Abstract
Background
Slowed Na+ current (INa) decay and enhanced late INa (INa-L) prolong the action potential duration (APD) and contribute to early afterdepolarizations (EADs). Cardiac resynchronization therapy (CRT) shortens APD compared to dyssynchronous heart failure (DHF), however, the role of altered Na+ channel gating in CRT remains unexplored.
Methods and Results
Adult dogs underwent left-bundle branch ablation and right atrial pacing (200 bpm) for 6 weeks (DHF) or 3 weeks followed by 3 weeks of biventricular pacing at the same rate (CRT). INa and INa-L were measured in left ventricular myocytes from non-failing (NF), DHF and CRT dogs. DHF shifted voltage dependence of INa availability by −3 mV compared to NF, enhanced intermediate inactivation and slowed recovery from inactivation. CRT reversed the DHF-induced voltage shift of availability, partially reversed enhanced intermediate inactivation but did not affect DHF-induced slowed recovery. DHF markedly increased INa-L compared to NF. CRT dramatically reduced DHF-induced enhanced INa-L, abbreviated the APD and suppressed EADs. CRT was associated with a global reduction in phosphorylated CaMKII, which has distinct effects on inactivation of cardiac Na+ channels. In a canine AP model, alterations of INa-L are sufficient to reproduce the effects on APD observed in DHF and CRT myocytes.
Conclusions
CRT improves DHF-induced alterations of Na+ channel function, especially suppression of INa-L, thus abbreviating the APD and reducing the frequency of EADs. Changes in the levels of phosphorylated CaMKII suggest a molecular pathway for regulation of INa by biventricular pacing of the failing heart.
Keywords: Na+ channels, resynchronization, heart failure, arrhythmias, electrophysiology
Introduction
Congestive heart failure is associated with profound abnormalities in both cardiac rhythm and contractile function. The hallmark of cells and tissues isolated from failing hearts independent of the cause is prolongation of action potential duration (APD) due to ion channel remodeling1, which is highly arrhythmogenic with frequent aberration of repolarization such as exaggerated spatial and temporal heterogeneity of repolarization, early (EADs) and delayed (DADs) afterdepolarizations.2
Ion channel remodeling, especially K+ channel down regulation has been consistently demonstrated in human3 and animal models of heart failure.4 Slowed Na+ current (INa) decay and enhanced late INa (INa-L) prolong the action potential (AP)5 and contribute to the high frequency of EADs.6 Increased INa-L and elevated cytosolic Na+ ([Na+]i) in heart failure is linked to the cellular Ca2+ overload via the reverse mode of the Na+-Ca2+ exchanger (NCX)7 and to reactive oxygen species (ROS) generation8, 9 as well as altered mitochondrial biogenesis. However, the role of Na+ current dysfunction in heart failure progression and arrhythmia predisposition is incompletely understood.
Dyssynchronous ventricular contraction in heart failure (DHF) alters the electrical phenotype and enhances the susceptibility to ventricular tachycardia or ventricular fibrillation (VT/VF). In patients who experience improvement in contractile synchrony, cardiac resynchronization therapy (CRT) improves symptoms, ventricular function and survival in patients with heart failure and is expected to reduce frequency of arrhythmias and number of appropriate ICD shocks.10 We and others have shown that CRT employing biventricular pacing in patients with heart failure and intraventricular conduction delays acutely increases left ventricular (LV) ejection fraction11–13 with a reduction in myocardial oxygen consumption.14 More recently we characterized the left ventricular regional remodeling of the cellular electrophysiology in DHF and CRT-induced electrical reverse remodeling.15 APs were significantly prolonged in DHF, particularly in cells isolated from the lateral LV wall; CRT significantly shortened the AP and reduced the LV regional heterogeneity in AP duration which is consistent with another more chronic model of CRT in non-ischemic cardiomyopathy 16 In this study, we hypothesized that CRT restores the DHF-induced altered Na channel gating and thus abbreviates the AP and suppresses EADs. We further explore the role of CaM/CaMKII signaling and oxidant stress, known to alter Na channel inactivation, in the salutary effect of CRT on the cellular electrophysiology of the failing ventricle.
Materials and Methods
Canine Tachypacing-Induced Heart Failure Model
All protocols followed the US department of Agriculture and National Institutes of Health guidelines, and were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. The canine models of DHF or CRT have been previously described and represent models of heart failure with dyssynchronous ventricular contraction (DHF) and synchronous contraction (CRT).17–19 In brief, adult male mongrel dogs underwent left bundle branch (LBB) radiofrequency ablation and the right atrial (RA) pacing (200 bpm) for 6 weeks (DHF dogs: n=7), or 3 weeks of RA pacing followed by 3 weeks of resynchronization by biventricular pacing at same pacing rate (CRT dogs: n=7).15 Control (non-failing: NF) dogs (n=4) underwent no tachypacing and no ablation.
Whole-cell patch clamp recording
Midmyocardial LV, LV anterior (ANT) and lateral (LTR) myocytes from normal (NF), DHF and CRT dogs were studied using the whole cell patch clamp. Ventricular myocytes were current and voltage clamped in the whole-cell configuration of the patch-clamp as previously described.15, 20 Voltage and current clamp control and data acquisition were performed using custom-written software. For the measurement of APs, ventricular myocytes were current clamped with borosilicate glass electrodes with tip resistances of 3.0–4.0 MΩ when filled with pipette solution, and the stimulation frequency was varied over cycle lengths of 2.0, 1.0, 0.5 and 0.25 sec. The steady-state APs were recorded and analyzed at least 1 min after pacing at each cycle length in standard Tyrode’s solution (37 °C).
INa recording was performed at room temperature with patch pipettes with tip resistances of 1–1.5 MΩ tip resistance when filled with pipette solution containing (in mM): NaCl 5, CsCl 40, glutamate 80, CsOH 80, Mg-ATP 5, EGTA 5, HEPES 10, CaCl2 1.5 (free [Ca2+] = 100 nM, pH 7.2 with CsOH, LJP = +5mV). The bath solution contained (in mM) NaCl 10, MgCl2 2, CsCl2 5, TEA-Cl 125, HEPES 20 (pH 7.4 with CsOH). In all experiments, recording was begun 10–15 min after establishment of the whole-cell mode to permit stabilization of current amplitude, voltage dependence and kinetics of gating. Standard voltage protocols were used for assessment of the voltage-dependence of activation/inactivation, recovery from inactivation and entry into inactivation. To determine the membrane potential of half inactivation (V1/2 ) and the slope factor k, steady state inactivation data were fit with a Boltzmann function of the form: I/Imax={1+exp[(V-V1/2)/k]}−1. Recovery from inactivation data were fit with a biexponential function of the form: I(t)/Imax=A1·exp(−t/ 1)+A2·exp(−t/ 2)+A3, using a nonlinear least squares minimization. The decay phase of the current during a voltage step was fit with biexponential function of the form: I(t)=Af·exp(−τ/ f)+ As·exp(−τ/ s), where Af and As are the fractions of fast and slow inactivating components, respectively. Persistent (late) INa was the tetrodotoxin (TTX) (30µM)-sensitive current elicited by 800 ms depolarizations to −20 mV (from −140 mV), from 100–500 ms after a depolarizing voltage step normalized to the peak INa.
