INTRODUCTION
Reversible phosphorylation of proteins and lipids is a key component of many cellular signaling pathways including those involved in cell growth, differentiation, proliferation, angiogenesis, apoptosis, cytoskeletal rearrangement, and metabolism (Matthews and Gerritsen, 2010). The process of phosphorylation is mediated via the action of kinases that catalyze the transfer of a phosphate group from a high energy donor, usually adenosine triphosphate (ATP), or to a lesser extent, GTP, to proteins or lipids. In proteins, the phosphate acceptor is the hydroxyl group of either a serine, threonine, or tyrosine residue. Phosphate acceptors can be located within the same molecule, e.g., autophosphorylation, or are proteins distinct from the kinase. Phosphorylation-dependent signaling can involve a complex cascade of networks. Some lipid head groups can also be phosphorylated, e.g., phosphoinositides, changing the signaling properties of the cell membrane in the local milieu.
The key role of kinases in disease pathophysiology is underscored by the finding that mutations in kinases can lead to dysfunctions in cellular signaling (Zhang et al., 2009). These, in turn, can lead to diabetes, cancer, cardiovascular, neurodegenerative, developmental, immune, and behavioral disorders (Lahiry et al., 2010). For this reason, both protein and lipid kinases are important clinical targets with a number of kinase inhibitors in clinical use or undergoing clinical trials. However, these inhibitors only target a small fraction of the kinome, with many clinically relevant kinases lacking validated inhibitors (Fedorov et al., 2010). The aim of this unit is to provide an overview of kinases and groups comprising the kinome, with a focus on protocols used to identify and evaluate potential kinase inhibitors.
THE KINOME
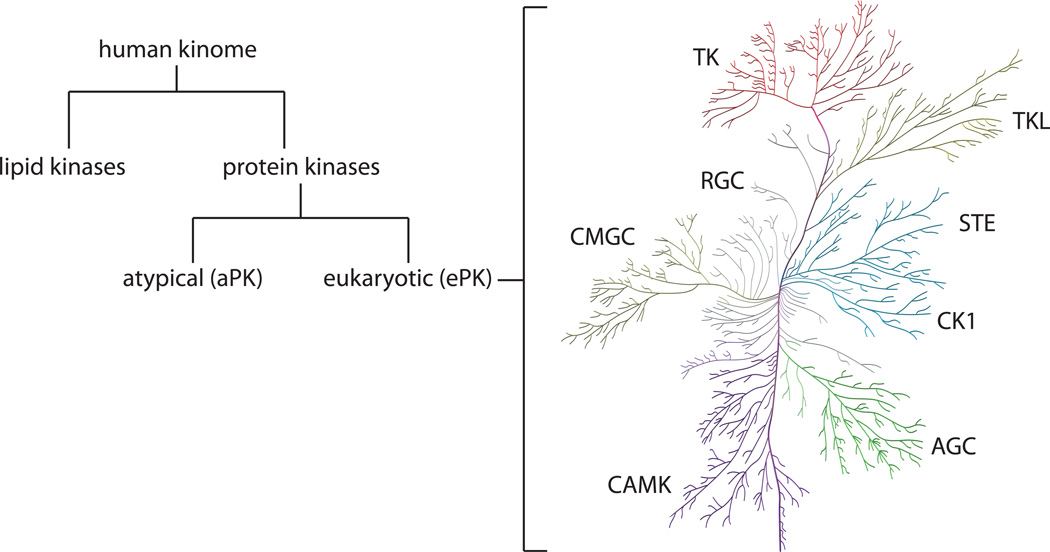
The human kinome contains 518 protein kinases that comprise 1.7% of human genes (Manning et al., 2002) and approximately 20 lipid kinases (Heath et al., 2003; Fabbro et al., 2012) (Figure 1). Of the protein kinases, 478 contain a eukaryotic protein kinase (ePK) domain. Although the remaining 40 kinases lack sequence similarity to the ePK domain, they are known to have kinase activity. For this reason they are referred to as the atypical protein kinases (aPKs) (Manning et al., 2002).
Figure 1. The human kinome.
The human kinome consists of lipid and protein kinases. Human ePKs are classified into groups based on sequence similarity in the kinase domain (Manning et al., 2002). For kinase group abbreviations, see text. The ePK protein kinome tree was prepared using the Reaction Biology Corporation Kinome Activity Mapper (www.reactionbiology.com) and was adapted from and reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com) based on Manning et al.
The ePKs are further classified into eight major groups based on sequence similarity within this domain. These are:
TK (tyrosine kinase)
TKL (tyrosine kinase-like)
STE (STE20, STE11, and STE7 related)
CK1 (casein kinase 1)
AGC (protein kinase A, protein kinase G, and protein kinase C related)
CAMK (Ca2+/calmodulin-dependent kinases)
CMGC (Cdk, MAPK, GSK, Cdk-like related)
RGC (receptor guanylyl cyclase)
TK group
The TK group consists of receptor TKs (RTKs) and non-receptor (cytosolic) TKs. RTKs are transmembrane proteins involved in signaling at the cell membrane. They bind to ligands in the extracellular milieu and transmit signals across the cell membrane into the cytoplasm. While the RTKs vary markedly in their extracellular domains, they all have intracellular kinase domains. Some RTKs possess C-terminal “tails” containing phosphorylation sites that mediate downstream signaling. Typically, the binding of ligands to the extracellular domain promotes receptor oligomerization leading to kinase activation on the intracellular face of the membrane. Examples of RTKs include the human epidermal growth factor receptor (HER/EGFR) family, the insulin receptor (IR) and the closely related insulin-like growth factor 1 receptor (IGF1-R), the platelet-derived growth factor receptors (PDGFRs), and the fibroblast growth factor receptors (FGFRs). All of these RTKs have been associated with cancer progression. This group contains the majority of targets for kinase inhibitors that are currently used clinically.
The remainder of the TK group is composed of non-receptor tyrosine kinases. Members of this group include the Src, Abl, and JAK kinases. The JAK kinases, in conjunction with the signal transducer and activator of transcription (STAT) proteins, are activated upon interaction with the C-terminal tails of RTKs. Their effects are mediated primarily through transcriptional regulation of gene expression. The Abl kinase can form a fusion with the breakpoint cluster region (BCR) to form the Bcr-Abl fusion protein (Shtivelman et al., 1985; Ben-Neriah et al., 1986; Chissoe et al., 1995). Levels of this dysregulated fusion protein correlate with decreased apoptosis and increased cell proliferation in chronic myelogenous leukemia (CML). It is the target of the first clinically approved kinase inhibitor, imatinib (Gleevec).
