Abstract
In the complex microcosm of a cell, information security and its faithful transmission are critical for maintaining internal stability. To achieve a coordinated response of all its parts to any stimulus the cell must protect the information received from potentially confounding signals. Physical segregation of the information transmission chain ensures that only the entities able to perform the encoded task have access to the relevant information. The cAMP intracellular signaling pathway is an important system for signal transmission responsible for the ancestral ‘flight or fight’ response and involved in the control of critical functions including frequency and strength of heart contraction, energy metabolism and gene transcription. It is becoming increasingly apparent that the cAMP signaling pathway uses compartmentalization as a strategy for coordinating the large number of key cellular functions under its control. Spatial confinement allows the formation of cAMP signaling “hot spots” at discrete subcellular domains in response to specific stimuli, bringing the information in proximity to the relevant effectors and their recipients, thus achieving specificity of action. In this report we discuss how the different constituents of the cAMP pathway are targeted and participate in the formation of cAMP compartmentalized signaling events. We illustrate a few examples of localized cAMP signaling, with a particular focus on the nucleus, the sarcoplasmic reticulum and the mitochondria. Finally, we discuss the therapeutic potential of interventions designed to perturb specific cAMP cascades locally.
Keywords: cAMP, compartmentalization, PKA, PDEs, AKAPs, signaling
1. Introduction
More than half a century after its discovery (Rall and Sutherland, 1958) 3′–5′-cyclic adenosine monophosphate (cAMP) remains the object of intense scientific interest (Beavo and Brunton, 2002; Perera and Nikolaev, 2013; Scott et al., 2012). Cyclic AMP is known to regulate many diverse and at times opposing cellular functions including, among others, gene transcription (Yamamoto et al., 1988), cell migration (Burdyga et al., 2013; Zimmerman et al., 2013), mitochondrial homeostasis (Di Benedetto et al., 2013a; Valsecchi et al., 2013), cell proliferation (Stork and Schmitt, 2002) and cell death (Suen et al., 2008; Andersen and Kornbluth, 2013). The cAMP signaling pathway comprises multiple components. G-protein coupled receptors (GPCRs) are activated at the plasma membrane on ligand binding. An active G-protein is released and in turn activates a membrane bound adenylyl cyclase (AC) to generate cAMP from ATP. cAMP can then bind and activate three main effector proteins: the cyclic-nucleotide-gated ion channels (CNG) (Matulef and Zagotta, 2003), the guanine-nucleotide exchange proteins activated by cAMP (EPAC) (Kawasaki et al., 1998) and the cAMP-dependent protein kinase (PKA) (Walsh et al., 1968; Taylor et al., 2013). The cAMP signal is then terminated by the actions of the cAMP-degrading enzymes phosphodiesterases (PDEs) (Manganiello and Degerman, 1999) while phosphatases can turn off the effects of PKA-mediated phosphorylation (Heijman et al., 2013)(Sim and Scott, 1999). As the effects of hormone signaling via cAMP were uncovered shortly after its discovery, it became obvious that the original view that receptors specify the spectrum of hormonal sensitivity of a cell and the substrates available for phosphorylation determine the response was too simplistic. It became clear instead that the same cell can simultaneously express multiple receptors that signal via cAMP as well as multiple targets of PKA, the phosphorylation of which can trigger very different functional outcomes. With this realization, it also became clear that a linear cascade -cAMP generation in response to external stimuli followed by activation of an effector and termination via degradation of the messenger by the phosphodiesterases (PDEs) - is inadequate to explain the ability of cAMP to convey the appropriate information with high fidelity in response to a multitude of extracellular stimuli. Studies conducted in the early eighties in the heart clearly demonstrated that cells can use cAMP to transduce the signal delivered by different hormones into distinct cellular functions. The experimental evidence in essence was that treatment with either prostaglandin E1 (PGE1) or the β-adrenergic receptor agonist isoproterenol (ISO) caused cAMP elevation in the heart, but only ISO triggered the expected effect (i.e. positive inotropy) whereas PGE1 had no effect on contractility, despite both stimuli produced similar amounts of second messenger and comparable levels of PKA activation. To explain these findings the hypothesis was put forward that the components of the cAMP signaling pathway are organized in such a way that cAMP is generated into specific intracellular spaces (Hayes et al., 1980).
These seminal studies first in whole tissue (Hayes et al., 1979, 1980) and then in cell preparations (Buxton and Brunton, 1983), provided the first indirect evidence of compartmentalized cAMP signals. Direct evidence for the existence and functional relevance of subcellular cAMP signaling events (Zaccolo and Pozzan, 2002; Nikolaev et al., 2006) came nearly 20 years later and consolidated the model of compartmentalized cAMP signaling (Zaccolo and Pozzan, 2002; Di Benedetto et al., 2008). The development of molecular tools for the detection of cAMP changes in real time in intact living cells (Berrera et al., 2008; Gesellchen et al., 2011; Stangherlin et al., 2014) greatly contributed to establish the notion of cAMP microdomains. Cyclic AMP and PKA-sensitive probes based on Fluorescence Resonance Energy Transfer (FRET) revolutionized the studies on cAMP signaling. These sensors take advantage of the cAMP binding domains present in PKA (Zaccolo et al., 2000; Zaccolo and Pozzan, 2002) or EPAC (Ponsioen et al., 2004; Nikolaev et al. 2004) and combine sensitivity of detection within the physiological levels with very high spatial and temporal resolution. Moreover, being genetically encoded, these tools can be easily targeted to different cellular compartments making it possible to detect cAMP at specific intracellular sites in real time. The high resolution of these probes allowed to establish that cAMP achieves specificity of action by virtue of a precise organization of the molecular components of its pathway in complex “signalosomes” located at defined cellular locations (Stangherlin and Zaccolo, 2012).
2. Compartmentalization of the cAMP/PKA signaling pathway components
While the evidence in support of compartmentalized cAMP signaling is growing, the newly developed technologies are unveiling an unanticipated level of sophistication of the mechanisms that generate and regulate cAMP functional compartments. In fact, it is now clear that generation of cAMP hotspots in subcellular microdomains involves a complex coordination of events at every step of the cAMP signaling cascade (Zaccolo, 2009; Houslay, 2010; Mika et al., 2012; Perera and Nikolaev, 2013).
2.1 G-protein coupled receptors
At any given moment cells are exposed to a myriad of extracellular stimuli, which act as “first messengers” and are detected by G protein-coupled receptors (GPCRs) at the plasma membrane. Upon ligand binding, GPCR undergo a conformational change resulting in activation of their associated heterotrimeric guanosine-binding proteins (G proteins). On activation, the Gα subunit of the trimeric G-protein is released and is free to associate and modulate the activity of ACs. Depending on the type of their associated Gα subunit, GPCRs can activate (Gαs) or inhibit (Gαi) cAMP production.
The large number of GPCRs confers significant level of diversity and specificity to cAMP signaling. The cell-specific pattern of GPRC expression certainly defines the ability of a particular cell to respond to extracellular stimuli as well as the intensity of the response. However, there is evidence that, within the same cell, distinct localization of individual GPCR at the plasma membrane may contribute to specificity of response. For example, in cardiac myocytes β1 adrenergic receptors (β1AR) are found in both caveolar and non-caveolar membrane fractions, whereas β2AR predominantly localize to caveolae. β2AR also appear to be able to exit caveolae upon activation (Rybin et al., 2000), thus adding a further potential element of dynamic regulation to the system (Patel et al., 2008; DiPilato and Zhang, 2009). An elegant study by Nikolaev et al. combining cAMP-sensitive FRET probes with scanning ion conductance microscopy showed that β1AR are evenly distributed on the plasmalemma of cardiac myocytes, whereas β2AR are found mainly in T-tubules and are absent from non-tubular areas of the membrane. This study also showed that β1AR generate a diffuse cAMP signal whereas the signal generated by β2AR is highly confined (Nikolaev et al., 2010). It is therefore tempting to speculate that the different localization of these receptors at the plasmalemma together with their ability to generate intracellular cAMP signals with distinct properties may contribute to the functional differences observed on selective activation of these receptors, with persistent β1AR stimulation evoking toxic effects, including myocyte apoptosis and hypertrophy, and persistent β2AR stimulation resulting in protective effects on the myocardium (Talan et al., 2011).