Molecular Analysis
Canine NaV1.5 steady state mRNA levels were measured by kinetic real time PCR of RNA in tissue isolated from the midmyocardial layer of LV anterior and lateral walls in NF, DHF and CRT dogs. NaV1.5, and CaMKII proteins were measured by Western immunoblotting. Detailed methods are provided in the online-only supplement.
Computational Model
Simulations were performed using a modified version of the canine coupled model incorporating local control of Ca2+-induced Ca2+ release 21. This model was modified by replacing its description of INa with a new Na+ channel representation, based on that of Grandi et al 22 (Figure S1). The model is further described in the on-line supplement.
Statistical Analysis
Differences among the three groups were compared by one-way ANOVA with Bonferroni's test. Bonferroni-corrected comparisons were performed only if the overall comparison was statistically significant. Two-group analysis was performed by unpaired or paired t-test as appropriate (Figure 3D and Figure 4B, respectively). Data were expressed as mean values ± SD or mean ± SEM as indicated in each of the figures. A value of p<0.05 was considered significant. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
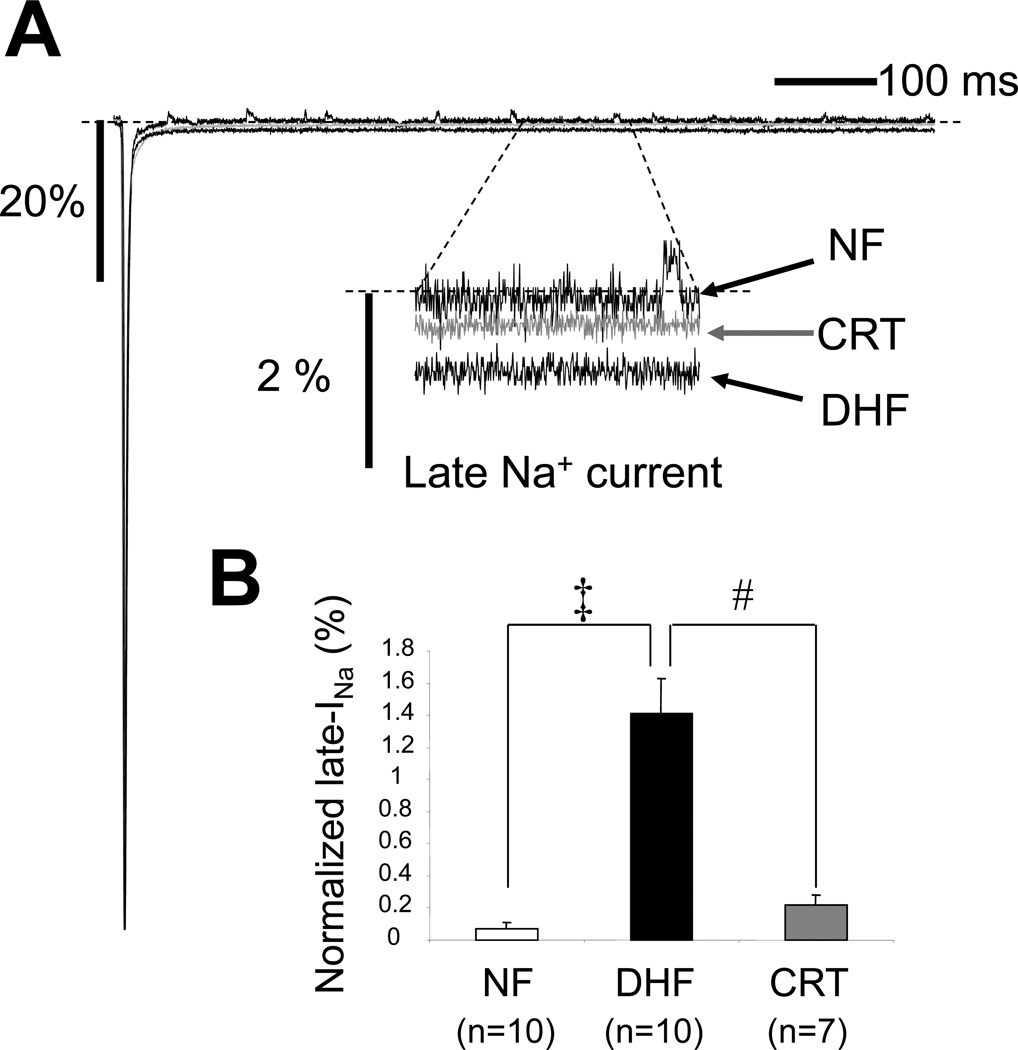
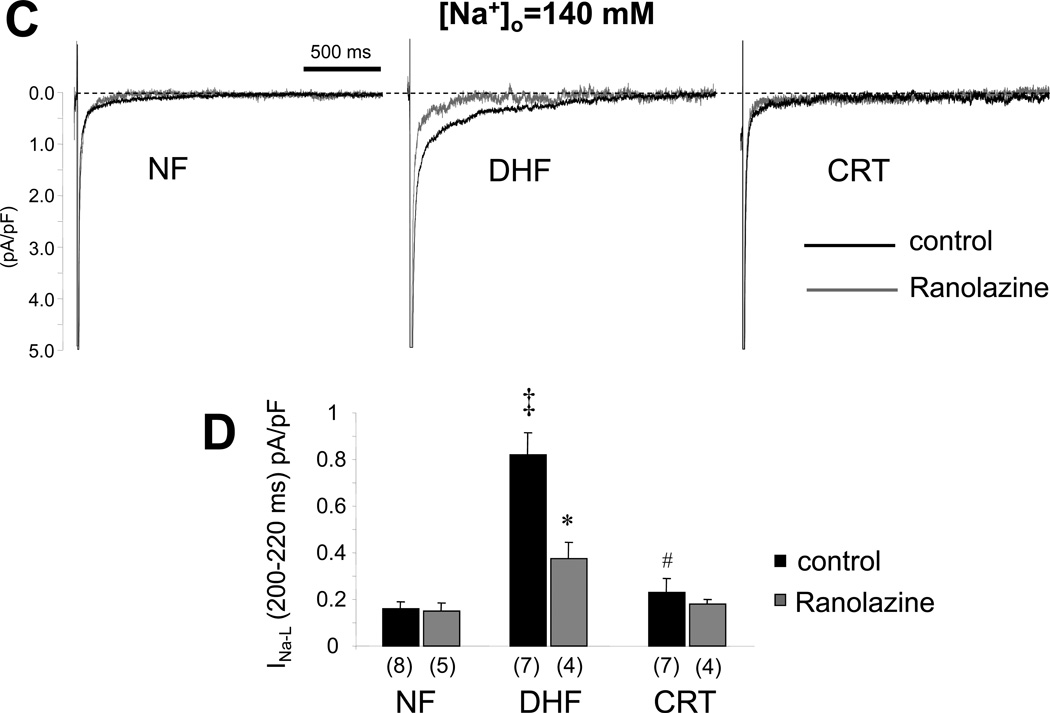
Figure 3.