TKL group
Members of the TKL group have sequences resembling those of the TK group. These are mostly serine/threonine kinases. Of all of the protein kinase groups, TKL is the most diverse, containing both nonreceptor and receptor kinases. Its members include the interleukin-1 (IL-1) receptor-associated kinase (IRAK), the RAF kinases, the LIM domain kinase (LIMK), and the transforming growth factor beta (TGFβ) receptors.
STE group
The STE kinases are classified into three families based on their homology to the yeast proteins STE20 (MAP4K), STE11 (MAP3K), and STE7 (MAP2K), which render yeast STErile if deleted. Prominent members of this family include the p21-activated kinases (Paks), which are critical regulators of diverse signaling pathways. Pak1 over-expression occurs in several types of tumors (Kumar et al., 2006).
CSNK1/CK1 group
The members of the CSNK1/CK1 group include casein kinase 1 (CSNK1/CK1) as well as homologous kinases. These enzymes are Ser/Thr kinases that are constitutively expressed. They phosphorylate a wide variety of substrates involved in cytoskeletal function and transcriptional regulation.
AGC group
The AGC group is named for the enzyme families PKA, PKG, and PKC (Pearce et al., 2010). The Akt (or PKB) family of kinases is particularly important for regulating cell growth, proliferation, protein synthesis, glucose metabolism, and survival. The Akt kinases are substrates of another AGC kinase, 3-phosphoinositide-dependent kinase 1 (PDK1), which contains a pleckstrin homology (PH) domain that facilitates its membrane recruitment. PDK1 also activates other AGC kinases, including p70S6 kinase, p90RSK, and SGK (Raimondi and Falasca, 2011).
CAMK group
Kinases belonging to this group are involved in calcium signaling and are basally autoinhibited. Binding of the Ca2+/calmodulin complex relieves the autoinhibition. A particularly relevant class of CAMK kinases is the cell cycle checkpoint kinases, CHK1 and CHK2. When these enzymes are activated by DNA damage, they initiate a phosphorylation cascade that leads to cell cycle arrest and DNA repair.
CMGC group
The CMGC group is a diverse group of kinases. Like the checkpoint kinases, the cyclin-dependent kinases (Cdks) are central regulators of cell cycle progression. Other important members of this kinase group are the mitogen-activated protein kinases (MAPKs) which are involved in cell proliferation, differentiation, and apoptosis, the glycogen synthase kinases (GSKs) which are involved in inflammation in addition to glycogen metabolism, and the Cdk-like kinases.
RGC group
The receptor guanylyl cyclases represent the smallest of the kinase groups. It consists entirely of pseudokinases that lack certain residues that are critical for phosphate transfer (Manning et al., 2002). As the name suggests, all of these enzymes are guanylyl cyclases, converting GTP to cyclic GMP.
Others
The remaining 83 ePK kinases identified by Manning et al. (2002) lack sufficient sequence similarity to any of the eight ePK groups described above. Members include CK2 and IκB kinases (IKKs). CK2 has hundreds of characterized substrates (Meggio and Pinna, 2003) and the unusual property that GTP as well as ATP can be used as the phosphate donor (Niefind et al., 1999). The IKKs (IKKα and IKKβ) are important in inflammation, forming a complex that phosphorylates the IκB protein. This allows the NFκB transcription factor to be translocated to the nucleus.
Atypical protein kinases (aPKs)
The aPKs contain kinase domains are distinct from the typical ePK domain but, nonetheless, have been demonstrated to display kinase activity (Manning et al., 2002). The aPKs should not be confused with ePKs that are classified as “others” (above). The ePKs have conserved elements in their kinase domain although they cannot be readily assigned to any of the eight conventional groups. Prominent aPKs include pyruvate dehydrogenase kinase, bromodomain kinases, BCR, and the phosphatidylinositol-3-kinase-related kinases or PIKKs. The latter are named for their shared homology with the kinase domain of the lipid kinase, phosphatidylinositol-3-kinase (PI3K), but are, nonetheless, protein kinases. This family includes the mammalian target of rapamycin (mTOR), a Ser/Thr kinase that forms complexes with several other proteins. The complex containing mTOR and Raptor (regulatory associated protein of mTOR) is known as mTORC1 (Foster et al., 2010). The complex containing mTOR and Rictor (rapamycin-insensitive companion of mTOR) is known as mTORC2 (Zhou and Huang, 2010). Each complex has a distinct set of substrates. As a member of these complexes, mTOR is associated with a number of pathways involved in protein expression, cell proliferation, motility, and growth.
Lipid kinases
In addition to the protein kinases, there are about 20 lipid kinases. Members of this class include sphingolipid kinase and the phosphoinositide kinases. Particularly relevant to signaling downstream of many RTKs are the Class IA PI3Ks which phosphorylate the 3-position of the inositol ring of phosphatidylinositol-4,5-bisphosphate (PIP2) to form PIP3. PIP3 can serve as a docking platform on the membrane for proteins that contain PH domains, such as the AGC group kinase, PDK1. PI3K itself is recruited to the membrane by binding phosphorylated tyrosine residues in receptor kinases or their substrates, such as the insulin receptor substrate-1 or IRS-1 (Denley et al., 2005; Jones et al., 1995). In this manner, PI3K couples extracellular signals, such as those generated by glucose or growth factors, to intracellular proteins, notably Akt, thereby inducing multiple cellular responses. Unsurprisingly, mutations in PI3K are associated with a number of cancers, including those found in the breast and colon (Wood et al., 2007).
THE ARCHITECTURE OF KINASES
The kinase reaction
The kinase reaction is complex, involving the binding of two substrates, ATP and a protein or lipid, and the transfer of the γ-phosphate from ATP to the protein or lipid substrate. The phosphorylated product and ADP are then released from the catalytic site. The kinase domain contains residues that facilitate the binding of substrates and magnesium, a required cofactor, as well as residues that enhance the formation and release of the products. To accomplish this, the ePK domain undergoes conformational changes between active (catalytically competent) and inactive states.
