Abstract
Objective
Cochlear implants (CIs) have provided some auditory function to hundreds of thousands of people around the world. Although traditionally carried out only in profoundly deaf patients, the eligibility criteria for implantation have recently been relaxed to include many partially-deaf patients with useful levels of hearing. These patients receive both electrical stimulation from their implant and acoustic stimulation via their residual hearing (electro-acoustic stimulation; EAS) and perform very well. It is unclear how EAS improves speech perception over electrical stimulation alone, and little evidence exists about the nature of the interactions between electric and acoustic stimuli. Furthermore, clinical results suggest that some patients that undergo cochlear implantation lose some, if not all, of their residual hearing, reducing the advantages of EAS over electrical stimulation alone. A reliable animal model with clinically-relevant partial deafness combined with clinical CIs is important to enable these issues to be studied. This manuscript outlines such a model that has been successfully used in our laboratory.
Approach
This manuscript outlines a battery of techniques used in our laboratory to generate, validate and examine an animal model of partial deafness and chronic CI use.
Main Result
Ototoxic deafening produced bilaterally symmetrical hearing thresholds in neonatal and adult animals. Electrical activation of the auditory system was confirmed, and all animals were chronically stimulated via adapted clinical CIs. Acoustic compound action potentials (CAPs) were obtained from partially-hearing cochleae, using the CI amplifier. Immunohistochemical analysis allows the effects of deafness and electrical stimulation on cell survival to be studied.
Significance
This animal model has applications in EAS research, including investigating the functional interactions between electric and acoustic stimulation, and the development of techniques to maintain residual hearing following cochlear implantation. The ability to record CAPs via the CI has clinical direct relevance for obtaining objective measures of residual hearing.
Keywords: electro-acoustic hearing, Cochlear implant, auditory prosthesis, partial deafness, chronic electrical stimulation, auditory function, residual hearing
1. Introduction
Significant hearing loss affects an estimated 278 million people worldwide (Tucci, Merson et al. 2010), of which most suffer from a partial hearing loss characterised by little or no hearing loss at frequencies below 1 kHz, and severe to profound hearing loss in the higher frequencies. The types of hearing loss seen in clinical populations are commonly caused by genetic factors (Smith, Bale et al. 2005), presbycusis (Gates and Mills 2005), noise damage (Daniel 2007) and drug ototoxicity (Schacht, Talaska et al. 2012), and manifest initially as damage to the basal hair cells, giving rise to the loss of high frequency hearing (Nadol 1993). Cochlear implants (CIs) have successfully restored some level of auditory function to more than three hundred thousand hearing impaired people worldwide (Roland and Tobey 2013). Improvements in technology and sound processing techniques have seen an increase in both the number of implantees and their satisfaction with the devices (Sampaio, Araujo et al. 2011). Recently, due to an easing of the cochlear implantation criteria, more patients with residual hearing are receiving CIs, allowing the use of both electrical stimulation provided by the device in the high frequency region of the cochlea and their remaining low frequency acoustic hearing (Talbot and Hartley 2008; McDermott, Sucher et al. 2009; Sampaio, Araujo et al. 2011). Due to the fact that both electric and acoustic stimuli act simultaneously upon the same system (i.e. the auditory pathway), there is the likelihood that these two input modalities will interact, either at the level of the cochlea or further along the auditory pathway. The majority of the published studies into electro-acoustic stimulation (EAS) to date have been psychophysical in nature, and have been carried out in human patients (Gstoettner, Kiefer et al. 2004; Turner, Gantz et al. 2004; Boëx, Baud et al. 2006; Carlyon, Long et al. 2007; Gstoettner, van de Heyning et al. 2008; Looi, McDermott et al. 2008; Talbot and Hartley 2008; Turner, Reiss et al. 2008; Simpson, McDermott et al. 2009; Gantz 2010; Von Ilberg, Baumann et al. 2011). These studies indicate that combined EAS provides benefits in pitch perception, speech perception in both quiet and noise and music perception to individuals with high frequency hearing loss compared to either electrical or acoustical stimulation alone (for review, see Talbot and Hartley, 2008).
Candidates for EAS are typically adult listeners who have experienced hearing loss post-lingually (Gstoettner, van de Heyning et al. 2008). However, newborn hearing screening programs target moderate and profound hearing, meaning that partial hearing loss is often not discovered until childhood. Treatment delay in this population can affect learning outcomes (Tharpe and Sladen 2008). It is likely that the population of children with partial hearing loss will exhibit similar beneficial effects by using EAS as those seen in patients with adult-onset deafness, suggesting that a rise in child EAS patients is likely to occur in the near future. Experimental models that successfully mimic both pre- and post-lingual partially-hearing, chronically stimulated clinical populations are crucial. Such models would improve our understanding of how bimodal information is integrated in the auditory pathway, which would lead to tailoring CI use to EAS users.
To date, studies investigating electro-acoustic interactions using animal models have overwhelmingly been acute (although see von Illberg et al., 1999), and have used normal-hearing animals (von Ilberg, Kierfer et al. 1999; Stronks, Versnel et al. 2010; Von Ilberg, Baumann et al. 2011), which have different physiological and perceptual conditions than clinical populations. In particular, the hair cells are functional along the entire length of the basilar membrane instead of only at the (low frequency encoding) apex, as in the case of partially-deaf individuals (Von Ilberg, Baumann et al. 2011). Although a few EAS studies using partially-deafened animals have been carried out (Coco, Epp et al. 2007; Fallon, Shepherd et al. 2009; Stronks, Versnel et al. 2011), there is a need for further animal research which combines acoustic stimulation with chronic electrical stimulation to determine the nature of the interactions between these two modalities in a clinically relevant partial deafness model. In order for these studies to be clinically relevant, it is important that the animal model have a similar hearing loss to that seen in the clinical population targeted for cochlear implantation (i. e. steeply sloping audiogram). Stimulation should also be provided by clinical devices in order to provide similar electrical stimulation to that used in the clinic.
Despite the development of EAS-specific electrodes, such as the Cochlear Ltd. Hybrid L or the Med-El FlexEAS electrodes (Turner, Gantz et al. 2004; Turner, Reiss et al. 2008; Sampaio, Araujo et al. 2011; Shepherd, Verhoeven et al. 2011), a significant number of CI patients (>30%) can experience a significant loss of their residual hearing after implantation (Gstoettner, Helbig et al. 2006; Skarzynski, Lorens et al. 2006). Maintaining the remaining, low frequency, acoustic hearing in implanted patients is critical for successful EAS outcomes, and a partially hearing chronic electrical stimulation model would enable investigation into the mechanisms responsible for CI-related loss of residual hearing, allowing the development of protective interventions.
