Abstract
Thirty years ago, glycerolipids captured the attention of biochemical researchers as novel cellular signaling entities. We now recognize that these biomolecules occupy signaling nodes critical to a number of physiological and pathological processes. Thus, glycerolipid-metabolizing enzymes present attractive targets for new therapies. A number of fields—ranging from neuroscience and cancer to diabetes and obesity—have elucidated the signaling properties of glycerolipids. The biochemical literature teems with newly emerging small molecule inhibitors capable of manipulating glycerolipid metabolism and signaling. This ever-expanding pool of chemical modulators appears daunting to those interested in exploiting glycerolipid-signaling pathways in their model system of choice. This review distills the current body of literature surrounding glycerolipid metabolism into a more approachable format, facilitating the application of small molecule inhibitors to novel systems.
Keywords: glycerolipids, lipase, metabolism, inhibitors, phospholipases, phospholipase C, phospholipase D, phospholipase A, autotaxin, lipid kinases, diacylglycerol kinase, PI3-kinase, monoacylglycerol lipase, diacylglycerol lipase, triacylglycerol lipase, hormone-sensitive lipase, adipose triglyceride lipase, CGI-58, PNPLA3, Lipin, phosphatidic acid phosphatase, fatty acyltransferase, glycerol-3-phosphate acyltransferase, lysophosphatidic acid acyltransferase, diacylglycerol acyltransferase
1. Introduction
With advances in mass spectrometry-based analysis of lipids the landscape of lipid biomolecule research has significantly broadened. Glycerol-based lipids are continually found playing prominent roles in human physiology and disease: from fat storage and metabolic disorders to survival pathways in cancer. As research efforts identified these core signaling nodes, chemical tools capable of interrupting lipid metabolism became essential tools for in depth characterization both in vitro and in vivo. Thus, the scientific literature is replete with studies on the medicinal chemistry and pharmacology associated with these inhibitors. This review focuses on the enzymes regulating the most critical biosynthetic steps of glycerolipids—for either signaling or fat storage—and distills the current body of literature to the most relevant and well-characterized inhibitors. Table 1 highlights these important tools and in this way serves as a summary of this review.
Table 1.
Tools for lipid signaling modulation
| Target | Compound Name | Isoform specificity | Mode of Inhibition | Shown In vivo efficacy | References |
|---|---|---|---|---|---|
| Phospholipase C | U73122 | unknown | Indirect | [21] | [19, 20] |
| 3013 | PI-PLC-β3,γ1,δ1 | Direct | ND | [24] | |
| 3017 | PI-PLC-β3,γ1,δ1 | Direct | ND | [24] | |
| Phospholipase D | Raloxifene | PLD1/2 | Direct | Yes – for ER | [77] |
| FIPI | PLD1/2 | Direct | [83] | [78] | |
| VU0359595 | PLD1 | Direct | ND | [85] | |
| VU0364739 | PLD2 | Direct | ND | [86] | |
| Phospholipase A | Varespladib methyl | sPLA2 | Direct active site | Yes [102] | [101] |
| Ecopladib | cPLA2 | Direct | Yes [100] | [103] | |
| Giripladib | cPLA2 | Direct | Yes [100] | [105] | |
| FKGK11 | iPLA2 | [107] | |||
| Darapladib | Lp-PLA1 | Direct | Yes [111] | [110] | |
| Autotaxin | S32826 | pan | Direct | ND | [125] |
| PF-8380 | [128] | [128] | |||
| HA130 | Direct: active site | ND | [129] | ||
| DAG Kinase | R59022 | Type I | allosteric | ND | [153] |
| PI3 Kinase | LY294002 | Pan | Direct: ATP competitive | ND | [163] |
| wortmannin | pan | Covalent irreversible | ND | [164] | |
| MAG Lipase | JZL-184 | Irreversible catalytic site inhibitor | Yes [182] | [182] | |
| JJKK048 | Irreversible catalytic site inhibitor | ND | [184] | ||
| DAG Lipase | KT172 | DAGLβ | Irreversible catalytic site inhibitor | Yes [189] | [189] |
| KT109 | DAGLβ | Yes [189] | [189] | ||
| Hormone Sensitive Lipase | Aventis 7600 | Reversible; transient inhibitor | ND | [225] | |
| CAY 10499 | [231] | ||||
| ATG Lipase | Atglistatin | Direct | Yes [249] | [249] | |
| GPAT | FSG67 | 1 and 2 | Substrate mimic | Yes [304] | [303] |
| LPAAT | CT-32228 | LPAAT-β | Direct binding | Yes [314] | [314] |
| DGAT | Niacin | DGAT2 | Noncompetitive | [341] | |
| AZD7687 | DGAT1 | Direct | Yes [347] | [347] | |
| LCQ-908 | DGAT1 | Direct | Yes [359] | [359] |
ND: Not determined, ER: Estrogen Receptor
Several of the targets discussed herein, have a long history in the literature, stretching as far back as the early 1900s in some cases. This review does not detail their long, complex history of biochemical and molecular characterization and will direct the reader to more comprehensive reviews where necessary. Rather, we hope it serves as an accessible, practical body of information for those unfamiliar with the medicinal chemistry efforts undertaken over the years. It is with this audience in mind that we highlight not only the capabilities of these small molecule inhibitors, but their limitations as well. In this way, we envision this review serving as a resource for the design and implementation of novel experiments, regardless of one’s specific field of study or technical expertise.
2. Phospholipases
2.1. Phospholipase C
2.1.1. Enzyme activity and regulation
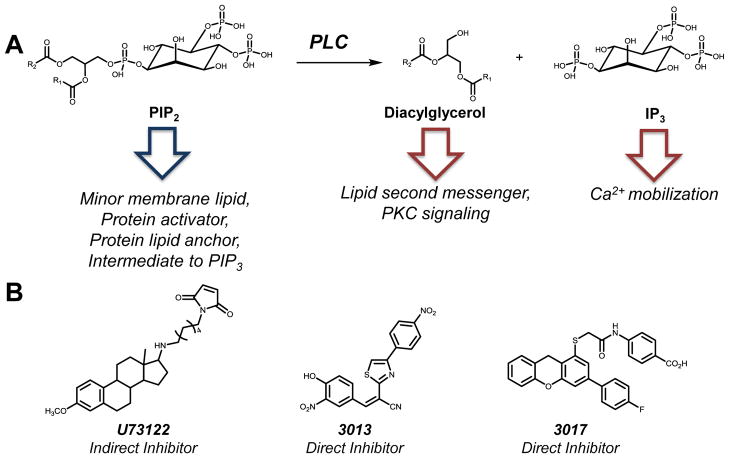
Phospholipase C (PLC) enzymes cleave phospholipids and produce diacylglycerol and the corresponding phospho- head group. Substrate specificity for either phosphatidylinositol-PLC (PI-PLC) or phosphatidylcholine-PLC (PC-PLC) defines the two main classes of PLCs. The cytosolic PI-PLC is the most well characterized class of PLC and localizes to the plasma membrane upon activation where it catalyzes the conversion of the minor membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2 or PIP2) into the lipid second messengers inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) (Fig. 1A). Both IP3 and DAG serve as signaling molecules for Ca2+ mobilization or protein kinase activation, respectively. These two signaling molecules are exceptionally versatile and control distinct signaling pathways, making them responsible for dozens of cellular processes [1, 2]. Cells tightly regulate PIP2 depletion due to its role in protein activation at the membrane. One important example is a minor form of phosphorylated PIP2, phosphatidylinositol 3,4,5-trisphosphate (PIP3), which controls crucial signaling cascades via the phosphoinositide 3 kinase (PI3K) pathway [3].
Figure 1.
Molecular trafficking and small molecule inhibitors of phospholipase C (PLC). A. The catalytic activity of PLC regulates cellular concentrations of PIP2, DAG and IP3. PIP2 is a minor component of cellular membranes but servers as an anchor for protein recruitment and subsequent activation. PLC degrades PIP2 into DAG, a second messenger at PKC, and IP3, which stimulates Ca2+ release. PLCs regulation of these three signaling nodes makes it an important drug target. B. U73122 is a modest inhibitor of PI-PLC and the most widely used, although has never been identified as a direct inhibitor of enzymatic activity. 3013 and 3017 are more recently identified mid-micromolar inhibitors of PI-PLC and empirical evidence suggests they directly inhibit PLC catalytic activity.
Mammals have 13 different PI-PLC enzymes subdivided into six different enzyme families: β,γ,δ,ε,ζ and η; each family is characterized by its unique mechanism of regulation and localization. Most major signaling events sit upstream of distinct PI-PLC isozymes. Growth factors, antigens and other extracellular stimuli activate PLCγ; extracellular stimuli, hormones, neurotransmitters, and chemosensory molecules activate PLCβ via heterotrimeric G-proteins [4]. Additionally, PLCε is activated downstream of Ras signaling affording this family of enzymes a unique role in cellular communication and signal transduction [5].
PI-PLC enzymes are highly conserved across phyla—bacteria, flies, and mammals all express PI-PLC isozymes. While the overall core structure of the PI-PLCs shows little variance between families, they share very little sequence homology. All family members contain pleckstrin homology domains (PH) (except PLCζ), EF hand motifs, X and Y domains, and a C2 domain [6]. Each of these core domains have important regulatory and catalytic functions for PLC [6]. PH domains mediate membrane recruitment and facilitate binding to both PI and PIP2. EF hand motifs bind Ca2+ ions, required for enzyme activity. X and Y domains dimerize, forming a triosephosphate isomerase (TIM) barrel, with the catalytic residues on the X portion of the TIM barrel. Finally, C2 domains, also essential for Ca2+ activation and anionic lipid binding, are found in repeating units of either 2 or 4 on the PI-PLCs, depending on the isoform. Other PI-PLC isoforms may contain more specialized regions, such as a Ras-GEF in PI-PLCε and PDZ-motifs found in β and η isoforms, believed to scaffold large protein complexes following G-protein coupled receptor (GPCR) activation [6].
Each isozyme class has unique signaling roles and tissue distribution. The β isoforms rely on Ca2+ release downstream of GPCR signaling for activation. Certain β isozymes actually serve as GTPase-activating-proteins, or GAPs, for Gα, which in turn activates other PLC isozymes [7]. PLCβ isoforms often localize to the nucleus but are also found in the cytosol. PLCβ1−/− animals have ocular and central nervous system (CNS) developmental deficiencies suggesting a critical role for PLCβ in the CNS [6]. Members of the PLCγ family are activated by receptor tyrosine kinases and cellular tyrosine kinases making them critical players in cell proliferation, and their expression in T and B lymphocytes suggests a role in immunity [8]. Ca2+ or Gαi/o and Gαq activate PLCδs and play roles in Alzheimer’s disease [9], fertilization [10], and balding [11]. Mammalian expression of PLCζ in sperm heads facilitates fertilization [2]. PLCη is expressed in the brain and neurons, resembles PLCδ in structure, sits downstream of several GPCRs and is activated by Ca2+ [12].
The largest and most broadly distributed PLC isoform, PLCε, interacts with Ras family member GTPases and functions as a guanine nucleotide exchange factor (GEF) [13]. Due to its close relationship with Ras family proteins, PLCε regulates cell proliferation, migration, signaling and disease processes such as those observed in cardiac hypertrophy [14]. Heterotrimeric G-proteins also activate PLCε following GPCR stimulation. The therapeutic potential for new compounds that modulate PLCs is considerable. For example, a recent report by Smrcka and colleagues [14] on the protective role played by PLCε in cardiac myocytes during stress-induced pathological hypertrophy has reinvigorated interest in the development of isoenzyme-selective modulators of PLCs.
PI-PLC cleaves PIP2 into the critical second messengers IP3 and DAG, distinguishing this protein family from other PLC isoforms. The varied tissue distribution, activation, and regulation of this enzyme suggest that it facilitates essential signal transduction cascades required for proper cellular function.
In addition to the PI-PLC enzyme family, a separate, phosphatidylcholine-specific Phospholipase C (PC-PLC), hydrolyzes PC to form phosphocholine and DAG [15]. PC-PLC was identified and cloned in bacteria but not yet identified in mammals. Evidence suggests that PC-PLC may rely on growth factors, cytokines, mitogens, and calcium for activation. These observations correlate with its implication in many cellular processes including proliferation, differentiation, metabolism, senescence, and apoptosis [16]. PC-PLC is likely responsible for elevation of ceramide production via DAG release resulting in macrophage activation [17]. In addition to its myriad of signaling roles, mounting evidence implicates PC-PLC activity in the expression of essential growth factor receptors like HER2 [18].
2.1.2. Tools for modulation of enzymatic activity
The integral role PLC isoforms play in key survival events makes them ideal targets for the development of future therapeutics. To better understand PLC as a therapeutic target, small molecule modulators of PLC catalytic activity were developed for further investigation.
The compound most often used in the literature to manipulate the PI-PLC isozymes is U73122 (Fig. 1B and Table 1). This sterol mimetic inhibits PI-PLC-dependent processes in human platelets and neutrophils albeit with only modest potency (IC50 values for collagen or thrombin induced platelet aggregation were 0.6 μM and 5 μM respectively) [19, 20]. Since its initial publication, more than 1,000 scientific papers utilize U73122 as a PI-PLC inhibitor, even showing efficacy in vivo [21]. In spite of its wide use, no evidence demonstrates U73122 as a direct modulator of enzyme activity. Several reports have detailed non-specific effects of U73122 on targets other than PLC; one study even suggests the compound is capable of stimulating certain PLC isoforms when used at micromolar concentrations [22]. Literature calls into question its isoform specificity and most reports refer to the compound as a pan-PLC inhibitor. However, some suggest it preferentially inhibits the β isoforms [6]. The indirect inhibition of PLC activity by U73122 should be carefully considered during experimental design.
Another less frequently employed PI-PLC inhibitor is the ether lysolipid mimic Edelfosine (ET-18-OCH3) [23]. Edelfosine directly inhibits the ability of PI-PLC to bind its PIP2 substrate in a mixed micelle. At high concentrations, Edelfosine inhibited other phospholipases (PC-PLC and PLD), likely an artifact of cellular toxicity rather than specific enzyme inhibition. Ether lysolipids, like Edelfosine have well documented cellular toxicity, which should be considered prior to its use in model systems [23].
A recent study by Zheng and colleagues yielded novel small molecule inhibitors of PLC following a high-throughput screen of over 6,000 compounds. Three direct inhibitors of PI-PLC were identified (ATA, 3013, and 3017) which inhibited the three isozymes tested (PLCβ3, γ1 and δ1), suggesting they may be pan-PLC inhibitors [24]. The authors verified the cellular activity of these three novel compounds by following IP3 accumulation upon receptor activation in intact cells. Importantly, ATA was less active than the other compounds in cellular assays due to poor cell penetration. While these compounds are direct and seem to inhibit multiple PLC isozymes, they only possess modest potency with reported IC50 values around 10 μM [24].
PC-PLC inhibitors are less common in the literature. The xanthate D609 was initially investigated for its antiviral properties [25]. Later studies documented D609 preferentially inhibited PC-PLC over PI-PLC enzymes but also inhibited sphingomyelin synthase (SMS) [16]. Initial reports suggested that this compound works through a competitive mode of inhibition [26]. Both in vitro and in vivo studies demonstrated that D609 inhibits cell cycle progression and arrests proliferation either through PC-PLC or SMS inhibition. These cellular activities may be the result of an overlapping set of one or more enzymes. The exact inhibitory target of D609 remains unknown, making the mechanism unclear [27]. In spite of its nebulous activity, D609 holds promise in cancer, artherosclerosis, and cerebral infarction studies, which may be the result of activity at multiple non-specific targets[16].
2.2. Phospholipase D
2.2.1. Enzyme activity and regulation
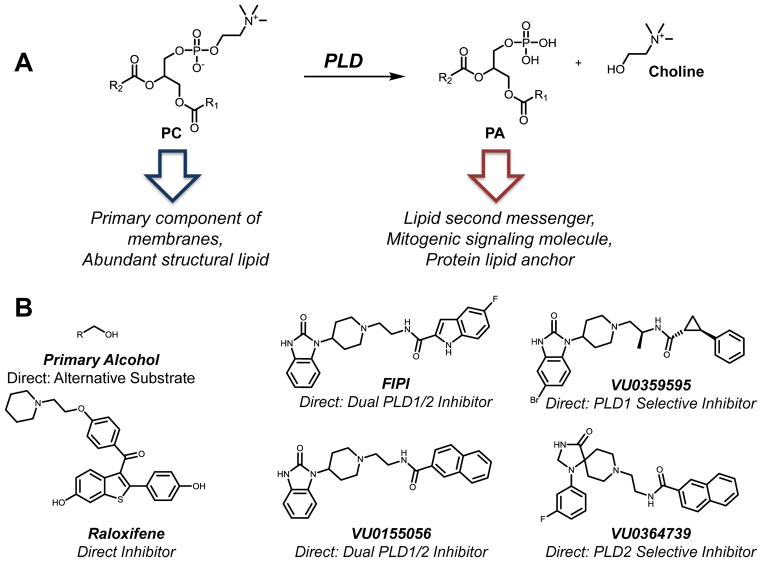
Phospholipase D (PLD) is a phosphodiesterase responsible for the hydrolysis of the cellular membrane lipid phosphatidylcholine (PC) into the lipid second messenger phosphatidic acid (PA) and free choline (Fig. 2A). PLD activity was first described in plants in the 1940s [28, 29] but PLD superfamily members have since been found in bacteria, viruses, yeast, worms, and mammals (for review [30, 31]). Members of the PLD superfamily often contain a conserved catalytic HKD motif (HxKxxxxDx6GSxN) [32] although there are non-HKD enzymes in this family as well. This review focuses on the conventional mammalian enzymes.
Figure 2.
Molecular trafficking and small molecule inhibitors of phospholipase D (PLD). A. PLD hydrolyzes phosphatidic esters, like phosphatidylcholine (PC), yielding phosphatidic acid (PA) and choline. PC is the primary component of cellular membranes while the PA is a vital second messenger within the cell regulating a number of downstream targets. Since PC is readily available within the cell, PLD plays a central role in rapid production of signaling PA. B. Small molecule inhibitors of PLD. Structural analogues of halopemide such as FIPI, VU0359595, VU0155056, and VU0364739 are potent, direct, and isoform-specific inhibitors of PLD activity while SERMs such as raloxifene are much less potent but provide a second structural scaffold for PLD inhibitor development. Primary alcohols limit PA production by PLD by competing with water as an enzymatic substrate and are thus not accurately described as PLD inhibitors.
Mammalian cells contain two major PLD isozymes, PLD1 and PLD2, each of which exhibit differences in localization, activity, and regulation [33]. PLD sits downstream of G-protein coupled receptors and receptor tyrosine kinases. Thus, cellular PLD activity responds to growth factor or hormone stimulation. The most commonly studied PLD-activating receptors are those within the immune system (e.g., IL-1, IL-8, Fcγ, fMLP and TLRs)[34], and those associated with uncontrolled cell growth (specifically growth factor receptors, EGFR [35] and PDGFR [36]).
Mammalian PLD1 and PLD2 isozymes are splice variants with 50% shared sequence homology. Both isoforms have four conserved domains which include Phox homology (PX) and Pleckstrin homology (PH) domains near the N-terminus and two HKD catalytic domains [30]. The PX domain contains anionic lipid binding sites that are known to bind phosphoinositides (PI(3,4,5)P3) which anchor PLD to the membrane [37]. This region may also facilitate protein-protein interactions. PH domains bind phosphoinositides and interact with small G-proteins such as Arf and Rho [38]. Some studies demonstrate that small G-proteins activate truncated PLD1 lacking the PH domain, suggesting an alternative recognition site [39]. Phosphoinositides, specifically PIP2 (PI(4,5)P2), are essential lipid cofactors required for PLD activity, which also bind the loop region of the enzyme between the two HKD catalytic motifs to further stimulate enzymatic activity [30].
PLD1 and PLD2 localize to distinct cellular compartments and possess different modes of regulation. The PLD1 isoform localizes to intracellular membranes such as the Golgi, early endosomes, or perinuclear vesicles. Upon stimulation, PLD1 translocates to late endosomes and the plasma membrane [40, 41] although this translocation event is cell-, expression-, and stimulation-dependent. Conversely, PLD2 constitutively binds to the plasma membrane [42]. Both PLD1 and PLD2 are palmitoylated and contain Ser/Thr phosphorylation sites which are not required for catalytic activity but facilitate membrane association [33].
In addition to differential subcellular localization, PLD1 and PLD2 have unique modes of regulation. PLD1 activity is robustly stimulated by Arf GTPases [43, 44], which only moderately increase PLD2 activity [43, 45]. Small G-proteins including those of the Rho family activate PLD1 catalytic activity [39, 46, 47]. Protein kinase C (PKC) is also a very well characterized activator of PLD. Phorbol esters, PMA, directly activate PKC and subsequently PLD (reviewed in [48]). PLD2 is less sensitive to protein stimulators and is constitutively active in cells.
The PLD catalytic product, PA, has a wide range of roles in many critical cellular functions including cell survival, proliferation, vesicular trafficking, and cytoskeletal reorganization. Because PLD sits downstream of several important signaling cascades, PA likely regulates the progression of specific disease states. Studies implicate PLD and PA in the progression of diabetes [49, 50], chronic inflammation [51], Alzheimer’s disease [52, 53], and several different types of cancer including gastric [54], breast [55, 56], colorectal [57], brain [58], and renal [59]. Thus, many recent reports suggested that small molecule inhibitors of PLD function are important compounds for therapeutic development [51, 60–62]. In light of the possible role of PLD as a therapeutic target, intense medicinal chemistry efforts focus on the development of modulators or inhibitors for this enzyme.
2.2.2. Tools for modulation of enzymatic activity
2.2.2.1. Alternative substrates
First identified in the 1960s, primary alcohols inhibit the ability of PLD to make the lipid product PA (Fig. 2B) [63, 64]. The PLD catalytic mechanism relies on water acting as a nucleophile in the cleavage of the phosphodiester bond and producing PA. In the presence of primary alcohols, such as butanol, the PLD enzymes produce phosphatidylbutanol (PtdBuOH) as an alternative product. PtdBuOH is a metabolically stable compound in the cell [65, 66] unlike PA, which is rapidly converted to other cellular lipids including diacylglycerol (DAG) and lysophosphatidic acid (LPA). However, native cellular metabolic pathways cannot recycle PtdBuOH like PA. Thus, referring to primary alcohols as PLD “inhibitors” is not technically accurate. Using primary alcohols as PLD inhibitor may elicit numerous cellular-signaling events and promote membrane instability; therefore the ramifications of using primary alcohols as PLD inhibitors should be carefully considered in experimental design and interpretation.