CRT reverses DHF-induced enhanced late Na current. A: Representative normalized TTX-sensitive currents in NF, DHF and CRT canine myocytes ([Na+]o= 10mM). Late INa was elicited by 800 ms depolarizations to −20 mV (from −140 mV) in the presence and absence of TTX. The average amplitude of the TTX-sensitive late current between 100 and 500 ms was normalized to the peak INa B: Bar plot of the magnitude of late current normalized to the peak for each condition in low external [Na+]. C: Representative late Na currents (INa-L) recorded in physiological Na+ concentration ([Na+]o=140mM) in absence (baseline) or presence of ranolazine (10µM) in myocytes from NF, DHF and CRT ventricles. INa-L was elicited by 2000 ms depolarization to −40 mV (from −140 mV). INa-L was measured as the average amplitude of the current between 200 and 220 ms. D: Bar plot of the magnitude of late current normalized to the peak for each condition in physiological external [Na+]. DHF increased INa-L in both low and physiological Na+ conditions, and CRT dramatically reduced the DHF-induced increase of INa-L. Ranolazine reduced INa-L in myocytes from DHF but not NF or CRT hearts. ‡ :p<0.01 vs. NF, #:p<0.01 vs. DHF by ANOVA with Bonferroni test. *: p<0.05 vs. control by unpaired t-test.
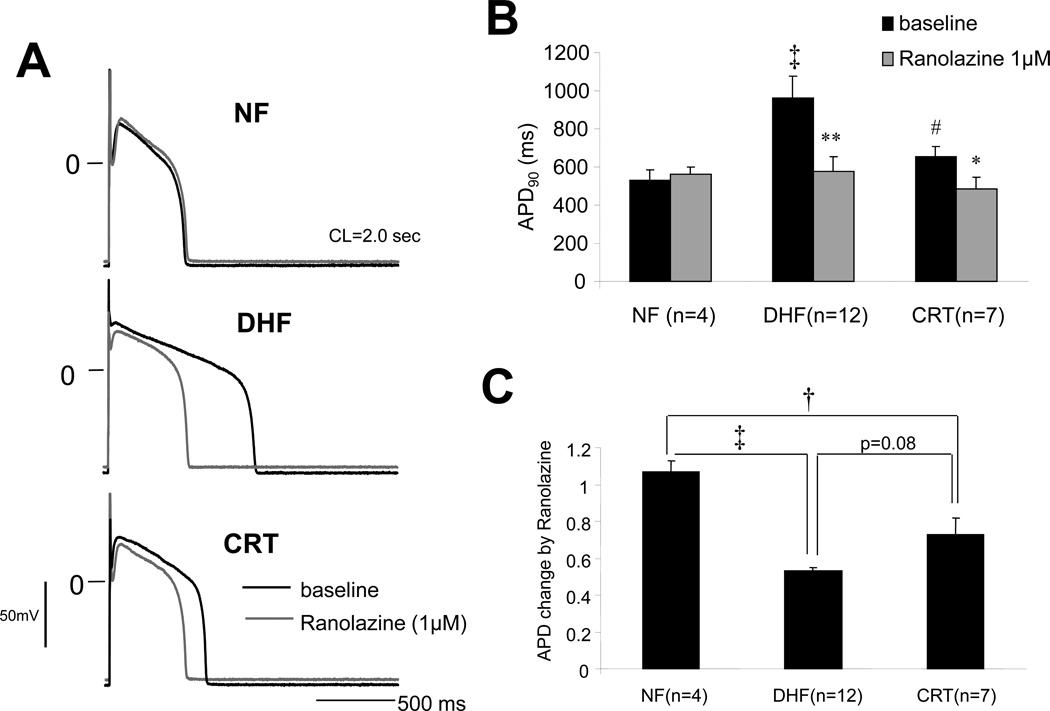
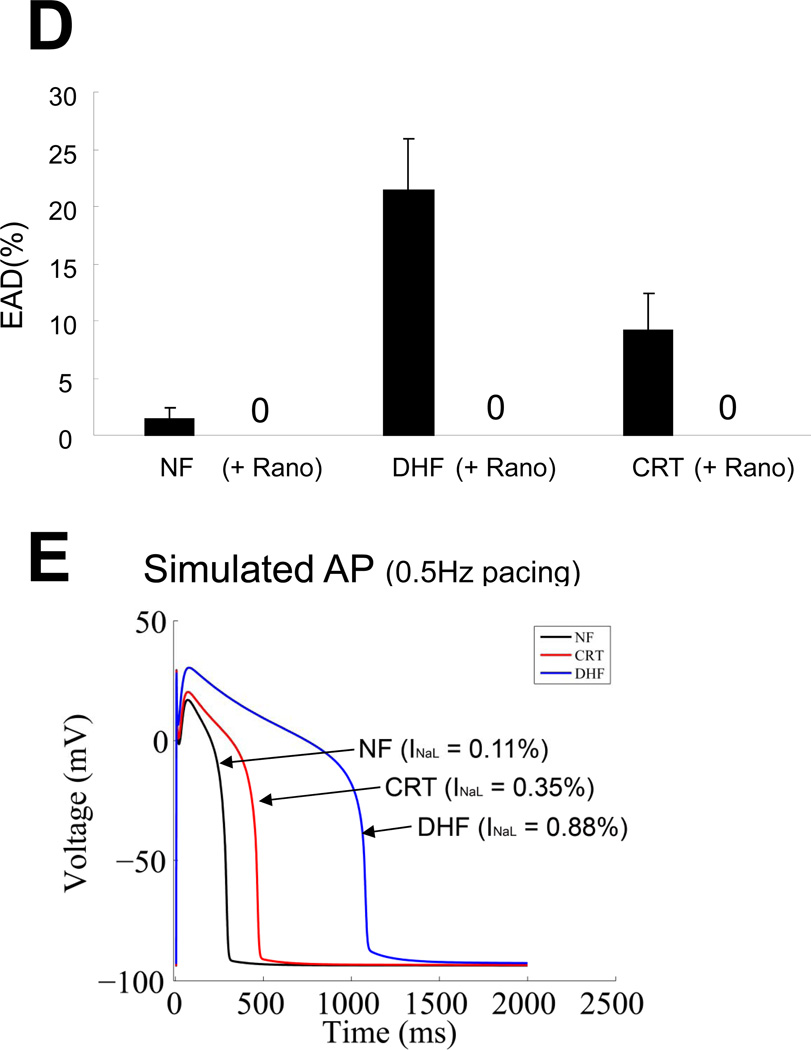
Figure 4.
CRT abbreviates DHF-induced prolongation of APD by reducing late Na current. A: Representative APs recorded from myocytes isolated from the lateral wall of the LV in NF, DHF and CRT canine ventricles at baseline and after ranolazine (1µM) at a paced cycle length of 2000 ms. B: Action potential duration at 90% repolarization (APD90) at baseline and after ranolazine at a paced CL of 2000 ms in NF, DHF and CRT, and C: Fractional change in APD90 by ranolazine in each group. D: Development of EADs at baseline and after ranolazine in ventricular myocytes from NF, DHF and CRT dogs. E: Alteration of INa-L in a canine mathematical AP model mimicking DHF and CRT reproduced the experimental effects of DHF and CRT on the APD. ‡ :p<0.01, †:p<0.05 vs. NF, #:p<0.01 vs. DHF by ANOVA with Bonferroni test. **:p<0.01, *: p<0.05 vs. baseline by paired t-test.