Deafness is typically induced in animal models by mechanical lesion, noise trauma or ototoxic deafening. Due to the nature of mechanical lesion (e.g. Rajan, Irvine et al. 1993), lesions can vary between animals and be asymmetrical when carried out bilaterally. Furthermore, cochlear mechanical trauma creates further issues when inserting a cochlear implant due to tissue growth, including new bone, making this deafening method unattractive for CI studies. Deafening by application of traumatic noise exposure can give rise to profound or partial hearing loss, depending upon the duration of stimulation and the nature of the deafening stimulus, although the degree of deafening is variable within and between animals (Eggermont and Komiya 2000; Sekiya, Viberg et al. 2012). A common experimental deafening technique is the use of ototoxic drugs, such as aminoglycosides, which have long been used to induce hair cell death (Chen 1983; Shepherd and Martin 1995; Leake, Hradek et al. 1999; Fallon, Irvine et al. 2009; Wise, Fallon et al. 2011). Aminoglycosides can be applied both systemically and topically (infused through the round window or through a cochleostomy; Hardie and Shepherd 1999), allowing both bilateral and unilateral deafening where appropriate. Systemic application provides reliable, predictable, and, importantly, bilaterally symmetrical hearing loss by destroying cochlear hair cells (Shepherd and Martin 1995) without the creation of tissue grown within the cochlea. This technique can be used to induce deafness in both neonatal and adult animals, providing models for both congenital and adult-onset deafness respectively.
This manuscript describes a neonatal and adult partial deafness animal model using cats, and details a wide range of experimental techniques, including the deafening procedure, the chronic use of clinical CI systems, and a range of chronic recording techniques to measure auditory function over time, which can be used to examine present and future clinical populations.
2. Methods
All procedures detailed in this paper were carried out under NHMRC Guidelines for Animal Experimentation, were in accordance with the guidelines laid down by the National Institutes of Health in the US regarding the care and use of animals for experimental procedures, and were approved by the Royal Victoria Eye and Ear Hospital Animal Ethics Committee, Melbourne, Australia. Experimental procedures are outlined in Table 1 and detailed below.
Table 1.
Experimental procedures undertaken for the two groups.
| Group | n | Age at deafening | Deafening technique | Deafness duration | Electrical stimulation |
|---|---|---|---|---|---|
| Neonatal deafening | 21 | 5 days | Single administration of kanamycin (s.c.) and ethacrynic acid (i.v.) | 8 months | No |
| Adult deafening | 10 | 7 months | Daily (s.c.) injections of kanamycin for < 1 month | 13 months | Unilateral |
s.c.: Subcutaneous; i.v. intravenous
2.1. Partial deafening procedure
Partial deafening was achieved in both neonatal and adult cats, providing a model of both congenital (which manifests from birth) and adult-onset partial hearing loss. All animals used in this study were deafened using kanamycin sulphate, an aminoglycoside antibiotic known to be ototoxic. The administration varied depending upon the animal’s age (neonate or adult; Table 1), and is detailed below.
2.1.1. Neonatal
The partial deafening procedure outlined here is based on results published by Shepherd and Martin (1995). Briefly, 21 neonatal cats (aged 5 days) were anaesthetised with gaseous isoflurane (3% in 1L/min oxygen). The cephalic vein, situated on the radial side of the forearm, was cannulated and the loop diuretic ethacrynic acid (1mg/ml, 25 mg/kg, dissolved in intravenous (i.v.) 0.9% saline) was slowly injected intravenously at a rate of approximately 2ml/min via a catheter. Kanamycin sulphate (300mg/kg, dissolved in 0.6ml 0.9% saline) was injected subcutaneously (s. c.). The animal was left to recover for a minimum of two hours prior to being returned to the maternal litter. Daily monitoring was carried out to ensure that no weight loss or lapses in the animal’s health occurred. Four weeks later, auditory brainstem responses (ABRs; see 2.1.3) were recorded to establish auditory thresholds.
2.1.2. Adult
Adult animals (n = 10) received daily kanamycin sulphate injections (200 mg/kg, dissolved in 3ml 0.9% saline; s. c.) for 19–30 days. To minimise discomfort, the site of the injection was varied each day. ABR thresholds and distortion-product otoacoustic emissions (DPOAEs) were measured (see 2.1.4) after 17 days of injections to determine the degree of hearing loss achieved. Subsequent daily kanamycin administration continued until a satisfactory hearing loss (> 80 dB HL for stimuli > 8 kHz) in the audiogram had been achieved (see 2.1.3).
2.1.3. Hearing thresholds
Tone-pip ABRs were recorded to determine the extent and frequency-dependence of each animal’s hearing loss. ABRs were recorded in a sound attenuated Faraday room following the same protocol for all animals, regardless of age. This procedure has been detailed elsewhere (Coco, Epp et al. 2007; Fallon, Irvine et al. 2009) and is included here for completeness. Animals were premedicated with xylazine (4mg/kg, s. c.) and sedated with ketamine (20mg/kg, intramuscular injection (i. m.)) before being placed on a heat pad to maintain core body temperature at 37°C ± 1°. Stimuli were tone pips (5 ms duration, 1 ms linear rise/fall, 25 stimuli/s; 0.5, 1, 2, 4, 8, 12, 24 and 36 kHz presented at 30 – 100 dB sound pressure level (SPL) in 5 dB SPL steps) generated by a custom-written IgorPro procedure (Wavemetrics, Lake Oswego, OR, USA), before being amplified by a TDT SA1 Stereo Power Amp (Tucker Davis Technologies, FL, USA) and presented via a calibrated 4″ Vifa XT25TG30-04 speaker (Vifa, Videbæk, Denmark) situated 10 cm from the pinna. The contralateral ear was blocked using earmould compression material (Otoform-k, Dreve-Otoplastik, GMBH, Unna, Germany). ABRs were recorded differentially via percutaneous stainless steel electrodes (vertex: positive; nape of neck: negative; with ground in flank) and amplified × 103 via an ISO-80 Bio-Amplifier (World Precision Instruments, Sarasota, FL). ABRs were then bandpass filtered (150 Hz to 3 kHz), averaged and displayed using IgorPro. Threshold was determined visually to be the lowest stimulus intensity required to elicit a clear response amplitude ≥ 0.2μV between 3.5–6 ms post-stimulus onset over two averaged traces (100 stimuli/each). To minimise instances of anaesthesia for adult deafened animals, ABRs were recorded no more frequently than once every 3 days during the deafening procedure.
2.1.4. DPOAEs
DPOAEs are acoustic emissions that occur when two pure tone frequencies in the mid-high frequencies are simultaneously presented to the cochlea and can be used as an indication of normal outer hair-cell (OHC) function (Probst, Lonsbury-Martin et al. 1991). DPOAEs were recorded in conjunction with ABRs in anaesthetised adult animals (see 2.1.3 for details of anaesthesia) before and during the deafening procedure to observe the deterioration of the OHC function over the treatment period. Stimuli were tone pip pairs (F1 and F2, F2/F1 ratio = 1.2; frequency range: 2–20 kHz; 70 dB SPL, 5 ms; 1ms rise/fall time; 25 stimuli/s), generated by a custom-written IgorPro procedure before being amplified by a TSTSA1 Stereo Power Amplifier and presented via two 4″ Vifa XT25TG30-04 speakers adapted to enable combined simultaneous closed-field presentation through a soft-tipped probe that sealed the ear canal. The largest DPOAE occurs at 2F1-F2, and it is at this frequency that responses were measured with an Etymotic ER-10B+ microphone (Etymotic Research, Inc., IL, USA) situated inside the soft probe tip adjacent to the opening of the stimulus-presenting tubes. The recording of DPOAEs was also attempted in a subset of neonatally deafened animals (n=8), but no such recordings were obtainable in this group.
