Abstract
Background
We assessed the acute and long-term impact of MI and stroke on post-event functional disability and cognition while controlling for survivors’ changes in functioning over the years before the event.
Methods and Results
Among participants in the nationally-representative Health and Retirement Study with linked Medicare data (1998-2010), we determined within-person changes in functional limitations (basic and instrumental activities of daily living) and cognitive impairment after hospitalization for stroke (n=432) and MI (n=450), controlling for pre-morbid functioning using fixed-effects regression. In persons without baseline impairments, an acute MI yielded a mean increase of 0.41 functional limitations (95% CI, 0.18-0.63) with a linear increase of 0.14 limitations/year in the following decade. These increases were 0.65 limitations (95% CI, 0.07-1.23) and 0.27 limitations/year afterwards for those with mild-to-moderate impairment at baseline. Stroke resulted in an increase of 2.07 (95% CI, 1.51-2.63) limitations due to the acute event and an increase of 0.15 limitations/year afterwards for those unimpaired at baseline. There were 2.65 new limitations (95% CI, 1.86-3.44) and 0.19/year afterwards for those with baseline mild-to-moderate impairment. Stroke hospitalization was associated with greater odds of moderate-to-severe cognitive impairment (odds ratio, 3.86; 95% CI, 2.10-7.11) at the time of the event, after adjustment for premorbid cognition but MI hospitalization was not.
Conclusions
In this population-based cohort, most MI and stroke hospitalizations were associated with significant increases in functional disability at the time of the event and in the decade afterwards. Survivors of MI and stroke warrant screening for functional disability over the long-term.
Keywords: myocardial infarction, functional disability, cognitive impairment
Introduction
Myocardial infarction (MI) and stroke are the leading vascular contributors to illness and death in the United States,1, 2 with hundreds of thousands of Americans experiencing one of these events annually.2 In 2010, 7.6 million US adults were MI survivors and 6.8 million were stroke survivors,2 and these prevalence rates are predicted to increase by up to 25% over the next two decades3 owing to treatment advances and growth of the population aged 65 or older.4-6 The chronic care of MI and stroke survivors will place huge burdens on families, health care systems, and public programs such as Medicare and Medicaid.
Disability accounts for a substantial amount of the health burden and costs due to MI and stroke.1 Although both MI and stroke experienced major improvements in acute care (e.g., thrombolysis, organized inpatient care) and secondary cardiovascular disease (CVD) prevention7 between 1990 and 2010, disability rates for MI fell nearly 15% but disability rates for stroke increased 40% during this period.1 Yet, few studies have compared acute and long-term changes in disability due to functional or cognitive problems after MI and stroke in a contemporary cohort of survivors.8
Importantly, declines in pre-morbid functioning may precede acute medical events and these declines may confound or even modify the effect of these events on functional and cognitive outcomes.9 While MI and stroke have been associated with acute increases in functional and cognitive disability,10-12 studies conflict on whether acute MI or stroke increase the rate of disability after controlling for individuals’ longitudinal changes in disability over time before the vascular event.13-18 Many studies suggest that average yearly changes in disability after MI or stroke are not significantly greater than those before MI or stroke.13-18 It is also not established whether pre-morbid functioning modifies the effect of acute MI or stroke on functional outcomes. If MI and stroke contribute to acute functional and cognitive disability and also new impairments in physical and cognitive functioning over the long-term, then clinicians and payers will need to consider interventions that identify and treat these problems beyond the immediate post-event period.
The present study used a longitudinal, nationally representative cohort of older Americans to compare the acute and long-term changes in functional disability, cognitive impairment, and depression after MI and stroke in a contemporary cohort of survivors. Our analyses focused on the pivotal role of pre-morbid functioning by controlling for and also stratifying by individuals’ longitudinal changes in function and cognition over time before the vascular event.
Methods
Data Source and Study Population
Study subjects were participants of the Health Retirement Study (HRS). The HRS is a nationally-representative, longitudinal study of 37,000 US residents aged 51 years or older.19 The HRS uses multi-stage area probability sampling from all US states and the District of Columbia, with oversampling of Blacks and Hispanics. Every 2 years since 1992, HRS participants have been interviewed regarding physical health and functioning, cognitive functioning, disability, health insurance, and other factors. The HRS uses standardized instruments to collect data on valid, generalizable measures that are applicable to stroke, MI, and a range of health conditions. The HRS achieves a high follow-up rate, ranging 85%-91% from 1998 to 2010 including proxies.19
Data regarding inpatient and outpatient medical services received, including dates of hospitalizations, were available from the Centers for Medicare and Medicaid Services (CMS) for those participants who were enrolled in Medicare fee-for-service from 1991 through 2007 and agreed to linkage. Approximately 80% of Medicare-eligible HRS participants consent to this linkage of their Medicare data. The HRS protocol was approved by the University of Michigan Institutional Review Board and all participants provided informed consent.