Results
Changes of INa availability by DHF and CRT
There is no statistically significance in the gating parameters between ANT and LTR cells in INa, this study focused on the difference in Na+ currents between NF, DHF and CRT conditions.
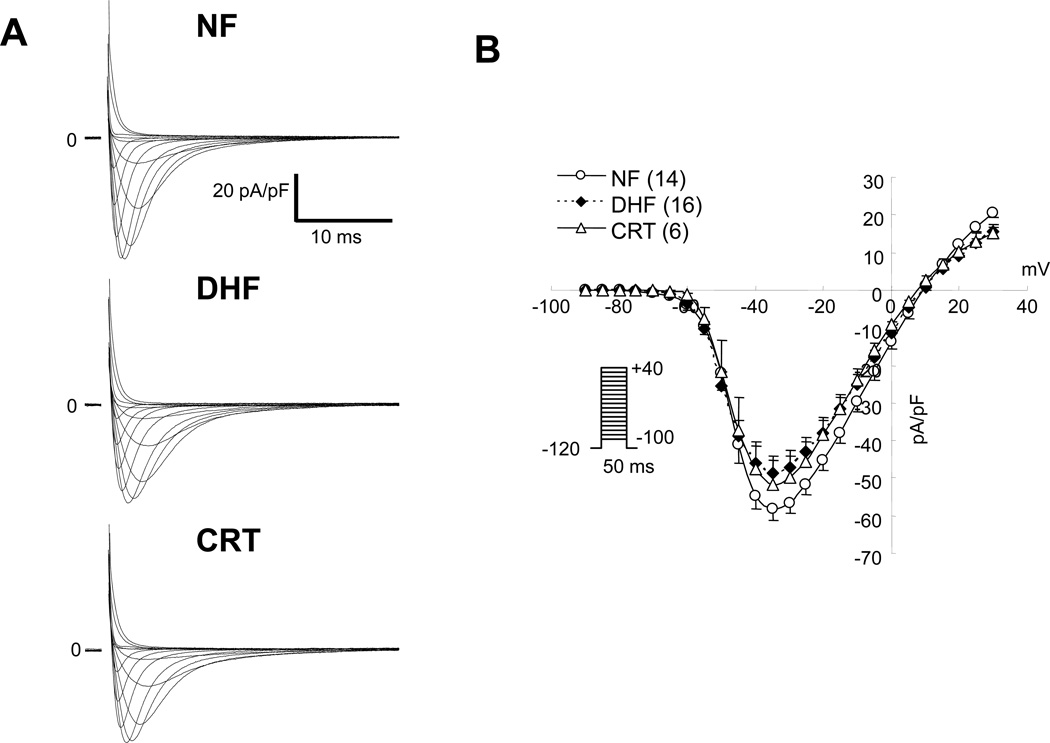
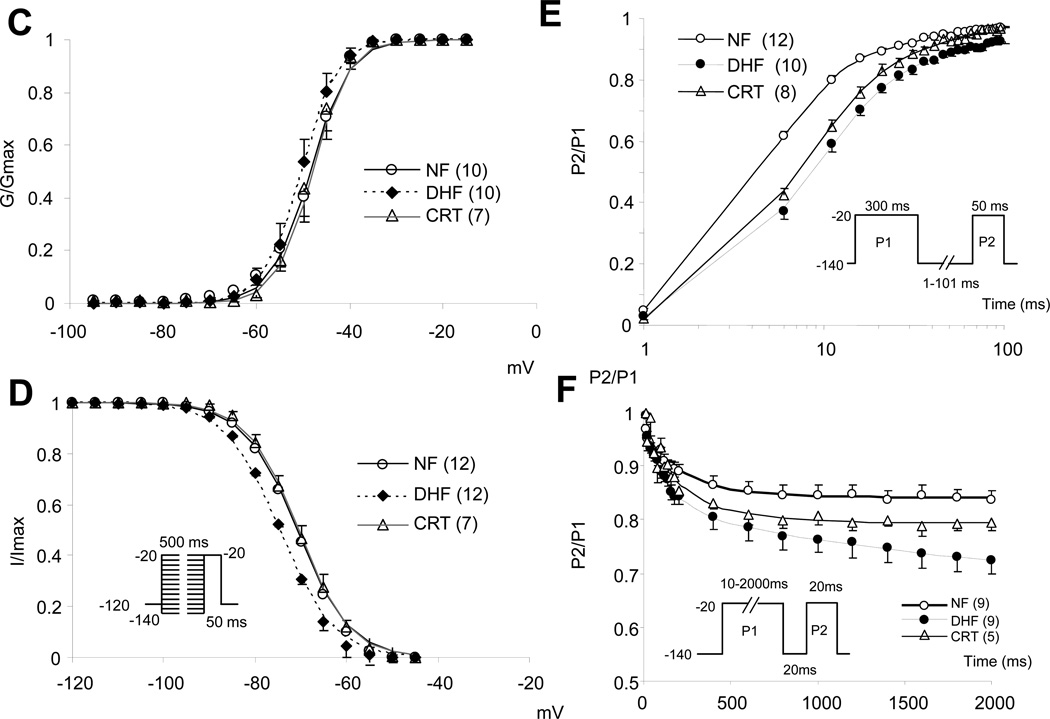
Figure 1A shows representative whole cell INa in ventricular myocytes from NF, DHF and CRT dogs. The current-voltage (I-V) relationship of cardiac INa revealed no significant difference in the peak and reversal potential of the INa between NF, DHF and CRT conditions (Figure 1B). Furthermore, no significant difference was found in the voltage-dependence of Na+ current activation in each group (Figure 1C, Table 1). On the other hand, DHF produced approximately a −3 mV shift in INa steady state inactivation compared with the NF dog (V1/2; − 74.5±3.2 vs. −71.1±3.8 mV, p<0.05) (Figure 1D, Table 1), whereas CRT normalized the DHF-mediated hyperpolarizing shift of steady state inactivation relationship (−70.6 ±.2.9 mV, p<0.05 vs. DHF).
Figure 1.
CRT partially reverses DHF-induced altered Na+ channel currents. A: Representative families of Na+currents (INa) in myocytes from non-failing (NF), DHF and CRT dogs. Cells were held at −120 mV and currents were elicited by 50 ms test pulses to potentials ranging from −100 to +40 mV. B: Peak current-voltage (I-V) relationships of INa in NF, DHF and CRT ventricular myocytes. C and D: The voltage-dependence of activation (G/Gmax) (C) and steady-state inactivation (I/Imax) of INa in NF, DHF and CRT myocytes (D), the data were fit with Boltzmann functions of the form: G/Gmax= 1/[1+exp(V1/2-V)k]. E: The time course of recovery from inactivation of INa F: Kinetics of entry into slow inactivated states of INa in NF, DHF and CRT ventricular myocytes. The values in parentheses are the number of cells studied in this and all remaining figures.
Table 1.