2.2. Adult cochlear implantation
Once partial deafness had been confirmed, all animals in the adult-deafened group (n=10) underwent unilateral cochlear implantation using the electrode array and techniques detailed below.
2.2.1. Electrode array for EAS
Cochlear Ltd. Hybrid L14 cat electrode arrays were used in this study (Cochlear Ltd., Sydney, Australia (Shepherd, Verhoeven et al. 2011)). These electrodes have 0.25–0.5 mm diameter platinum electrode half-bands situated along the 10.5 mm array. The arrays were modified from clinical human Hybrid L24 arrays to fit inside the cat cochlea by removal of the most basal 8 electrodes, thus making a 14-channel device (Shepherd, Verhoeven et al. 2011). Electrode insertion depth corresponds to ~50% the length of the cat scala tympani, representing a tip electrode position of around 4kHz if inserted so that the most basal electrode sits at the round window (Greenwood 1990; Shepherd, Verhoeven et al. 2011). As these arrays were designed with a soft tip to cause minimal insertion damage and are purposefully short to leave the apical region of the cochlea unimplanted, they are excellent for atraumatic use in animals with low frequency residual hearing.
2.2.2. Implantation surgery
The protocol for chronic cochlear implantation has been detailed elsewhere (Xu, Shepherd et al. 1997; Coco, Epp et al. 2007). Using aseptic techniques, the left cochlea was implanted with a Hybrid L14 electrode array. Each animal was sedated with atropine/axepromazine (ANAMAV, 0.05ml/kg s.c.) prior to induction of anaesthesia with gaseous isoflurane/oxygen. Each animal was then intubated and maintained at a surgical level of anaesthesia via a closed-loop anaesthetic machine for the duration of the procedure (isoflurane; 1–3%). A post-auricular incision was made and the soft tissue was blunt dissected until the bulla was exposed. A small hole was drilled in the bulla to expose the round window membrane which was subsequently incised with a 25 gauge needle. The electrode array was then inserted into the scala tympani making sure that all of the electrode contacts were inside the cochlea. The round window opening was closed with muscle fascia and the bulla was sealed with sterile polycarboxylate cement (Durelon, ESPE, Norristown, USA). The middle ear and ossicular pathway remain functionally intact during this procedure. The leadwire was then fixed to the animal’s skull by titanium clips and screws, before being fed subcutaneously to the nape of the animal’s neck where it exited the skin. All incisions were sutured in two layers. Post-surgical care included 5-day administering of antibiotics (0.05 ml, s.c.; Clavulox, Pfizer, New York, USA) and daily weight monitoring.
2.2.3. Stimulator and Sound processor
The chronic stimulation protocol has been detailed elsewhere (Fallon, Irvine et al. 2009) and is presented here briefly. Chronic intracochlear electrical stimulation was provided by a Nucleus Freedom speech processor via a clinical stimulator (Cochlear Ltd, Sydney, Australia) situated within a custom-made backpack that was worn by the animal. The processor was programmed to deliver biphasic current pulses at 500 pulses per second at each electrode. Stimulus levels were set relative to the electrically-evoked ABR (EABR) response for that electrode (see below). The speech processor was set to use the spectral peak (SPEAK) speech processing strategy with 8 maxima, where the 8 largest peaks of spectral energy are sampled at 500 Hz and pulses are applied to the electrodes corresponding to the relevant 8 frequency regions accordingly (Seligman and McDermott 1995).
2.2.4. Chronic electrical stimulation
Either monopolar or common ground stimulation modes were used for chronic intracochlear electrical stimulation. Monopolar stimulation required passing current pulses from one of the intracochlear electrodes to a large return ball electrode situated outside of the cochlea (Fallon, Shepherd et al. 2009). This stimulation regime is the most efficient and is therefore often preferred clinically (Seligman and Shepherd 2004). For monopolar stimulation, biphasic charge balanced current pulses (width: 25 μs and interphase gap: 8 μs) were used. Common ground stimulation refers to stimulation via one intracochlear electrode, with the return current carried via the remaining intracochlear electrodes. This stimulation regime resulted in higher non-auditory muscle response (‘myogenic’) thresholds, and was therefore selected if monopolar stimulation gave rise to myogenic responses at similar current levels to EABR threshold. In the common ground configuration, longer biphasic current pulses (100 μs; 50 μs interphase gap) were used reflecting the decreased efficiency compared to monopolar stimulation. Once activated, each animal received 24-hour per day stimulation, whilst in their cages and during play, providing chronic environmentally-derived stimulation that was consistent with that of a CI user in the same acoustic environment.
2.2.5. Electrical stimulation parameters
EABR-derived thresholds were used to determine Threshold (T) and Comfort (C) levels for each electrode, using T= EABR threshold −3 dB and C = EABR threshold +6 dB. Stimulation was well tolerated by the animals upon activation of the implant. In the rare cases where this was not the case, the C level was initially reduced and subsequently increased as the animal habituated to stimulation. Similarly, if myogenic responses were observed for a given electrode, the maximum delivered current (C level) was reduced below myogenic threshold at that electrode. This was typically still considerably above the EABR threshold, although if myogenic levels were determined to be at or similar to perceptual threshold for monopolar stimulation, common ground stimulation was used (see above).
2.3. Chronic stimulation and monitoring
All cochlear implants were stimulated and monitored using parameters available in the clinic, and these are briefly presented below. Further functional recordings not routinely undertaken in the clinic, such as ABRs and EABRs, were also carried out in all animals.
2.3.1. Electrode impedance
In order to check the status of implanted electrodes, impedances were measured in situ, weekly, throughout the chronic stimulation period using Custom Sound EP 2.0 (clinical software; Cochlear Ltd, Sydney, Australia).
2.3.2. Compound Action Potentials
2.3.2.1. Electrically-evoked compound action potentials (ECAP)
Neural Response Telemetry (NRT®) can be used to record from the auditory nerve using the implanted electrode array in monopolar configuration. In the clinic, NRT® is routinely used to obtain ECAPs, with the array being used for both stimulation and recording (Cohen, Richardson et al. 2003). This recording method is used to provide objective measures of electrical thresholds without the need for surface electrodes or anaesthesia, and, as large activity-driven responses can be obtained, the number of repetitions required for averaging the signal can be reduced to decrease recording time compared to ABRs. NRT®s were carried out in awake animals on a weekly basis for each chronically implanted animal using the Custom Sound software to monitor the electrical thresholds and provide functional recordings at more frequent intervals than the EABRs, which require anaesthesia.