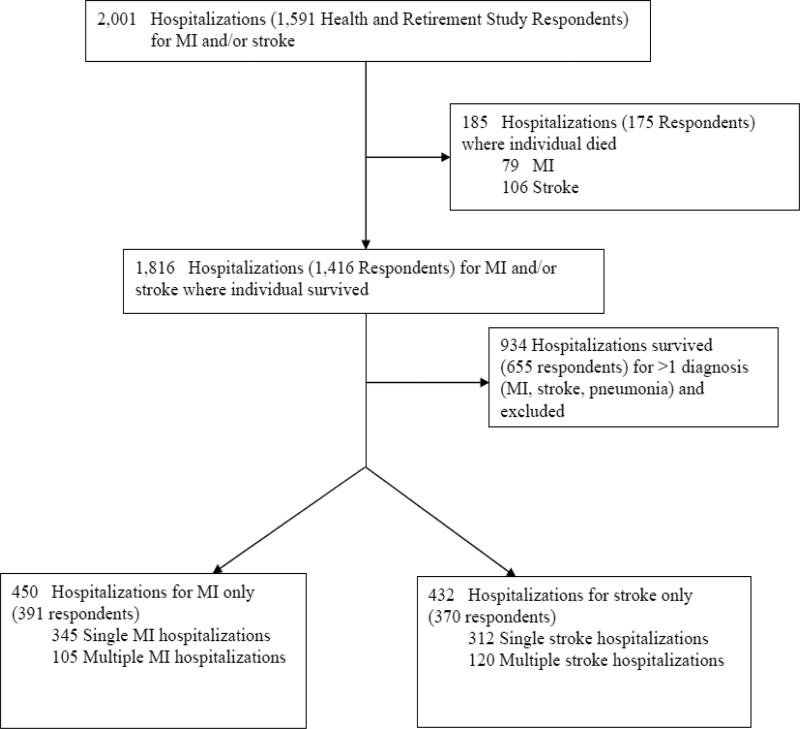
We identified all subjects hospitalized with a principal discharge diagnosis of MI or stroke during 1998 to 2007 using the linked Medicare data and valid ICD-9-CM codes.20, 21 We required hospitalizations to be ≥1 day and subjects to have ≥1 functional and cognitive measurement before and after the index MI or stroke hospitalization. To determine the unique effects of the exposures, MI hospitalization and stroke hospitalization, we excluded hospitalizations in which another vascular event or pneumonia was diagnosed and listed as a secondary discharge diagnosis (i.e., the MI group included individuals hospitalized with a principal discharge diagnosis of MI and no secondary diagnosis of stroke or pneumonia and the stroke group included individuals hospitalized with hospitalized for stroke). Figure 1 shows the derivation of the analytic cohort. All patients were followed through death or the 2010 HRS interview.
Figure 1.
Derivation of Analytic Cohort
Definitions of Outcome Measures
Functional Impairment
To ascertain functional disability, respondents (or their proxies) were asked if they required assistance with any of 6 activities of daily living (ADLs): walking, dressing, bathing, eating, getting into/out of bed, and toileting, and 5 instrumental activities of daily living (IADLs): preparing a hot meal, grocery shopping, making telephone calls, taking medicines, and managing money. We categorized the level of functional disability using thresholds utilized in prior HRS studies, defining baseline mild-to-moderate disability as 1 to 3 impairments in ADLs and IADLs, and baseline moderate-to-severe disability as ≥ 4 impairments.9
Cognitive Impairment
Cognitive impairment was assessed using versions of the modified Telephone Interview for Cognitive Status (TICS-m).22, 23 Proxies completed cognitive impairment assessments for patients unable to complete the interview themselves using the Informant Questionnaire on Cognitive Decline in the Elderly.24 Thresholds for mild-moderate and moderate-severe cognitive impairment were based on HRS research and the methods used for the Aging, Demographics, and Memory Study,25, 26 and have been validated against neuropsychiatric interviews.27
Depression
As a secondary outcome, we examined trajectories of depression before and after stroke and MI hospitalization, respectively. Depression was assessed at each HRS interview using an 8-item version of the Center for Epidemiologic Studies Depression Scale (CES-D)28 from patients, not proxies.29 We used a cutoff score of ≥ 4 to define substantial depressive symptoms because this threshold is comparable to the cutoff score of ≥ 16 on the full CES-D.29
Demographic and Clinical Characteristics
Information on demographics (e.g., age, race/ethnicity, sex, education, and marital/partnered status) and health-risk behaviors (e.g., alcohol use and smoking) came from HRS interviews. Baseline Charlson Comorbidity score30 and hospitalization-related characteristics (length of stay, intensive care unit or coronary care unit admission, and requirements for mechanical ventilation, major surgery, and dialysis) were obtained from Medicare claims.
Statistical Analysis
Multivariable regression analyses examined the adjusted associations between MI or stroke hospitalization and the 3 outcome measures. For analyses of functional status post-hospitalization for MI or stroke, our dependent variable was the total number of impairments in ADLs and IADLs. We conducted separate analyses for MI survivors and for stroke survivors. Initially, the change in functional status over time was graphed using piecewise regression models with one knot and one jump to account for the change in status at the time of the acute event (MI or stroke).31 We used fixed-effects regression to model trajectories of functional impairments over time with control for all stable patient characteristics (see online supplement).9, 32, 33 These models used only within-person variation over time to estimate the effect of hospitalization for each of our conditions of interest (i.e., patients serve as their own controls). We estimated three values with 95% confidence intervals: 1) the average change in functional impairments over time before the MI or stroke hospitalization (the pre-event slope or trajectory), 2) the acute change in functional impairments at the time of the MI or stroke hospitalization, and 3) the average change in functional impairments over time after the MI or stroke hospitalization (the post-event slope). We used all available information on the outcome measure for each participant to calculate these estimates. We also adjusted for post-hospitalization cognitive impairment to examine trajectories beyond that associated with cognitive impairment.
