Abstract
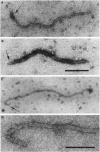
Elongated particles of simple RNA viruses of plants are composed of an RNA molecule coated with numerous identical capsid protein subunits to form a regular helical structure, of which tobacco mosaic virus is the archetype. Filamentous particles of the closterovirus beet yellow virus (BYV) reportedly contain approximately 4000 identical 22-kDa (p22) capsid protein subunits. The BYV genome encodes a 24-kDa protein (p24) that is structurally related to the p22. We searched for the p24 in BYV particles by using immunoelectron microscopy with specific antibodies against the recombinant p24 protein and its N-terminal peptide. A 75-nm segment at one end of the 1370-nm filamentous viral particle was found to be consistently labeled with both types of antibodies, thus indicating that p24 is indeed the second capsid protein and that the closterovirus particle, unlike those of other plant viruses with helical symmetry, has a "rattlesnake" rather than uniform structure.
Full text
PDF
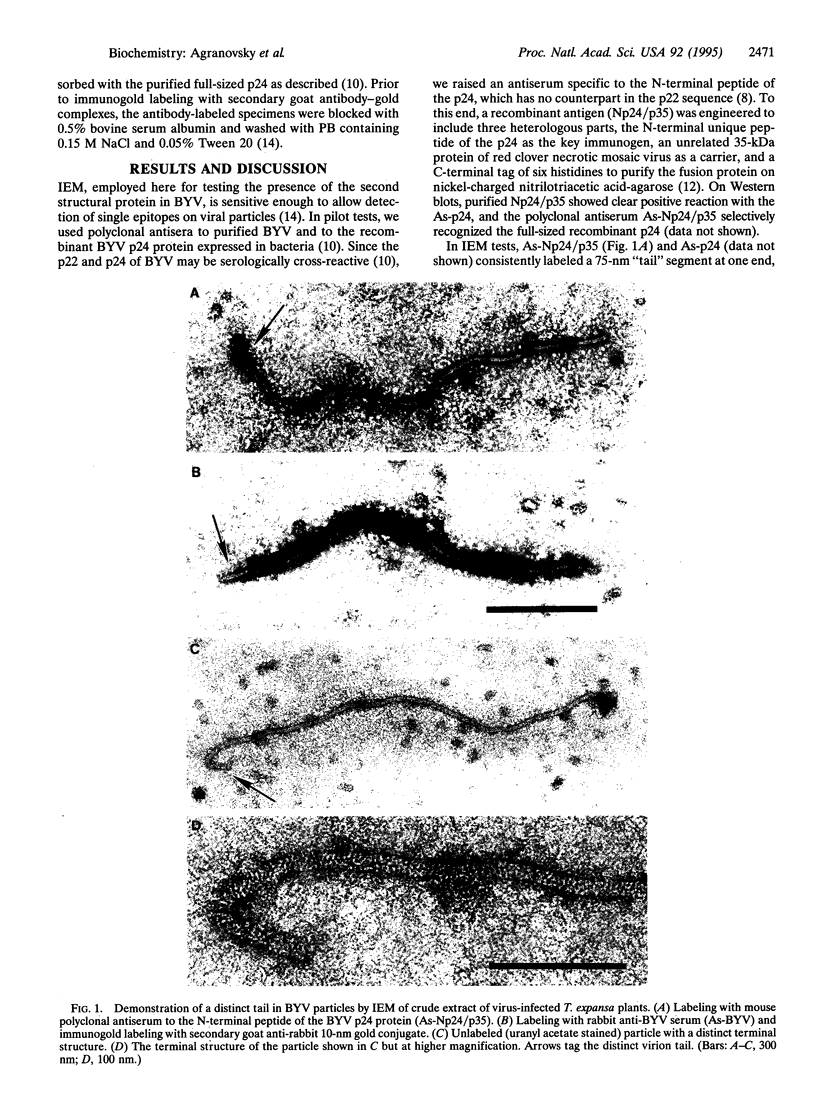
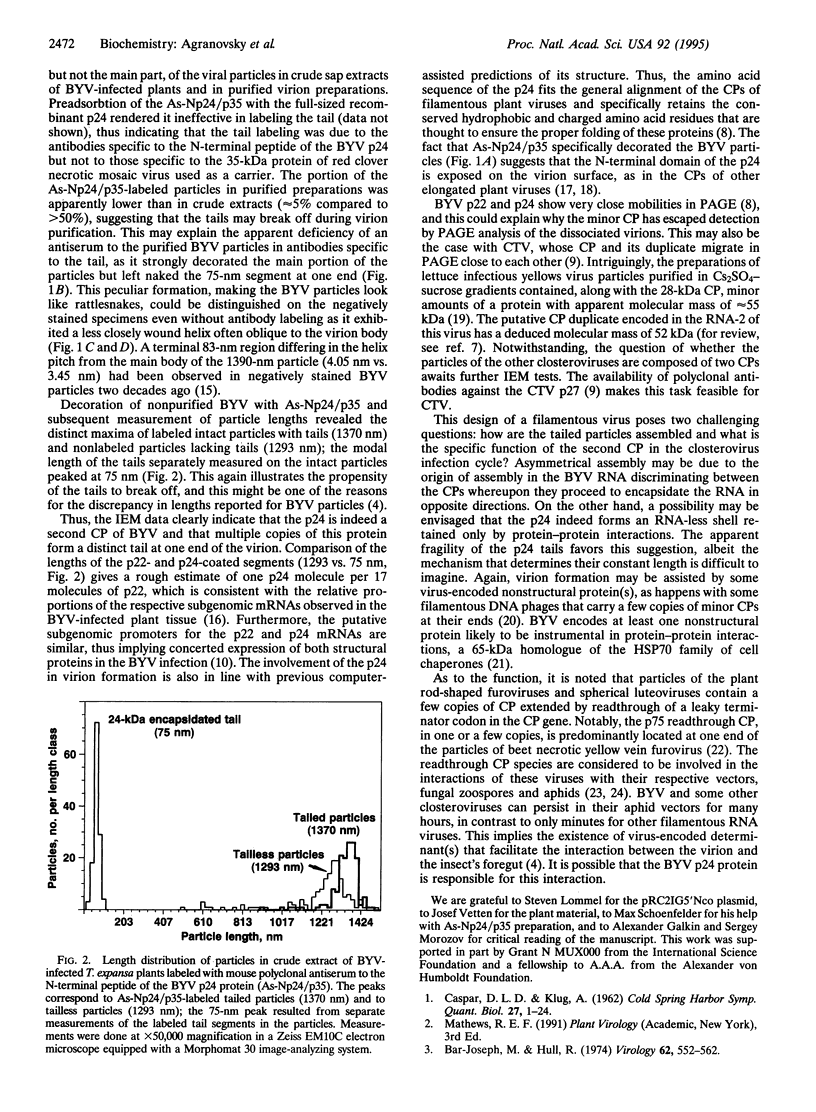

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranovsky A. A., Boyko V. P., Karasev A. V., Koonin E. V., Dolja V. V. Putative 65 kDa protein of beet yellows closterovirus is a homologue of HSP70 heat shock proteins. J Mol Biol. 1991 Feb 20;217(4):603–610. doi: 10.1016/0022-2836(91)90517-a. [DOI] [PubMed] [Google Scholar]
- Agranovsky A. A., Koenig R., Maiss E., Boyko V. P., Casper R., Atabekov J. G. Expression of the beet yellows closterovirus capsid protein and p24, a capsid protein homologue, in vitro and in vivo. J Gen Virol. 1994 Jun;75(Pt 6):1431–1439. doi: 10.1099/0022-1317-75-6-1431. [DOI] [PubMed] [Google Scholar]
- Agranovsky A. A., Koonin E. V., Boyko V. P., Maiss E., Frötschl R., Lunina N. A., Atabekov J. G. Beet yellows closterovirus: complete genome structure and identification of a leader papain-like thiol protease. Virology. 1994 Jan;198(1):311–324. doi: 10.1006/viro.1994.1034. [DOI] [PubMed] [Google Scholar]