2.3.2.2. Acoustically-evoked compound action potentials – laboratory amplifier
Compound action potentials (CAPs) can be recorded using the intracochlear electrode array and allows the acoustic threshold at the level of the auditory nerve to be obtained. This technique is particularly attractive when asymmetrical ABR thresholds are observed between the two ears, as CAPs, unlike ABRs, do not record activity evoked via the contralateral ear, which can contribute to the ABR threshold and produce artificially low auditory thresholds. CAP recordings are not routinely carried out in the clinic due to the need for percutaneous connection to the leadwire. The experimental recording set up is identical to that of ABR recording (see 1.1.3.), with the exception that instead of brainstem responses being recorded via percutaneous electrodes, CAPs are recorded differentially between the most basal intracochlear electrode and an extracochlear electrode. Signals are amplified and analysed in the same way as ABR recordings (see 2.1.3).
2.3.2.3. CAPs – implant amplifier
It is not currently possible to obtain acoustically-evoked CAPs in a clinical setting using a cochlear implant. We have developed a technique to do so using a similar setup to that used for NRT® to provide an objective measure of residual hearing in the clinic in a similar way to ECAPs. Stimuli and recording procedures were identical to those used for recording CAPs with the laboratory amplifier (see 2.3.2.2), with the exception that Nucleus Implant Communicator (NIC; Cochlear Ltd., Sydney, Australia) software was used to drive the CI to obtain acoustic stimulus-related auditory nerve activity. Due to current hardware constraints of the clinical CI system, CAPs were recorded over a 3.2 ms window centred according to expected response latency (2–5.2 ms). Although data presented here were recorded in anaesthetised animals, these recordings, like ECAPs, can be obtained in awake subjects (see 2.3.2.1).
2.3.3. ABRs
ABRs were recorded monthly to obtain full audiograms using the procedure detailed in section 2.1.3. For the purpose of data analysis, where thresholds were determined to be above 100 dB SPL (the maximum intensity delivered), threshold was recorded as 120 dB SPL.
2.3.4. EABRs
EABRs were recorded periodically throughout the chronic stimulation program to determine electrical thresholds in the auditory brainstem. EABRs are analogous to ABR recordings obtained with acoustic responses, offering a measure of functional threshold. Under xylazine (4mg/kg, s. c.) and ketamine (20mg/kg, i. m.) anaesthesia, biphasic current pulses were generated by a custom-written IgorPro procedure via a laboratory stimulator and delivered to the electrode of interest using either a monopolar or common ground electrode configuration. EABRs were recorded differentially in the same way as ABRs (vertex: positive; nape of neck: negative, and ground in animal’s flank) and amplified ×103 via an ISO-80 Bio-Amplifier (World Precision Instruments, Sarasota, FL), filtered, averaged and displayed via the IgorPro procedure. Threshold was determined visually to be the lowest stimulus intensity required to elicit a clear response with an amplitude ≥ 0.2μV. Electrical artefact was rejected following the sample and interpolate method described in Heffer and Fallon (2008).
Due to the partial nature of the hearing loss induced in these experimental animals, inner hair cells (IHCs) were still typically present in regions apical to the electrode array and electrical stimulation could give rise to long-latency ‘electrophonic’ responses. Electrophonic responses arise from the electrical stimulation caused motion of the basilar membrane, causing acoustic transduction in addition to the direct electrical stimulation of the spiral ganglion neurons (Hartmann, Topp et al. 1984; Shepherd and Javel 1997). In order to determine EABR thresholds in animals exhibiting electrophonic responses, a continuous broadband noise masker was presented simultaneously with the probe stimulus (amplitude: 80 dB SPL) in order to suppress the hair cell-mediated responses.
2.4. Tissue preparation
At the end of the experimental period, all animals were sacrificed via intracardiac perfusion of one litre of 0.9 % (wt/vol) normal saline at 37°C with 0.3% (vol/vol) heparin and 0.3% (vol/vol) sodium nitrite, followed by 4% paraformaldehyde. In all implanted animals, the electrode array was removed and the cochleae and brain were postfixed for one hour and washed 3 times in 0.1M phosphate buffered saline (PBS) in preparation for further processing.
2.5. Immunohistochemistry
Immunohistochemical processing to specifically label residual hair cells was carried out in one animal at the end of the experimental period, in order to demonstrate that this processing can be successfully applied in the cat. The animal was sacrificed and tissue was prepared as detailed above (2.4). The cochleae were decalcified in 10% (wt/vol) ethylene diamine tetra-acetic acid (EDTA) for two weeks, cryoprotected with 15%, then 30% sucrose overnight, and snap frozen in Tissue-Tek® optimum cutting temperature (OCT) compound (Saruka Finetek, Tokyo, Japan). Cochlear tissue was cryosectioned at 12 μm and mounted onto Superfrost slides (Menzel-Gläser, Braunschweig, Germany). Midmodiolar sections were washed in 0.1M PBS and then in PBS containing 0.2% Triton X. Slides were then soaked in a blocking solution containing PBS, 2% Goat Serum (Vector laboratories, Burlingame, USA) and 0.2% Triton X for 2 hours before overnight incubation in the primary antibodies at 4°C. Primary antibodies against Myosin VIIa (mouse, 1:200, Developmental Studies Hybridoma Bank, University of Iowa, USA), and Neurofilament 200 (Rabbit, 1:200. Millipore Corporation, Temecula, USA) were used to visualise both IHCs and OHCs, and the supporting cells of the organ of Corti (respectively). After washing in 0.1M PBS (3 × 5 min), the slides were incubated in the secondary antibodies at 1:200 (Alexa Fluor goat Anti-mouse 488 and goat anti-rabbit 568, Life Technologies Australia, Mulgrave, Australia) in PBS with 2% Goat serum and 0.2 % Triton X. Confocal images of cochlear sections were obtained using a Nikon A1R-A1 confocal microscope and processed using ImageJ (National Institute of Mental Health, Maryland, USA).
2.6. Statistics
Post-deafening ABR thresholds at each test frequency were subtracted from the pre-deafening thresholds (adult deafened) or the mean pre-deafening adult ABR thresholds (neonatal) to obtain dB HL (hearing loss). Analyses of variance (ANOVA) were then carried out in both groups using SPSS 18 (IBM, New York, USA) to determine the effects of kanamycin deafening and any differences between the neonatal and adult deafening techniques respectively. Repeated measures ANOVAs were also carried out in the adult, implanted group to determine the effects of electrode insertion on auditory thresholds, and differences between CAP and ABR thresholds, as well as differences in EABR thresholds for different stimulating electrodes. Bonferroni corrections were applied as required, and Greenhouse-Geisser corrections were used when sphericity could not be assumed. Comparison between ECAP and EABR thresholds were made using a linear mixed model, as NRT®s were not always available for comparison.