Kinase domain structure
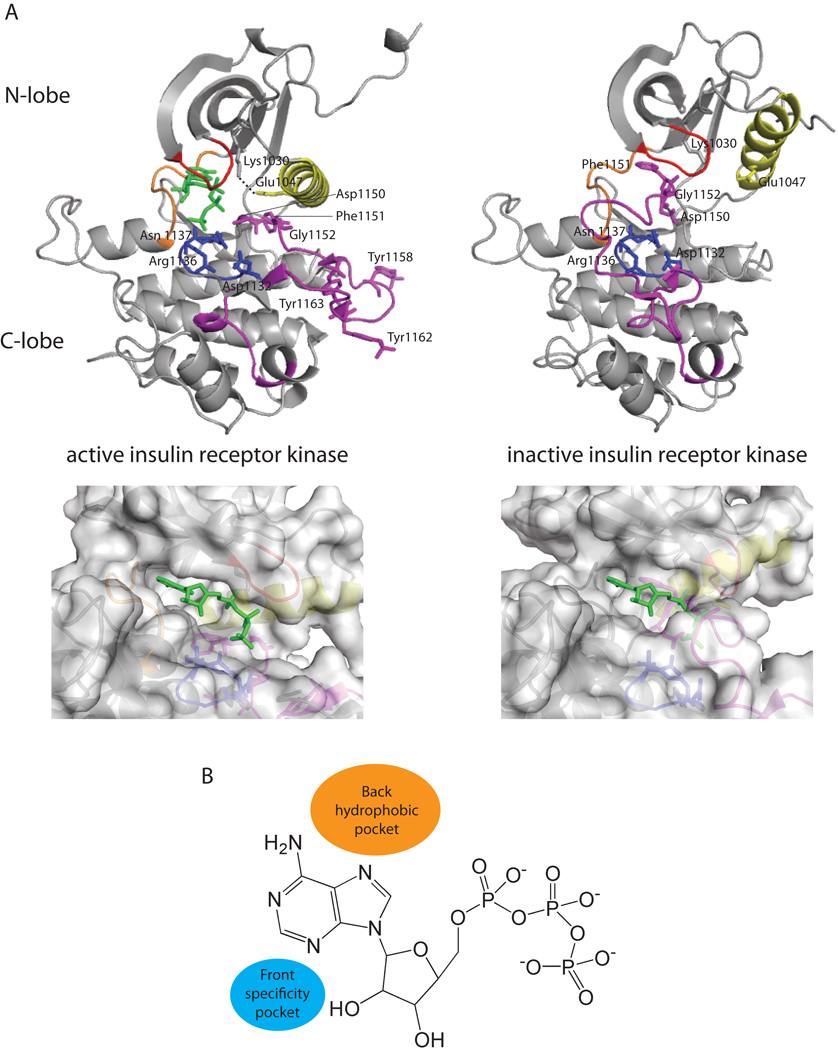
The ePK domain is approximately 250 amino acids in length and consists of two lobes. Referred to as the N-terminal lobe (N-lobe) and the C-terminal lobe (C-lobe), these are separated by a cleft that contains residues important for ATP binding as well as phosphate transfer (Figure 2A). The kinase domains of lipid kinases are similar to those of protein kinases. The N-lobe consists of one helix, known as the C-helix, and several β-strands. The C-lobe is predominantly α-helical and contains residues important for interacting with the phosphate acceptor. In some cases, residues in the N-lobe also mediate substrate recognition (Rennefahrt et al., 2007). A short linker known as the hinge region (orange segment in Figure 2A) separates these lobes and contains residues that hydrogen bond to the adenine ring of ATP.
Figure 2. The eukaryotic protein kinase domain.
(A) Top: The conformation of the inactive (PDB 1IRK) and active (PDB 1IR3) states of the kinase domain in IR. Elements of special significance are highlighted in color: C-helix (yellow), P-loop (red), hinge region (orange), catalytic loop (blue), activation loop (purple). The dashed line indicates the salt bridge between Glu1074 of the C-helix and Lys1030. Bottom: A close-up view of the surface of the ATP binding pocket for active and inactive IR. The binding of an ATP analog, AMP-PNP, is shown in green sticks. For ease of visualization, the conformation of AMP-PNP in active IR was docked into inactive IR where it clashes with the residues of the C-lobe. (B) Pockets adjacent to ATP that may also be exploited for inhibitor development.
The active states of kinases are highly conserved. The activation loop, flanked by the peptide motifs Asp-Phe-Gly (DFG triad motif; IR residues 1150–1152) and Ala-Pro-Glu (APE; IR residues 1177–1179), is located on the C-lobe of the kinase domain. In the active state, the activation loop is extended away from the ATP binding site (purple segment in Figure 2A). The Asp residue of the DFG motif coordinates a magnesium ion, which positions the phosphate groups of ATP for catalysis. Since the Asp side chain points toward the ATP binding site in the active state, this is termed the DFG-in conformation. The conformation of the activation loop in the active state is frequently stabilized by phosphorylation of multiple residues. In IR, these sites are Tyr1158, Tyr1162, and Tyr1163 (White et al., 1988; Zhang et al., 1991). Phosphorylation of the activation loop can be carried out by other kinases. This is the case for MAPK which is phosphorylated by a MAPK kinase (MAPKK), which in turn is activated by a MAPKK kinase (MAPKKK). Phosphorylation can also be carried out intramolecularly via cis-autophosphorylation, such as in the case of GSK3β (Lochhead et al., 2006). The activation loop of one kinase molecule can also be phosphorylated by another molecule of the same kinase via a process known as trans-autophosphorylation. Trans-autophosphorylation is observed for Chk2 and appears to be more prevalent than cis-autophosphorylation (Oliver et al., 2007).
Other important interactions for the stabilization of the active conformation of the kinase domain include a salt bridge between a Glu residue at the beginning of the C-helix (yellow segment in Figure 2A) of the N-lobe (Glu1047 in IR) and a Lys residue in the β3-strand of the N-lobe (Lys1030 in IR) (dashed line in Figure 2A). The Lys residue of this salt bridge can also stabilize the α and β phosphates of ATP. The phosphate-binding loop (P-loop; red segment in Figure 2A) of the N-lobe is glycine-rich and coordinates one of the phosphates of ATP. The catalytic loop (blue segment in Figure 2A) is located in the C-lobe and contains an Asp residue that interacts with the substrate hydroxyl group (Asp1132 in IR). Other residues (Asn1137 in IR) present in this catalytic loop help to orient this Asp residue. Also, a Lys or Arg residue in the catalytic loop (Arg1136 in IR) stabilizes the labile γ-phosphate of ATP.
Conformations of the inactive states of kinases vary more widely than those of the active states. In the inactive state, the activation loop usually occupies the substrate binding region and the ATP binding site is partially occluded (Figure 2A, bottom; compare active, left, with inactive, right). In many inactive kinases, the Asp residue of the DFG motif points away from the ATP binding site in the inactive state. This is known as the DFG-out conformation of the activation loop. Additionally, the salt bridge between the Glu of the C-helix and the Lys in the N-lobe is disrupted in the inactive state. The P-loop shifts due to a change in conformation of the DFG motif. The catalytic loop, however, does not change conformation significantly between the active and inactive states.