Similarly, we used multivariable conditional logistic regression to examine the impact of MI or stroke hospitalization on each outcome, development of moderate-to-severe cognitive impairment or the presence of substantial depressive symptoms. Trajectories reflect the change in the odds of developing each outcome over time before or after the event (e.g., the odds of developing cognitive impairment before MI per year). Given that survivors who develop cognitive impairment or new functional impairments after MI or stroke are also more likely to develop substantial depressive symptoms, we added time-dependent variables for incident mild-to-moderate cognitive impairment, moderate-to-severe cognitive impairment, and functional impairments to the model predicting the outcome of substantial depressive symptoms. Multivariable results are reported as adjusted odds ratios (aORs) and 95% confidence intervals
For the primary analyses, patients were allowed to have one or more hospitalization(s) for the vascular event (MI or stroke).
Sensitivity Analysis
We repeated analyses using only the first MI or stroke hospitalization for each patient. Since 8% of MI survivors and 11% of stroke survivors were missing post-hospitalization depression measurements because they required a proxy, we used non-response propensity score adjustment in our final depression model to quantify potential biases in our results.34 Analyses were performed with the IBM SPSS Statistics 18 (SPSS Inc., Chicago, IL) and STATA 11.2 (Stata Corporation, College Station, TX) statistical software programs.
Results
There were 391 individuals who experienced 450 hospitalizations for MI but not for stroke and 370 individuals who experienced 432 hospitalizations for stroke though not for MI in which individuals survived to complete ≥1 follow-up outcome assessment. Patients had up to 5 outcome measurements (range: 7.7-9.8 years) before hospitalization and up to 6 outcome measurements (range: 9.9-12.7 years) after hospitalization.
Characteristics of the patients and the hospitalizations have been reported previously.33 MI survivors were younger and more likely to be male, white, married, and former smokers compared with survivors of stroke hospitalization. Functional limitations, cognitive impairments, and depressive symptoms before the hospitalization were less common in survivors of MI hospitalization than in survivors of stroke hospitalization. Hospitalizations for MI more frequently involved major surgery and stays in ICUs or CCUs compared with hospitalizations for stroke. Survivors of stroke hospitalization had a greater likelihood of switching to proxy-respondent status than MI hospitalization survivors (28% vs. 8%).
Functional Outcomes
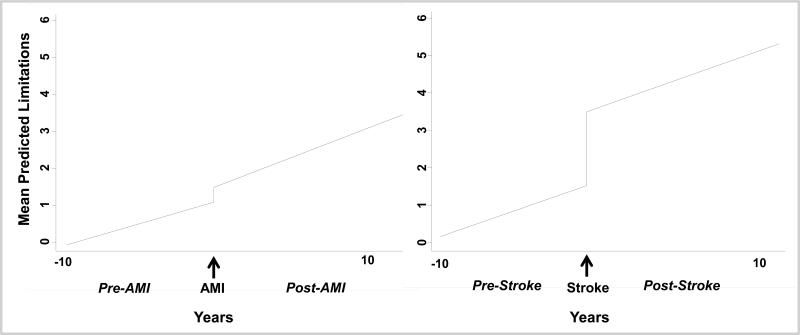
Figure 2 displays the adjusted change in functional status over time for both MI and stroke using fixed-effects regression. Overall, individuals experienced a mean of 0.40 additional limitations shortly after an MI (95% CI, 0.16-0.64), while patients who experienced a stroke had gained 1.97 additional limitations (95% CI, 1.56-2.39).
Figure 2. Piecewise Fixed-Effects Regression Models of Mean Predicted Functional Limitations before and after Acute Myocardial Infarction (AMI) and Stroke.
Plots are based on the fixed-effects regression model.
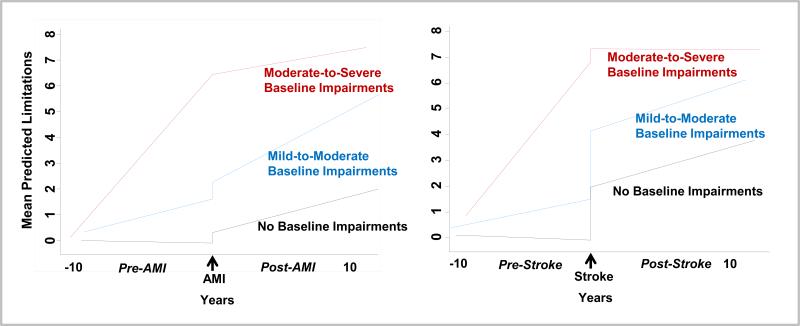
In fixed-effects regression, stratification by baseline functional status indicated that individuals with fewer limitations at baseline were most likely to experience additional limitations (Figure 3). For those without baseline impairment, there was a significant difference in trajectory slopes pre-to-post event for both MI (P<0.001) and stroke (P=0.003). This was also true for those with moderate-to-severe impairment at baseline for MI (P=0.004) and stroke (P<0.001). However, there was no difference in the pre- versus post-slopes for those with mild-to-moderate impairment at baseline for either MI (P=0.11) or stroke (P=0.49).