2.2.2.2. Indirect Inhibitors
Early PLD inhibitors relied on limiting enzyme activation rather than directly inhibiting catalytic activity. Several examples of these indirect PLD inhibitors are found throughout the literature. Calphostin C broadly inhibits protein kinases, like PKC (IC50 of 50 nM) [67], and subsequent PLD activation in intact cellular systems [68]. Resveratrol [69] and the closely related diethylstilbestrol [70] were both implicated as PLD inhibitors in human neutrophils, but direct catalytic inhibition of PLD itself was never shown. Honokiol, a natural product isolated from Magnolia grandiflora, inhibited PLD activity at micromolar concentrations in MDA-MB-231 cancer cell lines although not on purified enzymes, suggesting an indirect mechanism of action [71]. SCH420789 and SCH49210, novel fungal metabolites, displayed anti-PLD activity in intact cells, but displayed no evidence of direct PLD inhibition [72]. Taken together, these compounds do not work directly on PLD catalytic activity, but rather take advantage of the broad regulatory network upstream of these enzymes [73, 74]. Careful consideration should be made during experimental design and interpretation.
2.2.2.3. Direct Inhibition
Lipid mimics
Early PLD inhibitors also relied on alternative enzymatic substrates as a mode of enzyme inhibition. A lipid mimetic considered a direct inhibitor of PLD is (Z)-PSDP, the (Z)-isomer of presqualene diphosphate. This isoprenyl phosphate mimetic displayed inhibitory activity for both a bacterial PLD (Streptomyces chromofuscus PLD; scPLD), and the mammalian PLD1b isoform [75, 76]. A small structure activity relationship (SAR) study conducted around this compound found that both the isoprenoid chain and the diphosphate moiety are essential for enzyme inhibition. Data gathered in this study further suggested that (Z)-PSDP directly inhibited the mammalian enzyme, as inhibition was observed in partially purified membranes and intact cells (human neutrophils). The binding site of these analogues remains unknown.
Selective Estrogen Receptor Modulators
Eisen and Brown published a report identifying a class of selective estrogen receptor modulators (SERMs) as PLD inhibitors [77]. SERMs constitute an ideal class of lead compounds due to their extensive pharmacokinetic characterization. The most widely used SERM, raloxifene, inhibits PLD in whole cells and recombinant PLD1 and PLD2 protein with an IC50 value of approximately 4 μM on purified enzymes (Fig. 2B). Tamoxifen and 4-hydroxytamoxifen, another class of SERMs, were also assessed for their ability to modulate PLD activity. Tamoxifen stimulates PLD1 and PLD2, while 4-hydroxytamoxifen stimulated PLD1 and simultaneously inhibited PLD2 [77]. The molecular specifics of this interaction have yet to be fully defined.
Halopemide analogues
Identified as a PLD2 inhibitor through high throughput screening in 2007 [78], halopemide was initially developed by Janssen as a psychotropic agent for neuroscience applications in the late 1970s [79]. Monovich and colleagues detailed the synthesis and screening of 13 halopemide analogues as PLD2 inhibitors. This initial screen identified compound 4k [78] (later renamed FIPI), which inhibited PLD2 with an IC50 value of 20 nM using an unspecified in vitro assay (Fig. 2B and Table 1). While the authors detailed PLD2 inhibition in this initial publication, there was no mention of PLD1. Further characterization of FIPI revealed its ability to inhibit PLD1 and PLD2 both on purified enzymes and in intact cells [80]. Importantly, FIPI’s inhibition of PLD catalytic activity translates directly to decreases in cellular phenotypes regulated by PLD including PA production, cell spreading, chemotaxis [80], vesicle transport [81], and platelet aggregation [82]. Importantly, FIPI also inhibits PLD activity in vivo [83].
Following the initial report of halopemide as a PLD inhibitor, our labs published a series of subsequent studies exploring the PLD inhibitor activity associated with novel halopemide analogues. Our initial report used halopemide as a lead compound, developing a series of dual and isoform selective PLD inhibitors with a combinatorial library synthesis approach [84]. We identified a dual PLD1/2 compound (VU0155056, IC50 = 21 and 380 nM against cellular PLD1 and PLD2 respectively) (Fig. 2B) and modestly selective PLD1- (VU0155069, IC50 of 11 and 1800 nM against cellular PLD1 and PLD2) and PLD2-preferring compounds (VU0155072, IC50 of 1000 and 110 nM against cellular PLD1 and PLD2). These molecules directly inhibit PLD function in an in vitro assay using partially purified enzymes as well as in intact cells. Data suggests that the mechanism of inhibition occurs via an allosteric binding site on the enzyme rather than at the catalytic site. Our subsequent studies focused on structural modifications to increase target isoform selectivity; halogenation and chiral substitutions identified a highly selective inhibitor (VU0359595) (Fig. 2B and Table 1) with a 1700-fold preference for PLD1 over PLD2 with nanomolar potency (IC50=3.5 nM) at the target [85].
The development of a PLD2-preferring compound proved more difficult, requiring significant structural changes from lead compounds identified by our labs. Lavieri et al. reported the most potent and selective PLD2-preferring compound, VU0364739, which displays 75-fold selectivity for PLD2 over PLD1 with nanomolar potency (PLD2 IC50 of 20 nM)(Fig. 2B) [86]. Since this study, we identified PLD2-preferring compounds with lower PLD2 potency and minimal PLD1 activity; these compounds also have improved overall ancillary pharmacology (VU0285655 and ML298) [87, 88]. Our current studies focus primarily on improving the drug metabolism and pharmacokinetic properties of PLD inhibitors, enhancing overall dual isoform potency, and achieving better PLD2 selectivity [88].
Interest in PLD inhibition grew rapidly in the past few years. Currently, several direct small molecule inhibitors of PLD are commercially available, which are useful tools for the exploration of PLD’s role in disease models.
2.3. Phospholipase A
2.3.1. Enzyme activity and regulation
Phospholipase A enzymes hydrolyze the ester bonds of phospholipids at either the sn-1 (PLA1) or sn-2 position (PLA2) to generate lysophospholipids and free fatty acids. Organisms ranging from fungi to mammals display PLA1 enzymatic activity, which is a prominent component of venoms. However, the physiological relevance of PLA1 enzymes remains largely uncharacterized [89]. Currently there are nine PLA1 isozymes known in mammals, six extracellular and three intracellular, with no shared sequence homology between them [89]. Members of the PLA1 family exhibit broad substrate specificity, hydrolyzing lipids such as triacylglycerol, galactolipids, and phospholipids. Other PLA1 proteins have very specific substrate specificity and hydrolyze only phosphatidylserine (PS-PLA1) or phosphatidic acid (PA-PLA1). General inhibitors of this lipid enzyme class are not widely represented in the literature due in part to the large variation in family members. The literature is replete with reviews of PLA1-specific inhibitors [89, 90].
Phospholipase A2 catalyzes the hydrolysis of phospholipids at the sn-2 position, generating free fatty acids and lysophospholipids. Both of these products are important second messengers for cellular processes. The PLA2 class is large, containing more than 30 enzymes with PLA2 activity [91]. Thus, PLA2 enzymes are subcategorized into four major subclasses based on mechanism, localization, and structure: the secreted sPLA2, the cytosolic cPLA2, the Ca2+-independent iPLA2, and the lipoprotein associated Lp-PLA2 [92].
Secreted PLA2s were first identified in the literature in the 1890s as a component of cobra venom. All sPLA2 enzymes share general characteristics including an active site histidine, low molecular weight (13–15 kDa), Ca2+-required catalysis, and six conserved disulfide bonds [93]. Mammals express ten classes of sPLA2 that primarily function as antimicrobial agents, hydrolyzing negatively charged gram negative bacteria. In addition, these enzymes are implicated in arachidonic acid production and eicosanoid biosynthesis during inflammatory responses although this role is still debated [94]. While little is known about sn-2 fatty acyl chain specificity for sPLA2s, the enzymes prefer anionic phospholipids such as phosphatidylglycerol (PG), phosphatidylserine (PS), and phosphatidylethanolamine (PE) [95]. However, specific members of the sPLA2 family are more active against the cationic PC. The role of sPLA2s in the hydrolysis of PC may be relevant in the progression of atherosclerosis, as PC is the main phospholipid found in low density lipoprotein (LDL) particles [96].
Cytosolic PLA2s were identified in the late 1980s from human platelets and neutrophils [95]. There are six members of the cPLA2 family (α, β, γ, δ, ε, and ζ) which are structurally larger than the sPLA2s and do not possess the highly conserved disulfide bond network [93]. These enzymes contain a Ser/Asp catalytic dyad and are mechanistically serine acyltransferases, a large enzymatic family. While cPLA2s have broad substrate specificity they prefer arachidonic acid (AA) containing lipids. The arachidonate preference of these enzymes makes them key mediators of inflammatory responses [97], since AA is the essential fatty acid in prostaglandin biosynthesis. Cyclooxygenase (COX) enzymes convert AA released by cPLA2 into the core prostaglandin scaffold and are a key step in the inflammatory pathway [98].
There are six different types of Ca2+-independent cytosolic enzymes (iPLA2s): β, γ, δ, ε, ζ and η. These enzymes, also known as patatin-like phospholipase domain-containing lipases (PNPLA 9,8,6,3,2 &4) [91], rely on a characteristic Ser/Asp dyad to catalyze lipid hydrolysis. Mammalian iPLA2s are critical to lipid metabolism as they hydrolyze a broad array of lipids including triglycerides, phospholipids, and retinol esters [91].
Lipoprotein-associated PLA2s (Lp-PLA2s) centrally regulate lipid metabolism and inflammation. Lp-PLA2 circulates with lipoprotein particles, localizing onto arterial walls alongside low-density lipoprotein. Lp-PLA2 catalyzes the release of lysophosphatidylcholine and oxidized free fatty acids, promoting a local inflammatory response. Due to the importance of inflammation in atherosclerosis, Lp-PLA2 is recognized as a key therapeutic target in the treatment of cardiovascular disease [99].
2.3.2. Tools for modulation of enzymatic activity
Small molecule therapy development programs target PLA2 enzymes due to their central role in lipid metabolism and involvement in a host of physiological and disease processes. As such, the literature is replete with reviews on inhibitors of the PLA2 enzymes [95, 100]. Small molecule modulators of PLA2 range from phospholipid analogues, to natural products and peptide mimetics. Each subclass of PLA2 enzymes, secreted, cytosolic, Ca2+ independent, and lipoprotein-associated, has unique classes of chemical inhibitors. Due to the breadth and structural diversity of this enzymatic class and the sheer number of available inhibitors, we refer interested readers to excellent review articles from Dennis et al. [95] and Magrioti and Kokotos [100]. For the purposes of this review, we only highlight a select number of compounds.
Secreted PLA2s involvement in inflammatory responses established their therapeutic potential. For years, small molecules targeted the activity of this enzyme class. Lilly Research Laboratories developed the potent and selective sPLA2 inhibitor Varespladib (LY315920) with a low nanomolar IC50 value against recombinant human enzyme (9 nM against hGIIA PLA2) [101]. This commercially available compound targets the catalytic site of the enzyme and is selective over cPLA2s. The Lilly group later modified Varespladib to increase cell permeability through the addition of a methyl ester, which is rapidly hydrolyzed once inside the cell. Varespladib methyl was taken on to clinical trials for the treatment of severe sepsis but was terminated in phase II due to underperformance. However, Varespladib methyl remains in phase III clinical trials for the treatment of cardiovascular disease [95, 102]. Other small molecules commercially available for the inhibition of secreted PLA2 are reviewed elsewhere [95, 100].
The exploration of cytosolic PLA2 inhibitors as novel pharmaceutical agents for inflammatory diseases led to the generation of several potent and selective small molecules. Wyeth developed several submicromolar indole-based cPLA2 inhibitors including Ecopladib [103], Efipladib [104], and Giripladib [105]. Giripladib advanced to clinical trials for the treatment of osteoarthritis but terminated early because it could not improve upon the standard of care [100]. A recent study using Giripladib (PLA-695) showed that the compound sensitizes non-small cell lung cancer xenograft tumors in mice to radiation treatment and inhibits angiogenesis [106].
The pool of small molecules targeting the Ca2+-insensitive class of PLA2 enzymes is very limited. Lipid or peptide-like inhibitors of iPLA2 reported to be direct, reversible and specific showed poor pharmacokinetic profiles in vivo. Thus far the best iPLA2 inhibitors are the polyfluoro ketones, including the commercially available FKGK11, which directly inhibits iPLA2 activity [107]. Additional polyfluoro ketones (FKGK18 and GK187) with improved potency and selectivity have been reported, but are not commercially available [108, 109].
The Lp-PLA2-targeting Darapladib is a direct, reversible and highly potent small molecule inhibitor with sub-nanomolar potency (IC50=0.25 nM against rhLp-PLA2) [110]. Due to the involvement of Lp-PLA2 in atherosclerotic inflammation, Darapladib is currently in phase III clinical trials for treatment of cardiovascular disease [111].
2.4. Autotaxin
2.4.1. Enzyme activity and regulation
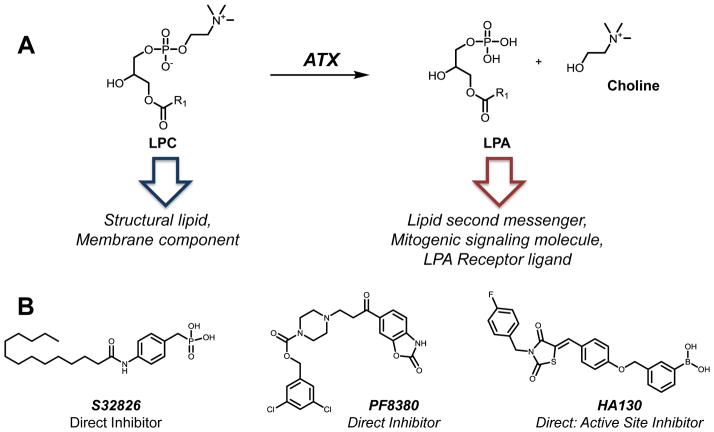
Autotaxin (ATX) belongs to the nucleotide pyrophosphatase/phosphodiesterase (NPP) family of enzymes responsible for the hydrolysis of phosphodiester bonds of nucleotides. Unlike other family members, however, ATX shows unique specificity for lipid substrates. ATX is a secreted lysophospholipase D that catalyzes the hydrolysis of lysophosphatidylcholine (LPC) into lysophosphatidic acid (LPA) and choline (Fig. 3A) [112]. ATX was initially identified in the 1990s as a motility factor capable of stimulating cell migration yet the catalytic activity remained unknown. Subsequent reports identified ATX as the prominent source of signaling-specific lysophosphatidic acid (LPA), a ligand for a subclass of lipid-binding GPCRs.
Figure 3.
Molecular trafficking and small molecule inhibitors of autotaxin (ATX). A. ATX is a lyso-PLD hydrolyzing lysophosphatidylcholine (LPC) to produce lysophosphatidic acid (LPA) and choline. Like PC, LPC is a membrane component and readily available in cells. The product of ATX catalytic activity, LPA, is an agonist at membrane-bound GPCRs known as the LPA receptors. The activity of LPA at its receptors grants the lipid an exceptional status as a mitogenic and survival signaling molecule. B. Inhibitors of ATX catalysis are thought to be ideal drug targets for hyper-proliferative diseases such as cancer. S32826 is a low-nanomolar inhibitor of ATX but its poor bioavailability limits its use in vivo. PF8380 is a commercially-available non-lipid ATX inhibitor developed by Pfizer. Along with the boronic acid HA130, both compounds inhibit ATX in whole blood and directly inhibit the enzyme at nanomolar IC50 values.
There are three isoforms of ATX resulting from alternative splicing: α, β, and γ. All three isoforms exhibit similar catalytic activities but differ in tissue distribution and expression, with ATXβ as the predominant and most well characterized isoform. ATX is initially expressed as a pre-proenzyme that contains an N-terminal secretion signal later removed by peptidase hydrolysis. The cleaved protein is then converted to its active form through the action of a proprotein convertase [113]. The catalytic activity of ATX relies on an interaction between a threonine residue and zinc ions in the active site. An adjacent hydrophobic pocket facilitates lipid substrate binding. Subsequent crystallization of mammalian ATX enzymes in 2011 provided clearer insight into the activation, mechanism, and potential inhibition of this enzyme [114, 115]. The large hydrophobic pocket of ATX is notoriously non-selective. While its most well-documented role is the production of LPA, ATX can also generate sphingosine 1-phosphate (S1P) from sphingosylphosphorylcholine (SPC), which is chemically distinct from its native substrate [116].
LPA, the catalytic product of ATX, is a potent agonist at six GPCRs. LPA mediates distinct physiological roles such as initiation of migration, proliferation and cell survival. The LPA receptors are divided into two subgroups: endothelial differentiation gene family (Edg) members and a less well characterized family more closely related to the purinergic receptors [117]. LPA receptor stimulation results in the activation of essential signal transduction pathways including the MAP Kinase cascade and PI3K signaling, each of which are key nodes in cancer pathogenesis [118]. In addition, LPA receptor stimulation also promotes the production of cytokines and growth factors, further contributors to survival [119]. Since the LPA receptors activate these critical survival pathways in the cell, LPA generated by ATX remains a major focus for cancer therapeutic development [120]. Several breast cancer lineages display aberrant LPA receptor and ATX expression, a sufficient mechanism to induce late onset invasive, metastatic breast cancers in transgenic mice [121]. ATX itself is differentially expressed in cancerous tissues compared to normally differentiated tissues and may function as a marker of cancer prognosis and a biomarker for cancer detection [112]. Importantly, overexpression of ATX in non-neoplastic diseases such as acute coronary syndrome and liver disease, suggests that modulators of this enzyme promote disease states other than cancer [122].
2.4.2. Tools for modulation of enzymatic activity
While overwhelming evidence implicates ATX and LPA in cancer, its direct relevance to disease remains in question since no cancer-specific mutations have yet been identified. Yet, it is clear that overexpression of the ATX-LPA receptor signaling nexus promotes tumor formation, angiogenesis, and metastasis. These established phenotypes brought ATX inhibitor development to the forefront of medicinal chemistry efforts. Recent reviews on the development of small molecule inhibitors of ATX give an in depth history and more complete picture of the field [116, 123]. This review focuses on some of the substantial developments.
Lipid mimics
Early ATX inhibitors disrupted the catalysis of ATX by focusing on the observation that ATX was sensitive to product inhibition by either LPA or S1P. Several LPA analogues are commonly described as ATX inhibitors although they display only modest potency and may have off-target effects on other LPA binding proteins [118]. Additional evidence suggests that lipid analogues are not ideal drug candidates since they could elicit effects through direct receptor modulation, independent of ATX inhibition. For example, FTY720, an S1P mimetic, inhibits ATX at micromolar doses but is a nanomolar functional antagonist at the S1P receptors. Although this compound does have reported anti-cancer activity at high concentrations, it is unclear which mode of action is responsible for those effects [124]. S32826 is a low nanomolar ATX inhibitor (IC50=9 nM) identified by Ferry and colleagues using a high throughput screen of several thousand compounds (Fig. 3B) [125]. While this compound was one of the first direct potent small molecule ATX inhibitors, it is not an ideal drug candidate due to poor in vivo stability, oral bioavailability, and possible off-target effects. Yet S32826 remains a potent tool compound to study ATX in vitro and in whole cells [125]. Other lipid-based small molecules identified as ATX modulators include cyclic-LPA analogues [126] and phosphonate-containing lysolipids [127].
Non-lipid inhibitors
Synthetic small molecule inhibitors of ATX show more promise than their lipid-based predecessors due to better DMPK profiles. PF-8380 is a commercially available small molecule inhibitor of ATX initially described in 2010 by Pfizer (Fig. 3B). Studies showed this molecule is a direct, potent ATX inhibitor with better pharmacodynamics than lipid-based molecules [128]. This report demonstrated that PF-8380 inhibited partially purified and cellular ATX activity as well as in vivo efficacy. Boronic acid-containing thiazolidinediones are another class of potent ATX inhibitors. One analogue, HA130, has an IC50 value in the low nanomolar range (Fig. 3B) [129, 130]. Importantly, these compounds along with PF-8380, all decrease LPA concentrations in plasma, an assay in which many ATX inhibitors have failed.
Fells and colleagues published a recent report detailing novel small molecule ATX inhibitors. A high throughput screen of more than 10,000 entities identified several novel low-nanomolar (IC50 values of 5–50 nM) ATX inhibitors [131]. Importantly, these novel leads also have no detectable receptor modulation at either the LPA or S1P receptors [131]. Taken together, these non-lipid based inhibitors may prove useful tools for further exploration of the role ATX and LPA play both in cells and in vivo; such experiments may lead to novel therapeutic compounds.
3. Lipid Kinases
3.1. Diacylglycerol Kinases
3.1.1. Enzyme activity and regulation
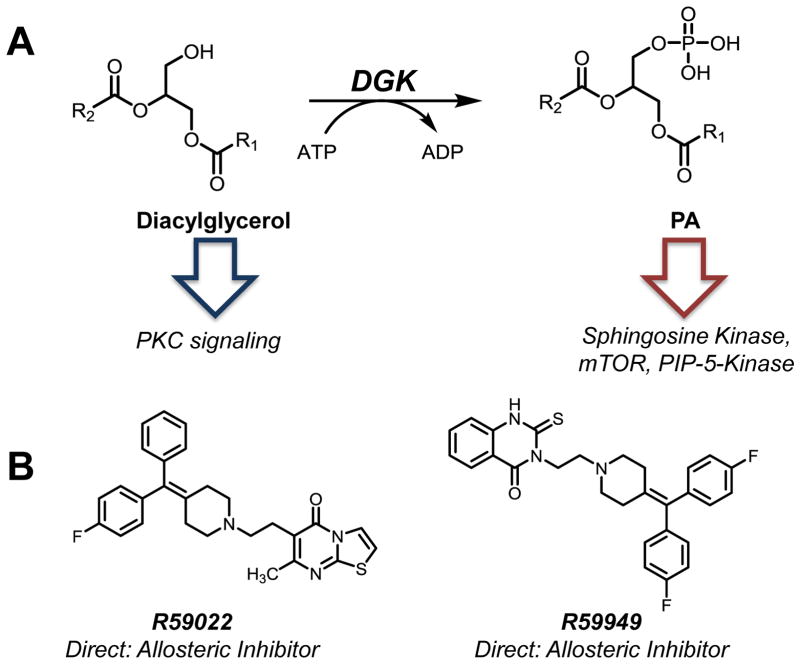
Diacylglycerol kinases (DGKs) catalyze the phosphorylation of diacylglycerol (DAG) to PA (Fig. 4A); most kingdoms of life—from bacteria and yeast to mammals—express DGK enzymes. Importantly, the specialization of DGK isoforms and their regulatory intricacies increase with organism complexity: bacteria and yeast express a single DGK enzyme while humans express ten isozymes, most possessing multiple variants [132, 133].