3. Results
3.1. Partial deafness model and monitoring techniques
3.1.1. Effects of kanamycin deafening- adult vs. neonatal animals
To produce a model of the partial hearing loss seen in clinical EAS candidates (Figure 1A) in adult animals, daily subcutaneous injections of kanamycin were delivered for up to 30 days (mean: 20.4 ± 1.8). Kanamycin injections caused an increase in hearing thresholds in a frequency-specific manner (p < 0.001). Greater hearing loss was seen at high frequencies (to ~ 60 dB HL; Figure 1B) and reduced loss below 8 kHz (< 30 dB HL). Neonatally deafened animals received a single systemic administration of kanamycin at 5 days of age. No significant interaction was found between frequency and group (Greenhouse-Geisser corrected; p = .129), suggesting that the different deafening procedures provided similar levels of hearing loss. The increased thresholds seen in the lower frequencies in all animals remained within the normal-hearing range (i.e. < −20 dB HL), and both groups are therefore considered normal hearing in this frequency range (< 4 kHz). Importantly, hearing thresholds were the same between the two ears (p > 0.05) indicating that the hearing loss was symmetrical. The stability of the hearing loss was tested in 5 neonatally-deafened animals over an 8-month period following completion of the kanamycin administration. No further changes in auditory thresholds occurred in these animals after the kanamycin-induced hearing loss had stabilised (RM ANOVA; p = 0.317; data not shown).
Figure 1.
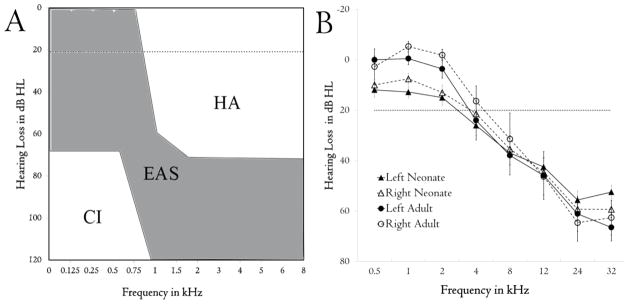
(A) Typical hearing ranges for hearing aid (HA), electro-acoustic (EAS) and cochlear implant only (CI) candidates. Adapted from Von Ilberg, Baumann et al. (2011). EAS recipients have significant hearing loss in higher frequencies. (B) Ototoxic deafening caused a bilaterally-symmetrical frequency-specific hearing loss (determined by ABR) in both neonatally- (n = 21) and adult-deafened (n = 10) animals. This loss is comparable to that seen in the clinical population seen in (A) for the frequency ranges available for each species. Lower frequencies had significantly greater hearing loss in the neonatal group compared to adults, although hearing remained within the boundaries of clinical normal hearing (horizontal dotted line).
3.1.2. DPOAEs
DPOAEs were recorded to determine the functional status of the OHCs. They were successfully obtained for frequencies above 4 kHz in normal hearing adult animals prior to the deafening regime. Below this frequency, we were unable to obtain DPOAEs, indicating a limitation of the recording technique, and not that OHCs in this region were not functional. DPOAE recordings were not obtained above 4 kHz at later sessions, after kanamycin administration, suggesting that, as anticipated, OHCs were damaged, over the mid-high frequency range tested, within the first 17 days of the deafening procedure.
3.1.3. Cochlear immunohistochemistry
The animal model presented here offers the opportunity to investigate the effects of cochlear pathology after partial hearing loss, as well as the effects of chronic intracochlear stimulation on the brain, making it a powerful research tool. To demonstrate successful immunochemical processing in the adult cat cochlea, midmodiolar cochlear sections were immunolabelled for Myosin VIIa (hair cells; Boeda et al., 2002) and Neurofilament (neuronal cytoskeleton; Berglund and Ryugo, 1991) in a partially-deafened cochlea. Both OHCs and IHCs were visible from myosin labelling in the organ of Corti in the apical turn, and supporting cells were also present (Figure 2A). The organ of Corti deteriorated further from the apex to the base, with gradual loss of OHCs, but maintenance of supporting structures in the upper basal turn (Figure 2B) and then absence of any hair cells or supporting structures at more basal locations (Figure 2C). These findings are consistent with the high-frequency partial hearing losses observed with ABRs and CAPs (see 3.1.1) and the absence of DPOAEs after deafening (see 3.1.2) and confirm that relevant immunohistochemical processing can be carried out in the cat providing an important research tool for establishing the mechanism(s) of residual hearing loss with CI use.
Figure 2.
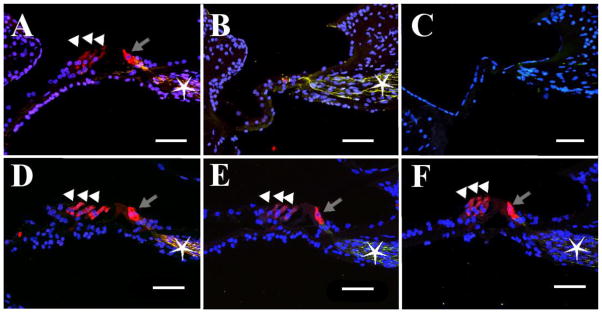
Midmodiolar section of an adult-deafened organ of Corti labelled for Myosin (red) and Neurofilament (green) and DAPI (blue). (A) Apical organ of Corti showing an IHC (in red, grey arrow), three OHCs (white arrowheads) and extensive nerve fibers (star). (B) Organ of Corti from the upper basal turn showing partial degeneration – no hair cells are present, but the nerve fibers remain (green, star). (C) Basilar membrane from the lower basal turn. The organ of Corti has entirely degenerated, as evidenced by a flattened sensory epithelium and lack of innervation. Duration of deafness = 13 months. (D–F). Organ of Corti of a normal-hearing control, taken from similar cochlear positions as A–C (respectively). Inner and outer hair cells are intact and fully innervated. Scale bar = 50 μm.
3.2. Cochlear implantation in adults
3.2.1. ABR vs. CAP
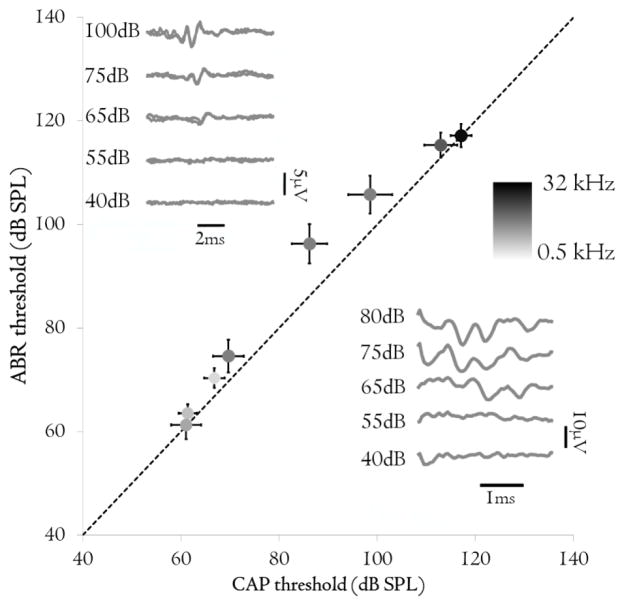
Hearing thresholds were recorded in all animals after implantation surgery using both ABRs and CAPs from the auditory nerve. Auditory thresholds obtained by CAPs were not significantly different to those obtained using ABRs (p = 0.586; Figure 3). As anticipated, similar CAP thresholds were observed between the laboratory system and the NRT® system. An example of a CAP recording obtained using NRT® system is presented in the inset in Figure 3.