Figure 3. Piecewise Fixed-Effects Regression Models of Mean Predicted Functional Limitations before and after Acute Myocardial Infarction and Stroke, Stratified by Baseline Functional Status.
Plots are based on the fixed-effects regression model.
We then performed fixed-effects regression models controlling for pre-event functional impairment. For individuals without baseline functional disability, the effect of an MI yielded a mean of 0.41 (95%CI, 0.18-0.63) new functional limitations, with a significant increase of 0.14 limitations/year in the decade following the MI (Table 1). Individuals with mild-to-moderate baseline disability gained 0.65 (95%CI, 0.07-1.23) new functional limitations with an acute MI which steadily increased by 0.27 limitations/year in the ensuing decade. Persons with moderate-to-severe impairment at baseline experienced a significant increase in the rate of impairments (0.65/year) prior to acute MI, which did not appreciably change after the event.
Table 1.
Adjusted Changes in Functional Limitations (95% Confidence Intervals) before and after Hospitalization for Myocardial Infarction and Stroke, Stratified by Baseline Physical Functioning
| Myocardial Infarction Survivors | Stroke Survivors | ||||||
|---|---|---|---|---|---|---|---|
| Functional Class before Hospitalization | Functional Class before Hospitalization | ||||||
| No Functional Impairment (n=277) | Mild-moderate Functional Impairment (n=124) | Moderate-severe Functional Impairment (n=49) | No Functional Impairment (n=217) | Mild-moderate Functional Impairment (n=135) | Moderate-severe Functional Impairment (n=80) | ||
| Change in functional limitations per year before MI | −0.01 (−0.04, 0.02) | 0.13 (0.04, 0.22) | 0.65 (0.50, 0.81) | Change in functional limitations per year before stroke | −0.02 (−0.07, 0.03) | 0.12 (0.05, 0.18) | 0.63 (0.35, 0.90) |
| P value | 0.57 | 0.005 | <0.001 | P value | 0.48 | 0.001 | <0.001 |
| Change in functional limitations at the time of the MI | 0.41 (0.18, 0.63) | 0.65 (0.07, 1.23) | −0.005 (−0.93, 0.92) | Change in functional limitations at the time of the stroke | 2.07 (1.51, 2.63) | 2.65 (1.86, 3.44) | 0.51 (−0.40, 1.43) |
| P value | <0.001 | 0.028 | 0.99 | P value | <0.001 | <0.001 | 0.27 |
| Change in functional limitations per year after MI | 0.14 (0.08, 0.20) | 0.27 (0.14, 0.41) | 0.10 (−0.21, 0.41) | Change in functional limitations per year after stroke | 0.15 (0.06, 0.25) | 0.19 (0.001, 0.37) | −0.003 (−0.23, 0.23) |
| P value | <0.001 | <0.001 | 0.51 | P value | 0.002 | 0.049 | 0.98 |
MI = myocardial infarction.
Functional class was defined by a subject's ability to perform basic and instrumental activities of daily living at the last interview before the hospitalization for MI or stroke.
Interpretive Example: As shown in the second column, there were 124 subjects hospitalized for myocardial infarction (MI) who had mild-to-moderate functional impairment at the most recent interview before the MI hospitalization. The first section of rows shows the change in functional limitations per year before MI. An average subject in the group of 124 with mild-moderate functional impairment at baseline had been gaining 0.13 new functional limitations per year (95% confidence interval, 0.04-0.22; P=0.005) before having an MI. The second section of rows shows the change in functional limitations at the time of the MI. An average subject in the group with mild-moderate impairment at baseline developed 0.65 new additional functional limitations at the time of the MI (95% confidence interval, 0.07-1.23; P=0.028). The third section of rows shows the long-term change in functional limitations per year after MI. Over the years following MI, an average subject in the group with mild-moderate impairment at baseline further gained 0.27 additional functional limitations per year (95% confidence interval, 0.14-0.41; P<0.001).
a Results of latent growth curve regression with individual-level fixed effects, controlling for all time-invariant characteristics of the patient. The absence of an association would be signified by acquiring 0 new ADL/IADL impairments. The within-patient R2 for patients with one or more MI hospitalizations were 0.15 for the no limitation group, 0.25 for the group with mild-to-moderate baseline limitations, and 0.39 for the group with moderate-to-severe limitations. The within-patient R2 for patients with one or more stroke hospitalizations were 0.31 for the no limitation group, 0.41 for the group with mild-to-moderate baseline limitations, and 0.30 for the group with moderate-to-severe limitations.
Functional impairment was more acutely affected by a stroke, with an additional 2.07 limitations experienced by those without baseline impairment and 2.65 limitations by those with mild-to-moderate baseline impairment. Furthermore, there was a steady increase for both of these groups in the rate of impairment over the decade following the stroke (0.15/year and 0.19/year, respectively). As found in MI, persons with moderate-to-severe baseline impairment experienced a rapid increase in the rate of functional impairment (0.63/year) prior to an acute stroke and no significant change in impairments at the time of, and in the decade after, the stroke.