Figure 4.
Molecular trafficking and small molecule inhibitors of diacylglycerol kinase (DGK). A. DGK phosphorylates DAG to produce PA. DAG is a vital second messenger and PA is a prominent activator of several signaling proteins including mTOR. The DGKs are broadly expressed and regulate a host of physiological processes. B. Two synthetic inhibitors of DGK have been identified: R59022 and R59949. Both inhibitors only target the Ca2+-dependent type I DGKs at low-micromolar concentrations.
The product of DGK enzymatic activity, PA, is an important signaling lipid with multiple functions. Enzymes as diverse as mTOR [134], sphingosine kinase [135], and phosphatidylinositol-4-phosphate 5-kinase [136] are all activated by PA. Yet the substrate of the DGK enzymes, DAG, is an important signaling molecule in its own right. DAG prominently regulates PKC, which regulates several signaling cascades [137]. Thus, the catalytic activity of the DGKs governs the concentrations of two critical classes of signaling molecules and places the DGKs at a unique metabolic nexus. The importance of DGKs in such diverse signaling pathways makes them immensely important targets for a variety of disease states and physiological processes, ranging from hyperproliferative diseases to neurotransmitter release.
Humans express a total of 10 DGK isoforms (α–κ), classified into five sub-families based on sequence homology. In most cases, these differences in sequence homology result in alternate modes of regulation. Type I DGKs include α, β, and γ and are characterized by their calcium-binding EF motif, making calcium an important regulator of these enzymes [138]. Empirical evidence also suggests that other lipids such as sphingosine may play an important regulatory role for the type I DGKs [139]. The second sub-family of DGKs, type II enzymes (δ, η, and κ), each possess a pleckstrin homology (PH) domain containing PKC phosphorylation sites for regulation. Phosphorylation of type II DGKs in the PH domain inhibits translocation to the plasma membrane thereby decreasing PA turnover [140]. DGKε is the only known type III isoform and does not contain an identifiable regulatory domain. However, empirical evidence suggests that anionic lipids negatively regulate enzymatic activity [141]. DGKζ and DGKι are classified as type IV DGKs and possess four ankyrin repeats and a myristoylated alanine-rich C-Kinase (MARCKS) phosphorylation site. MARCKS domains localize the type IV DGKs to the nucleus, a process negatively regulated by PKC phosphorylation [142]. RhoA association negatively regulates the enzymatic activity of DGKθ, the only known type V sub-family member. [143].
All tissues contain at least one member of each DGK sub-family with most tissues expressing multiple isozymes. However, the various DGK isoforms display stark contrasts in subcellular localization, suggesting that distinct pools of DAG and PA within specific cellular compartments perform discrete physiological roles despite catalyzing the same reaction. For instance, DGKβ closely associates with actin filaments and is vital for cytoskeletal processes [144], while DGKδ closely associates with endosomes [145]. DGKα localizes to membranes upon activation and regulates global DAG content in cells [146] while DGKε selectively phosphorylates arachidonoyl-containing substrates making it a critical regulator of the PI turnover cycle [147, 148]. DGKδ, regulates de novo fatty acid synthesis and lipogenesis; as a result, knockout mice perish shortly after birth[148]. In sum, the various DGK isozymes regulate a host of physiological processes, reviewed elsewhere in detail [132, 133].
3.1.2. Tools for modulation of enzymatic activity
The unique signaling juncture occupied by the DGKs and their broad signaling roles makes them ideal targets for small molecule inhibitors. Moreover, the apparent focus of distinct DGK isozymes to discrete cellular processes suggests that isozyme-specific inhibitors could be critical tools to study the role of PA and DAG signaling in specific cellular events. Yet, considering the impact small molecule inhibitors of DGK catalytic activity would have, these compounds remain underrepresented in the literature. However, small molecules were identified and used to characterize one subfamily of DGK isozymes.
Lipid-based inhibitors
Early characterization of DGK inhibitors focused on lipid analogues of DAG. In the development of novel in vitro DGK assays, Bell and coworkers identified dioctanoylethylene glycol and 1-monooleoylglycerol as mid-micromolar inhibitors of type I DGK enzymes [149]. This initial study surmised that dioctanoylethylene glycol would be an ideal small molecule inhibitor of the type I DGKs since its short-chain fatty acids granted moderate cell permeability. However, dioctanoylethylene glycol was sparsely used in early DGK inhibitor studies.
Non lipid-based inhibitors
Around the time of the characterization of structural analogues of DAG as DGK inhibitors, two small molecules were also developed. R59022 was identified from a screen of synthetic small molecules (Fig. 4B) [150]. Using activity from isolated membranes and the synthetic DAG 1-oleoyl-2-acylglycerol, the authors found R59022 to have a 3.8 μM IC50 value. Four years after the initial characterization of R59022, R59949 (a structural analogue) was identified as having an IC50 of 300 nM in platelet membranes (Fig. 4B and Table 1) [151]. However, a subsequent independent study reported R59949 actually had an IC50 value of 3 μM, nearly identical to R59022, in a head-to-head comparison in platelets [152].
Further characterization of R59022 and R59949 revealed that these compounds specifically inhibited Ca2+-binding (Type I) DGK isoforms. Analysis of DGKα truncation mutants revealed that R59949 did not bind to the Ca2+-binding motif. Rather, these compounds bind to an allosteric site adjacent to the active site, evidenced by altered Mg-ATP binding after R59949 administration [153]. The selectivity of these chemical tools allows one to specifically interrogate the role of the Type I DGKs in various diseases states and physiological processes. For example, a recent study found that DGKα negatively regulated interleukin production in anergic T cells through Ras. Administration of R59949 restored interleukin production in these cells [154].
R59022 and R59949 remain the best characterized diacylglycerol kinase inhibitors reported in the literature. However, other DGK family members play vital roles to intracellular signaling cascades. Our groups recently reported that DGKζ regulates large pools of PA downstream of the purinergic receptor P2Y6. In this way, DGKζ functions as a negative regulator of PLD activity through PKCα [155]. The ability of DGKζ to regulate known signaling nodes in disease processes makes it an attractive target for future lead compound development. This study highlights one of many examples implicating DGK isoforms other than type I in disease, and identifies DGK inhibition as an important area of development for future small molecule regulators of lipid metabolism.
3.2. PI3-Kinase
3.2.1. Enzyme activity and regulation
Perhaps the most well characterized and promising area for lipid metabolism and cancer research focuses on phosphatidylinositol 3-kinase (PI3K) and its downstream signaling cascade. The PI3K enzymes phosphorylate the 3′-hydroxyl group of phosphatidylinositols (PIs). This phosphorylation event regulates cell growth, migration, metabolism, angiogenesis, and survival [156]; other intracellular signaling proteins rely on the products of PI3K for activation. Importantly, many cancers display hyper-activation of the PI3K pathway, a key component to their survival [157]. There are 3 classes of PI3K enzymes some with additional isoforms. Denoted as classes I, II, and III, each class exhibits different substrate specificity [158]. Class I PI3K enzymes, the most relevant class for cancer survival, sit downstream of cell surface receptors and primarily phosphorylate PI(4,5)P2 to generate PI(3,4,5)P3. Class II PI3Ks produce PI(3)P and PI(3,4)P2 from PI and PI(4)P, respectively. Class III only contains one enzyme, Vps34p which converts PI into PI(3)P. In addition to PI3K, other classes of lipid kinases phosphorylate the remaining positions of PI(4′ and 5′) on the inositol ring, generating the other PIPn lipids.
Class I PI3K enzymes are heterodimers made up of a regulatory subunit, p85 for class IA or p101 for class IB, and a catalytic subunit, p110. Various regulatory subunit isoforms on the PI3Ks lead to differential activation by cell surface receptors, specifically receptor tyrosine kinases [159]. Class IA enzymes have a highly conserved catalytic domain, expressed as one of three isoforms, p110α, β, and δ. The p110 catalytic subunit is made up of a p85 binding region, a Ras-binding domain, a C2 domain, a PIK homology domain and a C-terminal catalytic domain [159]. The PIK domain is found in other kinases including mTOR, suggesting that these proteins are phylogenically related.
PI3K signaling pathways centrally sustain cell growth under a plethora of stimulation conditions. Receptor tyrosine kinase stimulation activates class I PI3Ks. Meanwhile, insulin, insulin growth factor, or platelet derived growth factor all trigger PI3K activity directly or through adaptor protein recruitment [159]. GPCRs also stimulate Class IB PI3K proteins through binding of the Gβγ subunit to the regulatory domain of PI3K.
The canonical PI3K-signaling pathway focuses on the production of PIP3, a critical lipid second messenger. The opposing actions of PI3K and PTEN (phosphatase and tensin homologue) balance this system; PTEN dephosphorylates PIP3 generating PIP2 and terminating PIP3 signaling. Aggressive cancers often display mutations in or complete loss of PTEN, leading to hyperactive PI3K signaling. The protein kinase Akt, considered the primary downstream effector of PI3K signaling, phosphorylates multiple target proteins affecting a broad range of cellular processes [159].
Class IA PI3Ks regulate cell survival, sitting downstream of growth factor receptors; many members of this signaling pathway work together in promoting tumor pathogenesis. In certain cancer types, specifically cervical or squamous cell lung cancer, mutations or amplifications in PI3K are present in 60% of cases [160]. The PI3K-Akt signaling nexus is often considered the major effector of metabolic insulin action though the insulin receptor and insulin-sensitive kinase activation. Proper metabolic control relies on the correct functioning of these signaling pathways. Dysregulation of these pathways often leads to impaired glucose homeostasis [161]. Because of its prevalence in cancer and diabetes progression, interest in PI3K as target of drug development grew substantially in the past decade.
3.2.2. Tools for modulation of enzymatic activity
Clinical trials
PI3K’s central role in cancer progression led to the development of several preclinical and clinical inhibitors. The broad cellular roles of PI3K make highly isoform-specific PI3K inhibitors preferable over pan-PI3K inhibitors. Ideally, isoform-specific PI3K inhibitors target the specific isozymes responsible for carcinogenic phenotypes, limiting the potential for on-target side effects. Indeed, specific inhibitors of p110α and p110δ are already in clinical trials for the treatment of hyper-proliferative diseases [162]. Many pharmaceutical companies relied on targeting the PI3K signaling pathway through either direct PI3K inhibitors (pan or isoform specific) or through targeting downstream effectors such as Akt and mTOR [156]. Early clinical trials demonstrated that agents targeting this pathway are generally well tolerated, safe, and effective in several tumor types. Importantly, tumor genotype and sensitivity to PI3K inhibition do not necessarily correlate [160].
Preclinical tool compounds
Although several new drug candidates entered clinical development, two major preclinical tool compounds remain useful to interrogate PI3K signaling in disease models: LY294002 [163] and wortmannin [164] (Table 1). These two compounds solidified PI3K as a viable drug target in early studies but their poor pharmacological properties limited their utility in vivo [162]. Lilly developed LY294002 in 1994 as an ATP-competitive small molecule inhibitor of PI3K, a class of molecules notoriously prone to off-target effects due to the sequence homology of ATP binding motifs across the genome [163]. The low potency of LY294002 toward PI3K (IC50=1.3 μM) makes this compound more therapeutically problematic; the higher doses needed for enzyme inhibition increase the likelihood of off-target kinase activity.
Wortmannin is a fungal metabolite that blocks PI3K function by covalently tethering to the enzyme’s ATP-binding site with a low nM IC50 value [165]. Due to the growing interest of PI3K in a host of disease processes, the number of commercially available, isoform-selective small molecule inhibitors of PI3K expanded appreciably in recent years [158]. Despite their similar mechanisms of action, x-ray crystallographic studies demonstrated that wortmannin and LY294002 bind to PI3K in distinct orientations. Elegant work by Williams and coworkers provided critical insights that have addressed the therapeutic potential of this target [166].
4. Lipases
4.1. Monoacylglycerol Lipase
4.1.1. Enzyme activity and regulation
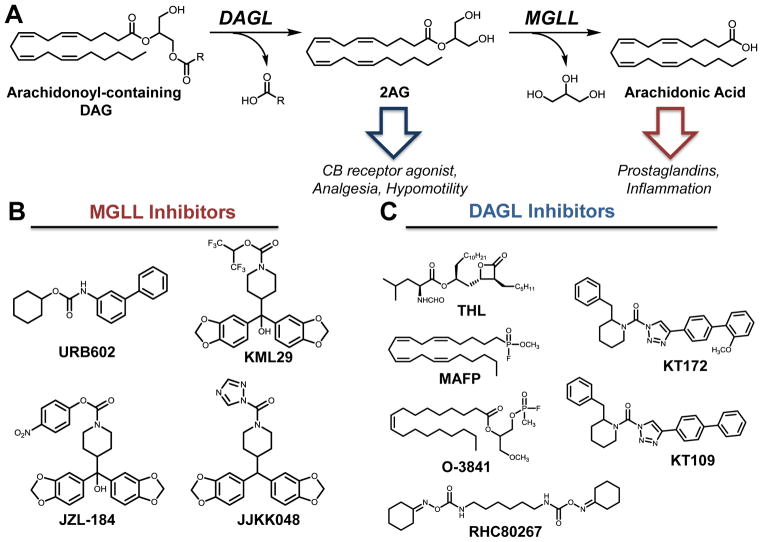
Monacylglycerol lipase (MGLL) facilitates the hydrolysis of monoacylglycerol (MAG) lipids to free fatty acids and glycerol, the final step in fatty acid catabolism (Fig. 5A). First cloned from mice, the MGLL gene is a relatively small enzyme with a molecular weight of 33 kDa [167]. Mammals only express one isoform of MGLL, which contains the characteristic serine hydrolase GXSXG catalytic motif in its active site. MGLL activity is detected in a variety of other tissues including brain, kidney, liver, heart, brown adipose tissue, and lung [168]. Primarily a cytosolic protein, MGLL can associate with membranes via a lid domain adjacent to the catalytic site [169]. Initially thought of as solely important to lipid metabolism, recent studies confirmed MGLL as a prominent regulator of three critical signaling nodes.
Figure 5.
Molecular trafficking and small molecule inhibitors of DAGL and MGLL. A. DAGL and MGLL regulate the production of 2AG and AA in cells. 2AG is a potent agonist at the EC receptors granting the lipid important signaling roles in the nervous system. In addition, DAGL and MGLL also regulate AA production in tissues in which they are expressed. The important signaling roles of both 2AG and AA have made DAGL and MGLL important targets for small molecule inhibitors. B. Small molecule inhibitors of MGLL are a short list. JZL-184 has been used extensively both in vitro and in vivo for the characterization of MGLL in the CNS and cancer models. C. Several DAGL inhibitors have been reported. The 1,2,3-triazoleureas KT172 and KT109 were recently used in vitro and in vivo to characterize the DAGLβ isoform and its role in inflammation.
MGLL activity negatively regulates cellular concentrations of the endocannabinoid 2-arachidonoylglycerol (2-AG). Along with anandamide, 2-AG is known as a retrograde synaptic suppressor, binding the endocannabinoid (EC) receptors, the same GPCRs responsible for binding, Δ9-tetrahydrocannabinol, the psychoactive component of marijuana [170]. When MGLL cDNA was transfected and overexpressed in neurons, 2-AG signaling decreased at the EC receptors, supporting the hypothesis that MGLL negatively regulates 2-AG concentrations [171]. These findings were later corroborated in vivo with a small molecule inhibitor of MGLL that demonstrated behavioral effects consistent with sustained cannabinoid receptor agonism [172].
A second critical signaling node regulated by MGLL activity centers on the arachidonic acid (AA) product of 2-AG hydrolysis by MGLL. AA is the essential fatty acid utilized for prostaglandin biosynthesis; oxidation of AA by the cyclooxygenase (COX) enzymes generates the core prostaglandin scaffold. COX enzymes are recognized as key regulators of inflammatory responses and are the targets of non-steroidal anti-inflammatory drugs [98]. As discussed previously, cPLA2 activity generates the critical pool of AA needed for COX-mediated prostaglandin biosynthesis in most tissues [173]. Indeed, cPLA2-knockout mice display altered levels of AA in nearly all tissues except, notably, the CNS [174]. A recent study demonstrated that MGLL activity on 2-AG regulated prostaglandin-specific AA production in the brain. Blockade of MGLL enzymatic activity with a small molecule inhibitor blunted LPS-induced inflammatory responses mediated by prostaglandins in neural tissues [175].
Aside from its roles in the nervous system regulating 2-AG and prostaglandin biosynthesis, MGLL also contributes to cancer malignancy. A recent study found that MGLL activity was upregulated in aggressive cancer types and that transfection of the MGLL gene into more benign tumor lines increased their aggressiveness and invasiveness [176]. This initial report found that MGLL regulated free fatty acids (FFAs) in aggressive cancer types and contributed to lipogenic phenotypes. A follow up study found that in addition to FFAs, MGLL regulation of 2-AG in prostate cancer regulated metastasis [177], identifying MGLL as a vital component to pro-survival signaling pathways in aggressive cancer types.
4.1.2. Tools for modulation of enzymatic activity
Early classes of MGLL inhibitors suffered from either insufficient potency or selectivity. The first reported MGLL-selective inhibitor, URB602 (Fig. 5B), was based on the structure of a previously identified carbamate-based inhibitor of fatty acid amide hydrolase (FAAH) [178], the primary enzyme responsible for the biosynthesis of the second endocannabinoid anandamide [179]. However, subsequent studies found URB602 lacked selectivity for MGLL over FAAH [180] as well as the potency necessary for in vivo characterization [181].
While initial MGLL inhibitors were not suitable chemical tools, the use of carbamates to disrupt the serine hydrolase activity of MGLL remained a fruitful strategy. These privileged scaffolds function by entering the enzyme’s active site and introducing an irreversibly-bound carbamate moiety (Fig. 6). Use of this strategy led to the identification of the MGLL-selective inhibitor JZL-184 (Fig. 5B and Table 1) and marked a turning point in MGLL studies. Identified through a screen of N-piperidine and N-piperidine-linked carbamate scaffolds, JZL-184 displayed an IC50 value of 10 nM at MGLL and nearly 5 μM at FAAH. Thus JZL-184 achieved 500-fold selectivity for regulating 2-AG-specific signaling pathways in the CNS [182]. Consistent with the proposed covalent binding mechanism, LC-MS studies detected the carbamate product of JZL-184 hydrolysis from tryptic residues of the MGLL active site [168]. JZL-184 is currently the standard chemical tool for the analysis of the physiological roles of MGLL both in vitro and in vivo and is currently commercially.
Figure 6.
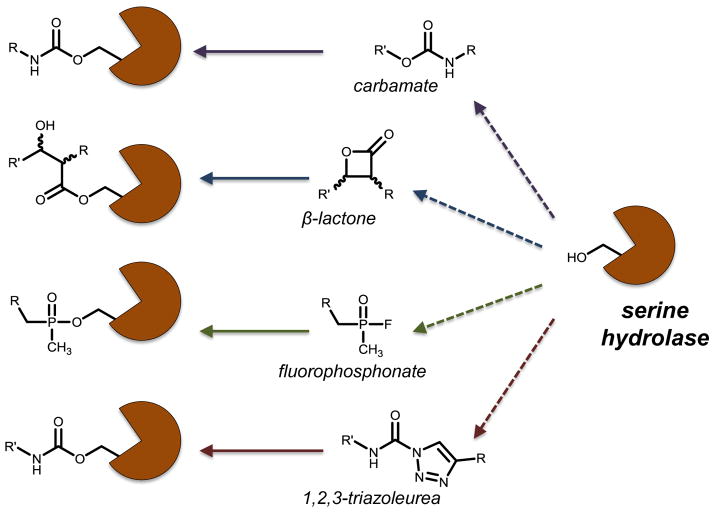
Mechanisms of serine hydrolase inhibition. Serine hydrolases are major regulators of lipid metabolism. MGLL, DAGL, HSL and ATGL all utilize a serine hydrolase catalytic motif to metabolize glycerolipids. Several classes of inhibitors of serine hydrolases have been developed to irreversibly bind to the active site of hydrolases by acting as electrophiles. Carbamates, β-lactones, fluorophosphonates and 1,2,3-triazole ureas are structurally distinct scaffolds but mechanistically inhibit serine hydrolases identically.
In spite of the successes of JZL-184, the compound does display some off-target activity against other serine hydrolase targets. Studies showed that prolonged doses of JZL-184 in vivo result in low levels of FAAH inhibition [168]. A new structural class of compounds identified in the Cravatt laboratory utilizes a hexafluoroisopropyl moiety as part of the carbamate scaffold (KML29). These compounds more closely resemble the structure and reactivity of 2-AG and thus display greater selectivity for MGLL [183]. A second, more recent class of MGLL inhibitors, based on a piperidine triazole urea scaffold, utilizes the same general strategy for irreversible serine hydrolase inhibition (Fig. 6). These series of compounds identified the most potent inhibitor of the enzyme yet reported: JJKK048 inhibits MGLL with an IC50 value of 0.2 nM (Fig. 5B) [184].
4.2. Diacylglycerol Lipase
4.2.1. Enzyme activity and regulation
Diacylglycerol lipase (DAGL) preferentially hydrolyzes DAG species at the sn-1 position yielding sn-2-substituted MAG and FFAs (Fig. 5A) [185]. DAGL exists as two isoforms, designated as DAGLα and DAGLβ [186]. The two isoforms of DAGL regulate tissue-specific pools of DAG metabolism, with DAGLα primarily localized to the nervous system and DAGLβ localized to peripheral tissues such as the liver [187, 188]. Although the two isozymes vary greatly in size, with DAGLα and DAGLβ having a 1042 and 672 amino acid sequences, respectively, they display extensive sequence homology and similar subcellular distribution [185].
The substrate specificity of the DAGL isoforms grants them two important signaling roles. The specificity of the DAGL enzymes for sn-1 hydrolysis of DAG species makes them tissue-specific producers of 2-AG, counteracting the activity of MGLL [186]. Due to its high level of expression in the nervous system, DAGLα is the primary regulator of 2-AG levels in the brain. Indeed, studies on DAGLα−/− mice revealed these animals lack retrograde synaptic suppression [188]. A higher level of DAGLβ expression outside the nervous system makes this isoform the critical regulator of 2-AG in peripheral tissues [187].
As with MGLL, a second, subtler role of DAGL lies in its ability to regulate tissue-specific pools of AA. Studies on DAGLβ−/− mice identified this isoform as the critical regulator of AA in liver [187]. Subsequent studies on DAGLβ in vivo revealed that decreases in AA pools in peripheral tissues led to decreased prostaglandin biosynthesis and suppressed local inflammatory responses [189].