There are deviations from the ePK kinase domain organization described above. Some RTKs, including PDGF, VEGF, KIT and colony-stimulating factor 1 receptors, have split kinase domains that contain an insert of about 70 amino acids in the C-lobe. This insert often undergoes autophosphorylation and is involved in interactions with other proteins (Kazlauskas and Cooper, 1989; Reedijk et al., 1992; Thommes et al., 1999; Matsumoto et al., 2005). Also, in a few kinases, there are relatively few structural differences between the active and inactive conformations of the kinase domain. The conformation of the activation loop of activated Cdc42-associated kinase (Ack) is unchanged due to phosphorylation. That is, in both the unphosphorylated and phosphorylated states a DFG-in conformation is observed for the activation loop (Lougheed et al., 2004).
Other domains of importance
In addition to the kinase domain, other domains may be present for regulation of kinase catalytic activity or substrate binding. Summarized below are some of the most common domains identified in Manning et al. (Manning et al., 2002). One of these, which is found in 44 protein kinases, is the AGC kinase C-terminal domain that contains two phosphorylation sites. In most of the AGC kinases that have this domain, both of these sites must be phosphorylated for kinase activity.
Other common kinase domains are those mediating protein-protein interactions, such as the SH3 and SH2 domains. The SH2 domains are involved in recognition of sequences containing phosphorylated Tyr residues. The SH3 domains recognize sequences containing proline residues (Pro) in the general form Pro-X-X-Pro, where X is any amino acid. These domains may also be involved in mediating autoinhibitory interactions with the kinase domain, such as the SH3 and SH2 domains of Src (Xu et al., 1999; Young et al., 2001). Other kinase protein interaction domains include immunoglobulin and fibronectin type III domains, which are located on the extracellular region of receptor kinases.
Kinases can also contain domains that mediate interactions with lipids. These include PH (pleckstrin homology), diacylglycerol binding, and C2 domains. One example is PDK-1, which has a PH domain allowing it to be recruited to PIP3-containing regions of the membrane. There, it can phosphorylate and activate another PH-domain- containing protein, the Ser/Thr kinase Akt, resulting in a number of downstream effects (Hanada et al., 2004).
TYPES OF INHIBITION
As most kinase inhibitors bind competitively to the ATP binding site of the kinase domain they are often rather non-selective with regard to kinases as a whole (Karaman et al., 2009; Anastassiadis et al., 2011; Davis et al., 2011). For instance, the kinase inhibitor staurosporine interacts with at least 20% of the kinases in the kinome (Tanramluk et al., 2009), which probably accounts, at least in part, for its toxicity. Highly conserved regions that have been exploited for the development of more selective kinase inhibitors include the adenine, ribose, and phosphate binding regions (Matthews and Gerritsen, 2010). Two adjacent regions that are less conserved are the back hydrophobic pocket and the front specificity pocket (Figure 2B). The back hydrophobic pocket is adjacent to the region in which the adenine ring of ATP binds. This pocket is obstructed when a constituent, known as the gatekeeper residue, has a large side chain such as Leu, Phe, or Met. Generally, inhibitors that interact with this pocket only bind to kinases with small gatekeeper residues. A second, smaller hydrophobic pocket, referred to as the front specificity pocket, is located on the opposite side of the adenine moiety.
All kinase inhibitors can be operationally classified as either ATP-competitive or non-ATP-competitive. Because many non-ATP-competitive inhibitors do not bind to the ATP binding site they are classified as allosteric kinase inhibitors. The highly conserved structure of the ATP binding pocket in the kinome is a major challenge for identifying selective kinase inhibitors as is the high intracellular ATP concentration that limits the potency of ATP competitive kinase inhibitors under physiological conditions (Knight and Shokat, 2005). In contrast, the non-ATP-competitive inhibitors are insensitive to ATP concentration. They may bind to non-conserved regions of the kinase, facilitating the design of more selective inhibitors (Garuti et al., 2010). These advantages can be taken one step further by inhibitors that covalently binding to their target kinase. The increased residence time of this class of inhibitor also has the potential to both enhance potency and selectivity (Singh, et al., 2010; Barf and Kaptein, 2012).
ATP-competitive inhibitors
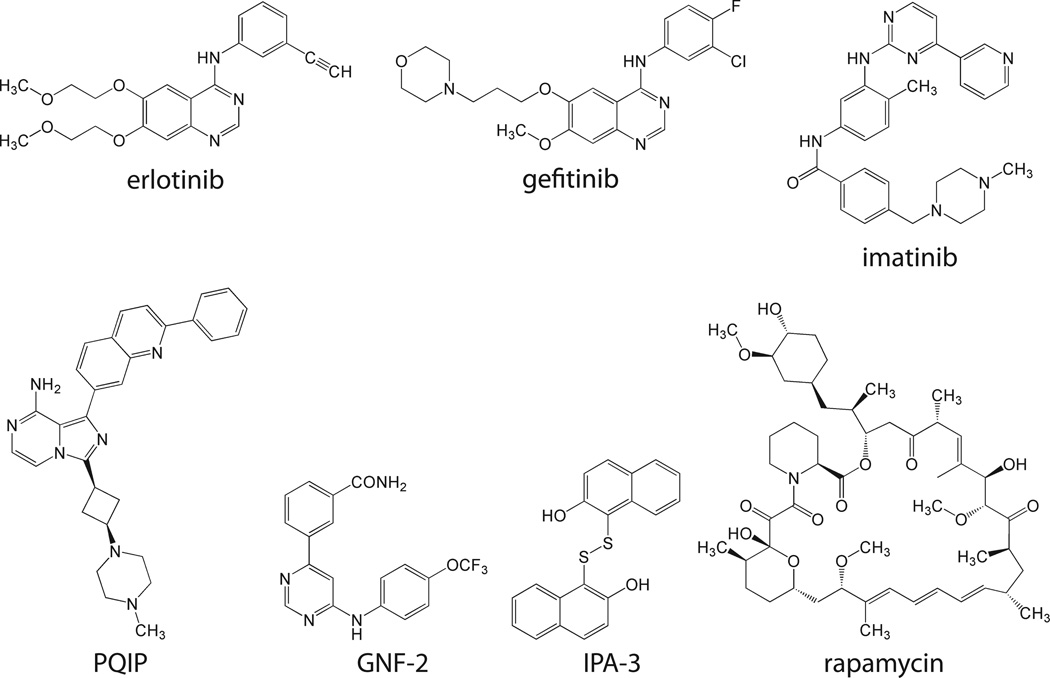
Kinase inhibitors having potencies that vary with ATP concentrations are referred to as ATP-competitive inhibitors and can be classified as either type I or type II inhibitors (Zuccotto et al., 2010). Type I inhibitors bind to the active/DFG-in conformation, whereas type II inhibitors bind to the inactive/DGF-out conformation (Liu and Gray, 2006). Examples of ATP-competitive inhibitors include erlotinib and gefitinib, which are structurally similar type I inhibitors that target EGFR (Figure 3). Both erlotinib and gefitinib have a quinazoline moiety that mimics the adenine base of ATP and aniline rings that bind to the back hydrophobic pocket of EGFR. It is therefore not surprising that the gatekeeper residue mutation Thr790Met is linked to both erlotinib and gefitinib resistance (Kobayashi et al., 2005; Pao et al., 2005). Both erlotinib and gefitinib are approved for clinical use in non-small cell lung cancer.