Cognitive Outcomes
Stroke hospitalization was associated with worse cognitive outcomes than MI hospitalization. The percentage of survivors with moderate-to-severe cognitive impairment increased from 19.6% at the interview just before stroke hospitalization to 30.2% at the first interview after stroke hospitalization (P<0.001); whereas, the percentage increased from 9.6% before MI hospitalization to 15.1% after MI hospitalization (P=0.01).
In fixed-effects regression controlling for cognitive impairment before the event, the odds of developing moderate-to-severe cognitive impairment at the time of the acute MI was increased but not significantly (aOR, 1.68; 95% CI, 0.91-3.10; P=0.10), whereas survivors of acute stroke had significantly greater odds of developing moderate-to-severe cognitive impairment (OR, 3.86; 95% CI, 2.10-7.11) (Table 2). In the decade following the MI or the stroke, we did not observe significantly greater odds of developing moderate-to-severe cognitive impairment over the long-term after controlling for the odds of developing cognitive impairment before or acutely after the event. However, before having an MI or stroke, these individuals experienced a significant annual increase in odds of developing moderate-to-severe cognitive impairment (OR=1.23 for MI and OR=1.52 for stroke).
Table 2.
Adjusted Odds Ratios (95% Confidence Intervals) for Moderate-to-Severe Cognitive Impairment and Substantial Depressive Symptoms over Timea
| Moderate-Severe Cognitive Impairment | Substantial Depressive Symptoms | ||||
|---|---|---|---|---|---|
| Odds Ratios (95% CI) | P Value | Odds Ratios (95% CI) | P Value | ||
| MI survivors | MI survivors | ||||
| Trajectory before MI, per year | 1.23 (1.04, 1.45) | 0.02 | Trajectory before MI, per year | 0.98 (0.88, 1.09) | 0.66 |
| Effect of MI | 1.68 (0.91, 3.10) | 0.10 | Effect of MI | 1.21 (0.70, 2.08) | 0.50 |
| Trajectory after MI, per year | 1.01 (0.89, 1.15) | 0.83 | Trajectory after MI, per year | 1.04 (0.92, 1.17) | 0.53 |
| Stroke survivors | Stroke survivors | ||||
| Trajectory before stroke, per year | 1.52 (1.25, 1.86) | <0.001 | Trajectory before stroke, per year | 0.97 (0.89, 1.06) | 0.52 |
| Effect of stroke | 3.86 (2.10, 7.11) | <0.001 | Effect of stroke | 1.35 (0.72, 2.56) | 0.35 |
| Trajectory after stroke, per year | 0.99 (0.89, 1.11) | 0.93 | Trajectory after stroke, per year | 0.95 (0.81, 1.10) | 0.48 |
Abbreviations: MI = myocardial infarction;
Trajectory reflects the change in the odds of developing cognitive impairment or depressive symptoms over time.
Results of latent growth curve regression with individual level fixed effects, controlling for all time-invariant characteristics of the patient. The analyses for substantial depressive symptoms post-MI and post-stroke are also adjusted for post-illness cognitive impairment, ADL/IADL impairments and non-response propensity scores. The absence of association would be signified by an odds ratio of 1.
Interpretive Example: With each passing year, older adults who survive one or more hospitalizations for stroke are more likely to develop moderate-to-severe cognitive impairment. After a stroke hospitalization, patients had 3.9-times greater odds of developing moderate-to-severe cognitive impairment than before their stroke hospitalization.
Depression Outcomes
In unadjusted analyses, stroke hospitalization was associated with a significant increase in the presence of substantial depressive symptoms but MI hospitalization was not. The percentage of survivors with substantial depressive symptoms increased from 16.4% at the interview just before stroke hospitalization to 22.0% at the first interview after stroke hospitalization (P=0.04); whereas, this percentage changed from 23.1% before MI to 26.9% (P=0.25) after MI.
After controlling for depressive symptoms before the event, survivors’ odds of developing substantial depressive symptoms were significantly increased at the time of stroke (aOR, 1.91; 95%CI, 1.04-3.50; P=0.04) though not at the time of acute MI (aOR, 1.36; 95%CI, 0.79-2.32; P=0.26). However, after controlling for post-event cognitive and functional impairment, neither acute MI nor stroke was associated with subsequent substantial depressive symptoms at the time of the acute event (Table 2). In the decade following the MI or the stroke, we did not observe significantly greater odds of developing depressive symptoms over the long-term after controlling for change in depressive symptoms before or acutely after the event whether models did or did not account for post-event cognitive and functional impairment. However, the odds of developing substantial depressive symptoms were 20% greater per each new functional limitation gained after MI (odds ratio, 1.20; 95% CI, 1.06, 1.37; P=0.005) and 34% greater per each new functional limitation gained after stroke (odds ratio, 1.34; 95% CI, 1.06-1.69; P=0.02) (Table 2).
Sensitivity Analyses
In analyses limited to the first hospitalization for MI or stroke, results were similar except some within-strata contrasts for functional impairments were no longer statistically significant and the magnitude of acute moderate-to-severe cognitive impairment after MI and stroke was lower (see online supplement). Results for substantial depressive symptoms were similar after adjusting for non-response propensity scores (see online supplement).