Functional effects of DHF and CRT on Na+ current recorded from canine ventricular myocytes
| Steady-state Inactivation (mV) |
Decay Time Const (ms) @-20mV |
Recovery from Inactivation (ms) |
Activation (mV) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1/2 | n | τfast | τslow | n | τfast | τ slow | n | V1/2 | n | |
| Non-Failing | −71.1 ± 3.8 | 12 | 1.29 ± 0.32 | 4.44 ± 3.62 | 12 | 4.54 ± 0.95 | 87.1 ± 62.3 | 12 | −48.8 ± 4.9 | 14 |
| (ANT) | −70.5 ± 3.2 | 6 | 1.38 ± 0.14 | 4.04 ± 2.81 | 6 | 4.15 ± 0.93 | 57.7 ± 16.7 | 6 | −48.0 ± 5.0 | 7 |
| (LTR) | −71.7 ± 4.5 | 6 | 1.18 ± 0.42 | 4.84 ± 4.52 | 6 | 4.95 ± 0.88 | 116.4 ± 78.6 | 6 | −49.5 ± 5.0 | 7 |
| DHF | −74.5 ± 3.2 † | 12 | 1.41 ± 0.18 | 13.91 ± 6.80 † | 13 | 7.59 ± 2.88 † | 87.0 ± 37.4 | 12 | −50.8 ± 4.0 | 16 |
| (ANT) | −74.0 ± 3.3 | 6 | 1.43 ± 0.18 | 17.65 ± 8.80 | 5 | 6.54 ± 4.45 | 81.6 ± 53.0 | 4 | −49.7 ± 5.1 | 8 |
| (LTR) | −75.6 ± 3.4 | 6 | 1.46 ± 0.13 | 11.24 ± 4.18 | 8 | 8.11 ± 1.92 | 90.1 ± 30.1 | 8 | −52.0 ± 2.1 | 8 |
| CRT | −70.6 ± 2.9 # | 7 | 1.46 ± 0.24 | 5.02 ± 4.37 # | 8 | 7.55 ± 1.76 † | 63.3 ± 31.5 | 8 | −48.6 ± 3.5 | 8 |
| (ANT) | −69.9 ± 3.5 | 4 | 1.53 ± 0.30 | 3.07 ± 1.39 | 5 | 7.32 ± 1.71 | 72.9 ± 34.3 | 5 | −47.9 ± 4.3 | 4 |
| (LTR) | −71.8 ± 0.6 | 3 | 1.34 ± 0.03 | 8.27 ± 6.14 | 3 | 7.93 ± 2.15 | 50.5 ± 28.0 | 3 | −49.5 ± 2.7 | 4 |
Mean ± SD
p<0.05 vs. NF,
p<0.05 vs. DHF by ANOVA with Bonferroni test
ANT: anterior, LTR: lateral
In DHF, INa had a slower recovery from inactivation, and with a hastened entry into inactivation as compared to NF (Figure 1E, Table 1). The fast time constant of recovery from inactivation (τfast) was significantly larger in DHF than in NF (τfast; 7.59 ± 2.88 vs. 4.54 ± 0.95 ms, p<0.05). Overall CRT did not significantly alter τfast compared to DHF (Table 1). Table 2 and Figure 1F summarizes the effect of DHF or CRT on the entry of cardiac Na+ channels into inactivated states. DHF increased the fraction of channels undergoing slow inactivation compared to NF cells (y0: 0.74 ± 0.08 DHF versus 0.83 ± 0.05 NF; p<0.05). Myocytes from CRT hearts exhibited intermediate entry into slow inactivated states with statistically significantly larger fraction of current entering intermediate inactivated states compared with cells from NF hearts. Consistent with a global rather than regional alteration, CRT-induced changes in INa kinetics and gating were similar in cells from the ANT and LTR walls
Table 2.
Effects of DHF and CRT on Entry into Inactivation of Na+ current
| y0 | A | τ | n | |
|---|---|---|---|---|
| Non-Failing | 0.83 ± 0.05 | 0.13 ± 0.05 | 248 ± 91 | 8 |
| (ANT) | 0.72 ± 0.09 | 0.29 ± 0.09 | 169 ± 48 | 4 |
| (LTR) | 0.72 ± 0.11 | 0.26 ± 0.11 | 222 ± 197 | 4 |
| DHF | 0.74 ± 0.08 † | 0.25 ± 0.09 † | 256 ± 171 | 9 |
| (ANT) | 0.79 ± 0.03 | 0.21 ± 0.02 | 256 ± 202 | 4 |
| (LTR) | 0.72 ± 0.08 | 0.26 ± 0.09 | 256 ± 178 | 5 |
| CRT | 0.79 ± 0.03 † | 0.20 ± 0.04 † | 175 ± 46 | 8 |
| (ANT) | 0.77 ± 0.02 | 0.22 ± 0.05 | 204 ± 66 | 4 |
| (LTR) | 0.81 ± 0.03 | 0.20 ± 0.03 | 156 ± 29 | 4 |
Mean ± SD
p<0.05 vs. NF by ANOVA with Bonferroni test, Abbreviations are shown in Table 1
The development of intermediate inactivation was fitted with single exponential functions: y (t) = y0 +A· exp (− t/i)
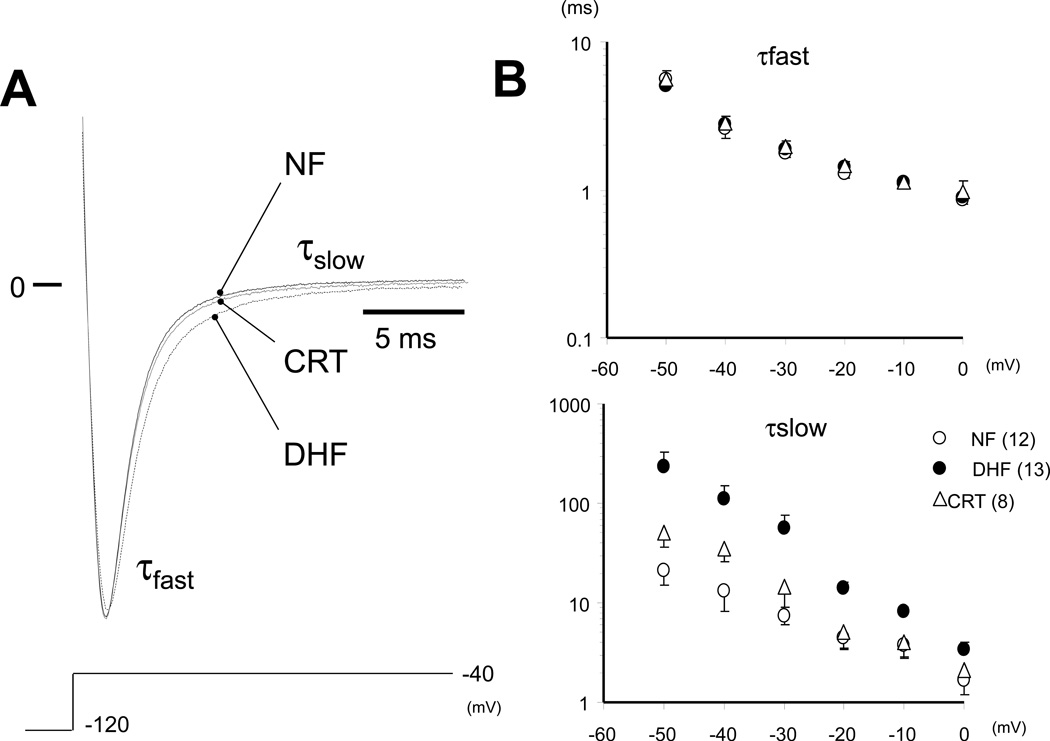
Changes of Decay time constant by DHF and CRT
As shown in Figure 2A, DHF slowed INa decay compared to NF, and CRT normalized the DHF- induced change of INa decay. The initial (τfast) and late (τslow) time constants of INa decay over a range of voltages are shown in Figure 2B and Table 1. There was no significant difference in the τfast between the groups, whereas τslow was significantly increased in DHF compared to NF (13.91 ± 6.80 vs. 4.44 ± 3.62 ms; p<0.05) and CRT normalized the DHF-induced change of INa decay (5.02 ± 4.37 ms). No significant difference was observed in these time constants between ANT and LTR cells.