In addition to differential expression and compartmentalization of receptors at the plasma membrane, the recently described ability of at least some GPCRs to signal after internalization (Calebiro et al., 2009, 2010; Irannejad et al., 2013) provides a novel exciting facet to the model of compartmentalized cAMP signaling. The canonical view is that internalization of GPCRs is part of the receptor desensitization process and involves receptor phosphorylation by G protein-coupled receptor kinases, recruitment of beta-arrestin and consequent GPCR internalization (Kamal et al., 2012). Once internalized, GPCRs can be re-exposed, in their inactive state, at the plasma membrane or, alternatively, can be targeted for degradation (Zhang and Eggert, 2013). Recent studies, however, have challenged this dogma by showing that internalized GPCRs maintain their ability to trigger cAMP production (Calebiro et al., 2009; Ferrandon et al., 2009; Irannejad et al., 2013). Calebiro et al. used intact thyroid follicles from a transgenic mouse with ubiquitous expression of a cAMP FRET-based sensor to demonstrate that internalized thyroid-stimulating hormone receptors (TSHR) produce cAMP signals that are distinct from those generated via activation of TSHR exposed at the cell surface (Calebiro et al., 2009). A further confirmation that GPCR-mediated signal transduction cascades are not triggered exclusively at the cell surface was provided by studies where a series of conformation-specific single-domain antibodies (nanobodies) able to discriminate between the active and inactive states of the β2-AR receptor were used as biosensors to provide direct evidence that internalized GPCRs are capable of generating cAMP signals in intact living cells (Irannejad et al., 2013). In the context of the compartmentalized cAMP signaling model, generation of cAMP by internalized GPCR is particularly attractive as it would provide a means to generate cAMP from intracellular “hotspots” and it would resolve the conundrum posed by the source of cAMP being localized at the plasma membrane and the requirement to selectively activate PKA subsets localized deep inside the cell. However, whether signaling from internalized GPCRs indeed leads to generation of cAMP microdomains and whether these are linked to specific functional outcomes remains to be determined.
2.2 Adenylyl cyclases
Recent studies using cAMP-sensitive FRET reporters have also challenged the idea that ACs are homogeneously distributed at the plasma membrane and that they constitute a uniform source of cAMP. In fact, ACs are emerging as key players in the generation of cAMP microdomains. There are ten distinct genes encoding for two classes of ACs, nine membrane bound (TmAC1 to TmAC9) and a soluble adenylyl cyclase (sAC) (Cooper and Crossthwaite, 2006; Tresguerres et al., 2011). A number of studies demonstrate that TmACs are not interchangeable, as individual isoforms are uniquely regulated. For example, TmAC1 and TmAC8 are Ca2+ activated whereas TmAC5 and TmAC6 are inhibited by Ca2+ (Cooper and Crossthwaite, 2006; Lefkimmiatis et al., 2009; Willoughby et al., 2012). Using differentially localized cAMP sensitive FRET-based reporters Wachten et al. showed that, in the pituitary cell line GH3B6, TmAC8 is not sensitive to vasoactive intestinal peptide (VIP) and is activated by Ca2+, whereas TmAC5 and TmAC6 respond to VIP with increased activity but their action is inhibited by Ca2+ (Wachten et al., 2010). Ca2+-dependent control is not the only mechanism involved in determining how TmACs link to downstream signaling pathways, as suggested by the observation that although TmAC5 and TmAC6 are both inhibited by Ca2+ deletion of tmAC5 appears to be protective against aging-induced cardiomyopathy (Yan et al., 2007) whereas deletion of TmAC6 does not protect against β-AR stimulation-induced cardiomyopathy and has been associated with increased mortality during stress (Tang et al., 2013). Evidence suggests that key to TmAC specific function is their organization in specific macromolecular complexes involving GPCRs (Dupré et al., 2007) and scaffolding proteins (Bauman et al., 2006; Piggott et al., 2008; Li et al., 2012). The specific interaction between TmAC and scaffolds belonging to the A-Kinase Anchoring proteins (AKAPs, see below) family has received particular attention. For example, the AKAP protein Yotiao was found to interact with a number of TmACs (AC1, AC2, and AC9) and to differentially regulate at least two of them (AC2 and AC9). Yotiao binds to the amino terminus of AC2 and this action does not alter the basal cyclase activity; however, it was found to significantly decrease AC2 activity in the presence of activators such as forskolin, Gαs or a combination of the two (Piggott et al., 2008). While interaction with Yotiao doesn’t appear to affect the activity of AC9, it was recently proposed that Yotiao acts as a facilitator for the formation of a macromolecular complex containing AC9 and the PKA-regulated Iks potassium channel (Li et al., 2012). The interaction of AC9 with Yotiao would thus bring the source of cAMP in close proximity to the final target, creating a truly autonomous cAMP mirodomain (Li et al., 2012; Efendiev et al., 2013).
sAC is also regulated by Ca2+ but, in contrast to TmAC, is not regulated by G proteins and is activated by bicarbonate (Tresguerres et al., 2011; Bitterman et al., 2013) and ATP (Zippin et al., 2013). Early after its discovery it was shown that sAC is not only cytosolic but can also localize at specific subcellular sites, including the nucleus, the midbody, the centrioles and mitochondria (Zippin et al., 2003). Acin-Perez et al. provided biochemical evidence that sAC is localized in the mitochondrial matrix and can produce cAMP in response to metabolically generated bicarbonate (Acin-Perez et al., 2009). Subsequent studies using cAMP-FRET reporters support the presence of sAC activity in the mitochondrial matrix in response to both Ca2+ (Di Benedetto et al., 2013a) and bicarbonate (Di Benedetto et al., 2013b; Lefkimmiatis et al., 2013). Given the impermeability of the inner mitochondrial membrane to cAMP (Acin-Perez et al., 2009; Di Benedetto et al., 2013b; Lefkimmiatis et al., 2013), these findings suggest that the mitochondrial matrix may host a cAMP signaling pathway that responds to metabolic changes and is independent of cytosolic cAMP signals.
2.3 Phosphodiesterases
Once generated, cAMP is degraded by a large and diverse family of enzymes called phosphodiesterases (PDEs) (Omori and Kotera, 2007). As the only mechanism responsible for termination of the cAMP signal, PDEs are critical components of the signaling cascade and have been shown to play an important role in compartmentalization of cAMP.
Because of its hydrophilic nature cAMP is in principle highly diffusible, with a diffusion coefficient experimentally calculated to be around 500μm2s−1 (Nikolaev et al., 2004). Fast diffusion would be expected to result in simultaneous and global activation of all cAMP effectors and is therefore in contrast with the notion of cAMP microdomains (Zaccolo, 2009, 2011; Houslay, 2010). While cAMP buffering (Chen et al., 1999) and cytosol viscosity (Feinstein et al., 2012) may have a role in slowing cAMP diffusion at particular subcellular locations, an important role in restricting cytosolic propagation of cAMP is played by PDEs.
There are 8 different families of cAMP-degrading PDEs (PDE1, 2, 3, 4, 7, 8, 10, 11). Each of these families may include multiple genes and a number of splice variants, thus enormously increasing the number of isoforms (Conti and Beavo, 2007; Zaccolo, 2009; Mika et al., 2012). Different PDE isoforms have different tissue distribution and most individual cells express multiple isoforms. The catalytic domain located at the carboxy-terminal region of each PDE gene (Conti and Beavo, 2007) is highly conserved. By contrast, the amino- terminus of these proteins is very diverse and confers unique regulatory properties and targeting sequences that result in tethering of individual PDEs to strategic subcellular sites (Zaccolo, 2009, 2011; Mika et al., 2012).