Discussion
In this nationally-representative cohort of older Americans, MI hospitalization was associated with significant acute and long-term increases in functional disability. We were able to demonstrate that MI and stroke each increase the rate of additional impairments in the following decade. This emphasizes the extent to which these acute vascular events are potential inflection points in patients’ lives. Clinically, this suggests that clinicians should have an increased index of suspicion that patients may need additional help with ADLs and IADLs over the years after MI and stroke. Scientifically, it suggests an urgent need to disentangle the extent to which this acute and also accelerated disability are the result of incomplete rehabilitation from the initial hospitalization, subsequent vascular events, behavioral changes, or other mechanisms.
Most studies have lacked information on individuals’ changes in disability over time before the MI or stroke hospitalization and consequently have controlled for baseline functioning using measures obtained during or soon after the hospitalization.10 However, many adults experience significant declines in functional disability over time before MI or stroke.13-17 Studies assessing changes in functional disability before and after MI and stroke in older adults have yielded mixed results.13-17 Some studies observed that MI increased the risk of a decline in physical functioning but that the effect was explained by comorbidity13 or limited to subjects with recent MI (<1 year)15. Other studies have found that the average rate of yearly increase in functional disability after MI or stroke did not change or lessened compared to changes in disability before the vascular event.14, 16, 17 We observed that acute MI is associated with increases in functional impairments years after the event, even after accounting for individuals’ changes in functional disability before the MI and acutely after the event. We likely detected a significant increase in rates of disability after MI and stroke because we had larger sample size, more outcome data before and after the vascular events, and included IADL changes.14, 16, 17
We also observed that stroke significantly increased the odds of survivors’ developing moderate-to-severe cognitive impairment acutely at the time of the event with a non-significant trend for MI to do so. After accounting for changes in cognitive impairment before and acutely after the vascular events, we did not detect a significant increase in survivors’ subsequent risk of cognitive impairment over the long-term, consistent with previous stroke research.18, 35 Our results may differ from others8, 36, 37 because of cohort differences and we studied incident MI and stroke events, used a different measure of cognitive impairment, and controlled for cognitive changes before the event and acutely at the time of the event. It is also possible that our measure of cognitive impairment was not sensitive to more subtle long-term changes in executive functioning, attention, or processing speed present after acute MI or stroke.
We did not detect a significant increase in the presence of substantial depressive symptoms after MI hospitalization38 when controlling for depressive symptoms prior to the acute event, similar to previous MI research.15 Still, we observed that having a higher number of functional impairments after acute MI or stroke was independently associated with substantial depressive symptoms, consistent with studies of other acute medical events like pneumonia and sepsis.32, 33 Taken together, these studies suggest that it is not the acute medical event itself but how disabling the event is that incrementally increases the risk of developing depression.
The key finding of our study—that functional disability mounts after an MI and stroke—has clinical implications. Clinical practice guidelines and quality improvement programs recommend assessments of function, cognition, and depression in stroke patients prior to discharge and also in the post-acute settings.39, 40 Our results suggest that survivors of MI merit functional assessment prior to hospital discharge because they may have acutely acquired functional impairments. Moreover, our results suggest that survivors of MI and stroke also warrant monitoring for mounting functional disability over the years after the event. Clinicians can screen survivors for difficulty performing ADLs or IADLs as these were our measures. Those survivors who acquire new functional impairments further warrant screening for depression because, in our study, the odds of developing substantial depressive symptoms increased 20% with each new functional limitation gained after MI and 34% greater per each new functional limitation gained after stroke.
We observed that progression of functional disability is relatively worse in some patients than in others. Survivors with fewer baseline limitations were most likely to gain functional limitations. These intriguing results should be interpreted with caution. We may not have detected significant changes in physical functioning after MI or stroke in patients with more baseline limitations because acute clinical events have less impact9 or our measures are relatively insensitive in patients with moderate-to-severe pre-existing functional disability. The patients with moderate-to-severe disability at baseline had steep functional declines over the years before MI or stroke and these slopes did not appear to change over the long-term after the events. Those with substantial functional decline before the MI or stroke event likely had CVD or other disabling clinical events before the study period while those with no disability at baseline may have experienced their first event. This vulnerable population warrants further study as to the reasons for, and prevention of, these steep functional declines.
The World Health Organization's International Classification of Functioning, Disability, and Health describes the complex interplay between a health condition like MI or stroke, functioning, and disability with contributions by pathophysiological processes, the impact of the health condition on the survivor, and contextual factors such as each survivor's personal and environmental resources.39, 41, 42 Survivors of MI or stroke may experience continued functional decline over the years after the event because of exacerbation of underlying neurodegenerative disease, subclinical vascular events (e.g, cerebral infarcts), or ongoing vascular and neuronal damage.43-45 Although we did not aim to identify the activities, behaviors, or contextual factors that predict long-term functional declines after MI or stroke, studies have shown that having Medicaid or no health insurance, lack of cardiac rehabilitation referral, and moderate-to-high perceived stress increase the risk for functional decline after MI.10, 16, 46 Conversely, vascular risk factor modification may reduce the long-term risk of cognitive impairment after stroke.47 Research is needed to better elucidate the mechanisms and risk factors for functional decline over the long-term after MI or stroke.
Since more adults are elderly and surviving MI and stroke, we need to better understand how to best care for an older population with CVD and functional or cognitive impairments. While much is known about the benefits and risks of rehabilitation, transitions of care, and pharmacological management in patients during the early months after MI or stroke, less is known about the benefits and risks of these interventions in functionally or cognitively disabled patients. How do we best apply or tailor effective CVD interventions to an older survivor population with functional or cognitive impairments? Research to identify efficacious, patient-centered clinical interventions and systems of care for the large and growing population of older adults with CVD and functional or cognitive impairments is urgently needed.