Figure 2.
CRT hastens the Na current decay slowing induced by DHF. A: Superimposed representative INa in NF, DHF and CRT elicited by 50ms depolarizations to −40 mV (from −120 mV) ([Na+]o= 10mM). The decay phase of the current during a voltage step was fit with a biexponential function of the form: I(t)= Af·exp(−τ/f) + As·exp(−τ/s), where Af and As are fractions of fast and slow inactivating components, respectively. B: Plots of the voltage-dependent change of the initial (τ fast) and late (τ slow) time constant of INa decay. CRT normalized DHF-induced prolongation of τ slow.
Change of late-INa (INa-L ) by DHF and CRT
Figure 3A shows the superimposed persistent INa-L in myocytes from NF, DHF and CRT canine ventricles ([Na+]o= 10mM). INa-L was elicited by 800 ms depolarizations to −20 mV (from −140 mV), and tetrodotoxin (TTX)-sensitive currents were normalized to peak INa. The current integral was calculated between 100 and 500 ms after the depolarizing pulse. As shown in Figure 3B and Table 3, DHF significantly increased INa-L compared to NF (1.33 ± 0.66 % vs. 0.09 ± 0.10 %, p<0.05), but this increase was virtually eliminated in CRT myocytes (0.42 ± 0.21 %, p<0.05 vs. DHF).
Table 3.
Effect of DHF and CRT on the peak and late Na+ current
| [Na+]o = 10 mM | [Na+]o = 140 mM | |||||
|---|---|---|---|---|---|---|
| Peak INa (pA/pF) | n | Late INa (%) | n | Late INa (pA/pF) | n | |
| Non-Failing | −60.3 ± 9.7 | 14 | 0.09 ± 0.10 | 10 | 0.17 ± 0.16 | 8 |
| (ANT) | −60.0 ± 11.7 | 7 | 0.07 ± 0.04 | 5 | 0.17 ± 0.09 | 4 |
| (LTR) | −60.6 ± 8.2 | 7 | 0.10 ± 0.14 | 5 | 0.16 ± 0.13 | 4 |
| DHF | −51.5 ± 16.9 | 16 | 1.32 ± 0.66 † | 10 | 0.82 ± 0.25† | 8 |
| (ANT) | −47.5 ± 21.3 | 8 | 1.34 ± 0.91 | 4 | 0.78 ± 0.18 | 4 |
| (LTR) | −54.3 ± 13.4 | 8 | 1.31 ± 0.53 | 6 | 0.83 ± 0.29 | 4 |
| CRT | −52.8 ± 15.9 | 8 | 0.42 ± 0.21 †# | 8 | 0.29 ± 0.14†# | 8 |
| (ANT) | −53.1 ± 20.4 | 4 | 0.54 ± 0.19 | 4 | 0.27 ± 0.20 | 4 |
| (LTR) | −52.4 ± 14.6 | 4 | 0.26 ± 0.09 | 4 | 0.31 ± 0.16 | 4 |
Mean ± SD
p<0.05 vs. NF,
p<0.05 vs. DHF by ANOVA with Bonferroni test
ANT: anterior, LTR: lateral
We then studied INa-L in physiological bath solution ([Na+]o=140 mM). INa-L was elicited by 2000 ms depolarizations to −40 mV (from −140 mV). INa-L was measured as the average amplitude of the current between 200 and 220 ms. DHF significantly increased INa-L in physiological Na+ conditions (0.16 ± 0.08 to 0.82 ± 0.26 pA/pF; p<0.05 vs. NF), and CRT dramatically reduced the DHF-induced increase of INa-L (0.23 ± 0.16 pA/pF; p<0.05 vs. DHF). To demonstrate the INa-L increase in heart failure, we measured INa in [Na+]o=140 mM at baseline and after ranolazine which is known to block INa-L. Ranolazine (1µM) reduced INa-L in myocytes from DHF (0.82±0.25 to 0.37±0.15 pA/pF; p<0.05) but not in myocytes isolated from NF or CRT hearts (Figure 3C and 3D). There were no regional differences in INa-L in ANT and LTR cells in either [Na+]o= 10mM and 140mM conditions.
APD prolongation and late-INa
Normal impulse formation and conduction depend on the fast inward INa. Also, an increase in INa-L can markedly prolong APD and promote polymorphic VT. Action potential prolongation in DHF is highly arrhythmogenic with frequent EADs that were not observed in myocytes isolated from NF hearts. CRT partially abbreviated the DHF-induced APD prolongation and reduced the frequency of EADs in cells isolated from both the anterior and lateral LV.4, 15 In order to understand the contribution of INa-L to prolongation of the AP in DHF and CRT, we added ranolazine to the bath solution during continuous AP recording. Shown in Figure 4A, ranolazine (1µM) did not change the APD in myocytes from NF, however it significantly shortened APD in cells isolated from DHF hearts (Figure 4B and 4C), consistent with an increase of INa-L having a substantial role in APD prolongation in DHF. Myocytes from CRT hearts had a reduced INa,L density compared with DHF thus ranolazine had less effect on APD and EADs in cells from these hearts compared with DHF (Figure 4D).
CRT has a number of effects on electrical remodeling in the failing heart including reversal of K+ channel down regulation and improvement of SR Ca handling.15 To determine if the changes in INa-L could explain the changes in APD, we used a mathematical model of the canine AP 21, 22. Increasing normalized INa-L from 0.11 to 0.88% of the peak INa reproduced APD prolongation similar to that observed in myocytes from DHF hearts. An intermediate normalized INa-L of 0.35% of peak current shortened the APD compared to DHF, similar to that observed in CRT. The simulations are consistent with changes of INa-L in heart failure contributing to APD prolongation in DHF and its abbreviation by CRT (Figure 4E).