Figure 3. Structures of inhibitors discussed in this review.
Another ATP-competitive inhibitor is imatinib (Figure 3), a type II agent that targets the Bcr-Abl kinase. Imatinib has a 2-phenylaminopyrimidine moiety that occupies the adenine pocket of the ATP binding site. The binding of imatinib to Abl kinase changes the orientation of the activation loop DFG motif. Additionally, the opening to the back hydrophobic pocket is widened, allowing the pyridine ring of imatinib to bind to this region. When imatinib binds, the activation loop occupies the substrate binding site (Schindler et al., 2000). In addition to CML, imatinib is approved as a treatment for several other leukemias, as well as gastrointestinal stromal tumors.
There are also ATP-competitive inhibitors that defy classification as either type I or type II inhibitors. These are exemplified by cis-3-[3-(4-methyl-piperazin-1-yl)-cyclobutyl]-1-(2-phenyl-quinolin-7-yl)-imidazo[1,5-a]pyrazin-8-ylamine (PQIP) (Figure 3), which targets IGF1R. Indeed, binding of PQIP displays characteristics of both type I and type II inhibition. The imadazopyrazinyl core mimics the adenine base and binds in the same pocket. This moiety forms two hydrogen bonds with the hinge region of the kinase domain. The quinolinyl moiety occupies the back hydrophobic pocket, packing against residues of the C-helix and the DFG motif, and hydrogen bonds with the Lys of the conserved Glu-Lys salt bridge. The activation loop is extended, but not in the same conformation observed for the fully activated IGF1R (Wu et al., 2008).
Non-ATP-competitive inhibitors
Kinase inhibitors that exhibit no change in potency at different ATP concentrations are referred to as non-ATP-competitive. Many of these are allosteric inhibitors and some even bind to regions outside of the kinase domain. Because these inhibitors often bind to regions unique to a particular kinase, they are often more selective than ATP-competitive inhibitors. Non-ATP competitive inhibitors often target the regulation of kinase activity. One example is 2,2’-dihydroxy-1,1’-dinapthyldisulfide (IPA-3) (Figure 3), an inhibitor of Pak1 that stabilizes the Pak1 autoinhibitory complex (Deacon et al., 2008; Viaud and Peterson, 2009). This compound was identified by its ability to inhibit Pak1 in the presence of high concentrations of ATP (Deacon et al., 2008). Autoinhibited Pak1 is a dimer. The regulatory domain of one molecule of the dimer binds to and inhibits the catalytic activity of the other dimer molecule. This autoinhibition is relieved upon binding to the p21 GTP-binding proteins Rac or Cdc42. IPA-3 binds covalently to the Pak1 regulatory domain, thereby preventing the binding of these activator proteins (Viaud and Peterson, 2009).
Rapamycin (sirolimus) (Figure 3) and its derivatives target the mTORC1 complex by forming heterodimers that interfere with kinase activation. Rapamycin binds to FK506 binding protein 12 (FKBP12), a petidyl-prolyl cis-trans isomerase. The FKBP12-rapamycin complex in turn binds to mTOR at the FKBP-rapamycin binding (FRB) domain. By binding mTOR, the FKBP-12-rapamycin complex interferes with mTOR regulation by Raptor. This inducible heterodimerization between FRB domains and FKBP12 has been exploited as an experimental tool (Geda et al., 2008). Rapamycin, a natural product from Streptomyces hygroscopicus is used to prevent rejection after kidney transplantation while its synthetic derivatives, everolimus and temsirolimus, are used clinically for the treatment of renal cell carcinoma.
Another example of an allosteric kinase inhibitor is GNF-2 (Figure 3), an alternative to imatinib in targeting the Bcr-Abl kinase by stabilizing its inactive state (Adrian et al., 2006). Several mutations in c-Abl are associated with imatinib resistance (Shah et al., 2002). Some of these mutated forms are sensitive to GNF-2 which inhibits Bcr-Abl by stabilizing an inactive conformation of the kinase domain (Adrian et al., 2006; Zhang et al., 2010). The wild-type c-Abl is myristoylated, with the binding of the myristoyl group to a site in the C-lobe stabilizing the inactive form of the kinase (Zhang et al., 2010). Because the Bcr-Abl fusion cannot be myristoylated, it lacks this autoinhibitory mechanism (Hantschel et al., 2003). As the binding of GNF-2 to Bcr-Abl mimics the binding of the myristoyl group to wild-type c-Abl it stabilizes the inactive state of Bcr-Abl (Zhang et al., 2010).
CONSIDERATIONS IN THE DEVELOPMENT OF KINASE INHIBITORS
Most kinase inhibitors developed thus far are ATP-competitive and show variable selectivity for this enzyme class (Anastassiadis et al., 2011; Davis et al., 2011). While drug discovery efforts are often aimed at developing agents that are selective for a particular target, the inhibition of several targets by a single compound is emerging as a promising therapeutic approach, particularly if inhibition of these targets synergistically promotes the desired therapeutic response. This is especially true in the treatment of cancer, where multiple, parallel kinase-mediated pathways can contribute to increased cell proliferation and some toxicity can be tolerated for the sake of therapeutic efficacy. Targeting a few select kinases may also lessen the likelihood of resistance or delay its development.
On the other hand, kinase inhibitors employed as research tools would be most useful if they were selective for single targets, as this characteristic would simplify the interpretation of experiments using these agents. Uncharacterized off-targets effects of a research tool greatly complicates the interpretation of experimental results (Anastassiadis et al., 2011). Bain et al. and Uitdehaag et al. provide recommendations for tool compounds to be used for the most selective inhibition of particular kinases of interest (Bain et al., 2007; Uitdehaag et al., 2012).