4.2.2. Tools for modulation of enzymatic activity
Given the important role of DAGL in 2-AG biosynthesis and retrograde synaptic suppression, inhibitors of DAGL traditionally focused on the inhibition of the DAGLα isoform. The first functionally-identified DAGL inhibitor, RHC80267, utilizes a cyclohexanone O-acetyl oxime moiety for irreversible enzyme inhibition, outlined in Fig. 6. RHC80267 decreased 2-AG levels in cortical neurons but was not evaluated against recombinant DAGL [190]. Later cloning of both DAGL isoforms confirmed that RHC80267 inhibited DAGL at 100 μM concentrations but could not distinguish between either of the DAGL isoforms [186]. High IC50 values and poor specificity make RHC80267 an unsuitable tool compound for interrogating DAGL enzymatic activity in native biological systems.
Fluorophosphonate (FP) lipid analogues are well known irreversible inhibitors of serine hydrolase activity. Studies on FP lipid analogues identified two additional DAGL inhibitors. Initial studies on the DAG-FP analogue O-3841 (Fig. 5C) reported an IC50 of 160 nM on the DAGLα isoform [191]. Following suit, reports surfaced of a second arachidonic acid fluorophosphonate inhibitor, known as MAFP. Not surprisingly, this compound also displayed substantial off-target activity towards MAGL [191]. FP lipid analogues are highly-reactive and bind non-selectively to a majority of serine hydrolases [192], calling into question their reliability as scaffolds for selective inhibitors. Indeed, activity-based protein profiling (ABPP) regularly takes advantage of this broad reactivity of lipid FP analogues to survey global cellular serine hydrolase activity [193, 194].
A third class of early DAGL inhibitors, based on tetrahydrolipstatin (THL, Orlistat), utilizes a beta-lactone functionality to irreversibly bind the DAGL active site [195]. Figure 6 describes the unique inhibitory mechanism of these analogues, reminiscent of β-lactam antibiotics. Later studies on THL raised suspicion of off-target effects at the CB receptors. Medicinal chemistry efforts have since focused on eliminating this activity from THL analogues. These studies identified three compounds having improved potency at DAGL over THL but with only modest gains in selectivity [196]. When evaluated by ABPP, this class of inhibitors proved more selective than the lipid-FP analogues but still displayed substantial off-target effects, making them unsuitable for in vivo use [193].
A recent study identified 1,2,3-triazoleureas as a dynamic scaffold for the inhibition of serine hydrolases. Like the carbamate moieties used in the development of MGLL inhibitors, 1,2,3-triazoleureas irreversibly bind to the active site of serine hydrolases (Fig. 6). Importantly, their streamlined, modular synthesis makes them amenable to rapid library expansion [197]. Using this strategy as a starting point, Cravatt and coworkers devised a fourth class of DAGL inhibitors. A recent report identified two 1,2,3-triazoleurea DAGLβ-selective inhibitors (KT109 and KT172), which displayed mid-nanomolar potencies with good selectivity over the other EC biosynthetic targets FAAH and MGLL [189]. However, KT109 and KT172 have prominent off-target activity against ABHD6. The negative control published alongside these inhibitors, KT195 displayed no activity toward either DAGLα or DAGLβ but retains the off-target effects on ABHD6 and is best used alongside KT109 and KT172 to study DAGL in vivo and in vitro.
4.3. Triacylglycerol Lipases
Triacylglycerol (TAG) is the primary mode of fat storage in animals, which is rapidly hydrolyzed to FFAs and DAG by the hydrolytic function of TAG lipases. Multiple enzymes possess TAG lipase activity. Initially, lipid biochemists postulated that hormone sensitive lipase (HSL) solely regulated TAG hydrolysis in adipose tissue. However, the development of HSL knockout mice revealed that DAG accumulated independently of HSL activity, suggesting alternative routes of TAG hydrolysis [198]. Following this observation, researchers identified a second catalyst of initial TAG hydrolysis: adipose TAG lipase (ATGL). Subsequent studies later found that the protein comparative gene identification 58 (CGI-58) significantly enhances ATGL activity. Together, these three enzymes regulate TAG catabolism and are potential targets for therapeutic development.
4.3.1. Hormone-sensitive lipase
4.3.1.1. Enzyme activity and regulation
Hormone-sensitive lipase (HSL) is a neutral intracellular lipase first identified in adipose tissue in the 1960s [199]. HSL hydrolyzes TAG and DAG and releases free fatty acids from adipose tissue (Fig. 7A). Outside of acylglycerols, HSL can also hydrolyze cholesterol esters, retinyl esters, steroid esters and p-nitrophenyl esters [200]. Studies identified three isoforms of HSL, which range in size from 84–130 KDa [201–203]. Adipose tissue, macrophages [204, 205], adrenal tissues [206], cardiac tissue [207] and skeletal muscle [208] all express the 84kD variant of HSL. Pancreatic islet cells express the 84 and 89 KDa isoforms of HSL [203], while mature testis express the larger (~120–130 KDa) isoforms, where they contribute to normal spermiogenesis [202, 209]. HSL exists as homodimer and all known isoforms contain the catalytic C-terminal domain while the N-terminal domain differs between isoforms. These differences account for the various protein-protein and protein-lipid interactions of HSL [210].
Figure 7.
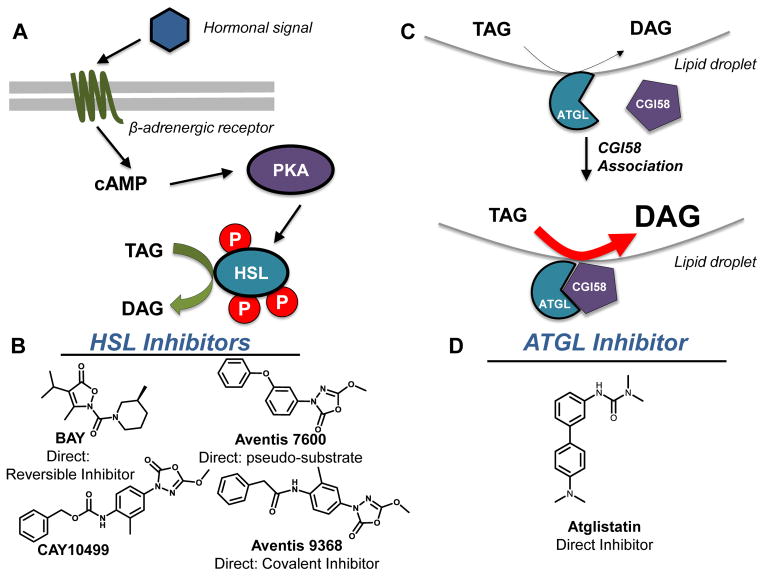
Differential regulation of HSL and ATGL for TAG metabolism. A. HSL sits downstream of the β-adrenergic receptor where hormonal cues activate PKA which stimulates HSL activity after phosphorylation. B. Due to their central role in TAG metabolism, small molecule inhibitors of HSL have been developed. Aventis’ 7600 and 9368 compounds inhibit HSL at nanomolar concentrations C. AGTL relies on its association with a catalytically-inactive serine hydrolase, CGI-58, to increase hydrolytic activity. ATGL displays a basal level of TAG hydrolysis which increases dramatically upon CGI-58 binding. D. Atglistatin is a recently reported ATGL inhibitor.
Hormones and neurotransmitters prominently regulate fat storage in mammals and HSL is the primary mechanism through which hormone signaling mediates lipolysis [211–213]. Following agonism of the β-adrenergic receptor, increased cAMP production and protein kinase A (PKA) activation phosphorylate HSL at three serine residues (563, 659 and 660), which results in enzyme activation (Fig. 7A) [214, 215]. Conversely the anti-lipolytic function of insulin arises from its ability to decrease cAMP levels, thereby terminating PKA activation by HSL [212]. Lipolytic hormones, known as catecholamines, independently regulate HSL activity through the mitogen-activated protein kinase (MAPK) pathway. Specifically, extracellular signal-regulated kinase (ERK), downstream of MAPK, phosphorylates HSL on Ser600, thereby increasing HSL activity [213, 216]. Importantly, serine phosphorylation only activates HSL toward TAG and cholesteryl ester hydrolysis; HSL hydrolysis of DAG and MAG are not affected by these signaling events [217]. Owing to its broad lipolytic activity and diverse expression, altered HSL activity is associated with several physiological disorders including familial combined hyperlipidemia [218], metabolic syndrome and diabetes [219], obesity [220], and cancer [221], suggesting a therapeutic role for HSL-modulating small molecules.
4.3.1.2. Tools for modulation of enzymatic activity
“Double feedback” inhibition
Early reports implicated intermediate lipid metabolites (oleoyl CoA, oleic acid, and 2-monopalmitoylglycerol) as HSL inhibitors by preventing accumulation of free fatty acids through direct enzyme inhibition and activation of an inhibitory kinase [222]. Oleoyl CoA inhibits HSL with an IC50 of 0.1 μM, while oleic acid inactivates the enzyme with a 0.5 μM IC50 and 2-monopalmitoylglycerol at 500 μM. Importantly, fatty acids inhibit adenylate cyclase and thus prevent accumulation of cyclic AMP [223], thereby reducing HSL activation by PKA. These two mechanisms work in concert to decrease fatty acid production and accumulation from HSL, yet the details of their interconnected relationship remain unclear.
Substrate mimetics
In the early 2000s Vertesy and colleagues isolated cyclipostins from Streptomyces sp. DSM 13381 mycelium. After chemical characterization, the research team assessed each compound’s HSL inhibitory activity. Four of the isolated compounds showed outstanding HSL inhibition with an IC50 of 20–40 nM in both partially purified enzymes and in cultured adipocytes. Cyclipostins show structural and physicochemical properties similar to those of neutral lipids and data suggest they operate as a substrate mimetic [224].
Small molecule inhibitors
Recent studies identified several additional classes of HSL small molecule inhibitors [225–230]. A series of 3-phenyl-5-alkoxy-1,3,4-oxadiazol-2-ones were developed and tested against recombinant human HSL by Aventis. Compound Aventis 9368 inhibits HSL with an IC50 of 90 nM and functions through the introduction of an irreversible, covalent acyl-enzyme complex. Another member of this family, compound Aventis 7600 is a pseudo-substrate demonstrating a reversible mechanism of inhibition [225]. The only commercially available inhibitor CAY10499 is based on oxydiazolyl carbamate (Fig. 7B) [231].
High-throughput screening carried out by Bayer identified HSL inhibitors based on a 2H-isoxazol-5-one scaffold, with an IC50 of 6 nM against the recombinant human HSL in intact cells [226]. Further optimization of lead compounds developed a potent, reversible, and selective HSL inhibitor BAY [227] based on 5-(2H)-isoxazolonyl urea. Pyrollopyrazinediones are another class of heterocyclic compounds tested as HSL inhibitors developed by Ontogene [228] with low micromolar potencies (IC50 of 0.2–1.3 μM). Novo Nordisk published work on carbamoyl triazole-based compounds as potent HSL inhibitors [232], the structure of their inhibitor NNC 76-0079 remains unpublished despite its use in the literature. Low nanomolar potency (IC50=1–5 nM) HSL inhibitors centered on a variety of N-aminomorpholine and phenols with a lipophilic substituent at the 4-position of the molecule’s aromatic moiety [229]. Optimization of the carbamate-based inhibitors resulted in the synthesis of two selective HSL inhibitors, 4-hydroxymethyl-piperidine-1-carboxylic acid 4-(5-trifluoromethylpyridin-2-yloxy)-phenyl ester and 4-hydroxy-piperidine-1-carboxylic acid 4-(5-trifluoromethylpyridin-2-yloxy)-phenyl ester with IC50s of 110 and 500 nM, respectively [230].
4.3.2. Adipose triglyceride lipase (ATGL)
4.3.2.1. Enzyme activity and regulation
Adipose triacylglycerol lipase (ATGL) catalyzes the hydrolysis of TAG to DAG and FA during basal and hormone stimulated conditions (Fig. 7C) [233–236]. Identified in 2004 by three independent laboratories, ATGL is known by different names – ATGL or desnutrin in mice [233, 234] and calcium-independent phospholipase A2ζ in humans [235]. Unlike HSL, ATGL associates with lipid droplets (LD) and possesses high substrate specificity for triacylglycerol. While the human and mouse ATGL homologues vary in size (506 and 486 amino acids, respectively), they share 86% sequence homology [233]. The N-terminal domain contains an α/β-hydrolase fold domain and a ‘patatin domain,’ both of which correlate to acyl hydrolase activity. The C-terminus contains a lipid-binding domain, rich in hydrophobic residues, which are critical for ATGL localization to LDs [236, 237]. White and brown adipose tissue [233], heart, testis, skeletal muscle, normal retina, and portions of the gastrointestinal tract [238–241] all express ATGL. This enzyme is the rate-limiting enzyme for TAG hydrolysis and is critical for TAG metabolism in tissues where it is expressed [239, 242, 243].
Nutritional signals regulate ATGL function [234, 238], with insulin being the most potent regulator [244]. Full activation of ATGL requires CGI-58 [245], but perilipin also plays a role in the regulation of basal and stimulated TAG hydrolysis, serving as a protein coordinator in recruitment to LDs [246]. Elevated circulating FAs and lipids are associated with metabolic disorders such as obesity and type 2 diabetes. Since ATGL is the critical regulator of TAG break down and FA release, it stands as a promising pharmacological target for the treatment of several diseases.
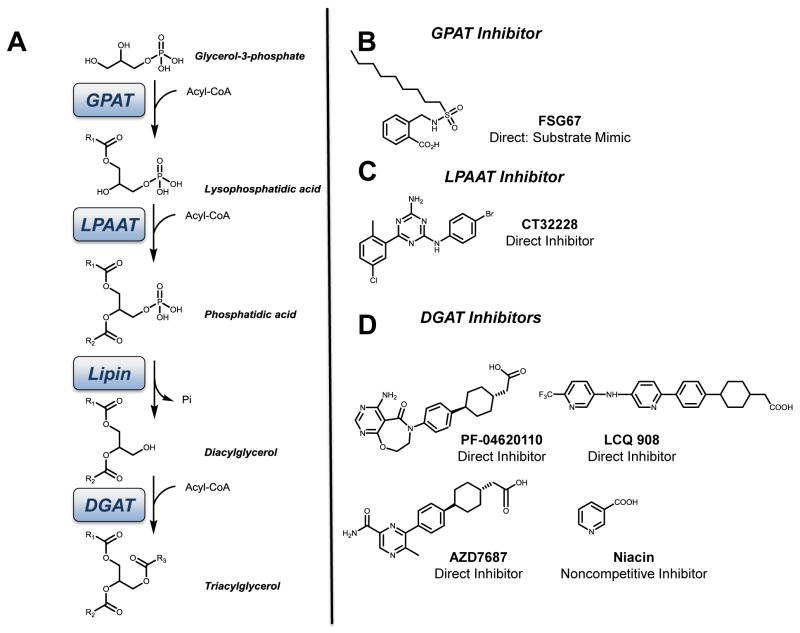
4.3.2.2. Tools for modulation of enzymatic activity
Bromoenol lactone (BEL, (E)-6-(bromomethylene)-3-(1-naphtalenyl)-2H-tetrahydropyran-2-one) is a suicide inhibitor of all known iPLA2s and inhibits ATGL, formally an iPLA2, with an IC50 of 0.5 μM [247]. The University of Graz (Austria) offers ATGL inhibitors with various structures for treatment of type 2 diabetes and tumor-induced cachexia by way of ATGL and HSL inhibition [248]. A recently developed ATGL inhibitor, Atglistatin (Fig. 7D and Table 1) directly inhibits ATGL with an IC50 of 0.7 μM and no toxicity up to 50 μM [249]. Importantly, Atglistatin exhibits high selectivity for ATGL with virtually no effects on other metabolic lipases.
4.3.3. CGI-58 (ABHD5)
4.3.3.1. Enzyme activity and regulation
The recently identified perilipin-binding protein, ABHD5 (α/β Hydrolase domain-containing protein 5), also known as comparative gene identification 58 (CGI-58)[250, 251], formally belongs to the ever-growing superfamily of serine hydrolases. This enzyme family contains a variety of members including hydrolases, lipases, hormone precursors or transporters, activators of other proteins, as well as lyases [252, 253]. Other lipase members of this family include two less characterized lipases hydrolyzing 2-arachidonoylglycerol – ABHD6 and ABHD12 [254, 255]. Human CGI-58 is a 38 kDa protein, which despite containing conserved sequences with other members of the esterase/lipase/thioesterase subfamily, does not display lipase activity [245, 256]. Interest in CGI-58 grew rapidly after mutations to this enzyme correlated to a severe neutral lipid-storage disorder known as Chanarin-Dorfman syndrome [257]. Since CGI-58 itself does not exhibit TAG hydrolytic activity, the prevailing hypothesis states that the enzyme serves as an activator of ATGL. Indeed, several studies demonstrated that CGI-58 regulates cellular TAG levels by modulating ATGL activity (Fig. 7C) [245, 258–260]. Recent reports in mice lacking CGI-58 described the existence of ATGL-independent functions for the enzyme. Specifically, CGI-58 activated lipases other than ATGL in the skin and knockdown of CGI-58 protected animals against high fat diet-induced obesity [261, 262]. Structural studies on the enzyme also revealed the lack of a complete catalytic triad [245], resulting in no lipase activity, while the carboxyl terminus of mouse CGI-58 contains an acyltransferase consensus sequence [263]. These and other studies confirmed a secondary role of CGI-58: a lysophosphatidic acid acyltransferase (LPAAT) [263–265]. CGI-58/ABHD5 is widely expressed in a variety of tissues including adipose tissue, skin, liver, skeletal muscle and brain [266].
4.3.3.2. Tools for modulation of enzymatic activity
There are no reported inhibitors of CGI-58/ABHD5 at the time of this review. However, compounds that could selectively disrupt the ability of CGI-58 to activate ATGL could prove to be useful tools for studying lipid-storage disorders and have become high priority targets.
4.3.4. PNPLA3
4.3.4.1. Enzyme activity and regulation
Patatin-like phospholipase domain-containing proteins (PNPLA) are a family of lipid hydrolases with diverse substrate specificity. The nine mammalian PNPLA family members possess hydrolytic activity against triacylglycerols, phospholipids, and retinyl esters [267, 268]. Yet, some PNPLA enzymes exhibit hydrolase activity but not phospholipase activity, rendering the PNPLA moniker misleading. Mice carrying either gain- or loss-of-function PNPLA mutations revealed essential roles for this enzyme in vivo: energy homeostasis, sepsis induction, triglyceride metabolism, and neuronal integrity among others [268].
PNPLA3, also known as adiponutrin, is a 53 kDa member of the PNPLA family with an undefined biochemical function [269]. Originally identified in 2001 by Baulande et al., PNPLA3 is a membrane-associated protein expressed at very low levels in a variety of tissues but significantly upregulated during adipocyte differentiation and in response to feeding.[269]. This observation suggests a critical role for PNPLA3 in energy mobilization and lipid storage [241]. Subsequent studies found that human PNPLA3 hydrolyzed TAG in vitro [235] and possessed transacylase activity and modest PLA2 activity. Interestingly, overexpression of PNPLA3 does not affect global levels of TAG, suggesting an alternative role for this protein or a highly conserved compensatory mechanism in the cell [238].
The Dallas Heart Study led by Hobbs and colleagues identified mutations in PNPLA3, both protective and detrimental, in individuals from different ethnic groups [270]. A genetic polymorphism in PNPLA3 (I148M) correlated to increased fat accumulation in the Hispanic population, which resulted in the highest prevalence of hepatic injury and steatosis [271]. A second polymorphism of PNPLA3 (S453I) correlated to lower hepatic fat content in African Americans, a group with the lowest risk of hepatic disease. Taken together, these data suggest that PNPLA3 is an important regulator of liver disease with key mutations differentially affecting ethnic groups.
Studies on the loss-of-function I148M mutation revealed that triglyceride accumulation in the liver is directly linked to normal PNPLA3 activity [272]. Studies in mice found that overexpression of wild-type PNPLA3 does not lower hepatic TAG levels, unlike overexpression of ATGL or HSL. However, overexpression of the I148M mutant did lead to increased hepatic TAG content [273]. These contradicting data convolute the exact physiological role of PNPLA3. One hypothesis suggests that the I148M mutation may interfere with the activity of another TAG hydrolase, such as ATGL or HSL in hepatic cells. Alternatively, this mutation could contribute to sequestration of other proteins and cofactors required for TAG homeostasis in the liver [274]. Despite detailed analysis of functional mutants, the precise role of PNPLA3 in hepatic metabolism remains unclear.
Consistent with the fatty liver phenotype, three metabolic phenotypes define PNPLA3 I148M transgenic mice: increased formation of FA and TAG, impaired TAG hydrolysis, and depletion of long polyunsaturated TAGs in the liver. These observations suggest a second role for PNPLA3 in the remodeling of TAG species within lipid droplets as they accumulate [275]. Another recent report from the Zechner lab suggests a different role for PNPLA3 as a nutrient-dependent LPAAT [276]. High-sucrose diets in wild-type mice substantially upregulated PNPLA3 expression in mouse liver, which was accompanied by increased LPAAT activity. These studies further explore the role of the mutant PNPLA3 I148M in cellular lipid accumulation, and are in accord with the proposed disease progression of non-alcoholic fatty liver disease in subjects carrying the I148M polymorphism.
4.3.4.2. Tools for modulation of enzymatic activity
Despite the emerging importance of PNPLA3 in metabolic disorders and lipid biosynthesis, no chemical modulators or inhibitors of PNPLA3 exist at the time of this review.
5. Lipid Phosphatases
5.1. Lipin (Phosphatidic Acid Phosphatase)
5.1.1. Enzyme activity and regulation
In the de novo biosynthesis of TAG, lipin catalyzes the penultimate step dephosphorylating PA to produce DAG (Fig. 8A) [277]. Kennedy first detected phosphatidic acid phosphatase activity from mitochondrial preparations in the 1950s [278], but the lipin enzyme remained uncharacterized until 2001 [279]. Mammals express three lipin isoforms (lipin1-3) each with differing tissue expression profiles. A broad set of tissues express lipin1 but the enzyme is predominantly found in the muscle and adipose tissue of humans. Lipin2 and 3 have much lower levels of expression with lipin2 localized to brain, liver, placenta, and adipose tissue while lipin3 is broadly expressed albeit at lower levels [280].
Figure 8.