Evidence of the involvement of PDEs in the generation of compartmentalized cAMP signals was provided in the early nineties by Hohl and Li. These authors showed that roughly half of the cAMP generated upon treatment of cardiac myocytes with ISO is found in the particulate fraction whereas when ISO stimulation is combined with inhibition of PDEs, total cAMP increases nearly 4 fold but, surprisingly, the fraction of messenger found in the particulate fraction is reduced from 45% to about 20%. These findings were interpreted as the result of confinement of cAMP to the particulate fraction as a consequence of a high cAMP degrading activity in the cytosol and hinted to a role for PDEs in maintaining cAMP microdomains (Hohl and Li, 1991). A more direct confirmation of the key role played by PDEs in shaping intracellular cAMP microdomains came with the use of cAMP-sensitive FRET-based sensors. Using such tools it was possible to visualize for the first time cAMP gradients in living rat neonatal cardiomyocytes and to show that these gradients are dependent on PDE activity (Zaccolo and Pozzan, 2002). Subsequent studies using isoform-specific PDE inhibitors showed that, in cardiac myocytes, marginal inhibition of PDE4 produces a significant increase in the level of cAMP generated in response to catecholamines whereas total inhibition of PDE3 is insufficient to produce similar cAMP elevations, indicating preferential coupling of individual PDEs with the signaling cascade triggered by specific GPCR (Mongillo et al., 2004). Functional coupling of localized PDEs to the local cAMP signal generated by specific hormones was also demonstrated in HEK293 cells where the spatial displacement of endogenous PDE isoforms by exogenously expressed catalytically inactive mutants of PDE enzymes was shown to be sufficient to disrupt cAMP gradients, thus demonstrating the critical role of PDE tethering to specific subcellular sites in shaping intracellular pools of second messenger (Terrin et al., 2006). In another study, displacement of PDE4D3 from the centrosome resulted in selective increase in cAMP at that site and alteration of cell cycle progression (Terrin et al., 2012).
2.4 A Kinase Anchoring Proteins (AKAPs)
The main effector of cAMP is PKA. In its inactive form PKA is a tetramer composed of two regulatory (R) and two catalytic (C) subunits. There are four genes encoding for distinct regulatory subunits (RIα, RIβ, RIIα and RIIβ) and three genes encoding the catalytic subunits (Cα, Cβ and Cγ). The latter are responsible for the enzymatic actions of PKA and phosphorylate preferentially targets containing the [R/K][R/K/X]X[pS/pT] motif. In the absence of cAMP a dimer of R subunits binds and suppresses the activity of two C subunits (Taylor et al., 2013). cAMP binds to the R subunits causing a conformational change that relieves the inhibitory effect on the C subunits allowing phosphorylation of PKA targets (Taylor et al., 2005). The role of the R subunits is not limited to inhibition of the C counterparts as they are also responsible for binding of the PKA holoenzyme to specific A kinase-anchoring proteins (AKAPs). AKAPs serve a critical role in compartmentalized cAMP signaling as they constrains PKA to specific subcellular locations in physical proximity to PKA targets (Marx et al., 2002; Hulme et al., 2003; Kritzer et al., 2012; Scott et al., 2012; Tröger et al., 2012).
The PKA-AKAP interaction occurs via binding of a relatively short amino acid sequence called the dimerization-docking domain (D/D). This domain, located at the N-terminus of the R subunit, binds to a cognate amphipathic domain found in the majority of known AKAPs. This short (14–18aa) domain is the most conserved feature among the over 50 members identified to date of the AKAP family of proteins (Scott et al., 2012). These scaffolding proteins function as the basis for the generation of focal cAMP signaling domains or signalosomes, as they can anchor not only PKA but also multiple other components of the cAMP pathway including receptors (Malbon et al., 2004; Malbon, 2007), ACs (Piggott et al., 2008; Dessauer, 2009), phosphatases (Dodge-Kafka et al., 2010) and PDEs (Scott et al., 2012). The majority of AKAPs preferentially bind the R subunits type II, however there are also AKAPs that bind both RI and RII and AKAPs that selectively bind R subunits type I (Kovanich et al., 2010; Means et al., 2011; Scott et al., 2012). An important contribution to our understanding of the functional significance of signaling by AKAP-anchored PKA subsets came from the development of a series of disruptor peptides that have been used to displace PKA from its anchor sites on AKAPs and to assess the resulting cellular effect (Carr et al., 1992). Of particular interest are peptides that can selectively perturb the interaction between AKAP and either PKA type I or PKA type II. For instance, the fragment called RIAD (RI anchoring disruptor) displays a 1000-fold higher affinity for type I PKA over type II (Carlson et al., 2006) while the disruptor called SuperAKAP-IS is 10000-fold more selective for RII (Gold et al., 2006). More recent additions to the experimental toolkit are disruptors peptides engineered with the aim to displace PKA from individual AKAPs (Gold et al., 2013a). One important drawback for the use of these inhibitor peptides is their limited diffusion and stability. In order to overcome these issues an alternative strategy, involving small molecules such as terpyridines, has recently been developed. Terpyridines and their derivatives can interfere with AKAP-PKA interactions, they display higher cell permeability and have been shown to be more stable than peptides, although the latter remain more selective (Christian et al., 2011; Tröger et al., 2012; Schäfer et al., 2013).
Anchoring of PKA subsets to different AKAPs determines a unique phosphorylation pattern downstream of the kinase, as demonstrated for example by work by Di Benedetto et al. In these studies cAMP sensitive FRET-based sensors were targeted to RI- or RII-binding AKAPs aiming to assess the extent of cAMP changes around these different PKA-AKAP signaling complexes in cardiac myocytes. Fluorescence recovery after photobleaching (FRAP) was used to demonstrate that in these cells both PKA type I and PKA type II are predominantly anchored to subcellular sites. Most importantly, it was found that ISO stimulation generated a cAMP response that was selectively confined to PKA type II- binding AKAPs. This response was associated to phosphorylation of phospholamban (PLN) and troponin I and was sensitive to the PDE4-selective inhibitor rolipram, suggesting the presence of PDE4 in the same compartment. In contrast, the domain defined by the PKA type I-binding AKAPs responded to prostaglandin 1 and glucagon stimulation, was sensitive to PDE2-specific inhibitors but activation of the PKA type I pool did not result in phosphorylation of PLN and troponin I (Di Benedetto et al., 2008). Intriguingly, the location of some AKAPs can change in response to cellular states altering the functional outcome of cAMP signals. An interesting example with pathophysiological significance is the targeting of AKAPs to lipid rafts. Under normal conditions these proteins are found within the lipid rafts where they coordinate important cellular functions such as antigen presentation (Schillace et al., 2011), AC activity (Delint-Ramirez et al., 2011) and cytoskeletal remodeling (Su et al., 2013). During increased ROS production lipids are oxidized and their biophysical properties drastically change (Jin et al., 2011) resulting in release of the AKAPs from the lipid rafts with potential effects on downstream function. For example AKAP12 was shown to be important for membrane targeting and suppression of the metastatic effects of the oncogenic src-tyrosine kinase (Su et al., 2013) and it has been suggested that oxidative stress can alter AKAP12 and this may release src from lipid rafts thus enhancing the metastatic potential of the cell.
Another mechanism through which oxidative stress may affect PKA signaling is via formation of disulfide bonds in type I PKA and consequent cAMP-independent activation of the enzyme (Brennan et al., 2006; Burgoyne and Eaton, 2009, 2013). Cyclic AMP-independent activation of PKA has been proposed also for PKA type II. According to this paradigm signaling via AKAP-tethered PKA provides the possibility for the C subunits to phosphorylate a target protein without dissociating from the R subunits (Akimoto et al., 2013; Taylor et al., 2013), a feature that would restrict the range of action of PKA and would thus contribute to generate a compartmentalized signal. Evidence in support of this mechanism was recently reported by Smith and collaborators using the AKAP18γ-PKA type II complex as a model (Smith et al., 2013). Although the concept of cAMP-independent activation of PKA is intriguing, its relevance to cell physiology remains to be established.