Compound selectivity is related to both potency and target residence time (Barf and Kaptein, 2012). If a compound is used at a concentration that is much higher than its potency, an increase in off-target effects can be anticipated (Knight and Shokat, 2005). To maximize selectivity it is therefore best to employ concentrations at or below those needed to fully inhibit the targeted enzyme. While potency is easily measured in vitro or in vivo, the measurement of selectivity on a large scale can be both challenging and resource intensive (Karaman et al., 2008).
ASSAY FORMATS FOR MEASURING KINASE-INHIBITOR INTERACTIONS
There are a number of assays for measuring kinase inhibition. One of the more standard kinase assays is the in vitro filter-binding procedure. For this assay, a reaction mixture consisting of buffer, the protein/peptide substrate, γ-phosphate radiolabeled ATP, and the kinase is prepared and the reaction is allowed to proceed for a given period of time. The reaction is spotted on filter paper and washed thoroughly to remove any remaining free γ-phosphate-labeled ATP. Substrate and covalently bound radiolabeled phosphate remains attached to the filter paper during these washes. The radiolabeled substrate is then quantified. The filter-binding assay is a convenient method for monitoring peptide phosphorylation. Phosphorylation of larger protein substrates can also be monitored by analyzing reaction products on a SDS-PAGE gel to separate protein substrates from ATP.
Many other assay formats have been developed to monitor kinase-inhibitor interactions. Recent advances in automated sample preparation have also made it possible to perform these assays quickly for large-scale selectivity screening. These tests can be divided into two groups; binding assays and functional assays.
Binding assays
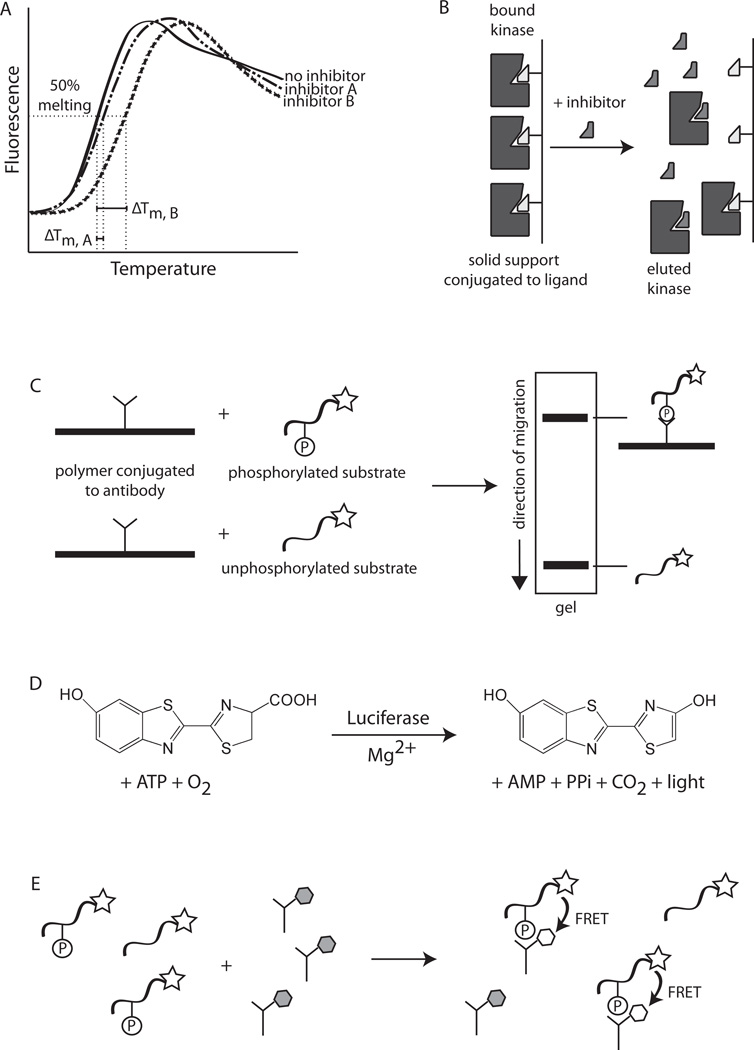
In binding assays the physical interaction between the inhibitor candidate and the kinase is measured. One type of binding assay is a melting temperature (Tm) shift assay (Figure 4A). As temperature increases, proteins unfold or “melt.” At much higher temperatures, proteins often aggregate in a non-native manner. The Tm of a protein is the temperature at which the protein is 50% unfolded. This may be measured by monitoring changes in the fluorescence emission spectrum of intrinsic tryptophan residues. Extrinsic probes, such as dyes that bind to exposed hydrophobic regions of unfolded proteins, can also be employed to monitor protein melting. For kinase inhibitor screening, the melting curves in the absence and presence of the inhibitor are measured. Generally, inhibitors that bind more tightly to the kinase stabilize the enzyme and increase the Tm (Fedorov et al., 2007; Vedadi et al., 2006).
Figure 4. Assay formats for measuring kinase-inhibitor interactions.
(A) Melting temperature assay. In this test the shift in the melting temperature (ΔTm) correlates with the strength of the kinase-inhibitor interaction. In this example, inhibitor B interacts more potently with the kinase than inhibitor A since ΔTm, B is greater than ΔTm, A. (B) Competition binding assay. A ligand that binds the kinase is affixed on a solid support. Free inhibitor is passed over the solid support and competes with binding to the kinase, causing the enzyme to be “eluted” from the solid support. The concentration of the eluted kinase is measured and is an indicator of inhibitor binding. (C) Electrophoretic mobility assay. A polymer is conjugated to a phosphospecific antibody for the phosphorylation site of interest. Phosphorylated substrate binds the polymer and therefore migrates slower than unphosphorylated substrate that is not bound to the polymer. Visualization is possible if the substrate is conjugated to a fluorescent probe (indicated by the star). (D) ATP detection assay. The amount of unhydrolyzed ATP following a kinase reaction is assayed by coupling the reaction to the luciferase reaction. Luciferase requires ATP to oxidize luciferin to form oxyluciferin and to emit light. (E) Proximity assay. The substrate is conjugated to a fluorescent donor (star). The products of a kinase reaction utilizing this substrate are incubated with an antibody conjugated to a fluorescent acceptor (filled hexagon). Phosphorylated substrates bind the antibody and result in FRET (indicated by arrows and the open hexagons).