De novo TAG biosynthetic pathway and inhibitors. A. TAG biosynthesis is derived from sequential acylation of glycerol-3-phosphate by a series of acyltransferases and dephosphorylation by lipin. B. FSG67 is the only reported inhibitor of GPAT activity with an IC50 value of 25 μM. C. CT32228 is one of a small number of LPAAT inhibitors with a mid-nanomolar IC50 but off target toxicity limits its use in vivo. D. DGAT inhibitors are well-represented in the literature because of DGATs position in the TAG biosynthetic pathway.
Lipin1 is the most biochemically and pathophysiologically characterized member of the lipin family. Genetic studies on the fatty liver dystrophic (fld) mouse identified the lipin gene. fld mice exhibit extreme hypertriglyceridemia, developmental retardation, early mortality, and abnormal adipose tissue development [281]. Interestingly, these animals maintained normal TAG levels in the liver. This characteristic fld phenotype was subsequently linked to a loss-of-function mutation in the lipin1 protein [279]. Later studies found that lipin2 compensates for the defective lipin1 in hepatocytes [282].
Given the importance of lipin to anabolic metabolism of fats, researchers postulated that nutrient-sensing mechanisms regulated the activity of this enzyme. Less than a year after the initial characterization of the lipin gene from fld mice, Lawrence and coworkers identified several mTORC1-specific phosphorylation sites on the lipin protein, placing it under the umbrella of insulin signaling [283]. In spite of these findings, regulation of lipin activity is not conducted strictly via post-translational modifications. Rather, sub-cellular translocation of lipin results in phosphorylation and increases enzymatic activity [284]. Carefully controlled expression mechanisms also account for regulations in lipin activity. For example peroxisome proliferator-activated receptor γ (PPARγ) regulates lipin1 expression to increase fatty acid oxidation and oxidative phosphorylation in the mitochondria [285].
Loss-of-function lipin1 mutations in humans do not exhibit the extreme lipodystrophy observed in fld mice. However, recent studies demonstrated that perturbations in lipin1 expression in humans result in insulin-insensitivity and poor glucose metabolism [286–288]. Thus small molecule activators of lipin1 activity may prove an important class of small molecule chemical tools to study the role of lipin in normal metabolic flux.
5.1.2. Tools for modulation of enzymatic activity
Metabolic disorders such as lipodystrophy and type 2 diabetes mellitus are mounting health crises facing the developed world. Identification of loss-of-function lipin1 mutants in the fld mouse demonstrates the enzyme’s critical role in hepatic TAG production and circulating lipid levels. Hormonal and transcriptional regulators critical to energy balance, PPARγ and insulin, are known modulators of the expression and activity of lipin1. Taken together, these studies suggest that lipin1 is central to lipid metabolism and energy balance in vivo.
Despite the growing importance of lipin1 to metabolic homeostasis, no small molecules capable of inhibiting the enzymatic activity of lipin currently exist. Interestingly, these enzymes regulate the same biosynthetic equilibrium as the DGKs, cellular concentrations of PA and DAG, which are critical lipid second messengers in cells. While genetic perturbation studies identified critical roles of lipin in metabolic development, the ability to acutely inhibit the activity of this enzyme may reveal new and important roles for lipin in physiological processes.
6. Fatty acyltransferases
The de novo biosynthesis of triacylglycerol involves a series of fatty acyltransferase enzymes working in a stepwise fashion resulting in the primary energy storage molecule in eukaryotic cells (Fig. 8A). Biosynthesis begins with the sequential acylation of glycerol-3-phosphate (G3P) at the sn-1 position by the enzyme glycerol-3-phosphate acyltransferase (GPAT) [289]; the enzyme uses acyl-CoAs as lipid donors to form LPA. Step two is the acylation of the sn-2 position of LPA with acyl-CoA by 1-acylglycerol-3-phosphate acyltransferase, also known as lysophosphatidic acid acyltransferase (LPAAT), to generate PA. After dephosphorylation by lipin, the resulting DAG is finally esterified by DAG acyltransferase (DGAT) to produce TAG.
6.1. GPAT
6.2.1. Enzyme activity and regulation
First described by Kennedy [290], GPAT commences de novo phospholipid and TAG biosynthesis by esterifying G3P with fatty acyl CoA at the sn-1 position (Fig. 8A). Initial studies identified two GPAT isoforms that differed in cellular and tissue localization as well as substrate preference [291, 292]. Further study identified two additional GPAT isoforms, each as a unique gene product [289, 293]. GPAT1 is an intrinsic membrane protein primarily located in the mitochondria of hepatic cells [294] and preferentially acetylates G3P with saturated fatty acyl-CoAs [293], particularly 16:0. The enzyme regulates hepatic TAG levels by controlling TAG biosynthesis [295]. GPAT1−/− mice, despite being obese, display 60% less TAG content in the liver compared to their wild-type littermates [296]. Gene expression, primarily facilitated by SREBP-1c, regulates GPAT1 expression in response to nutritional and hormonal cues. During periods of fasting and decreased fat storage, GPAT1 activity also decreases in the liver [297].
GPAT2, the second mitochondrial isoform, displays no obvious substrate preference. The enzyme has 72% sequence similarity to GPAT1 and an estimated mass of 89 KDa. GPAT2 is much more highly expressed in testis than GPAT1 and is likely to have a specialized function there [297]. Experimental evidence suggests that GPAT2 expression in the liver occurs independently of nutritional fluxes [297].
The recently identified GPAT3 and 4 associate closely with the endoplasmic reticulum [298–300]. Both exhibit similar levels of expression in adipose tissues, liver, and heart. However, 3T3-L1 differentiating adipocytes specifically induce high levels of GPAT3 [301]. GPAT3 and 4 lack the extended C-terminal region found in GPAT1 and 2, suggesting important differences in protein regulation.
6.1.2. Tools for modulation of enzymatic activity
Of the four isoforms, only GPAT1 exhibits strong substrate specificity. In addition, GPAT1 is the only isoform regulated on a transcriptional level by both diet and exercise. Chronic high fat diet induces GPAT1 overexpression resulting in increased phospholipid biosynthesis and TAG accumulation. Meanwhile physical exercise decreases GPAT1 activity in the liver and adipose tissue [302]. Thus, inhibition of this enzyme should result in decreased TAG levels and serve as a treatment for metabolic disorders.
Small molecule inhibitors
The first report of design and synthesis of small molecules for GPAT inhibition identified several compounds based on sulfonamidobenzoic acid [303]. While several classes of compounds were synthesized and screened for activity, only 2-(nonylsulfonamido)benzoic acid (FSG67) showed a moderate inhibitory effect with an IC50 of ~25 μM in an in vitro assay (Fig. 8B). When administered to obese mice on a high fat diet, FSG67 decreased body weight and food intake [304]. A second class of GPAT inhibitors attempted to mimic the structure of fatty acyl-CoAs with alkylsulfonamide and carboxylate groups. Unfortunately, these compounds did not significantly inhibit GPAT catalytic activity [305].
6.2. Lysophosphatidic Acyltransferase (LPAAT)
6.2.1. Enzyme activity and regulation
A number of mechanisms account for total PA production in the cell. De novo biosynthesis relies on the sn-2 acylation of LPA by LPAAT (Fig. 8A). Kennedy published the first reports of LPAAT activity in the early 1960s [306, 307], but the six human LPAAT isoforms (α-ζ) remained uncharacterized until the late 1990s [308]. The α and β isoforms are the most well studied LPAAT isozymes [309] and possess relatively low molecular weights (31.7 and 30.9 KDa, respectively [308]). Humans express LPAATα in most tissues but the enzyme is more abundant in brain, heart, spleen, thymus, kidney, and skeletal muscle [308]. Expression of the β isoform is limited to the liver, pancreas, adipose tissue and heart. Interestingly, select tumor types overexpress LPAATβ, specifically lung, colon, cervix, brain, breast, ovary, and prostate [308, 310, 311]. The α isomer preferentially adds 14:0, 16:0, and 18:2 fatty acyl CoAs to LPA while the β isomer preferentially adds 18:1, 18:2, and 18:3 fatty acyl CoAs [309]. Specific mechanisms for LPAAT regulation remain uncharacterized.
6.2.2. Tools for modulation of enzymatic activity
The elevated level of LPAATβ in oncogenic tissues suggests a unique role of this isoform in disease pathogenicity. Investigations into the sources of PA within cells (PLD, DGK, LPAAT) imply that LPAATβ generates a significant pool of PA involved in survival signaling. Indeed, studies modulating LPAATβ function demonstrate that certain cancers rely on this activity for survival [311, 312].
Small molecule inhibitors
Existing LPAATβ inhibitors arose from work conducted by Cell Therapeutics, Inc. [311–317]. High-throughput screening of small molecule libraries identified a number of isoform-selective inhibitors based on aryldiaminotriazines, which displayed IC50 values in the nanomolar range [311]. Another second group of inhibitors based on triazine and pyrimidine core structures are also quite potent. The most potent triazine found in this study was CT-32228 (Fig. 8C), which had an IC50 of 0.06 μM whereas CT-32521, the most potent pyrimidine-based inhibitor displays an IC50 of 0.16 μM [314]. Compound CT-32228 inhibits proliferation of several non-Hodgkins lymphoma cell lines in vitro with IC50 values between 0.025 to 0.05 μM. However, relevant doses of CT-32228 in vivo resulted in off-target toxicity, making the compound unsuitable for clinical development [314].
Further studies identified three additional structural classes of small molecules based on 2-arylbenzoxazole, 2-arylbenzothiazole, and 2-arylbenzimidazole scaffolds [316]. Despite most of these analogues showing potent antiproliferative properties in breast and prostate carcinoma cell lines in vitro, decreased growth effect phenotypes required relatively high concentrations (IC50s 15–50 μM), making in vivo experimentation challenging.
6.3. DGAT
6.3.1. Enzyme activity and regulation
Whether TAG is synthesized through the canonical “Kennedy pathway” [318] or the MAG pathway [319], TAG biosynthesis requires DGAT activity (Fig. 8A) [289, 291, 320]. Two DGAT isoforms exist in eukaryotes, both localized to the endoplasmic reticulum. Tissues specializing in TAG storage and biosynthesis display elevated DGAT1 and 2 mRNA levels expression, which correlates to increased TAG production [289]. There are significant differences between the two isoforms: DGAT1 belongs to the acyl-CoA:cholesterol acyltransferase (ACAT) enzyme family, while DGAT2 is part of a 7 member family more closely related to the MGAT enzymes.
DGAT1 is a 55 kDa protein abundantly expressed in adipose tissue, small intestine, liver, and mammary glands [321]. This versatile enzyme catalyzes DAG, retinol and wax esterification due to its broad acyltransferase activity [320]. The DGAT2 isozyme was not identified until 2001 [322] and is smaller than DGAT1 with a molecular mass of 40–44 kDa but contains the conserved amino acid sequences essential for neutral lipid binding. Unlike DGAT1, DGAT2 has greater substrate specificity and does not catalyze the synthesis of wax or retinyl esters. There is no obvious substrate preference for either isoform with respect to chain length and unsaturation of acyl CoAs. Both isoforms exhibit similar expression patterns, mostly in tissues and organs that generate large amounts of TAG and are regulated by transcriptional regulators of adipogenesis. Nutritional and hormonal cues regulate DGAT1 and 2 activities at the transcriptional level [323, 324].
Dysregulation of either DGAT enzyme contributes to metabolic disorders such as diabetes, liver steatosis, obesity, and insulin resistance. DGAT’s role in energy metabolism, leptin sensitivity, glucose metabolism [325], lipotoxicity [326], and inflammation [327] makes it an important therapeutic target. While a DGAT1 deficiency results in reduced TAG production and resistance to high-fat diet-induced obesity [328], loss of DGAT2 is lethal in neonatal mice [329]. These observations suggest that DGAT1 and 2 have non-redundant, non-compensatory functions in spite of overlapping catalytic activity and tissue expression. Wurie and colleagues published a report suggesting that DGAT2 acts upstream of DGAT1 and serves as the rate-limiting step for de novo TAG synthesis. Meanwhile, DGAT1 is largely responsible for the production of TAG via DAG re-esterification [330].
6.3.2. Tools for modulation of enzymatic activity
Due to their involvement in a wide range of metabolic diseases, the DGAT enzymes are now a major target for pharmacological intervention. Coleman et al. [289] and Dow [331] published excellent reviews that are especially useful in regards to DGAT enzymatic structure, function, and regulation as well as inhibition. There is a profound interest in investigating natural and synthetic inhibitors of this enzyme, resulting in a large number of reports in the literature.
Natural products as inhibitors
De novo TAG biosynthesis and metabolism are essential physiological processes in mammals, illustrated by the neonatal lethality seen in DGAT2−/− mice [330]. However, perturbation of DGAT1 activity may serve as an ideal strategy for the treatment for metabolic disorders such as diabetes and obesity [327, 332]. A number of natural products isolated from microorganisms, plants, and fish oils possess DGAT inhibitory effects with IC50 values in the μM range (50–200 μM) [333–340]. Eight prenylflavonoids extracted from the bark of Erythrina senegalensis were tested against DGAT activity in vitro and six compounds displayed inhibitory activity (IC50 from 1.1 to 15 μg/ml) [339]. Four sesquiterpenoids isolated from the flower buds of Tussilago farfara inhibited DGAT1 activity in rat liver and HepG2 microsomes in the mid to high micromolar range (IC50 from 18 to ~300 μM) [340]. Two xantohumols (one newly identified) from the hops of Humulus lupulus exhibited modest DGAT activity in rat liver microsomes with IC50 values of 50 and 194 μM [333].
The only tested inhibitor of DGAT2, niacin (vitamin B3), which pharmacologically reduces levels of plasma TAG and LDL, is active in an in vitro assay with an IC50 of 0.1 mM [341]. The primary therapeutic effect of niacin is mediated through G protein-coupled receptors (GPR109A/B).
Small molecule inhibitors
Medicinal chemistry efforts focused primarily on DGAT1 inhibition. Together, academic research labs and pharmaceutical companies report a variety of DGAT1-selective inhibitors. However a single DGAT2-prefering small molecule inhibitor exists, possessing IC50 values towards hDGAT2 and hDGAT1 of 8.5 and 70.8 μM, respectively [330].
Pharmaceutical companies licensed a number of potent compounds targeting DGAT1 inhibition in the last decade [342–349]. The two major chemical scaffolds that led to effective inhibitors are based on lipophillic carboxylic acids and were disclosed almost simultaneously in 2004 by Bayer and Japan Tobacco/Tularik(Amgen). Bayer published inhibitors based on arylamine keto acids and introduced BAY 74-4113 [342], while Japan Tobacco/Tularik introduced aminopyrimidinooxazine phenyl cyclohexyl acetic acid-based T863 [344, 345]. Both compounds displayed low nanomolar inhibition against DGAT1 catalytic activity (IC50 of 83 nM for BAY 74-4113 and 72 nM for T863) but showed photoinstability and potential toxicity. Improvements to bioavailability and potency of the leading Bayer structure by Abbott resulted in a compound used in animal preclinical studies [343]. Pfizer redesigned their approach to DGAT inhibitors based on the lead from T863 which yielded an improved compound (PF-04620110) currently advancing to human clinical trials for treatment of type 2 diabetes [346]. Another research team from Astra Zeneca utilizing high throughput screening techniques identified a compound with improved properties (AZD 7687) [347, 348] which is presently in phase I human clinical trials for treatment of obesity and type 2 diabetes. A second compound, AZD 3988, is also being evaluated as a clinical candidate [349].
Efforts from other pharmaceutical companies continue to expand the base of DGAT inhibitors based on novel structural scaffolds. Bristol-Myers Squibb introduced a triazolo compound with a low nanomolar IC50=7 nM [350], Hoffman-La Roche expanded the class of amide-based inhibitors, and reported phenyl methyloxazole carboxamide derivatives as potent DGAT1 inhibitors [351]. Sanofi- Aventis published thiadiazoles as competitive DGAT1 inhibitors, based on three different scaffolds with IC50 values below 100 nM [352]. Recent work continues to discover improved analogues of potent carboxylic acid-based DGAT inhibitors [353] and including novel scaffolding elements such as benzimidazole [354] or other amide based isoxasole-, oxazole-, oxadiazole-, and thiazole-containing heteroaryl analogs of biaryl ureas with low nanomolar inhibitory properties [349, 355–358].
Thus far, the most potent inhibitors of DGAT remain phenylcyclohexylacetic acids containing varied heterocyclic cores. These compounds display low-nanomolar potency, good selectivity and solubility as well as excellent oral pharmacokinetic properties. However, only a few of these compounds advanced to clinical trials [331]: LCQ-908 from Novartis [359], PF-04620110 from Pfizer [346], and AZD7687 from AstraZeneca [347].
7. Conclusions
Successful delineation of signaling pathways in physiological and diseases in vitro and in vivo depends upon the selection of the appropriate tool. Usually, chemical modulators must discriminate between multiple enzyme isoforms since each may regulate independent pools of signaling lipids. Monumental medicinal chemistry efforts have sought to address these questions of inhibitor potency and selectivity throughout the years. These efforts resulted in a number of compounds that manipulate glycerolipid-signaling pathways in model systems. Navigating the resulting deluge of information is a challenge even for those familiar with the field. This review provides a manageable view of the current published knowledge base, highlighting some of the more useful and applicable chemical tools.
Metabolic disorders such as lipodystrophy and type 2 diabetes remain the largest health problems facing developed countries such as the United States. Lipid metabolism directly affects the progression of these diseases in vivo. Unfortunately, these global metabolic disorders propagate through complex and integrated signaling circuits, which are difficult to recreate in vitro since they often include compensatory redundancies. Glycerolipid-metabolizing enzymes such as lipin, ATGL and HSL clearly contribute to these signaling circuits—their dysfunction facilitates disease progression. Similar mechanisms are at work in hyper-proliferative diseases such as cancer: growth factors and hormonal signaling simultaneously regulate cancer progression, lipid metabolism, and glycerolipid-signaling circuits. As a guiding principle, cancer should be viewed as a systematic dysfunction of metabolic processes. Given the complexity of these metabolic disorders—both in cancer and in metabolic syndrome—their detailed characterization is only possible in vivo. Currently, a major limitation of glycerolipid biosynthetic inhibitors is their bioavailability and DMPK properties. Moving forward, medicinal chemistry efforts must focus on the optimization of in vivo properties of inhibitors to characterize the role of lipid metabolism in vivo. Perhaps this next wave of glycerolipid biosynthetic inhibitors will provide scaffolds for the next generation of therapeutics combating metabolic disorders and cancer.
Highlights.
Glycerolipid signaling pathways play important role in human physiology and disease
Glycerolipid metabolizing enzymes are attractive targets for therapeutic intervention
Chemical modulators interrupting glycerolipid signaling are essential tools in research
Acknowledgments
The authors acknowledge partial support from the McDonnell Foundation for Brain Cancer Research, Bixler-Johnson-Mayes Endowed Chair (to HAB), William K. Warren Jr. Chair in Medicine (to CWL), NIH Grant U54 MH084659, and NIEHS Grant P01-ES013125.