3. Cyclic AMP microdomains
As evidence is being unveiled of the specific cellular processes regulated by individual cAMP pools, an emerging theme is that unique cAMP signaling domains are associated with different organelles. This is perhaps not surprising, given that organelles are physical compartments designated for very specific tasks. A few examples are discussed below.
3.1 Nucleus
One of the well recognized actions of cAMP is the regulation of transcription via PKA-dependent activation of the cAMP-responsive element binding protein (CREB) (Yamamoto et al., 1988; Altarejos and Montminy, 2011). The sequence of events that culminates with CREB activation involves cAMP binding to PKA holoenzyme, dissociation of active C subunits and their translocation to the nucleus via diffusion (Harootunian et al., 1993). Once in the nucleus, PKA phosphorylates CREB at serine 133 facilitating its interaction with CREB binding protein and enhancing the transcription of its target genes. The signal is then terminated by binding of the C subunit to protein kinase inhibitor (PKI), export of the resulting complex from the nucleus (Taylor et al., 2005; Dalton and Dewey, 2006) and dephopshorylation of CREB by Ser/Thr phosphatases (PP1 and PP2A) (Yamamoto et al., 1988; Altarejos and Montminy, 2011). The kinetics of this process are predicted to be relatively slow, due to the time required for C subunits to migrate to the nucleus.
The nucleus is promptly reached by cAMP generated at the plasma membrane by TmACs, indicating that the nuclear envelope is not an obstacle to cAMP diffusion (Terrin et al., 2006). Other studies, however, have suggested that sAC may be an alternative nuclear source of cAMP (Zippin et al., 2003). Recently Sample and colleagues combined live cell imaging techniques with computational modeling and provided evidence pointing to the presence in the nucleus of HEK-293 cells of a sAC-responsive PKA subpopulation (Sample et al., 2012). Based on these studies a model was proposed where in addition to translocation of C subunits to the nucleus in response to TmAC-produced cAMP, the nucleus contains a functional pool of dormant PKA; when cytosolic cAMP reaches the nucleus PDE4 keeps its levels below the activation threshold of the local PKA holoenzyme but when cAMP is produced locally and above that threshold, activation of the local PKA can occur resulting in faster activation kinetics (Sample et al., 2012). However, another study in primary rat neonatal cardiac myocytes failed to confirm the presence of a pool of nuclear PKA holoenzyme that is activated by cAMP locally and provided evidence for the different kinetics of nuclear PKA activity being limited by diffusion and PKI-dependent inhibition of nuclear C subunits (Yang et al., 2013).
3.2 Sarcoplasmic reticulum
In the cardiovascular system cAMP generated via adrenergic stimulation increases the rate and force of heart contraction and increases relaxation. A key function under the control of the cAMP cascade in the heart is diastolic Ca2+ reuptake via the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2) (Movsesian, 1998). cAMP does not exert its regulatory actions directly on SERCA, but on its associated protein PLN. In resting conditions SERCA2 is inhibited by unphopshorylated PLN. In the presence of catecholamines PLN is phosphorylated by PKA and its inhibitory effect on SERCA2 is released, allowing more efficient refilling of the SR and consequent faster decrease of free cytosolic Ca2+ (MacLennan and Kranias, 2003). Using a multidisciplinary approach Lygren and colleagues demonstrated that PLN phopshorylation depends on a SR-located cAMP cascade organized by AKAP18δ (Henn et al., 2004). These authors found that AKAP18δ acts as a scaffold to tether PKA to the SR and in proximity to PLN and SERCA2 (Lygren et al., 2007). The importance of SR-bound PKA was demonstrated by disrupting the AKAP18δ-PLN interaction, resulting in a 50% decrease in the cAMP-dependent phosphorylation of PLN and in a 50% decrease in the rate of Ca2+ re-uptake in the SR, demonstrating that SERCA2 regulation relies largely on the local pool of PKA (Lygren et al., 2007). An independent study by Kerfant and colleagues proposed that the cAMP signals that control SERCA2 activity are shaped by both PDE3 and PDE4 (Kerfant et al., 2007).
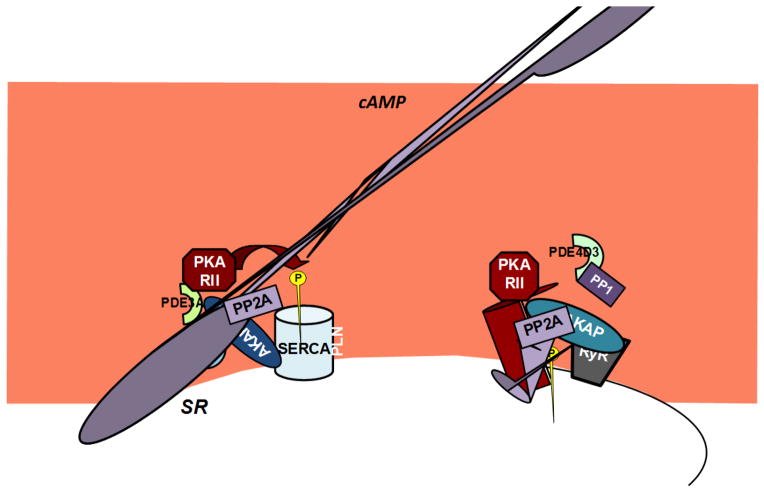
Mice deficient for PDE4 (PDE4−/−) display enhanced myocardial contractility and elevated PLN phosphorylation suggesting that PDE4 participates in the regulation of local cAMP levels and consequently SERCA2 activity (Beca et al., 2011). The role of PDE3 at this site was also assessed in a recent study, using knock out mice deficient for PDE3A (PDE3A−/−) or for PDE3B (PDE3B−/−). Pharmacological inhibition of all PDE3 isoforms in PDE3A−/− mice had no effect on cardiac contractility whereas inhibition of PDE3 in the PDE3B−/− animals showed an increase similar to their wild type littermates. These experiments suggest that PDE3A is the PDE3 isoform contributing to the cAMP signals that regulate SERCA2 function (Beca et al., 2013). Immunoprecipitation and liquid chromatography-tandem mass spectroscopy experiments showed that PDE3A is in complex with SERCA2 while co-immunoprecipitation experiments confirmed that PDE3A interacts also with PLN, AKAP18, PKA-RII and protein phopshatase type 2A (Beca et al., 2013). Taken together these studies strongly support the existence at the SR of a localized cAMP microdomain designated to the regulation of PLN phosphorylation and SERCA2 activity in response to adrenergic stimuli (figure 1).
Figure 1. cAMP-PKA signalosomes at the Sarcoplasmic Reticulum.
The schematic shows two signalosomes found at the SR, formed around AKAP18δ and mAKAP (left side and right side respectively). AKAP18δ recruits PKA type II near the complex SERCA/PLN. In basal conditions PDE3A and PDE4D maintain cAMP levels low and PKA inactive. In response to increased cAMP levels (e.g. during catecolamine-dependent signaling) PKA is activated and free to phosphorylate PLN. Phopshorylated PLN can not efficiently repress the function of SERCA allowing for more efficient Ca2+ refill of the SR. The local signal is terminated by the coordinated action of PDEs and protein phopshatases (PP2A). Similarly, mAKAP acts as platform for a cAMP-responsive signalosome comprised of PKA type II; PDE4D3; PP1 and PP2A. This multiprotein complex was shown to localize in proximity and phophorylate the Ca2+-channel ryanodine receptor.