In a competition binding assay, a potential inhibitor competes with another ligand for binding to a kinase (Figure 4B). Several different assay formats of this type are available. One uses immobilized ligand bound to kinase (Fabian et al., 2005). The potential inhibitor is added to compete with the immobilized ligand for kinase binding. Inhibitors that bind more tightly to the kinase compete with ligand binding. A variation of this assay utilizes fluorescently-labeled ligands that are bound to kinase in solution (Vaasa et al., 2009). The release of labeled ligand by competing inhibitor molecules can be monitored by measuring changes in the fluorescence anisotropy of the ligand. Free ligands are less anisotropic than ligands bound to kinase molecules.
An advantage of binding assays is that no substrate is required. Thus, in cases where the substrate is unknown or unavailable, these assay formats may be the only option for assessing interactions with inhibitors. The primary disadvantage of binding assays is that binding may not result in inhibition of catalytic activity.
Functional assays
Functional assays, such as the filter-binding assay, measure changes in kinase catalytic activity, thereby revealing whether the interaction with a test agent results in kinase inhibition. An modification of this assay separates phosphorylated and unphosphorylated substrate by electrophoresis as a consequence of the additional negative charge conferred by the phosphate group. Other types of electrophoretic assays use polymers conjugated to phosphate-binding moieties (Figure 4C). These polymers bind to the phosphorylated substrates and reduce their electrophoretic mobility, allowing for separation from unphosphorylated substrate (Miick et al., 2005).
In other types of functional assays ATP hydrolysis is measured as either the loss of ATP or the appearance of ADP. ATP may be assayed by measuring light production in an enzyme-coupled assay employing luciferase (Figure 4D), which utilizes ATP to oxidize luciferin to form oxyluciferin. This reaction emits light. The coupled luciferase assay can also be employed to measure ADP formation. After the kinase reaction, the unreacted ATP is depleted and the kinase activity terminated. The ADP formed by the kinase reaction is then converted to ATP which is quantified using the coupled luciferase assay (Zegzouti et al., 2009). Alternatively, ADP formation may be measured using antibody-based methods. One of these involves antibodies that are prebound with fluorescently-labeled ADP. Production of ADP in the kinase reaction competes with the fluorescently-labeled ADP for binding to the antibody. The displacement of the fluorescently-labeled ADP from the antibody alters its fluorescence polarization (Huss et al., 2007).
Electrophoretic assays and those involving the measurement of ATP and ADP concentrations are, however, not real-time assays. Rather, they are endpoint measurements taken after the kinase reaction is allowed to proceed for a given amount of time. Phosphorylation can be monitored in real-time using proximity assays. An example is detection of the phosphorylated substrate using fluorescence resonance energy transfer (FRET) (Figure 4E) (Moshinsky et al., 2003). In these assays, the substrate is conjugated to a fluorescent donor moiety. An antibody raised against the phosphorylated substrate is conjugated to a fluorescent acceptor moiety which yields a FRET signal only after phosphorylation, when the donor and acceptor moieties are brought together on the phosphorylated peptide substrate.
The above tests are only a sampling of the numerous methods available for measuring kinase-inhibitor interactions. Functional assays are generally preferred over binding assays as the former reveal whether the interaction with the test agent affects enzyme activity, and they allow for the kinetic measurement of the kinase-inhibitor interactions. Furthermore, the mode of inhibition (ATP-competitive or non-ATP-competitive) can be determined with these assays. While cell-based assays to monitor kinase inhibition in the cellular context have also been developed, a description of these tests is beyond the scope of this review.
DISCUSSION
Some of the assays discussed in this unit are employed for the large-scale measurement of kinase-inhibitor interactions. Libraries containing the majority of the kinases in the human kinome are now available, facilitating inhibitor selectivity profiling. Recently, many commercially available kinase inhibitors were subjected to selectivity profiling against the majority of human kinases (Anastassiadis et al., 2011; Davis et al., 2011). Several unanticipated off-target effects were identified and, in some cases, inhibitors designed to inhibit one particular target were found to inhibit another kinase more potently. Such selectivity profiling has proven to be valuable in predicting the potential effect of these agents on signaling pathways. These same technologies can be employed to characterize the target selectivity of novel kinase inhibitors.
It has been estimated that about one-third of drug discovery in the pharmaceutical industry is focused on the development of kinase inhibitors (Matthews and Gerritsen, 2010). Despite these efforts, however, there are still only a limited number of FDA-approved kinase inhibitors. Although a great deal of progress has been made in developing kinase inhibitors, most kinases still do not have well-validated inhibitors. The measurement of kinase-inhibitor interactions, in conjunction with X-ray crystallography of these complexes and cell-based measurements of inhibition, will greatly aid in the development and optimization of new inhibitors for this important class of enzymes.
ACKNOWLEDGEMENTS
The authors’ work is supported by a W. W. Smith Charitable Trust Award and by the NIH (GM083025). K.C.D. is supported by NIH T32 CA009035-36.