Figure 3.
ABR thresholds plotted against CAP threshold for adult deafened animals (n = 10). Dotted line is the line of equality. Marker shades indicate the stimulus frequency. Error bars indicate standard error of mean (s.e.m.). Inset: representative example of ABR (left) and CAP recorded using the NRT® system (right) in response to a 2 kHz pure tone, note different scales. The acoustic thresholds were the same (65 dB SPL).
3.2.2. Increased hearing thresholds with electrode insertion
Insertion of the CI resulted in an increase in ABR thresholds, one month post-implantation, on the side of the implant (mean: 18.2 dB HL ± 1.67 dB HL; p < 0.001) for the frequencies that had residual hearing after the ototoxic deafening procedure (< 12 kHz). This further hearing loss was similar across frequencies (p = 0.785), and usable acoustic thresholds (55–100 dB SPL) were maintained.
3.3. Electrical thresholds
3.3.1. EABRs
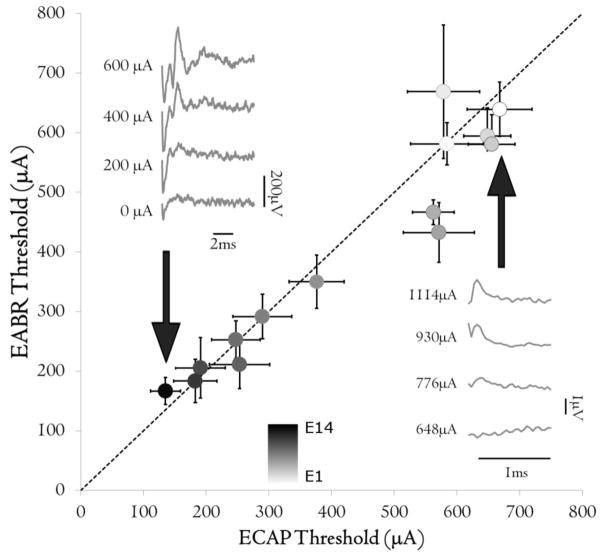
Electrical thresholds varied with electrode position across all animals (p < 0.001), with thresholds decreasing from the most basal (E1) to apical (E14) electrodes, similarly to previous findings in profoundly deafened cats (Figure 4A; Fallon et al. 2009a). Some attrition of available stimulating electrodes occurred due to damage to the percutaneous leadwire and/or connectors. At the final recording session, electrical stimulation could be provided on 77.7% (±7.3%) of electrodes across animals, compared to with 94.6% (±2.2%) in the initial recording session.
Figure 4.
Mean EABR thresholds plotted against mean ECAP thresholds in adult-deafened animals. Dotted line indicates the line of equality. Thresholds were not significantly different between the two measures, but varied with stimulating electrode indicating that ECAP and EABR provide comparable results. Inset: representative example of EABR (left) in response to stimulation from electrode 14; and ECAP in response to stimulation from electrode 2, recorded using the NRT® system (right). Note different scales. Error bars indicate standard error of the mean.
3.3.2. ECAPs
ECAPs were recorded in chronically-stimulated adult-deafened animals using NRT®. We were unable to record ECAPs in some animals (such as those that were stimulated in the common ground paradigm, and those with high electrode impedances). However, for those electrodes for which it was possible to record an ECAP, thresholds were not significantly different to EABR thresholds (p = 0.338; Figure 4).
4. Discussion
The increase in the number of CI patients with residual hearing calls for a reliable, predictable and stable experimental model to address two key issues in EAS, notably; the integration of acoustic and electric information that occurs in EAS patients that may underlie the improved performance in these patients; and the loss of residual hearing experienced in a significant proportion of EAS patients. In this paper, we describe such a model using ototoxic deafening to obtain a partial hearing loss, similar to that seen in clinical populations before cochlear implantation, in either adult or neonatal cats. Importantly, this hearing loss is stable and bilaterally symmetrical, allowing different manipulations to be carried out in each cochlea and contrasted for effects, providing a within-animal control. The ability to partially deafen cats as either adults or neonates permits the effects of hearing loss at these different stages in development to be explored.
The use of different deafening approaches for neonatal and adult animals relates to the maturity of the cochlea at the time of deafening. The cat is an altricial animal, born with an immature auditory system which develops over the first two weeks after birth (Pujol and Marty 1970). Hair cells mature sequentially from the base to the apex, and this property allows the ablation of high-frequency specific cells before the more apical cells develop, to create a partial hearing loss with properties determined by the cochlear developmental stage (Pujol and Marty 1970; Shepherd and Martin 1995). As well as developing first, basal hair cells are also more susceptible to ototoxic damage than those located in more apical regions. The adult partial deafening technique described here relies upon hair cells in low-frequency region being more robust to this deafening. Daily doses of ototoxic agents are administered until sufficient basal hair cells are damaged to produce the desired high-frequency hearing loss.
Partial hearing animals can be atraumatically implanted with a Cochlear Hybrid L14 electrode array (Shepherd, Verhoeven et al. 2011), and receive chronic intracochlear electrical stimulation with environmentally-derived stimuli from a clinical cochlear implant system. The use of a clinical cochlear implant and speech processor allows clinically-relevant stimulation and monitoring of cochlear implant function in this species.
Although common in the mouse (Taylor, Jagger et al. 2012), rat (Gross, Stute et al. 2010) and guinea pig (Altschuler, Hoffman et al. 1985; Wise, Richardson et al. 2005; Coleman, Hardman et al. 2006; Coleman, Rickard et al. 2009), immunohistochemistry is seldom performed in the cat cochlea (but see Wise, Fallon et al. 2011). We have shown that immunohistochemical processing can be carried out in the cochlea of the cat, with a focus upon the organ of Corti. The use of this technique provides the potential for investigation into the cytochemical changes that occur during chronic intracochlear electrical stimulation. The labelling of hair cells, in particular, enables the exploration of potential mechanisms for the loss of residual hearing commonly observed after cochlear implantation. The loss of measurable DPOAEs (> 4 kHz) after ototoxic deafening is a functional reflection of the cochlear anatomy seen in partial hearing animals: a gradual deterioration of the organ of Corti from the apex to the base of the cochlea (Fig. 2., Leake, Kuntz et al. 1997). Generally speaking, the functional recordings of ABR and CAPs also coincide well with the cochlear anatomy revealed by the cellular labelling. These findings corroborate those seen in previous studies published from our group (Coco, Epp et al. 2007; Fallon, Shepherd et al. 2009) and elsewhere (Leake, Kuntz et al. 1997; Wu, Sha et al. 2000).