The SR hosts another cAMP signaling domain organized around mAKAP and including the Ca2+ release channels ryanodine receptors, protein phopshatase 1, protein phosphatase 2A and PKA type II (Marx et al., 2000) (figure 1). The functional role of this signaling domain was suggested by experiments showing that genetic ablation PDE4D aggravates the post-infarction cardiac phenotype and by the observation that in human failing hearts there is reduced association of PDE4D3 with Ryanodine receptors (Lehnart et al., 2005).
3.3 Mitochondria
Another organelle at which cAMP signaling appears to be uniquely regulated is the mitochondrion. In fact, every mitochondrial compartment, the outer mitochondrial membrane (OMM), the inter membrane space (IMS) and the mitochondrial matrix, potentially hosts a distinct and functionally segregated cAMP microdomain. Evidence shows that these submitochondrial cascades differ in most of the defining aspects of local cAMP signaling, including source of cAMP, effectors involved and terminators of the response (figure 2).
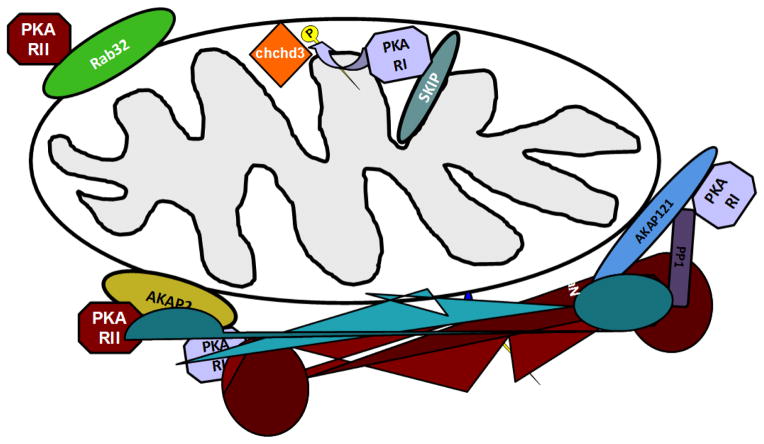
Figure 2. Mitochondrial AKAPs.
The inter membrane space contains the PKA type I-specific AKAP named SKIP. PKA tethered at the inter membrane space via SKIP can phopshorylate the protein chchd3. At the outer mitochondrial membrane are found 3 different AKAPs, Rab32; AKAP2 and AKAP121. AKAP2 and AKAP121 can form complexes with PKA type I and type II while Rab32 can complex only with PKA type II. One of the well-characterized targets of AKAP121-bound PKA is the fission regulator Drp1. Phopshorylated forms of Drp1 can not promotes mitochondrial fission therefore high PKA activity at the outer mitochondrial membrane blocks mitochondrial fission resulting in elongated organelles.
In an early work DiPilato and colleagues used FRET-based cAMP sensitive sensors to measure intramitochondrial messenger variations and concluded that cytosolic cAMP could reach every mitochondrial compartment including the matrix (DiPilato et al., 2004). These findings were later challenged by Acin-Perez and colleagues who, using a biochemical approach, provided evidence that the inner mitochondrial membrane is not permeable to cytosolic cAMP and proposed that the second messenger is generated in situ by a local isoform of sAC (Acin-Perez et al., 2009). The notion that cytosolic cAMP cannot reach the mitochondrial matrix was confirmed in more recent studies using FRET-based biosensors showing that cAMP is generated locally in the matrix in response to bicarbonate or Ca2+ (Di Benedetto et al., 2013b; Lefkimmiatis et al., 2013). Thus, the inner mitochondrial membrane appears to act as a barrier to cAMP diffusion with the resulting possibility that cAMP signaling at the OMM and IMS may be independent from cAMP signaling in the matrix (Lefkimmiatis et al., 2013; Valsecchi et al., 2013).
It has been proposed that mitochondria contain two of the cAMP effectors, EPAC and PKA (Schwoch et al., 1990; Sardanelli et al., 2006; Chen et al., 2008). EPAC appears to localize to the mitochondria in a cell cycle-dependent manner; however, its precise submitochondrial localization and its actions are still unknown (Qiao et al., 2002). Recent data showing that treatment of isolated mitochondria with EPAC-specific activators has no effect on oxygen consumption and oxidative phosphorylation activity would suggest that this protein, if present in the mitochondrial matrix, does not affect respiration (Acin-Perez et al., 2009). PKA, on the other hand, has been described to be present in all mitochondrial compartments. The localization at the OMM and IMS is achieved via binding to a number of different AKAPs.
At least three different AKAPs have been found to associate with the outer mitochondrial membrane: AKAP1 (which comprises a number of splice variants) (Affaitati et al., 2003; Feliciello et al., 2005), Rab32 (Alto et al., 2002; Bui et al., 2010) and AKAP2 (Wang et al., 2001). The presence of several AKAPs at this site suggests that OMM is an important cAMP microdomain, an hypothesis that is supported by recent work showing that PKA actions at the OMM persist longer than in the cytosol possibly as a consequence of local enrichment in the kinase and/or of a lower phosphatase activity at this site (Lefkimmiatis et al., 2013). PKA tethered to the OMM is activated by cytosolic cAMP and participates in the control of both mitochondrial morphology and mitochondrial function. OMM-PKA has been linked to a cell survival pathway via phosphorylation and inhibition of the pro-apoptotic protein BAD (Harada et al., 1999). Additionally, PKA at the OMM is known to phosohorylate Dynamin-1-like protein (Drp1), a large GTP-ase involved in regulating mitochondrial fission-fusion balance (figure 2). PKA-mediated phosphorylation of Drp1 at Ser637 blocks its ability to promote fission resulting in elongated mitochondria and enhanced cell survival in a neuronal cell model (Chang and Blackstone, 2007; Cribbs and Strack, 2007; Dagda et al., 2011; Merrill et al., 2011; Slupe et al., 2013; Merrill and Strack, 2014). The pro-survival actions of AKAP121-tethered PKA are also supported by its effects on ROS production. In cardiac myocytes downregulation or displacement of AKAP121 resulted in mitochondrial dysfunction, increased ROS production and cell death (Livigni et al., 2006; Perrino et al., 2010). Taken together these data suggest that OMM-tethered PKA might be an important regulator of mitochondrial function.
Interestingly, it has been reported that during ischemia or acidosis sAC translocates to the mitochondria where presumably it activates a local pool of PKA enhancing the activity of the pro-apoptotic BAX protein (Kumar et al., 2009; Appukuttan et al., 2012), suggesting the possibility that distinct mitochondrial pools of cAMP may have opposing effects on cell death and survival.
A novel AKAP was recently described in the IMS (Means et al., 2011). This protein called sphingosine kinase interacting protein (SKIP) was shown to interact specifically with PKA regulatory subunits type I (Kovanich et al., 2010; Means et al., 2011). This feature is of particular interest since the RI subunits have higher affinity for cAMP compared to RII subunits. As a consequence the IMS-PKA population might be responsive to levels of free cAMP that are not sufficient to activate the OMM-tethered pool of PKA. IMS-PKA has been shown to phosphorylate ChChd3, a mitochondrial protein that resides in the IMM facing the IMS. Since ChChd3 is an important regulator of cristae maintenance and energy production(Darshi et al., 2011) it will be interesting to understand how PKA phosphorylation influences the actions of this protein.
4. Implications for drug development
It is now clear that localized cAMP signaling is critical for function in many cell types. It is therefore expected that deregulation of compartmentalized cAMP signals might result in disease. This prediction is supported by a number of in vitro studies (Nikolaev et al., 2010; Perera and Nikolaev, 2013) and an increasing number of experiments in animal models (MacLennan and Kranias, 2003; Beca et al., 2011, 2013). For instance, several common pathologies have been associated with disruption of local AKAP/PKA signaling including cataract, diabetes, cancer and cardiovascular disease, among others (Zakhary et al., 2000; Chen et al., 2007; Wirtenberger et al., 2007; Gold et al., 2013b). As our understanding of the mechanisms that underlie compartmentalized signaling builds up, ideas are also developing about how to selectively manipulate this signaling pathway locally for therapeutic purposes. For example, targeting the interface between interacting proteins at a specific site in order to perturb the local branch of the signaling cascade would provide much greater selectivity than perturbing the entire cascade by acting on the receptor at the cell surface (Houslay et al., 2005; Zaccolo, 2011).