Our study has some potential limitations. Our results are generalizable to older, community-dwelling survivors of MI and stroke including those who move to nursing homes. Although survivors were sampled from a nationally-representative cohort, the small numbers, particularly within functional strata, may limit generalizability. Still, our study was larger than other studies of this topic. Although the HRS-Medicare data do not include the results of testing for cardiac biomarkers, the principal and secondary diagnoses of MI and dates for a hospitalization are highly accurate in CMS records. Our analysis does not include individuals with out-of-hospital or severe events that do not survive hospitalization. The use of proxies by survivors during longitudinal follow-up is a potential limitation. Although 28% of stroke survivors and 8% of MI survivors converted to proxy-respondents at some point during follow-up, sensitivity analyses with non-response propensity score adjustment found similar results for the depression outcome.
Our estimates of declines in physical and cognitive functioning may be underestimated because of survivor bias and outmigration due to death (i.e., death of patients with greater disability at baseline or patients with severe deficits after MI or stroke). We were unable to examine the role of clinical variables such as disease severity, treatments, atrial fibrillation, or revascularization surgery. However, among patients with coronary artery disease, long-term cognitive decline may occur similarly in those who do and do not undergo surgery11 and physical disability after one year may be less in those undergoing surgery compared to percutaneous coronary intervention.48 We did not identify personal and environmental factors associated with long-term functional declines after MI or stroke. Although we did not study disease-specific functional measures, we studied functional and cognitive impairments using standardized, valid instruments that are commonly used in older adults with vascular and non-vascular conditions. Our outcome measures represent clinically meaningful changes in patient-centered outcomes: physical and cognitive functioning.
Conclusion
The population of MI and stroke survivors is substantial and growing. Given improved event survival, the increasing number of older adults, and recent disability trends, the chronic care of MI and stroke survivors will prove costly. In this nationally-representative cohort of older Americans, we found that hospitalizations for MI and stroke were associated with significant acute and long-term increases in functional disability. Our results suggest that survivors of MI merit functional assessment prior to hospital discharge because they may have acutely acquired functional impairments. Moreover, our results suggest that survivors of MI and stroke also warrant monitoring for mounting functional disability over the years after the event.
“What is Known”
Myocardial infarction (MI) and stroke may have long-term impact on post-event functional disability.
Many studies have suggested that longitudinal changes in functional disability after MI are not greater than those before MI.
“What this Article Adds”
In this nationally-representative cohort of older Americans, we found that hospitalizations for MI and stroke were associated with significant acute and long-term increases in functional disability after controlling for survivors’ changes in functioning over the years before the event.
Our results suggest that survivors of MI merit functional assessment prior to hospital discharge because they may have acutely acquired functional impairments.
Moreover, our results suggest that survivors of MI and stroke also warrant monitoring for mounting functional disability over the years after the event.
Acknowledgements
We thank Tish Shapiro and Mohammed Kabeto for their excellent programming. The Health and Retirement Study is conducted by the Institute for Social Research at the University of Michigan.
The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Funding
The Health and Retirement Study is sponsored by the National Institute on Aging (U01 AG09740) and the Social Security Administration. Dr. Levine received research support from the NIH (P30DK092926 and K23AG040278). Dr. Davydow received research support from the NIH (KL2 TR000421). Dr. Iwashyna received research support from the NIH (K08, HL091249) and VA HSR&D (IIR 11-109).
Footnotes
Author Contributions
DL, DD, and TI - conception and design
TI and KL - acquisition of data
DL, DD, CH, KL, MR and TI - analysis and interpretation of data
DL and DD - drafting of the manuscript
DL, DD, CH, KL, MR and TI - critical revision of the manuscript for important intellectual content
DL, DD, and TI - statistical analysis
MR - administrative, technical, or material support
DL, DD, CH, KL, MR and TI - supervision
Disclosures
None.