CaMKII
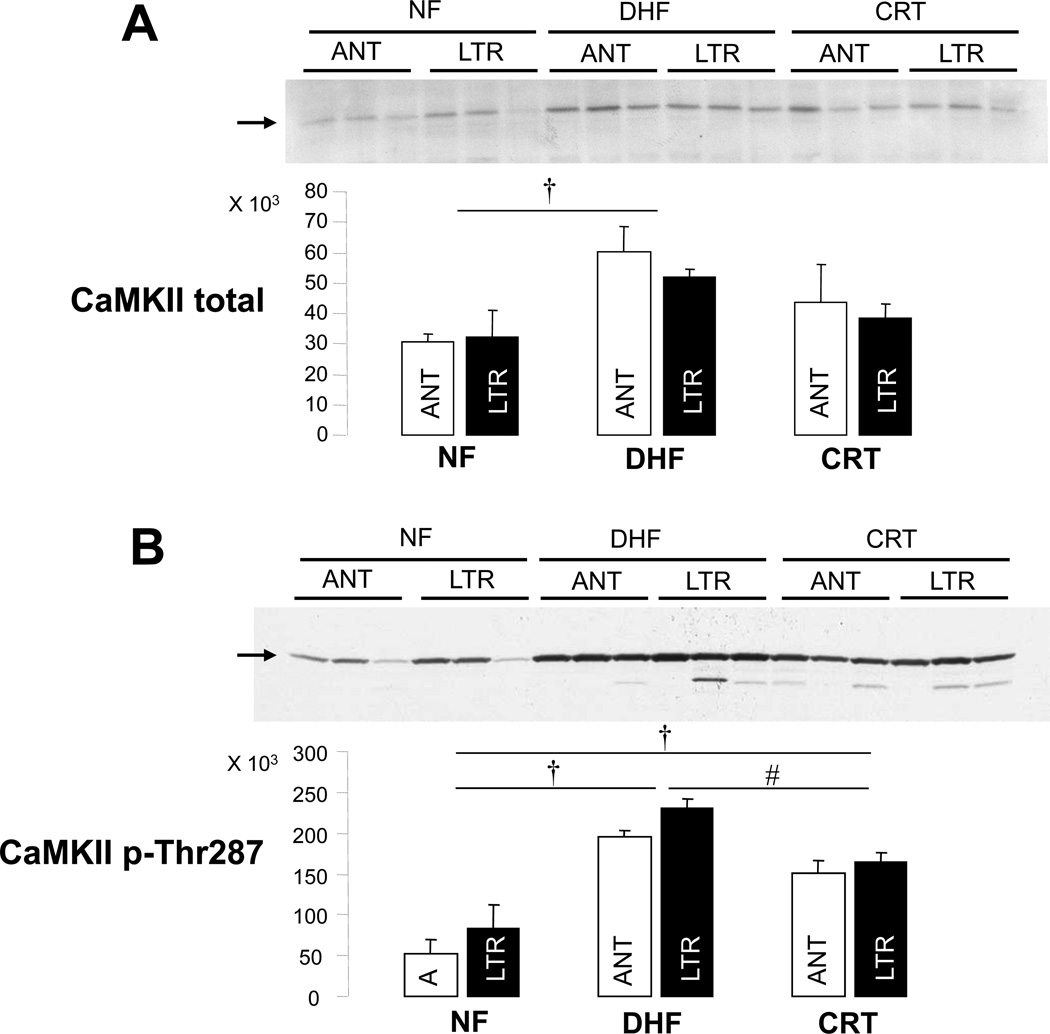
Heart failure is associated with global23 and regional19, 24 increases in calcium (Ca2+) -calmodulin dependent protein kinase II (CaMKII) activity that are partially reversed by CRT.19 CaMKII modulates the function of Na currents producing an increase in INa-L,20, 25 therefore, we propose that changes in CaMKII expression in myocytes are linked to alteration in the Na current phenotype in NF, DHF and CRT myocytes. As shown in Figure 5, DHF increased total and phosphorylated (pThr287) CaMKII compared to NF. Notably CRT significantly reduced the DHF-induced increase of CaMKII pThr287 with a trend in a reduction in total CaMKII. No significant regional differences between ANT and LTR regions of the LV were found in any group.
Figure 5.
CRT partially normalizes the DHF-induced increase of CaMKII. A: Total CaMKII protein expression in NF, DHF and CRT canine ventricle. B: Phosphorylation of CaMKII (p-Thr 287) in NF, DHF and CRT ventricular myocardium. ANT: anterior, LTR: lateral myocardium. † :p<0.05 vs. NF, #:p<0.05 vs. DHF by ANOVA with Bonferroni test.
Discussion
The failing heart is characterized by a number of changes in its electrophysiological function, prominently prolongation of the AP and delayed repolarization. This is attributed to a number of changes in ionic and transporter currents described in heart failure3, 4 and these prominently include changes in the density and gating of the Na+ current.26, 27 We previously reported that resynchronization of mechanical contraction by biventricular pacing of the failing heart, despite persistence of global LV dysfunction, reverses detrimental remodeling of ion channel function and calcium handling.15 In this study we demonstrated that CRT reverses the DHF-induced alterations of the Na+ current and in particular attenuates the increase of INa-L associated with DHF, thus abbreviating the APD and reducing the frequency of EADs. The changes in Na+ channel gating are associated with a reduction in activated, autophosphorylated CaMKII in CRT.
INa remodeling in DHF and CRT
Normal impulse formation and conduction as well APD depend on the inward INa. Studies of INa regulation in heart failure have been somewhat variable and model dependent. In this study we found peak current was not significantly altered, though others have reported down regulation in a similar model.27 However, we did observe slowing of the decay and rise in late current, and this has been observed in a canine myocardial infarction model of heart failure and in failing human hearts.26, 28, 29 An increase in the late component of Na+ current (INa-L) can markedly prolong APD and facilitate the generation of EADs and promote polymorphic VT.30 Therefore, changes in INa density and kinetics may predispose to arrhythmias either by disrupting conduction and/or prolonging repolarization. In this study, the increase of INa-L by DHF prolonged APD, while CRT partially normalized INa-L, and abbreviated the APD.
Ranolazine is an inhibitor of a number of ion channels with relative selectivity for INa-L and antiarrhythmic effects in vitro.31, 32 In an ischemic canine model of HF, ranolazine improved abnormal repolarization and contraction in LV myocytes.33 Consistent with CRT modifying INa-L as key mechanism for electrical reverse remodeling, we found that ranolazine suppresses INa-L and, at a low dose, completely normalized the APD and prevented EADs in acutely isolated DHF myocytes. In cells from NF and CRT hearts with significantly less INa-L, the drug had little effect on the APD. Simulations of the experimentally determined changes in the magnitude of INa-L in a canine AP model for each condition predicted prolongation of the APD in DHF, albeit larger than that observed experimentally. The quantitative contribution of the increase in INa-L to APD is complex, but reproduces the AP prolongation in DHF and the partial reversal of the APD in CRT myocytes. The data, which are consistent with a global reduction in APD and hastening of repolarization by CRT, suggest a mechanism for the antiarrhythmic properties of this pacing therapy. The data do not allow us to speak to the role of ranolazine as an antiarrhythmic therapy in this model since we used a concentration of the drug designed to affect only INa-L and not other changes in the Na current or other currents that are remodeled in heart failure.