Abbreviations
- PLC
phospholipase C
- PLD
phospholipase D
- PLA
phospholipase A
- PtdBuOH
phosphatidylbutanol
- PC
phosphatidylcholine
- PA
phosphatidic acid
- PG
phosphatidylglycerol
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- LPC
lysophosphatidylcholine
- LPA
lysophosphatidic acid
- ATX
autotaxin
- PIP2
phosphatidylinositol bisphosphate
- DAG
diacylglycerol
- AA
arachidonic acid
- 2-AG
2-arachidonoylglycerol
- FA
fatty acid
- FFA
free fatty acid
- DGK
diacylglycerol kinase
- PI 3-kinase
phosphatidylinositol 3-kinase
- MGLL
monoacylglycerol lipase
- DAGL
diacylglycerol lipase
- TAG
triacylglycerol
- HSL
hormone-sensitive lipase
- ATGL
adipose triacylglycerol lipase
- CGI-58
comparative gene identification 58
- ADHD5
α/β hydrolase domain-containing protein 5
- LPAAT
lysophosphatidic acid acyltransferase
- PNPLA
patatin-like phospholipase domain-containing proteins
- GPAT
glycerol-3-phosphate acyltransferase
- DGAT
diacylglycerol acyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 2.Kadamur G, Ross EM. Mammalian phospholipase C. Annu Rev Physiol. 2013;75:127–154. doi: 10.1146/annurev-physiol-030212-183750. [DOI] [PubMed] [Google Scholar]
- 3.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 4.Harden TK, Waldo GL, Hicks SN, Sondek J. Mechanism of activation and inactivation of Gq/phospholipase C-beta signaling nodes. Chem Rev. 2011;111:6120–6129. doi: 10.1021/cr200209p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunney TD, Katan M. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 2006;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Vines CM. Phospholipase C. Adv Exp Med Biol. 2012;740:235–254. doi: 10.1007/978-94-007-2888-2_10. [DOI] [PubMed] [Google Scholar]
- 7.Berstein G, Blank JL, Jhon DY, Exton JH, Rhee SG, Ross EM. Phospholipase C-beta 1 is a GTPase-activating protein for Gq/11, its physiologic regulator. Cell. 1992;70:411–418. doi: 10.1016/0092-8674(92)90165-9. [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft S, Thomas GM. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J. 1992;288:1–14. doi: 10.1042/bj2880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimohama S, Homma Y, Suenaga T, Fujimoto S, Taniguchi T, Araki W, Yamaoka Y, Takenawa T, Kimura J. Aberrant accumulation of phospholipase C-delta in Alzheimer brains. Am J Pathol. 1991;139:737–742. [PMC free article] [PubMed] [Google Scholar]
- 10.Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol. 2003;161:79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Ichinohe M, Hirata M, Matsuura H, Fujiwara T, Igarashi T, Nakahara M, Yamaguchi H, Yasugi S, Takenawa T, Fukami K. Phospholipase C-delta1 is an essential molecule downstream of Foxn1, the gene responsible for the nude mutation, in normal hair development. FASEB J. 2008;22:841–849. doi: 10.1096/fj.07-9239com. [DOI] [PubMed] [Google Scholar]
- 12.Kim JK, Choi JW, Lim S, Kwon O, Seo JK, Ryu SH, Suh PG. Phospholipase C-eta1 is activated by intracellular Ca(2+) mobilization and enhances GPCRs/PLC/Ca(2+) signaling. Cell Signal. 2011;23:1022–1029. doi: 10.1016/j.cellsig.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–227. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 16.Adibhatla RM, Hatcher JF, Gusain A. Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature. Neurochem Res. 2012;37:671–679. doi: 10.1007/s11064-011-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuschieri J, Billgren J, Maier RV. Phosphatidylcholine-specific phospholipase C (PC-PLC) is required for LPS-mediated macrophage activation through CD14. J Leukoc Biol. 2006;80:407–414. doi: 10.1189/jlb.1105622. [DOI] [PubMed] [Google Scholar]
- 18.Paris L, Cecchetti S, Spadaro F, Abalsamo L, Lugini L, Pisanu ME, Iorio E, Natali PG, Ramoni C, Podo F. Inhibition of phosphatidylcholine-specific phospholipase C downregulates HER2 overexpression on plasma membrane of breast cancer cells. Breast Cancer Res. 2010;12:R27. doi: 10.1186/bcr2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 20.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 21.Hou C, Kirchner T, Singer M, Matheis M, Argentieri D, Cavender D. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-di one (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- 22.Klein RR, Bourdon DM, Costales CL, Wagner CD, White WL, Williams JD, Hicks SN, Sondek J, Thakker DR. Direct activation of human phospholipase C by its well known inhibitor u73122. J Biol Chem. 2011;286:12407–12416. doi: 10.1074/jbc.M110.191783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52:2835–2840. [PubMed] [Google Scholar]
- 24.Huang W, Barrett M, Hajicek N, Hicks S, Harden TK, Sondek J, Zhang Q. Small molecule inhibitors of phospholipase C from a novel high-throughput screen. J Biol Chem. 2013;288:5840–5848. doi: 10.1074/jbc.M112.422501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amtmann E, Muller-Decker K, Hoss A, Schalasta G, Doppler C, Sauer G. Synergistic antiviral effect of xanthates and ionic detergents. Biochem Pharmacol. 1987;36:1545–1549. doi: 10.1016/0006-2952(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 26.Amtmann E. The antiviral, antitumoural xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phospholipase C. Drugs Exp Clin Res. 1996;22:287–294. [PubMed] [Google Scholar]
- 27.Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J Biol Chem. 1998;273:14550–14559. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan DJ, Chaikoff IL. A new phospholipide-splitting enzyme specific for the ester linkage between the nitrogenous base and the phosphoric acid grouping. J Biol Chem. 1947;169:699–705. [PubMed] [Google Scholar]
- 29.Hanahan DJ, Chaikoff IL. The phosphorus-containing lipides of the carrot. J Biol Chem. 1947;168:233–240. [PubMed] [Google Scholar]
- 30.Selvy PE, Lavieri RR, Lindsley CW, Brown HA. Phospholipase D: Enzymology, functionality, and chemical modulation. Chem Rev. 2011;111:6064–6119. doi: 10.1021/cr200296t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott M, Wakelam MJ, Morris AJ. Phospholipase D. Biochem Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 32.Ponting CP, Kerr ID. A novel family of phospholipase D homologues that includes phospholipid synthases and putative endonucleases: Identification of duplicated repeats and potential active site residues. Protein Sci. 1996;5:914–922. doi: 10.1002/pro.5560050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Exton JH. Regulation of phospholipase D. FEBS letters. 2002;531:58–61. doi: 10.1016/s0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- 34.Melendez AJ, Allen JM. Phospholipase D and immune receptor signalling. Semin Immunol. 2002;14:49–55. doi: 10.1006/smim.2001.0341. [DOI] [PubMed] [Google Scholar]
- 35.Cook SJ, Wakelam MJ. Epidermal growth factor increases sn-1,2-diacylglycerol levels and activates phospholipase D-catalysed phosphatidylcholine breakdown in Swiss 3T3 cells in the absence of inositol-lipid hydrolysis. Biochem J. 1992;285:247–253. doi: 10.1042/bj2850247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plevin R, Cook SJ, Palmer S, Wakelam MJ. Multiple sources of sn-1,2-diacylglycerol in platelet-derived-growth-factor-stimulated Swiss 3T3 fibroblasts. Evidence for activation of phosphoinositidase C and phosphatidylcholine-specific phospholipase D. Biochem J. 1991;279:559–565. doi: 10.1042/bj2790559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato TK, Overduin M, Emr SD. Location, location, location: membrane targeting directed by PX domains. Science. 2001;294:1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- 38.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henage LG, Exton JH, Brown HA. Kinetic analysis of a mammalian phospholipase D: Allosteric modulation by monomeric GTPases, protein kinase C, and polyphophoinositides. J Biol Chem. 2006;281:3408–3417. doi: 10.1074/jbc.M508800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes WE, Parker PJ. Endosomal localization of phospholipase D 1a and 1b is defined by the C-termini of the proteins, and is independent of activity. Biochem J. 2001;356:727–736. doi: 10.1042/0264-6021:3560727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du G, Altshuller YM, Vitale N, Huang P, Chasserot-Golaz S, Morris AJ, Bader MF, Frohman MA. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J Cell Biol. 2003;162:305–315. doi: 10.1083/jcb.200302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 43.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 44.Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 45.Sung TC, Altshuller YM, Morris AJ, Frohman MA. Molecular analysis of mammalian phospholipase D2. J Biol Chem. 1999;274:494–502. doi: 10.1074/jbc.274.1.494. [DOI] [PubMed] [Google Scholar]
- 46.Singer WD, Brown HA, Bokoch GM, Sternweis PC. Resolved phospholipase D activity is modulated by cytosolic factors other than Arf. J Biol Chem. 1995;270:14944–14950. doi: 10.1074/jbc.270.25.14944. [DOI] [PubMed] [Google Scholar]
- 47.Malcolm KC, Ross AH, Qiu RG, Symons M, Exton JH. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994;269:25951–25954. [PubMed] [Google Scholar]
- 48.Exton JH. New developments in phospholipase D. J Biol Chem. 1997;272:15579–15582. doi: 10.1074/jbc.272.25.15579. [DOI] [PubMed] [Google Scholar]
- 49.Ortmeyer J, Mohsenin V. Inhibition of phospholipase D and superoxide generation by glucose in diabetic neutrophils. Life Sci. 1996;59:255–262. doi: 10.1016/0024-3205(96)00284-6. [DOI] [PubMed] [Google Scholar]
- 50.Lee HY, Yea K, Kim J, Lee BD, Chae YC, Kim HS, Lee DW, Kim SH, Cho JH, Jin CJ, Koh DS, Park KS, Suh PG, Ryu SH. Epidermal growth factor increases insulin secretion and lowers blood glucose in diabetic mice. J Cell Mol Med. 2008;12:1593–1604. doi: 10.1111/j.1582-4934.2007.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steed PM, Chow AH. Intracellular signaling by phospholipase D as a therapeutic target. Curr Pharm Biotechnol. 2001;2:241–256. doi: 10.2174/1389201013378644. [DOI] [PubMed] [Google Scholar]
- 52.Cai D, Zhong M, Wang R, Netzer WJ, Shields D, Zheng H, Sisodia SS, Foster DA, Gorelick FS, Xu H, Greengard P. Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons. Proc Natl Acad Sci U S A. 2006;103:1936–1940. doi: 10.1073/pnas.0510710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, Wenk MR, Arancio O, Di Paolo G. Phospholipase d2 ablation ameliorates Alzheimer’s disease-linked synaptic dysfunction and cognitive deficits. J Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uchida N, Okamura S, Kuwano H. Phospholipase D activity in human gastric carcinoma. Anticancer Res. 1999;19:671–675. [PubMed] [Google Scholar]
- 55.Noh DY, Ahn SJ, Lee RA, Park IA, Kim JH, Suh PG, Ryu SH, Lee KH, Han JS. Overexpression of phospholipase D1 in human breast cancer tissues. Cancer Lett. 2000;161:207–214. doi: 10.1016/s0304-3835(00)00612-1. [DOI] [PubMed] [Google Scholar]
- 56.Uchida N, Okamura S, Nagamachi Y, Yamashita S. Increased phospholipase D activity in human breast cancer. J Cancer Res Clin Oncol. 1997;123:280–285. doi: 10.1007/BF01208639. [DOI] [PubMed] [Google Scholar]
- 57.Yamada Y, Hamajima N, Kato T, Iwata H, Yamamura Y, Shinoda M, Suyama M, Mitsudomi T, Tajima K, Kusakabe S, Yoshida H, Banno Y, Akao Y, Tanaka M, Nozawa Y. Association of a polymorphism of the phospholipase D2 gene with the prevalence of colorectal cancer. J Mol Med. 2003;81:126–131. doi: 10.1007/s00109-002-0411-x. [DOI] [PubMed] [Google Scholar]
- 58.Park MH, Ahn BH, Hong YK, Min do S. Overexpression of phospholipase D enhances matrix metalloproteinase-2 expression and glioma cell invasion via protein kinase C and protein kinase A/NF-kappaB/Sp1-mediated signaling pathways. Carcinogenesis. 2009;30:356–365. doi: 10.1093/carcin/bgn287. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Ehara H, Akao Y, Shamoto M, Nakagawa Y, Banno Y, Deguchi T, Ohishi N, Yagi K, Nozawa Y. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem Biophys Res Commun. 2000;278:140–143. doi: 10.1006/bbrc.2000.3719. [DOI] [PubMed] [Google Scholar]
- 60.Su W, Chen Q, Frohman MA. Targeting phospholipase D with small-molecule inhibitors as a potential therapeutic approach for cancer metastasis. Future Oncol. 2009;5:1477–1486. doi: 10.2217/fon.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang P, Frohman MA. The potential for phospholipase D as a new therapeutic target. Expert Opin Ther Targets. 2007;11:707–716. doi: 10.1517/14728222.11.5.707. [DOI] [PubMed] [Google Scholar]
- 62.Lindsley CW, Brown HA. Phospholipase D as a therapeutic target in brain disorders. Neuropsychopharmacology. 2012;37:301–302. doi: 10.1038/npp.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawson RM. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967;102:205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang SF, Freer S, Benson AA. Transphosphatidylation by phospholipase D. J Biol Chem. 1967;242:477–484. [PubMed] [Google Scholar]
- 65.Randall RW, Bonser RW, Thompson NT, Garland LG. A novel and sensitive assay for phospholipase D in intact cells. FEBS Lett. 1990;264:87–90. doi: 10.1016/0014-5793(90)80772-b. [DOI] [PubMed] [Google Scholar]
- 66.Seidler L, Kaszkin M, Kinzel V. Primary alcohols and phosphatidylcholine metabolism in rat brain synaptosomal membranes via phospholipase D. Pharmacol Toxicol. 1996;78:249–253. doi: 10.1111/j.1600-0773.1996.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 68.Zheng XL, Gui Y, Du G, Frohman MA, Peng DQ. Calphostin-C induction of vascular smooth muscle cell apoptosis proceeds through phospholipase D and microtubule inhibition. J Biol Chem. 2004;279:7112–7118. doi: 10.1074/jbc.M310721200. [DOI] [PubMed] [Google Scholar]
- 69.Tou J, Urbizo C. Resveratrol inhibits the formation of phosphatidic acid and diglyceride in chemotactic peptide- or phorbol ester-stimulated human neutrophils. Cell Signal. 2001;13:191–197. doi: 10.1016/s0898-6568(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 70.Tou JS, Urbizo C. Diethylstilbestrol inhibits phospholipase D activity and degranulation by stimulated human neutrophils. Steroids. 2008;73:216–221. doi: 10.1016/j.steroids.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Garcia A, Zheng Y, Zhao C, Toschi A, Fan J, Shraibman N, Brown HA, Bar-Sagi D, Foster DA, Arbiser JL. Honokiol suppresses survival signals mediated by Ras-dependent phospholipase D activity in human cancer cells. Clin Cancer Res. 2008;14:4267–4274. doi: 10.1158/1078-0432.CCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puar MS, Barrabee E, Hallade M, Patel M. Sch 420789: a novel fungal metabolite with phospholipase D inhibitory activity. J Antibiot. 2000;53:837–838. doi: 10.7164/antibiotics.53.837. [DOI] [PubMed] [Google Scholar]
- 73.Pai JK, Frank EA, Blood C, Chu M. Novel ketoepoxides block phospholipase D activation and tumor cell invasion. Anticancer Drug Des. 1994;9:363–372. [PubMed] [Google Scholar]
- 74.McDonald LA, Barbieri LR, Bernan VS, Janso J, Lassota P, Carter GT. 07H239-A, a new cytotoxic eremophilane sesquiterpene from the marine-derived Xylariaceous fungus LL-07H239. J Nat Prod. 2004;67:1565–1567. doi: 10.1021/np049924g. [DOI] [PubMed] [Google Scholar]
- 75.Levy BD, Hickey L, Morris AJ, Larvie M, Keledjian R, Petasis NA, Bannenberg G, Serhan CN. Novel polyisoprenyl phosphates block phospholipase D and human neutrophil activation in vitro and murine peritoneal inflammation in vivo. Br J Pharmacol. 2005;146:344–351. doi: 10.1038/sj.bjp.0706338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop’ signaling switch for aspirin-triggered lipoxin A4. FASEB J. 1999;13:903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 77.Eisen SF, Brown HA. Selective estrogen receptor (ER) modulators differentially regulate phospholipase D catalytic activity in ER-negative breast cancer cells. Mol Pharmacol. 2002;62:911–920. doi: 10.1124/mol.62.4.911. [DOI] [PubMed] [Google Scholar]
- 78.Monovich L, Mugrage B, Quadros E, Toscano K, Tommasi R, LaVoie S, Liu E, Du Z, LaSala D, Boyar W, Steed P. Optimization of halopemide for phospholipase D2 inhibition. Bioorg Med Chem Lett. 2007;17:2310–2311. doi: 10.1016/j.bmcl.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 79.Loonen AJ, van Wijngaarden I, Janssen PA, Soudijn W. Regional localization of halopemide, a new psychotropic agent, in the rat brain. Eur J Pharmacol. 1978;50:403–408. doi: 10.1016/0014-2999(78)90146-2. [DOI] [PubMed] [Google Scholar]
- 80.Su W, Yeku O, Olepu S, Genna A, Park JS, Ren H, Du G, Gelb MH, Morris AJ, Frohman MA. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol Pharmacol. 2009;75:437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faugaret D, Chouinard FC, Harbour D, Elazreq MA, Bourgoin SG. An essential role for phospholipase D in the recruitment of vesicle amine transport protein-1 to membranes in human neutrophils. Biochem Pharmacol. 2011;81:144–156. doi: 10.1016/j.bcp.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Elvers M, Grenegård M, Khoshjabinzadeh H, Münzer P, Borst O, Tian H, Di Paolo G, Lang F, Gawaz M, Lindahl TL, Fälker K. A novel role for phospholipase D as an endogenous negative regulator of platelet sensitivity. Cell Signal. 2012;24:1743–1752. doi: 10.1016/j.cellsig.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 83.Stegner D, Thielmann I, Kraft P, Frohman MA, Stoll G, Nieswandt B. Pharmacological inhibition of phospholipase D protects mice from occlusive thrombus formation and ischemic stroke--brief report. Arterioscler Thromb Vasc Biol. 2013;33:2212–2217. doi: 10.1161/ATVBAHA.113.302030. [DOI] [PubMed] [Google Scholar]
- 84.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis JA, Scott SA, Lavieri R, Buck JR, Selvy PE, Stoops SL, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity. Bioorg Med Chem Lett. 2009;19:1916–1920. doi: 10.1016/j.bmcl.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavieri RR, Scott SA, Selvy PE, Kim K, Jadhav S, Morrison RD, Daniels JS, Brown HA, Lindsley CW. Design, synthesis, and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)ethyl)benzamides: discovery of an isoform-selective small molecule phospholipase D2 inhibitor. J Med Chem. 2010;53:6706–6719. doi: 10.1021/jm100814g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavieri RR, Scott SA, Lewis JA, Selvy PE, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part II. Identification of the 1,3,8-triazaspiro[4,5]decan-4-one privileged structure that engenders PLD2 selectivity. Bioorg Med Chem Lett. 2009;19:2240–2243. doi: 10.1016/j.bmcl.2009.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Reilly MC, Scott SA, Brown KA, Oguin TH, 3rd, Thomas PG, Daniels JS, Morrison R, Brown HA, Lindsley CW. Development of dual PLD1/2 and PLD2 selective inhibitors from a common 1,3,8-Triazaspiro[4.5]decane Core: discovery of Ml298 and Ml299 that decrease invasive migration in U87-MG glioblastoma cells. J Med Chem. 2013;56:2695–2699. doi: 10.1021/jm301782e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 2007;89:197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Aoki J, Nagai Y, Hosono H, Inoue K, Arai H. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim Biophys Acta. 2002;1582:26–32. doi: 10.1016/s1388-1981(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 91.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 92.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 95.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karabina SA, Gora S, Atout R, Ninio E. Extracellular phospholipases in atherosclerosis. Biochimie. 2010;92:594–600. doi: 10.1016/j.biochi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov. 2003;2:179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 99.Carlquist JF, Muhlestein JB, Anderson JL. Lipoprotein-associated phospholipase A2: a new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn. 2007;7:511–517. doi: 10.1586/14737159.7.5.511. [DOI] [PubMed] [Google Scholar]
- 100.Magrioti V, Kokotos G. Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin Ther Pat. 2010;20:1–18. doi: 10.1517/13543770903463905. [DOI] [PubMed] [Google Scholar]
- 101.Snyder DW, Bach NJ, Dillard RD, Draheim SE, Carlson DG, Fox N, Roehm NW, Armstrong CT, Chang CH, Hartley LW, Johnson LM, Roman CR, Smith AC, Song M, Fleisch JH. Pharmacology of LY315920/S-5920, [[3-(aminooxoacetyl)-2-ethyl-1- (phenylmethyl)-1H-indol-4-yl]oxy] acetate, a potent and selective secretory phospholipase A2 inhibitor: A new class of anti-inflammatory drugs, SPI. J Pharmacol Exp Ther. 1999;288:1117–1124. [PubMed] [Google Scholar]
- 102.Karakas M, Koenig W. Varespladib methyl, an oral phospholipase A2 inhibitor for the potential treatment of coronary artery disease. IDrugs. 2009;12:585–592. [PubMed] [Google Scholar]
- 103.Lee KL, Foley MA, Chen L, Behnke ML, Lovering FE, Kirincich SJ, Wang W, Shim J, Tam S, Shen MW, Khor S, Xu X, Goodwin DG, Ramarao MK, Nickerson-Nutter C, Donahue F, Ku MS, Clark JD, McKew JC. Discovery of Ecopladib, an indole inhibitor of cytosolic phospholipase A2alpha. J Med Chem. 2007;50:1380–1400. doi: 10.1021/jm061131z. [DOI] [PubMed] [Google Scholar]
- 104.McKew JC, Lee KL, Shen MW, Thakker P, Foley MA, Behnke ML, Hu B, Sum FW, Tam S, Hu Y, Chen L, Kirincich SJ, Michalak R, Thomason J, Ipek M, Wu K, Wooder L, Ramarao MK, Murphy EA, Goodwin DG, Albert L, Xu X, Donahue F, Ku MS, Keith J, Nickerson-Nutter CL, Abraham WM, Williams C, Hegen M, Clark JD. Indole cytosolic phospholipase A2 alpha inhibitors: discovery and in vitro and in vivo characterization of 4-{3-[5-chloro-2-(2-{[(3,4-dichlorobenzyl)sulfonyl]amino}ethyl)-1-(diphenylmethyl)-1H-indol-3-yl]propyl}benzoic acid, efipladib. J Med Chem. 2008;51:3388–3413. doi: 10.1021/jm701467e. [DOI] [PubMed] [Google Scholar]
- 105.McKew JC, Lee KL, Chen L, Vargas R, Clark JD, Williams C, Clerin V, Marusic S, Pong K. Inhibitors of cytosolic phospholipase A2. U.S. Patent, editor. 7557135B2. USA: US. 2006
- 106.Thotala D, Craft JM, Ferraro DJ, Kotipatruni RP, Bhave SR, Jaboin JJ, Hallahan DE. Cytosolic PhospholipaseA2 Inhibition with PLA-695 Radiosensitizes Tumors in Lung Cancer Animal Models. PLoS One. 2013;8:e69688. doi: 10.1371/journal.pone.0069688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baskakis C, Magrioti V, Cotton N, Stephens D, Constantinou-Kokotou V, Dennis EA, Kokotos G. Synthesis of polyfluoro ketones for selective inhibition of human phospholipase A2 enzymes. J Med Chem. 2008;51:8027–8037. doi: 10.1021/jm800649q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kokotos G, Hsu YH, Burke JE, Baskakis C, Kokotos CG, Magrioti V, Dennis EA. Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2. J Med Chem. 2010;53:3602–3610. doi: 10.1021/jm901872v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magrioti V, Nikolaou A, Smyrniotou A, Shah I, Constantinou-Kokotou V, Dennis EA, Kokotos G. New potent and selective polyfluoroalkyl ketone inhibitors of GVIA calcium-independent phospholipase A. Bioorg Med Chem. 2013;21:5823–5829. doi: 10.1016/j.bmc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blackie JA, Bloomer JC, Brown MJ, Cheng HY, Hammond B, Hickey DM, Ife RJ, Leach CA, Lewis VA, Macphee CH, Milliner KJ, Moores KE, Pinto IL, Smith SA, Stansfield IG, Stanway SJ, Taylor MA, Theobald CJ. The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg Med Chem Lett. 2003;13:1067–1070. doi: 10.1016/s0960-894x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Garcia HM, Oemrawsingh RM, Brugaletta S, Vranckx P, Shannon J, Davies R, Boersma E, Serruys PW. Darapladib effect on circulating high sensitive troponin in patients with acute coronary syndromes. Atherosclerosis. 2012;225:142–147. doi: 10.1016/j.atherosclerosis.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 112.Boutin JA, Ferry G. Autotaxin. Cell Mol Life Sci. 2009;66:3009–3021. doi: 10.1007/s00018-009-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jansen S, Stefan C, Creemers JW, Waelkens E, Van Eynde A, Stalmans W, Bollen M. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J Cell Sci. 2005;118:3081–3089. doi: 10.1242/jcs.02438. [DOI] [PubMed] [Google Scholar]