LITERATURE CITED
- Adrian FJ, Ding Q, Sim T, Velentza A, Sloan C, Liu Y, Zhang G, Hur W, Ding S, Manley P, Mestan J, Fabbro D, Gray NS. Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nat. Chem. Biol. 2006;2:95–102. doi: 10.1038/nchembio760. [DOI] [PubMed] [Google Scholar]
- Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barf T, Kaptein A. Irreversible protein kinase inhibitors: balancing the benefits and risks. J. Med. Chem. 2012;55:6243–6262. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Chissoe SL, Bodenteich A, Wang YF, Wang YP, Burian D, Clifton SW, Crabtree J, Freeman A, Iyer K, Jian L, et al. Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics. 1995;27:67–82. doi: 10.1006/geno.1995.1008. [DOI] [PubMed] [Google Scholar]
- Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011;29:1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16:421–439. doi: 10.1016/j.cytogfr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Fabbro D, Cowan-Jacob SW, Mobitz H, Martiny-Baron G. Targeting cancer with small-molecular-weight kinase inhibitors. Methods Mol. Biol. 2012;795:1–34. doi: 10.1007/978-1-61779-337-0_1. [DOI] [PubMed] [Google Scholar]
- Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Fedorov O, Marsden B, Pogacic V, Rellos P, Muller S, Bullock AN, Schwaller J, Sundstrom M, Knapp S. A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20523–20528. doi: 10.1073/pnas.0708800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov O, Muller S, Knapp S. The (un)targeted cancer kinome. Nat. Chem. Biol. 2010;6:166–169. doi: 10.1038/nchembio.297. [DOI] [PubMed] [Google Scholar]
- Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, Roux PP, Ballif BA, Fingar DC. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garuti L, Roberti M, Bottegoni G. Non-ATP competitive protein kinase inhibitors. Curr. Med. Chem. 2010;17:2804–2821. doi: 10.2174/092986710791859333. [DOI] [PubMed] [Google Scholar]
- Geda P, Patury S, Ma J, Bharucha N, Dobry CJ, Lawson SK, Gestwicki JE, Kumar A. A small molecule-directed approach to control protein localization and function. Yeast. 2008;25:577–594. doi: 10.1002/yea.1610. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Nagar B, Guettler S, Kretzschmar J, Dorey K, Kuriyan J, Superti-Furga G. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845–857. doi: 10.1016/s0092-8674(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Heath CM, Stahl PD, Barbieri MA. Lipid kinases play crucial and multiple roles in membrane trafficking and signaling. Histol. Histopathol. 2003;18:989–998. doi: 10.14670/HH-18.989. [DOI] [PubMed] [Google Scholar]
- Huss KL, Blonigen PE, Campbell RM. Development of a Transcreener kinase assay for protein kinase A and demonstration of concordance of data with a filter-binding assay format. J. Biomol. Screen. 2007;12:578–584. doi: 10.1177/1087057107300221. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Cooper JA. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989;58:1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem. Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat. Rev. Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat. Rev. Genet. 2010;11:60–74. doi: 10.1038/nrg2707. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Lougheed JC, Chen RH, Mak P, Stout TJ. Crystal structures of the phosphorylated and unphosphorylated kinase domains of the Cdc42-associated tyrosine kinase ACK1. J. Biol. Chem. 2004;279:44039–44045. doi: 10.1074/jbc.M406703200. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Matthews DJ, Gerritsen ME. Targeting Protein Kinases for Cancer Therapy. Hoboken, N.J.: John Wiley & Sons; 2010. [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Miick SM, Jalali S, Dwyer BP, Havens J, Thomas D, Jimenez MA, Simpson MT, Zile B, Huss KL, Campbell RM. Development of a microplate-based, electrophoretic fluorescent protein kinase a assay: comparison with filter-binding and fluorescence polarization assay formats. J. Biomol. Screen. 2005;10:329–338. doi: 10.1177/1087057104272909. [DOI] [PubMed] [Google Scholar]
- Moshinsky DJ, Ruslim L, Blake RA, Tang F. A widely applicable, high-throughput TR-FRET assay for the measurement of kinase autophosphorylation: VEGFR-2 as a prototype. J. Biomol. Screen. 2003;8:447–452. doi: 10.1177/1087057103255282. [DOI] [PubMed] [Google Scholar]
- Niefind K, Putter M, Guerra B, Issinger OG, Schomburg D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nat. Struct. Biol. 1999;6:1100–1103. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- Oliver AW, Knapp S, Pearl LH. Activation segment exchange: a common mechanism of kinase autophosphorylation? Trends Biochem. Sci. 2007;32:351–356. doi: 10.1016/j.tibs.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nature Rev Mol. Cell. Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Raimondi C, Falasca M. Targeting PDK1 in cancer. Curr. Med. Chem. 2011;18:2763–2769. doi: 10.2174/092986711796011238. [DOI] [PubMed] [Google Scholar]
- Reedijk M, Liu X, van der Geer P, Letwin K, Waterfield MD, Hunter T, Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3'-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J. Biol. Chem. 2007;282:15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Singh J, Petter RD, Kluge AF. Targeted covalent drugs of the kinase family. Curr. Opin. Chem. Biol. 2010;14:475–480. doi: 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- Tanramluk D, Schreyer A, Pitt WR, Blundell TL. On the origins of enzyme inhibitor selectivity and promiscuity: a case study of protein kinase binding to staurosporine. Chem. Biol. Drug Des. 2009;74:16–24. doi: 10.1111/j.1747-0285.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommes K, Lennartsson J, Carlberg M, Ronnstrand L. Identification of Tyr-703 and Tyr-936 as the primary association sites for Grb2 and Grb7 in the c-Kit/stem cell factor receptor. Biochem. J. 1999;341(Pt 1):211–216. [PMC free article] [PubMed] [Google Scholar]
- Uitdehaag JC, Verkaar F, Alwan H, de Man J, Buijsman RC, Zaman GJ. A guide to picking the most selective kinase inhibitor tool compounds for pharmacological validation of drug targets. Br. J. Pharmacol. 2012;166:858–876. doi: 10.1111/j.1476-5381.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaasa A, Viil I, Enkvist E, Viht K, Raidaru G, Lavogina D, Uri A. High-affinity bisubstrate probe for fluorescence anisotropy binding/displacement assays with protein kinases PKA and ROCK. Anal. Biochem. 2009;385:85–93. doi: 10.1016/j.ab.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Vedadi M, Niesen FH, Allali-Hassani A, Fedorov OY, Finerty PJ, Jr., Wasney GA, Yeung R, Arrowsmith C, Ball LJ, Berglund H, Hui R, Marsden BD, Nordlund P, Sundstrom M, Weigelt J, Edwards AM. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15835–15840. doi: 10.1073/pnas.0605224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol. Cancer Ther. 2009;8:2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J. Biol. Chem. 1988;263:2969–2980. [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Wu J, Li W, Craddock BP, Foreman KW, Mulvihill MJ, Ji QS, Miller WT, Hubbard SR. Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor. EMBO J. 2008;27:1985–1994. doi: 10.1038/emboj.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001;105:115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Zegzouti H, Zdanovskaia M, Hsiao K, Goueli SA. ADP-Glo: A Bioluminescent and homogeneous ADP monitoring assay for kinases. Assay Drug Dev. Technol. 2009;7:560–572. doi: 10.1089/adt.2009.0222. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tavare JM, Ellis L, Roth RA. The regulatory role of known tyrosine autophosphorylation sites of the insulin receptor kinase domain. An assessment by replacement with neutral and negatively charged amino acids. J. Biol. Chem. 1991;266:990–996. [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- Zhang J, Adrian FJ, Jahnke W, Cowan-Jacob SW, Li AG, Iacob RE, Sim T, Powers J, Dierks C, Sun F, Guo GR, Ding Q, Okram B, Choi Y, Wojciechowski A, Deng X, Liu G, Fendrich G, Strauss A, Vajpai N, Grzesiek S, Tuntland T, Liu Y, Bursulaya B, Azam M, Manley PW, Engen JR, Daley GQ, Warmuth M, Gray NS. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang S. The complexes of mammalian target of rapamycin. Curr. Protein Pept. Sci. 2010;11:409–424. doi: 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotto F, Ardini E, Casale E, Angiolini M. Through the “Gatekeeper Door”: Exploiting the Active Kinase Conformation. J. Med. Chem. 2010;53:2681–2694. doi: 10.1021/jm901443h. [DOI] [PubMed] [Google Scholar]