In this manuscript, all CI data were obtained in adult-deafened animals which accurately model the large number of patients with post-lingual partial deafness that use combined EAS. However, the partial neonatal deafness model also lends itself well to chronic electrical stimulation studies (Fallon, Irvine et al. 2009; Fallon, Shepherd et al. 2009; Wise, Fallon et al. 2011) and allows for the exploration of early integration of electrical and residual acoustic signals on brain plasticity and hearing, in a model of a clinical population that is set to increase in coming years.
Auditory and electrical thresholds were monitored via ABR and CAP and via EABR and ECAP recordings, respectively. As the partial hearing, chronic electrical stimulation model presented here serves to inform about effects of EAS use in the clinic, it is crucial that our diagnostic techniques be directly comparable to those used in the clinical setting. As such, routine clinical procedures such as the collection of DPOAEs and ECAPs enable a complete clinical test battery for direct comparison with human patients, but with the benefit of an animal model providing the capacity to manipulate intracochlear stimulation paradigms, carry out drug testing to improve cochlear sensory cell survival, and collect histological tissues for subsequent analysis. In the case of asymmetrical hearing loss, there is the concern when using ABRs as a measure for functional thresholds that responses from the contralateral ear due to acoustic bleed via bone conduction could contribute to the recorded threshold (although this can be reduced using a contralateral noise masker; Hardie and Shepherd 1999). Recording acoustic CAPs from the auditory nerve does not suffer from contralateral effects as the recording electrode is situated inside the target cochlea, and therefore becomes attractive as both a research and clinical technique. Hearing thresholds were the same when determined by ABR or CAP, emphasising that CAPs are an appropriate metric to use as an alternative to ABR. To date, the recording of CAPs in EAS patients has not been undertaken in the clinic, as recording requires coupling to a laboratory amplifier which is not possible with the fully implanted clinical devices. However, as indicated above (2.3.2.1.), contemporary cochlear implants possess the ability to record from the auditory nerve, a technique used extensively to obtain ECAPs in patients (Cohen, Richardson et al. 2003). With the developing trend to implant patients with residual hearing, the use of the in-built implant amplifier to determine auditory thresholds becomes even more compelling. In particular, the acquisition of CAPs in response to acoustic stimulation is possible, giving a non-invasive, objective, fast and reliable way to record peripheral auditory thresholds. In terms of animal research, ECAPs are routinely recorded via NRT® technology in our lab without the need for, or use of, sedation or anaesthetics, and the application of the NRT® technology to acoustic stimulation provides a convenient way to monitor hearing thresholds following cochlear implantation. To our knowledge, we present the first description of acoustically-driven CAP responses acquired using the CI amplifier. This technique can also be adapted for use in the clinic, for implanted patients with ipsilateral residual hearing.
Aside from the general effects of chronic intracochlear electrical stimulation on residual acoustic thresholds, these animals can be used in single- and multi-unit recording studies to determine any plastic effects of combined EAS on neural plasticity (Fallon, Irvine et al. 2009; Fallon, Shepherd et al. 2009; Fallon, Shepherd et al. 2013). As the signals from each modality interact at some level, carrying out such experiments will allow us to determine where these interactions occur and shape interventions to target these areas specifically.
Our finding that implantation of a cochlear implant negatively affects residual hearing in adult-deafened animals echoes findings in the clinic, and may be due to chronic inflammation following cochlear implantation (Gstoettner, Kiefer et al. 2004; Kiefer, Gstoettner et al. 2004; Skarzynski, Lorens et al. 2012), although a detailed analysis of histological data will be required to confirm this. It is generally accepted that the benefit of EAS stimulation outweighs the partial loss of residual hearing (Gantz 2010; Von Ilberg, Baumann et al. 2011; Skarzynski, Lorens et al. 2012). Combined with the development of the EAS-specific electrode arrays (Cochlear Ltd. Hybrid L and Med-L FLEXEAS), designed to minimise insertion trauma and maximise the use of residual hearing (Turner, Gantz et al. 2004; Turner, Reiss et al. 2008; Sampaio, Araujo et al. 2011; Shepherd, Verhoeven et al. 2011) it is likely that instances of implantation into patients with residual hearing will continue to rise. The animal model presented here offers the potential to carry out in-depth studies into the effects of cochlear implantation on residual hearing, in particular determining the mechanisms of this loss as well as allowing the development of clinically-relevant strategies to stop this loss occurring such as cell-based therapies (Pettingill, Wise et al. 2011; Wise, Fallon et al. 2011) and, ultimately, improve outcomes in clinical populations.
Acknowledgments
We would like to thank Ms. H Feng for electrode fabrication, Ms. N. Critch, A. Morley and A. Neil for animal care and Dr. S. Peirce for veterinary advice. This work was funded by the NHMRC, NIH Contract HHS-N-263-2007-00053-C and Cochlear Ltd. The Bionics Institute acknowledges the support it receives from the Victorian Government via its Operational Infrastructure Support Scheme.