- 114.Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA, Houben AJ, van Zeijl L, Jansen S, Andries M, Hall T, Pegg LE, Benson TE, Kasiem M, Harlos K, Kooi CW, Smyth SS, Ovaa H, Bollen M, Morris AJ, Moolenaar WH, Perrakis A. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol. 2011;18:198–204. doi: 10.1038/nsmb.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishimasu H, Okudaira S, Hama K, Mihara E, Dohmae N, Inoue A, Ishitani R, Takagi J, Aoki J, Nureki O. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol. 2011;18:205–212. doi: 10.1038/nsmb.1998. [DOI] [PubMed] [Google Scholar]
- 116.Albers HM, Ovaa H. Chemical evolution of autotaxin inhibitors. Chem Rev. 2012;112:2593–2603. doi: 10.1021/cr2003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 118.Houben AJ, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011;30:557–565. doi: 10.1007/s10555-011-9319-7. [DOI] [PubMed] [Google Scholar]
- 119.Stortelers C, Kerkhoven R, Moolenaar WH. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics. 2008;9:387. doi: 10.1186/1471-2164-9-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Federico L, Pamuklar Z, Smyth SS, Morris AJ. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr Drug Targets. 2008;9:698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Willier S, Butt E, Grunewald TG. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: A focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol Cell. 2013:317–333. doi: 10.1111/boc.201300011. [DOI] [PubMed] [Google Scholar]
- 123.Barbayianni E, Magrioti V, Moutevelis-Minakakis P, Kokotos G. Autotaxin inhibitors: a patent review. Expert Opin Ther Pat. 2013 doi: 10.1517/13543776.2013.796364. [DOI] [PubMed] [Google Scholar]
- 124.van Meeteren LA, Brinkmann V, Saulnier-Blache JS, Lynch KR, Moolenaar WH. Anticancer activity of FTY720: phosphorylated FTY720 inhibits autotaxin, a metastasis-enhancing and angiogenic lysophospholipase D. Cancer Lett. 2008;266:203–208. doi: 10.1016/j.canlet.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferry G, Moulharat N, Pradere JP, Desos P, Try A, Genton A, Giganti A, Beucher-Gaudin M, Lonchampt M, Bertrand M, Saulnier-Blache JS, Tucker GC, Cordi A, Boutin JA. S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J Pharmacol Exp Ther. 2008;327:809–819. doi: 10.1124/jpet.108.141911. [DOI] [PubMed] [Google Scholar]
- 126.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, Uchiyama A, Murakami-Murofushi K, Koh E, Bandle RW, Byun HS, Bittman R, Fan D, Murph M, Mills GB, Tigyi G. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, Tigyi G, Prestwich GD. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2007;2:679–690. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gierse J, Thorarensen A, Beltey K, Bradshaw-Pierce E, Cortes-Burgos L, Hall T, Johnston A, Murphy M, Nemirovskiy O, Ogawa S, Pegg L, Pelc M, Prinsen M, Schnute M, Wendling J, Wene S, Weinberg R, Wittwer A, Zweifel B, Masferrer J. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J Pharmacol Exp Ther. 2010;334:310–317. doi: 10.1124/jpet.110.165845. [DOI] [PubMed] [Google Scholar]
- 129.Albers HM, van Meeteren LA, Egan DA, van Tilburg EW, Moolenaar WH, Ovaa H. Discovery and optimization of boronic acid based inhibitors of autotaxin. J Med Chem. 2010;53:4958–4967. doi: 10.1021/jm1005012. [DOI] [PubMed] [Google Scholar]
- 130.Albers HM, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris AJ, Smyth SS, Moolenaar WH, Ovaa H. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci U S A. 2010;107:7257–7262. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fells JI, Lee SC, Fujiwara Y, Norman DD, Lim KG, Tsukahara R, Liu J, Patil R, Miller DD, Kirby RJ, Nelson S, Seibel W, Papoian R, Parrill AL, Baker DL, Bittman R, Tigyi G. Hits of a high-throughput screen identify the hydrophobic pocket of autotaxin/lysophospholipase D as an inhibitory surface. Mol Pharmacol. 2013;84:415–424. doi: 10.1124/mol.113.087080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Topham MK, Epand RM. Mammalian diacylglycerol kinases: Molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta. 2009;90:416–424. doi: 10.1016/j.bbagen.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shulga YV, Topham MK, Epand RM. Regulation and functions of diacylglycerol kinases. Chem Rev. 2011;111:6186–6208. doi: 10.1021/cr1004106. [DOI] [PubMed] [Google Scholar]
- 134.Ávila-Flores A, Santos T, Rincón E, Mérida I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280:10091–10099. doi: 10.1074/jbc.M412296200. [DOI] [PubMed] [Google Scholar]
- 135.Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J Biol Chem. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 136.Luo B, Prescott SM, Topham MK. Diacylglycerol kinase zeta regulates phosphatidylinositol 4-phosphate 5-kinase Ialpha by a novel mechanism. Cell Signal. 2004;16:891–897. doi: 10.1016/j.cellsig.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 137.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamada K, Sakane F, Matsushima N, Kanoh H. EF-hand motifs of alpha, beta and gamma isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. The Biochem J. 1997;321:59–64. doi: 10.1042/bj3210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sakane F, Yamada K, Kanoh H. Different effects of sphingosine, R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS letters. 1989;255:409–413. doi: 10.1016/0014-5793(89)81134-2. [DOI] [PubMed] [Google Scholar]
- 140.Takeuchi H, Kanematsu T, Misumi Y, Sakane F, Konishi H, Kikkawa U, Watanabe Y, Katan M, Hirata M. Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase and a new 130 kDa protein. Biochim Biophys Acta. 1997;1359:275–285. doi: 10.1016/s0167-4889(97)00109-2. [DOI] [PubMed] [Google Scholar]
- 141.Thirugnanam S, Topham MK, Epand RM. Physiological implications of the contrasting modulation of the activities of the epsilon- and zeta-isoforms of diacylglycerol kinase. Biochemistry. 2001;40:10607–10613. doi: 10.1021/bi010609s. [DOI] [PubMed] [Google Scholar]
- 142.Luo B, Prescott SM, Topham MK. Association of diacylglycerol kinase zeta with protein kinase C alpha: spatial regulation of diacylglycerol signaling. J Cell Biol. 2003;160:929–937. doi: 10.1083/jcb.200208120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Houssa B, de Widt J, Kranenburg O, Moolenaar WH, van Blitterswijk WJ. Diacylglycerol kinase theta binds to and is negatively regulated by active RhoA. J Biol Chem. 1999;274:6820–6822. doi: 10.1074/jbc.274.11.6820. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi N, Hozumi Y, Ito T, Hosoya T, Kondo H, Goto K. Differential subcellular targeting and activity-dependent subcellular localization of diacylglycerol kinase isozymes in transfected cells. Eur J Cell Biol. 2007;86:433–444. doi: 10.1016/j.ejcb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Murakami T, Sakane F, Imai S, Houkin K, Kanoh H. Identification and characterization of two splice variants of human diacylglycerol kinase eta. J Biol Chem. 2003;278:34364–34372. doi: 10.1074/jbc.M301542200. [DOI] [PubMed] [Google Scholar]
- 146.Milne SB, Ivanova PT, Armstrong MD, Myers DS, Lubarda J, Shulga YV, Topham MK, Brown HA, Epand RM. Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry. 2008;47:9372–9379. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lung M, Shulga YV, Ivanova PT, Myers DS, Milne SB, Brown HA, Topham MK, Epand RM. Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J Biol Chem. 2009;284:31062–31073. doi: 10.1074/jbc.M109.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shulga YV, Loukov D, Ivanova PT, Milne SB, Myers DS, Hatch GM, Umeh G, Jalan D, Fullerton MD, Steinberg GR, Topham MK, Brown HA, Epand RM. Diacylglycerol kinase delta promotes lipogenesis. Biochemistry. 2013;52:7766–7776. doi: 10.1021/bi401178y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bishop WR, Ganong BR, Bell RM. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986;261:6993–7000. [PubMed] [Google Scholar]
- 150.de Chaffoy de Courcelles DC, Roevens P, Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985;260:15762–15770. [PubMed] [Google Scholar]
- 151.de Chaffoy de Courcelles D, Roevens P, Van Belle H, Kennis L, Somers Y, De Clerck F. The role of endogenously formed diacylglycerol in the propagation and termination of platelet activation. A biochemical and functional analysis using the novel diacylglycerol kinase inhibitor, R 59 949. J Biol Chem. 1989;264:3274–3285. [PubMed] [Google Scholar]
- 152.Rodriguez-Linares B, Walker T, Watson S. The diacylglycerol kinase inhibitor, R59949, potentiates secretion but not increased phosphorylation of a 47 kDalton protein in human platelets. Biochem Pharmacol. 1991;41:835–838. doi: 10.1016/0006-2952(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 153.Jiang Y, Sakane F, Kanoh H, Walsh JP. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharmacol. 2000;59:763–772. doi: 10.1016/s0006-2952(99)00395-0. [DOI] [PubMed] [Google Scholar]
- 154.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 155.Scott SA, Xiang Y, Mathews TP, Plumley HC, Myers DS, Armstrong MD, Tallman KA, O’Reilly MC, Lindsley CW, Brown HA. Regulation of Phospholipase D Activity and Phosphatidic Acid Production Following Purinergic (P2Y6) Receptor Stimulation. J Biol Chem. 2013 doi: 10.1074/jbc.M113.451708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sadeghi N, Gerber DE. Targeting the PI3K pathway for cancer therapy. Future Med Chem. 2012;4:1153–1169. doi: 10.4155/fmc.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Brit J Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wymann MP, Schultz C. The chemical biology of phosphoinositide 3-kinases. Chembiochem. 2012;13:2022–2035. doi: 10.1002/cbic.201200089. [DOI] [PubMed] [Google Scholar]
- 159.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 160.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 161.Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med. 2012;14:e1. doi: 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- 162.Kurtz JE, Ray-Coquard I. PI3 kinase inhibitors in the clinic: an update. Anticancer Res. 2012;32:2463–2470. [PubMed] [Google Scholar]
- 163.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 164.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, Vlahos CJ. Wortmannin, a Potent and Selective Inhibitor of Phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 165.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 167.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 168.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V, Berne P, Michot N, Cheuret D, Hoornaert C, Mathieu M. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396:663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 170.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 171.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Nat Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2008;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–117. doi: 10.1194/jlr.m200298-jlr200. [DOI] [PubMed] [Google Scholar]
- 175.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng S, Cravatt BF. Monoacylglycerol Lipase Regulates a Fatty Acid Network that Promotes Cancer Pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nomura D, Lombardi D, Chang J, Niessen S, Ward A, Long J, Hoover H, Cravatt B. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 179.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 180.Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–191. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Long JZ, Jin X, Adibekian A, Li W, Cravatt BF. Characterization of tunable piperidine and piperazine carbamates as inhibitors of endocannabinoid hydrolases. J Med Chem. 2010;53:1830–1842. doi: 10.1021/jm9016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chang J, Niphakis M, Lum K, Iii AC, Wang C, Matthews M, Niessen S, Buczynski M, Parsons L, Cravatt B. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aaltonen N, Savinainen J, Ribas C, Rönkkö J, Kuusisto A, Korhonen J, Navia-Paldanius D, Häyrinen J, Takabe P, Käsnänen H, Pantsar T, Laitinen T, Lehtonen M, Pasonen-Seppänen S, Poso A, Nevalainen T, Laitinen J. Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase. Chem Biol. 2013;20:379–390. doi: 10.1016/j.chembiol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 185.Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89:112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 186.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 189.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol. 2012;8:999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 191.Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, Appendino G, Saturnino C, Martin B, Razdan R, Di Marzo V. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta. 2006;1761:205–212. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 192.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: the serine hydrolases. Proc Nat Acad Sci U S A. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hoover HS, Blankman JL, Niessen S, Cravatt BF. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg Med Chem Letts. 2008;18:5838–5841. doi: 10.1016/j.bmcl.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Leung D, Hardouin C, Boger DL, Cravatt BF. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat Biotechnol. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- 195.Lee MW, Kraemer FB, Severson DL. Characterization of a partially purified diacylglycerol lipase from bovine aorta. Biochim Biophys Acta. 1995;1254:311–318. doi: 10.1016/0005-2760(94)00193-3. [DOI] [PubMed] [Google Scholar]
- 196.Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, Di Marzo V. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem. 2008;51:6970–6979. doi: 10.1021/jm800978m. [DOI] [PubMed] [Google Scholar]
- 197.Adibekian A, Martin BR, Wang C, Hsu KL, Bachovchin DA, Niessen S, Hoover H, Cravatt BF. Click-generated triazole ureas as ultrapotent in vivo-active serine hydrolase inhibitors. Nat Chem Biol. 2011;7:469–478. doi: 10.1038/nchembio.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 199.Vaughan M, Berger JE, Steinberg D. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J Biol Chem. 1964;239:401–409. [PubMed] [Google Scholar]
- 200.Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 201.Holm C, Kirchgessner TG, Svenson KL, Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C, Sparkes RS, Mohandas T, et al. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science. 1988;241:1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 202.Holst LS, Langin D, Mulder H, Laurell H, Grober J, Bergh A, Mohrenweiser HW, Edgren G, Holm C. Molecular cloning, genomic organization, and expression of a testicular isoform of hormone-sensitive lipase. Genomics. 1996;35:441–447. doi: 10.1006/geno.1996.0383. [DOI] [PubMed] [Google Scholar]
- 203.Mulder H, Holst LS, Svensson H, Degerman E, Sundler F, Ahren B, Rorsman P, Holm C. Hormone-sensitive lipase, the rate-limiting enzyme in triglyceride hydrolysis, is expressed and active in beta-cells. Diabetes. 1999;48:228–232. doi: 10.2337/diabetes.48.1.228. [DOI] [PubMed] [Google Scholar]
- 204.Small CA, Rogers MP, Goodacre JA, Yeaman SJ. Phosphorylation and activation of hormone-sensitive lipase in isolated macrophages. FEBS Lett. 1991;279:323–326. doi: 10.1016/0014-5793(91)80179-7. [DOI] [PubMed] [Google Scholar]
- 205.Khoo JC, Reue K, Steinberg D, Schotz MC. Expression of hormone-sensitive lipase mRNA in macrophages. J Lipid Res. 1993;34:1969–1974. [PubMed] [Google Scholar]
- 206.Kraemer FB, Shen WJ, Natu V, Patel S, Osuga J, Ishibashi S, Azhar S. Adrenal neutral cholesteryl ester hydrolase: identification, subcellular distribution, and sex differences. Endocrinology. 2002;143:801–806. doi: 10.1210/endo.143.3.8693. [DOI] [PubMed] [Google Scholar]
- 207.Kraemer FB, Patel S, Saedi MS, Sztalryd C. Detection of hormone-sensitive lipase in various tissues. I. Expression of an HSL/bacterial fusion protein and generation of anti-HSL antibodies. J Lipid Res. 1993;34:663–671. [PubMed] [Google Scholar]
- 208.Peters SJ, Dyck DJ, Bonen A, Spriet LL. Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am J Physiol Endocrinol Metab. 1998;275:E300–E309. doi: 10.1152/ajpendo.1998.275.2.E300. [DOI] [PubMed] [Google Scholar]
- 209.Kraemer FB, Tavangar K, Hoffman AR. Developmental regulation of hormone-sensitive lipase mRNA in the rat: changes in steroidogenic tissues. J Lipid Res. 1991;32:1303–1310. [PubMed] [Google Scholar]
- 210.Østerlund T, Beussman DJ, Julenius K, Poon PH, Linse S, Shabanowitz J, Hunt DF, Schotz MC, Derewenda ZS, Holm C. Domain identification of hormone-sensitive lipase by circular dichroism and fluorescence spectroscopy, limited proteolysis, and mass spectrometry. J Biol Chem. 1999;274:15382–15388. doi: 10.1074/jbc.274.22.15382. [DOI] [PubMed] [Google Scholar]
- 211.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 212.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 213.Carmen GY, Víctor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 214.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 215.Belfrage P, Fredrikson G, Nilsson NO, Stralfors P. Regulation of adipose tissue lipolysis: phosphorylation of hormones sensitive lipase in intact rat adipocytes. FEBS Lett. 1980;111:120–124. doi: 10.1016/0014-5793(80)80775-7. [DOI] [PubMed] [Google Scholar]
- 216.Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 217.Yeaman SJ. Hormone-sensitive lipase--a multipurpose enzyme in lipid metabolism. Biochim Biophys Acta. 1990;1052:128–132. doi: 10.1016/0167-4889(90)90067-n. [DOI] [PubMed] [Google Scholar]
- 218.Reynisdottir S, Eriksson M, Angelin B, Arner P. Impaired activation of adipocyte lipolysis in familial combined hyperlipidemia. J Clin Invest. 1995;95:2161–2169. doi: 10.1172/JCI117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Klannemark M, Orho M, Langin D, Laurell H, Holm C, Reynisdottir S, Arner P, Groop L. The putative role of the hormone-sensitive lipase gene in the pathogenesis of Type II diabetes mellitus and abdominal obesity. Diabetologia. 1998;41:1516–1522. doi: 10.1007/s001250051099. [DOI] [PubMed] [Google Scholar]
- 220.Hellström L, Langin D, Reynisdottir S, Dauzats M, Arner P. Adipocyte lipolysis in normal weight subjects with obesity among first-degree relatives. Diabetologia. 1996;39:921–928. doi: 10.1007/BF00403911. [DOI] [PubMed] [Google Scholar]
- 221.Thompson MP, Cooper ST, Parry BR, Tuckey JA. Increased expression of the mRNA for hormone-sensitive lipase in adipose tissue of cancer patients. Biochim Biophys Acta. 1993;1180:236–242. doi: 10.1016/0925-4439(93)90044-2. [DOI] [PubMed] [Google Scholar]
- 222.Jepson CA, Yeaman SJ. Inhibition of hormone-sensitive lipase by intermediary lipid metabolites. FEBS Lett. 1992;310:197–200. doi: 10.1016/0014-5793(92)81328-j. [DOI] [PubMed] [Google Scholar]
- 223.Fain JN, Shepherd RE. Free fatty acids as feedback regulators of adenylate cyclase and cyclic 3′:5′-AMP accumulation in rat fat cells. J Biol Chem. 1975;250:6586–6592. [PubMed] [Google Scholar]
- 224.Vertesy L, Beck B, Bronstrup M, Ehrlich K, Kurz M, Muller G, Schummer D, Seibert G. Cyclipostins, novel hormone-sensitive lipase inhibitors from Streptomyces sp. DSM 13381. II. Isolation, structure elucidation and biological properties. J Antibiot. 2002;55:480–494. doi: 10.7164/antibiotics.55.480. [DOI] [PubMed] [Google Scholar]
- 225.Ali YB, Verger R, Carriere F, Petry S, Muller G, Abousalham A. The molecular mechanism of human hormone-sensitive lipase inhibition by substituted 3-phenyl-5-alkoxy-1,3,4-oxadiazol-2-ones. Biochimie. 2012;94:137–145. doi: 10.1016/j.biochi.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 226.Lowe DB, Magnuson S, Qi N, Campbell AM, Cook J, Hong Z, Wang M, Rodriguez M, Achebe F, Kluender H, Wong WC, Bullock WH, Salhanick AI, Witman-Jones T, Bowling ME, Keiper C, Clairmont KB. In vitro SAR of (5-(2H)-isoxazolonyl) ureas, potent inhibitors of hormone-sensitive lipase. Bioorg Med Chem Lett. 2004;14:3155–3159. doi: 10.1016/j.bmcl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 227.Claus TH, Lowe DB, Liang Y, Salhanick AI, Lubeski CK, Yang L, Lemoine L, Zhu J, Clairmont KB. Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J Pharmacol Exp Ther. 2005;315:1396–1402. doi: 10.1124/jpet.105.086926. [DOI] [PubMed] [Google Scholar]
- 228.Slee DH, Bhat AS, Nguyen TN, Kish M, Lundeen K, Newman MJ, McConnell SJ. Pyrrolopyrazinedione-based inhibitors of human hormone-sensitive lipase. J Med Chem. 2003;46:1120–1122. doi: 10.1021/jm020460y. [DOI] [PubMed] [Google Scholar]
- 229.de Jong JC, Sørensen LG, Tornqvist H, Jacobsen P. Carbazates as potent inhibitors of hormone-sensitive lipase. Bioorg Med Chem Lett. 2004;14:1741–1744. doi: 10.1016/j.bmcl.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 230.Ebdrup S, Refsgaard HH, Fledelius C, Jacobsen P. Synthesis and structure-activity relationship for a novel class of potent and selective carbamate-based inhibitors of hormone selective lipase with acute in vivo antilipolytic effects. J Med Chem. 2007;50:5449–5456. doi: 10.1021/jm0607653. [DOI] [PubMed] [Google Scholar]
- 231.Ben-Ali Y, Muller G. Kinetic characterization and specific inhibition of hormone-sensitive lipase. 46th International Conference on the Bioscience of Lipids; 2005. [Google Scholar]
- 232.Ebdrup S, Sørensen LG, Olsen OH, Jacobsen P. Synthesis and structure-activity relationship for a novel class of potent and selective carbamoyl-triazole based inhibitors of hormone sensitive lipase. J Med Chem. 2004;47:400–410. doi: 10.1021/jm031004s. [DOI] [PubMed] [Google Scholar]
- 233.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 234.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 235.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 236.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, Zechner R, Zimmermann R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 237.Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, Ueno N, Sasaki S, Sawada F, Fujii M, Matoba Y, Sumiyoshi S, Kawate H, Takayanagi R. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93:2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- 238.Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- 239.Watt MJ, van Denderen BJ, Castelli LA, Bruce CR, Hoy AJ, Kraegen EW, Macaulay L, Kemp BE. Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol Endocrinol. 2008;22:1200–1212. doi: 10.1210/me.2007-0485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 240.Watt MJ, Steinberg GR. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J. 2008;414:313–325. doi: 10.1042/BJ20080305. [DOI] [PubMed] [Google Scholar]