References
- 1.The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.McGovern PG, Jacobs DR, Jr., Shahar E, Arnett DK, Folsom AR, Blackburn H, Luepker RV. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: The minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 6.He WS, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau, Current Population Reports. US Government Printing Office; Washington DC: 2005. 65+ in the United States: 2005. pp. P23–209. [Google Scholar]
- 7.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, Hernandez AF, Peterson ED, Schwamm LH, Committee GW-SS. Investigators. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get with the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 8.Volonghi I, Pendlebury ST, Welch SJ, Mehta Z, Rothwell PM. Cognitive outcomes after acute coronary syndrome: A population based comparison with transient ischaemic attack and minor stroke. Heart. 2013;99:1509–1514. doi: 10.1136/heartjnl-2013-304207. [DOI] [PubMed] [Google Scholar]
- 9.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson JA, Arnold SV, Reid KJ, Gill TM, Rich MW, Masoudi FA, Spertus JA, Krumholz HM, Alexander KP. Physical function and independence 1 year after myocardial infarction: Observations from the translational research investigating underlying disparities in recovery from acute myocardial infarction: Patients' health status registry. Am Heart J. 2012;163:790–796. doi: 10.1016/j.ahj.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 12.Moroney JT, Bagiella E, Tatemichi TK, Paik MC, Stern Y, Desmond DW. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology. 1997;48:1317–1325. doi: 10.1212/wnl.48.5.1317. [DOI] [PubMed] [Google Scholar]
- 13.Covinsky KE, Hilton J, Lindquist K, Dudley RA. Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care. 2006;44:149–157. doi: 10.1097/01.mlr.0000196955.99704.64. [DOI] [PubMed] [Google Scholar]
- 14.Mendes de Leon CF, Bang W, Bienias JL, Glass TA, Vaccarino V, Kasl SV. Changes in disability before and after myocardial infarction in older adults. Arch Intern Med. 2005;165:763–768. doi: 10.1001/archinte.165.7.763. [DOI] [PubMed] [Google Scholar]
- 15.Mendes de Leon CF, Krumholz HM, Vaccarino V, Williams CS, Glass TA, Berkman LF, Kas SV. A population-based perspective of changes in health-related quality of life after myocardial infarction in older men and women. J Clin Epidemiol. 1998;51:609–616. doi: 10.1016/s0895-4356(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 16.Dhamoon MS, Moon YP, Paik MC, Sacco RL, Elkind MS. Trajectory of functional decline before and after ischemic stroke: The northern manhattan study. Stroke. 2012;43:2180–2184. doi: 10.1161/STROKEAHA.112.658922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capistrant BD, Wang Q, Liu SY, Glymour MM. Stroke-associated differences in rates of activity of daily living loss emerge years before stroke onset. J Am Geriatr Soc. 2013;61:931–938. doi: 10.1111/jgs.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Capistrant BD, Ehntholt A, Glymour MM. Long-term rate of change in memory functioning before and after stroke onset. Stroke. 2012;43:2561–2566. doi: 10.1161/STROKEAHA.112.661587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: The Health and Retirement Study (HRS). Int J Epidemioly. 2014;43:576–85. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 22.Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JCS. Properties of the telephone interview for cognitive status - application in epidemiologic and longitudinal-studies. Neuropsy Neuropsy Be. 1994;7:235–241. [Google Scholar]
- 23.Welsh KA, Breitner JCS, Magruderhabib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsy Neuropsy Be. 1993;6:103–110. [Google Scholar]
- 24.Jorm AF. The informant questionnaire on cognitive decline in the elderly (iqcode): A review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 25.Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB. Trends in the prevalence and mortality of cognitive impairment in the united states: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NH, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Weir DR, Willis RJ. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 27.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The ces-d scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. Survey Research Center; Ann Arbor, Michigan: 2000. [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell MN. Interpreting and visualizing regression models using stata. Stata Press Publication; College Station, TX: 2012. [Google Scholar]
- 32.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: A prospective cohort study of older americans. Am J Geriatr Psychiatry. 2013;21:887–97. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med. 2013;126:615–624. e615. doi: 10.1016/j.amjmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao RS, Sigurdson AJ, Doody MM, Graubard BI. An application of a weighting method to adjust for nonresponse in standardized incidence ratio analysis of cohort studies. Ann Epidemiol. 2005;15:129–136. doi: 10.1016/j.annepidem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 36.Petrovitch H, White L, Masaki KH, Ross GW, Abbott RD, Rodriguez BL, Lu G, Burchfiel CM, Blanchette PL, Curb JD. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am J Cardiol. 1998;81:1017–1021. doi: 10.1016/s0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 37.Haring B, Leng X, Robinson J, Johnson KC, Jackson RD, Beyth R, Wactawski-Wende J, von Ballmoos MW, Goveas JS, Kuller LH, Wassertheil-Smoller S. Cardiovascular disease and cognitive decline in postmenopausal women: Results from the women's health initiative memory study. JAHA. 2013;2:e000369. doi: 10.1161/JAHA.113.000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Echols MR, O'Connor CM. Depression after myocardial infarction. Curr Heart Fail Rep. 2010;7:185–193. doi: 10.1007/s11897-010-0024-6. [DOI] [PubMed] [Google Scholar]
- 39.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA. American Heart Association Council on Cardiovascular N, the Stroke C. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the american heart association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 40. [July 31, 2014]; Http://www.Jointcommission.Org/assets/1/18/dsc_csc_chap.Pdf.
- 41.International classification of functioning, disability and health (icf) World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 42.Iwashyna TJ, Netzer G. The burdens of survivorship: An approach to thinking about long-term outcomes after critical illness. Sem Respir Crit Care Med. 2012;33:327–338. doi: 10.1055/s-0032-1321982. [DOI] [PubMed] [Google Scholar]
- 43.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of alzheimer disease. The Nun study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 44.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: Inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 45.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 46.Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: Long-term mortality and health status outcomes. J Am Coll Cardiol. 2012;60:1756–1763. doi: 10.1016/j.jacc.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douiri A, McKevitt C, Emmett ES, Rudd AG, Wolfe CD. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation. 2013;128:1341–1348. doi: 10.1161/CIRCULATIONAHA.113.002236. [DOI] [PubMed] [Google Scholar]
- 48.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ, Investigators FT. Quality of life after pci vs cabg among patients with diabetes and multivessel coronary artery disease: A randomized clinical trial. JAMA. 2013;310:1581–1590. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]