CaMKII and Oxidant Stress
Among the many signaling changes in the failing heart, an increase in CaMKII protein and activity has been regularly observed.34 A mechanistic link between the increase in CaMKII activity and INa-L that is observed in human and canine heart failure is suggested by recent studies showing that intracellular CaMKII signaling increases INa-L and Na+ influx in failing myocytes by slowing inactivation kinetics and shifting steady state inactivation.20, 25,35 In our study, consistent with these previous results, DHF is associated with an increase CaMKII activity; moreover, CRT partially normalizes CaMKII activity indexed by a reduction in the autophosphorylated forms of the enzyme (Figure 5), while at the same time reducing the amplitude of INa-L. The data support a critical role of CaMKII and its regulation in the remodeling and reverse electrical remodeling of ventricular myocyte electrophysiology by DHF and CRT respectively.19, 36–38
Oxidative stress is linked to the progression of heart failure, and mitochondria are critical source of ROS in failing myocardium. Blocking INa-L reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction.9, 39 Oxidative stress-induced afterdepolarizations are directly linked to CaMKII activity.40, 41 Furthermore, Kohlhaas et al suggest that increased [Na+]i in heart failure promotes ROS formation by reducing mitochondrial Ca2+ uptake.42, 43 Cardiac resynchronization of the failing heart potently alters the mitochondrial proteome with an associated improvement in mitochondrial function reflected in an increase in the respiratory control index.44 Moreover, DHF is associated with global and region activation of stress-related proteins, and CRT reduces stress kinase activation.19, 24 We did not directly measure ROS in these studies; however, our findings suggest a mechanism of remodeling in DHF characterized by a ROS and CaMKII mediated increase of INa-L, and that is reversed by a reduction in CaMKII activity decreasing INa-L and suppressing EADs in CRT.
Limitations
One limitation in this study is the absence of a direct measurement of oxidant levels in the tissue; however, our recent study demonstrating that resynchronization improved mitochondria oxidative phosphorylation coupling44 suggests that CRT may suppress ROS level in the heart failure. Second, as we have shown previously, CRT improves multiple ion channel and Ca handling abnormalities in DHF15 and the functional effects on cardiac electrophysiology are complex and interrelated. Therefore, we used computer simulations to quantify the effect of reverse remodeling of Na+ current in the context of the changes in other ionic currents and transporters in our models of DHF and CRT. An isolated change in INa-L in the simulated AP is sufficient to dramatically alter the duration consistent with a significant contribution of INa-L to prolongation of AP in the failing heart but is certainly not only change that influences the APD in DHF and CRT.
Clinical Implications
CRT is a remarkable therapy that improves symptoms and survival in a subset of patients with heart failure associated with dyssynchronous mechanical contraction. The mechanical act of resynchronizing contraction by pacing has significant effects on cellular signaling in the failing heart that are associated with not only improved mechanical performance but also enhanced energetic efficiency. This provides an explanation for the mechanism of action of CRT in heart failure and identified pathways that are central to improving cardiac function and which could be approached pharmacologically in patients that do not response to CRT. In addition, a role for INa-L in the AP prolongation associated with DHF and its reversal by CRT in the setting of improved mechanical and electrical performance of the heart suggests a pathogenic role of the late Na+ current in arrhythmias in heart failure that could be addressed not only by CRT, but pharmacological agents that target this current component.
Supplementary Material
Acknowledgments
The authors thank Yanli Tian, Deborah DiSilvestre, and Victoria Halperin for excellent technical support.
Funding Sources: The work was supported by NIH P01 HL 077180 (to Drs. Kass and Tomaselli) and HL 072488 (to Dr. Tomaselli). Gordon Tomaselli is the Michel Mirowski M.D. Professor in Cardiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 4.Kääb S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 5.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 6.Kiyosue T, Arita M. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res. 1989;64:389–397. doi: 10.1161/01.res.64.2.389. [DOI] [PubMed] [Google Scholar]
- 7.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H. Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 10.Ermis C, Seutter R, Zhu AX, Benditt LC, VanHeel L, Sakaguchi S, Lurie KG, Lu F, Benditt DG. Impact of upgrade to cardiac resynchronization therapy on ventricular arrhythmia frequency in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:2258–2263. doi: 10.1016/j.jacc.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 11.Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, Boschat J, Benditt DG, Lurie KG. Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation. 1997;96:3273–3277. doi: 10.1161/01.cir.96.10.3273. [DOI] [PubMed] [Google Scholar]
- 12.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 13.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 14.Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, Hare JM, Kass DA. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 15.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O’Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–1230. doi: 10.1161/CIRCULATIONAHA.108.794834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishijima Y, Sridhar A, Viatchenko-Karpinski S, Shaw C, Bonagura JD, Abraham WT, Joshi MS, Bauer JA, Hamlin RL, Györke S, Feldman DS, Carnes CA. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007;81:1152–1159. doi: 10.1016/j.lfs.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–1763. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 18.Spragg DD, Akar FG, Helm RH, Tunin RS, Tomaselli GF, Kass DA. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67:77–86. doi: 10.1016/j.cardiores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 20.Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O’Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenstein JL, Hinch R, Winslow RL. Mechanisms of excitation-contraction coupling in an integrative model of the cardiac ventricular myocyte. Biophys J. 2006;90:77–91. doi: 10.1529/biophysj.105.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 24.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]
- 25.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maltsev VA, Sabbab HN, Undrovinas AI. Down-regulation of sodium current in chronic heart failure: effect of long-term therapy with carvedilol. Cell Mol Life Sci. 2002;59:1561–1568. doi: 10.1007/s00018-002-8529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Maltsev VA, Sabbah HN, Tanimura M, Lesch M, Goldstein S, Undrovinas AI. Relationship between action potential, contraction-relaxation pattern, and intracellular Ca2+ transient in cardiomyocytes of dogs with chronic heart failure. Cell Mol Life Sci. 1998;54:597–605. doi: 10.1007/s000180050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pu J, Boyden PA. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res. 1997;81:110–119. doi: 10.1161/01.res.81.1.110. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittköpper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of Elevated Ca2+/Calmodulin-Dependent Protein Kinase II Improves Contractility in Human Failing Myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 35.Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294:H1597–H1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saba S, Mehdi H, Mathier MA, Islam MZ, Salama G, London B. Effect of right ventricular versus biventricular pacing on electrical remodeling in the normal heart. Circ Arrhythm Electrophysiol. 2010;3:79–87. doi: 10.1161/CIRCEP.109.889741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xi Y, Wu G, Yang L, Han K, Du Y, Wang T, Lei X, Bai X, Ma A. Increased late sodium currents are related to transcription of neuronal isoforms in a pressure-overload model. Eur J Heart Fail. 2009;11:749–757. doi: 10.1093/eurjhf/hfp092. [DOI] [PubMed] [Google Scholar]
- 38.Shang LL, Pfahnl AE, Sanyal S, Jiao Z, Allen J, Banach K, Fahrenbach J, Weiss D, Taylor WR, Zafari AM, Dudley SC., Jr Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101:1146–1154. doi: 10.1161/CIRCRESAHA.107.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma JH, Luo AT, Zhang PH. Effect of hydrogen peroxide on persistent sodium current in guinea pig ventricular myocytes. Acta Pharmacol Sin. 2005;26:828–834. doi: 10.1111/j.1745-7254.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 40.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O’Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, Van Eyk JE. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3:78–87. doi: 10.1161/CIRCGENETICS.109.871236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.