- 241.Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ. Characterization of the human patatin-like phospholipase family. J Lipid Res. 2006;47:1940–1949. doi: 10.1194/jlr.M600185-JLR200. [DOI] [PubMed] [Google Scholar]
- 242.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 244.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 245.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 246.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 247.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, Crawford SE. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 248.Zimmerman R, Zechner R, Haemmerle G, Hofler G, Das S, Lass A. Modulation of adipose triglyceride lipase for prevention and treatment of cachexia, loss of weight and muscle atrophy and methods of screening thereof. 0230943. US Patent. 2012
- 249.Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, Ivkovic J, Heier C, Mrak I, Lass A, Hofler G, Fledelius C, Zechner R, Zimmermann R, Breinbauer R. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol. 2013;9:785–787. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 251.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 252.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 253.Lenfant N, Hotelier T, Bourne Y, Marchot P, Chatonnet A. Proteins with an alpha/beta hydrolase fold: Relationships between subfamilies in an ever-growing superfamily. Chem Biol Interact. 2013;203:266–268. doi: 10.1016/j.cbi.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 254.Simon GM, Cravatt BF. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J Biol Chem. 2010;285:11051–11055. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Navia-Paldanius D, Savinainen JR, Laitinen JT. Biochemical and pharmacological characterization of human alpha/beta-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12) J Lipid Res. 2012;53:2413–2424. doi: 10.1194/jlr.M030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 257.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud’homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, Eder C, Kumari M, Schoiswohl G, Wolinski H, Kohlwein SD, Zechner R, Zimmermann R, Oberer M. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–12298. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 261.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, Liu X, Graham MJ, Lee R, Crooke R, Shulman GI, Xue B, Shi H, Yu L. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lord CC, Betters JL, Ivanova PT, Milne SB, Myers DS, Madenspacher J, Thomas G, Chung S, Liu M, Davis MA, Lee RG, Crooke RM, Graham MJ, Parks JS, Brasaemle DL, Fessler MB, Brown HA, Brown JM. CGI-58/ABHD5-derived signaling lipids regulate systemic inflammation and insulin action. Diabetes. 2012;61:355–363. doi: 10.2337/db11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yamaguchi T. Crucial role of CGI-58/alpha/beta hydrolase domain-containing protein 5 in lipid metabolism. Biol Pharm Bull. 2010;33:342–345. doi: 10.1248/bpb.33.342. [DOI] [PubMed] [Google Scholar]
- 267.Andrews DL, Beames B, Summers MD, Park WD. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem J. 1988;252:199–206. doi: 10.1042/bj2520199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50:S63–68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- 270.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 271.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Browning JD, Cohen JC, Hobbs HH. Patatin-like phospholipase domain-containing 3 and the pathogenesis and progression of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1189–1192. doi: 10.1002/hep.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, Cohen JC, Hobbs HH. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest. 2012;122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, Wongsiriroj N, Nagy HM, Ivanova PT, Scott SA, Knittelfelder O, Rechberger GN, Birner-Gruenberger R, Eder S, Brown HA, Haemmerle G, Oberer M, Lass A, Kershaw EE, Zimmermann R, Zechner R. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Harris TE, Finck BN. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab. 2011;22:226–233. doi: 10.1016/j.tem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kennedy EP, Smith SW, Weiss SB. New synthesis of lecithin in an isolated enzyme system. Nature. 1956;178:594–595. doi: 10.1038/178594a0. [DOI] [PubMed] [Google Scholar]
- 279.Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 280.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 281.Langner CA, Birkenmeier EH, Ben-Zeev O, Schotz MC, Sweet HO, Davisson MT, Gordon JI. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J Biol Chem. 1989;264:7994–8003. [PubMed] [Google Scholar]
- 282.Gropler MC, Harris TE, Hall AM, Wolins NE, Gross RW, Han X, Chen Z, Finck BN. Lipin 2 is a liver-enriched phosphatidate phosphohydrolase enzyme that is dynamically regulated by fasting and obesity in mice. J Biol Chem. 2009;284:6763–6772. doi: 10.1074/jbc.M807882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Huffman TA, Mothe-Satney I, Lawrence JC. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Nat Acad Sci U S A. 2002;99:1047–1052. doi: 10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, Lawrence JC. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282:277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 285.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 286.Suviolahti E, Reue K, Cantor RM, Phan J, Gentile M, Naukkarinen J, Soro-Paavonen A, Oksanen L, Kaprio J, Rissanen A, Salomaa V, Kontula K, Taskinen MR, Pajukanta P, Peltonen L. Cross-species analyses implicate Lipin 1 involvement in human glucose metabolism. Hum Mol Genet. 2006;15:377–386. doi: 10.1093/hmg/ddi448. [DOI] [PubMed] [Google Scholar]
- 287.Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN, Phan J, Spencer HJ, McGehee RE, Reue K, Kern PA. Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor gamma activation. Diabetes. 2006;55:2811–2818. doi: 10.2337/db05-1688. [DOI] [PubMed] [Google Scholar]
- 288.Croce MA, Eagon JC, LaRiviere LL, Korenblat KM, Klein S, Finck BN. Hepatic lipin 1beta expression is diminished in insulin-resistant obese subjects and is reactivated by marked weight loss. Diabetes. 2007;56:2395–2399. doi: 10.2337/db07-0480. [DOI] [PubMed] [Google Scholar]
- 289.Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem Rev. 2011;111:6359–6386. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kennedy EP. Biosynthesis of complex lipids. Fed Proc. 1961;20:934–940. [PubMed] [Google Scholar]
- 291.Dircks L, Sul HS. Acyltransferases of de novo glycerophospholipid biosynthesis. Prog Lipid Res. 1999;38:461–479. doi: 10.1016/s0163-7827(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 292.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 293.Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res. 2008;49:2079–2088. doi: 10.1194/jlr.R800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 294.Pellon-Maison M, Montanaro MA, Coleman RA, Gonzalez-Baro MR. Mitochondrial glycerol-3-P acyltransferase 1 is most active in outer mitochondrial membrane but not in mitochondrial associated vesicles (MAV) Biochim Biophys Acta. 2007;1771:830–838. doi: 10.1016/j.bbalip.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Karlsson EA, Wang S, Shi Q, Coleman RA, Beck MA. Glycerol-3-phosphate acyltransferase 1 is essential for the immune response to infection with coxsackievirus B3 in mice. J Nutr. 2009;139:779–783. doi: 10.3945/jn.108.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hammond LE, Albright CD, He L, Rusyn I, Watkins SM, Doughman SD, Lemasters JJ, Coleman RA. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol. 2007;82:210–219. doi: 10.1016/j.yexmp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang S, Lee DP, Gong N, Schwerbrock NM, Mashek DG, Gonzalez-Baro MR, Stapleton C, Li LO, Lewin TM, Coleman RA. Cloning and functional characterization of a novel mitochondrial N-ethylmaleimide-sensitive glycerol-3-phosphate acyltransferase (GPAT2) Arch Biochem Biophys. 2007;465:347–358. doi: 10.1016/j.abb.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cao J, Li JL, Li D, Tobin JF, Gimeno RE. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc Natl Acad Sci U S A. 2006;103:19695–19700. doi: 10.1073/pnas.0609140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nagle CA, Vergnes L, Dejong H, Wang S, Lewin TM, Reue K, Coleman RA. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6−/− mice. J Lipid Res. 2008;49:823–831. doi: 10.1194/jlr.M700592-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chen YQ, Kuo MS, Li S, Bui HH, Peake DA, Sanders PE, Thibodeaux SJ, Chu S, Qian YW, Zhao Y, Bredt DS, Moller DE, Konrad RJ, Beigneux AP, Young SG, Cao G. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J Biol Chem. 2008;283:10048–10057. doi: 10.1074/jbc.M708151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 303.Wydysh EA, Medghalchi SM, Vadlamudi A, Townsend CA. Design and synthesis of small molecule glycerol 3-phosphate acyltransferase inhibitors. J Med Chem. 2009;52:3317–3327. doi: 10.1021/jm900251a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kuhajda FP, Aja S, Tu Y, Han WF, Medghalchi SM, El Meskini R, Landree LE, Peterson JM, Daniels K, Wong K, Wydysh EA, Townsend CA, Ronnett GV. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2011;301:R116–130. doi: 10.1152/ajpregu.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wydysh EA, Vadlamudi A, Medghalchi SM, Townsend CA. Design, synthesis, and biological evaluation of conformationally constrained glycerol 3-phosphate acyltransferase inhibitors. Bioorg Med Chem. 2010;18:6470–6479. doi: 10.1016/j.bmc.2010.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kanfer J, Kennedy EP. Metabolism and Function of Bacterial Lipids. I. Metabolism of Phospholipids in Escherichia Coli B. J Biol Chem. 1963;238:2919–2922. [PubMed] [Google Scholar]
- 307.Kanfer J, Kennedy EP. Metabolism and Function of Bacterial Lipids. II. Biosynthesis of Phospholipids in Escherichia Coli. J Biol Chem. 1964;239:1720–1726. [PubMed] [Google Scholar]
- 308.West J, Tompkins CK, Balantac N, Nudelman E, Meengs B, White T, Bursten S, Coleman J, Kumar A, Singer JW, Leung DW. Cloning and expression of two human lysophosphatidic acid acyltransferase cDNAs that enhance cytokine-induced signaling responses in cells. DNA Cell Biol. 1997;16:691–701. doi: 10.1089/dna.1997.16.691. [DOI] [PubMed] [Google Scholar]
- 309.Leung DW. The structure and functions of human lysophosphatidic acid acyltransferases. Front Biosci. 2001;6:D944–953. doi: 10.2741/leung. [DOI] [PubMed] [Google Scholar]
- 310.Eberhardt C, Gray PW, Tjoelker LW. Human lysophosphatidic acid acyltransferase. cDNA cloning, expression, and localization to chromosome 9q34.3. J Biol Chem. 1997;272:20299–20305. doi: 10.1074/jbc.272.32.20299. [DOI] [PubMed] [Google Scholar]
- 311.Bonham L, Leung DW, White T, Hollenback D, Klein P, Tulinsky J, Coon M, de Vries P, Singer JW. Lysophosphatidic acid acyltransferase-beta: a novel target for induction of tumour cell apoptosis. Expert Opin Ther Targets. 2003;7:643–661. doi: 10.1517/14728222.7.5.643. [DOI] [PubMed] [Google Scholar]
- 312.Coon M, Ball A, Pound J, Ap S, Hollenback D, White T, Tulinsky J, Bonham L, Morrison DK, Finney R, Singer JW. Inhibition of lysophosphatidic acid acyltransferase beta disrupts proliferative and survival signals in normal cells and induces apoptosis of tumor cells. Mol Cancer Ther. 2003;2:1067–1078. [PubMed] [Google Scholar]
- 313.Hollenback D, Bonham L, Law L, Rossnagle E, Romero L, Carew H, Tompkins CK, Leung DW, Singer JW, White T. Substrate specificity of lysophosphatidic acid acyltransferase beta -- evidence from membrane and whole cell assays. J Lipid Res. 2006;47:593–604. doi: 10.1194/jlr.M500435-JLR200. [DOI] [PubMed] [Google Scholar]
- 314.Pagel JM, Laugen C, Bonham L, Hackman RC, Hockenbery DM, Bhatt R, Hollenback D, Carew H, Singer JW, Press OW. Induction of apoptosis using inhibitors of lysophosphatidic acid acyltransferase-beta and anti-CD20 monoclonal antibodies for treatment of human non-Hodgkin’s lymphomas. Clin Cancer Res. 2005;11:4857–4866. doi: 10.1158/1078-0432.CCR-04-2352. [DOI] [PubMed] [Google Scholar]
- 315.Hideshima T, Chauhan D, Hayashi T, Podar K, Akiyama M, Mitsiades C, INM, Gong B, Bonham L, de Vries P, Munshi N, Richardson PG, Singer JW, Anderson KC. Antitumor activity of lysophosphatidic acid acyltransferase-beta inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. 2003;63:8428–8436. [PubMed] [Google Scholar]
- 316.Gong B, Hong F, Kohm C, Jenkins S, Tulinsky J, Bhatt R, De Vries P, Singer JW, Klein P. Synthesis, SAR, and antitumor properties of diamino-C, N-diarylpyrimidine positional isomers: inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett. 2004;14:2303–2308. doi: 10.1016/j.bmcl.2004.01.104. [DOI] [PubMed] [Google Scholar]
- 317.Gong B, Hong F, Kohm C, Bonham L, Klein P. Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett. 2004;14:1455–1459. doi: 10.1016/j.bmcl.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 318.Kennedy EP. Metabolism of lipids. Annu Rev Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- 319.Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem. 1980;49:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]
- 320.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 322.Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ. DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem. 2001;276:38862–38869. doi: 10.1074/jbc.M106168200. [DOI] [PubMed] [Google Scholar]
- 323.Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- 324.Haagsman HP, de Haas CG, Geelen MJ, van Golde LM. Regulation of triacylglycerol synthesis in the liver: a decrease in diacylglycerol acyltransferase activity after treatment of isolated rat hepatocytes with glucagon. Biochim Biophys Acta. 1981;664:74–81. doi: 10.1016/0005-2760(81)90029-1. [DOI] [PubMed] [Google Scholar]
- 325.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 328.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 329.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 330.Wurie HR, Buckett L, Zammit VA. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 2012;279:3033–3047. doi: 10.1111/j.1742-4658.2012.08684.x. [DOI] [PubMed] [Google Scholar]
- 331.Dow RL. Acyl-CoA; diacylglycerol acyltransferase-1 inhibition as an approach to the treatment of type 2 diabetes. In: Jones RM, Thurston DE, Rotelle D, Guiccione S, Martinez A, Fox D, Gannelin R, editors. New therapeutic strategies for type2 diabetes: small molecule approaches. RSC; Cambridge, UK: 2012. pp. 215–249. [Google Scholar]
- 332.Matsuda D, Tomoda H. DGAT inhibitors for obesity. Curr Opin Investig Drugs. 2007;8:836–841. [PubMed] [Google Scholar]
- 333.Tabata N, Ito M, Tomoda H, Omura S. Xanthohumols, diacylglycerol acyltransferase inhibitors, from Humulus lupulus. Phytochemistry. 1997;46:683–687. doi: 10.1016/s0031-9422(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 334.Tomoda H, Ohyama Y, Abe T, Tabata N, Namikoshi M, Yamaguchi Y, Masuma R, Omura S. Roselipins, inhibitors of diacylglycerol acyltransferase, produced by Gliocladium roseum KF-1040. J Antibiot. 1999;52:689–694. doi: 10.7164/antibiotics.52.689. [DOI] [PubMed] [Google Scholar]
- 335.Chung MY, Rho MC, Ko JS, Ryu SY, Jeune KH, Kim K, Lee HS, Kim YK. In vitro inhibition of diacylglycerol acyltransferase by prenylflavonoids from Sophora flavescens. Planta Med. 2004;70:258–260. doi: 10.1055/s-2004-815545. [DOI] [PubMed] [Google Scholar]
- 336.Lee SW, Kim K, Rho MC, Chung MY, Kim YH, Lee S, Lee HS, Kim YK. New Polyacetylenes, DGAT inhibitors from the roots of Panax ginseng. Planta Med. 2004;70:197–200. doi: 10.1055/s-2004-815534. [DOI] [PubMed] [Google Scholar]
- 337.Ko JS, Ryu SY, Kim YS, Chung MY, Kang JS, Rho MC, Lee HS, Kim YK. Inhibitory activity of diacylglycerol acyltransferase by tanshinones from the root of Salvia miltiorrhiza. Arch Pharm Res. 2002;25:446–448. doi: 10.1007/BF02976599. [DOI] [PubMed] [Google Scholar]
- 338.Rustan AC, Nossen JO, Christiansen EN, Drevon CA. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J Lipid Res. 1988;29:1417–1426. [PubMed] [Google Scholar]
- 339.Oh WK, Lee C, Seo JH, Chung MY, Cui L, Fomum ZT, Kang JS, Lee HS. Diacylglycerol acyltransferase-inhibitory compounds from Erythrina senegalensis. Arch Pharm Res. 2009;32:43–47. doi: 10.1007/s12272-009-1116-2. [DOI] [PubMed] [Google Scholar]
- 340.Park HR, Yoo MY, Seo JH, Kim IS, Kim NY, Kang JY, Cui L, Lee CS, Lee CH, Lee HS. Sesquiterpenoids isolated from the flower buds of Tussilago farfara L. inhibit diacylglycerol acyltransferase. J Agric Food Chem. 2008;56:10493–10497. doi: 10.1021/jf801978r. [DOI] [PubMed] [Google Scholar]
- 341.Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res. 2004;45:1835–1845. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 342.Smith R, Campbell AM, Coish M, Dai J, Lowe D, O’Connor S, Su N, Wang G, Zhang M, Zhu L. Preparation and use of aryl alkyl acid derivatives for the treatment of obesity. 0224997 A1. US Pat. 2004
- 343.Zhao G, Souers AJ, Voorbach M, Falls HD, Droz B, Brodjian S, Lau YY, Iyengar RR, Gao J, Judd AS, Wagaw SH, Ravn MM, Engstrom KM, Lynch JK, Mulhern MM, Freeman J, Dayton BD, Wang X, Grihalde N, Fry D, Beno DW, Marsh KC, Su Z, Diaz GJ, Collins CA, Sham H, Reilly RM, Brune ME, Kym PR. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J Med Chem. 2008;51:380–383. doi: 10.1021/jm7013887. [DOI] [PubMed] [Google Scholar]
- 344.Fox B, Furukawa N, Hao X, Ilo K, Inaba T, Jackson S, Kayser F. Fused bicyclic nitrogen containing heterocycles. 047755. WO Pat. 2004
- 345.Fox BM, Iio K, Li K, Choi R, Inaba T, Jackson S, Sagawa S, Shan B, Tanaka M, Yoshida A, Kayser F. Discovery of pyrrolopyridazines as novel DGAT1 inhibitors. Bioorg Med Chem Lett. 2010;20:6030–6033. doi: 10.1016/j.bmcl.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 346.Dow RL, Munchhoff MJ. 4-Amino-7,8-dihydropyrido[4,3-D]pyrimidin-5(6H)-one derivatives. 0197590 A1. US Pat. 2010
- 347.Barlind JG, Bauer UA, Birch AM, Birtles S, Buckett LK, Butlin RJ, Davies RD, Eriksson JW, Hammond CD, Hovland R, Johannesson P, Johansson MJ, Kemmitt PD, Lindmark BT, Morentin Gutierrez P, Noeske TA, Nordin A, O’Donnell CJ, Petersson AU, Redzic A, Turnbull AV, Vinblad J. Design and optimization of pyrazinecarboxamide-based inhibitors of diacylglycerol acyltransferase 1 (DGAT1) leading to a clinical candidate dimethylpyrazinecarboxamide phenylcyclohexylacetic acid (AZD7687) J Med Chem. 2012;55:10610–10629. doi: 10.1021/jm301296t. [DOI] [PubMed] [Google Scholar]
- 348.Birch AM, Pal S, Pettersen A, Tomkinson GP. 1,3,4-Oxadiazole derivatives and their uses to treat diabetes. 070343. WO Pat. 2010
- 349.McCoull W, Addie MS, Birch AM, Birtles S, Buckett LK, Butlin RJ, Bowker SS, Boyd S, Chapman S, Davies RD, Donald CS, Green CP, Jenner C, Kemmitt PD, Leach AG, Moody GC, Gutierrez PM, Newcombe NJ, Nowak T, Packer MJ, Plowright AT, Revill J, Schofield P, Sheldon C, Stokes S, Turnbull AV, Wang SJ, Whalley DP, Wood JM. Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. Bioorg Med Chem Lett. 2012;22:3873–3878. doi: 10.1016/j.bmcl.2012.04.117. [DOI] [PubMed] [Google Scholar]
- 350.Huang Y, Sun C, Lawrence MR, Ewing WR, Turdi H. Triazolo compounds useful as DGAT1 inhibitors. 126624. WO Pat. 2009
- 351.Qian Y, Wertheimer SJ, Ahmad M, Cheung AW, Firooznia F, Hamilton MM, Hayden S, Li S, Marcopulos N, McDermott L, Tan J, Yun W, Guo L, Pamidimukkala A, Chen Y, Huang KS, Ramsey GB, Whittard T, Conde-Knape K, Taub R, Rondinone CM, Tilley J, Bolin D. Discovery of orally active carboxylic acid derivatives of 2-phenyl-5-trifluoromethyloxazole-4-carboxamide as potent diacylglycerol acyltransferase-1 inhibitors for the potential treatment of obesity and diabetes. J Med Chem. 2011;54:2433–2446. doi: 10.1021/jm101580m. [DOI] [PubMed] [Google Scholar]
- 352.Mougenot P, Namane C, Fett E, Camy F, Dadji-Faihun R, Langot G, Monseau C, Onofri B, Pacquet F, Pascal C, Crespin O, Ben-Hassine M, Ragot JL, Van-Pham T, Philippo C, Chatelain-Egger F, Peron P, Le Bail JC, Guillot E, Chamiot-Clerc P, Chabanaud MA, Pruniaux MP, Schmidt F, Venier O, Nicolai E, Viviani F. Thiadiazoles as new inhibitors of diacylglycerol acyltransferase type 1. Bioorg Med Chem Lett. 2012;22:2497–2502. doi: 10.1016/j.bmcl.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 353.Bali U, Barba O, Dawson G, Gattrell WT, Horswill JG, Pan DA, Procter MJ, Rasamison CM, Sambrook Smith CP, Taylor-Warne A, Wong-Kai-In P. Design and synthesis of potent carboxylic acid DGAT1 inhibitors with high cell permeability. Bioorg Med Chem Lett. 2012;22:824–828. doi: 10.1016/j.bmcl.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 354.Lee K, Goo JI, Jung HY, Kim M, Boovanahalli SK, Park HR, Kim MO, Kim DH, Lee HS, Choi Y. Discovery of a novel series of benzimidazole derivatives as diacylglycerol acyltransferase inhibitors. Bioorg Med Chem Lett. 2012;22:7456–7460. doi: 10.1016/j.bmcl.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 355.Motiwala H, Kandre S, Birar V, Kadam KS, Rodge A, Jadhav RD, Mahesh Kumar Reddy M, Brahma MK, Deshmukh NJ, Dixit A, Doshi L, Gupte A, Gangopadhyay AK, Vishwakarma RA, Srinivasan S, Sharma M, Nemmani KV, Sharma R. Exploration of pyridine containing heteroaryl analogs of biaryl ureas as DGAT1 inhibitors. Bioorg Med Chem Lett. 2011;21:5812–5817. doi: 10.1016/j.bmcl.2011.07.109. [DOI] [PubMed] [Google Scholar]
- 356.Jadhav RD, Kadam KS, Kandre S, Guha T, Reddy MM, Brahma MK, Deshmukh NJ, Dixit A, Doshi L, Potdar N, Enose AA, Vishwakarma RA, Sivaramakrishnan H, Srinivasan S, Nemmani KV, Gupte A, Gangopadhyay AK, Sharma R. Synthesis and biological evaluation of isoxazole, oxazole, and oxadiazole containing heteroaryl analogs of biaryl ureas as DGAT1 inhibitors. Eur J Med Chem. 2012;54:324–342. doi: 10.1016/j.ejmech.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 357.Kadam KS, Jadhav RD, Kandre S, Guha T, Reddy MM, Brahma MK, Deshmukh NJ, Dixit A, Doshi L, Srinivasan S, Devle J, Damre A, Nemmani KV, Gupte A, Sharma R. Evaluation of thiazole containing biaryl analogs as diacylglycerol acyltransferase 1 (DGAT1) inhibitors. Eur J Med Chem. 2013;65:337–347. doi: 10.1016/j.ejmech.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 358.Nakada Y, Aicher TD, Le Huerou Y, Turner T, Pratt SA, Gonzales SS, Boyd SA, Miki H, Yamamoto T, Yamaguchi H, Kato K, Kitamura S. Novel acyl coenzyme A (CoA): diacylglycerol acyltransferase-1 inhibitors: synthesis and biological activities of diacylethylenediamine derivatives. Bioorg Med Chem. 2010;18:2785–2795. doi: 10.1016/j.bmc.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 359.Serano-Wu MH, Kwan YS, Liu W. New compounds. 126957 A2 WO Pat. 2007