In the case of localized cAMP signalosomes interference with protein-protein interaction could provide a way to fine-tune the local signal (Tröger et al., 2012). For instance displacement of a resident PDE would result in augmentation of local cAMP concentrations and increased activation of PKA, whereas removal of PKA would result in selective decrease in the level of cAMP-dependent phosphorylation of neighboring targets (Hundsrucker and Klussmann, 2008; Lee et al., 2013).
An example for the potential therapeutic value of manipulating localized cAMP signals is provided by the study of the small heat shock protein 20 (Hsp20). During an ischemic insult increased PKA-mediated phosphorylation of Hsp20 confers cardioprotection (Qian et al., 2009). Recently it was shown that in cardiac myocytes Hsp20 phopshorylation is under the control of a compartmentalized cAMP signaling cascade and that Hsp20 can directly sequester components of the signaling pathway, namely members of the PDE4 family (Sin et al., 2011). Disruption of the interaction between Hsp20 and PDE4 with a competing peptide was shown to result in higher cAMP availability in the microenvironment surrounding Hsp20 and in its increased phopshorylation by PKA. In the presence of hypertrophic stimuli disruption of the interaction between Hsp20 and PDE4 isoforms was sufficient to increase phopshorylation of Hsp20 at the PKA site serine 16 and to protect from hypertrophy in an in vitro model (Sin et al., 2011).
The site of interaction with Hsp20 has been mapped within the catalytic domain of PDE4 and therefore a disrupting peptide could not distinguish between different PDE4 isoforms. This is not the case for a different class of peptides able to disrupt selectively the interaction of the PDE4D5 isoform with its two interacting partner proteins, receptor for activated C-kinase 1 (RACK1) and β–arrestin. In fact, disruptor peptides that selectively interfere with the binding of PDE4D5 with either RACK1 or β–arrestin (Bolger et al., 2006; Smith et al., 2007) have been used in a clinically relevant model of cancer cell polarity and migration. RACK1 interacts with the protein focal adhesion kinase (FAK) at nascent protrusive structures that form as cells spread onto extracellular matrix. The role of RACK1 would be to act as an intermediary scaffold bringing PDE4D5 into the complex. Using selective disruptors of the RACK1-PDE4D5 interaction it was shown that local PDE4D5 activity is crucial for these cells to acquire an maintain a “spread” phenotype (Serrels et al., 2010). By directly comparing the two peptides these authors found that contrary to the PDE4D5-β–arrestin disruptor, that had no effect on the polarity and migration of cancer cells, the PDE4D5-RACK1 disrupting peptide inhibited both cell polarity and migration (Serrels et al., 2010).
Although peptides have proven invaluable research tools, their restricted membrane permeability and poor oral availability limit their use for therapeutic purposes. The limatations of disruptor peptides may be overcome by screening for small molecules that selectively disrupt protein-protein interactions, an alternative strategy that has already been employed to locally manipulate the cAMP/PKA signaling pathway (Christian et al., 2011).
Targeting protein-protein interactions is a promising therapeutic venue. However, a major drawback to its development is the lack of a sensitive, potent assay to be used in a high throughput setup. In the case of compartmentalized cAMP signalling such an assay would be optimal if combined with sensitive live cell imaging tools. A challenge for the future is therefore the development of robust reporters able to efficiently measure cAMP variations at specific multi molecular complexes and their applications in high throughput systems. The development of such tools would allow screening of large number of molecules for their actions to different cAMP microdomains providing the means for the identification of molecules with unprecedented specificity of action.
Pharmacological treatment targeting the cAMP signaling cascade has been so far largely based on global inhibition or activation of the pathway and has often been associated with adverse secondary off target effects. The accumulating information on the mechanisms that underlie local signalling of this messenger provides now an opportunity for the development of site and action specific therapeutic interventions.
Acknowledgments
This work was supported by the British Heart Foundation (PG/10/75/28537 and RG/12/3/29423) and the NSF-NIH CRCNS program (NIH R01 AA18060) to MZ and a British Heart Foundation Centre of Research Excellence Intermediate Fellowship (RE/08/004) to KL.
Abbreviations
- cAMP
3′–5′-cyclic adenosine monophosphate
- GPCRs
G-protein coupled receptors
- EPAC
Guanine-nucleotide exchange proteins activated by cAMP
- PKA
cAMP-dependent protein kinase
- PDEs
Phosphodiesterases
- FRET
Fluorescence Resonance Energy Transfer
- G proteins
Heterotrimeric guanosine-binding proteins
- βAR
β adrenergic receptors
- AKAPs
A kinase-anchoring proteins
- R
PKA regulatory subunits
- C
PKA catalytic subunits
- CREB
Cyclic AMP-responsive element binding protein
- PLN
Phospholamban
- SERCA2
Sarcoplasmic reticulum (SR) Ca2+-ATPase
- Ca2+
Calcium
- SR
Sarcoplasmic reticulum
- OMM
Outer mitochondrial membrane
- IMS
Inter membrane space
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–76. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, et al. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem. 2003;278:4286–94. doi: 10.1074/jbc.M209941200. [DOI] [PubMed] [Google Scholar]
- Akimoto M, Selvaratnam R, McNicholl ET, Verma G, Taylor SS, Melacini G. Signaling through dynamic linkers as revealed by PKA. Proc Natl Acad Sci U S A. 2013;110:14231–6. doi: 10.1073/pnas.1312644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell. 2013;49:399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appukuttan A, Kasseckert Sa, Micoogullari M, Flacke JP, Kumar S, Woste A, et al. Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovasc Res. 2012;93:340–9. doi: 10.1093/cvr/cvr306. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, et al. Dynamic Regulation of cAMP Synthesis through Anchored PKA-Adenylyl Cyclase V / VI Complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, et al. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium ATPase type 2a signaling complexes in mouse heart. Circ Res. 2013;112:289–97. doi: 10.1161/CIRCRESAHA.111.300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beca S, Helli PB, Simpson Ja, Zhao D, Farman GP, Jones PP, et al. Phosphodiesterase 4D regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ Res. 2011;109:1024–30. doi: 10.1161/CIRCRESAHA.111.250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto G, Pendin D, Greotti E, Pizzo P, Pozzan T. Ca2+ and cAMP crosstalk in mitochondria. J Physiol. 2013a;00:1–8. doi: 10.1113/jphysiol.2013.259135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013b;17:965–75. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, et al. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–44. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- Berrera M, Dodoni G, Monterisi S, Pertegato V, Zamparo I, Zaccolo M. A toolkit for real-time detection of cAMP: insights into compartmentalized signaling. Handb Exp Pharmacol. 2008:285–298. doi: 10.1007/978-3-540-72843-6_12. [DOI] [PubMed] [Google Scholar]
- Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane Adenylyl Cyclases. J Pharmacol Exp Ther. 2013;347:589–98. doi: 10.1124/jpet.113.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger GB, Baillie GS, Li X, Lynch MJ, Herzyk P, Mohamed A, et al. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem J. 2006;398:23–36. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, et al. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281:21827–36. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- Bui M, Gilady SY, Fitzsimmons REB, Benson MD, Lynes EM, Gesson K, et al. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga A, Conant A, Haynes L, Zhang J, Jalink K, Sutton R, et al. cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: Effects of PKA and EPAC. Biochim Biophys Acta. 2013;1833:2664–2672. doi: 10.1016/j.bbamcr.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284:29260–8. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, Eaton P. Detecting disulfide-bound complexes and the oxidative regulation of cyclic nucleotide-dependent protein kinases by H2O2. Methods Enzymol. 2013;528:111–28. doi: 10.1016/B978-0-12-405881-1.00007-0. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258:10233–9. [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Lohse MJ. Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J Mol Endocrinol. 2010;45:1–8. doi: 10.1677/JME-10-0014. [DOI] [PubMed] [Google Scholar]
- Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Taskén K, et al. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem. 2006;281:21535–45. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–7. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chen C, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J. 1999;76:2861–2867. doi: 10.1016/S0006-3495(99)77440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shi X, Padmanabhan R, Wang Q, Wu Z, Stevenson SC, et al. Identification of novel modulators of mitochondrial function by a genome-wide RNAi screen in Drosophila melanogaster. Genome Res. 2008;18:123–136. doi: 10.1101/gr.6940108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian F, Szaszák M, Friedl S, Drewianka S, Lorenz D, Goncalves A, et al. Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J Biol Chem. 2011;286:9079–96. doi: 10.1074/jbc.M110.160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and Physiology of Cyclic Nucleotide Phosphodiesterases: Essential Components in Cyclic Nucleotide Signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Cooper DMF, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27:426–31. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Gusdon aM, Pien I, Strack S, Green S, Li C, et al. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011;18:1914–23. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GD, Dewey WL. Protein kinase inhibitor peptide (PKI): a family of endogenous neuropeptides that modulate neuronal cAMP-dependent protein kinase function. Neuropeptides. 2006;40:23–34. doi: 10.1016/j.npep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins Ga, et al. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem. 2011;286:2918–32. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delint-Ramirez I, Willoughby D, Hammond GVR, Ayling LJ, Cooper DMF. Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8. J Biol Chem. 2011;286:32962–75. doi: 10.1074/jbc.M111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76:935–941. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–8. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato LM, Zhang J. The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst. 2009;5:832–837. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010;285:11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré DJ, Baragli A, Rebois RV, Ethier N, Hébert TE. Signalling complexes associated with adenylyl cyclase II are assembled during their biosynthesis. Cell Signal. 2007;19:481–9. doi: 10.1016/j.cellsig.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Bavencoffe A, Hu H, Zhu MX, Dessauer CW. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem. 2013;288:3929–37. doi: 10.1074/jbc.M112.428144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein WP, Zhu B, Leavesley SJ, Sayner SL, Rich TC. Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells. Am J Physiol Cell Physiol. 2012;302:C839–52. doi: 10.1152/ajpcell.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–87. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–42. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesellchen F, Stangherlin A, Surdo N, Terrin A, Zoccarato A, Zaccolo M. Measuring spatiotemporal dynamics of cyclic AMP signaling in real-time using FRET-based biosensors. Methods Mol Biol. 2011;746:297–316. doi: 10.1007/978-1-61779-126-0_16. [DOI] [PubMed] [Google Scholar]
- Gold MG, Fowler DM, Means CK, Pawson CT, Stephany JJ, Langeberg LK, et al. Engineering A-kinase anchoring protein (AKAP)-selective regulatory subunits of protein kinase A (PKA) through structure-based phage selection. J Biol Chem. 2013a;288:17111–21. doi: 10.1074/jbc.M112.447326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Gonen T, Scott JD. Local cAMP signaling in disease at a glance. J Cell Sci. 2013b;126:4537–43. doi: 10.1242/jcs.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Taskén K, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–95. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–22. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- Harootunian aT, Adams SR, Wen W, Meinkoth JL, Taylor SS, Tsien RY. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. Mol Biol Cell. 1993;4:993–1002. doi: 10.1091/mbc.4.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci U S A. 1979;76:1570–1574. doi: 10.1073/pnas.76.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, Mayer SE. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980;255:5113–5119. [PubMed] [Google Scholar]
- Heijman J, Dewenter M, El-Armouche A, Dobrev D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol. 2013;64:90–8. doi: 10.1016/j.yjmcc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Henn V, Edemir B, Stefan E, Wiesner B, Lorenz D, Theilig F, et al. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem. 2004;279:26654–26665. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- Hohl CM, Li QA. Compartmentation of cAMP in adult canine ventricular myocytes. Relation to single-cell free Ca2+ transients. Circ Res. 1991;69:1369–1379. doi: 10.1161/01.res.69.5.1369. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KYJ. Keynote review: Phosphodiesterase-4 as a therapeutic target preclinical and clinical pharmacology. Drug Discov Today. 2005;10:1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Lin TWC, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase- anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundsrucker C, Klussmann E. Direct AKAP-mediated protein-protein interactions as potential drug targets. Handb Exp Pharmacol. 2008:483–503. doi: 10.1007/978-3-540-72843-6_20. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–8. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Zhou F, Katirai F, Li PL. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid Redox Signal. 2011;15:1043–83. doi: 10.1089/ars.2010.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal Fa, Travers JG, Blaxall BC. G protein-coupled receptor kinases in cardiovascular disease: why “where” matters. Trends Cardiovasc Med. 2012;22:213–9. doi: 10.1016/j.tcm.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kerfant BG, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai S, Chen SRW, et al. PI3Kgamma is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circ Res. 2007;101:400–8. doi: 10.1161/CIRCRESAHA.107.156422. [DOI] [PubMed] [Google Scholar]
- Kovanich D, van der Heyden MaG, Aye TT, van Veen TaB, Heck AJR, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chembiochem. 2010;11:963–71. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- Kritzer MD, Li J, Dodge-Kafka K, Kapiloff MS. AKAPs: the architectural underpinnings of local cAMP signaling. J Mol Cell Cardiol. 2012;52:351–8. doi: 10.1016/j.yjmcc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284:14760–8. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LCY, Maurice DH, Baillie GS. Targeting protein-protein interactions within the cyclic AMP signaling system as a therapeutic strategy for cardiovascular disease. Future Med Chem. 2013;5:451–64. doi: 10.4155/fmc.12.216. [DOI] [PubMed] [Google Scholar]
- Lefkimmiatis K, Leronni D, Hofer AM. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol. 2013;202:453–62. doi: 10.1083/jcb.201303159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen L, Kass RS, Dessauer CW. The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J Biol Chem. 2012;287:29815–24. doi: 10.1074/jbc.M112.380568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, et al. Mitochondrial AKAP121 Links cAMP and src Signaling to Oxidative Metabolism. Mol Biol Cell. 2006;17:263–271. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–7. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Malbon CC. A-kinase anchoring proteins: trafficking in G-protein-coupled receptors and the proteins that regulate receptor biology. Curr Opin Drug Discov Devel. 2007;10:573–579. [PubMed] [Google Scholar]
- Malbon CC, Tao J, Wang H. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem J. 2004;379:1–9. doi: 10.1042/BJ20031648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost. 1999;82:407–11. [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci U S A. 2011;108:E1227–35. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill Ra, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, et al. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol. 2011;9:e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill Ra, Strack S. Mitochondria: A kinase anchoring protein 1, a signaling platform for mitochondrial form and function. Int J Biochem Cell Biol. 2014;48C:92–96. doi: 10.1016/j.biocel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika D, Leroy J, Vandecasteele G, Fischmeister R. PDEs create local domains of cAMP signaling. J Mol Cell Cardiol. 2012;52:323–9. doi: 10.1016/j.yjmcc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, et al. Fluorescence Resonance Energy Transfer Based Analysis of cAMP Dynamics in Live Neonatal Rat Cardiac Myocytes Reveals Distinct Functions of Compartmentalized Phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- Movsesian MA. cAMP-mediated signal transduction and sarcoplasmic reticulum function in heart failure. Ann N Y Acad Sci. 1998;853:231–239. doi: 10.1111/j.1749-6632.1998.tb08271.x. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–8. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–91. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–7. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- Perera RK, Nikolaev VO. Compartmentation of cAMP signalling in cardiomyocytes in health and disease. Acta Physiol (Oxf) 2013;207:650–62. doi: 10.1111/apha.12077. [DOI] [PubMed] [Google Scholar]