- Bahner I., Lamb J., Mayo M. A., Hay R. T. Expression of the genome of potato leafroll virus: readthrough of the coat protein termination codon in vivo. J Gen Virol. 1990 Oct;71(Pt 10):2251–2256. doi: 10.1099/0022-1317-71-10-2251. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph M., Garnsey S. M., Gonsalves D. The closteroviruses: a distinct group of elongated plant viruses. Adv Virus Res. 1979;25:93–168. doi: 10.1016/s0065-3527(08)60569-2. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph M., Hull R. Purification and partial characterization of sugar beet yellows virus. Virology. 1974 Dec;62(2):552–562. doi: 10.1016/0042-6822(74)90415-2. [DOI] [PubMed] [Google Scholar]
- Boyko V. P., Karasev A. V., Agranovsky A. A., Koonin E. V., Dolja V. V. Coat protein gene duplication in a filamentous RNA virus of plants. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9156–9160. doi: 10.1073/pnas.89.19.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Febres V. J., Pappu H. R., Anderson E. J., Pappu S. S., Lee R. F., Niblett C. L. The diverged copy of the citrus tristeza virus coat protein is expressed in vivo. Virology. 1994 May 15;201(1):178–181. doi: 10.1006/viro.1994.1282. [DOI] [PubMed] [Google Scholar]
- Giesman-Cookmeyer D., Lommel S. A. Alanine scanning mutagenesis of a plant virus movement protein identifies three functional domains. Plant Cell. 1993 Aug;5(8):973–982. doi: 10.1105/tpc.5.8.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberlé A. M., Stussi-Garaud C., Schmitt C., Garaud J. C., Richards K. E., Guilley H., Jonard G. Detection by immunogold labelling of P75 readthrough protein near an extremity of beet necrotic yellow vein virus particles. Arch Virol. 1994;134(1-2):195–203. doi: 10.1007/BF01379118. [DOI] [PubMed] [Google Scholar]
- Klaassen V. A., Boeshore M., Dolja V. V., Falk B. W. Partial characterization of the lettuce infectious yellows virus genomic RNAs, identification of the coat protein gene and comparison of its amino acid sequence with those of other filamentous RNA plant viruses. J Gen Virol. 1994 Jul;75(Pt 7):1525–1533. doi: 10.1099/0022-1317-75-7-1525. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Russel M. Protein-protein interactions during filamentous phage assembly. J Mol Biol. 1993 Jun 5;231(3):689–697. doi: 10.1006/jmbi.1993.1320. [DOI] [PubMed] [Google Scholar]