References
- Altschuler RA, Hoffman DW, et al. Localization of dynorphin B-like and alpha-neoendorphin-like immunoreactivities in the guinea pig organ of Corti. Hearing research. 1985;17(3):249–258. doi: 10.1016/0378-5955(85)90069-3. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK. Neurofilament antibodies and spiral ganglion neurons of the mammalian cochlea. The Journal of comparative neurology. 1991;306(3):393–408. doi: 10.1002/cne.903060304. [DOI] [PubMed] [Google Scholar]
- Boeda B, El-Amraoui A, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. The EMBO journal. 2002;21(24):6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boëx C, Baud L, et al. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. Journal of the Association for Research in Otolaryngology; JARO. 2006;7(2):110. doi: 10.1007/s10162-005-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Long CJ, et al. Concurrent sound segregation in electric and acoustic hearing. Journal of the Association for Research in Otolaryngology: JARO. 2007;8(1):119–133. doi: 10.1007/s10162-006-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS. The sensitive period for ototoxicity of kanamycin in mice: morphological evidence. European archives of oto-rhino-laryngology. 1983;238(3):217. doi: 10.1007/BF00453932. [DOI] [PubMed] [Google Scholar]
- Coco A, Epp SB, et al. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hearing Research. 2007;225(1–2):60. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LT, Richardson LM, et al. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hearing research. 2003;179(1–2):72–87. doi: 10.1016/s0378-5955(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Coleman B, Hardman J, et al. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell transplantation. 2006;15(5):369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Rickard NA, et al. A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. Journal of Neuroscience Methods. 2009;176(2):144–151. doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. Noise and hearing loss: a review. The Journal of school health. 2007;77(5):225–231. doi: 10.1111/j.1746-1561.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hearing Research. 2000;142(1–2):89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DR, et al. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. The Journal of Comparative Neurology. 2009;512(1):101–114. doi: 10.1002/cne.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Shepherd RK, et al. Effects of neonatal partial deafness and chronic intracochlear electrical stimulation on auditory and electrical response characteristics in primary auditory cortex. Hearing research. 2009;257(1–2):93–105. doi: 10.1016/j.heares.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Shepherd RK, et al. Effects of chronic cochlear electrical stimulation after an extended period of profound deafness on primary auditory cortex organization in cats. The European journal of neuroscience. 2013 doi: 10.1111/ejn.12445. [DOI] [PubMed] [Google Scholar]
- Gantz BJ. Combining acoustic and electrical hearing. The Laryngoscope. 2010;113(10):1726. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species--29 years later. The Journal of the Acoustical Society of America. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Gross J, Stute K, et al. Expression of prestin and Gata-3,-2,-1 mRNA in the rat organ of Corti during the postnatal period and in culture. Hearing research. 2010;261(1–2):9–21. doi: 10.1016/j.heares.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Gstoettner W, Kiefer J, et al. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta oto-laryngologica. 2004;124(4):348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, Helbig S, et al. Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiology & neuro-otology. 2006;11 (Suppl 1):49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, van de Heyning P, et al. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta oto-laryngologica. 2008;128(9):968–975. doi: 10.1080/00016480701805471. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hearing research. 1999;128(1–2):147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, et al. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hearing Research. 1984;13(1):47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Heffer LF, Fallon JB. A novel stimulus artifact removal technique for high-rate electrical stimulation. Journal of neuroscience methods. 2008;170(2):277–284. doi: 10.1016/j.jneumeth.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta oto-laryngologica. 2004;124(3):272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, et al. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. Journal of Comparative Neurology. 1999;412(4):543. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Kuntz AL, et al. Cochlear pathology induced by aminoglycoside ototoxicity during postnatal maturation in cats. Hearing research. 1997;113(1–2):117–132. doi: 10.1016/s0378-5955(97)00133-0. [DOI] [PubMed] [Google Scholar]
- Looi V, McDermott H, et al. The effect of cochlear implantation on music perception by adults with usable pre-operative acoustic hearing. International journal of audiology. 2008;47(5):257–268. doi: 10.1080/14992020801955237. [DOI] [PubMed] [Google Scholar]
- McDermott H, Sucher C, et al. Electro-acoustic stimulation. Acoustic and electric pitch comparisons. Audiology & neuro-otology. 2009;14(Suppl 1):2–7. doi: 10.1159/000206489. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Hearing loss. The New England journal of medicine. 1993;329(15):1092–1102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Wise AK, et al. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PloS one. 2011;6(4):e18733. doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, et al. A review of otoacoustic emissions. The Journal of the Acoustical Society of America. 1991;89(5):2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Pujol R, Marty R. Postnatal maturation in the cochlea of the cat. The Journal of comparative neurology. 1970;139(1):115–118. doi: 10.1002/cne.901390108. [DOI] [PubMed] [Google Scholar]
- Roland PS, Tobey E. A tribute to a remarkably sound solution. Cell. 2013;154(6):1175–1177. doi: 10.1016/j.cell.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Sampaio AL, Araujo MF, et al. New criteria of indication and selection of patients to cochlear implant. International journal of otolaryngology. 2011;2011:573968. doi: 10.1155/2011/573968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, et al. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anatomical record. 2012;295(11):1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Viberg A, et al. Trauma-specific insults to the cochlear nucleus in the rat. Journal of neuroscience research. 2012;90(10):1924–1931. doi: 10.1002/jnr.23093. [DOI] [PubMed] [Google Scholar]
- Seligman P, McDermott H. Architecture of the Spectra 22 speech processor. The annals of otology, rhinology & laryngology Supplement. 1995;166:139–141. [PubMed] [Google Scholar]
- Seligman PM, Shepherd RK. Cochlear Implants. In: HKW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Vol. 2. Singapore: World Scientific; 2004. pp. 878–904. [Google Scholar]
- Shepherd R, Verhoeven K, et al. Hearing Research. 2011. An improved cochlear implant electrode array for use in experimental studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hearing research. 1997;108(1–2):112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Martin RL. Onset of ototoxicity in the cat is related to onset of auditory function. Hearing Research. 1995;92(1–2):131–142. doi: 10.1016/0378-5955(95)00211-1. [DOI] [PubMed] [Google Scholar]
- Simpson A, McDermott HJ, et al. Comparison of two frequency-to-electrode maps for acoustic-electric stimulation. International journal of audiology. 2009;48(2):63–73. doi: 10.1080/14992020802452184. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, et al. Partial deafness treatment with the nucleus straight research array cochlear implant. Audiology & neuro-otology. 2012;17(2):82–91. doi: 10.1159/000329366. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, et al. Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss. Acta oto-laryngologica. 2006;126(9):934–940. doi: 10.1080/00016480600606632. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Bale JF, Jr, et al. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- Stronks HC, Versnel H, et al. Effects of electrical stimulation on the acoustically evoked auditory-nerve response in guinea pigs with a high-frequency hearing loss. Hearing research. 2011;272(1–2):95–107. doi: 10.1016/j.heares.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Stronks HC, Versnel H, et al. Suppression of the acoustically evoked auditory-nerve response by electrical stimulation in the cochlea of the guinea pig. Hearing research. 2010;259(1–2):64–74. doi: 10.1016/j.heares.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Talbot KN, Hartley DE. Combined electro-acoustic stimulation: a beneficial union? Clinical otolaryngology: official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2008;33(6):536–545. doi: 10.1111/j.1749-4486.2008.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, et al. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PloS one. 2012;7(1):e30577. doi: 10.1371/journal.pone.0030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharpe AM, Sladen DP. Causation of permanent unilateral and mild bilateral hearing loss in children. Trends in amplification. 2008;12(1):17–25. doi: 10.1177/1084713807313085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci D, Merson MH, et al. A summary of the literature on global hearing impairment: current status and priorities for action. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010;31(1):31–41. doi: 10.1097/mao.0b013e3181c0eaec. [DOI] [PubMed] [Google Scholar]
- Turner CW, Gantz BJ, et al. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. The Journal of the Acoustical Society of America. 2004;115(4):1729. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- Turner CW, Reiss LA, et al. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hearing research. 2008;242(1–2):164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ilberg C, Kierfer J, et al. Electric-acoustic stimulation of the auditory system. ORL. 1999;61(6):334. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Von Ilberg CA, Baumann U, et al. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiology & neuro-otology. 2011;16(2):1. doi: 10.1159/000327765. [DOI] [PubMed] [Google Scholar]
- Wise AK, Fallon JB, et al. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics: the Journal of the American Society for Experimental Neuro Therapeutics. 2011;8(4):774–787. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, et al. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. The Journal of Comparative Neurology. 2005;487(2):147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha S, et al. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiology & neuro-otology. 2000;7(3):171. doi: 10.1159/000058305. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, et al. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hearing Research. 1997;105(1–2):1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]