- Perrino C, Feliciello A, Schiattarella GG, Esposito G, Guerriero R, Zaccaro L, et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovasc Res. 2010;88:101–10. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- Piggott La, Bauman AL, Scott JD, Dessauer CW. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A. 2008;105:13835–40. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–80. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, et al. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–31. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Mei FC, Popov VL, Vergara La, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–6. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem. 1958;232:1065–76. [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Sample V, Dipilato LM, Yang JH, Ni Q, Saucerman JJ, Zhang J. Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat Chem Biol. 2012;8:375–82. doi: 10.1038/nchembio.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardanelli AM, Signorile A, Nuzzi R, De Rasmo D, Technikova-Dobrova Z, Drahota Z, et al. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–6. doi: 10.1016/j.febslet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Schäfer G, Milić J, Eldahshan A, Götz F, Zühlke K, Schillinger C, et al. Highly Functionalized Terpyridines as Competitive Inhibitors of AKAP-PKA Interactions. Angew Chem Int Ed Engl. 2013:12187–12191. doi: 10.1002/anie.201304686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillace RV, Miller CL, Carr DW. AKAPs in lipid rafts are required for optimal antigen presentation by dendritic cells. Immunol Cell Biol. 2011;89:650–8. doi: 10.1038/icb.2010.148. [DOI] [PubMed] [Google Scholar]
- Schwoch G, Trinczek B, Bode C. Localization of catalytic and regulatory subunits of cyclic AMP-dependent protein kinases in mitochondria from various rat tissues. Biochem J. 1990;270:181–8. doi: 10.1042/bj2700181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Dessauer CW, Tasken K. Creating Order from Chaos: Cellular Regulation by Kinase Anchoring. Annu Rev Pharmacol Toxicol. 2012;53:187–210. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, et al. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–92. doi: 10.1016/j.cub.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Sim AT, Scott JD. Targeting of PKA, PKC and protein phosphatases to cellular microdomains. Cell Calcium. 1999;26:209–217. doi: 10.1054/ceca.1999.0072. [DOI] [PubMed] [Google Scholar]
- Sin YY, Edwards HV, Li X, Day JP, Christian F, Dunlop aJ, et al. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the β-agonist induced hypertrophic response in cardiac myocytes. J Mol Cell Cardiol. 2011;50:872–83. doi: 10.1016/j.yjmcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Slupe AM, Merrill Ra, Flippo KH, Lobas Ma, Houtman JCD, Strack S. A calcineurin docking motif (LxVP) in dynamin-related protein 1 contributes to mitochondrial fragmentation and ischemic neuronal injury. J Biol Chem. 2013;288:12353–65. doi: 10.1074/jbc.M113.459677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, et al. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. Elife. 2013;2:1–19. doi: 10.7554/eLife.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Baillie GS, Hyde EI, Li X, Houslay TM, McCahill A, et al. 1H NMR structural and functional characterisation of a cAMP-specific phosphodiesterase-4D5 (PDE4D5) N-terminal region peptide that disrupts PDE4D5 interaction with the signalling scaffold proteins, beta-arrestin and RACK1. Cell Signal. 2007;19:2612–24. doi: 10.1016/j.cellsig.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Stangherlin A, Koschinski A, Terrin A, Zoccarato A, Jiang H, Fields LA, et al. Analysis of Compartmentalized cAMP: A Method to Compare Signals from Differently Targeted FRET Reporters. Methods Mol Biol. 2014;1071:59–71. doi: 10.1007/978-1-62703-622-1_5. [DOI] [PubMed] [Google Scholar]
- Stangherlin A, Zaccolo M. Phosphodiesterases and subcellular compartmentalized cAMP signaling in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2012;302:H379–90. doi: 10.1152/ajpheart.00766.2011. [DOI] [PubMed] [Google Scholar]
- Stork PJS, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–66. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Su B, Gao L, Meng F, Guo LW, Rothschild J, Gelman IH. Adhesion-mediated cytoskeletal remodeling is controlled by the direct scaffolding of Src from FAK complexes to lipid rafts by SSeCKS/AKAP12. Oncogene. 2013;32:2016–26. doi: 10.1038/onc.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talan MI, Ahmet I, Xiao RP, Lakatta EG. β2 AR Agonists in Treatment of Chronic Heart Failure: Long Path to Translation. J Mol Cell Cardiol. 2011;51:529–533. doi: 10.1016/j.yjmcc.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Lai NC, Wright AT, Gao MH, Lee P, Guo T, et al. Adenylyl cyclase 6 deletion increases mortality during sustained β-adrenergic receptor stimulation. J Mol Cell Cardiol. 2013;60:60–7. doi: 10.1016/j.yjmcc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, et al. Dynamics of signaling by PKA. Biochim Biophys Acta. 2005;1754:25–37. doi: 10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta. 2013;1834:1271–8. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, et al. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–51. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin A, Monterisi S, Stangherlin A, Zoccarato A, Koschinski A, Surdo NC, et al. PKA and PDE4D3 anchoring to AKAP9 provides distinct regulation of cAMP signals at the centrosome. J Cell Biol. 2012;198:607–21. doi: 10.1083/jcb.201201059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79:1277–88. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröger J, Moutty MC, Skroblin P, Klussmann E. A-kinase anchoring proteins as potential drug targets. Br J Pharmacol. 2012;166:420–33. doi: 10.1111/j.1476-5381.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology (Bethesda) 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachten S, Masada N, Ayling LJ, Ciruela A, Nikolaev VO, Lohse MJ, et al. Distinct pools of cAMP centre on different isoforms of adenylyl cyclase in pituitary-derived GH3B6 cells. J Cell Sci. 2010;123:95–106. doi: 10.1242/jcs.058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–3765. [PubMed] [Google Scholar]
- Wang L, Sunahara RK, Krumins A, Perkins G, Crochiere ML, Mackey M, et al. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc Natl Acad Sci U S A. 2001;98:3220–3225. doi: 10.1073/pnas.051633398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, et al. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- Wirtenberger M, Schmutzhard J, Hemminki K, Meindl A, Sutter C, Schmutzler RK, et al. The functional genetic variant Ile646Val located in the kinase binding domain of the A-kinase anchoring protein 10 is associated with familial breast cancer. Carcinogenesis. 2007;28:423–426. doi: 10.1093/carcin/bgl164. [DOI] [PubMed] [Google Scholar]
- Yamamoto KK, Gonzalez GA, Biggs WH, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Yang JH, Polanowska-Grabowska RK, Smith JS, Shields CW, Saucerman JJ. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to β-adrenergic signaling. J Mol Cell Cardiol. 2013;66:83–93. doi: 10.1016/j.yjmcc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M. Spatial control of cAMP signalling in health and disease. Curr Opin Pharmacol. 2011;11:649–55. doi: 10.1016/j.coph.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu Pa, et al. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2000;2:25–9. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–5. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zakhary DR, Moravec CS, Bond M. Regulation of PKA binding to AKAPs in the heart: alterations in human heart failure. Circulation. 2000;101:1459–1464. doi: 10.1161/01.cir.101.12.1459. [DOI] [PubMed] [Google Scholar]
- Zhang X, Eggert US. Non-traditional roles of G protein-coupled receptors in basic cell biology. Mol Biosyst. 2013;9:586–95. doi: 10.1039/c2mb25429h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman NP, Roy I, Hauser AD, Wilson JM, Williams CL, Dwinell MB. Cyclic AMP regulates the migration and invasion potential of human pancreatic cancer cells. Mol Carcinog. 2013 doi: 10.1002/mc.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman Da, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–4. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, et al. CO2/HCO3- and Calcium regulated Soluble Adenylyl Cyclase as a Physiological ATP Sensor. J Biol Chem. 2013;288:33283–91. doi: 10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]