo box gene, Hox-2.6, related to the Drosophila Deformed gene. Genes Dev. 1988 Nov;2(11):1424–1438. doi: 10.1101/gad.2.11.1424. [DOI] [PubMed] [Google Scholar]
- Gérard M., Duboule D., Zákány J. Structure and activity of regulatory elements involved in the activation of the Hoxd-11 gene during late gastrulation. EMBO J. 1993 Sep;12(9):3539–3550. doi: 10.1002/j.1460-2075.1993.tb06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Kappen C., Ruddle F. H. Evolution of a regulatory gene family: HOM/HOX genes. Curr Opin Genet Dev. 1993 Dec;3(6):931–938. doi: 10.1016/0959-437x(93)90016-i. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994 Aug 18;370(6490):567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Kafatos F. C. Regulatory elements controlling chorion gene expression are conserved between flies and moths. Nature. 1985 Oct 3;317(6036):453–456. doi: 10.1038/317453a0. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Spoerel N., Leviten M., Kafatos F. C. A short 5'-flanking DNA region is sufficient for developmentally correct expression of moth chorion genes in Drosophila. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7987–7991. doi: 10.1073/pnas.84.22.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. Muscle differentiation. Curr Opin Cell Biol. 1992 Dec;4(6):923–928. doi: 10.1016/0955-0674(92)90119-w. [DOI] [PubMed] [Google Scholar]
- Moses K., Heberlein U., Ashburner M. The Adh gene promoters of Drosophila melanogaster and Drosophila orena are functionally conserved and share features of sequence structure and nuclease-protected sites. Mol Cell Biol. 1990 Feb;10(2):539–548. doi: 10.1128/mcb.10.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D. A. Evolutionary genetics of fish. Adv Genet. 1991;29:119–228. doi: 10.1016/s0065-2660(08)60108-x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., Wegner J., Jegalian B. G., DeRobertis E. M., Püschel A. W. Specific activation of mammalian Hox promoters in mosaic transgenic zebrafish. Genes Dev. 1992 Apr;6(4):591–598. doi: 10.1101/gad.6.4.591. [DOI] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]